| FEIS Home Page |
 |
|
| Figure 1. Mule deer on Deer Flat National Wildlife Refuge, Idaho. Photo courtesy of Addison Mohler, USFWS. |
Mule deer group:
Odocoileus hemionus subsp. hemionus (Rafinesque), Rocky mountain mule deer [121,215,363]
Odocoileus hemionus subsp. californicus (Caton), California mule deer [121,215,363]
Odocoileus hemionus subsp. cerrosensis Merriam, Cedros Island mule deer [363]
Odocoileus hemionus subsp. eremicus (Mearns), desert mule deer [215]
Odocoileus hemionus subsp. fuliginatus (Cowan), southern mule deer [121,215,363]
Odocoileus hemionus subsp. peninsulae (Lydekker), peninsula mule deer [121,215]
Odocoileus hemionus subsp. sheldoni Goldman, Tiburon Island mule deer [121,363]
The taxonomic status of the Cedros Island [215,347] and Tiburon Island [121,215,347] mule deer is in doubt, and the Inyo mule deer (O. hemionus subsp. inyoensis (Cowan)) [121,363] is generally no longer recognized as a distinct subspecies [215,347].
Black-tailed deer group:
Odocoileus hemionus subsp. columbianus (Richardson), Columbian black-tailed deer [121,215,363]
Odocoileus hemionus subsp. sitkensis Merriam, Sitka black-tailed deer [121,215,363]
Subspecies are distinguished by body size, pelage color, skull form and dentition, size and shape of antlers, behavior, and geographical distribution [5,119,121,215,347]. However, the distinction of North American subspecies has been brought into question by genetic analyses. Cronin and others [84] found variation in mitochondrial DNA between mule deer and black-tailed deer groups but not between Columbian black-tailed deer and Sitka black-tailed deer. Translocations have led to intermixing of subspecies in some areas [347], and subspecies may interbreed where they coexist [83,84]. See Geist [121] for more information about subspecies distinctions.
Mule deer and white-tailed deer (O. virginianus) may hybridize where their ranges overlap [83,85,155,316], although hybrids are rare in the wild [121]. The survival of hybrids in captivity [7] and in the wild [121] is poor. For more information about mule deer and white-tailed deer hybridization, see Geist [121].
This review synthesizes information about mule deer and black-tailed deer at the group level, when possible. Collectively, they are referred to as mule deer throughout this review. In some publications the term "deer" was used to describe mule deer and white-tailed deer in combination. In those cases, this review does the same.
SYNONYMS: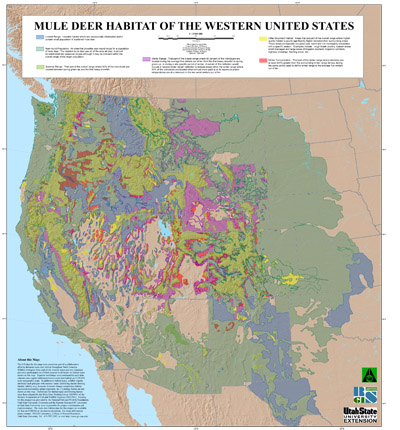 |
Figure 2. Mule deer distribution and habitat in the western United States. Map courtesy of Remote Sensing and GIS Laboratory, Mule Deer of the Western United States. 2005. Utah State University, Logan, Utah. http://www.gis.usu.edu. Click on map for a larger image. |
Mule deer are native to western North America. Scattered populations occur as far east as western Minnesota and Iowa. In Mexico, they occur south to Baja California (including some islands in the Sea of Cortez) and the southern end of the Mexican Plateau. They have been introduced in Hawaii and several islands in Prince William Sound [215]. Major gaps in mule deer distribution occur in the Mojave and Sonoran deserts in southeastern California, southern Nevada, southwestern Arizona, and northwestern Sonora, Mexico; the high-elevation or cold deserts and plains grasslands of northeastern Arizona and southeastern Utah; the Central Valley of California; and probably the Great Salt Lake desert region. Otherwise, mule deer occur in all of the biomes of western North America north of central Mexico, except the arctic tundra [7,345]. Within the mule deer's distribution, black-tailed deer occur along the northern Pacific Coast from central California north to southern Alaska [215].
States and provinces:
United States: AK, AZ, CA, CO, ID, KS, MT, NE, NV, NM, ND, OK, OR, SD, TX, UT, WA, WY
Canada: AB, BC, MB, NT, SK, YT [234]
Mexico [215]
PLANT COMMUNITIES:
Mule deer are the most widely distributed and abundant of all large mammal species in western North America. They occur in diverse habitats from moist, dense coniferous forests to dry, open plains and deserts, and alpine habitats [129]. A review stated that "the multitudinous habitats of the mule and black-tailed deer are so diverse as to defy generalization" [347]. Mule deer occur in tallgrass, mixed-grass, and shortgrass prairies of the Great Plains, in shrublands, woodlands, and forests of the Rocky Mountains, and in sagebrush (Artemisia spp.) communities, pinyon-juniper (Pinus-Juniperus spp.) woodlands, and desert scrub of the Southwest. They are absent, however, from many desert communities of the Southwest because succulent forage occurs too infrequently to maintain populations [347]. In terms of elevation, they occur from coastal communities up to subalpine and alpine communities [119,215,347]. See the Fire Regime Table for a list of plant communities in which mule deer may occur and information on the fire regimes associated with those communities.
Canadian boreal forest: Mule deer occur in boreal forests of the 4 major forest types: quaking aspen (Populus tremuloides) parkland; mixed woodlands of quaking aspen, balsam poplar (P. balsamea), paper birch (Betula papyrifera), resin birch (B. glandulosa), white spruce (Picea glauca), and balsam fir (Abies balsamea); lower foothills dominated by lodgepole pine (Pinus contorta), quaking aspen, and balsam fir with white spruce and black spruce (Picea mariana); and northern foothills of black spruce, white spruce, subalpine fir (A. lasiocarpa), and pine (Pinus spp.). The boreal forest-subarctic woodland ecotone marks the northern limits of mule deer's distribution. In the boreal forest ecosystem, mule deer prefer open grassland-parkland types [347]. In Yukon, mule deer habitats are largely open, south-facing grassy slopes bordered by quaking aspen, and recently burned areas. Mule deer expanded their natural range into Yukon around the 1930s [152].
Alaska, Pacific Northwest, and California: In Alaska, black-tailed deer occur in Sitka spruce (Picea sitchensis), western redcedar (Thuja plicata), and western hemlock (Tsuga heterophylla) forests. Inland these forests transition to alpine tundra [135]. In coastal regions of northern California and southern Oregon, redwood (Sequoia sempervirens) and Douglas-fir (Pseudotsuga menziesii) dominate black-tailed deer habitats. To the north, throughout habitats in Washington, British Columbia, and Alaska, Sitka spruce, western redcedar, and western hemlock dominate [135,215,347]. Inland in northern California, Oregon, Washington, and British Columbia, black-tailed deer occur in western redcedar, incense-cedar (Calocedrus decurrens), western hemlock, and/or Douglas-fir forests. On the Coast Ranges, they occur in Douglas-fir and silver fir (A. alba) forests [135]. In the Cascades Range, silver fir-Douglas-fir, subalpine fir-mountain hemlock (T. mertensiana), and ponderosa pine (Pinus ponderosa)-shrub forests are common mule deer habitats, whereas in the Sierra Nevada, mule deer commonly occur in mixed-conifer forests (white fir (A. concolor), incense-cedar, sugar pine (P. lambertiana), ponderosa pine, and Douglas-fir), red fir (Abies magnifica) forests, and subalpine forests (lodgepole pine, whitebark pine (P. albicaulis), foxtail pine (P. balfouriana), and/or mountain hemlock) [347,348]. Throughout coastal and inland California, mule and black-tailed deer occur in mixed-evergreen forests comprised of incense-cedar, Pacific madrone (Arbutus menziesii), California bay (Umbellularia californica), Coulter pine (P. coulteri), canyon live oak (Quercus chrysolepis), and/or coast live oak (Q. agrifolia) [52,347]. Throughout California, mule deer are particularly common in oak woodlands and chaparral. California oak woodlands are dominated by a mixture of oaks including coast live oak, canyon live oak, blue oak (Q. douglasii), valley oak (Q. lobata), and interior live oak (Q. wislizeni), and pines such as gray pine (Pinus sabiniana) and Coulter pine [347]. California chaparral communities occupied may be monospecific communities dominated by chamise (Adenostoma fasciculatum), manzanita (Arctostaphylos spp.), or ceanothus (Ceanothus spp.) or diverse mixtures with oaks (Quercus spp.) and other shrubs [340,347]. From California north to Washington and east to Wyoming, mule deer occur in sagebrush steppe. Antelope bitterbrush (Purshia tridentata) and snowberry (Symphoricarpos spp.) are important shrubs browsed by mule deer in this ecosystem and may be codominant in some areas [287,347].
Southwest: Mule deer occur in a range of habitats in the Southwest, including desert shrublands at the lowest elevations, semidesert shrubland-grassland communities, chaparral, mountain shrub, woodlands at midelevations, and some forests at high elevations [347]. Desert grasslands without shrubs do not have mule deer unless they contain rugged topography or riparian areas. Dry washes are important to mule deer in semidesert grasslands because they provide food as well as resting, escape, and travel cover throughout the year. In parts of the Mojave, Sonoran, and Chihuahuan deserts, mule deer are restricted almost entirely to riparian habitats [290]. In the Sonoran Desert, they are most abundant on upper bajadas in desert scrub either in or near ecotones with interior chaparral, grassland, or woodland [340]. Interior Arizona chaparral provides good year-round habitat for mule deer [290,345].
Great Basin: In the Great Basin, mule deer occur in semidesert shrublands of sagebrush, saltbush (Atriplex spp.), Stansbury cliffrose (Purshia mexicana var. stansburiana), and winterfat (Krascheninnikovia lanata) [215,347]. At high elevations, mule deer occur in juniper-pinyon woodlands and in forests of lodgepole, ponderosa, Great Basin bristlecone (P. longaeva), and/or limber (P. flexilis) pine [101,347]. Great Basin conifer woodland [53,290] and Great Basin montane scrubland [54] also provide good habitat for mule deer. Pinyon-juniper woodlands are used as year-long mule deer rangeland but are particularly valuable as winter rangeland [290,305]. Montane and subalpine conifer forests, particularly those near mountain meadows or quaking aspen communities, serve primarily as summer rangelands [51,290].
Rocky Mountains: In northern mountainous areas of the West, montane and subalpine forest communities dominate summer ranges and open, shrub-dominated slopes and ridges are the primary winter rangeland [215]. Forests of quaking aspen, grand fir (A. grandis), western larch (Larix laricina), western white pine (P. monticola), western redcedar, and/or western hemlock, meadows, and alpine communities are common mule deer habitats. In valleys and piedmonts of the Rocky Mountain region, grasslands and open ponderosa pine forests are common mule deer habitats [347,348]. Sagebrush steppe, juniper-pinyon woodland, and true mountain-mahogany (Cercocarpus montanus)/oak scrub are the most extensive winter rangeland types in northern mountainous regions [66,348].
Mule deer use a wide variety of habitats in southern mountainous areas. The "most significant" are sagebrush, juniper-pinyon woodland, mountain shrub, montane forest, and subalpine forest. At low elevations, big sagebrush (A. tridentata), juniper (e.g., oneseed (Juniperus monosperma), Utah (J. osteosperma), alligator (J. deppeana), and Rocky Mountain juniper (J. scopulorum)), and pinyon occur in various combinations. The true mountain-mahogany/oak shrub type and many pine and Douglas-fir types occur at midelevations. In subalpine forests, subalpine fir, blue spruce (P. pungens), and Engelmann spruce (P. engelmannii) are most prevalent to the south and lodgepole pine, subalpine fir, and Engelmann spruce are most prevalent to the north. Mule deer occur in quaking aspen habitats in montane and lower subalpine zones [347].
Great Plains: In the prairies of the northern United States and southern Canada, rough, forested, or nonforested breaks along river drainages, badlands, and shrubby stream courses and draws—especially near agricultural lands—provide mule deer habitat. Level and rolling grasslands provide poor habitat for mule deer [215,287,347]. Mule deer populations diminish abruptly at the transition from shortgrass or mixed-grass prairie to tallgrass prairie [347]. Mule deer occur in the quaking aspen parklands of Alberta and Saskatchewan [287]. Mule deer prefer shrubby draws dominated by western snowberry (S. occidentalis), common snowberry (S. albus), silver buffaloberry (Shepherida argentea), chokecherry (Prunus virginiana), golden currant (Ribes aureum), and rose (Rosa spp.); hardwood draws dominated by green ash (Fraxinus pennsylvanica), American elm (Ulmus americana), boxelder (Acer negundo), and hackberry (Celtis occidentalis); and floodplains dominated by eastern cottonwood (Populus deltoides) with willow (Salix spp.) and saltcedar (Tamarix spp.) in the understory. Slopes with Rocky Mountain juniper are also important [287,288]. In Prairie County, Montana, over all seasons and years, mule deer generally used sagebrush-grasslands, bunchgrass prairies, badlands, mesic shrublands, and hardwood draws more than expected based on availability [365].Courtship and mating: One review described mule deer as polygamous, with bucks wandering about extensively and seeking individual does in estrus [215]. Other reviews described them as polygynous [7,119], with a tending bond system where a doe seeks out a dominant buck and the buck tends to the doe until she is bred [7,119,121]. The largest bucks with the largest antlers are dominant and breed most often [7,215].
The breeding season (rut) begins as early as September and ends as late as March, depending upon location [7,215]. Within a given location, however, breeding tends to occur within a short period [7]. For example, in the Missouri River Breaks region of eastern Montana, 75% of pregnant does conceived between 21 November and 1 December [129]. A single buck may breed many females, and a single doe may breed several times during a single estrous period [58,215]. Yearling (1- to 1.5-year-old) females tend to breed 3 to 4 weeks later than adults [14,102].
The interval between estrous periods ranges from 22 to 29 days, although in black-tailed deer the estrous cycle may shorter. True estrus lasts 24 to 36 hours [5,7,58,215]. As many as 5 estrous periods may occur when does repeatedly fail to conceive [7,102].
Reproduction and development: Gestation ranges from 183 to 218 days [7,58,215]. In the northern part of the mule deer's distribution, parturition occurs primarily from late May to mid-July [215]. In the southern part, parturition occurs primarily in July and August [215,290]. Extreme birth dates occur as early as mid-May and as late as early October [7]. Like the rut, fawning periods tend to occur within a short period. In Utah (Robinette and others 1977 cited in [5]) and Colorado (Anderson and Medina 1967 cited in [5]), for example, about 85% of fawns were born within a 32-day period. However, a long fawning period may be more typical in arid areas of the Southwest where rainy seasons are unpredictable. In Arizona, fawning coincided with the summer monsoon season and in one study ranged from 5 August to 5 October [115].
Growth: As parturition approaches, pregnant females move to fawning areas. Does with fawns may remain in these areas until late summer or fall [215]. Fawns weigh from 4.5 to 11 pounds (2.0-5.0 kg) [5,7,215]. Singletons weigh more than fawns from litters with ≥2 fawns [5]. Males and females tend to weigh the same at birth [5], although among sets of twins of opposite sex, males may be heavier [7]. A fawn's birth weight may affect its survival. A review stated that the effect of a maternal doe's physical condition on fawn birth weights is unclear. However, females in good physical condition the year before parturition may have a shorter gestation and/or give birth earlier than females in poor physical condition [215].
Newborn fawns hide and may be separated from their mothers for long periods. The hiding period lasts for 6 to 8 weeks [119]. Fawns begin to consume green vegetation at 2 weeks old and are weaned in fall, when their mothers breed again [7,14,102,215]. Young remain with their mothers until the following spring, when their mothers drive them away before giving birth [215]. For more information, see Social behavior.
Fawns grow rapidly, with males tending to be heavier than females. Yearling females weigh about 10% less than yearling males on average [215]. Anderson and others [6] concluded that male mule deer on the Roosevelt National Forest continued to gain weight throughout their lives, whereas females reached their maximum body weight at about 8 years old. In contrast, Mackie (1964 cited in [215]) concluded that males in Montana gained weight until at least 7.5 years old, whereas female weight changed little after 2.5 years old.
Most mule deer attain sexual maturity and can breed as yearlings [215]. However, yearling males are frequently prevented from mating by older males [215]. Fawns may become pregnant, but this is rare in the wild [215,322]. The age at first parturition is influenced by nutritional condition. In severely malnourished populations, the age at first parturition may be ≥3.5 years old [215].
Pregnancy and twinning rates: Adult mule deer commonly produce twins, whereas yearlings usually produce singletons; triplets and quadruplets are rare [5,77,215]. Pregnancy rates are influenced by local environmental conditions and nutritional status of does. In the Missouri River Breaks region of Montana, mule deer produced an average of 44% singletons, 55% twins, and 1% triplets during 12 years. During times with the poorest range conditions, mule deer produced 75% singletons, 25% twins, and no triplets. During times with the best conditions, they produced 27% singletons, 70% twins, and 3% triplets [129]. According to a review, triplets are born mainly to >4-year-olds [5].
Pregnancy rates range from 70% to >90% among adult (≥1.5-year-old) females. Yearlings typically have lower pregnancy rates than adults [215]. At Hopland Research and Extension Center, California, wild black-tailed deer fawns bred when the population was experimentally reduced from 25 to 10 black-tailed deer/km². The author suggested that the reduced population density resulted in rapid body growth and early maturation [227]. In the Missouri River Breaks region, 4-, 5-, and 6-year-old mule deer females were the most productive segment of the population. Reproduction declined sharply at 7 years old. Females ≥8 years old had reproductive levels similar to that of 3-year-olds, which were 12% to 21% lower than levels of 4-, 5-, and 6-year olds [130]. Reproduction in males may decline at 7 years old [5].
The number of fawns per doe varies with range quality [293]. The number of mule deer fawns per doe on "poor" Utah juniper/big sagebrush rangeland in south-central Utah was 64% of that on "good" Utah juniper/big sagebrush rangeland in southern Idaho [164]. In California chaparral, the black-tailed deer fawn:doe ratio was <85:100 in dense, tall chaparral. It was 147:100 in areas where repeated prescribed fires and seeding of grasses and legumes resulted in a mosaic of grasslands with scattered areas of dense chaparral, and it was 116:100 in an area burned in a summer wildfire 2 years previously that had large areas of small shrubs and very little herbaceous cover [320,322]. Taber [323] suggested that a diet low in protein and phosphorus resulted in low ovulation and reproductive rates of black-tailed deer living in the dense chaparral.
In the arid Southwest, the abundance of forage is frequently controlled by seasonal rainfall, and Hungerford [156] suggested that precipitation during the summer may be the most important factor regulating mule deer fawn production and survival. On the Kaibab Plateau in Arizona, a dry summer resulted in the lowest fawn production on record (28 fawns:100 does) [319]. In another study, fawn production on the Kaibab National Forest, north-central Arizona, averaged 90% 5 years after about 10% of the mule deer summer rangeland was treated. Slash piles were burned and grasses, legumes, and shrubs were seeded in forested areas. Some forested areas were also logged. Meadows, which were historically overgrazed, were disked and seeded. Fawn production during the 5 years prior to treatments averaged 66%. The author suggested the increased fawn production was a result of increased forage productivity due to the treatments [156].
Social behavior: Social structure in mule deer is organized around family groups consisting ≥2 generations of related females and their male and female offspring [215]. Bucks older than yearlings are absent from these groups [102]. Adult bucks may form groups, although they often remain solitary [97,102]. In late spring and early summer, adult females drive off previous year's young and isolate themselves in fawning areas, where they remain until late summer or fall [102,119,215]. Other associations during summer include small groups of adult males, mixed yearling-adult male groups, and groups of nonreproductive adult females that may have lost young-of-the-year. In late summer and fall, mixed family groups re-form. In late fall, winter, and spring, mule deer may concentrate in large groups on winter rangelands. Winter aggregations may be comprised of >100 individuals [97,102,215]. The largest groups form in the northern part of the species' distribution in areas where deep snow restricts access to forage. Large groups may also form in early spring where green, succulent forage is available [215]. Group size may be related to presence of cover. On the eastern and western slopes of the Cascades Range in southwestern Washington, the size of black-tailed deer groups was inversely related to canopy cover. Group sizes in open grassland-woodland ranges on the east side were significantly larger than those in forested rangelands on the west side [28]. Within groups, the largest and oldest individuals tend to be dominant [102,215].
Movements and home range: Mule deer may inhabit the same range throughout the year or migrate to separate summer-fall and winter ranges [159,215]. Migratory mule deer are generally found in mountainous regions, where they move up and down along elevational gradients in response to weather and seasonal changes in vegetation. Transitional ranges are used in spring and fall as mule deer move between summer and winter ranges [159,215]. Nonmigratory individuals tend to occur at low elevations year-round [135]. In the prairies of the northern United States and southern Canada, mule deer tend to be nonmigratory but exhibit local shifts in habitats among seasons [215]. In other regions, a single population may be comprised of migratory and nonmigratory individuals. Individuals generally retain the same ranges from year to year and travel the same routes between ranges [159,215].
Daily activity: Mule deer are active throughout the day and night [102,327]. According to reviews, they are most active in early morning, late afternoon, and early evening [102,241].Seasonal movements and migration: Mule deer may be year-long residents in foothills, occupy seasonal ranges that include footslopes in winter and adjacent mountain slopes in summer and fall, or migrate between distinct, widely separated winter-spring and summer-fall ranges. Mule deer may move from high-elevation montane ranges in summer to low-elevation ranges in fall and winter [215]. In mountainous regions of the West, most mule deer are migratory, spending summer in the mountains and fall in foothills and valleys. Some mule deer live at low elevations year-round and in the absence of snow, most migratory mule deer remain at high-elevation summer ranges during winter [66,159,348]. Generally when snow is deep, mule deer move down from high elevations; move up from areas of low relief to slopes of southern or western exposure or to windswept ridges; or move into forests where snow is shallow. In regions with snow, fall migration usually depends upon the timing of snowfall and the depth of snow on summer and transitional ranges, whereas timing of spring migration is associated with snowmelt, the appearance of succulent forage on transitional and summer ranges, and perhaps the timing of parturition [7,135,215]. In the arid Southwest, mule deer may migrate in response to rainfall patterns [7].
Not all mule deer in a population migrate. Only 7% of female mule deer migrated in the Rocky Mountain foothills of eastern Colorado [183]. In the Poudre Canyon area of the Roosevelt National Forest, about 80% of mule deer were migratory [204]. In the Green River Basin of western Wyoming, 95% of mule deer were migratory [285]. In populations of north-central Colorado, the average proportion of mule deer that migrated was 52% but ranged from 0% to 100% [75].
Reviews stated that migration distances vary from <1 mile up to nearly 100 miles (2-160 km) [135,215]. Migrations may take from 4 to 7 weeks [7]. In the Green River Basin, mule deer took 9 to 13 weeks to complete their migration in spring and fall, spending 4 to 5 months each year on midelevation transitional ranges [285].
Dispersal: Mule deer are most likely to disperse during the fawning period or during the rut [119]. One- to 2-year-old mule deer are most likely to disperse. Males are more likely to disperse than females [59,215,274,288,365]. In a migratory population in west-central Utah, few mule deer dispersed as fawns, but 60% of yearling males and 35% of yearling females apparently dispersed by 16 months old [274]. In the Missouri River Breaks, 70% of yearling males and 16% of yearling females dispersed [129].
Dispersal distances vary but are typically short. On Vancouver Island and in western Washington, male black-tailed deer dispersed 9.4 miles (15.2 km) and females dispersed 7.6 miles (12.2 km) on average. The maximum dispersal distances were 20 miles (32 km) for a male and 19 miles (30 km) for a female [59]. In Prairie County, Montana, migration distances ranged from 7 to 87 miles (11-140 km) for males and 8 to 16 miles (12-26 km) for females. Wood and others [365] suggested that long migration distances may be more characteristic of mule deer in patchy environments where suitable habitats are widely separated. The longest dispersal distance reported as of this writing (2012) was for a male mule deer in west-central Utah that dispersed at least 150 miles (240 km) [274].
Home range: Adult mule deer establish and traditionally use seasonal or year-round home ranges. According to reviews, mean annual home range sizes for mule deer vary from 74 to 34,220 acres (30-13,850 ha) [7,135,215]. Nonmigratory black-tailed deer have among the smallest home ranges [324].
According to a review, mule deer in "open, simple, and more variable habitats" tend to have larger home ranges than those in "closed, diverse, and stable environments" [215]. For example, in open, nonforested northern prairie habitat in Prairie County, Montana, year-long home ranges of migratory and nonmigratory adult mule deer ranged from 452 to 16,306 acres (183-6,599 ha) [365], whereas in forested prairie breaks habitat, year-long home ranges of migratory and nonmigratory adult mule deer were smaller, ranging from 114 to 8,253 acres (46-3,340 ha) [129]. According to Wood and others [365], seasonal home ranges tend to be small among mule deer in mesic mountainous habitats and large in more xeric, desert habitats. In the northern Great Plains in South Dakota, mule deer occupying rough terrain had smaller home ranges than those in relatively level terrain [288]. In Prairie County, Montana, nonmigratory mule deer had small home ranges and relatively high local population densities in areas with interspersed hardwood draws and badlands. Mule deer ranged over larger areas and occurred at lower densities in areas where badlands and hardwood draws were lacking or more widely separated [365]. In arid mixed woodland-grassland communities in north-central New Mexico, larger home ranges tended to have a greater proportion of grasslands (r²=0.18, P=0.037) [29].
Males tend to have larger home ranges than females [7,135,215]. In the Bridger Mountains of Montana, winter home ranges averaged 613 acres (248 ha) for males and 603 acres (244 ha) for females. Summer home ranges averaged 593 acres (240 ha) for males and 437 acres (177 ha) for females [243]. In the Missouri River Breaks region, the presence of fawns-at-side was the most important factor in determining home range sizes of adult females, with adult females with fawns-at-side having smaller home ranges than nonreproductive females. Home range sizes of adult females were not related to population density, forage condition, or age of females [129].
Seasonal movements and daily activity patterns may be strongly influenced by air temperature, wind, and/or snow depths [92,215]. In Colorado, mule deer tended to move within their winter habitats to areas with temperatures of 16 to 45 °F (-9 to 7 °C). When temperatures were below 16 °F, they tended to increase their metabolic rate (Mautz and others 1985 cited in [215]). During cold weather mule deer tend to use south-facing slopes, and during warm weather they tend to use north-facing slopes (see Topography) [92]. High winds appear to influence mule deer movements only during periods of cold weather, when mule deer seek shelter and reduce their activities [92,215]. According to Mackie and others [215], snow depth probably has the most influence on mule deer movements. Deep snow makes forage less accessible, increases energy expenditure, and may increase an animal's vulnerability to predation [78,135,237,348]. Snow depths of about 12 inches (30 cm) generally impede mule deer movement, especially that of young animals, and may cause mule deer to move to areas with less snow [78,135,204,215].
In regions with snow, winter ranges are often smaller than summer ranges. In the Bridger Mountains of Montana, average snow depths and patterns restricted mule deer to <20% of their total year-round range in winter. Under severe snow conditions only 20% to 50% of the winter range was considered usable [243]. Gilbert and others [122] concluded that because snow was too deep (>18 inches (46 cm)) in Middle Park, Colorado, during 2 of 3 winters, >90% of mule deer winter range was uninhabitable. In cold and snowy periods on Vancouver Island, mule deer used <40% of the area used during mild winters (McNay and Doyle 1987 cited in [58]).
In the arid Southwest, summer ranges may be smaller than winter ranges because of limited water. In Maricopa County, Arizona, mule deer had smaller home ranges in summer than winter, possibly due to greater dependence on limited water sources during periods of hot weather [115]. The 400,000-acre (162,000 ha) summer range of the Kaibab mule deer herd in Arizona was smaller than its winter range [156].
Fidelity to traditional home ranges can be so great that deer will "starve to death" rather than travel "a few kilometers" to abundant forage (Dasmann and Taber 1956 cited in [135]). During a fire, mule deer may not leave their home ranges even as their home ranges burn. If they do leave, they typically return soon after fire. Shantz [291] noted that mule deer and white-tailed deer returned to their home ranges so soon after fire that they burned their feet. Humphrey (1926 personal communication cited in [291]) noted that mule deer on the Manti-La Sal National Forest, Utah, returned to their burned home ranges "even though the forage was practically all destroyed". For more information, see Travel patterns. Although mule deer are unlikely to explore unfamiliar but favorable areas even if these areas are only a few kilometers away, they may shift their use of an area over time to exploit changes in resource availability [58]. Livestock grazing may affect the use of mule deer home ranges. For more information, see Livestock grazing.
Population density: According to a review, mule deer population density ranges from <0.1 to >29 mule deer/km² [215]. Winter concentrations in mountainous areas may be up to 130 mule deer/km² [243]. Mule deer densities in prairie habitats tend to be low [215]. In open northern prairie and plains habitats, population density in spring ranged from 0.6 to 3.3 mule deer/km² [365]. In prairie breaks and badlands, where topography and habitat are more diverse, density is usually higher, ranging from 1.4 to 4.4 mule deer/km² in spring [129]. In desert shrublands in Arizona, density ranged from 5.5 to 10.3 mule deer/km² (Smith and others 1969 cited in [215]). In mountainous areas, habitats tend to be heterogeneous and support high mule deer densities. In mountain-foothill type rangelands in Utah, density was about 16 mule deer/km² (Robinette and others 1977 cited in [215]). Hanson and McCulloch (1955 cited in [345]) considered the carrying capacity of mule deer in Arizona chaparral to be 4 to 5 mule deer/km². Mule deer in California chaparral have medium population densities (4-8 mule deer/km²) [324,327]. If oak woodland is interspersed with chaparral, density may be as high as 22 to 28 mule deer/km² [324]. In coastal forests in California, density tends to be about 2 to 8 mule deer/km², but in areas opened by logging or fire, density may be as high as 15 to 19 mule deer/km² [324]. For more information, see Logging and Indirect Fire Effects. Desert shrubland and grassland habitats tend to be "marginal" for mule deer, and wide oscillations in population density are typical, with low maximum densities compared with those in other habitats [340]. In the Southwest, mule deer populations fluctuate with annual precipitation (see Malnutrition and weather).
Life span and survival: According to reviews, the oldest male from a captive population was 22 years old, and the oldest female was 16 years old [215]. In wild populations, the oldest male was 19 years old, and the oldest female was 20 years old [7]. However, in wild populations, males seldom live longer than 8 years, and females seldom live longer than 14 years [215].
Predators: Major predators of mule deer include coyotes (Canis latrans), mountain lions (Puma concolor), gray wolves (Canis lupus), bobcats (Lynx rufus), brown bears (Ursus arctos), American black bears (Ursus americanus), and humans [7,78,121,215]. Golden eagles (Aquila chrysaetos) are common predators of young [7,78,215]. Predators may kill mule deer of all sexes and ages and in all physical conditions [44,78,154]. For more information, see Predation risk.Diseases and parasites: Numerous bacterial diseases and parasites infest mule deer and may cause mortality. Occasional epizootics in wild populations have been responsible for high mortality in some populations [144]. Fire may indirectly affect the prevalence of diseases and parasites in mule deer (see Diseases and parasites) in the Indirect Fire Effects section. For a comprehensive review of diseases and parasites that infest mule deer, see Hibler [144]. Mule deer may be more vulnerable to the detrimental effects of parasites and diseases when malnourished [32].
Malnutrition and weather: Malnutrition is often the leading cause of mule deer deaths. On Utah juniper-big sagebrush rangelands in southeastern Utah, winter mule deer mortality varied inversely with the amount of available browse [277]. Prolonged, continuous snow cover may result in substantial mortality due to malnutrition and starvation [78,88]. For example, in Oregon, a mule deer die-off occurred after snow covered the Cedar Creek Enclosure for >50 consecutive days; the die-off occurred about 23 years after the last wildfire [148].
In the prairies of the northern Great Plains, annual variations in amount and timing of precipitation influence vegetation production and thus mule deer mortality due to malnutrition [365]. In Prairie County, Montana, overwinter fawn mortality rates of mule deer were positively correlated with winter severity during 4 of 12 years (r=0.94, P=0.03). During the other years, fawn survival rates appeared to be mainly influenced by drought and poor forage the prior summer. Total amount of precipitation occurring in the area from July to April prior to fawning and percent of fawns in the population in spring were positively correlated (r=0.76, P=0.01) during the 12 years [365].
In the arid Southwest, precipitation may indirectly affect mule deer mortality through its effects on plant productivity [217,219,347]. A study in the Sonoran Desert of California found positive correlations between rainfall and the proportion of mule deer in good physical condition (r=0.60, P=0.064) and fair physical condition (r=0.70, P=0.017), whereas the proportion of mule deer in poor physical condition was negatively correlated with rainfall (r= -0.72, P=0.20) [218]. In the Trans-Pecos region of Texas, abundance of adult mule deer (R=0.645, P≤0.001) and fawn production (R=0.553, P≤0.003) were correlated to the Palmer Hydrologic Drought Index, indicating that the mule deer population was negatively affected by drought [352]. At Three Bar Wildlife Area in Arizona, survival of mule deer fawns in a given year varied with total rainfall during the previous winter. This relationship appeared to result mainly from the influence of precipitation on production of winter-growing forbs: Variation in forb production accounted for about 75% of the total variation in fawn survival during the 8-year study [303]. A severe, year-long drought in desert grassland of southeastern Arizona caused an apparent decline in local mule deer and white-tailed deer populations [11].
Black-tailed deer may have high mortality during hot, dry summers in California chaparral [14,90,323,324]. In this habitat, there is typically an abrupt decline in forage quality in June and July as vegetation desiccates. During dry summers, black-tailed deer mortality may be high if forage desiccates early, while fawns are nursing. In addition, years of low acorn production may lead to high mortality in August and September. Conversely, because the acorn drop is coincident with the breeding period, years of good acorn production may improve breeding conditions of bucks and does, resulting in increased fawn production [14,323].
