| FEIS Home Page |
 |
||
 Image 2: Western poison-ivy. Photo courtesy of Dave Powell, USDA Forest Service, Bugwood.org.
Image 2: Western poison-ivy. Photo courtesy of Dave Powell, USDA Forest Service, Bugwood.org. |
||
| Image 1: Eastern poison-ivy. Photo courtesy of David J. Moorhead, University of Georgia, Bugwood.org. |
for Toxicodendron radicans:
eastern poison-ivy
common poison-ivy
for Toxicodendron rydbergii:
western poison-ivy
Rydberg's poison-ivy
Toxicodendron radicans (L.) Kuntze, eastern poison-ivy [40,87,88,92,124,137,168,266,281,283].
Toxicodendron rydbergii (Small ex. Rydb.) Greene, western poison-ivy [29,38,47,83,132,156,240,263].
Nine subspecies of eastern poison ivy are recognized globally, 6 of which occur in the United States:
Toxicodendron radicans subsp. radicans (L.) Kuntze [55,88,132,156,247,283]
Toxicodendron radicans subsp. divaricatum (Greene) Gillis [83,263]
Toxicodendron radicans subsp. eximium (Greene) Gillis [55,132,202,263]
Toxicodendron radicans subsp. negundo (Greene) Gillis [55,92,132,240,263,268,283]
Toxicodendron radicans subsp. pubens (Engelm. ex Watson) [55,263,283]
Toxicodendron radicans subsp. verrucosum (Scheele) Gillis [55,83,92,132,263]
Eastern and western poison-ivy are morphologically plastic [81] and intergrade with one another [83,87,171]. They occasionally hybridize where their ranges overlap [47,83,92,268]. Eastern poison-ivy may hybridize with Atlantic poison-oak (T. pubescens), and western poison-ivy may hybridize with Pacific poison-oak (T. diversilobum) [83].
Older literature often does not distinguish between eastern and western poison-ivy, and because the 2 species' geographic distributions overlap, it is sometimes impossible to determine to which species older literature is referring. In this review, species are referred to by their common names, when possible, and "poison-ivies" refers to both species.
SYNONYMS:for Toxicodendron radicans subsp. divaricatum (Greene) Gillis:
Toxicodendron radicans var. divaricatum (Greene) F. A. Barkley [83,133]
Toxicodendron divaricatum Greene [83,133]
Rhus radicans var. divaricata (Greene) Fern. [83,278]
for Toxicodendron radicans subsp. eximium (Greene) Gillis:
Toxicodendron radicans var. eximia (Greene) F. A. Barkley [83,202,266]
Toxicodendron eximium Greene [83,202]
Rhus eximium (Greene) Standl. [202]
Rhus eximia (Greene) Standl. [83]
Rhus toxicodendron var. eximia (Greene) McNair [83,132]
for Toxicodendron radicans subsp. negundo (Greene) Gillis:
Toxicodendron radicans var. negundo (Greene) Reveal [55,87,132,230,283]
Toxicodendron radicans var. vulgaris forma negundo (Greene) Fern. [266]
Toxicodendron negundo Greene [223,240,283]
Rhus radicans var. vulgaris (Michaux) de Candolle forma negundo (Greene) Fern. [79,204,240,243,244]
Rhus toxicodendron subsp. negundo (Greene) Gates [240]
for Toxicodendron radicans subsp. pubens (Engelm. ex Watson):
Toxicodendron radicans subsp. pubens (Engelm.) Gillis [92]
Toxicodendron radicans subsp. pubens (Engelm. ex Watson) Gillis [132]
Toxicodendron radicans var. pubens (Engelm. ex Watson) Reveal [132,283]
Rhus toxicodendron var. pubens Engelm. ex Watson [283]
for Toxicodendron radicans subsp. verrucosum (Scheele) Gillis:
Toxicodendron radicans var. verrucosa (Scheele) F. A. Barkley [266]
Toxicodendron radicans var. verrucosum (Scheele) F. A. Barkley [83,132]
Toxicodendron verrucosum (Scheele) Greene [83]
Rhus radicans var. verrucosa (Scheele) Fern. [83]
Rhus verricosa Scheele [83,132]
for Toxicodendron rydbergii (Small ex Rydb.) Greene:
Toxicodendron rydbergii (Small) Greene [87,92,274,275]
Toxicodendron radicans var. rydbergii (Small ex Rydb.) Erskine [47,83,132,263]
Toxicodendron radicans var. rydbergii (Small) Erskine [274,275]
Toxicodendron radicans var. rydbergii (Small) Rehd. [266]
Toxicodendron longipes Greene [47,83,274,275]
Toxicodendron desertorum Lunell [83,92,132,240,263]
Rhus rydbergii Small ex Rydb. [47,83,275]
Rhus rydbergii Small [223]
Rhus radicans var. rydbergii (Small) Rydb. [156,214,223,244,268,275]
Rhus radicans var. rydbergii (Small) Rehd. [79,133]
Rhus radicans var. rydbergii (Small ex Rydb.) Rehd. [47,83,132]
Rhus radicans var. vulgaris (Michaux) de Condolle [132,263]
Rhus toxicodendron var. rydbergii (Small) Garrett [223,274,275]
Rhus toxicodendron var. rydbergii (Small ex Rydb.) Rehd. [263]
Rhus toxicodendron var. rydbergii (Small ex Rydb.) Garrett [83]
Rhus toxicodendron var. vulgaris (Michaux) [132,263]
| A. | B. |
 |
 |
| Distribution of eastern poison-ivy (A) and western poison-ivy (B). Maps courtesy of USDA, NRCS. 2012. The PLANTS Database. National Plant Data Team, Greensboro, NC. (2012, 3 January). | |
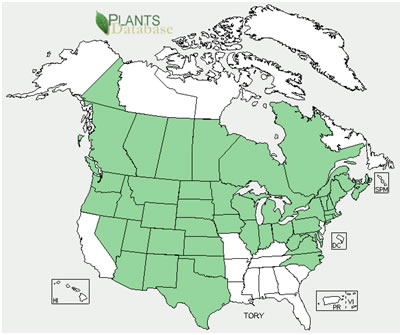
Eastern and western poison-ivy's geographic ranges overlap in the Midwest and Northeast. Together, they are native to every state except California, Alaska, and Hawaii and to every province except Nunavut, the Northwest Territories, and Newfoundland and Labrador. Eastern poison-ivy also occurs on Bermuda and the western Bahamas and in Mexico, Central America, Japan, China, Taiwan, and Russia [47,82,87,88]. It has been introduced in Africa, Europe, New Zealand, and Australia [83,166,283]. In the West, western poison-ivy occurs as far west as the eastern side of the Cascade Range in Washington, Oregon, and southern British Columbia. In the East, disjunct western poison-ivy populations occur at high elevations in the southern Appalachian Mountains in Pennsylvania, West Virginia, and Virginia [47,81,87,223,266].