Fawn survival: Fawn mortality is generally higher than that of adults. It is often highest at or immediately following parturition [58,77,77,163,215,283]. According to a review, 25% to 30% of fawns are commonly dead by fall, 50% more by early winter, and up to 75% or more by spring [215]. For example, in the declining North Kings River mule deer herd in oak-grassland and chaparral habitats in California, about 50% to 70% of fawns died within the first month of life, whereas winter fawn losses were "minor". The largest cause of mortality was coyote predation. The authors hypothesized that low fawn recruitment and population decline were due to reduction in the occurrence of fire and other disturbances on summer and transitional ranges that led to a decline in nutritional quality of mule deer forage during the last trimester of pregnancy and the lactation period [283].
Fawn survival may be related to fawn weight and gender, litter size, and other factors. In Colorado, Idaho, and Montana, where the average annual mortality of mule deer fawns was 66%, the heaviest fawns at the start of winter had the highest overwinter survival (P<0.001). Predation and malnutrition accounted for most deaths [338]. On 3 winter ranges in southwestern Idaho, the probability of winter fawn mortality increased with lower body mass (P=0.007) and being male (P=0.018) [31]. In the scablands of eastern Washington, twin fawns had a risk of dying 2.6 times higher than that of single fawns [163]. In north-central New Mexico, mule deer fawn survival was related to birth mass, birth date, litter size, maternal body fat, and total and seasonal precipitation (P<0.009 for all variables). The authors concluded that fawn survival was driven in part by an interaction between total and seasonal precipitation and effects of these factors on plant production, with consequential effects on female nutrition, and ultimately, fawn birth attributes [198].
Weather may affect fawn survival. In several plant communities in central Oregon, mule deer fawn survival during winter was closely related to temperature, wind, and snow cover and depth. Fawn survival decreased as the combination of these factors increased in severity (Leckenby and Adams 1986 cited in [106]). In Prairie County, Montana, there were significant relationships between the total amount of precipitation occurring from July to April prior to fawning and percent of fawns in the population the following winter (r=0.75, P=0.01) and spring (r=0.76, P=0.01). Average or greater precipitation during summer apparently resulted in relatively good summer forage conditions during those years, leading fawns to have ample fat reserves that could carry them through even severe winters. On the other hand, fawn winter mortality rates were relatively high in years with extreme drought and resultant poor summer forage, even when winters following the drought were mild [365].
Several other studies reported relationships between precipitation or forage production on summer ranges and herd productivity or population fluctuations (e.g., [129,164,250,303]).
Cover is important in fawning areas. Cattle (Bos primigenius) grazing may result in a loss of hiding cover for fawns, possibly increasing fawn predation mortality [197]. See Livestock grazing for more information.
Diet: Along the continuum from grazers to browsers, mule deer are classified as intermediate or mixed feeders and can switch from a diet composed primarily of grasses and forbs to one primarily of browse [7,119,216]. Mule deer are opportunistic, concentrate selectors. Compared with other ruminants, they have small rumens and gut lengths relative to body size; thus, they must eat small volumes of high-quality, easily digested food [121,192,215]. Mule deer consume the stalks, flowers, fruits, and seeds of grasses and forbs. They eat the buds, fruits, seeds (particularly acorns), stems, leaves, and bark of trees and shrubs [88,119,215,293]. They also eat fungi, lichens, algae, mosses, and ferns [135,185,215,293,340]. Cacti and other succulents may be seasonally important in mule deer diets in some areas [215,340]. Mule deer may eat aquatic vegetation, but according to Cowan [81], they do not normally feed in water >8 inches (20 cm) deep. Mule deer can only access forage that is <5 feet (1.5 m) tall [119,145,327]. They avoid dense thickets when feeding [81,102,141,145,181,327]. Reviews of mule deer diets are available: [88,185,340,348].
The foods eaten by mule deer are extremely varied [119]. For example, a review listed 202 species of trees and shrubs, 484 species of forbs, and 84 species of graminoids eaten by Rocky Mountain mule deer [185], which occur from Yukon and Alberta south to Arizona and Texas [185]. In the Southwest, a review of 12 studies across Arizona, New Mexico, and southwestern Texas found that 327 plant species were consumed by mule deer [290]. Although mule deer utilize a large number of species, relatively few make up a large part of their diet. For example, in Colorado pinyon-juniper woodland in Fort Bayard, New Mexico, tame mule deer sampled 113 of the 194 plant species found in the area, but only 10 comprised ≥92% of the diet [157].
Forbs and grasses are the most important mule deer forages during the growing season in most regions, whereas browse is often most important during the dormant season [41,42,88,101,241,290,327,330,340]. In the coastal forests of southern Vancouver Island, mule deer annual diets consisted of 67% browse, 15% lichens (mostly beard lichens (Usnea spp.)), 11% forbs, 5% fungi, and 2% graminoids, ferns, horsetails (Equisetum spp.), and quillworts (Isoetes spp.). Mule deer fed on 92% of available browse species, 64% of forbs, 56% of graminoids, and 74% of ferns, horsetails, and quillworts throughout the year [81]. In conifer forest in the North Coast Ranges of California, browse, including acorns, were eaten consistently throughout the year (48% of annual diet), forbs were eaten mostly in summer (28% of annual diet), and graminoids were important in cool months (24% of annual diet) (California Wildlife Investigations Laboratory cited in [88]). Forb quality and quantity appeared to increase the productivity of 2 mule deer herds in Utah. On a summer range with forage that was 52% forbs, a herd averaged greater carcass weights and greater fawn production than a herd on a range where only 12% of forage was forbs [250].
Typical genera browsed by black-tailed deer along the northern Pacific Coast include maple (Acer spp.), alder (Alnus spp.), wintergreen (Gaultheria spp.), currant (Ribes spp.), blackberry (Rubus spp.), willow (Salix spp.), elderberry (Sambucus spp.), and huckleberry (Vaccinium spp.) [324]. "Staple" forage plants for black-tailed deer in California chaparral include chamise, interior live oak, and wedgeleaf ceanothus (Ceanothus cuneatus) [36]. Oak foliage and acorns are important in regions where they occur [10,36,48,57,236,327]. In the Great Basin, antelope bitterbrush, mountain-mahogany (Cercocarpus spp.), sagebrush, and juniper are major components of the mule deer's winter diet [41,42,290,348,354]. Other important browse plants include ceanothus, manzanita, serviceberry (Amelanchier spp.), desert peach (Prunus andersonii), and rose [41]. Forage species important to Rocky Mountain mule deer are shown below [185].
Table 1. Forage species most valuable to Rocky Mountain mule deer in at least one season [185] |
|
| Browse | |
| antelope bitterbrush | |
| big sagebrush | |
| chokecherry | |
| curlleaf mountain-mahogany (Cercocarpus ledifolius) | |
| Gambel oak (Quercus gambelii) | |
| hollyleaved barberry (Mahonia aquifolium) | |
| ponderosa pine | |
| quaking aspen | |
| rabbitbrush (Chrysothamnus spp.) | |
| Rocky Mountain juniper | |
| rose | |
| Saskatoon serviceberry (Amelanchier alnifolia) | |
| snowberry | |
| skunkbush (Rhus trilobata) | |
| snowbrush ceanothus (Ceanothus velutinus) | |
| true mountain-mahogany | |
| willow | |
| Graminoids | |
| bluegrass (Poa spp.) | |
| brome (Bromus spp.) | |
| fescue (Festuca spp.) | |
| sedge (Carex spp.) | |
| wheatgrass (Agropyron spp., sensu lato) | |
| wildrye (Elymus spp.) | |
| Forbs | |
| alfalfa (Medicago spp.) | |
| aster (Asteraceae) | |
| balsamroot (Balsamorhiza spp.) | |
| beardtongue (Penstemon spp.) | |
| buckwheat (Eriogonum spp.) | |
| cinquefoil (Potentilla spp.) | |
| clover (Trifolium spp.) | |
| dandelion (Taraxacum spp.) | |
| fleabane (Erigeron spp.) | |
| lupine (Lupinus spp.) | |
| phlox (Phlox spp.) | |
| pussytoes (Antennaria spp.) | |
| sagebrush vetch (Vicia spp.) | |
| thistle (Cirsium spp.) | |
| yarrow (Achillea spp.) | |
Forage preferences of mule deer vary among rangelands, seasons, and years, and appear strongly related to forage availability and plant phenology [41,215,327,348]. When green and succulent, forbs and grasses are selected over browse, but as forbs and grasses dry up, browse becomes increasingly important in the diet [41,215,236,241,327,348]. Leafless twigs are consumed only when other forage is scarce [348]. Severson and Medina [290] ranked mule deer food preferences in the Southwest from highest to lowest as follows: 1) fruits, flowers, and mushrooms; 2) new green herbage, particularly forbs and new leaves of deciduous shrubs; 3) new twigs and mature green herbaceous material; 4) new leaves and twigs of evergreen species, and 5) mature leaves and twigs of evergreen species. In general, mule deer forage is most abundant during the growing season and declines progressively in quantity, variety, and quality after annual growth ceases. On most mule deer rangelands, succulent forage is scarce in winter. However, in the mediterranean climatic region of California, succulent forage is abundant during spring, late fall, and winter and relatively scarce in summer. In some areas, mule deer forage is green and available year-round [88]. A review of mule deer diets in the Chihuahuan Desert reported that browse dominated diets in dry years and forbs dominated diets in wet years [340]. During a drought year in southeastern Arizona, mule deer and white-tailed deer diets changed from succulent deciduous forage to drought-tolerant evergreen species. The author suggested that competition with livestock may accentuate the effects of drought on mule deer and white-tailed deer diets [11]. See Livestock grazing for more information.
Deep snow makes forage less accessible to mule deer [348]. For example, on a treeless area of the Tillamook Burn, Crouch [87] estimated that 12 inches (30 cm) of snow would reduce available forage for black-tailed deer from about 224 kg/ha to 34 kg/ha [87]. Mule deer may paw through snow to feed, but they prefer to feed where there is no snow [119]. When other forage is buried by deep snow, conifer browse and arboreal lichens are important in mule deer diets in many regions [13,58,124,134,215,355].
Fire may affect mule deer diet composition. After a September prescribed fire on the east slope of the Colorado Front Range, mule deer diets in a montane shrub community contained more grass and less browse on burned plots compared with controls for 2 postfire years. In a montane grassland community, mule deer diets contained more grass and less browse on burned plots relative to controls during the 1st postfire year, but during the 2nd postfire year, there were no differences in grass and browse content [306]. For more information, see Indirect Fire Effects.
Nutrition: Protein content in preferred mule deer browse changes seasonally, reaching its lowest levels in winter. The time of year in which browse plants reach their highest nutritional level varies with the plant species [109,340]. Nutrients in mule deer forage species also change with seral stage [133]. Thus, diversity in browse composition and age is important for mule deer nutrition. For a review of the chemical composition of mule deer forage, see Kufeld and others [185].
Mule deer may select the relatively more nutritious foods from among those available (e.g., [223,325,327]). In interior Arizona chaparral near Globe, Arizona, combined mule deer and white-tailed deer use of sprouting shrubs during the first spring after a mid-September prescribed fire was positively related to moisture levels and crude protein content and negatively related to crude fiber content. For example, heavy use of true mountain-mahogany during the spring was associated with highest crude protein values and comparatively low crude fiber values, and selection of Wright silktassel (Garrya wrightii) during the summer growing season coincided with increases in moisture and crude protein and decreases in crude fiber [267]. However, in the Tillamook Burn in February—approximately 24 years after the 1951 fire in an area that had been salvaged logged—mule deer did not appear to select among 6 forage species (cascara (Rhamnus purshiana), red huckleberry (V. parvifolium), Douglas-fir, California hazelnut (Corylus cornuta subsp. californica), red alder (Alnus rubra), or vine maple (Acer circinatum)) based upon chemical composition, except that moisture tended to be higher in the most preferred species [262]. For more information, see Indirect Fire Effects.
Mule deer foraging effects: Because mule deer forage selectively, they can influence plant species composition and diversity by consuming palatable species, which may allow unpalatable species to gain dominance [80,135,315]. They can influence rates of nutrient cycling by altering litter quantity and quality and via urination and defecation. Also, mule deer may affect growth of stems and leaves and alter levels of plant nutrition [80]. Deer exert cascading effects on animals both by competing directly for resources with other herbivores and by indirectly modifying the composition and physical structure of habitats [3,80,264]. Reviews describing mule deer foraging effects are available: [24,80,220]. For information about mule deer effects on postfire succession, see Mule deer interactions with fuels and fire effects.
PREFERRED HABITAT: |
| Figure 3. Mule deer herd on a snowy slope. Photo courtesy of David Heffernan, USFWS. |
During hot weather, mule deer in the Southwest tend to forage on north- or west-facing slopes in dense vegetation, to bed in shade, and to seek shelter in washes [290]. In Lake County, California, black-tailed deer use mostly south-facing slopes in winter. In late spring they use mostly cool, northern exposures until fall, although they sometimes move to streambeds during hot weather and use south-facing slopes at night. They also move up and down elevation throughout the day and year, using areas near ridgelines in cool weather and deep canyon bottoms during hot weather [14,327]. In Fort Bayard, New Mexico, mule deer used all slopes throughout the year. The author concluded that a diversity of slopes and aspects likely benefited mule deer by providing diverse forage and protection from weather [157].
Cover: Mule deer require cover for security, thermal protection, and snow interception [34,58,102,119,215,347]. Cover influences the energetic costs of maintaining body temperature; the abundance of forage; security from predators and humans; and the costs of movement through snow [58]. According to a review, concealment cover is provided by vegetation within 7 feet (2 m) of the ground. Olson [241] described patches of concealment cover as "any vegetation capable of hiding 90% of a mule deer from human view at a distance ≤200 feet (60 m)". Conifers and other evergreen plants provide some of the best cover for mule deer in winter [241,287]. Topographic features such as boulders, river breaks, irregular topography, and ledges also provide concealment cover for mule deer [58,253]. Thermal cover is provided by vegetation and topography that ameliorate temperature and wind. During high wind, mule deer seek pockets of calm air below the crests of hills and in dense forests [102,119]. The importance of thermal cover varies with season, weather, and the age, size, and nutritional condition of the animal [58]. Based upon a simulation model using data from 14 years in shrubsteppe and shrub-woodland winter ranges in Colorado, mule deer doe and fawn thermal cover requirements under severe winter weather conditions were inversely correlated with the physical condition of the animal. However, under less than severe weather conditions, enhancing thermal cover on shrubsteppe and shrub-woodland winter ranges appeared unlikely to improve mule deer condition, although loss of cover could markedly alter patterns of mortality [150].
Snow interception cover is provided by forest and shrub canopy cover and topography. According to Bunnell [58], the degree of canopy closure has the most influence on the proportion of snowfall that will be intercepted by a forest >30 feet (10 m) tall. Forest stands with high canopy closure have shallower snow beneath, which reduces the cost of movement and increases forage availability [58]. In coastal British Columbia, mean snow depths across 4 mountain hemlock stands of different ages decreased linearly with mean canopy closure (r²=0.87, P<0.05). The stands included a 200-year-old forest with 60% canopy closure, an 80-year-old stand with 90% canopy closure, a 20-year-old stand with 36% canopy closure, and a recently clearcut stand with 0% canopy closure. The 2 oldest stands had less snow and a harder crust than the recent clearcut. Thus, mean black-tailed deer sinking depths were lower in those stands [60]. Clearcuts in western redcedar-western hemlock-western white pine forests in northern Idaho accumulated more snow than mature forest. However, spring snow melted faster from clearcuts than mature forest. Clearcuts on south-facing slopes had exposed forage soonest in spring [138]. A sagebrush canopy intercepts snow in addition to providing frequently melted-out areas around large plants, allowing for access to forage. Near Dubois, Wyoming, big sagebrush stands with about 50% cover accumulated more snow than open grasslands. In spring, snowmelt began earlier and proceeded at greater rates in and adjacent to big sagebrush crowns [158]. Because the amount and duration of snow varies within and among years, the value of canopy cover to mule deer also varies [253].
Mule deer are attracted to canopy openings by abundant forage but may make little use of the centers of large openings because of distance from cover [351]. See Edge habitat for more information.
Foraging sites: Mule deer forage-site selection is based in part on forage quantity and nutritional quality, which are influenced by plant species composition, plant phenology and related changes in nutrition, site characteristics (soil, shade, and topography), successional stage, grazing and browsing pressure (see Livestock grazing), and weather. Mule deer forage-site selection is also affected by predation risk and proximity of foraging sites to drinking water and habitats providing cover.
Weather affects mule deer forage availability and thus foraging-site selection throughout the year. For example, black-tailed deer along the northern Pacific Coast in British Columbia and southeastern Alaska largely depend on canopy cover in mature forests [135,347,350]. A review of black-tailed deer habitats in southeastern Alaska, where black-tailed deer used forests year-round, stated that the 2 most important features of forest vegetation for black-tailed deer are a productive understory of high-quality forage and an overstory that intercepts and/or redistributes enough snow that understory forage remains available throughout the winter [135]. Patterns of forest use by black-tailed deer in southeastern Alaska shift through the winter and spring with changes in snow conditions and plant phenology [135]. In high snow areas in British Columbia and Alaska, "critical" winter rangelands include areas at low elevations; areas with southern aspects on moderate to steep (40%-100%) slopes; forests with multiple canopy layers; small, interspersed openings; and dense forest patches with well-developed crowns that intercept snow [58]. Black-tailed deer in areas of deep snow along the northern Pacific Coast largely depend upon old-growth western hemlock-Sitka spruce stands of moderate to high volume (≥20,000 board feet/acre) with an understory of huckleberry, bunchberry, and strawberryleaf raspberry (Rubus pedatus) due to the high degree of snow interception by the canopy and high-quality forage in the understory. In areas of low snow, however, forest stands with more open canopies and lower densities may be relatively more important [135]. In spring, the most important habitats for black-tailed deer are those with early snowmelt because these areas provide abundant, early succulent vegetation. Open areas such as clearcuts, rocky outcrops, and open forests have rapid snowmelt and early initiation of spring growth [58]. Wet sites, particularly those with patches of skunk cabbage (Lysichiton americanus), also provide abundant early new growth and are important spring foraging habitats [135].
Mature chaparral stands provide essential cover and forage for mule deer during parts of the year [345]. Mule deer summer foraging sites in California chaparral include riparian areas, seeps, springs, streams, and ponds. In fall, foraging sites include stream bottoms, ridge tops, and northern slopes. In winter, mule deer forage on south slopes and sheltered ridges [14]. A review stated that mule deer carrying capacities in chaparral are largely determined by weather and its effects on forage quality and quantity. "Good deer years" have weather conditions that promote herbaceous forage production and acorn production and/or extend forage succulence through the summer and fall [14]. Diversity of habitats is important to mule deer in California chaparral. Biswell [39] stated water availability, combined with chamise chaparral on south-facing slopes and mixed chaparral on north-facing slopes and in drainages, favored black-tailed deer in Lake County, California, because the combination provided diverse browse. In California oak woodlands, black-tailed deer use all successional stages, but Anderson and Pasquinelli [10] considered oak woodlands with abundant seedlings and saplings most important.
Successional status of mule deer habitats: Mule deer occur in habitats in all stages of succession. With perhaps the exception of black-tailed deer that reside along the northern Pacific Coast, mule deer generally benefit from early successional vegetation that establishes after logging or fire [215,347]. Many mule deer forage species are characteristic of seral plant communities created after fire and other disturbances [91,236]. Disturbances that open the forest canopy and create early successional plant communities may provide deer with more forage than closed-canopy, old-growth forests in regions where snow does not become too deep [88]. Where snow is deep, mule deer forage in the understories of forests with structurally diverse, multilayered canopies, such as old-growth forests [58]. In general, the length of time that successional vegetation benefits mule deer varies with the type of disturbance, habitat type, soils, climate, and other factors [147,215]. For more information, see Habitat management and Indirect Fire Effects.
Edge habitat: Edge habitat is generally considered important to deer because of high habitat diversity within ecotones and easy access to more than one habitat type [27,176]. Mule deer commonly use edges between burned and unburned habitats (see Size and shape of burned areas). Their use of edge habitats varies depending upon the interspersion of habitats providing forage and cover. A review stated that studies showing an apparent preference by deer for edge habitats tended to be conducted in areas where forage and cover were not available within the same habitat or where forage and cover habitats were not well interspersed. Studies finding little response of deer to edges tended to be in areas that had a high degree of interspersion of forage habitats and cover habitats or had a fine-grained interspersion where forage and cover were available in the same habitat [176].
Age and gender: Adult male and adult female mule deer may select habitats differently, whereas young males and young females use similar habitats as adult females [286]. During their 1st or 2nd year, young males typically shift from habitats used by females to those used by adult males. Often, this shift is made during the fawning or rutting periods (see Dispersal and Social behavior). Female mule deer may select habitats with more ground cover than males (King and Smith 1980, Main and Coblentz 1996, cited in [233]). In the Southwest, males may be found at higher elevations than females [286,290]. For example, in Arizona adult females always used habitats in the Picacho Mountains, whereas adult males used the creosotebush-velvet mesquite (Larrea tridentata-Prosopis velutina) flats surrounding the mountains. Adult males and adult females were generally found together only during the rut [286]. However, in the eastern Sierra Nevada, male and female mule deer selected habitats similarly [258].
Predation risk: A review stated antipredator strategies of mule deer include early detection and outmaneuvering of predators; avoidance of areas frequented by predators; formation of groups with other mule deer; and restriction of movements to areas close to cover or escape terrain (e.g., steep slopes, riverbanks, and areas with low obstacles such as deadfall). Mule deer may also defend themselves against predators such as bobcats and coyotes [119,121]. Geist [121] suggested that the mule deer's antipredator strategies in part determine the species' preference for areas with broken terrain and steep slopes with obstacles.
Presence of predators may alter mule deer habitat use, movements, diet, and behavior (e.g., [4,239,258]). At the Three Bar Wildlife Area, summering female mule deer in enclosures with coyotes used areas with denser vegetation than females in enclosures without coyotes. Four habitats occurred in the enclosures: burned and unburned interior Arizona chaparral and burned and unburned Sonoran desertscrub. Burns resulted from a severe spring wildfire 4 years earlier. Vegetation was denser in burned and unburned chaparral than in desertscrub. Thus, mule deer may have selected chaparral for its escape and hiding cover from coyotes. However, they may have selected it for its greater thermal and security cover or for its greater forb biomass [239]. In the eastern Sierra Nevada, where mountain lions accounted for 68% of predator-caused mortality, male and female mule deer selected locations at higher elevations with more antelope bitterbrush, an important winter forage species, than random locations. Mountain lion kill sites were in relatively more open locations than locations in which mule deer foraged (P<0.05 for all variables). Therefore, mule deer did not appear to be confronted with a trade-off between predation risk and forage abundance when selecting habitat [258].
Predation risk from human hunting may also alter mule deer habitat use, movements, diet, and behavior [78]. For example, near Fort Collins, Colorado, hunting resulted in mule deer moving to areas with dense cover within their home ranges [182].
Other factors:
Interspecific interactions:
Mule deer habitat use may be indirectly affected by that of other wildlife. Some researchers concluded that habitat selection by mule deer could be explained largely by avoidance of areas used by elk (Cervus elaphus) (e.g., [114,161,222,313]). See
Mule deer, other ungulate, and fire interactions
for more information. Apparently, elk's ability to utilize a greater variety of forage gives elk a competitive advantage over mule deer [74,214]. For a review of interrelationships between mule deer and other wildlife, see the review by Mackie [216]. Mule deer habitat use may also be affected by that of livestock, particularly cattle. For more information, see
Livestock grazing.
Coarse woody debris: Mule deer may avoid areas with abundant coarse woody debris. See Logging slash and Physical barriers for more information.
Water: According to reviews, mule deer are well-adapted to cope with limited amounts of drinking water. In most of the species' range, water is usually not a factor limiting mule deer distribution and abundance [215,290], but in arid regions the local distribution of mule deer is influenced by the location of water [129,137,215,290,347]. For example, in arid regions of California, Arizona, New Mexico, and Texas, they are typically found within 1.6 miles (2.5 km) of water sources, particularly during dry periods [47,215,280,290,319,345,367]. Water developments have apparently benefited many mule deer populations in the Southwest (see Water management) [280]. For a more detailed review of mule deer use of water in the Southwest, see Severson and Medina [290].
 |
| Figure 4. Mule deer buck at Bosque del Apache National Wildlife Refuge, Socorro County, New Mexico. Photo courtesy of Robert Sivinski, CalPhotos. |
Fawning areas: During and soon after parturition, female mule deer prefer areas with concealment cover, such as areas with dense vegetation [102]. Reviews stated that "ideal" fawning habitat for mule deer in Wyoming, Oregon, and Washington includes small areas (1-5 acres (0.4-2.0 ha)) of low shrubs or small trees 2 to 6 feet (0.6-1.8 m) tall, with about 50% canopy cover, slopes <15%, and water within 600 feet (180 m) [40,241]. During fawning on Steens Mountain, Oregon, preferred habitat was in mountain big sagebrush (Artemisia tridentata subsp. vaseyana) with a canopy closure >23%. Forty percent canopy closure was most preferred. Although woodland communities were not preferred, most fawning sites were within 164 feet (50 m) of a quaking aspen grove or juniper woodland (Sheehy 1978 cited in [106]). In mixed-conifer forest of Tehama County, California, habitat heterogeneity seemed to be the "key" to quality fawn rearing habitat, with fawns selecting forests with 26- to 39-foot (8-12 m) tall trees, 20% to 39% canopy cover, and stumps, downed trees, or the bases of trees or snags as bedding sites [358]. In California chaparral, black-tailed deer sought dense cover to bear their fawns [14,92,327]. In Oregon, female mule deer selected gentle, south-facing slopes dominated by ponderosa pine and avoided permanent water sources prior to parturition, but after parturition they selected relatively open portions of fir (Abies spp.) forests on steep north-facing slopes (likely to avoid predation) and habitats close to permanent water sources [199]. In Maricopa County, Arizona, most fawn locations were on upper, steep slopes in mountainous terrain, possibly to avoid coyotes, which tended to hunt on gentle or flat terrain. Mule deer also selected fawning areas close to water. The average distance of fawning areas to water was 1.8 miles (2.8 km) [115]. Because mule deer often select dense areas of trees and shrubs as protective cover during and after parturition, fire and other disturbances that reduce cover may reduce fawning habitat for mule deer.
MANAGEMENT CONSIDERATIONS:Threats to mule deer populations include overharvesting, increased human disturbance, and nonnative invasive plants:
Human disturbance: Mule deer may habituate to human presence and become nuisances in some areas. However, human development generally reduces mule deer use of developed areas [215,347].
Nonnative invasive plants: Spread of nonnative invasive grasses and forbs may harm or benefit mule deer. Some researchers found that mule deer commonly consume nonnative invasive plants, including spotted knapweed (Centaurea maculosa) (e.g., [50,105,190,206,244,270,278,368]). Along the Selway River in Idaho, mule deer ate spotted knapweed seedheads, particularly when snow was on the ground, because the seedheads were easily obtainable above the snow. In fact, they were one of the few herbaceous plants readily available to mule deer in open areas when snow was >12 inches (30 cm) deep. Mule deer also ate large amounts of spotted knapweed rosettes, particularly in spring after snowmelt [368]. Mule deer consumed green shoots of cheatgrass and rosettes of tumble mustard (Sisymbrium altissimum) in late winter and spring after several wild and prescribed fires in Lava Beds National Monument, California [261].
Some sources suggested that the carrying capacity of rangeland for mule deer and livestock may be reduced by nonnative invasive plants that displace more palatable native grasses and forbs (e.g., [46,105,206,271]). In the Bitterroot Valley, Montana, mule deer rarely used spotted knapweed-dominated open areas [360]. However, along the Selway River in Idaho, where densities ranged from 0.03 to 0.17 mule deer/ha during winter, spotted knapweed infestations of xeric south and west-facing slopes on year-round rangeland did not appear to affect mule deer carrying capacity in winter when compared with Saskatoon serviceberry/bunchgrass-sedge shrubfields [368]. Wright and Kelsey [368] attributed differences between the studies to lower mule deer densities, greater availability of agricultural lands, and less snow cover in the Bitterroot Valley study.
Mule deer may contribute to the spread of nonnative invasive plants by ingesting, transporting, and disseminating viable seeds of nonnative invasive plants in their feces [25,89,139,240,244,342,343]. In maritime chamise-La Purissima manzanita (Arctostaphylos purissima) chaparral habitats in Santa Barbara County, California, mule deer dispersed seeds of hottentot fig (Carpobrotus edulis), a nonnative invasive plant, into recently burned areas [89].
The spread of cheatgrass has important indirect effects on mule deer and other wildlife by increasing fuel loads and fire frequency, which may alter the structure and composition of native plant communities. Because sagebrush communities provide important winter rangelands for mule deer and sagebrush is easily killed by fire, cheatgrass invasion may be particularly detrimental to mule deer in sagebrush habitats [270,371]. Buildup of medusahead (Taeniatherum caput-medusae) litter may also lead to increased fuel loads and more frequent fires in low sagebrush (Artemisia arbuscula) and other sagebrush communities [271]. For more information, see FEIS reviews of cheatgrass, medusahead, and other species of interest.
Habitat management: Disturbance can produce habitat for mule deer by favoring forage growth and by creating ecotones between areas of dense cover and more open feeding areas. Conversely, loss of cover over large areas can be detrimental to mule deer [135,215]. Several researchers suggested that resource managers may need to consider proximity of food, cover, and water before implementing actions that may impact mule deer habitats [153,215,216].
Prescribed fire: For information on the use of prescribed fire in mule habitats, see Fire Management Considerations.
Logging: With the exception of black-tailed deer in British Columbia and southeastern Alaska, which are dependent upon canopy cover in mature forests [134,135,215,325,350], mule deer generally benefit from early successional vegetation that establishes after logging and other disturbances [135,215]. Logging may benefit mule deer because early-seral habitats often contain a greater variety, quantity, and quality of mule deer forage than mature forests (e.g., [39,72,98,137,269,290,304,310,325,349]). However, forage quantity and quality may not increase immediately in logged areas and may last only 20 to 30 years (e.g., [134,147,215,269,310,350]). In addition, cover may be reduced [310]. A review stated that clearcutting of old-growth forests in southeastern Alaska has 4 potential effects may decrease the carrying capacity of habitat for black-tailed deer: 1) sun-grown plants in open clearcuts may have lower digestible protein concentrations than shade-grown plants in forests; 2) large amounts of logging slash may increase energy costs of locomotion for black-tailed deer and reduce the area of usable habitat; 3) snow may accumulate and persist more in open clearcuts than in forests; and 4) understory production may be reduced to extremely low levels when the conifer canopy closes; this may occur at about 20 to 30 years after logging and persist for 100 years or more [134].
In general, the duration of logging benefits to mule deer varies with forest type, soils, climate, and other factors. Use of prescribed fire, herbicides, soil scarification, planting of seeds and seedlings, and other site preparation may shorten or lengthen the time a logged site is used by mule deer [134,147,215]. For example, burning young clearcuts in southeastern Alaska may benefit black-tailed deer by reducing shrub and conifer biomass and increasing the diversity of herbaceous forage plants, thus potentially delaying conifer canopy closure [134]. In addition, succession following clearcutting may be affected by heavy mule deer browsing. For more information, see Mule deer foraging effects. Mule deer use of logged areas is modified by opening size, logging slash, predisturbance movement patterns, and weather, particularly snow depth. Reviews of logging effects on mule deer are available: [72,134,351].
Opening size: The size and distribution of clearcuts in space and time are important to mule deer; this is likely also true of burned sites. In the boreal forest zone in western Alberta, the size and dispersion of 2- to 9-year-old clearcut blocks and type of treatment best explained mule deer and white-tailed deer use of clearcuts (R²=0.21, P<0.01). Deer showed a strong preference for clearcut blocks that were <40 acres (16 ha); because they preferred areas within clearcuts that were <330 feet (100 m) from cover, they favored configurations that provided a high degree of edge per unit area. They also preferred clearcuts that were scarified or scarified and burned under prescription compared with untreated clearcuts. The authors suggested that such treatments may have led to greater abundance of preferred herbaceous species and reduced logging slash, which benefited deer. Clearcut blocks in clumped patterns appeared unfavorable [334]. Some authors suggested that many small, scattered, irregularly shaped clearcuts may be preferable to fewer, large, block-shaped clearcuts because multiple small treatments would contact the home ranges of more mule deer [135,334]. A review stated that deer used natural and created openings in ponderosa pine forests similarly, particularly when thinned stands occurred nearby, but in dense stands, deer likely benefited from small openings [72]. For more information, see Edge habitat. See the review by Wallmo and Schoen [351] for management recommendations regarding sizes of clearcut openings in various regions.
Logging slash: Depending upon its density, logging slash may be a benefit or a detriment to mule deer. Reviews stated that abundant logging slash generally impedes mule deer movements and may act as a barrier to mule deer use of clearcut openings and selectively logged areas [63,135,351]. Conversely, some logging slash can provide cover for mule deer [351]. In quaking aspen stands on the Apache and Coconino National Forests, Arizona, deer use was lower in thinned quaking aspen stands without slash removal despite greater density of perennial grasses, forbs, and quaking aspen sprouts in these stands compared to unthinned stands. Apparently, the amount of woody debris in thinned stands prohibited deer use [268]. In southeastern Alaska, dense logging debris apparently impeded black-tailed deer use of 1- to 2-year-old clearcuts in western hemlock-Sitka spruce forest. Dense logging debris continued to impede black-tailed deer until 15 to 20 years after logging [350]. Mule deer pellet group counts in clearcut strips in subalpine lodgepole pine and spruce-fir forest in Colorado were less than those on adjacent uncut sites during the first year after logging, possibly due to the deep tangle of residual slash and disturbance of logging operations. However, 10 years after strip clearcutting, pellet group counts were 2 times higher on clearcut strips than on adjacent uncut strips [344]. In a selectively cut ponderosa pine forest in Arizona, deer pellet groups were more numerous where slash was undisturbed after logging. Slash abundance was 1.7 times greater where slash was undisturbed than where it was piled and burned, but forbs were more abundant where slash was piled and burned, which should have attracted deer. The author suggested that deer may have preferred the site where slash was undisturbed because the slash provided protective cover [266]. In north-central Arizona, mule deer use was higher on clearcuts where the slash had been piled and burned than on clearcuts where slash had been piled but not burned [189]. In Arizona, Neff (1980 cited in [72]) found that deer showed no preference for either the presence or absence of slash in small (1-10 acres (0.4-4.0 ha)) openings in ponderosa pine stands.
A review recommended prescribed fire in southeastern Alaskan forests after clearcutting to reduce logging slash, reduce shrub biomass, favor herbs, reduce conifer regeneration, and prolong the useful life of clearcuts for mule deer, at least in snow-free seasons or areas [135]. In juniper woodlands in Texas, Bryant [57] suggested that 10% to 15% of cleared areas should contain slash to provide cover for mule deer. Black-tailed deer may also benefit from reduced logging slash that potentially impedes their movements [134]. For more information, see Indirect Fire Effects.
Logging and weather interactions: Mule deer may not use clearcuts because of deep snow compared to forests [357]. In the interior western redcedar-western hemlock subzone near Horsefly, British Columbia, mule deer tracks during a year of low snowfall were half as abundant in clearcuts as in uncut forest. Snow was 17 inches (44 cm) deep in openings and just 10 inches (26 cm) deep in forests, suggesting that deep snow in clearcuts may have reduced forage access and thus use of clearcuts [357]. For more information, see Foraging sites.
Other treatments:
Sagebrush and pinyon-juniper:
Removal of shrubs and trees in sagebrush and pinyon-juniper ecosystems is a common management practice on mule deer rangelands. In sagebrush and pinyon-juniper ecosystems, large areas have been treated mechanically, with prescribed fire, or with herbicides to try to convert them to grass-shrub or grass types [66,347]. Such treatments in sagebrush communities may reduce important winter forage for mule deer [91]. Partial or complete removal of trees in pinyon-juniper communities may result in substantial increases in production of grasses, forbs, and shrubs, which could potentially increase mule deer carrying capacity [66], but according to a review, this practice produces mixed results. Some studies showed increased mule deer use of treated pinyon-juniper sites, typically due to greater amounts of forage and browse on treated areas, while other studies did not show increased mule deer of treated areas, typically because of reduced cover in these areas [2]. On the Zuni Indian Reservation in western New Mexico and eastern Arizona, mule deer pellet groups increased with the number of pinyon and juniper trees removed (R²=0.95, P=0.03) [2]. A similar increase did not occur on 2- to 24-year-old pinyon-juniper rangelands in Utah that were chained and seeded with grasses, forbs, and shrubs. The authors suggested that treated sites were used despite decreased cover because of increased forage [299]. At Fort Bayard, New Mexico, mule deer abundance in Colorado pinyon (Pinus edulis)-juniper habitat was higher before than after tree removal. Mule deer forage increased after tree removal, but the authors concluded that the absence of cover reduced mule deer use [294]. In a review, Phillips [256] stated that chained pinyon-juniper stands did not benefit mule deer and other wild ungulates until trees and shrubs established [299]. Other researchers reported that treatment of pinyon-juniper rangelands did not affect mule deer habitat use. Mechanical and herbicide treatments on 5,200 acres (2,100 ha) of pinyon-juniper rangeland in Arizona resulted in no differences in mule deer use of the area (Neff 1980 cited in [290]).
Mule deer use pinyon-juniper woodlands in all stages of succession [2]. How long each stage is utilized depends in part on site, composition of the understory prior to disturbance, the type of disturbance, weather conditions, postdisturbance treatments such as seeding, and livestock grazing [290]. In general, the usefulness of pinyon-juniper habitats to mule deer declines as the understory and midstory decline [295]. Based upon studies in west-central Utah, posttreatment production of forbs and grasses generally diminishes to pretreatment levels in <20 years; shrubs increase up to 40 years after treatment; and at 40 years, juniper and pinyon again dominate the site [21]. In Nevada, annual and perennial forbs dominated for 1 to 2 years after canopy removal; perennial grasses dominated in the 2nd year and reached maximum abundance in the 4th year. Shrubs reached a peak after grasses, between the 1st and 3rd posttreatment years, and trees regained dominance in <15 years [329].
According to a review, published literature is "nearly unanimous" in recommendations for pinyon-juniper management for mule deer and other wild ungulates: 1) keep openings small and close to escape cover, usually 0.1 to 0.2 mile (0.16-0.32 km) maximum; 2) locate projects near areas of historical big game usage; and 3) leave browse plants untreated or reestablish following treatments [2]. These authors provide management recommendations for mule deer and other ungulates in pinyon-juniper communities: [57,290,295]. See Great Basin woodlands for information about fire effects on mule deer in pinyon-juniper communities. For a review of the effects of management practices in sagebrush steppe on mule deer—including topics not discussed here such as management of sagebrush with herbicides, fertilizing sagebrush habitats, and reseeding after sagebrush reduction—see Carpenter and Wallmo [66]. See Great Basin shrublands for information about fire effects on mule deer in sagebrush communities.
Gambel oak: Gambel oak is an important mule deer forage species; both its mast and browse are used extensively. It may form almost pure stands in some areas. Because of its growth habit, however, it often forms impenetrable thickets that are too tall or inaccessible for mule deer [66,186]. Methods used to treat Gambel oak communities include prescribed fire, logging, and herbicides. Clearcutting patches in Gambel oak habitat may produce abundant browse because of Gambel oak's sprouting ability, but this would temporarily reduce acorns. Selective cutting, in which the best acorn-producing trees are left, was recommended by Severson and Medina [290] to ensure both browse and acorn production at a single location. A review stated that treating Gambel oak stands with prescribed fire or mechanical methods may increase mule deer use of treated stands up to 4 times but that use declines as time since treatment increases and stands become dense and inaccessible [186]. In Colorado, dense Gambel oak stands were sprayed with herbicides. Two years after spraying, grasses increased 44% compared with pretreatment levels, while shrubs decreased 29% and forbs decreased 15%. Five years after spraying, grasses were 17% below pretreatment levels and shrubs were 7% below pretreatment levels. Consequently, herbicide treatment was considered beneficial to mule deer for only a brief time, and frequent retreatment was considered necessary to maintain high-quality habitat for mule deer [180]. See Southwest shrublands for information about fire effects on mule deer in Gambel oak communities.
Livestock grazing: Influences of livestock grazing on mule deer can be detrimental, neutral, or beneficial [67,153,216,290]. Grazing, as well as the physical presence of cattle, domestic sheep (Ovis aries), and other livestock can have negative impacts on mule deer not only by reducing forage and changing ratios of live to dead plant material [8,153,290,361,362], but by causing changes in movements and behavior and altering activity budgets [67,67,169,216,263,290]. However, some researchers reported few or no effects of livestock grazing on mule deer in areas where livestock grazing intensity was low or moderate [18,67,153,290]. Livestock grazing in many perennial grasslands historically increased shrub and annual grass-forb types, potentially benefiting mule deer (see Threats) [153,216,290]. In other areas, heavy livestock grazing reduced shrubs and herbs important as mule deer forage [216]. Reviews noted that livestock grazing on the northern Great Plains has extensively reduced or eliminated hardwood tree and shrub cover along drainageways, which has limited the occurrence of mule deer in some areas [287,290]. In the Sierra Nevada, cattle grazing during peak fawning season reduced hiding cover for fawns in both quaking aspen and meadow-riparian habitats compared with areas without cattle grazing [169,197]. Reviews stated that livestock management practices and factors that may affect mule deer include weather, topography, water availability, rangeland type, grazing intensity, animal distribution, livestock species, grazing system, and timing and duration of grazing. Timing of livestock grazing was considered particularly important to mule deer, which are particularly susceptible to adverse effects during fawning [17,67,153,169,215,216,290,290].
Water management: On some arid ranges of the Southwest, water development and better distribution of water sources for livestock can benefit mule deer by permitting year-round or seasonal use of rangelands from which they may have been excluded by a lack of free water (see Water) [153,215,216,280]. Mule deer numbers increased during the 5 years following development of permanent water sources on areas of Fort Stanton, New Mexico, that had little or no free water previously. In one area, use increased from <1.6 to >13 mule deer/mile² in 5 years. In another area, use increased from 14.2 to 19.2 mule deer/mile² in 1 year, dropped to 9.4 mule deer/mile² in the 4th year when water sources deteriorated, and increased to 22.1 mule deer/mile² in the 5th year when water was again available [367]. However, water developments may concentrate livestock and deer, leading to degradation of some habitats [153,215,216]. Furthermore, redistribution of livestock through water development may increase overlap of mule deer and livestock on areas previously occupied by mule deer but not livestock [153,216]. For more information, see the review by Mackie and others [215].
Population management: Mule deer are hunted by humans throughout their range [78]. Hunting can alter population density, sex ratios, behavior, movements, and life span [78,327]. Historically, overhunting has reduced mule deer populations (See Threats). See Connelly [78] for a review of hunting effects on mule deer populations.| Tillamook Burn: The Tillamook Burn in western Oregon resulted from a series of 4 wildfires that occurred in 1933, 1939, 1945, and 1951 and burned and reburned approximately 300,000 acres (120,000 ha) of old-growth Sitka spruce, western hemlock, Douglas-fir, and western redcedar forests [110,147]. Much of the conifer regeneration that established from seed after the 1933 fire was killed by subsequent fires. Thus, conifer seedlings were scarce following the reburns. Timber salvage operations, which removed snags and most live trees, began in 1934 and continued until 1960. Planting and seeding of conifers began in 1949 [147]. See Diet and Indirect Fire Effects for more information on fire effects on mule deer habitat in this area. |
Large mammal mortality is most likely when fire fronts are wide and fast moving, fires are actively crowning, and thick ground smoke occurs [117,236]. Large fires may be more likely to result in death, injury, or eventual starvation of deer than small fires because large fires remove more protective cover and temporarily reduce forage (see Size and shape of burned areas) [284]. Nichols and Menke [236] noted that mule deer are mobile and generally able to flee chaparral fires, but large, rapidly moving wildfires may trap and kill them. A researcher observed some incidences of direct fire mortality of black-tailed deer following the 1939 Tillamook Fire in Oregon. The author also noted that although exact losses were not recorded for the 1933 Tillamook Fire, "it is reasonable to assume that destroying 84% of the cover in the 401-mile² (1,039 km²) area in a 20-hour period resulted in substantial wildlife losses" [147]. Necropsies revealed the primary cause of death of mule deer and other large mammals during the 1988 Greater Yellowstone Area fires was asphyxiation by smoke inhalation [117]. A mule deer in southern California chaparral apparently died of asphyxiation or heat during the 1957 Malibu area fire [69]. Shantz [291] noted many instances where the feet of deer were burned, thus crippling the animals.
As with other ungulates such as moose (Alces americanus) and elk, the number of fatalities caused by fire is likely related to season, population density, habitat type, terrain, fuel load, and prevailing winds [70,297]. Mule deer fawns may be most vulnerable to fire-caused mortality in spring during the hiding period (see Growth), when they are relatively immobile. Nichols and Menke [236] commented that mule deer fawns may be more susceptible to fire mortality because they cannot flee as quickly as adults. However, Collins [73] stated that young-of-the-year of most mammals, including deer, would have been able to escape an early-August mixed-severity wildfire on the Salmon National Forest, Idaho, in part because considerable escape terrain was available in the form of rock outcrops and slides. Sizer (1921 cited in [291]) noted that deer that hemmed against a rock bluff during the Mazatzal Fire on the Tonto National Forest in Arizona were able to escape the fire.
General observations suggest that mule deer use areas during and soon after fire (e.g., [107,117,196,291]). During an early August mixed-severity wildfire on the Salmon National Forest, deer were occasionally seen in areas still burning. For example, a doe and fawn were seen standing beneath a burning snag [73]. French and French [117] observed no large mammals fleeing a fire, and most appeared "indifferent" even to crowning fires.
INDIRECT FIRE EFFECTS:Prefire stand age and species composition play an important role in plant response to fire in boreal forests [210,213]. The benefits of burning to moose, and possibly mule deer, may peak 20 to 25 years after stand-replacing fire and last less than 50 years [210]. See the FEIS review of moose for more information on fire effects on moose browse in boreal forests. Although mule deer and moose may consume many of the same browse species in this region, such browse is likely to grow out of reach of mule deer and thus become inaccessible to mule deer before becoming inaccessible to moose, which are taller [45]. In addition, herbaceous plants in postfire successional communities tend to be more important to mule deer than to moose.
Mule deer browse species may be more nutritious in early than late succession [282]. Stand-replacing fire in boreal forest often increases the protein, phosphorus, calcium, magnesium, and potassium content of woody browse for up to 3 postfire growing seasons [210].
Pacific NorthwestPacific Northwest grasslands: Fire in grasslands may increase palatability and accessibility of some grasses. In big sagebrush/bluebunch wheatgrass (Pseudoroegneria spicata) and Douglas-fir/bluebunch wheatgrass communities of interior British Columbia, the proportion of available bluebunch wheatgrass used by tame mule deer in April was highest the spring after a November prescribed fire. It was lowest in unburned control areas. However, total use of bluebunch wheatgrass by mule deer was less on burned areas than unburned areas because availability was less. The authors suggested that mule deer foraged preferentially on bluebunch wheatgrass in the burn despite its relatively low abundance there because of its improved palatability and accessibility (due to removal of litter) [362].
Pacific Northwest forests: Agree [1] described black-tailed deer response to postfire succession in western hemlock-Douglas-fir forests of the Pacific Northwest. In the 1st year after fire, shrubs sprout and conifer seedlings are abundant. Black-tailed deer populations generally increase on the burned area, particularly on the perimeter. Twenty years later, the understory is dense and saplings are heavily browsed; black-tailed deer populations continue to expand. As the canopy closes, the lack of sunlight in the understory reduces shrub and herb cover and black-tailed deer populations decline. After 200 to 250 years, the forest begins to resemble old growth, with small openings caused by disease and windthrow [1]; at this time, black-tailed deer populations presumably increase. Cowan [81] reported that the carrying capacity of black-tailed deer in coastal conifer forests on Vancouver Island increased gradually to a peak in approximately 10 to 15 years after fire. However, data provided by Bendell [27] showed that black-tailed deer populations peaked during postfire years 1 and 2 and then declined the subsequent 13 years. His study was based upon hunter success following a 31,000-acre (12,500 ha) August wildfire in the Sayward Forest on the east coast of Vancouver Island. No data were available regarding prefire hunter success or hunter success in control areas [27].
| Figure 5. Mule deer harvested per hunter during the 13 years following a large wildfire on Vancouver Island. No data were collected during postfire year 8 [27]. |
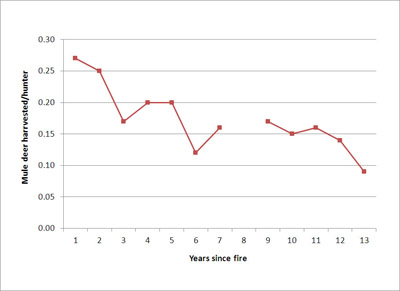 |
Population density immediately following the 1939 Tillamook Fire was <1 black-tailed deer/mile² [110]. At this time, <5% of the area within the burn perimeter supported green trees [147]. During postfire years 1 to 3, when black-tailed deer were protected from hunting, populations increased to >15 black-tailed deer/mile². Most of the population increase was attributed to an increased number of births and decreased mortality, but some was also likely due to immigration from adjacent areas of poorer forage. Protein in black-tailed deer browse (e.g., vine maple, salmonberry, thimbleberry, red alder, red huckleberry, and red elderberry (Sambucus racemosa)) on the Tillamook Burn increased after the 1939 fire and then declined. For example, protein content in vine maple was 12.8% 3 years after the fire and 9.3% 6 years after the fire [109]. A comparison of adult black-tailed deer body weights in 1943, 4 years following the 1939 fire, and again in 1966, 27 years following the 1939 fire, showed that as the seral vegetation developed in the burned area and black-tailed deer populations increased, black-tailed deer body weights declined [147].
Postlogging practices employed in mule deer habitats in the Pacific Northwest often include prescribed fire, and black-tailed deer populations may be higher on logged and burned sites than on untreated sites. On the western Olympic Peninsula, Washington, black-tailed deer and elk used 2 clearcuts in western hemlock forest that were burned in a "patchy" prescribed fire in late May. Some plots were also fertilized, seeded with grasses and forbs, and planted with western hemlock, western redcedar, and Douglas-fir seedlings. It took 2 years for the treatments to be completed. During postfire year 2, mule deer and elk pellet group densities were higher on unburned plots than burned plots. During postfire years 3 and 4, pellet group densities were higher on burned than unburned plots, coincident with peak forage grass production in burned areas. During postfire year 5, when grass production declined markedly, pellet group densities were higher on unburned than burned plots [195]. On the Clemons Tree Farm in coastal Washington, black-tailed deer densities peaked in midsuccessional Douglas-fir-western hemlock forests after logging and prescribed fire. High densities in midsuccession were thought to occur because of use of these forests for cover (Table 2) [55]. In contrast, at the Starkey Experimental Forest and Range in northeastern Oregon, within a 30-mile² (78 km²) area enclosed by a fence, fuels in western spruce budworm (Choristoneura occidentalis)-killed grand fir-Douglas-fir forests were reduced by thinning and broadcast burning or slash pile burning in September or October. Between 1 and 5 years after treatment, patterns of stand use indicated no effects of fuels reduction treatments on ≥2-year-old does; mule deer either avoided treated areas or used all stand types proportional to their availability across seasons (spring and summer) and scales within the 19,300-acre (7,800 ha) landscape. The authors suggested that control stands likely provided better foraging opportunities than treated stands, particularly during hot summer months, because of rapid senescence of understory vegetation in areas with open canopies [200].
| Table 2. Black-tailed deer density in 4 seral stages of Douglas-fir-western hemlock forests on the Clemons Tree Farm in coastal Washington [55] | ||
| Black-tailed deer/mile² | Seral stage | Description |
| 35 | soon after disturbance* | recently logged and burned forest that was "just starting to produce deer forage" |
| 34 | early succession | 10 to 25 years after logging and burning when the food supply peaked |
| 57 | midsuccession* | logged and burned forests in midsuccession "when the forests had reached, or passed, their peak productivity" |
| 36 | late succession* | dense, mature 2nd-growth or old-growth forests with low forage productivity |
| *Time since disturbance not specified. | ||
Postlogging site preparation practices may benefit mule deer in the short term by increasing forage quality. As mentioned earlier, protein in black-tailed deer browse generally increased after the 1939 Tillamook Fire, then declined [109]. However, forage quantity and/or quality may not always increase on burned areas. Throughout western Washington, there was little difference in January protein content of black-tailed deer foods (e.g., trailing blackberry, salal (Gaultheria shallon), western redcedar, blue huckleberry (Gaylussacia frondosa), and red huckleberry) from 4 seral stages of Douglas-fir-western hemlock forests. The seral stages ranged from recently logged and burned forests to mature forests. The author stated that he may not have detected a difference because of high variability among samples and small sample sizes [55].
Increased forage abundance on logged and burned sites may increase black-tailed deer body condition. In coastal Douglas-fir, western hemlock, and spruce forests of western Oregon, harvested black-tailed deer bucks on recently burned forest averaged 213 pounds (97 kg), whereas harvested bucks in unburned, closed-canopy forest averaged only 125 pounds (57 kg). The protein content of black-tailed deer's most preferred browse (e.g., salal, red alder, salmonberry, thimbleberry, and red huckleberry) was higher on the burned forest than the unburned forest and may have contributed to the greater weight of black-tailed deer in logged and burned forest [109].
California California shrublandsAccording to a review, burning effects on chaparral vegetation depend on time of year. The quality of annual growth on unburned shrubs in chaparral is highest (15%-20% crude protein, depending on species) during rapid growth in spring and declines during the dry season when plants are dormant (4%-15% crude protein, depending upon species). If an area is burned in August or September, there is usually some sprouting in November or earlier, but most growth begins in April or May. Sprout growth continues during the early summer, when sprouts are high in protein (20%-30%) and moisture. Protein levels decline thereafter but remain higher than those in unburned chaparral vegetation until the 2nd postfire summer. If the fire occurs in spring, sprouting typically occurs within a few weeks after the fire. Protein levels following spring burning follow the same seasonal trend as those on fall-burned areas but are typically much higher than that of the fall-burned areas. This may be due to ash deposition and resultant abundant nitrogen after spring burning. In fall-burned areas, the ash has been leached away before it is used in spring. Unless growth of shrubs is controlled by moderate browsing or other means, many sprouting shrubs become unpalatable or unavailable to black-tailed deer within a few postfire years [170].
Lake County studies: A series of related studies reported nonmigratory black-tailed deer densities in chamise and mixed chaparral following fire in the North Coast Ranges of Lake County, California (e.g., [34,36,37,90,320,322,323,326,327]). These studies examined black-tailed deer use of 3 habitats: 1) mature, dense chaparral, with nearly pure chamise stands on south-facing slopes and mixed chaparral stands with interior live oak on north-facing slopes (hereafter, mature chaparral); 2) a large area of chamise and mixed chaparral burned in a September 1948 wildfire with scarce unburned islands (hereafter, burned chaparral); and 3) treated chaparral habitat with an "opened brushland" of interspersed patches of dense shrubs and herbs (hereafter, treated chaparral). The latter area was burned in a series of small prescribed fires, then seeded with native and nonnative herbs. Some of the area was burned again by a wildfire in August 1948 [36,90,326]. Burned and treated chaparral had higher quantity and quality of black-tailed deer forage than mature chaparral. Because of herbs seeded on the treated chaparral site, the average yearly proportion of grasses and forbs in the diets of black-tailed deer 1 year after fire was highest there (45%). It was higher in burned (14%) than mature chaparral (10%). Protein content in annual black-tailed deer diets averaged 17% on burned and 14% on treated chaparral 1 year after the fires, but only 9% on mature chaparral. All 3 habitats had a similar seasonal pattern, with black-tailed deer diets highest in protein in spring and lowest in fall after shrubs had ceased growing and grasses and forbs were mainly dry. The authors suggested that black-tailed deer diets in burned and treated chaparral had higher protein content than mature chaparral because of the higher proportion of high-protein, herbaceous forage in winter and early spring in these habitats. Shrubs in these habitats were shorter, and leaves, which were higher in protein than stems, were more available [327].
Black-tailed deer densities in burned and treated chaparral peaked during the1st postfire summer, then declined. In the burned chaparral, black-tailed deer were sparse in September immediately after fire because of lack of food. Density increased in burned chaparral in October and November after shrubs had sprouted [326]. It peaked at 120 black-tailed deer/mile² the 1st postfire year as black-tailed deer emigrated from surrounding areas and increased productivity [90,326]. Fawn production and survival increased in the burned chaparral during the 1st postfire spring and summer, probably due to increased herbaceous forage and accessibility of shrubs [90]. Production was higher in burned chaparral (1.15 fawns/doe) than in nearby mature chaparral (0.7 fawn/doe) the 2nd postfire spring [14,321,322]. However, the population declined the 2nd postfire summer because reduced forage quantity and nutritional quality led to many animals dying of malnutrition during July and August [90,326]. The population decreased in subsequent years (Table 3) [90,321,326]. Trends in black-tailed deer densities in treated chaparral were similar to those in burned chaparral. The July following treatments, black-tailed deer densities peaked at 131/mile² then declined to 82/mile² the 5th postfire summer (Table 3) [326]. Overall, black-tailed deer densities were higher in treated than burned chaparral because seeding of herbs in treated chaparral led to higher forage production [323]. In addition, higher black-tailed deer density in the treated chaparral kept sprouts hedged and accessible longer [36]. Bissell [32,34] noted that although densities in mature chaparral were lower than those in burned and treated chaparral (about 30 black-tailed deer/mile² [326]), mature chaparral provided more acorns, an important fall food. This suggests the importance of habitat heterogeneity to black-tailed deer in chaparral habitats.
| Table 3. Black-tailed deer population density in burned and treated chaparral in Lake County, California [326] | ||
| Summers since fire | Black-tailed deer/mile² |
|
| Burned chaparral | Treated chaparral |
|
| 1* | 120 | 131 |
| 2 | 106 | 112 |
| 3 | 52 | 103 |
| 4 | 44 | 85 |
| 5 | no data | 82 |
| *Black-tailed deer density in nearby, mature chaparral during this summer was 30/mile². | ||
Black-tailed deer body weight may improve following fire. During the hunting season in Lake County, California, bucks from treated and burned chaparral tended to be heavier than bucks in mature chaparral. Doe weights tended to be highest in treated chaparral, intermediate in burned chaparral, and lowest in mature chaparral. Researchers suggested that black-tailed deer body weight was higher in treated and burned chaparral because of higher-quality summer diets compared with mature chaparral [34,36,327].
In contrast to studies in Lake County in the North Coast Ranges, a study in the Central Coast Ranges found no increase in black-tailed deer population size after prescribed fire in chaparral. Instead, the density of black-tailed deer was significantly higher in oak woodlands and grasslands than in burned chaparral for all seasons (P<0.05 for all comparisons), except the 2nd growing season after fire. Density in chaparral did not increase until the 2nd growing season after fire, and then declined to prefire numbers within 6 months. Density increased again during the 3rd postfire growing season, but the increase was not as great as the previous growing season. No significant change in fawn survival occurred after the fire, and the increase in density during the growing season was attributed to female groups moving into chaparral from oak woodlands rather than to an increase in population size. The results demonstrated that burning chaparral may not result in increased black-tailed deer numbers. The lack of an effect of burning was attributed to habitat heterogeneity and the juxtaposition of chaparral habitats near oak woodlands and grasslands in this Central Coast Range site [174].
Other California shrublands: Dasmann [94] stated that fire offers no short-term benefits to mule deer on winter rangelands on the east side of the Sierra Nevada because it removes important winter browse and cover. However, in the Owens Valley, California, a study reviewed the effects of 3 wildfires and 2 prescribed fires on mule deer winter rangelands dominated by blackbrush (Coleogyne ramosissima) and concluded that fire increased plant species diversity and mule deer carrying capacity on most blackbrush rangelands during the first "few" years after fire by increasing annuals, shrub seedlings, and sprouts [26].
California woodlands: Black-tailed deer pellet group counts in spring were 1.7 times higher on burned sites than adjacent unburned sites after a prescribed fire near Trinity Reservoir, northern California. The vegetation type was a gray pine-Oregon white oak-California black oak (Quercus garryana-Quercus kelloggii)/wedgeleaf ceanothus-true mountain-mahogany woodland. Black-tailed deer appeared to be attracted to the burned area due to a flush of new herbaceous growth in early spring. By postfire year 4, however, pellet group counts were similar among burned and unburned areas [167]. For more information about black-tailed deer use of oak habitats after fire, see California shrublands.
California forests: In California forests, mule deer may benefit from availability of both burned and unburned areas. On the western slope of the Sierra Nevada—in areas within a giant sequoia (Sequoiadendron giganteum) grove that were treated by cutting, piling, and prescribed burning—mule deer forage was more abundant and utilized more heavily by mule deer on treated than untreated, control areas. In addition, many mule deer forage species (e.g., California hazelnut, ceanothus, canyon live oak, Sierra mountain misery (Chamaebatia foliolosa), and sedge) were higher in protein on treated than untreated areas. The results were attributed in part to increased sunlight on treated areas. The authors concluded that prescribed burning favored the germination of seeds of the shrubs most valuable for mule deer. However, the optimal mineral balance resulted from mule deer feeding on both treated and untreated sites because some mule deer forage species, such as Pacific dogwood (Cornus nuttallii), had higher calcium and phosphorus levels on shady, control sites than on sunny, burned sites [191].
The benefits of logging and prescribed fire to black-tailed deer may be short-lived. According to a review, black-tailed deer density in redwood forests of northern California increases following fire and logging from about 3/mile² to >70/mile², with the greatest increase occurring from about 5 to 10 years after disturbance. After 10 years, shrub and tree cover becomes dense or grows out of reach and protein content declines. Black-tailed deer then decrease in number until 10 years after disturbance, when they reach their original low levels [91].
Southwest Southwest grasslands: Fires may improve palatability of plants in southwestern grasslands. Fires that burn off the spines from cacti (cholla (Cylindropuntia spp.), pricklypear (Opuntia spp.), and barrel cactus (Ferocactus spp.)) make cacti more palatable and/or available as forage for mule deer [210,221]. In grazed southwestern shrubsteppe south of Tucson, Arizona, deer were attracted "almost immediately" to an area that was burned under prescription in November, in part because of the attractiveness of pricklypear. Nearly all pricklypears from which spines were burned were consumed by deer and other animals "within a few weeks" [221]. In desert grasslands, old growth of tobosa (Pleuraphis mutica), big sacaton (Sporobolus wrightii), and Johnson grass (Sorghum halepense) is relatively coarse and unpalatable to mule deer, white-tailed deer, and other ungulates, but their new postfire growth is succulent and readily eaten [341].Fires at the grassland-woodland ecotone may remove woody vegetation without increasing ground cover [210], which may be detrimental to mule deer. For more information, see FEIS reviews of species of interest.
Southwest shrublands
Interior Arizona chaparral:
Mule deer are common in interior Arizona chaparral [143,290,319,345]. Because most shrub dominants sprout after fire or germinate quickly from seeds after fire, fire in this community may increase forage for mule deer [62,143]. Forbs and grasses develop rapidly after fire and are generally abundant for 3 or 4 years, followed by an abrupt drop to prefire levels in 2 to 3 more years, with forbs declining more rapidly than grasses. The decrease in herbs is associated with an increase in shrubs over time. Shrubs generally recover rapidly and dominate the site in about 5 years, regaining prefire values approximately 11 years after fire [290,345]. In the Mingus Mountain area, forb production peaked at about 281 pounds/acre in the 3rd postfire growing season after an 18,000-acre (7,300 ha) June wildfire, while grasses peaked at 213 pounds/acre in the 5th postfire growing season. Shrub cover and biomass were still increasing 6 years after the fire, when the study ended [246]. In the Three Bar Wildlife Area, forb and grass production was about 217 to 325 pounds/acre in the 5th and 6th postfire growing seasons and 109 to 110 pounds/acre in the 7th and 10th postfire growing seasons [143]. Longevity of increases in mule deer forage quantity in interior Arizona chaparral varies with productivity of the site. In interior chaparral in east-central Arizona that was seeded with nonnative weeping lovegrass (Eragrostis curvula) following a severe prescribed fire, shrub growth in the burned area was the most rapid the first 2 years following fire; by postfire year 5, shrub density was equal to that on the unburned control [62].
Burning may increase the nutrient content of mule deer browse in interior Arizona chaparral. Protein content of deer browse in recently burned areas in 3 regions of Arizona was generally higher than that in unburned areas but declined over time. Protein contents of plants on 9-month-old and 3-year-old burned sites were similar to those on adjacent, unburned sites, indicating that the effects of burning on plant nutritive quality were short-lived. Browse use by deer was much greater on burned than unburned sites [319].
Gambel oak: Mule deer consume Gambel oak leaves, twigs, and acorns [9,254]. For example, along the Wasatch Front in Utah, Gambel oak may provide up to 75% of the available winter browse [254]. Although important to wintering mule deer in terms of forage availability and palatability, Gambel oak ranks low in nutritional value [254]. It sprouts after fire, and fire in Gambel oak communities may result in abundant, succulent browse for mule deer. On the Uinta National Forest, Utah, examination of Gambel oak stands that had been burned 3 and 15 years prior to the study indicated that burned stands recovered to unburned control levels in 6 to 35 years, with stands at low elevations recovering faster than stands at high elevations (r=0.99, P<0.01). Mule deer wintered at low elevations, where postfire recovery of Gambel oak was most rapid [186].
Fire in Gambel oak communities may not always result in abundant browse. Two, 5, and 10 years after a fall mixed-severity prescribed fire in Gambel oak rangeland in western Colorado, total vegetation production decreased 17%, 27%, and 19%, respectively, compared to prefire levels. Most of the reduction occurred among shrubs, particularly big sagebrush and common snowberry. They decreased 38% during the 2nd and 5th postfire years and 46% after 10 years compared with prefire levels, partially as a result of dry weather. Relative to prefire levels, seeded and native grasses decreased by 5% after 2 years and 29% after 5 years, but they increased by 76% after 10 years. Forbs increased by 14%, 2%, and 17% after 2, 5, and 10 and postfire years, respectively [181].
Mule deer in Gambel oak rangelands in western Colorado may not have responded to prescribed fire and other treatments because of heavy hunting pressure [181]. They occurred at similar densities in treated (see treatments in Table 4) and untreated sites within Gambel oak rangelands 2, 5, and 10 years after treatment, except that densities were reduced 61% on chained sites 2 years after treatment. Burned sites had patches of unburned or partially burned vegetation, which increased mule deer access to forage and provided escape cover and shade. In sprayed areas, dead material remained standing for the first few years, leaving a thicket only slightly more open and accessible than before spraying. During posttreatment years 5 and 10, accessibility decreased further as dead materials fell against shrubs. Mule deer use of chained stands may have been reduced because chaining treatments reduced cover the most. During posttreatment year 10, chained areas were still open and accessible but cover had increased. The authors noted that hunting pressure on mule deer was very heavy during posttreatment year 2 [181].
| Table 4. Mule deer use of prescribed burned, herbicide sprayed, or chained Gambel oak rangelands in western Colorado 2, 5, and 10 years after treatments [181] | |||||||
| Years since treatment | Control | Treatment |
|||||
Prescribed fire* |
Herbicide spraying |
Chaining |
|||||
| Mule deer density** | Mule deer density |
% change mule deer density*** | Mule deer density | % change mule deer density | Mule deer density | % change mule deer density | |
| 2 | 19.1 | 16.7 | -13 | 15.0 | -21 | 7.4 | -61**** |
| 5 | 17.6 | 24.2 | +38 | 25.9 | +47 | 24.1 | +37 |
| 10 | 18.1 | 15.6 | -14 | 26.0 | +44 | 14.1 | -22 |
| *An October mixed-severity fire. | |||||||
| **Number of mule deer/2.59 km². Based on mule deer pellet group counts and the average deer defecation rate of 13 groups/day. | |||||||
| ***Calculated as the percent difference between treatments and controls. | |||||||
| ****Denotes significance at P≤0.10. | |||||||
Fire may increase nutrition of plants in Gambel oak communities. Two growing seasons after a fall mixed-severity prescribed fire in Gambel oak rangeland in Colorado, zinc and copper levels were higher in forbs, grasses, and shrubs on burned than unburned sites. However, no differences were found in the protein, lignin, calcium, or phosphorus content of these plants on burned and unburned sites [181]. Less than 1 year after a 1,270-acre (514 ha) August wildfire burned Gambel oak and mountain big sagebrush on southwest-facing slopes in north-central Utah, nutrient values (protein, phosphorus, and in vitro digestibility) of Gambel oak buds and stems from burned stands were significantly higher than those of unburned stands (P<0.001). However, tannin content of the sprouts was also higher than that in unburned stands, and overall forage value of Gambel oak to wintering mule deer was relatively low [254]. In southwestern mixed-conifer forest on the Lincoln National Forest, New Mexico, the nutritional quality of wavyleaf oak (Quercus × pauciloba), Gambel oak, and alligator juniper—all important mule deer browse—was generally higher on burned sites 2 to 4 years after a severe April wildfire than on unburned sites. However, the authors cautioned that differences were unlikely to be biologically significant to mule deer. Nutritional quality of browse varied with the plant species' responses to fire, plant parts sampled (leaves or stems), and the season sampled (winter, fall, or spring) [228].
According to a review, whether mule deer use a Gambel oak community after fire depends on the structure of the community and type of vegetation present on adjacent areas as well as the type of fire and size of the burned area. Mule deer may benefit most from mixed-severity fires that create openings in very dense stands and increase herbaceous plant production in the understory [254]. In north-central Utah, mule deer use of Gambel oak browse on burned and unburned sites was similar. The authors suggested that mule deer may have used the sites similarly despite increased nutritional quality of browse on burned sites because cover on burned sites was reduced, important browse species such as mountain big sagebrush and antelope bitterbrush were reduced, and tannin content of forage was increased on burned sites. They suggested that burning may temporarily improve forage nutritional quality on Gambel oak rangelands but that, without repeated fire, the postfire proliferation of Gambel oak sprouts may ultimately result in dense, less usable Gambel oak forage and fewer understory species. Moreover, mule deer habitat quality may be reduced by the reduction of "fire-susceptible" browse species such as mountain big sagebrush and antelope bitterbrush [254].
Too frequent fire in Gambel oak communities may be deleterious to mule deer by reducing acorn crops because few acorns are produced from small diameter stems. See the FEIS review of Gambel oak.
Great Basin Great Basin shrublands: Fire may have short-term negative effects on mule deer in the Great Basin and other parts of the Intermountain West by reducing cover and important winter forage species such as sagebrush, antelope bitterbrush, desert bitterbrush (Purshia glandulosa), and Stansbury cliffrose [126,142,146]. Klebenow [172] noted that mule deer avoided large burned areas on a Nevada winter range until antelope bitterbrush and other shrubs recovered, which often took 15 years. The carrying capacity of California's Lassen-Washoe winter rangelands was reduced after large wildfires in the 1980s increased mule deer susceptibility to winter mortality because of loss of shrub cover. Small openings in sagebrush communities may favor mule deer by increasing habitat heterogeneity, but large fires that reduce heterogeneity and cover are typically detrimental [339]. However, lack of fire for long periods in Great Basin shrublands may reduce shrubs as succession proceeds to pinyon and juniper [146], thus reducing important winter forage.Antelope bitterbrush and sagebrush, particularly big sagebrush, are important mule deer browse species in the Great Basin and elsewhere that may be reduced by fire. Antelope bitterbrush's response to fire is variable, depending upon genetics and site characteristics, but it is likely to decrease following even low-severity fire. In south-central Oregon, antelope bitterbrush declined following low-severity fire and showed little increase in cover during the subsequent 5 to 6 years [61]. In southwestern Montana, 8 years after a prescribed fire in an antelope bitterbrush-mountain big sagebrush-bluebunch wheatgrass community, antelope bitterbrush density did not differ between burned and unburned sites, but antelope bitterbrush cover, flower production, and seed production were less on burned than unburned sites. Mountain big sagebrush density and cover were less on burned sites, while total herbaceous cover was similar on all sites [116].
Frequent fire could reduce the amount of browse available to mule deer by eliminating antelope bitterbrush from large areas. In an antelope bitterbrush-cheatgrass community in south-central Washington, vegetation production, cover, and species composition were similar on unburned areas and areas burned in wildfires in 1963 and again in 1970, except that antelope bitterbrush and big sagebrush were killed by burning and had not established in the 8 years following the 1970 fire [272].
A summer wildfire in Wasatch County, Utah, killed most antelope bitterbrush plants, and postfire growth was slower for plants within the burned area. The number of mule deer pellet groups was higher in unburned areas (16-36 groups/100 feet²) than burned areas (4-8 groups/100 feet²) 4 years after the fire. Burned areas also had fewer big sagebrush plants than unburned areas, so the difference in mule deer use between burned and unburned areas appeared to be due to reduced forage availability and probably deeper snow on burned areas [142]. In south-central Oregon, antelope bitterbrush declined following low-severity fire and showed little increase in cover during the subsequent 5 to 6 years. The authors suggested that burning in a mosaic pattern may enhance antelope bitterbrush regeneration, and thus mule deer habitat, by maintaining scattered, unburned plants as seed sources [61].
Klebenow [172] provided the following generalizations about mule deer use of antelope bitterbrush communities after fire: Initially, mule deer tend to avoid large burned areas, particularly in winter, although they may be attracted to some burned areas in early spring when green vegetation first becomes available. From 10 to 15 years after fire, mule deer may again use the burned area if cover of antelope bitterbrush and other shrubs ranges from about 10% to 15%. The timing of antelope bitterbrush reestablishment varies, depending in part on the distribution of seed caches by rodents, weather, and cattle use of the burned area [172]. The effects of fire on antelope bitterbrush, and thus mule deer, depend in part on fire severity and timing. Severe late summer fires in Idaho killed 66% of the antelope bitterbrush, while a moderate-severity spring fire in Montana killed only 33%. A summer fire of moderate severity in Oregon killed an entire stand of antelope bitterbrush [127]. See the FEIS review of antelope bitterbrush for more information.
Some researchers reported increases in herb and/or shrub productivity and nutritional quality after fires on sagebrush rangelands [302,336]. In south-central Wyoming , a study investigating effects of 2 wildfires and 3 prescribed fires on production of herbs and shrubs in mesic, high-elevation big sagebrush communities found production of perennial herbs on burned sites averaged twice that on unburned controls by postfire year 3, while production of annual herbs varied little 2 to 3 years after fire. Productivity of important browse species increased and generally compensated for mortality, which was <15% for Saskatoon serviceberry, 55% for antelope bitterbrush, and 25% for true mountain-mahogany. Crude protein content of herbs from late spring through early fall was significantly higher on burns for 2 years after fire (P≤0.05). The authors speculated that the consistent increases in plant productivity and nutrient concentrations across all burned sites resulted from the mesic conditions [79]. A review by Nielson and Hinckley [238] stated that mule deer prefer rangelands containing a variety of herbs and shrubs to those dominated by big sagebrush, noting that when more palatable shrubs are available in winter, mule deer use of big sagebrush decreases.
A review stated that on mule deer sagebrush-grassland winter rangelands "it is possible to have short-term and long-term benefits (from fire), but there is a great possibility of both short-term and long-term losses as well". The author described effects of fire in antelope bitterbrush and sagebrush communities as follows [172]:
Short-term effects (<15 years):The effect of prescribed fire in pinyon-juniper woodlands on mule deer depends on what plant species were present in the understory prior to fire and whether deep snow occurs in the area [41,111,192,225,290]. Everett [111] cautioned that prefire composition in pinyon-juniper stands will most likely determine the postfire plant composition. If sprouting shrubs are present before fire, they will likely come back following fire. If sprouting shrubs are not present, perennial grasses are likely to develop [41]. In pinyon-juniper woodlands adjacent to interior Arizona chaparral stands with similar shrub composition, prescribed fire may result in increased production of sprouting shrubs and increased herbaceous growth. In the Coconino and Kaibab plateaus in Arizona, however, most understory shrubs (for example, Stansbury cliffrose and big sagebrush) associated with pinyon-juniper woodlands are easily killed by prescribed fire. However, herbage production may increase [290]. On the Hualapai Indian Reservation in Arizona, herbage production increased significantly after prescribed and wildfires in pinyon-juniper communities. As a result, mule deer used burned areas more than unburned areas during most of fall and winter (P<0.10). Stands burned in prescribed fires ranged from 4 to 12 years old, and a stand burned in a wildfire was 15 years old. Because snow was not deep in this study area, shrubs were not critical winter forage items. In addition, precipitous terrain provided cover for mule deer. After 13 to 15 years, browse plants were more abundant in unburned areas, but grasses and forbs were more abundant on burned areas [225].
In pinyon-juniper woodlands, mule deer often concentrate their activities near edges of burned areas. In eastern Nevada and eastern California, an evaluation of 11 wildfire burns in pinyon-juniper communities ranging from 2 to 115 years old found that mule deer pellet groups and tracks did not occur inside recently burned areas in a grass-forb stage of succession; instead, pellet groups were concentrated within 330 feet (100 m) of the burned edge in the unburned pinyon-juniper woodland. On burned areas in a shrub-dominated stage of succession, more deer pellet groups were found within the burned area away from the edge than within 160 feet (50 m) of the edge. Big sagebrush and steep and broken topography substituted for tree cover in these areas [307]. In pinyon-juniper woodland on the Hualapai Indian Reservation, mule deer used the edges of burned areas more frequently than they used the interiors of burned areas or unburned areas. Pellet group accumulation rates were highest in burned areas 1,320 to 2,640 feet (400-800 m) from unburned areas [225].
See Other treatments for more information on mule deer use of pinyon-juniper woodlands.
Great Basin forests:
Ponderosa pine:
Fire in ponderosa pine stands may benefit mule deer by increasing understory vegetation quantity and nutritional quality [33,289,290]. According to a review, nutrient responses in ponderosa pine in Arizona vary with the type of understory, age and structure of the forest, and season. However, prescribed fire generally increases nutrient availability and concentrations, which improves forage quality for mule deer and other wild ungulates for at least the 1st postfire growing season [289]. A study in Arizona ponderosa pine found that in the 1st growing season after fire, crude protein, phosphorus, and in vitro digestible dry matter were higher in mule deer forage from areas burned in a severe May wildfire than in adjacent unburned controls. Increases in phosphorus and digestible dry matter lasted to the 2nd postfire year but increases in protein did not. By the end of the 2nd growing season, however, there were no differences in nutritional content of mule deer forage between burned areas and unburned controls [249].
Mule deer use of ponderosa pine stands may increase after burning in response to increased forage production and edge, although responses vary in the first few postfire years. Near Flagstaff, Arizona, deer use of a ponderosa pine forest that had been burned in a high-severity May wildfire increased for the first 2 years after the fire, then became "inconsistent" during the 3rd year, possibly due to reinstated cattle grazing on the burned areas [177]. In another ponderosa pine forest in Arizona, mule deer use of burned areas decreased in all seasons the 1st postfire year. Summer and fall use then increased 2.5 times more than use on an unburned control through the rest of the 20-year study. In winter and spring, however, mule deer use returned to control levels for a few postfire years, then increased to 120 times more than that of the control at the end of the study. Increased use was likely the result of increased amount of edge and forage production, especially forbs and ceanothus, on the burned areas [205]. In a recently logged ponderosa pine forest on the Coconino National Forest that burned in a May wildfire, deer pellet densities were higher in a moderate-severity burned area during postfire summers 1 to 3 than in an unburned control. However, pellet group densities were higher in the control than in a high-severity burned area during postfire summers 1 and 2. During postfire summer 3, pellet group densities were higher in the high-severity burned area than the control (Table 5). The result was attributed to the production of palatable herbaceous species on burned areas. Herbaceous plant production was similar on all sites during the 1st postfire summer (range: 452-582 pounds/acre). During the 3rd postfire summer, production averaged 1,651 pounds/acre on the high-severity burned area, 1,275 pounds/acre on the moderate-severity burned area, and only 559 pounds/acre on the unburned control [64].
| Table 5. Mean deer pellet groups/acre in a logged and burned ponderosa pine forest on the Coconino National Forest, Arizona, 1 to 3 summers after wildfire [64] | |||
| Summers since fire | Moderate-severity fire | High-severity fire | Unburned control |
| 1 | 1,001 | 257 | 672 |
| 2 | 398 | 191 | 267 |
| 3 | 363 | 262 | 116 |
Although total biomass of grasses and forbs often increases in ponderosa pine forest after fire, the quantity of useable mule deer forage may actually be less on burned areas if species composition shifts to relatively unpalatable species [210]. In the short term, prescribed understory burning failed to improve herbaceous forage production for deer in ponderosa pine stands near Flagstaff, Arizona. Although herbaceous plant production increased dramatically, nonnative common mullein (Verbascum thapsus), an unpalatable species, dominated the understory 1 year after fire [113].
Quaking aspen: Quaking aspen communities are important summer and fall rangelands for migratory mule deer throughout the West [203,248,290]. Leckenby and others [193] rated quaking aspen communities in the Great Basin shrubsteppe ecosystem as 2nd only to riparian zones in value to mule deer. According to a review, quaking aspen is among the top 10 preferred browse species for mule deer, and understory species in quaking aspen communities provide abundant forage [203]. Another review stated that prescribed fire in quaking aspen parklands may benefit mule deer and white-tailed deer by: 1) top-killing sprouting woody plants; 2) providing a seedbed for establishment of forage species; and 3) increasing the nutrient level and digestibility of browse and herbs the first 2 years after burning [19]. The effects of prescribed fire on quaking aspen stands and fire's resulting effect on mule deer depend, in part, upon the amount of postfire quaking aspen sprouting. Young quaking aspen trees are more likely to sprout than old trees [290]. See the FEIS review of quaking aspen for more detailed information. Maximum sprout densities are typically realized the 1st and 2nd postfire years, followed by a gradual decline [290]. Abundant mule deer browse is typically available for 5 to 8 years following burning, at which time leafy crowns typically grow beyond the reach of mule deer. Small burned areas or clearcuts may attract concentrations of mule deer and other browsing animals, to the point where quaking aspen browse is eliminated (see Mule deer interactions with fuels and fire effects) [248,290]. Thinning quaking aspen stands, rather than burning or clearcutting, may promote herbaceous understory production rather than quaking aspen sprouting [290]. Mature quaking aspen stands may provide better cover for mule deer than clearcut stands [333]. See these reviews on managing quaking aspen for mule deer and other wildlife: [248,333].
Rocky MountainsIn contrast, fire in Rocky Mountain shrublands may be detrimental to mule deer by removing important browse. For example, a Wyoming big sagebrush (Artemisia tridentata subsp. wyomingensis)/bluebunch wheatgrass winter rangeland on the East Fork of the Salmon River in Idaho was burned under prescription in fall. Grass production decreased slightly the 1st year after burning but returned to prefire levels 2 years afterwards. Frequency of bluebunch wheatgrass was reduced for 2 years but returned to prefire levels by the 3rd postfire year. However, Wyoming big sagebrush, a preferred mule deer browse species, was removed from the site for at least 4 years after burning [252].
Rocky Mountain forests: Lyon [207] provided a generalized description of mule deer and white-tailed deer response to postfire succession in forests in the northern Rocky Mountains: Immediately following a severe fire, the landscape may appear barren and provide little forage for deer. As early as the 1st growing season after fire, some woody seedlings may appear, and plants not killed by fire may sprout. Within the first few postfire years, forbs and grasses dominate the area, and shrub cover increases. As shrub cover increases, forbs and grasses decrease. If the shrubs are palatable to deer, they can provide abundant deer forage. Shrub dominance may continue from 10 to 100 years after fire, but shrubs are eventually displaced by trees. In mature forests, understory vegetation is typically sparse and provides little forage for deer [207]. Plant succession on large, severely burned areas may be slow compared with that on small burns because of low plant survival within burned areas and remoteness of seed sources [207,235]. A review stated that the positive effects of fire on deer forage generally last <30 years [210]. An "intense" prescribed fire in Douglas-fir forest in Idaho improved forage for mule deer and elk; the improvement was expected to last >20 years [208].
Mule deer may benefit from the development of shrubfields resulting from fire in forests of the Rocky Mountains and elsewhere (e.g., [15,16,222]). On a mule deer ponderosa pine-Douglas-fir winter rangeland in the Rattlesnake River drainage near Missoula, Montana, a wildfire in 1919 resulted in shrubfields dominated by snowbrush ceanothus and mallow ninebark (Physocarpus malvaceus). About 40 years after the fire, the shrubfields were developing into forests, and mule deer populations appeared to be declining as a result [171]. Large fires in Idaho during 1910, 1919, and 1934 created extensive shrubfields in mule deer winter range. In the 1950s, a helicopter survey of the North Fork Clearwater River drainage found that 68% of winter rangeland for mule deer burned between 1889 and 1949, with 41% of the area burned in 1919. In the Selway River drainage, fires burned 32% of the mule deer winter rangeland (Norberg and Trout 1957 cited in [370]). The postfire successional shrubfields appeared to favor mule deer [370]. Mule deer use of postfire successional shrubfields may decrease as vegetation grows tall and out of reach. In the Lochsa River area, Idaho, prescribed fires in spring and fall decreased shrub height to a level accessible to mule deer and elk. Four years after burning, 3 primary mule deer and elk browse species (Rocky Mountain maple (Acer glabrum), willow, and Saskatoon serviceberry) averaged nearly 10.5 feet (3.2 m) tall, with about 80% of their twig production within reach of the ungulates [194].
Most studies of fire effects in Rocky Mountain forests indicate that burning favors mule deer by increasing forage. A review reported that grass and forb biomass generally increases the first 5 to 10 years following stand-replacing fires in Rocky Mountain forests [210]. Quaking aspen stands are important mule deer foraging habitats in the Rocky Mountains and throughout the West (see Great Basin forests) [203,248,290]. Grass and forb biomass decreased the 1st growing season after fire in quaking aspen stands in western Wyoming but increased the 2nd and 3rd growing seasons to above prefire levels. On severely burned sites, grass recovered more slowly than forbs [23]. Mule deer primarily foraged in burned habitats 3 winters after an August mixed-severity wildfire. The fire burned 2,700 acres (1,100 ha) of mule and white-tailed deer winter rangeland in the Selway-Bitterroot Wilderness, Idaho. Mule deer used burned Douglas-fir/mallow ninebark and ponderosa pine/bluebunch wheatgrass communities more frequently than their availability during winter. In spring, mule deer still preferred burned Douglas-fir/mallow ninebark and ponderosa pine/bluebunch wheatgrass communities but increased use of other habitats, including burned grand fir/white clintonia (Clintonia umbellulata) communities. Shrubs that sprouted after fire, particularly Saskatoon serviceberry, mallow ninebark, and Scouler willow (Salix scouleriana), were most heavily used by mule deer [166].
Burning may increase the palatability of mule deer forage in Rocky Mountain forests. In the Selway-Bitterroot Wilderness, Idaho, mule deer used mallow ninebark more on burned areas than on unburned areas. The authors suggested that mallow ninebark palatability was increased by burning [166].
Snags may provide important cover for mule deer in burned areas. In a conifer forest near Laramie and Saratoga, Wyoming, mule deer pellet groups were 1.3 to 9.3 times greater on plots burned in wildfires than on nearby, similarly-aged clearcut plots 5 and 10 years after disturbance. This was attributed in part to a greater variety of forage species and to greater hiding cover provided by snags in burned areas (633-722 snags/ha) compared to clearcuts (0-66 snags/ha) [96]. Eight years after a severe August wildfire in a lodgepole pine forest near Fort Collins, Colorado, mule deer used the burned area more than adjacent, unburned lodgepole pine stands from September to June (P=0.01), apparently because of abundant cover provided by snags and abundant seeded grasses in the burned area [279].
Although fire may create snags that provide cover, fire may also remove important cover from Rocky Mountain forests. Deer pellet group counts were "negligible" during the winter and spring immediately following the Moose Creek Fire on the Salmon National Forest, and they were substantially reduced from prefire counts during postfire year 1. Prefire cover within and adjacent to the burned area was limited due to previous logging and the natural sparseness of the forest. The fire removed much of the remaining cover, and only one "sizeable" patch of cover remained. The author noted that despite road closures, hunting pressure on deer using the burn during the fall immediately after the fire was high. The fire was a mixed-severity August wildfire in a mosaic of curlleaf mountain-mahogany/bluebunch wheatgrass, bluebunch wheatgrass/needle-and-thread grass, spiny grease bush (Glossopetalon spinescens), mountain big sagebrush, ponderosa pine, and Douglas-fir communities [73]. Lack of cover and forage in burned areas may limit mule deer use. One year after a 83,500-acre (334,800 ha), mixed-severity August to September wildfire in the southern Black Hills of west-central South Dakota and northeastern Wyoming, all radio-collared mule deer selected ponderosa pine/true mountain-mahogany/Rocky Mountain juniper habitat with >70% cover and a grass-forb and shrub understory for foraging and bedding. Meadow habitats were avoided. Burned ponderosa pine habitat was used in proportion to availability. During summer, radio-collared mule deer selected unburned ponderosa pine habitat and avoided burned ponderosa pine, unburned ponderosa pine/quaking aspen, and meadow habitats [104].
Postlogging site preparation in mule deer habitats in the Rocky Mountains may include prescribed fire, and mule deer browse production may be higher on clearcut and burned sites than on unburned clearcut sites. In Mineral County, Montana, mule deer browse production was higher 5 to 6 years after clearcutting and burning under prescription (48.4 pounds/acre) than after clearcutting alone (20.8 pounds/acre). The result was attributed to increased production of redstem ceanothus (Ceanothus sanguineus) and evergreen ceanothus (C. arboreus), both of which had abundant sprouting on clearcut and burned areas [356].
Postfire seeding of conifers may reduce the amount of time that abundant mule deer forage is available. In western Montana, mule deer browse biomass 37 years after fire was lowest within Douglas-fir/mallow ninebark habitat that was seeded with Douglas-fir soon after fire. It was highest in unseeded postfire successional shrublands (Table 6) [356].
| Table 6. Mule deer forage biomass (pounds/acre) on 2 sites in western Montana, 37 to 39 years after wildfire [356] | ||||
| Location | Years of fire | Years since last fire | Stand characteristics | Forage biomass |
| Boyd Mountain | 1910, 1931 | 37 | dense tree canopy as a result of seeding with conifers after fire | 1 |
| 1910 | 37 | no forest overstory and dense shrubs | 91 | |
| Tamarack Creek | 1929 | 39 | no forest overstory and dense shrubs, particularly evergreen ceanothus | 496 |
Nutritional quality of mule deer forage species in the Rocky Mountains may increase, decrease, or remain unchanged by burning. Any effects are usually short-lived. One year after prescribed fire in western redcedar/Oregon boxwood (Paxistima myrsinites) communities on mule deer winter range in northern Idaho, 4 browse species (redstem ceanothus, willow, serviceberry, and Rocky Mountain maple combined) were higher in moisture and crude protein content on burned sites than unburned sites. However, the effect was absent during postfire years 2 and 3 [15]. In western larch-Douglas-fir stands in Montana that had been burned with "light, moderate, or hot" understory fires 3 years previously, nutrient content of plants was compared with samples from stands not burned for 70 years. Sodium levels were higher for several mule deer forage species (heartleaf arnica (Arnica cordifolia), fireweed (Chamerion angustifolium), and white spirea (Spiraea betulifolia)) in stands where at least half the duff was consumed by fire (i.e., moderate or severe fires). Iron levels were significantly greater in some forage species on burned than unburned sites (heartleaf arnica, dwarf bilberry (Vaccinium caespitosum)). Calcium (white spirea, fireweed) and phosphorus (white spirea) levels were significantly lower on burned than unburned sites (P≤0.05 for all variables). Of the species tested, none that occurred in both burned and unburned sites (e.g., heartleaf arnica, dwarf bilberry, white spirea, and fireweed) showed significant differences in nitrogen, manganese, or copper content due to burning [309]. During the 4 years following a late-summer wildfire in a xeric ponderosa pine forest and adjacent montane grasslands used as winter-spring rangeland by mule deer in the Selway-Bitterroot Wilderness in Idaho, mineral concentration in herbaceous plants tended to be similar between burned and unburned sites. However, nitrogen and potassium were lower on burned sites than unburned controls the first year following the fire [229].
Better nutrition on burns may lead to increased body condition of mule deer. After a 83,500-acre (334,800 ha), mixed-severity August to September wildfire in the Black Hills of west-central South Dakota, increased mule deer body condition during the 2nd and 3rd postfire years was attributed to increased nutritional quality of forage in burned areas [353].
Fire may shift plant composition in communities, which may harm or benefit mule deer. In western larch-Douglas-fir stands in Montana, unburned control sites were covered by palatable mule deer forage species such as white spirea, huckleberry (Vaccinium spp.), twinflower (Linnaea borealis), and grasses, whereas sites burned in a "hot" understory prescribed fire were largely covered by Marchantia polymorpha—a liverwort with unknown palatability to mule deer—and fireweed, which is a highly palatable and nutritious mule deer forage species. Species composition of sites burned with a "light" understory fire was similar to that of unburned control sites [309]. Mule deer populations may not increase after a fire if the fire does not increase palatable forage. Over 5,400 miles² (14,100 km²) burned in the 1988 Greater Yellowstone Area fires, including 42% of Yellowstone National Park [49,296]. Mule deer populations declined 19% during the first winter after the fires. Singer and others [297] speculated that mule deer may have declined because they were consuming less palatable shrubs after the fires.
Great Plains Great Plains grasslands: Historically, mule deer in the Great Plains were largely confined to breaks and rough terrain, where shrubs were protected from fire [146]. According to a review, tree and shrub cover in the Great Plains is limiting to mule deer populations, so fire that removes available cover may have a negative effect on mule deer. However, fire that results in sprouting of trees and shrubs may benefit mule deer by increasing browse, and after such plants are tall enough, cover [290]. In parts of the northern Great Plains where snow is deep, optimum mule deer habitat may not be reached until 30 or more years after fire, when woody plants used as cover have developed on grassland sites. However, the absence of fire from grasslands for >50 years may result in tree encroachment, canopy closure, and reduction of herbs and shrubs, thus reducing mule deer habitat quality [146].In the Great Plains, grassland fire may result in early green-up of warm-season grasses, improved seed germination, and increased production of grasses and forbs [210], which may be beneficial to mule deer on summer rangelands. Many studies that reported increased production of forage after fire also described some circumstances under which production was reduced. In general, fires followed by drought and fires in areas averaging <11 inches (300 mm) of summer rainfall may result in decreased forage production for mule deer and other ungulates [210]. On plains rough fescue (Festuca hallii) grasslands, prescribed fire may reduce litter, making plains rough fescue more palatable to mule deer. If burning is done in a "wet" year, prescribed fire may maintain a stable forage supply, but if done in a "dry" year, prescribed fire can reduce forage production for 1 to 4 years [19]. See the FEIS review of rough fescue for more information.
Great Plains woodlands and forests: In the Great Plains, hardwood and shrubby draws, cottonwood (Populus spp.) floodplains, and north-slope Rocky Mountain juniper communities are important mule deer habitats [287]. According to a review, fires in draws and floodplains may result in sprouting of trees and shrubs important to mule deer as food and cover (e.g., chokecherry, silver buffaloberry, green ash, boxelder, and bur oak (Quercus macrocarpa)). Small fires in Rocky Mountain juniper communities likely benefit mule deer by increasing the number of openings, improving shrub growth, and increasing plant diversity [287]. In the Missouri River Breaks region, Eichhorn and Watts [108] measured plant succession over 10 years following wildfires that occurred 1 to 28 years prior to the start of the study. In Douglas-fir-Rocky Mountain juniper stands, grass cover was significantly reduced during the first 3 postfire years compared to unburned controls (P<0.05) but was greater than in unburned controls during postfire years 8 to 28. Forb cover peaked during postfire year 3 and then decreased, but it remained above unburned controls in all years (P<0.05). Shrubs "steadily" increased after burning. Total shrub cover exceeded that on unburned controls during postfire years 5 to 28 (P<0.05). Cover of sprouting shrubs such as chokecherry, rose, and snowberry, however, exceeded that on unburned controls after the 1st postfire year. Rocky Mountain juniper was "eliminated" on all burned areas during postfire years 1 to 28. The study sites had "little or no" livestock grazing pressure [108].
In the Missouri River Breaks region, mule deer likely benefit from increased grasses and forbs on burned areas as long as some cover is retained. Prescribed fires conducted over 2 weeks from late May to early June resulted in a 1,100-acre (445 ha) mosaic of unburned, lightly burned, and severely burned areas. Mule deer thermal cover did not differ among unburned, lightly burned, and severely burned areas. However, hiding cover was significantly less on lightly burned (32%) and severely burned areas (21%) than on unburned areas (45%) 1 year after the fire (P<0.05 for all variables). Grass cover did not differ among burned areas during the 1st and 2nd postfire summers. Forb cover was greater in lightly and severely burned areas during the 1st postfire year. During the 2nd postfire year, forb cover was similar in unburned, lightly burned, and severely burned areas in May and June, but in July and August, forb cover in burned areas exceeded that of the unburned control. Browse cover decreased to <1% in lightly burned and severely burned areas during the 1st and 2nd postfire summers, while unburned areas had significantly more browse. Mule deer pellet group counts indicated the mule deer used all treatments similarly during the 1st and 2nd postfire years, and habitats were not selected or avoided based on cover or forage availability [366].
Mule deer interactions with fuels and fire effects: Mule deer affect vegetation development and plant productivity after fire. Total herb production on deer and elk rangeland in Arizona increased 3-fold on both understory and stand-replacement burned plots compared with unburned control plots after mixed-severity wildfire in a ponderosa pine forest. Total herb production remained higher in understory-burned plots than in control plots for at least 9 years after fire. However, it was higher in stand-replacement burned plots than in control stands for only 2 years after fire, possibly because of heavy grazing by livestock and wildlife [242]. On Quail Ridge, California, prescribed fire in blue oak woodlands resulted in blue oak and interior live oak sprouting. However, heavy postfire browsing by mule deer reduced sprout production: 95% of oak sprouts were grazed by mule deer [12]. In west-central Montana, Saskatoon serviceberry showed considerable damage from deer browsing following a severe wildfire in a Douglas-fir forest [82]. Postfire mule deer and elk browsing pressure influences the response of quaking aspen stands to fire (e.g., [118,165,203,232]). In the Grand Canyon, Fule and others [118] concluded that high mule deer herbivory reduced quaking aspen regeneration for 60 years, leading to white fir- and Douglas-fir-dominated forests with fuels able to support stand-replacing fire. The authors hypothesized that had excessive mule deer herbivory not occurred, the fuel structures of quaking aspen-ponderosa pine forests would probably be less susceptible to crown fire and more conducive to long-term ponderosa pine survival. They noted, however, that white fir may still have come to dominate the stand in the long-term absence of fire [118].
Fire severity may influence the effect of mule deer browsing on postfire plant growth. On the Hastings Reservation in Monterey County, California, browsing was heavier on postfire growth of plants that had all of their stems consumed by fire than on postfire growth of plants where old, charred stems remained. Chamise plants with old stems remaining had stems with significantly longer root crown sprouts over an 8-year period following an August wildfire (P<0.001) [95].
Mule deer in most cases do not alter the successional trajectory of large burned areas, but the course of succession may be altered in small burned areas that receive heavy browsing pressure, particularly if other early seral habitats in the landscape are sparse [232,248,290]. For example, Mueggler and Bartos [232] noted that mule deer browsing prevented quaking aspen regeneration in small clearcuts and in an uncut quaking aspen forest on summer rangeland in southern Utah, but quaking aspen in nearby large burned areas regenerated successfully. They suggested that burned areas or clearcuts <5 acres (2 ha) would concentrate mule deer use, reducing quaking aspen regeneration [232]. In Colorado, logging of several large (15-20 acres (6-8 ha)) areas at one time resulted in successful quaking aspen regeneration despite large mule deer and elk populations (Crouch 1983 cited in [24]). See Lotan and others [203] for suggestions regarding size of burned areas and burning intervals for deer in quaking aspen stands.
Prior to fire, mule deer selection of preferred forage may alter the abundance and kinds of fuels [209]. By removing fine fuels, mule deer may reduce the likelihood of surface fires. They may also enhance the development of unpalatable trees that may act as ladder fuels [364]. Wisdom and others [364] made the following generalizations regarding the interactions between fires and herbivory by mule deer, elk, and cattle in forested landscapes in western North America:Mule deer, other ungulate, and fire interactions: Presence of other ungulates in burned areas may affect mule deer use. In Banff and Jasper National Parks, mule deer, moose, and bighorn sheep were abundant and elk were sparse. Following a large wildfire that increased grasslands and shrublands, elk increased in abundance but mule deer, moose, and bighorn sheep populations decreased. The author concluded that fire favored elk over the other species [114]. Large fires in northern Idaho during 1910, 1919, and 1934 created expansive shrubfields in mule deer and elk winter rangelands. Initially, mule deer and elk populations increased, but mule deer populations declined as elk populations further increased (Norberg and Trout 1957 cited in [370]). Mule deer used postfire successional shrubfields in subalpine fir-Engelmann spruce forests of Glacier National Park, Montana. However, their populations gradually declined on some of these sites as elk populations grew [222]. In contrast, Long and others [200] found no evidence that adult female mule deer avoided grand fir-Douglas-fir forests that were thinned and broadcast burned or slash pile burned 1 to 5 years after treatment because of elk selection for these stands. For more information, see Interspecific interactions.
Livestock presence in burned areas: Because burns attract livestock as well as mule deer [181,209,221,273,290,291,332], fire could increase the potential for mule deer-livestock interactions. There were more mule deer pellet groups inside than outside a cattle exclosure in southern California chamise chaparral 1 and 2 years after a prescribed fire. This suggested that cattle displaced mule deer from burned areas. When cattle were removed during the 3rd postfire year, mule deer pellet group counts were similar throughout the burned area (inside and outside the exclosure), and mule deer use of the burned area increased 23% to 76%. The authors suggested, however, that because of increased cover, mule deer use of the burned area may have increased during the 3rd postfire year even if cattle remained on the burned area [332]. For more information, see Livestock grazing.
Travel patterns: Use of burned areas by mule deer may be influenced by their movements prior to fire. Some researchers reported that mule deer do not readily use burned areas outside of their home ranges [325]. In California chaparral, black-tailed deer did not use burned areas that were 1,500 to 3,000 feet (460-910 m) away despite the presence of abundant and nutritious sprouting shrubs. Instead, most remained in their home ranges and many died from malnutrition [92]. In Lava Beds National Monument, migratory and nonmigratory mule deer occupied the same winter ranges after wild and prescribed fires as they did prior to the fires. Does with home ranges adjacent to burned areas were not attracted to the burned areas, and does with burned areas in their home ranges did not expand their home ranges to include more of the burned area. Thus, they showed a strong fidelity to their home ranges regardless of availability of burned habitat. However, the winter after the fires was mild, with snow always <7 inches (18 cm) deep, and does concentrated in burned areas in late winter and early spring. Does used topographical features in the burned areas as cover [261]. Gibbens (1963 cited in [14]) reported that black-tailed deer detected treated chaparral habitats (see item 3 in Lake County studies) up to 0.75 mile (1.2 km) away, with some individuals shifting home ranges to use the site. A study in Utah reported large shifts in movement due to fire. In west-central Utah, mule deer temporarily moved 2 to 3 miles (3-5 km) to adjacent unburned rangelands in response to a 15,000-acre (6,100 ha) September wildfire on their usual winter rangeland. They returned to their usual winter rangeland after the vegetation grew back [274]. For more information, see Home range.
Physical barriers: Postfire accumulations of deadfall might discourage use of burned habitats by mule deer, white-tailed deer, and other ungulates by creating impassable areas. Burning may also remove such obstructions in some habitats and allow mule deer and other wildlife to move about and access forage more easily [209,255]. Gates (1968 cited in [27]) showed that mule deer in coastal British Columbia used burned and debris-free areas more frequently than those that contained unburned logging slash. The author, however, could not rule out the possibility that mule deer simply preferred the forage on burned areas. Prescribed burning in quaking aspen/Saskatoon serviceberry-mountain snowberry (Symphoricarpos oreophilus) stands in southeastern Idaho reduced obstructions in the understory, which increased accessibility to forage by mule deer and other ungulates for at least 2 postfire years [43]. Charred stems in chaparral may form barriers that cause black-tailed deer to avoid feeding on sprouts [327]. DeCalesta [99] noted that deer cannot feed easily on young plants growing within logging slash.
Weather and use of burned areas: Snow depth, duration, and crusting are often different in burned than unburned forest, which may affect mule deer movements and use of burned forest [209]. Generally, the amount of snow that reaches the ground is less in unburned forest because of interception by the canopy. Where melting occurs in tree crowns, dripping water further reduces the depth of snow on the ground. Since temperatures fluctuate less in a forest and winds are reduced, any crust that forms on the snow tends to remain. Snow may persist longer in a forest than on an open burned area because the forest shields the snow from sunlight and insulates the ground. When trees are removed by burning or logging, deeper snow, alternating crusting and thawing, and shorter duration of snow cover may result. Blackened soil on burns may accelerate snowmelt. Mule deer generally leave a burned area when the snow is soft and deep and live in the surrounding forest where the snow is relatively hard and shallow, even when abundant food occurs on the burned area [27]. However, early snowmelt and green-up on burned areas in spring may benefit mule deer [27,175].
Size and shape of burned areas: Several small fires may be more beneficial to mule deer than one large fire because of increased edge habitat. A review of published and unpublished reports by students and faculty at the University of Nevada stated that while mule deer occurred 0.25 mile (0.40 km) into a large (4-mile² (10 km²)) burned area on winter range in the Jack's Valley Habitat Management Area near Carson City, use of antelope bitterbrush was concentrated at the edge of the burn, within only about 450 feet (140 m) inside and outside of the burned area [173]. Because mule deer often prefer to feed close to cover, they may not use interiors of large burned areas [236]. Immediately following large, stand-replacing fires in California chaparral, mule deer grazed no farther than 300 feet (90 m) from cover [14].
Large fires may be detrimental to mule deer in the short term by removing cover [36]. Dubreuil [104] suggested that selection for unburned habitat by mule deer 1 year after a 83,500-acre (334,800 ha), mixed-severity August to September wildfire in the southern Black Hills was related to the relative lack of cover and forage in burned areas. In the North Coast Ranges of California, mule deer densities were higher during the first 5 postfire years in "opened brushland" consisting of scattered islands of shrubs and herb-dominated openings than in a large burned area with few shrub islands. The authors suggested that the lack of cover in the large burned area kept mule deer densities lower [36]. For more information, see Fire size.
The elimination of vegetation by fire over a large area may result in initial food shortages for mule deer. Wildfire that denudes low chaparral areas, often critical to mule deer populations for winter forage, can lead to overgrazing and starvation [236]. The shortage of food after a chaparral fire is usually short-lived, however, because sprouting often begins within a few days after fire and provides excellent forage. For more information, see California shrublands.
Diseases and parasites: Fire may reduce the numbers of external and internal parasites that affect mule deer and other animals [27,146,209], although the effect is likely brief. Fires can reduce winter tick (Dermacentor albipictus) populations [144]. After a May prescribed fire in mature quaking aspen forest and willow habitat in Elk Island National Park, Alberta, the number of engorged adult female ticks and larvae immediately declined. Winter tick survival was highest where the burn was patchy and duff consumption was least [103]. According to a review, the number of winter ticks killed also depends on the habitat type and season of burning because most ticks are found on the tops of shrubs in spring [146]. Although winter tick populations may be reduced in the short term, fire's long-term effects on winter tick populations were unknown as of this writing (2012).
Fire in wetland habitats may help reduce giant liver fluke (Fascioloides magna) populations, which may be detrimental to mule deer and white-tailed deer [327]. Giant liver flukes have a complex life cycle that involves an intermediate aquatic snail host for the embryonic stage, aquatic vegetation for the larval-cyst stage, and an ungulate host for the juvenile and adult stages [331]. In east-central Alberta, deer, elk, moose, and American bison (Bos bison) populations were heavily infected with giant liver flukes (Swales 1936 cited in [331]). In order to control these infestations, dead aquatic vegetation was burned and aquatic snails were controlled with chemicals. This "apparently eradicated" giant live flukes in ungulates in the area; limited examinations found no giant liver flukes in deer harvested by hunters (Stock 1978, Pybus 1990b, cited in [331]). Following the Tillamook fires in Oregon, black-tailed deer were free of liver flukes (Fascioliasis) and lungworms (Strongylida) that had been prevalent before the fires. Apparently, fire had killed the dryland snail that is the intermediate host for liver flukes and some lungworms [160,369].
FIRE REGIMES:Fire exclusion beginning in the early 1900s resulted in increased tree density in formerly open rangelands in much of the Southwest and Great Basin. This may have increased habitat for mule deer from what occurred historically (see Threats). Clark and Starkey [71] cautioned that although rangeland ecosystems evolved with fire, they are not in their "natural" condition today because of a legacy of fire exclusion, livestock grazing, agricultural development, introduction of nonnative invasive plants, construction of roads and other barriers, and other factors.
FIRE MANAGEMENT CONSIDERATIONS:Because most of the mule deer's annual diet is browse, enhancement of browse is "key" to providing sufficient mule deer forage throughout the year [292]. While many forage species for mule deer increase after fire, others decrease. For example, 40 acres (16 ha) of mature quaking aspen-Engelmann spruce forest, Douglas-fir/mallow ninebark forest, and hawthorn (Crataegus spp.) shrubland were clearcut and burned in early spring in the Absaroka Range in south-central Montana. Saskatoon serviceberry and chokecherry were prominent understory shrubs. Two years after the fire, density of quaking aspen and willows had increased compared to prefire levels due to sprouting, but Saskatoon serviceberry and chokecherry densities were reduced [123].
Some researchers cautioned against using prescribed fire in oak habitats to avoid removing important mule deer forage and/or cover. Removal of mature, acorn-producing oak trees was often cited as detrimental to mule deer. Anderson (1969 cited in [66]) considered Gambel oak rangeland in Colorado to be important mule deer habitat. He cautioned against any treatment of Gambel oak as a general policy because of the importance of its acorns and browse to mule deer. Kruse [178] suggested using prescribed fire in Gambel oak woodlands on poor-quality sites to enhance brushy growth but avoiding prescribed fire use on better-quality sites with mature oaks. Taber and Dasmann [327] cautioned against using prescribed fire in California oak woodlands. They suggested that oaks be left untreated to provide acorns for black-tailed deer [327]. Guidelines for managing interior Arizona chaparral for mule deer included leaving Sonoran scrub oak (Quercus turbinella) untreated (USDA Forest Service 1970 cited in [345]). For more information, see Southwest shrublands.
Because fire may reduce important cover, several authors cautioned against using prescribed fire in mule deer habitats where cover is limiting. This is a management concern particularly on mule deer winter rangelands in desert grasslands, plains grasslands, prairie, southwestern shrubsteppe, pinyon-juniper woodlands, sagebrush, and desert shrub communities of the Great Plains, Southwest, and Great Basin regions [112,172]. Fairchild [112] suggested that particular attention be paid to maintaining cover in "key" wintering areas where mule deer concentrate. Suminski [318] cautioned against using prescribed fire and other treatments in areas containing sagebrush, antelope bitterbrush, and/or Stansbury cliffrose unless "excess" winter rangeland was present, because these species are important as winter forage and cover, and they are easily killed by fire. For more information, see Great Basin shrublands.
Most authors recommend creating a mosaic of burned and unburned habitats within a landscape to benefit mule deer (e.g., [57,146,292]). In a review of fire effects on ungulates in the northern Great Plains, Higgins and others [146] stated that "optimum" benefits of fire occur where fire creates a mosaic pattern of burned and unburned vegetation that provides new forage growth, seasonal habitats, and vegetation in early to late stages of succession. Nichols and Menke [236] stated that in general, optimal wildlife habitat is created in California chaparral when fire results in a mosaic of different age classes of shrubs interspersed with grassland. In high-elevation big sagebrush communities in Wyoming, Cook and others [79] recommended creating a mosaic of different-aged burned and unburned habitats for mule deer and other ungulates, burning a treatment unit once every 15 to 25 years. According to Stevens [311], diversity of food and cover over short distances is the key to enhancing mule deer populations in big sagebrush areas, with the distribution and pattern of shrub stands being more important than the quantity of shrubs [311]. Mule deer use pinyon-juniper woodlands in all stages of succession. Using prescribed fire or mechanical methods to create a mosaic of different successional stages in large areas of homogeneous pinyon-juniper woodlands may benefit mule deer by providing forage in early successional stands and mature forests nearby for cover (see Great Basin woodlands) [290]. Clark and Starkey [71] suggested that because mule deer forage heavily upon shrubs on most western winter rangelands and consume mostly herbaceous plants in spring and summer, prescribed fires to improve forage quality should provide a mosaic of burned and unburned areas. Prescribed fire may be less beneficial to mule deer in areas already having a mosaic of habitats. In an area with sparse herbaceous forage in the surrounding landscape in Lava Beds National Monument, average mule deer pellet group density 2 years after a prescribed fire was higher in burned areas than prior to the fire. However, in an area where herbaceous forage was abundant in the surrounding landscape, average mule deer pellet group density was similar before and 2 years after the fire [261].
Gruell [127] listed several factors that influence postfire plant composition, including the severity, size, and season of the fire, fuel type, prefire vegetation composition, and postfire foraging intensity (see Mule deer interactions with fuels and fire effects).
Fire timing: Prefire vegetation composition and season of burning may affect the availability of mule deer forage, particularly during the 1st postfire growing season. Several studies have compared effects of spring and fall burning on mule deer in California chaparral. In general, spring burning may be most beneficial to mule deer in this habitat because sprouts typically appear in 3 to 4 weeks, producing highly nutritious forage during both the dry summer months and the 1st postfire winter. Burning in spring also tends to favor crown-sprouting species such as chamise, a "staple" black-tailed deer food [170]. Biswell and others [34,36] cautioned that if a fire occurs in California chaparral after September, there may be little to no crown sprouting until the following spring, which would leave little to no cover or forage for overwintering black-tailed deer. Burning in fall may also favor shrubs that establish only by seed [320,327] and thus take longer to provide substantial mule deer forage. Fall fires and spring fires that occur prior to mid-March may stimulate germination from seeds as well as sprouting. Some important browse species reproduce from seed after fire, including wedgeleaf ceanothus, wavyleaf ceanothus (Ceanothus foliosus), and Stanford's manzanita (Arctostaphylos stanfordiana) [293]. Biswell and others [34,36] noted the importance of spring burning to black-tailed deer browse production but suggested use of fall burning in some areas to increase forage diversity. For more information, see California shrublands. In high-elevation big sagebrush communities in Wyoming, Cook and others [79] recommended burning in spring to minimize damage to shrubs and perennials and minimize first-year increases in weedy annual species. For more information, see the Fire Case Study on antelope bitterbrush. See also Great Basin shrublands.
Fire type: Very frequent fires in California chaparral may reduce important browse species [35,36]. A review of fire in California chaparral concluded that reburning 1 to 3 years after a fire may cause high mortality of sprouting plants such as chamise. Shrub mortality may increase when shrubs are heavily browsed by mule deer or livestock after reburning (see Mule deer interactions with fuels and fire effects) [38]. Reburning that occurs after plants have matured and produced seeds, which may be 15 years or longer, is most likely to maintain wedgeleaf ceanothus and other highly palatable, nonsprouting species in chaparral. Reburning before this point may reduce these species, and thus reduce carrying capacity for mule deer [35]. According to a review by Kinucan [170], the chaparral canopy reaches full development about 12 years after fire. Fires <9 years apart tend to kill seedlings, weaken sprouting response, and retard seed production [170]. Frequent fires in interior Arizona chaparral may reduce or eliminate some browse species for mule deer, including true mountain-mahogany, Wright silktassel, and hollyleaf buckthorn (Rhamnus ilicifolia) [259,290].
Patchy burns may be best for mule deer. After a wildfire in San Antonio Canyon, California, burned areas with "scant cover" were sparsely occupied by mule deer, while many mule deer (35% of observations) occurred in a burned area that had some cover remaining, even though it comprised only about 12% of the study area (Bartholomew 1942 personal communication cited in [291]). According to a review, patchy burns in mountain shrublands and chaparral rangelands have the greatest value to mule deer [153]. Other authors refer to the value of creating a mixture of early and late-successional habitats [43,54,146,209,239]. For more information, see Size and shape of burned areas.
Fire size: Regardless of habitat, small burns are often considered better for mule deer because portions of large burns may be left entirely unused by mule deer [9,27,41,93,236,291]. Miller [230] suggested that if burned areas are too large in chaparral habitats, most shrubs grow out of reach quickly, whereas small burned areas may provide browse for longer due to hedging by mule deer. Shantz [291] considered burning in small patches best for wildlife in general. The reduction of vegetation by fire over a large area may cause short-term food shortages for mule deer [236,284]. For example, large stand-replacing wildfires in chaparral habitats may reduce critical mule deer winter rangelands, which could lead to overgrazing and starvation during the first postfire winter [236]. Bendell [27] hypothesized that mule deer may benefit most from small fires because they result in more edge and greater interspersion of habitats than one large fire. However, Gates (1968 cited in [27]) noted that black-tailed deer in 2 areas on the east coast of Vancouver Island had similar population densities despite contrasting amounts of edge and interspersion resulting from wildfires and patchy clearcut logging over large areas. Although a large fire could potentially reduce the interspersion of food and cover for mule deer by producing uniform vegetation, reviews stated that fires rarely burn evenly and typically result in a mosaic of vegetation beneficial for deer [27,209].
Small burns provide some advantages over large burns, but a disadvantage is that heavy deer browsing may reduce or eliminate preferred sprouting trees and shrubs from small burns [291]. For example, several authors noted that small burned areas or clearcuts in quaking aspen forests may concentrate mule deer and other browsing animals to the point where quaking aspen browse is eliminated (see Mule deer interactions with fuels and fire effects) [248,290]. For this reason, Brown [56] suggested burning multiple small areas within a landscape to disperse animals. Alternatively, a single, large fire that creates a mosaic of vegetation may create favorable mule deer habitat while still dispersing animals [56]. In bluebunch wheatgrass grasslands in the Rocky Mountains, concentrations of animals on small burned areas may increase interspecific competition by increasing diet overlap between mule deer, elk, and bighorn sheep [162,306].
Other considerations: Because travel patterns of mule deer prior to fire may affect postfire use, Carpenter and Wallmo [66] suggested it is important that habitat management efforts for mule deer be based on the particular movement patterns and needs of the individuals making up that population. Dasmann [92] suggested that because black-tailed deer may not use a burned area outside of their home range, numerous small burns spaced within about 0.5 mile (0.8 km) of one another will benefit more black-tailed deer than a few large, widely spaced burns (see Fire size). He also suggested that because black-tailed deer use different aspects at different times of day and year (see Topography), prescribed fires should be spaced to include a variety of aspects, including southern and northern exposure, and slope positions, including ridgetops and canyon bottoms [92]. In California chaparral, black-tailed deer consume different forage species at different times of year. During summer, species growing on cool, north-facing slopes and streambeds are used. In late fall, black-tailed deer move to warm, south-facing slopes where they consume herbaceous plants and chamise [170]. Thus, prescribed fires in cold stream bottoms and on north-facing slopes are unlikely to benefit black-tailed deer on winter rangelands, whereas prescribed fires on south-facing slopes may be beneficial [327]. Urness (1974 cited in [345]) recommended treating interior Arizona chaparral on various slopes and aspects due to the changing seasonal requirements of mule deer, while maintaining sufficient cover for security.
Prescribed burning and its associated human activities may reduce mule deer populations in the short term by increasing their vulnerability to hunting. Two years after an October mixed-severity prescribed fire in Gambel oak shrublands in western Colorado, mule deer pellet group densities during fall, winter, and spring were 25% less than before the fire. This was due, in part, to heavy hunting pressure and lack of cover after the fire [184]. Mule deer in Gambel oak rangelands in western Colorado may not have benefited from prescribed burning because of heavy hunting pressure [181]. In coastal Oregon, declining black-tailed deer populations protected from hunting increased following fire. The author cautioned that fire and logging may increase mule deer herds only if they are protected from hunting [110]. Sampson [284] cautioned that hunting restrictions may be needed to maintain populations in burned areas due to greater hunter success.
Proximity of habitats to water may affect their use after fire, particularly in the Southwest. Biswell and others [36] suggested that mule deer might not respond positively to postfire habitat improvements in areas where water is lacking. See Water management for more information.
The presence of cattle and other livestock may reduce the benefits of prescribed fire to mule deer. Tiller and others [273,332] suggested that because cattle and mule deer compete for space within chamise chaparral burned under prescription on the San Bernardino National Forest, California, cattle should be excluded during the 1st and 2nd postfire years. See Livestock presence in burned areas for more information.
Fire affects the spread of nonnative invasive plants, which may be beneficial or detrimental to mule deer. For more information on mule deer use of nonnative invasive plants, see Nonnative invasive plants. See also FEIS reviews of nonnative invasive plant species of interest.
Mule deer may affect postfire succession. For more information, see Mule deer interactions with fuels and fire effects.
Fire may influence interspecific interactions. For example, mule deer may avoid postfire successional communities if elk are present (see Mule deer, other ungulate, and fire interactions). Asherin [15] suggested using several small prescribed fires scattered across winter range in order to reduce interspecific interactions and disperse browsing pressure across burned and adjacent unburned areas.
In California chaparral, fuel breaks may provide travel ways, increase interspersion of herbaceous vegetation, and increase edge that benefits mule deer [93,145,291].
For a 2002 analysis of the economic costs and benefits of implementing a prescribed burning program in southern California for increasing mule deer and other big game habitat, see Loomis and others [202].
The following table provides fire regime information that may be relevant to mule deer habitats. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
| Fire regime information on vegetation communities in which mule deer may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [188], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||||||||
|
|||||||||||||
| Pacific Northwest | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Pacific Northwest Grassland | |||||||||||||
| Alpine and subalpine meadows and grasslands | Replacement | 68% | 350 | 200 | 500 | ||||||||
| Mixed | 32% | 750 | 500 | >1,000 | |||||||||
| Bluebunch wheatgrass | Replacement | 47% | 18 | 5 | 20 | ||||||||
| Mixed | 53% | 16 | 5 | 20 | |||||||||
| Idaho fescue grasslands | Replacement | 76% | 40 | ||||||||||
| Mixed | 24% | 125 | |||||||||||
| Marsh | Replacement | 74% | 7 | ||||||||||
| Mixed | 26% | 20 | |||||||||||
| Pacific Northwest Shrubland | |||||||||||||
| Low sagebrush | Replacement | 41% | 180 | ||||||||||
| Mixed | 59% | 125 | |||||||||||
| Mountain big sagebrush (cool sagebrush) | Replacement | 100% | 20 | 10 | 40 | ||||||||
| Salt desert scrubland | Replacement | 13% | 200 | 100 | 300 | ||||||||
| Mixed | 87% | 31 | 20 | 100 | |||||||||
| Salt desert shrub | Replacement | 50% | >1,000 | 500 | >1,000 | ||||||||
| Mixed | 50% | >1,000 | 500 | >1,000 | |||||||||
| Wyoming big sagebrush semidesert | Replacement | 86% | 200 | 30 | 200 | ||||||||
| Mixed | 9% | >1,000 | 20 | ||||||||||
| Surface or low | 5% | >1,000 | 20 | ||||||||||
| Wyoming big sagebrush steppe | Replacement | 89% | 92 | 30 | 120 | ||||||||
| Mixed | 11% | 714 | 120 | ||||||||||
| Pacific Northwest Woodland | |||||||||||||
| Oregon white oak | Replacement | 3% | 275 | ||||||||||
| Mixed | 19% | 50 | |||||||||||
| Surface or low | 78% | 12.5 | |||||||||||
| Oregon white oak-ponderosa pine | Replacement | 16% | 125 | 100 | 300 | ||||||||
| Mixed | 2% | 900 | 50 | ||||||||||
| Surface or low | 81% | 25 | 5 | 30 | |||||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||||
| Mixed | 17% | 60 | |||||||||||
| Surface or low | 78% | 13 | |||||||||||
| Ponderosa pine savannah (ultramafic) | Replacement | 7% | 200 | 100 | 300 | ||||||||
| Surface or low | 93% | 15 | 10 | 20 | |||||||||
| Subalpine woodland | Replacement | 21% | 300 | 200 | 400 | ||||||||
| Mixed | 79% | 80 | 35 | 120 | |||||||||
| Western juniper (pumice) | Replacement | 33% | >1,000 | ||||||||||
| Mixed | 67% | 500 | |||||||||||
| Pacific Northwest Forested | |||||||||||||
| California mixed evergreen (northern California and southern Oregon) | Replacement | 6% | 150 | 100 | 200 | ||||||||
| Mixed | 29% | 33 | 15 | 50 | |||||||||
| Surface or low | 64% | 15 | 5 | 30 | |||||||||
| Douglas-fir (Willamette Valley foothills) | Replacement | 18% | 150 | 100 | 400 | ||||||||
| Mixed | 29% | 90 | 40 | 150 | |||||||||
| Surface or low | 53% | 50 | 20 | 80 | |||||||||
| Douglas-fir-western hemlock (dry mesic) | Replacement | 25% | 300 | 250 | 500 | ||||||||
| Mixed | 75% | 100 | 50 | 150 | |||||||||
| Douglas-fir-western hemlock (wet mesic) | Replacement | 71% | 400 | ||||||||||
| Mixed | 29% | >1,000 | |||||||||||
| Lodgepole pine (pumice soils) | Replacement | 78% | 125 | 65 | 200 | ||||||||
| Mixed | 22% | 450 | 45 | 85 | |||||||||
| Mixed conifer (eastside dry) | Replacement | 14% | 115 | 70 | 200 | ||||||||
| Mixed | 21% | 75 | 70 | 175 | |||||||||
| Surface or low | 64% | 25 | 20 | 25 | |||||||||
| Mixed conifer (eastside mesic) | Replacement | 35% | 200 | ||||||||||
| Mixed | 47% | 150 | |||||||||||
| Surface or low | 18% | 400 | |||||||||||
| Mixed conifer (southwestern Oregon) | Replacement | 4% | 400 | ||||||||||
| Mixed | 29% | 50 | |||||||||||
| Surface or low | 67% | 22 | |||||||||||
| Mountain hemlock | Replacement | 93% | 750 | 500 | >1,000 | ||||||||
| Mixed | 7% | >1,000 | |||||||||||
| Oregon coastal tanoak | Replacement | 10% | 250 | ||||||||||
| Mixed | 90% | 28 | 15 | 40 | |||||||||
| Ponderosa pine (xeric) | Replacement | 37% | 130 | ||||||||||
| Mixed | 48% | 100 | |||||||||||
| Surface or low | 16% | 300 | |||||||||||
| Ponderosa pine, dry (mesic) | Replacement | 5% | 125 | ||||||||||
| Mixed | 13% | 50 | |||||||||||
| Surface or low | 82% | 8 | |||||||||||
| Pacific silver fir (low elevation) | Replacement | 46% | 350 | 100 | 800 | ||||||||
| Mixed | 54% | 300 | 100 | 400 | |||||||||
| Pacific silver fir (high elevation) | Replacement | 69% | 500 | ||||||||||
| Mixed | 31% | >1,000 | |||||||||||
| Red fir | Replacement | 20% | 400 | 150 | 400 | ||||||||
| Mixed | 80% | 100 | 80 | 130 | |||||||||
| Sitka spruce-western hemlock | Replacement | 100% | 700 | 300 | >1,000 | ||||||||
| Spruce-fir | Replacement | 84% | 135 | 80 | 270 | ||||||||
| Mixed | 16% | 700 | 285 | >1,000 | |||||||||
| Subalpine fir | Replacement | 81% | 185 | 150 | 300 | ||||||||
| Mixed | 19% | 800 | 500 | >1,000 | |||||||||
| California | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| California Grassland | |||||||||||||
| Alpine meadows and barrens | Replacement | 100% | 200 | 200 | 400 | ||||||||
| California grassland | Replacement | 100% | 2 | 1 | 3 | ||||||||
| Herbaceous wetland | Replacement | 70% | 15 | ||||||||||
| Mixed | 30% | 35 | |||||||||||
| Wet mountain meadow-lodgepole pine (subalpine) | Replacement | 21% | 100 | ||||||||||
| Mixed | 10% | 200 | |||||||||||
| Surface or low | 69% | 30 | |||||||||||
| California Shrubland | |||||||||||||
| Chaparral | Replacement | 100% | 50 | 30 | 125 | ||||||||
| Coastal sage scrub | Replacement | 100% | 50 | 20 | 150 | ||||||||
| Coastal sage scrub-coastal prairie | Replacement | 8% | 40 | 8 | 900 | ||||||||
| Mixed | 31% | 10 | 1 | 900 | |||||||||
| Surface or low | 62% | 5 | 1 | 6 | |||||||||
| Montane chaparral | Replacement | 34% | 95 | ||||||||||
| Mixed | 66% | 50 | |||||||||||
| Saltbush | Replacement | 70% | 100 | 60 | 200 | ||||||||
| Mixed | 30% | 235 | 10 | ||||||||||
| California Woodland | |||||||||||||
| California oak woodlands | Replacement | 8% | 120 | ||||||||||
| Mixed | 2% | 500 | |||||||||||
| Surface or low | 91% | 10 | |||||||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||||
| Mixed | 17% | 60 | |||||||||||
| Surface or low | 78% | 13 | |||||||||||
| California Forested | |||||||||||||
| Aspen with conifer | Replacement | 24% | 155 | 50 | 300 | ||||||||
| Mixed | 15% | 240 | |||||||||||
| Surface or low | 61% | 60 | |||||||||||
| California mixed evergreen | Replacement | 10% | 140 | 65 | 700 | ||||||||
| Mixed | 58% | 25 | 10 | 33 | |||||||||
| Surface or low | 32% | 45 | 7 | ||||||||||
| Coast redwood | Replacement | 2% | ≥1,000 | ||||||||||
| Surface or low | 98% | 20 | |||||||||||
| Jeffrey pine | Replacement | 9% | 250 | ||||||||||
| Mixed | 17% | 130 | |||||||||||
| Surface or low | 74% | 30 | |||||||||||
| Interior white fir (northeastern California) | Replacement | 47% | 145 | ||||||||||
| Mixed | 32% | 210 | |||||||||||
| Surface or low | 21% | 325 | |||||||||||
| Mixed conifer (north slopes) | Replacement | 5% | 250 | ||||||||||
| Mixed | 7% | 200 | |||||||||||
| Surface or low | 88% | 15 | 10 | 40 | |||||||||
| Mixed conifer (south slopes) | Replacement | 4% | 200 | ||||||||||
| Mixed | 16% | 50 | |||||||||||
| Surface or low | 80% | 10 | |||||||||||
| Mixed evergreen-bigcone Douglas-fir (southern coastal) | Replacement | 29% | 250 | ||||||||||
| Mixed | 71% | 100 | |||||||||||
| Red fir-western white pine | Replacement | 16% | 250 | ||||||||||
| Mixed | 65% | 60 | 25 | 80 | |||||||||
| Surface or low | 19% | 200 | |||||||||||
| Red fir-white fir | Replacement | 13% | 200 | 125 | 500 | ||||||||
| Mixed | 36% | 70 | |||||||||||
| Surface or low | 51% | 50 | 15 | 50 | |||||||||
| Sierra Nevada lodgepole pine (cold wet upper montane) | Replacement | 23% | 150 | 37 | 764 | ||||||||
| Mixed | 70% | 50 | |||||||||||
| Surface or low | 7% | 500 | |||||||||||
| Sierra Nevada lodgepole pine (dry subalpine) | Replacement | 11% | 250 | 31 | 500 | ||||||||
| Mixed | 45% | 60 | 31 | 350 | |||||||||
| Surface or low | 45% | 60 | 9 | 350 | |||||||||
| Southwest | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Southwest Grassland | |||||||||||||
| Desert grassland | Replacement | 85% | 12 | ||||||||||
| Surface or low | 15% | 67 | |||||||||||
| Desert grassland with shrubs and trees | Replacement | 85% | 12 | ||||||||||
| Mixed | 15% | 70 | |||||||||||
| Montane and subalpine grasslands | Replacement | 55% | 18 | 10 | 100 | ||||||||
| Surface or low | 45% | 22 | |||||||||||
| Montane and subalpine grasslands with shrubs or trees | Replacement | 30% | 70 | 10 | 100 | ||||||||
| Surface or low | 70% | 30 | |||||||||||
| Plains mesa grassland | Replacement | 81% | 20 | 3 | 30 | ||||||||
| Mixed | 19% | 85 | 3 | 150 | |||||||||
| Plains mesa grassland with shrubs or trees | Replacement | 76% | 20 | ||||||||||
| Mixed | 24% | 65 | |||||||||||
| Shortgrass prairie | Replacement | 87% | 12 | 2 | 35 | ||||||||
| Mixed | 13% | 80 | |||||||||||
| Shortgrass prairie with shrubs | Replacement | 80% | 15 | 2 | 35 | ||||||||
| Mixed | 20% | 60 | |||||||||||
| Shortgrass prairie with trees | Replacement | 80% | 15 | 2 | 35 | ||||||||
| Mixed | 20% | 60 | |||||||||||
| Southwest Shrubland | |||||||||||||
| Desert shrubland without grass | Replacement | 52% | 150 | ||||||||||
| Mixed | 48% | 165 | |||||||||||
| Gambel oak | Replacement | 75% | 50 | ||||||||||
| Mixed | 25% | 150 | |||||||||||
| Interior Arizona chaparral | Replacement | 100% | 125 | 60 | 150 | ||||||||
| Low sagebrush shrubland | Replacement | 100% | 125 | 60 | 150 | ||||||||
| Mountain-mahogany shrubland | Replacement | 73% | 75 | ||||||||||
| Mixed | 27% | 200 | |||||||||||
| Mountain sagebrush (cool sagebrush) | Replacement | 75% | 100 | ||||||||||
| Mixed | 25% | 300 | |||||||||||
| Salt desert scrubland | Replacement | 13% | 200 | 100 | 300 | ||||||||
| Mixed | 87% | 31 | 20 | 100 | |||||||||
| Southwestern shrub steppe | Replacement | 72% | 14 | 8 | 15 | ||||||||
| Mixed | 13% | 75 | 70 | 80 | |||||||||
| Surface or low | 15% | 69 | 60 | 100 | |||||||||
| Southwestern shrub steppe with trees | Replacement | 52% | 17 | 10 | 25 | ||||||||
| Mixed | 22% | 40 | 25 | 50 | |||||||||
| Surface or low | 25% | 35 | 25 | 100 | |||||||||
| Southwest Woodland | |||||||||||||
| Bristlecone-limber pine (Southwest) | Replacement | 67% | 500 | ||||||||||
| Surface or low | 33% | >1,000 | |||||||||||
| Madrean oak-conifer woodland | Replacement | 16% | 65 | 25 | |||||||||
| Mixed | 8% | 140 | 5 | ||||||||||
| Surface or low | 76% | 14 | 1 | 20 | |||||||||
| Mesquite bosques | Replacement | 32% | 135 | ||||||||||
| Mixed | 67% | 65 | |||||||||||
| Pinyon-juniper (mixed fire regime) | Replacement | 29% | 430 | ||||||||||
| Mixed | 65% | 192 | |||||||||||
| Surface or low | 6% | >1,000 | |||||||||||
| Pinyon-juniper (rare replacement fire regime) | Replacement | 76% | 526 | ||||||||||
| Mixed | 20% | >1,000 | |||||||||||
| Surface or low | 4% | >1,000 | |||||||||||
| Ponderosa pine/grassland (Southwest) | Replacement | 3% | 300 | ||||||||||
| Surface or low | 97% | 10 | |||||||||||
| Riparian deciduous woodland | Replacement | 50% | 110 | 15 | 200 | ||||||||
| Mixed | 20% | 275 | 25 | ||||||||||
| Surface or low | 30% | 180 | 10 | ||||||||||
| Southwest Forested | |||||||||||||
| Aspen, stable without conifers | Replacement | 81% | 150 | 50 | 300 | ||||||||
| Surface or low | 19% | 650 | 600 | >1,000 | |||||||||
| Aspen with spruce-fir | Replacement | 38% | 75 | 40 | 90 | ||||||||
| Mixed | 38% | 75 | 40 | ||||||||||
| Surface or low | 23% | 125 | 30 | 250 | |||||||||
| Lodgepole pine (Central Rocky Mountains, infrequent fire) | Replacement | 82% | 300 | 250 | 500 | ||||||||
| Surface or low | 18% | >1,000 | >1,000 | >1,000 | |||||||||
| Ponderosa pine-Douglas-fir (southern Rockies) | Replacement | 15% | 460 | ||||||||||
| Mixed | 43% | 160 | |||||||||||
| Surface or low | 43% | 160 | |||||||||||
| Ponderosa pine-Gambel oak (southern Rockies and Southwest) | Replacement | 8% | 300 | ||||||||||
| Surface or low | 92% | 25 | 10 | 30 | |||||||||
| Riparian forest with conifers | Replacement | 100% | 435 | 300 | 550 | ||||||||
| Southwest mixed conifer (cool, moist with aspen) | Replacement | 29% | 200 | 80 | 200 | ||||||||
| Mixed | 35% | 165 | 35 | ||||||||||
| Surface or low | 36% | 160 | 10 | ||||||||||
| Southwest mixed conifer (warm, dry with aspen) | Replacement | 7% | 300 | ||||||||||
| Mixed | 13% | 150 | 80 | 200 | |||||||||
| Surface or low | 80% | 25 | 2 | 70 | |||||||||
| Spruce-fir | Replacement | 96% | 210 | 150 | |||||||||
| Mixed | 4% | >1,000 | 35 | >1,000 | |||||||||
| Great Basin | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Great Basin Grassland | |||||||||||||
| Great Basin grassland | Replacement | 33% | 75 | 40 | 110 | ||||||||
| Mixed | 67% | 37 | 20 | 54 | |||||||||
| Mountain meadow (mesic to dry) | Replacement | 66% | 31 | 15 | 45 | ||||||||
| Mixed | 34% | 59 | 30 | 90 | |||||||||
| Great Basin Shrubland | |||||||||||||
| Basin big sagebrush | Replacement | 80% | 50 | 10 | 100 | ||||||||
| Mixed | 20% | 200 | 50 | 300 | |||||||||
| Black and low sagebrushes | Replacement | 33% | 243 | 100 | |||||||||
| Mixed | 67% | 119 | 75 | 140 | |||||||||
| Black and low sagebrushes with trees | Replacement | 37% | 227 | 150 | 290 | ||||||||
| Mixed | 63% | 136 | 50 | 190 | |||||||||
| Blackbrush | Replacement | 100% | 833 | 100 | >1,000 | ||||||||
| Creosotebush shrublands with grasses | Replacement | 57% | 588 | 300 | >1,000 | ||||||||
| Mixed | 43% | 769 | 300 | >1,000 | |||||||||
| Curlleaf mountain-mahogany | Replacement | 31% | 250 | 100 | 500 | ||||||||
| Mixed | 37% | 212 | 50 | ||||||||||
| Surface or low | 31% | 250 | 50 | ||||||||||
| Gambel oak | Replacement | 75% | 50 | ||||||||||
| Mixed | 25% | 150 | |||||||||||
| Interior Arizona chaparral | Replacement | 88% | 46 | 25 | 100 | ||||||||
| Mixed | 12% | 350 | |||||||||||
| Montane chaparral | Replacement | 37% | 93 | ||||||||||
| Mixed | 63% | 54 | |||||||||||
| Mountain big sagebrush | Replacement | 100% | 48 | 15 | 100 | ||||||||
| Mountain big sagebrush (cool sagebrush) | Replacement | 75% | 100 | ||||||||||
| Mixed | 25% | 300 | |||||||||||
| Mountain big sagebrush with conifers | Replacement | 100% | 49 | 15 | 100 | ||||||||
| Mountain shrubland with trees | Replacement | 22% | 105 | 100 | 200 | ||||||||
| Mixed | 78% | 29 | 25 | 100 | |||||||||
| Salt desert scrubland | Replacement | 13% | 200 | 100 | 300 | ||||||||
| Mixed | 87% | 31 | 20 | 100 | |||||||||
| Salt desert shrub | Replacement | 50% | >1,000 | 500 | >1,000 | ||||||||
| Mixed | 50% | >1,000 | 500 | >1,000 | |||||||||
| Wyoming big sagebrush semidesert | Replacement | 86% | 200 | 30 | 200 | ||||||||
| Mixed | 9% | >1,000 | 20 | >1,000 | |||||||||
| Surface or low | 5% | >1,000 | 20 | >1,000 | |||||||||
| Wyoming big sagebrush semidesert with trees | Replacement | 84% | 137 | 30 | 200 | ||||||||
| Mixed | 11% | >1,000 | 20 | >1,000 | |||||||||
| Surface or low | 5% | >1,000 | 20 | >1,000 | |||||||||
| Wyoming big sagebrush steppe | Replacement | 89% | 92 | 30 | 120 | ||||||||
| Mixed | 11% | 714 | 120 | ||||||||||
| Great Basin Woodland | |||||||||||||
| Juniper and pinyon-juniper steppe woodland | Replacement | 20% | 333 | 100 | >1,000 | ||||||||
| Mixed | 31% | 217 | 100 | >1,000 | |||||||||
| Surface or low | 49% | 135 | 100 | ||||||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||||
| Mixed | 17% | 60 | |||||||||||
| Surface or low | 78% | 13 | |||||||||||
| Great Basin Forested | |||||||||||||
| Aspen with conifer (low to midelevations) | Replacement | 53% | 61 | 20 | |||||||||
| Mixed | 24% | 137 | 10 | ||||||||||
| Surface or low | 23% | 143 | 10 | ||||||||||
| Aspen with conifer (high elevations) | Replacement | 47% | 76 | 40 | |||||||||
| Mixed | 18% | 196 | 10 | ||||||||||
| Surface or low | 35% | 100 | 10 | ||||||||||
| Aspen-cottonwood, stable aspen without conifers | Replacement | 31% | 96 | 50 | 300 | ||||||||
| Surface or low | 69% | 44 | 20 | 60 | |||||||||
| Aspen, stable without conifers | Replacement | 81% | 150 | 50 | 300 | ||||||||
| Surface or low | 19% | 650 | 600 | >1,000 | |||||||||
| Aspen with spruce-fir | Replacement | 38% | 75 | 40 | 90 | ||||||||
| Mixed | 38% | 75 | 40 | ||||||||||
| Surface or low | 23% | 125 | 30 | 250 | |||||||||
| Douglas-fir (Great Basin, dry) | Replacement | 12% | 90 | 600 | |||||||||
| Mixed | 14% | 76 | 45 | ||||||||||
| Surface or low | 75% | 14 | 10 | 50 | |||||||||
| Douglas-fir (interior, warm mesic) | Replacement | 28% | 170 | 80 | 400 | ||||||||
| Mixed | 72% | 65 | 50 | 250 | |||||||||
| Ponderosa pine-Douglas-fir | Replacement | 10% | 250 | >1,000 | |||||||||
| Mixed | 51% | 50 | 50 | 130 | |||||||||
| Surface or low | 39% | 65 | 15 | ||||||||||
| Ponderosa pine, interior | Replacement | 5% | 161 | 800 | |||||||||
| Mixed | 10% | 80 | 50 | 80 | |||||||||
| Surface or low | 86% | 9 | 8 | 10 | |||||||||
| Spruce-fir-pine (subalpine) | Replacement | 98% | 217 | 75 | 300 | ||||||||
| Mixed | 2% | >1,000 | |||||||||||
| Northern and Central Rockies | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northern and Central Rockies Grassland | |||||||||||||
| Mountain grassland | Replacement | 60% | 20 | 10 | |||||||||
| Mixed | 40% | 30 | |||||||||||
| Northern prairie grassland | Replacement | 55% | 22 | 2 | 40 | ||||||||
| Mixed | 45% | 27 | 10 | 50 | |||||||||
| Northern and Central Rockies Shrubland | |||||||||||||
| Basin big sagebrush | Replacement | 60% | 100 | 10 | 150 | ||||||||
| Mixed | 40% | 150 | |||||||||||
| Riparian (Wyoming) | Mixed | 100% | 100 | 25 | 500 | ||||||||
| Low sagebrush shrubland | Replacement | 100% | 125 | 60 | 150 | ||||||||
| Mountain big sagebrush steppe and shrubland | Replacement | 100% | 70 | 30 | 200 | ||||||||
| Mountain shrub, nonsagebrush | Replacement | 80% | 100 | 20 | 150 | ||||||||
| Mixed | 20% | 400 | |||||||||||
| Salt desert shrub | Replacement | 50% | >1,000 | 500 | >1,000 | ||||||||
| Mixed | 50% | >1,000 | 500 | >1,000 | |||||||||
| Wyoming big sagebrush | Replacement | 63% | 145 | 80 | 240 | ||||||||
| Mixed | 37% | 250 | |||||||||||
| Northern and Central Rockies Woodland | |||||||||||||
| Ancient juniper | Replacement | 100% | 750 | 200 | >1,000 | ||||||||
| Northern and Central Rockies Forested | |||||||||||||
| Douglas-fir (cold) | Replacement | 31% | 145 | 75 | 250 | ||||||||
| Mixed | 69% | 65 | 35 | 150 | |||||||||
| Douglas-fir (warm mesic interior) | Replacement | 28% | 170 | 80 | 400 | ||||||||
| Mixed | 72% | 65 | 50 | 250 | |||||||||
| Douglas-fir (xeric interior) | Replacement | 12% | 165 | 100 | 300 | ||||||||
| Mixed | 19% | 100 | 30 | 100 | |||||||||
| Surface or low | 69% | 28 | 15 | 40 | |||||||||
| Grand fir-Douglas-fir-western larch mix | Replacement | 29% | 150 | 100 | 200 | ||||||||
| Mixed | 71% | 60 | 3 | 75 | |||||||||
| Grand fir-lodgepole pine-western larch-Douglas-fir | Replacement | 31% | 220 | 50 | 250 | ||||||||
| Mixed | 69% | 100 | 35 | 150 | |||||||||
| Lodgepole pine, lower subalpine | Replacement | 73% | 170 | 50 | 200 | ||||||||
| Mixed | 27% | 450 | 40 | 500 | |||||||||
| Lodgepole pine, persistent | Replacement | 89% | 450 | 300 | 600 | ||||||||
| Mixed | 11% | >1,000 | |||||||||||
| Lower subalpine (Wyoming and Central Rockies) | Replacement | 100% | 175 | 30 | 300 | ||||||||
| Mixed-conifer upland western redcedar-western hemlock | Replacement | 67% | 225 | 150 | 300 | ||||||||
| Mixed | 33% | 450 | 35 | 500 | |||||||||
| Ponderosa pine (Black Hills, low elevation) | Replacement | 7% | 300 | 200 | 400 | ||||||||
| Mixed | 21% | 100 | 50 | 400 | |||||||||
| Surface or low | 71% | 30 | 5 | 50 | |||||||||
| Ponderosa pine (Black Hills, high elevation) | Replacement | 12% | 300 | ||||||||||
| Mixed | 18% | 200 | |||||||||||
| Surface or low | 71% | 50 | |||||||||||
| Ponderosa pine (Northern and Central Rockies) | Replacement | 4% | 300 | 100 | >1,000 | ||||||||
| Mixed | 19% | 60 | 50 | 200 | |||||||||
| Surface or low | 77% | 15 | 3 | 30 | |||||||||
| Ponderosa pine (Northern Great Plains) | Replacement | 5% | 300 | ||||||||||
| Mixed | 20% | 75 | |||||||||||
| Surface or low | 75% | 20 | 10 | 40 | |||||||||
| Ponderosa pine-Douglas-fir | Replacement | 10% | 250 | >1,000 | |||||||||
| Mixed | 51% | 50 | 50 | 130 | |||||||||
| Surface or low | 39% | 65 | 15 | ||||||||||
| Western larch-lodgepole pine-Douglas-fir | Replacement | 33% | 200 | 50 | 250 | ||||||||
| Mixed | 67% | 100 | 20 | 140 | |||||||||
| Whitebark pine-lodgepole pine (upper subalpine, Northern and Central Rockies) | Replacement | 38% | 360 | ||||||||||
| Mixed | 62% | 225 | |||||||||||
| Upper subalpine spruce-fir (Central Rockies) | Replacement | 100% | 300 | 100 | 600 | ||||||||
| Western redcedar | Replacement | 87% | 385 | 75 | >1,000 | ||||||||
| Mixed | 13% | >1,000 | 25 | ||||||||||
| Northern Great Plains | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northern Plains Grassland | |||||||||||||
| Central tallgrass prairie | Replacement | 75% | 5 | 3 | 5 | ||||||||
| Mixed | 11% | 34 | 1 | 100 | |||||||||
| Surface or low | 13% | 28 | 1 | 50 | |||||||||
| Nebraska Sandhills prairie | Replacement | 58% | 11 | 2 | 20 | ||||||||
| Mixed | 32% | 20 | |||||||||||
| Surface or low | 10% | 67 | |||||||||||
| Northern mixed-grass prairie | Replacement | 67% | 15 | 8 | 25 | ||||||||
| Mixed | 33% | 30 | 15 | 35 | |||||||||
| Northern tallgrass prairie | Replacement | 90% | 6.5 | 1 | 25 | ||||||||
| Mixed | 9% | 63 | |||||||||||
| Surface or low | 2% | 303 | |||||||||||
| Oak savanna | Replacement | 7% | 44 | ||||||||||
| Mixed | 17% | 18 | |||||||||||
| Surface or low | 76% | 4 | |||||||||||
| Southern mixed-grass prairie | Replacement | 100% | 9 | 1 | 10 | ||||||||
| Northern Plains Woodland | |||||||||||||
| Great Plains floodplain | Replacement | 100% | 500 | ||||||||||
| Northern Great Plains wooded draws and ravines | Replacement | 38% | 45 | 30 | 100 | ||||||||
| Mixed | 18% | 94 | |||||||||||
| Surface or low | 43% | 40 | 10 | ||||||||||
| Oak woodland | Replacement | 2% | 450 | ||||||||||
| Surface or low | 98% | 7.5 | |||||||||||
| South-central US | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| South-central US Grassland | |||||||||||||
| Desert grassland | Replacement | 82% | 8 | ||||||||||
| Mixed | 18% | 37 | |||||||||||
| Southern shortgrass or mixed-grass prairie | Replacement | 100% | 8 | 1 | 10 | ||||||||
| South-central US Shrubland | |||||||||||||
| Shinnery oak-mixed grass | Replacement | 96% | 7 | ||||||||||
| Mixed | 4% | 150 | |||||||||||
| Shinnery oak-tallgrass | Replacement | 93% | 7 | ||||||||||
| Mixed | 7% | 100 | |||||||||||
| Southwestern shrub steppe | Replacement | 76% | 12 | ||||||||||
| Mixed | 24% | 37 | |||||||||||
| South-central US Woodland | |||||||||||||
| Mesquite savanna | Replacement | 5% | 100 | ||||||||||
| Mixed | 4% | 150 | |||||||||||
| Surface or low | 91% | 6 | |||||||||||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [136,187]. |
|||||||||||||
1. Agee, James K. 1981. Fire effects on Pacific Northwest forests: flora, fuels, and fauna. In: Conference proceedings: annual meeting of the Northwest Forest Fire Council; 1981 November 23-24; Portland, OR. [Place of publication unknown]:[Northwest Forest Fire Council]: 54-66. [29752]
2. Albert, Steven K.; Luna, Nelson; Chopito, Albert L. 1995. Deer, small mammal, and songbird use of thinned pinon-juniper plots: preliminary results. In: Shaw, Douglas W.; Aldon, Earl F.; LoSapio, Carol, technical coordinators. Desired future conditions for pinon-juniper ecosystems: Proceedings of the symposium; 1994 August 8-12; Flagstaff, AZ. Gen. Tech. Rep. RM-258. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 54-64. [24797]
3. Allombert, Sylvian; Gaston, Anthony J.; Martin, Jean-Louis. 2005. A natural experiment on the impact of overabundant deer on songbird populations. Biological Conservation. 126(1): 1-13. [54519]
4. Altendorf, Kelly B.; Laundre, John W.; Lopez Gonzalez, Carlos A.; Brown, Joel S. 2001. Assessing effects of predation risk on foraging behavior of mule deer. Journal of Mammalogy. 82(2): 430-439. [49151]
5. Anderson, Allen E. 1981. Morphological and physiological characteristics. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 27-98. [84940]
6. Anderson, Allen E.; Medin, Dean E.; Bowden, David C. 1974. Growth and morphometry of the carcass, selected bones, organs, and glands of mule deer. Wildlife Monographs. 39: 3-122. [85688]
7. Anderson, Allen E.; Wallmo, Olof C. 1984. Odocoileus hemionus. Mammalian Species. 219: 1-9. [84978]
8. Anderson, E. William; Franzen, David L.; Melland, Jack E. 1990. Forage quality as influenced by prescribed grazing. In: Severson, Kieth E., tech. coord. Can livestock be used as a tool to enhance wildlife habitat? 43rd annual meeting of the Society for Range Management; 1990 February 13; Reno, NV. Gen. Tech. Rep. RM-194. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 56-70. [85214]
9. Anderson, Loren. 1994. Chapter VII - terrestrial wildlife and habitat. In: Miller, Melanie, ed. Fire effects guide. PMS 481/NFES 2394. Boise, ID: National Wildfire Coordinating Group, Prescribed Fire and Fire Effects Working Team: VII: 1-16. [69984]
10. Anderson, Melanie Vael; Pasquinelli, Renee L. 1984. Ecology and management of the northern oak woodland community, Sonoma County, California. Rohnert Park, CA: Sonoma State University. 125 p. Thesis. [68830]
11. Anthony, Robert G. 1976. Influence of drought on diets and numbers of desert deer. The Journal of Wildlife Management. 40(1): 140-144. [11558]
12. Arevalo, Jose Ramon; Alvarez, Pelayo; Narvaez, Nelmi; Walker, Kenny. 2009. The effetcs of fire on the regeneration of a Quercus douglasii stand in Quail Ridge Reserve, Berryessa Valley (California). Journal of Forest Research. 14(2): 81-87. [84234]
13. Armleder, H. M.; Dawson, R. J. 1992. Logging on mule deer winter range: An integrated management approach. The Forestry Chronicle. 68(1): 132-137. [18573]
14. Ashcraft, G. C. 1979. Effects of fire on deer in chaparral. Cal-Neva Wildlife Transactions. (1979): 177-189. [5995]
15. Asherin, Duane A. 1973. Prescribed burning effects on nutrition, production and big game use of key northern Idaho browse species. Moscow, ID: University of Idaho. 96 p. Dissertation. [360]
16. Asherin, Duane A. 1975. Changes in elk use and available browse production on north Idaho winter ranges following prescribed burning. In: Hieb, Susan R., ed. Proceedings, elk logging-roads symposium; 1975 December 16-17; Moscow, ID. Moscow, ID: University of Idaho: 122-134. [17049]
17. Austin, Dennis D. 2000. Managing livestock grazing for mule deer (Odocoileus hemionus) on winter range in the Great Basin. Western North American Naturalist. 60(2): 198-203. [85623]
18. Austin, Dennis D.; Urness, Philip J. 1986. Effects of cattle grazing on mule deer diet and area selection. Journal of Range Management. 39(1): 18-21. [364]
19. Bailey, Arthur W. 1978. Prescribed burning as an important tool for Canadian rangelands. In: McAvoy, S. D. A. M.; Gordon, R. C., co-chairs. Fire and range management; 1978 April; Regina, SK. Regina, SK: Saskatchewan Department of Agriculture: 15-27. [18390]
20. Ballard, Warren B.; Lutz, Daryl; Keegan, Thomas W.; Carpenter, Len H.; deVos, James C., Jr. 2001. Deer-predator relationships: a review of recent North American studies with emphasis on mule and black-tailed deer. Wildlife Society Bulletin. 29(1): 99-115. [85630]
21. Barney, Milo A.; Frischknecht, Neil C. 1974. Vegetation changes following fire in the pinyon-juniper type of west-central Utah. Journal of Range Management. 27(2): 91-96. [397]
22. Bartmann, Richard M. 1986. Growth rates of mule deer fetuses under different winter conditions. Great Basin Naturalist. 46(2): 245-248. [85634]
23. Bartos, D. L.; Mueggler, W. F. 1981. Early succession in aspen communities following fire in western Wyoming. Journal of Range Management. 34(4): 315-318. [5100]
24. Bartos, Dale L. 2007. Aspen. In: Hood, Sharon M.; Miller, Melanie, eds. Fire ecology and management of the major ecosystems of southern Utah. Gen. Tech. Rep. RMRS-GTR-202. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 39-55. [71079]
25. Bartuszebige, Anne M.; Endress, Bryan A. 2008. Do ungulates facilitate native and exotic plant spread? Seed dispersal by cattle, elk and deer in northeastern Oregon. Journal of Arid Environments. 72(6): 904-913. [70508]
26. Bates, Patricia A. 1983. Prescribed burning blackbrush for deer habitat improvement. Cal-Neva Wildlife Transactions. (1983): 174-182. [4458]
27. Bendell, J. F. 1974. Effects of fire on birds and mammals. In: Kozlowski, T. T.; Ahlgren, C. E., eds. Fire and ecosystems. New York: Academic Press: 73-138. [16447]
28. Bender, Louis C. 2000. Relationships between social group size of Colombian black-tailed deer and habitat cover in Washington. Northwestern Naturalist. 81(2): 49-53. [85610]
29. Bender, Louis C.; Lomas, Laurie A.; Kamienski, Tomas. 2007. Habitat effects on condition of doe mule deer in arid mixed woodland-grassland. Rangeland Ecology and Management. 60(3): 277-284. [85653]
30. Bird, Ralph D. 1961. Ecology of the aspen parkland of western Canada in relation to land use. Contribution No. 27. Ottawa: Canada Department of Agriculture, Research Branch. 153 p. [15620]
31. Bishop, Chad J.; Unsworth, James W.; Garton, Edward O. 2005. Mule deer survival among adjacent populations in southwest Idaho. The Journal of Wildlife Management. 69(1): 311-321. [85719]
32. Bissell, Harold Deane. 1951. Nutritive value of winter deer browse with respect to burning and growth stage. Berkeley, CA: University of California. 31 p. Thesis. [17046]
33. Biswell, H. H. 1960. Prescribed burning and other methods of deer range improvement in ponderosa pine in California. In: Proceedings, annual meeting of the Society of American Foresters; 1959 November 15-19; San Francisco, CA. Bethesda, MD: Society of American Foresters: 102-105. [5269]
34. Biswell, H. H. 1961. Manipulation of chamise brush for deer range improvement. California Fish and Game. 47(2): 125-144. [6366]
35. Biswell, H. H.; Gilman, J. H. 1961. Brush management in relation to fire and other environmental factors on the Tehama deer winter range. California Fish and Game. 47(4): 357-389. [6275]
36. Biswell, H. H.; Taber, R. D.; Hedrick, D. W.; Schultz, A. M. 1952. Management of chamise brushlands for game in the North Coast region of California. California Fish and Game. 38(4): 453-484. [13673]
37. Biswell, H. H.; Taber, R. D.; Schultz, A. M. 1953. Managing brushland for game: opening and later management of chamise brushland improve conditions for production of deer, other game. California Agriculture. 7(2): 5. [17024]
38. Biswell, Harold H. 1974. Effects of fire on chaparral. In: Kozlowski, T. T.; Ahlgren, C. E., eds. Fire and ecosystems. New York: Academic Press: 321-364. [14542]
39. Biswell, Harold H. 1989. Prescribed burning in California wildlands vegetation management. Berkeley, CA: University of California Press. 255 p. [63320]
40. Black, Hugh; Scherzinger, Richard J.; Thomas, Jack Ward. 1976. Relationships of Rocky Mountain elk and Rocky Mountain mule deer habitat to timber management in the Blue Mountains of Oregon and Washington. In: Hieb, S. R., ed. Elk-logging-roads: Proceedings of the symposium; 1975 December 16-17; Moscow, ID. Moscow, ID: University of Idaho: 11-31. [14367]
41. Blackburn, W. H.; Beall, R.; Bruner, A.; Klebenow, D.; Mason, R.; Roundy, B.; Stager, W.; Ward, K. 1975. Controlled fire as a management tool in the pinyon-juniper woodland, Nevada. Annual Progress Report FY 1975. Unpublished report on file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 77 p. [453]
42. Blaisdell, James P.; Murray, Robert B.; McArthur, E. Durant. 1982. Managing Intermountain rangelands--sagebrush-grass ranges. Gen. Tech. Rep. INT-134. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 41 p. [467]
43. Blank, Deborah L. 1984. Forage quality of burned and nonburned aspen communities. Logan, UT: Utah State University. 74 p. Thesis. [471]
44. Bleich, Vernon C.; Taylor, Timothy J. 1998. Survivorship and cause-specific mortality in five populations of mule deer. Great Basin Naturalist. 58(3): 265-272. [85638]
45. Blower, Dan. 1982. Key winter forage plants for B.C. ungulates. Victoria, BC: British Columbia Ministry of the Environment, Terrestrial Studies Branch. 57 p. [17065]
46. Bodurtha, Timothy S.; Peek, James P.; Lauer, Jerry L. 1989. Mule deer habitat use related to succession in a bunchgrass community. The Journal of Wildlife Management. 53(2): 314-319. [6677]
47. Boroski, Brian B.; Mossman, Archie S. 1996. Distribution of mule deer in relation to water sources in northern California. The Journal of Wildlife Management. 60(4): 770-776. [85535]
48. Bowyer, R. Terry; Bleich, Vernon C. 1980. Ecological relationships between southern mule deer and California black oak. In: Plumb, Timothy R., technical coordinator. Proceedings of the symposium on the ecology, management, and utilization of California oaks; 1979 June 26-28; Claremont, CA. Gen. Tech. Rep. PSW-44. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 292-296. [7049]
49. Boyce, Mark S.; Merrill, Evelyn H. 1996. Predicting effects of 1988 fires on ungulates in Yellowstone National Park. In: Effects of grazing by wild ungulates in Yellowstone National Park. Technical Report NPS/NRYELL/NRTR/96-10. Washington, DC: U.S. Department of the Interior, National Park Service, Yellowstone National Park: 361-365. [30344]
50. Brandt, C. A.; Rickard, W. H. 1994. Alien taxa in the North American shrub-steppe four decades after cessation of livestock grazing and cultivation agriculture. Biological Conservation. 68(2): 95-105. [23456]
51. Brown, David E. 1982. Alpine and subalpine grasslands. In: Brown, David E., ed. Biotic communities of the American Southwest--United States and Mexico. Desert Plants. 4(1-4): 109-111. [8894]
52. Brown, David E. 1982. Californian evergreen forest and woodland. In: Brown, David E., ed. Biotic communities of the American Southwest--United States and Mexico. Desert Plants. 4(1-4): 66-69. [8887]
53. Brown, David E. 1982. Great Basin conifer woodland. In: Brown, David E., ed. Biotic communities of the American Southwest--United States and Mexico. Desert Plants. 4(1-4): 52-57. [535]
54. Brown, David E. 1982. Great Basin montane scrubland. In: Brown, David E., ed. Biotic communities of the American Southwest--United States and Mexico. Desert Plants. 4(1-4): 83-84. [8890]
55. Brown, Ellsworth R. 1961. The black-tailed deer of western Washington. Biological Bulletin No. 13. Olympia, WA: Washington State Game Commission. 124 p. [8843]
56. Brown, James K. 1985. Fire effects and application of prescribed fire in aspen. In: Saunders, Ken; Durham, Jack; [and others], eds. Rangeland fire effects: Proceedings of the symposium; 1984 November 27-29; Boise, ID. Boise, ID: U.S. Department of the Interior, Bureau of Land Management, Idaho State Office: 38-47. [3658]
57. Bryant, Fred C. 1991. Managed habitats for deer in juniper woodlands of west Texas. In: Rodiek, Jon E.; Bolen, Eric G., eds. Wildlife and habitats in managed landscapes. Island Press: Washington, DC: 56-75. [85242]
58. Bunnell, F. L. 1990. Ecology of black-tailed deer. In: Nyberg, J. B.; Janz, D. W., tech. eds. Deer and elk habitats in coastal forests of southern British Columbia. Special report series 5. Victoria, BC: British Columbia Ministry of Forests, Research Branch: 31-63. In cooperation with Wildlife Habitat Canada. [84971]
59. Bunnell, F. L.; Harestad, A. S. 1983. Dispersal and dispersion of black-tailed deer: models and observations. Journal of Mammalogy. 64(2): 201-209. [84932]
60. Bunnell, Fred L.; Hovey, Fred W.; McNay, R. Scott; Parker, Kathy L. 1990. Forest cover, snow conditions, and black-tailed deer sinking depths. Canadian Journal of Zoology. 68(11): 2403-2408. [85546]
61. Busse, Matt D.; Simon, Steve A.; Riegel, Gregg M. 2000. Tree-growth and understory responses to low-severity prescribed burning in thinned Pinus ponderosa forests of central Oregon. Forest Science. 46(2): 258-268. [42058]
62. Cable, Dwight R. 1957. Recovery of chaparral following burning and seeding in central Arizona. Res. Note. No. 28. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 6 p. [6342]
63. Campbell, Dan L. 1982. Influence of site preparation on animal use and animal damage to tree seedlings. In: Baumgartner, David M., compiler. Site preparation and fuels management on steep terrain: Proceedings of a symposium; 1982 February 15-17; Spokane, WA. Pullman, WA: Washington State University, Cooperative Extension: 93-101. [18536]
64. Campbell, R. E.; Baker, M. B., Jr.; Ffolliott, P. F.; Larson, F. R.; Avery, C. C. 1977. Wildfire effects on a ponderosa pine ecosystem: an Arizona case study. Res. Pap. RM-191. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 12 p. [4715]
65. Carbyn, L. N. 1975. Factors influencing activity patterns of ungulates at mineral licks. Canadian Journal of Zoology. 53(4): 378-384. [85537]
66. Carpenter, Len H.; Wallmo, Olof C. 1981. Rocky Mountain and Intermountain habitats: Part 2. Habitat evaluation and management. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 399-422. [14383]
67. Chaikina, Natalia A.; Ruckstuhl, Kathreen E. 2006. The effect of cattle grazing on native ungulates: the good, the bad, and the ugly. Rangelands. 28(3): 8-14. [63224]
68. Chatelain Edward Frank. 1947. Food preferences of the Columbian black-tailed deer Odocoileus hemionus columbianus (Richardson) on the Tillamook Burn, Oregon. Corvallis, OR: Oregon State College. 64 p. Thesis. [85105]
69. Chew, Robert M.; Butterworth, Bernard B.; Grechman, Richard. 1959. The effects of fire on the small mammal populations of chaparral. Journal of Mammalogy. 40(2): 253. [2703]
70. Child, Kenneth N. 2007. Incidental mortality. In: Franzmann, Albert W.; Schwartz, Charles C.; McCabe, Richard E., eds. Ecology and management of the North American moose. 2nd ed. Boulder, CO: University Press of Colorado: 275-302. [79101]
71. Clark, Robert G.; Starkey, Edward E. 1990. Use of prescribed fire in rangeland ecosystems. In: Walstad, John D.; Radosevich, Steven R.; Sandberg, David V., eds. Natural and prescribed fire in Pacific Northwest forests. Corvallis, OR: Oregon State University Press: 81-91. [46959]
72. Clary, Warren P. 1987. Overview of ponderosa pine bunchgrass ecology and wildlife habitat enhancement with emphasis on southwestern United States. In: Fisser, Herbert G., ed. Wyoming shrublands: Proceedings, 16th Wyoming shrub ecology workshop; 1987 May 26-27; Sundance, WY. Laramie, WY: University of Wyoming, Department of Range Management, Wyoming Shrub Ecology Workshop: 11-21. [13913]
73. Collins, Thomas C. 1980. A report on the Moose Creek Fire of August, 1979. North Fork, ID: U.S. Department of Agriculture, Forest Service, Salmon National Forest, North Fork Range District. Unpublished report on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 27 p. [+ appendices]. [666]
74. Collins, William B.; Urness, Philip J. 1983. Feeding behavior and habitat selection of mule deer and elk on northern Utah summer range. The Journal of Wildlife Management. 47(3): 646-663. [6915]
75. Conner, Mary M.; Miller, Michael W. 2004. Movement patterns and spatial epidemiology of a prion disease in mule deer population units. Ecological Applications. 14(6): 1870-1881. [85692]
76. Connolly, Guy E. 1978. Predators and predator control. In: Schmidt, John L.; Gilbert, Douglas L., eds. Big game of North America. Harrisburg, PA: Stackpole Books: 369-394. [85702]
77. Connolly, Guy E. 1981. Assessing populations. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 287-346. [84945]
78. Connolly, Guy E. 1981. Limiting factors and population regulation. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 245-286. [84944]
79. Cook, John G.; Hershey, Terry J.; Irwin, Larry L. 1994. Vegetative response to burning on Wyoming mountain-shrub big game ranges. Journal of Range Management. 47(4): 296-302. [23449]
80. Cote, Steeve D.; Rooney, Thomas P.; Tremblay, Jean-Pierre; Dussault, Christian; Waller, Donald M. 2004. Ecological impacts of deer overabundance. Annual Review of Ecology and Systematics. 35: 113-147. [85547]
81. Cowan, Ian McTaggart. 1945. The ecological relationships of the food of the Columbian black-tailed deer, Odocoileus hemionus columbianus (Richardson), in the coast forest region of southern Vancouver Island, British Columbia. Ecological Monographs. 15(2): 110-139. [16006]
82. Crane, M. F.; Habeck, James R.; Fischer, William C. 1983. Early postfire revegetation in a western Montana Douglas-fir forest. Res. Pap. INT-319. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 29 p. [710]
83. Cronin, Matthew A. 1991. Mitochondrial and nuclear genetic relationships of deer (Odocoileus spp.) in western North America. Canadian Journal of Zoology. 69(5): 1270-1279. [84928]
84. Cronin, Matthew A. 1992. Intraspecific variation in mitochondrial DNA of North American cervids. Journal of Mammalogy. 73(1): 70-82. [78057]
85. Cronin, Matthew A.; Vyse, Ernest R.; Cameron, David G. 1988. Genetic relationships between mule deer and white-tailed deer in Montana. The Journal of Wildlife Management. 52(2): 320-328. [84925]
86. Crouch, Glenn L. 1966. Preferences of black-tailed deer for native forage and Douglas-fir seedlings. The Journal of Wildlife Management. 30(3): 471-475. [8881]
87. Crouch, Glenn L. 1968. Forage availability in relation to browsing of Douglas-fir seedlings by black-tailed deer. The Journal of Wildlife Management. 32(3): 542-553. [16105]
88. Crouch, Glenn L. 1981. Coniferous forest habitats. Part 1. Food habits and nutrition. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 423-433. [84949]
89. D'Antonio, Carla M. D.; Odion, Dennis C.; Tyler, Claudia M. 1993. Invasion of maritime chaparral by the introduced succulent Carpobrotus edulis. Oecologia. 95(1): 14-21. [25949]
90. Dasmann, Raymond F. 1956. Fluctuations in a deer population in California chaparral. In: Trefethen, James B., ed. Transactions of the 21st North American wildlife conference; 1956 March 5-7; New Orleans: LA. Washington DC: Wildlife Management Institute: 487-499. [84935]
91. Dasmann, Raymond F; Dasmann, William P. 1963. Mule deer in relation to a climatic gradient. The Journal of Wildlife Management. 27(2): 196-202. [14003]
92. Dasmann, Raymond Fredric. 1954. Ecology and social behavior of a population of the Columbian black-tailed deer. Berkeley, CA: University of California. 155 p. Dissertation. [17402]
93. Dasmann, W.; Hubbard, R.; MacGregor, W. G.; Smith, A. E. 1968. Evaluation of the wildlife results from fuel breaks, browseways, and type conversions. In: Proceedings, California Tall Timbers fire ecology conference; 1967 November 9-10; Hoberg, CA. No. 7. Tallahassee, FL: Tall Timbers Research Station: 179-193. [31000]
94. Dasmann, William P. 1950. Basic deer management. California Fish and Game. 36(3): 251-284. [16713]
95. Davis, John. 1967. Some effects of deer browsing on chamise sprouts after fire. The American Midland Naturalist. 77(1): 234-238. [11745]
96. Davis, Peter R. 1977. Cervid response to forest fire and clearcutting in southeastern Wyoming. The Journal of Wildlife Management. 41(4): 785-788. [224]
97. de Vos, A.; Brokx, P.; Geist, V. 1967. A review of social behavior of the North American cervids during the reproductive period. The American Midland Naturalist. 77(2): 390-417. [78496]
98. Dealy, J. Edward. 1975. Management of lodgepole pine ecosystems for range and wildlife. In: Baumgartner, David M., ed. Management of lodgepole pine ecosystems: Symposium proceedings; 1973 October 9-11; Pullman, WA. Volume 2. Pullman, WA: Washington State University Cooperative Extension Service: 556-568. [7807]
99. deCalesta, David S. 1990. Impact of prescribed burning on damage to conifers by wildlife. In: Walstad, John D.; Radosevich, Steven R.; Sandberg, David V., eds. Natural and prescribed fire in Pacific Northwest forests. Corvallis, OR: Oregon State University Press: 105-110. [47585]
100. Demarchi, Dennis A.; Lofts, Susan. 1985. The effects of spring burning on the productivity and nutrient concentration of several shrub species in the southern Rocky Mountain Trench. MOE Technical Report 19. Victoria, BC: British Columbia Ministry of Environment, Wildlife Branch, Wildlife Habitat and Inventory Section. 89 p. [28269]
101. Deschamp, Joseph A.; Urness, Philip J.; Austin, Dennis D. 1979. Summer diets of mule deer from lodgepole pine habitats. The Journal of Wildlife Management. 43(1): 154-161. [4524]
102. Dorrance, Michael J. 1967. A literature review on behavior of mule deer. Special Report Number 7. [Denver, CO]: Colorado Department of Game, Fish, and Parks, Game Research Division; Colorado Cooperative Wildlife Research Unit. 26 p. [85019]
103. Drew, Mark L.; Samuel, W. M.; Lukiwski, G. M.; Willman, J. N. 1985. An evaluation of burning for control of winter ticks, Dermacentor albipictus, in central Alberta. Journal of Wildlife Diseases. 21(3): 313-315. [79624]
104. Dubreuil, Robert P. 2003. Habitat selection of white-tailed and mule deer in the southern Black Hills, South Dakota. Brookings, SD: South Dakota State University. 212 p. Thesis. [85337]
105. Duncan, Celestine A. 2005. Diffuse knapweed--Centaurea diffusa Lam. In: Duncan, Celestine L.; Clark, Janet K., eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. WSSA Special Publication. Lawrence, KS: Weed Science Society of America: 26-35. [60229]
106. Eddleman, Lee E.; Miller, Patricia M.; Miller, Richard F.; Dysart, Patricia L. 1994. Western juniper woodlands (of the Pacific Northwest): Science assessment. Walla Walla, WA: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Interior Columbia Basin Ecosystem Management Project. 131 p. Available online: http://www.icbemp.gov/science/eddleman.pdf [2010, June 22]. [27969]
107. Edwards, R. Y. 1954. Fire and the decline of a mountain caribou herd. The Journal of Wildlife Management. 18(4): 521-526. [8394]
108. Eichhorn, Larry C.; Watts, C. Robert. 1984. Plant succession on burns in the river breaks of central Montana. Proceedings, Montana Academy of Science. 43: 21-34. [15478]
109. Einarsen, Arthur S. 1946. Crude protein determination of deer food as an applied management technique. Transactions, 11th North American Wildlife Conference. 11: 309-312. [17031]
110. Einarsen, Arthur S. 1946. Management of black-tailed deer. The Journal of Wildlife Management. 10(1): 54-59. [8727]
111. Everett, Richard L., compiler. 1987. Proceedings--pinyon-juniper conference; 1986 January 13- 16; Reno, NV. General Technical Report INT-215. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 581 p. [891]
112. Fairchild, John A. 1999. Pinyon-juniper chaining design guidelines for big game winter range enhancement projects. In: Monsen, Stephen B.; Stevens, Richard, compilers. Proceedings: ecology and management of pinyon-juniper communities within the Interior West: Sustaining and restoring a diverse ecosystem; 1997 September 15-18; Provo, UT. Proceedings RMRS-P-9. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 278-280. [30565]
113. Ffolliott, Peter F.; Guertin, D. Phillip. 1990. Prescribed fire in Arizona ponderosa pine forests: a 24-year case study. In: Krammes, J. S., tech. coord. Effects of fire management of southwestern natural resources: Proceedings of the symposium; 1988 November 15-17; Tucson, AZ. Gen. Tech. Rep. RM-191. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 250-254. [11300]
114. Flook, Donald R. 1964. Range relationships of some ungulates native to Banff and Jasper National Parks, Alberta. In: Crisp, D. J., ed. Grazing in terrestrial and marine environments: A symposium of the British Ecological Society: Proceedings; 1962 April 11-14; Bangor, UK. No. 4. Oxford: Blackwell Scientific Publications: 119-128. [15688]
115. Fox, Kevin B.; Krausman, Paul R. 1994. Fawning habitat of desert mule deer. The Southwestern Naturalist. 39(3): 269-275. [24007]
116. Fraas, W. Wyatt; Wambolt, Carl L.; Frisina, Michael R. 1992. Prescribed fire effects on a bitterbrush--mountain big sagebrush--bluebunch wheatgrass community. In: Clary, Warren P.; McArthur, E. Durant; Bedunah, Don; Wambolt, Carl L., comps. Proceedings--symposium on ecology and management of riparian shrub communities; 1991 May 29-31; Sun Valley, ID. Gen. Tech. Rep. INT-289. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 212-216. [19124]
117. French, Marilynn Gibbs; French, Steven P. 1996. Large mammal mortality in the 1988 Yellowstone fires. In: Greenlee, Jason, ed. The ecological implications of fire in Greater Yellowstone: Proceedings, 2nd biennial conference on the Greater Yellowstone Ecosystem; 1993 September 19-21; Yellowstone National Park, WY. Fairfield, WA: International Association of Wildland Fire: 113-115. [27835]
118. Fule, Peter A.; Covington, W. Wallace; Moore, Margaret M.; Heinlein, Thomas A.; Waltz, Amy E. M. 2002. Natural variability in forests of the Grand Canyon, USA. Journal of Biogeography. 29(1): 31-47. [45921]
119. Geist, Valerius. 1981. Behavior: adaptive strategies in mule deer. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 157-224. [84942]
120. Geist, Valerius. 1998. Red deer. In: Deer of the world: Their evolution, behaviour, and ecology. Mechanicsburg, PA: Stackpole Books: 170-222. [78069]
121. Geist, Valerius. 1998. White-tailed deer and mule deer. In: Deer of the world: Their evolution, behaviour, and ecology. Mechanicsburg, PA: Stackpole Books: 255-414. [85316]
122. Gilbert, Paul F.; Wallmo, Olof C.; Gill, R. Bruce. 1970. Effect of snow depth on mule deer in Middle Park, Colorado. The Journal of Wildlife Management. 34(1): 15-23. [85658]
123. Gordon, Floyd A. 1976. Spring burning in an aspen-conifer stand for maintenance of moose habitat, West Boulder River, Montana. In: Proceedings, Montana Tall Timbers fire ecology conference and Intermountain Fire Research Council fire and land management symposium; 1974 October 8-10; Missoula, MT. No. 14. Tallahassee, FL: Tall Timbers Research Station: 501-538. [13529]
124. Gray, Robert W.; MacKenzie, Kenneth L. 2004. Mule deer browse species response to thinning and burning in interior Douglas-fir forests in British Columbia. In: Engstrom, R. Todd; Galley, Krista E. M.; de Groot, William J., eds. Fire in temperate, boreal, and montane ecosystems: Proceedings of the 22nd Tall Timbers fire ecology conference: an international symposium; 2001 October 15-18; Kananaskis Village, AB. No. 22. Tallahassee, FL: Tall Timbers Research: 265. Abstract. [52333]
125. Greater Yellowstone Coordinating Committee. 1988. Greater Yellowstone Area fire situation, 1988. Final report. Billings, MT: U.S. Department of Agriculture, Forest Service, Custer National Forest. 207 p. [38771]
126. Griffith, Brad; Peek, James M. 1989. Mule deer use of seral stage and habitat type in bitterbrush communities. The Journal of Wildlife Management. 53(3): 636-642. [8408]
127. Gruell, George E. 1986. Post-1900 mule deer irruptions in the Intermountain West: principle cause and influences. Gen. Tech. Rep. INT-206. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 37 p. [1049]
128. Hall, E. Raymond. 1981. Dama hemionus: Black-tailed deer or mule deer. In: The mammals of North America. 2nd ed. Vol. 2. New York: John Wiley & Sons: 1088-1091. [54718]
129. Hamlin, Kenneth L.; Mackie, Richard J. 1989. Mule deer in the Missouri River Breaks, Montana: A study of population dynamics in a fluctuating environment. Final Report. Helena, MT: Montana Department of Fish, Wildlife, and Parks. 401 p. [84930]
130. Hamlin, Kenneth L.; Mackie, Richard J. 1991. Age-specific reproduction and mortality in female mule deer: an implication of population dynamics. In: Bobek, B.; Perzanowski, K.; Regelin, W., eds. Global trends in wildlife management: Transactions of the 18th International Union of Game Biology congress. Volume 1; 1987 August 23-29; Krakow, Poland. Krakow-Warszawa, Poland: Swiat Press: 569-573. [84931]
131. Hamlin, Kenneth L.; Riley, Shawn J; Pyrah, Duane; Dood, Arnold R.; Mackie, Richard J. 1984. Relationships among mule deer fawn mortality, coyotes, and alternate prey species during summer. The Journal of Wildlife Management. 48(2): 489-499. [85704]
132. Hanley, Thomas A. 1986. Physical and chemical response of understory vegetation to deer use in southeastern Alaska. Canadian Journal of Forest Research. 17(3): 195-199. [85920]
133. Hanley, Thomas A.; Cates, Rex G.; Van Horne, Beatrice; McKendrick, Jay D. 1987. Forest stand-age related differences in apparent nutritional quality of forage for deer in southeastern Alaska. In: Provenza, Frederick D.; Flinders, Jerran T.; McArthur, E. Durant, compilers. Proceedings--symposium on plant-herbivore interactions; 1985 August 7-9; Snowbird, UT. Gen. Tech. Rep. INT-222. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 9-17. [7395]
134. Hanley, Thomas A.; Robbins, Charles T.; Spalinger, Donald E. 1989. Forest habitats and the nutritional ecology of Sitka black-tailed deer: a research synthesis with implications for forest management. Gen. Tech. Rep. PNW-GTR-230. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 52 p. [7509]
135. Hanley, Thomas P. 1984. Relationships between Sitka black-tailed deer and their habitat. Gen. Tech. Rep. PNW-168. Portland, OR: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 21 p. [14397]
136. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org. [81749]
137. Happe, Patricia J.; Jenkins, Kurt J.; Starkey, Edward E.; Sharrow, Steven H. 1990. Nutritional quality and tannin astringency of browse in clear-cuts and old-growth forests. The Journal of Wildlife Management. 54(4): 557-566. [13290]
138. Haupt, Harold F. 1979. Effects of timber cutting and revegetation on snow accumulation and melt in north Idaho. Res. Pap. INT-224. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 14 p. [12884]
139. Heady, Harold F. 1954. Viable seed recovered from fecal pellets of sheep and deer. Journal of Range Management. 7(6): 259-261. [25224]
140. Hebblewhite, M.; Munro, R. H.; Merrill, E. H. 2009. Trophic consequence of postfire logging in a wolf-ungulate system. Forest Ecology and Management. 257(3): 1053-1062. [74080]
141. Hedrick, D. W.; Biswell, H. H.; Schultz, A. M. 1953. Response of brush seedlings to sprays of 2,4-D and 2,4,5-T on burned chamise areas. California Fish and Game. Sacramento, CA: California Department of Fish and Game. 39(4): 497-505. [34932]
142. Hefner, P.; Clark, R. G.; Britton, C. M. 1980. Seasonal flammability of big sagebrush and western juniper foliage. In: Research in rangeland management--1980 progress report. Special Report 586. Corvallis, OR: Oregon State University, Agricultural Experiment Station: 3-6. In cooperation with: U.S. Department of Agriculture, SEA-AR. [2713]
143. Hibbert, Alden R.; Davis, Edwin A.; Scholl, David G. 1974. Chaparral conversion potential in Arizona. Part I: water yield response and effects on other resources. Res. Pap. RM-126. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 36 p. [1144]
144. Hibler, Charles P. 1981. Diseases. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 129-156. [14390]
145. Hiehle, Jack L. 1962. Improving chamise brushlands for deer and other game. Sacramento, CA: California Department of Fish and Game. 21 p. [17167]
146. Higgins, Kenneth F.; Kruse, Arnold D.; Piehl, James L. 1989. Effects of fire in the Northern Great Plains. Ext. Circ. EC-761. Brookings, SD: South Dakota State University, Cooperative Extension Service; South Dakota Cooperative Fish and Wildlife Research Unit. 47 p. [14749]
147. Hines, William W. 1973. Black-tailed deer populations and Douglas-fir reforestation in the Tillamook Burn, Oregon. Game Research Report No. 3. Final report: Federal Aid to Wildlife Restoration--Project W-51-R. Corvallis, OR: Oregon State Game Commission, Research Division. 59 p. [8431]
148. Hines, William W. 1975. Black-tailed deer behavior and population dynamics in the Tillamook Burn, Oregon. Wildlife Research Report Number 5. Corvallis, OR: Oregon Wildlife Commission. 31 p. [84962]
149. Hobbs, N. T.; Spowart, R. A. 1984. Effects of prescribed fire on nutrition of mountain sheep and mule deer during winter and spring. The Journal of Wildlife Management. 48(2): 551-560. [4485]
150. Hobbs, N. Thompson. 1989. Linking energy balance to survival in mule deer: development and test of a simulation model. Wildlife Monographs. 101: 3-39. [85909]
151. Hobbs, N. Thompson; Swift, David M. 1985. Estimates of habitat carrying capacity incorporating explicit nutritional constraints. The Journal of Wildlife Management. 49(3): 814-822. [85247]
152. Hoefs, Manfred. 2001. Mule, Odocoileus hemionus, and white-tailed, O. virgianus, deer in the Yukon. Canadian Field-Naturalist. 115(2): 296-300. [85143]
153. Holechek, Jerry L. 1982. Managing rangelands for mule deer. Rangelands. 4(1): 25-28. [10500]
154. Hornocker, M. G. 1970. An analysis of mountain lion predation upon mule deer and elk in the Idaho Primitive Area. Wildlife Monographs No. 21. 39 p. [17924]
155. Hughes, Glenys A.; Carr, Steven M. 1993. Reciprocal hybridization between white-tailed deer (Odocoileus virginianus) and mule deer (O. hemionus) in western Canada: evidence from serum albumin and mtDNA sequences. Canadian Journal of Zoology. 71(3): 524-530. [84927]
156. Hungerford, C. R. 1970. Response of Kaibab mule deer to management of summer range. The Journal of Wildlife Management. 34(40): 852-862. [1219]
157. Hunt, Duston Lamar, Jr. 1978. Diet and habitat utilization of tame mule deer in a pinyon-juniper woodland. Las Cruces, NM: New Mexico State University. 82 p. Thesis. [85057]
158. Hutchison, Boyd A. 1965. Snow accumulation and disappearance influenced by big sagebrush. Research Note RM-46. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 7 p. [85912]
159. Hygnstrom, Scott E.; Groepper, Scott R.; VerCauteren, Kurt C.; Frost, Chuck J.; Boner, Justin R.; Kinsell, Travis C.; Clements, Greg M. 2008. Literature review of mule deer and white-tailed deer movements in western and midwestern landscapes. Great Plains Research: A Journal of Natural and Social Sciences. Paper 962: 219-231. [85432]
160. Isaac, Leo A. 1963. Fire--a tool not a blanket rule in Douglas-fir ecology. In: Proceedings, 2nd annual Tall Timbers fire ecology conference; 1963 March 14-15; Tallahassee, FL. Tallahassee, FL: Tall Timbers Research Station: 1-17. [10700]
161. Johnson, Bruce K.; Kern, John W.; Wisdom, Michael J.; Findholt, Scott L.; Kie, John G. 2000. Resource selection and spatial separation of mule deer and elk during spring. The Journal of Wildlife Management. 64(3): 685-697. [85936]
162. Johnson, Craig A. 1989. Early spring prescribed burning of big game winter range in the Snake River Canyon of westcentral Idaho. In: Baumgartner, David M.; Breuer, David W.; Zamora, Benjamin A.; Neuenschwander, Leon F.; Wakimoto, Ronald H., comps. Prescribed fire in the Intermountain region: Forest site preparation and range improvement: Symposium proceedings; 1986 March 3-5; Spokane, WA. Pullman, WA: Washington State University, Department of Natural Resources, Cooperative Extension: 151-155. [11263]
163. Johnstone-Yellin, Tamara L.; Shipley, Lisa A.; Myers, Woodrow L.; Robinson, Hugh S. 2009. To twin or not to twin? Trade-offs in litter size and fawn survival in mule deer. Journal of Mammalogy. 90(2): 453-460. [85713]
164. Julander, Odell; Robinette, W. Leslie; Jones, Dale A. 1961. Relation of summer range condition to mule deer herd productivity. The Journal of Wildlife Management. 25(1): 54-60. [85508]
165. Kay, Charles E.; Bartos, Dale L. 2000. Ungulate herbivory on Utah aspen: assessment of longterm exclosures. Journal of Range Management. 53(2): 145-153. [36034]
166. Keay, Jeffrey A.; Peek, James M. 1980. Relationships between fires and winter habitat of deer in Idaho. The Journal of Wildlife Management. 44(2): 372-380. [125]
167. Kie, John G. 1984. Deer habitat use after prescribed burning in northern California. Res. Note PSW-369. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 3 p. [14393]
168. Kie, John G. 1996. The effects of cattle grazing on optimal foraging in mule deer (Odocoileus hemionus). Forest Ecology and Management. 88(1-2): 131-138. [27298]
169. Kie, John G.; Evans, Charles J.; Loft, Eric R.; Menke, John W. 1991. Foraging behavior by mule deer: the influence of cattle grazing. The Journal of Wildlife Management. 55(4): 665-674. [85616]
170. Kinucan, Edith Seyfert. 1965. Deer utilization of postfire chaparral shrubs and fire history of the San Gabriel Mountains. Los Angeles, CA: California State College, Los Angeles. 61 p. Thesis. [11163]
171. Klebenow, Donald A. 1965. A montane forest winter deer habitat in western Montana. The Journal of Wildlife Management. 29(1): 27-33. [8430]
172. Klebenow, Donald A. 1985. Big game response to fire in sagebrush-grass rangelands. In: Saunders, Ken; Durham, Jack; [and others], eds. Rangeland fire effects: Proceedings of the symposium; 1984 November 27-29; Boise, ID. Boise, ID: U.S. Department of the Interior, Bureau of Land Management, Idaho State Office: 53-57. [1347]
173. Klebenow, Donald A.; Beall, Robert C. 1978. Fire impacts on birds and mammals on Great Basin rangelands. In: Johnson, Carl, general chairman. Proceedings of the 1977 rangeland management and fire symposium; 1977 November 1-3; Casper, WY. Missoula, MT: University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station: 59-62. [31169]
174. Klinger, Robert C.; Kutilek, Michael J.; Shellhammer, Howard S. 1989. Population responses of black-tailed deer to prescribed burning. The Journal of Wildlife Management. 53(4): 863-871. [10686]
175. Kramp, Betty A.; Patton, David R.; Brady, Ward W. 1983. The effects of fire on wildlife habitat and species. Wildlife Unit Tech. Rep. RUN WILD: Wildlife/habitat relationships. Albuquerque, NM: U.S. Department of Agriculture, Forest Service, Southwestern Region, Wildlife Unit. 29 p. [152]
176. Kremsater, Laurie L.; Bunnell, Fred L. 1992. Testing responses to forest edges: the example of black-tailed deer. Canadian Journal of Zoology. 70(12): 2426-2435. [85545]
177. Kruse, William H. 1972. Effects of wildfire on elk and deer use of a ponderosa pine forest. Res. Note RM-226. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 4 p. [5045]
178. Kruse, William H. 1992. Quantifying wildlife habitats within Gambel oak/forest/woodland vegetation associations in Arizona. In: Ffolliott, Peter F.; Gottfried, Gerald J.; Bennett, Duane A.; Hernandez C., Victor Manuel; Ortega-Rubio, Alfred; Hamre, R. H., tech. coords. Ecology and management of oak and associated woodlands: perspectives in the southwestern United States and northern Mexico: Proceedings; 1992 April 27-30; Sierra Vista, AZ. Gen. Tech. Rep. RM-218. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 182-186. [19762]
179. Kucera, Thomas E. 1991. Adaptive variation in sex ratios of offspring in nutritionally stressed mule deer. Journal of Mammalogy. 72(4): 745-749. [85433]
180. Kufeld, Roland C. 1977. Improving Gambel oak ranges for elk and mule deer by spraying with 2,4,5-TP. Journal of Range Management. 30(1): 53-57. [85924]
181. Kufeld, Roland C. 1983. Responses of elk, mule deer, cattle, and vegetation to burning, spraying and chaining of Gambel oak rangeland. Tech. Publ. 34. Fort Collins, CO: Colorado Division of Wildlife. 47 p. [253]
182. Kufeld, Roland C.; Bowden, David C.; Schrupp, Donald L. 1988. Influence of hunting on movements of female mule deer. Journal of Range Management. 41(1): 70-72. [85761]
183. Kufeld, Roland C.; Bowden, David C.; Schrupp, Donald L. 1989. Distribution and movements of female mule deer in the Rocky Mountain foothills. The Journal of Wildlife Management. 53(4): 871-877. [85691]
184. Kufeld, Roland C.; Stewart, Larry. 1975. Experimental improvement of oakbrush on deer, elk and cattle ranges--Hightower Mountain. Project No. W-101-R-17: Game Range Investigations. Work Plan No. 4: Job No. 3. Job Progress Report: April 1, 1974 through March 31, 1975. Denver, CO: Colorado Department of Fish and Game: 25-92. [16427]
185. Kufeld, Roland C.; Wallmo, O. C.; Feddema, Charles. 1973. Foods of the Rocky Mountain mule deer. Res. Pap. RM-111. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 31 p. [1387]
186. Kunzler, L. M.; Harper, K. T. 1980. Recovery of Gambel oak after fire in central Utah. The Great Basin Naturalist. 40(2): 127-130. [1389]
187. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: https://www.landfire.gov/downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
188. LANDFIRE Rapid Assessment. 2007. Rapid assessment reference condition models, [Online]. In: LANDFIRE. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; The Nature Conservancy (Producers). Available: https://www.landfire.gov/models_EW.php [2008, April 18] [66533]
189. Larson, Frederick R.; Ffolliott, Peter F.; Thill, Ronald E.; Clary, Warren P. 1977. Animal use of ponderosa pine forest openings. The Journal of Wildlife Management. 41(4): 782-784. [5111]
190. Lavelle, Darlene Anne. 1986. Use and preference of spotted knapweed (Centaurea maculosa) by elk (Cervus elaphus) and mule deer (Odocoileus hemionus) on two winter ranges in western Montana. Missoula, MT: University of Montana. 72 p. Thesis. [37896]
191. Lawrence, George; Biswell, Harold. 1972. Effect of forest manipulation on deer habitat in giant sequoia. The Journal of Wildlife Management. 36(2): 595-605. [41671]
192. Leckenby, Donavin A. 1978. Western juniper management for mule deer. In: Martin, Robert E.; Dealy, J. Edward; Caraher, David L., eds. Proceedings of the western juniper ecology and management workshop; 1977 January; Bend, OR. Gen. Tech. Rep. PNW-74. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: 137-161. [1430]
193. Leckenby, Donavin A.; Sheehy, Dennis P.; Nellis, Carl H.; Scherzinger, Richard J.; Luman, Ira D.; Elmore, Wayne; Lemos, James C.; Doughty, Larry; Trainer, Charles E. 1982. Wildlife habitats in managed rangelands--the Great Basin of southeastern Oregon: mule deer. Gen. Tech. Rep. PNW-139. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 40 p. [1432]
194. Leege, Thomas A. 1972. Northern elk ranges improved by burning. Idaho Wildlife Review. 24(4): 7-10. [16753]
195. Lehmkuhl, John F. 2002. The effects of spring burning and grass seeding in forest clearcuts on native plants and conifer seedlings in coastal Washington. Northwest Science. 76(1): 46-60. [82417]
196. Leopold, Aldo. 1923. Wild followers of the forest: The effect of forest fires on game and fish--the relation of forests to game conservation. American Forestry. 29(357): 515-510, 568. [250]
197. Loft, Eric R.; Menke, John W.; Kie, John G.; Bertram, Ron C. 1987. Influence of cattle stocking rate on the structural profile of deer hiding cover. The Journal of Wildlife Management. 51(3): 655-664. [85866]
198. Lomas, Laurie A.; Bender, Louis C. 2007. Survival and cause-specific mortality of neonatal mule deer fawns, north-central New Mexico. The Journal of Wildlife Management. 71(3): 884-894. [85935]
199. Long, Ryan A.; Kie, John G.; Bowyer, R. Terry; Hurley, Mark A. 2009. Resource selection and movements by female mule deer Odocoileus hemionus: effects of reproductive stage. Wildlife Biology. 15(3): 288-298. [85538]
200. Long, Ryan A.; Rachlow, Janet L.; Kie, John G. 2008. Effects of season and scale on response of elk and mule deer to habitat manipulation. The Journal of Wildlife Management. 72(5): 1133-1142. [82412]
201. Longhurst, W. H.; Garton, E. O.; Heady, H. F.; Connolly, G. E. 1976. The California deer decline and possibilities for restoration. In: Yoakum, Jim, ed. Cal-Neva wildlife transactions 1976: Annual meeting for the western section of the Wildlife Society and the California-Nevada chapter of the American Fisheries Society; 1976 January 29-31; Fresno, CA. Bethesda, MD: The Wildlife Society; The American Fisheries Society: 74-103. [68843]
202. Loomis, John; Griffin, Dana; Wu, Ellen; Gonzalez-Caban, Armando. 2002. Estimating the economic value of big game habitat production from prescribed fire using a time series approach. Journal of Forest Economics. 8(2): 119-129. [47107]
203. Lotan, James E.; Brown, James K., compilers. 1984. Fire's effects on wildlife habitat--symposium proceedings; 1984 March 21; Missoula, MT. General Technical Report INT- 186. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 96 p. [1476]
204. Loveless, Charles M. 1967. Ecological characteristics of a mule deer winter range. Technical Publication 20. Denver, CO: Colorado Game, Fish, and Parks Department. 124 p. [84938]
205. Lowe, Philip O.; Ffolliott, Peter F.; Dieterich, John H.; Patton, David R. 1978. Determining potential wildlife benefits from wildfire in Arizona ponderosa pine forests. Gen. Tech. Rep. RM-52. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 12 p. [4481]
206. Lym, Rodney G.; Duncan, Celestine A. 2005. Canada thistle--Cirsium arvense (L.) Scop. In: Duncan, Celestine L.; Clark, Janet K., eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. WSSA Special Publication. Lawrence, KS: Weed Science Society of America: 69-83. [60234]
207. Lyon, L. Jack. 1966. Problems of habitat management for deer and elk in the northern forests. Res. Pap. INT-24. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 15 p. [8426]
208. Lyon, L. Jack. 1971. Vegetal development following prescribed burning of Douglas-fir in south-central Idaho. Res. Pap. INT-105. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 30 p. [1495]
209. Lyon, L. Jack; Crawford, Hewlette S.; Czuhai, Eugene; Fredriksen, Richard L.; Harlow, Richard F.; Metz, Louis J.; Pearson, Henry A. 1978. Effects of fire on fauna: a state-of-knowledge review--National fire effects workshop; 1978 April 10-14; Denver, CO. Gen. Tech. Rep. WO-6. Washington, DC: U.S. Department of Agriculture, Forest Service. 41 p. [25066]
210. Lyon, L. Jack; Hooper, Robert G.; Telfer, Edmund S.; Schreiner, David Scott. 2000. Fire effects on wildlife foods. In: Smith, Jane Kapler, ed. Wildland fire in ecosystems: Effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 51-58. [44448]
211. Lyon, L. Jack; Huff, Mark H.; Telfer, Edmund S.; Schreiner, David Scott; Smith, Jane Kapler. 2000. Fire effects on animal populations. In: Smith, Jane Kapler, ed. Wildland fire in ecosystems: Effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 25-34. [44436]
212. Lyon, L. Jack; Telfer, Edmund S.; Schreiner, David Scott. 2000. Direct effects of fire and animal responses. In: Smith, Jane Kapler, ed. Wildland fire in ecosystems: Effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 17-23. [44435]
213. MacCracken, James G.; Viereck, Leslie A. 1990. Browse regrowth and use by moose after fire in interior Alaska. Northwest Science. 64(1): 11-18. [10803]
214. Mackie, Richard J. 1970. Range ecology and relations of mule deer, elk, and cattle in the Missouri River Breaks, Montana. Wildlife Monographs No. 20. Washington, DC: The Wildlife Society. 79 p. [5897]
215. Mackie, Richard J.; Kie, John G.; Pac, David F.; Hamlin, Kenneth L. 2003. Mule deer (Odocoileus hemionus). In: Feldhamer, George A.; Thompson, Bruce C.; Chapman, Joseph A., eds. Wild mammals of North America: Biology, management, and conservation. 2nd ed. Baltimore, MD: Johns Hopkins University Press: 889-905. [82121]
216. Mackie, Richie J. 1981. Interspecific relationships. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 487-508. [84943]
217. Marshal, J. P.; Krausman, P. R.; Bleich, V. C. 2005. Dynamics of mule deer forage in the Sonoran Desert. Journal of Arid Environments. 60(4): 593-609. [85932]
218. Marshal, Jason P.; Krausman, Paul R.; Bleich, Vernon C. 2008. Body condition of mule deer in the Sonoran Desert is related to rainfall. The Southwestern Naturalist. 53(3): 311-318. [85933]
219. Marshal, Jason P.; Krausman, Paul R.; Bleich, Vernon C.; Ballard, Warren B.; McKeever, Jane S. 2002. Rainfall, El Nino, and dynamics of mule deer in the Sonoran Desert, California. The Journal of Wildlife Management. 66(4): 1283-1289. [85930]
220. Martin, Jean-Louis; Baltzinger, Christophe. 2002. Interaction among deer browsing, hunting, and tree regeneration. Candian Journal of Forestry Research. 32(7): 1254-1264. [43079]
221. Martin, S. Clark. 1983. Responses of semidesert grasses and shrubs to fall burning. Journal of Range Management. 36(5): 604-610. [1539]
222. Martinka, C. J. 1976. Fire and elk in Glacier National Park. In: Proceedings, Tall Timbers fire ecology conference and fire and land management symposium; 1974 October 8-10; Missoula, MT. No. 14. Tallahassee, FL: Tall Timbers Research Station: 377-389. [7523]
223. McArthur, C.; Robbins, C. T.; Hagerman, A. E.; Hanley, T. A. 1993. Diet selection by a ruminant generalist browser in relation to plant chemistry. Canadian Journal of Zoology. 71(11): 2236-2243. [85648]
224. McCulloch, C. Y; Wallmo, O. C.; Ffolliott, P. F. 1965. Acorn yield of Gambel oak in northern Arizona. Res. Note RM-48. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 2 p. [35813]
225. McCulloch, Clay Y. 1969. Some effects of wildfire on deer habitat in pinyon-juniper woodland. The Journal of Wildlife Management. 33(4): 778-784. [1594]
226. McCullough, Dale R. 1964. Relationship of weather to migratory movements of black-tailed deer. Ecology. 45(2): 249-256. [85544]
227. McCullough, Dale R. 1997. Breeding by female fawns in black-tailed deer. Wildlife Society Bulletin. 25(2): 296-297. [85718]
228. Meneely, Scott C.; Schemnitz, Sanford D. 1981. Chemical composition and in vitro digestibility of deer browse three years after a wildfire. The Southwestern Naturalist. 26(4): 365-374. [85068]
229. Merrill, Evelyn H.; Mayland, Henry F.; Peek, James M. 1980. Effects of a fall wildfire on herbaceous vegetation on xeric sites in the Selway-Bitterroot Wilderness, Idaho. Journal of Range Management. 33(5): 363-367. [1642]
230. Miller, Howard A. 1963. Use of fire in wildlife management. In: Proceedings, 2nd annual Tall Timbers fire ecology conference; 1963 March 14-15; Tallahassee, FL. Tallahassee, FL: Tall Timbers Research Station: 19-30. [17921]
231. Mills, James N. 1986. Herbivores and early postfire succession in southern California chaparral. Ecology. 67(6): 1637-1649. [5405]
232. Mueggler, W. F.; Bartos, D. L. 1977. Grindstone Flat and Big Flat exclosures--a 41-year record of changes in clearcut aspen communities. INT-195. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 16 p. [5105]
233. Mysterud, A.; Ostbye, E. 1999. Cover as a habitat element for temperate ungulates: effects on habitat selection and demography. Wildlife Society Bulletin. 27(2): 385-394. [78526]
234. NatureServe. 2013. NatureServe Explorer: An online encyclopedia of life, [Online]. Version 7.1. Arlington, VA: NatureServe (Producer). Available http://www.natureserve.org/explorer. [69873]
235. Nelson, Jack R. 1976. Forest fire and big game in the Pacific Northwest. In: Proceedings, annual Tall Timbers fire ecology conference: Pacific Northwest; 1974 October 16-17; Portland, OR. No. 15. Tallahassee, FL: Tall Timbers Research Station: 85-102. [6464]
236. Nichols, R.; Menke, J. 1984. Effects of chaparral shrubland fire on terrestrial wildlife. In: DeVries, Johannes J., ed. Shrublands in California: literature review and research needed for management. Contribution No. 191. Davis, CA: University of California, Water Resources Center: 74-97. [5706]
237. Nicholson, Matthew C.; Bowyer, R. Terry; Kie, John G. 1997. Habitat selection and survival of mule deer: tradeoffs associated with migration. Journal of Mammalogy. 78(2): 483-504. [85628]
238. Nielsen, Darwin B.; Hinckley, Stan D. 1975. Economic and environmental impacts of sagebrush control on Utah's rangelands--a review and analysis. Research Report No. 25. Logan, UT: Utah State University, Agricultural Experiment Station. 27 p. [16931]
239. O'Brien, Chantal S.; Krausman, Paul R.; Boyd, Hilary M.; Ballard, Warren B.; Cunningham, Stan C.; Devos, James C., Jr. 2010. Influence of coyotes on habitat use by mule deer following a wildfire. California Fish and Game. 96(1): 7-22. [85104]
240. Olson, Bret E. 1999. Grazing and weeds. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 85-96. [35714]
241. Olson, Rich. 1992. Mule deer habitat requirements and management in Wyoming. B-965. Laramie, WY: University of Wyoming, Cooperative Extension Service. 15 p. [20679]
242. Oswald, Brian P.; Covington, W. Wallace. 1983. Changes in understory production following a wildfire in southwestern ponderosa pine. Journal of Range Management. 36(4): 507-509. [5663]
243. Pac, David F.; Mackie, Richard J.; Jorgensen, Henry E. 1991. Mule deer population organization, behavior and dynamics in a northern Rocky Mountain environment. Final Report. [Helena, MT]: Montana Department of Fish, Wildlife and Parks. 316 p. [85698]
244. Parks, Catherine G.; Endress, Bryan A.; Vavra, Martin; McInnis, Michael L.; Naylor, Bridgett J. 2008. Cattle, deer, and elk grazing of the invasive plant sulfur cinquefoil. Natural Areas Journal. 28(4): 404-409. [83522]
245. Pase, Charles P. 1982. Californian (coastal) chaparral. In: Brown, David E., ed. Biotic communities of the American Southwest--United States and Mexico. Desert Plants. 4(1-4): 91-94. [8891]
246. Pase, Charles P.; Pond, Floyd W. 1964. Vegetation changes following the Mingus Mountain burn. Res. Note RM-18. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 8 p. [5700]
247. Patton, David R.; Gordon, Janet. 1995. Fire, habitats, and wildlife. Final report. Flagstaff, AZ: U.S. Department of Agriculture, Forest Service, Coconino National Forest. Unpublished report on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 85 p. [61019]
248. Patton, David R.; Jones, John R. 1977. Managing aspen for wildlife in the Southwest. Gen. Tech. Rep. RM-37. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 7 p. [5410]
249. Pearson, H. A.; Davis, J. R.; Schubert, G. H. 1972. Effects of wildfire on timber and forage production in Arizona. Journal of Range Management. 25(4): 250-253. [5664]
250. Pederson, Jordan C.; Harper, K. T. 1978. Factors influencing productivity of two mule deer herds in Utah. Journal of Range Management. 31(2): 105-110. [4544]
251. Pederson, Jordan C.; Harper, K. T. 1984. Does summer range quality influence sex ratios among mule deer fawns in Utah? Journal of Range Management. 37(1): 64-66. [85636]
252. Peek, James M.; Riggs, Robert A.; Lauer, Jerry L. 1979. Evaluation of fall burning on bighorn sheep winter range. Journal of Range Management. 32(6): 430-432. [1863]
253. Peek, James M.; Scott, Michael D.; Nelson, Louis J.; Pierce, D. John. 1982. Role of cover in habitat management for big game in northwestern United States. Transactions, 47th North American Wildlife and Natural Resources Conference. Washington, DC: Wildlife Management Institute. 47: 363-373. [13901]
254. Pendleton, Rosemary L.; Wagstaff, Fred J.; Welch, Bruce L. 1992. Winter nutrient content and deer use of Gambel oak twigs in north central Utah. The Great Basin Naturalist. 52(4): 293-299. [21136]
255. Phillips, T. A. 1973. The effects of fire on vegetation and wildlife on a lodgepole pine burn in Chamberlain Basin, Idaho. Range Improvement Notes. 18(1): 1-9. [16548]
256. Phillips, T. A. 1977. An analysis of pinyon-juniper chaining projects in the Intermountain Region: 1954 - 1975. Range Improvement Notes/ September 1977. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Region. 20 p. [1880]
257. Picton, Harold; Mackie, Richard J. 1980. Single species island biogeography and Montana mule deer. Biological Conservation. 19(1): 41-49. [85542]
258. Pierce, Becky M.; Bowyer, R. Terry; Bleich, Vernon C. 2004. Habitat selection by mule deer: forage benefits or risk of predation? The Journal of Wildlife Management. 68(3): 533-541. [66863]
259. Pond, Floyd W.; Cable, Dwight R. 1960. Effect of heat treatment on sprout production of some shrubs of the chaparral in central Arizona. Journal of Range Management. 13(6): 313-317. [260]
260. Potts, Jennifer B.; Marino, Eva; Stephens, Scott L. 2010. Chaparral shrub recovery after fuel reduction: a comparison of prescribed fire and mastication techniques. Plant Ecology. 210(2): 303-315. [82367]
261. Purcell, Alice; Schnoes, Roger; Starkey, Edward. 1982. The effects of prescribed burning on mule deer in Lava Beds National Monument. In: Starkey, Edward E.; Franklin, Jerry F.; Matthews, Jean W. Proceedings of the second conference on scientific research in the National Parks; 1979 November; San Francisco, CA. Corvallis, OR: Oregon State University: 111-120. [85100]
262. Radwan, M. A.; Crouch, G. L. 1974. Plant characteristics related to feeding preference by black-tailed deer. The Journal of Wildlife Management. 38(1): 32-41. [64401]
263. Ragotzkie, Kim E.; Bailey, James A. 1991. Desert mule deer use of grazed and ungrazed habitats. Journal of Range Management. 44(5): 487-490. [16573]
264. Rambo, Jennie L.; Faeth, Stanley H. 1999. Effect of vertebrate grazing on plant and insect community structure. Conservation Biology. 13(5): 1047-1054. [51843]
265. Reed, Dale F. 1981. Conflicts with civilization. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 509-536. [14384]
266. Reynolds, Hudson G. 1966. Slash cleanup in a ponderosa pine forest affects use by deer and cattle. Research Note RM-64. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 3 p. [39274]
267. Reynolds, Hudson G. 1967. Chemical constituents and deer use of some crown sprouts in Arizona chaparral. Journal of Forestry. 65(12): 905-908. [12057]
268. Reynolds, Hudson G. 1969. Aspen grove use by deer, elk, and cattle in southwestern coniferous forests. Res. Note RM-138. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 3 p. [16745]
269. Reynolds, Hudson G. 1969. Improvement of deer habitat on southwestern forest lands. Journal of Forestry. 67(11): 803-805. [16544]
270. Rice, Peter M. 2005. Downy brome--Bromus tectorum L. In: Duncan, Celestine L.; Clark, Janet K., eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. WSSA Special Publication. Lawrence, KS: Weed Science Society of America: 147-170. [60251]
271. Rice, Peter M. 2005. Medusahead--Taeniatherum caput-medusae (L.) Nevski. In: Duncan, Celestine L.; Clark, Janet K., eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. WSSA Special Publication. Lawrence, KS: Weed Science Society of America: 171-178. [60252]
272. Rickard, W. H.; Sauer, R. H. 1982. Primary production and canopy cover in bitterbrush-cheatgrass communities. Northwest Science. 56(3): 250-256. [85312]
273. Roberts, Thomas A.; Tiller, Ronald L. 1985. Mule deer and cattle responses to a prescribed burn. Wildlife Society Bulletin. 13(3): 248-252. [5978]
274. Robinette, W. Leslie. 1966. Mule deer home range and dispersal in Utah. The Journal of Wildlife Management. 30(2): 335-349. [84968]
275. Robinette, W. Leslie; Baer, C. Harold; Pillmore, Richard E.; Knittle, C. Edward. 1973. Effects of nutritional change on captive mule deer. The Journal of Wildlife Management. 37(3): 312-326. [85689]
276. Robinette, W. Leslie; Gashwiler, Jay S.; Low, Jessop B.; Jones, Dale A. 1957. Differential mortality by sex and age among mule deer. The Journal of Wildlife Management. 21(1): 1-16. [85664]
277. Robinette, W. Leslie; Julander, Odell; Gashwiler, Jay S.; Smith, Justin G. 1952. Winter mortality of mule deer in Utah in relation to range condition. The Journal of Wildlife Management. 16(3): 289-299. [85715]
278. Roche, Ben F., Jr.; Roche, Cindy Talbott. 1999. Diffuse knapweed. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 217-230. [35725]
279. Roppe, Jerry A.; Hein, Dale. 1978. Effects of fire on wildlife in a lodgepole pine forest. The Southwestern Naturalist. 23(2): 279-287. [261]
280. Rosenstock, Steven S.; Ballard, Warren B.; Devos, James C., Jr. 1999. Viewpoint: benefits and impacts of wildlife water developments. Journal of Range Management. 52(4): 302-311. [85536]
281. Rost, Gregory R.; Bailey, James A. 1979. Distribution of mule deer and elk in relation to roads. The Journal of Wildlife Management. 43(3): 634-641. [85541]
282. Rowe, J. S.; Scotter, G. W. 1973. Fire in the boreal forest. Quaternary Research. 3(3): 444-464. [72]
283. Salwasser, Hal; Holl, Stephen A.; Ashcraft, Gordan A. 1978. Fawn production and survival in the North Kings River deer herd. California Fish and Game. 64(1): 38-52. [84957]
284. Sampson, Arthur W. 1944. Plant succession on burned chaparral lands in northern California. Bull. 65. Berkeley, CA: University of California, College of Agriculture, Agricultural Experiment Station. 144 p. [2050]
285. Sawyer, Hall; Lindzey, Fred; McWhirter, Doug. 2005. Mule deer and pronghorn migration in western Wyoming. Wildlife Society Bulletin. 33(4): 1266-1273. [85543]
286. Scarbrough, David L.; Krausman, Paul R. 1988. Sexual segregation by desert mule deer. The Southwestern Naturalist. 33(2): 157-165. [5250]
287. Severson, K. E. 1981. Plains habitats. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 459-486. [84950]
288. Severson, Keith E.; Carter, Arthur V. 1978. Movement and habitat use by mule deer in the northern great plains, South Dakota. In: Hyder, Donald N., ed. Proceedings of the 1st international rangelands congress; 1978 August 14-18; Denver, CO. Denver, CO: Society for Range Management: 466-468. [84969]
289. Severson, Kieth E. 1987. Deer and elk nutrition in Rocky Mountain ponderosa pine forests. In: Fisser, Herbert G., ed. Wyoming shrublands: Proceedings of the 16th Wyoming shrub ecology workshop; 1987 May 26-27; Sundance, WY. Laramie, WY: University of Wyoming, Department of Range Management, Wyoming Shrub Ecology Workshop: 23-27. [13914]
290. Severson, Kieth E.; Medina, Alvin L. 1983. Deer and elk habitat management in the Southwest. Journal of Range Management. Monograph No. 2. Denver, CO: Society for Range Management. 64 p. [2110]
291. Shantz, H. L. 1947. The use of fire as a tool in the management of the brush ranges of California. Sacramento, CA: State of California, Department of Natural Resources, Division of Forestry. 156 p. [36305]
292. Shepperd, Wayne D.; Battaglia, Michael A. 2002. Ecology, silviculture, and management of Black Hills ponderosa pine. Gen. Tech. Rep. RMRS-GTR-97. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 112 p. [44794]
293. Short, Henry L. 1981. Nutrition and metabolism. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 99-128. [84941]
294. Short, Henry L.; Evans, Wain; Boeker, Erwin L. 1977. The use of natural and modified pinyon pine-juniper woodlands by deer and elk. The Journal of Wildlife Management. 41(3): 543-559. [12036]
295. Short, Henry L.; McCulloch, Clay Y. 1977. Managing pinyon-juniper ranges for wildlife. Gen. Tech. Rep. RM-47. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 10 p. [2137]
296. Singer, F. J.; Harter, M. K. 1996. Comparative effects of elk herbivory and 1988 fires on northern Yellowstone National Park grasslands. Ecological Applications. 6(1): 185-199. [26712]
297. Singer, Francis J.; Schreier, William; Oppenheim, Jill; Garton, Edward O. 1989. Drought, fires, and large mammals. BioScience. 39(10): 716-722. [67678]
298. Skogland, Terje. 1991. What are the effects of predators on large ungulate populations? Oikos. 61(3): 401-411. [85633]
299. Skousen, J. G.; Davis, J. N.; Brotherson, J. D. 1989. Pinyon-juniper chaining and seeding for big game in central Utah. Journal of Range Management. 42(2): 98-104. [1297]
300. Smith, J. G. 1949. Deer forage observations in Utah. The Journal of Wildlife Management. 13(3): 314-315. [9758]
301. Smith, Jane Kapler, ed. 2000. Wildland fire in ecosystems: Effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 83 p. [44460]
302. Smith, Michael A. 1985. Prescribed burning of big sagebrush in Wyoming. In: Fisser, Herbert G., ed. Wyoming shrublands: Proceedings, 14th Wyoming shrub ecology workshop; 1985 May 29-30; Rock Springs, WY. Laramie, WY: University of Wyoming, Department of Range Management, Wyoming Shrub Ecology Workshop: 41-45. [13910]
303. Smith, Ronald H.; Lecount, Albert. 1979. Some factors affecting survival of desert mule deer fawns. The Journal of Wildlife Management. 43(3): 657-665. [85706]
304. Smithey, Douglas A.; Wisdom, Michael J.; Hines, William W. 1985. Roosevelt elk and black-tailed deer response to habitat changes related to old-growth forest conversion in southwestern Oregon. In: Nelson, R. Wayne, ed. Proceedings of the 1984 western states and provinces elk workshop; 1984 April 17-19; Edmonton, AB. Edmonton, AB: Alberta Fish and Wildlife Division; Western Association of Fish & Wildlife Agencies: 41-55. [82288]
305. Sosebee, Ronald E.; Britton, Carlton M.; Bryant, Fred C.; Wester, David Bsolela. 1999. Noxious brush and weed control research at Texas Tech University. In: Wester, David B.; Britton, Carlton M., eds. Research highlights - 1999: Noxious brush and weed control: Range, wildlife, and fisheries management. Volume 30. Lubbock, TX: Texas Tech University, College of Agricultural Sciences and Natural Resources: 6-13. [35496]
306. Spowart, Richard A.; Hobbs, N. Thompson. 1985. Effects of fire on diet overlap between mule deer and mountain sheep. The Journal of Wildlife Management. 49(4): 942-946. [2207]
307. Stager, D.Waive. 1977. Mule deer response to successional changes in the pinyon-juniper vegetation type after wildfire. Reno, NV: University of Nevada. 50 p. Thesis. [85339]
308. Stark, N. 1980. Light burning and the nutrient value of forage. Res. Note INT-280. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 7 p. [2223]
309. Stark, N.; Steele, R. 1977. Nutrient content of forest shrubs following burning. American Journal of Botany. 64(10): 1218-1224. [2224]
310. Stelfox, J. G.; Lynch, G. M.; McGillis, J. R. 1976. Effects of clearcut logging on wild ungulates in the central Albertan foothills. The Forestry Chronicle. 52(2): 65-70. [13506]
311. Stevens, Richard. 2004. Incorporating wildlife habitat needs into restoration and rehabilitation projects. In: Monsen, Stephen B.; Stevens, Richard; Shaw, Nancy L., comps. Restoring western ranges and wildlands. Gen. Tech. Rep. RMRS-GTR-136-vol. 1. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 155-174. [52826]
312. Stewart, Kelley M.; Bowyer, R. Terry; Dick, Brian L.; Kie, John G. 2011. Effects of density dependence on diet composition of North American elk Cervus elaphus and mule deer Odocoileus hemionus: an experimental manipulation. Wildlife Biology. 17(4): 417-430. [85657]
313. Stewart, Kelley M.; Bowyer, R. Terry; Kie, John G.; Cimon, Norman J.; Johnson, Bruce K. 2002. Temporospatial distributions of elk, mule deer, and cattle: resource partitioning and competitive displacement. Journal of Mammalogy. 83(1): 229-244. [45202]
314. Stewart, Kelley M.; Bowyer, R. Terry; Kie, John G.; Hurley, Mark A. 2010. Spatial distribution of mule deer and North American elk: resource partitioning in a sagebrush steppe environment. The American Midland Naturalist. 163(2): 400-412. [85540]
315. Stockton, Stephen A.; Allombert, Sylvain; Gaston, Anthony J.; Martin, Jean-Louis. 2005. A natural experiment on the effects of high deer densities on the native flora of coastal temperate rain forests. Biological Conversation. 126(1): 118-128. [85554]
316. Stubblefield, Suzy S.; Warren, Robert J.; Murphy, Brian R. 1986. Hybridization of free-ranging white-tailed and mule deer in Texas. The Journal of Wildlife Management. 50(4): 688-690. [84926]
317. Sullivan, Thomas P.; Sullivan, Druscilla S.; Lindgren, Pontus M. F.; Ransome, Douglas B. 2007. Long-term responses of ecosystem components to stand thinning in young lodgepole pine forest IV. Relative habitat use by mammalian herbivores. Forest Ecology and Management. 240(1-3): 32-41. [66054]
318. Suminski, Rita R. 1993. Management implications for mule deer winter range in northern pinon-juniper. In: Aldon, Earl F.; Shaw, Douglas W., technical coordinators. Managing pinon-juniper ecosystems for sustainability and social needs: Proceedings; 1993 April 26-30; Santa Fe, NM. Gen. Tech. Rep. RM-236. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 133-139. [22864]
319. Swank, Wendell G. 1958. The mule deer in Arizona chaparral. Wildlife Bulletin No. 3. Phoenix, AZ: State of Arizona, Game and Fish Department. 109 p. [12327]
320. Taber, Richard D. 1952. Game range revegetation in California brushlands. Proceedings, 32nd Annual Conference of Western Association of State Game and Fish Commissioners. 32: 136-140. [16670]
321. Taber, Richard D. 1953. Production, mortality, and yield of black-tailed deer on chaparral range. Proceedings, 33rd Annual Conference of Western Association of Game and Fish Commissioners. 33: 28-131. [16666]
322. Taber, Richard D. 1953. Studies of black-tailed deer reproduction on three chaparral cover types. California Fish and Game. 39(2): 177-186. [16373]
323. Taber, Richard D. 1956. Deer nutrition and population dynamics in the north Coast Range of California. In: Transactions, 21st North American Wildlife Conference. 21: 159-172. [16311]
324. Taber, Richard D. 1961. The black-tailed deer: A review of ecology and management. La Terre et La Vie. 2: 221-245. [17027]
325. Taber, Richard D. 1973. Effects of even-age forest management on big game. In: Hermann, Richard K.; Lavender, Denis P., eds. Even-age management: Proceedings of a symposium; 1972 August 1; [Corvallis, OR]. Paper 848. Corvallis, OR: Oregon State University, School of Forestry: 59-74. [16240]
326. Taber, Richard D.; Dasmann, Raymond F. 1957. The dynamics of three natural populations of the deer Odocoileus hemionus columbianus. Ecology. 38(2): 233-246. [14007]
327. Taber, Richard D.; Dasmann, Raymond F. 1958. The black-tailed deer of the chaparral: Its life history and management in the north Coast Range of California. Game Bulletin No. 8. Sacramento, CA: State of California, Department of Fish and Game, Game Management Branch. 166 p. [16312]
328. Taber, Richard D.; Murphy, James L. 1971. Controlled fire in the management of North American deer. In: The scientific management of animal and plant communities for conservation: Proceedings, 11th symposium of the British Ecological Society; 1970 July 7-9; Norwich, Great Britian. Oxford: Blackwell Scientific Publications: 425-435. [16732]
329. Tausch, Robin J.; Tueller, Paul T. 1977. Plant succession following chaining of pinyon-juniper woodlands in eastern Nevada. Journal of Range Management. 30(1): 44-49. [2305]
330. The Wildlife Society, Nevada Chapter. 1998. Influence of fire on wildlife habitat in the Great Basin: a position statement - August 16, 1998. Transactions, Western Section of the Wildlife Society. 34: 42-57. [35093]
331. Thorne, E. Tom; Williams, Elizabeth S.; Samuel, William M.; Kistner, T. P. 2002. Diseases and parasites. In: Toweill, Dale E.; Thomas, Jack Ward, eds. North American elk: ecology and management. 1st ed. Washington, DC: Smithsonian Institution Press: 351-388. [81797]
332. Tiller, Ronald L.; Roberts, Thomas A.; Quinn, Ronald D. 1986. Deer and cattle interactions following a prescribed burn in chaparral. Transactions of the Western Section of the Wildlife Society. 22: 75-79. [85311]
333. Timmermann, H. R. 1991. Ungulates and aspen management. In: Navratil, S.; Chapman, P. B., eds. Aspen management for the 21st century: Proceedings of a symposium; 1990 November 20-21; Edmonton, AB. Edmonton, AB: Forestry Canada, Northwest Region, Northern Forestry Centre; Poplar Council of Canada: 99-110. [18550]
334. Tomm, H. O.; Beck, J. A., Jr.; Hudson, R. J. 1981. Response of wild ungulates to logging practices in Alberta. Canadian Journal of Forest Research. 11(3): 606-614. [85750]
335. Trueblood, Jack. 1992. Burnt deer, elk range gets help. Idaho Wildlife. [Volume unknown]: 23. [20794]
336. U.S. Department of Agriculture, Forest Service, Northern Region. 1973. U.S.D.A. Forest Service environmental statement: burning for control of big sagebrush. Unpublished draft supplied by Steve Yurich, Regional Forester, U.S. Department of Agriculture, Forest Service, Region 1. 70 p. [2379]
337. U.S. Department of the Interior, Fish and Wildlife Service. 2016. Endangered Species Program, [Online]. Available: http://www.fws.gov/endangered/. [86564]
338. Unsworth, James W.; Pac, David F.; White, Gary C.; Bartmann, Richard M. 1999. Mule deer survival in Colorado, Idaho, and Montana. The Journal of Wildlife Management. 63(1): 315-326. [84937]
339. Updike, Douglas R.; Loft, Eric R.; Hall, Frank A. 1990. Wildfires on big sagebrush/antelope bitterbrush range in northeastern California: implications for deer populations. In: McArthur, E. Durant; Romney, Evan M.; Smith, Stanley D.; Tueller, Paul T., compilers. Proceedings--symposium on cheatgrass invasion, shrub die-off, and other aspects of shrub biology and management; 1989 April 5-7; Las Vegas, NV. Gen. Tech. Rep. INT-276. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 41-46. [12734]
340. Urness, Philip J. 1981. Desert and chaparral habitats. Part 1. Food habits and nutrition. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 347-365. [14389]
341. Wagle, R. F. 1981. Fire: its effects on plant succession and wildlife in the Southwest. Some effects of fire on plant succession and variability in the Southwest from a wildlife management viewpoint. RR 281. Tucson, AZ: University of Arizona. 82 p. [4031]
342. Wald, Eric J.; Kronberg, Scott L.; Larson, Gary E.; Johnson, W. Carter. 2005. Dispersal of leafy spurge (Euphorbia esula L.) seeds in the feces of wildlife. The American Midland Naturalist. 154(2): 342-357. [60036]
343. Wallander, Roseann T.; Olson, Bret E.; Lacey, John R. 1995. Spotted knapweed seed viability after passing through sheep and mule deer. Journal of Range Management. 48(2): 145-149. [37447]
344. Wallmo, O. C. 1969. Response of deer to alternate strip clearcutting lodgepole pine and spruce-fir timber in Colorado. Res. Note RM-141. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 4 p. [14398]
345. Wallmo, O. C.; LeCount, A.; Brownlee, S. L. 1981. Desert and chaparral habitats. Part II. Habitat evaluation and management. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 366-386. [84947]
346. Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press. 605 p. [In cooperation with U.S. Department of Agriculture, Forest Service]. [14392]
347. Wallmo, Olof C. 1981. Mule and black-tailed deer distribution and habitats. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 1-26. [14391]
348. Wallmo, Olof C.; Regelin, Wayne L. 1981. Rocky Mountain and Intermountain habitats: Part 1. Food habits and nutrition. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 387-398. [14387]
349. Wallmo, Olof C.; Regelin, Wayne L.; Reichert, Donald W. 1972. Forage use by mule deer relative to logging in Colorado. The Journal of Wildlife Management. 36(4): 1025-1033. [4486]
350. Wallmo, Olof C.; Schoen, John W. 1980. Response of deer to secondary forest succession in southeast Alaska. Forest Science. 26(3): 448-462. [14394]
351. Wallmo, Olof C.; Schoen, John W. 1981. Coniferous forest habitats. Part 2. Forest management for deer. In: Wallmo, Olof C., ed. 1981. Mule and black-tailed deer of North America. Lincoln, NE: University of Nebraska Press: 434-448. [14386]
352. Walser, Ryan E. 2006. Influence of precipitation and the effects of season change on desert mule deer populations in Trans-pecos, Texas. Alpine, TX: Sul Ross State University. 40 p. Thesis. [84973]
353. Walter, W. D.; Zimmerman, T. J.; Leslie, D. M., Jr.; Jenks, J. A. 2009. Dietary response of sympatric deer to fire using stable isotope analysis of liver tissue. Wildlife Biology in Practice. 5(2): 128-135. [84974]
354. Wambolt, Carl L. 1998. Sagebrush and ungulate relationships on Yellowstone's Northern Range. Wildlife Society Bulletin. 26(3): 429-437. [75521]
355. Ward, Richard L.; Marcum, C. Les. 2005. Lichen litterfall consumption by wintering deer and elk in western Montana. The Journal of Wildlife Management. 69(3): 1081-1089. [85613]
356. Warner, Ralph. 1970. Some aspects of browse production in relation to timber harvest methods and succession in western Montana. Final report. Project No. W-98-R-9: Job No. B-17.1. Washington, DC: Department of the Interior, Bureau of Sport Fisheries and Wildlife, Region 1. 79 p. [39468]
357. Waterhouse, Michaela. 2009. Silvicultural systems on a deep snowpack, mule deer winter range in the central interior of British Columbia: 10-year update. Extension Note 89. Victoria, BC: British Columbia Ministry of Forests and Range Forest Science Program. 8 p. [85763]
358. Welker, Heather J. 1986. Fawn rearing habitat of the Lake Hollow deer herd, Tehama County, California. California Fish and Game. 72(2): 94-98. [85144]
359. White, Gary C.; Freddy, David J.; Gill, R. Bruce; Ellenberger, John H. 2001. Effect of adult sex ratio on mule deer and elk productivity in Colorado. The Journal of Wildlife Management. 65(3): 543-551. [83249]
360. Willard, E. Earl; Bedunah, J; Marcum, C. Les; Lavelle, Darlene. 1988. Use of spotted knapweed by elk and deer in winter. Montana Forest and Conservation Experiment Station Biennial Report 1987-1988. Missoula, MT: University of Montana, School of Forestry: 34. [6579]
361. Willms, W.; Bailey, A. W.; McLean, A. 1980. Effect of burning or clipping Agropyron spicatum in the autumn on the spring foraging behaviour of mule deer and cattle. Journal of Applied Ecology. 17: 69-84. [2572]
362. Willms, W.; Bailey, A. W.; McLean, A.; Tucker, R. 1980. The effects of fall grazing or burning bluebunch wheatgrass range on forage selection by deer and cattle in spring. Canadian Journal of Animal Science. 80: 113-122. [2576]
363. Wilson, Don E.; Reeder, DeeAnn M., eds. 2005. Mammal species of the world: A taxonomic and geographic reference, [Online]. 3rd ed. Baltimore, MD: Johns Hopkins University Press. 2,142 p. Washington, DC: Smithsonian National Museum of Natural History, Department of Vertebrate Zoology, Division of Mammals; American Society of Mammalogists (Producers). Available: http://www.vertebrates.si.edu/msw/mswcfapp/msw/index.cfm [69038]
364. Wisdom, Michael J.; Vavra, Martin; Boyd, Jennifer M.; Hemstrom, Miles A.; Ager, Alan A.; Johnson, Bruce K. 2006. Understanding ungulate herbivory--episodic disturbance effects on vegetation dynamics: knowledge gaps and management needs. Wildlife Society Bulletin. 34(2): 283-292. [82462]
365. Wood, Alan K.; Mackie, Richard J.; Hamlin, Kenneth L. 1989. Ecology of sympatric populations of mule deer and white-tailed deer in a prairie environment. Bozeman, MT: Montana Department of Fish, Wildlife, and Parks, Wildlife Division. 97 p. [84933]
366. Wood, Christopher Karl. 2004. The effects of prescribed burning on deer and elk habitat parameters in Montana's Missouri River Breaks. Bozeman, MT: Montana State University. 68 p. Thesis. [63765]
367. Wood, John E.; Bickle, Thomas S.; Evans, Wainright; Germany, James C.; Howard, Volney W., Jr. 1970. The Fort Stanton mule deer herd (some ecological and life history characteristics with special emphasis on the use of water). Bulletin 567. Las Cruces, NM: New Mexico State University Agricultural Experiment Station. 32 p. [84961]
368. Wright, Anthony L.; Kelsey, Rick G. 1997. Effects of spotted knapweed on a cervid winter-spring range in Idaho. Journal of Range Management. 50(5): 487-496. [27926]
369. Wright, Henry A. 1974. Range burning. Journal of Range Management. 27(1): 5-11. [2613]
370. Yeo, Jeffrey J.; Peek, James M. 1994. Successional patterns of antlered game in cedar-hemlock forests. In: Baumgartner, David M.; Lotan, James E.; Tonn, Jonalea R., compilers. Interior cedar-hemlock-white pine forests: ecology and management: Symposium proceedings; 1993 March 2-4; Spokane, WA. Pullman, WA: Washington State University, Department of Natural Resources: 199-205. [25803]
371. Young, James A.; Clements, Charlie D. 2009. Cheatgrass: Fire and forage on the range. Reno, NV: University of Nevada Press. 348 p. [75666]
372. Zimmerman, Teresa J.; Jenks, Jonathan A.; Leslie, David M., Jr. 2006. Gastrointestinal morphology of female white-tailed deer and mule deer: effects of fire, reproduction, and feeding type. Journal of Mammalogy. 87(3): 598-605. [85309]