Eastern poison-ivy:
States and provinces (as of 2012 [263]):
United States: AL, AR, AZ, CT, DC, DE, FL, GA, IA, IL, IN, KS, KY, LA, MA, MD, ME, MI, MN, MO, MS, NC, NE, NH, NJ, NY, OH, OK, PA, RI, SC, SD, TN, TX, VA, VT, WI, WV
Canada: NB, NS, ON, QC
Mexico [83,88,133,202,266,278]
Western poison-ivy:
States and provinces (as of 2012 [263]):
United States: AZ, CO, CT, IA, ID, IL, IN, KS, MA, MD, ME, MI, MN, MT, NC, ND, NE, NH, NM, NV, NY, OH, OK, OR, PA, RI, SD, TX, UT, VA, VT, WA, WI, WV, WY
Canada: AB, BC, MB, NB, NS, ON, PE, QC, SK, YT
Elevation: Across their distribution in the United States and Canada, poison-ivies occur from sea level on the Atlantic coast, to 3,510 feet (1,070 m) in the Appalachian Mountains, to 3,770 feet (1,150 m) in Oregon, and to 8,500 feet (2,590 m) in New Mexico (e.g., [38,59,84,108,165,202,212,230,246,275]). In West Virginia [244] and New York [140], eastern poison-ivy was most abundant at low elevations. Disjunct western poison-ivy populations occur only at high elevations in the southern Appalachian Mountains [47,87,223,266].
Soils and moisture:
Soils:
Poison-ivies grow best in fertile, moist but well-drained soils, although they tolerate a wide range of fertility, moisture, and other conditions. They occur on soils ranging from xeric, shallow rocky soils with southern exposure, to mesic soils on northerly and sheltered exposures, to saturated soils in seeps [216]. Poison-ivies grow in talus and in crevices on steep cliffs as well as in deep soils (>3 feet (1 m)) [174].
Texture: Poison-ivies grows in soils of all textures, including clays, silts, loams, and sands (e.g., [10,33,44,75,80,85,98,130,234,240]). According to Mulligan and Junkins [171], western poison-ivy generally occurs on sandier soils than eastern poison-ivy. Poison-ivies also occur in areas dominated by boulders, stones, cobbles, and gravels [70,130,148,198,223,234,240,268], including talus slopes, cliffs, and rocky ridges [174,234].
pH and parent materials: Poison-ivies occur in extremely acidic to moderately alkaline soils. Eastern poison-ivy occurred in soils with pH ranging from 3.6 to 6.5 [13,80,83]. It increased as soil pH increased in pondcypress (Taxodium distichum var. imbricarium) domes in north-central Florida [169]. Western poison-ivy generally occurs in more alkaline soils than eastern poison-ivy [84,171]. It occurred in soils with pH ranging from 5.7 to 8.4 [75,85,130]. Poison-ivies occur in soils derived from most parent materials (e.g., [75,83,91,130,140,160,174,214,216]).
Nutrients: Poison-ivies occur in "rich" soils [84,133,240] as well as in nutrient-poor soils (e.g., [12,33,98]). They were dominant in the understory of a white oak-northern pin oak/leadplant (Quercus ellipsoidalis/Amorpha canescens) community in Wisconsin that had dry, fine sands with poor to medium nutrient content [138].
Poison-ivies appear to prefer soils with high calcium content. Eastern poison-ivy appears tolerant of high phosphorus levels. In the laboratory, seeds of poison-ivies in a calcium-poor solution germinated but seedlings died soon after [84]. Eastern poison-ivy increased with increased soil calcium in pondcypress heads in north-central Florida [169]. In Florida, eastern poison-ivy was an important component of 100-year-old baldcypress (Taxodium distichum var. distichum) stands with high phosphorus levels [179].
Moisture: Poison-ivies occur on very dry to very wet sites and on poorly drained to well-drained soils (e.g., [33,80,110,112,157,174,216,248]), but generally they prefer well-drained, mesic soils (e.g., [2,47,70,123,135,158,214,234,250,260]). For example, in mixed-mesophytic forest in Indiana, eastern poison-ivy occurred on some xeric sites but had highest cover on mesic sites [123]. In mixed-oak (Quercus spp.) forest in southern Ohio, poison-ivies occurred with lower frequency on xeric sites (10.3%) than on intermediate (16.9%) or mesic (15.1%) sites [127].
Throughout their ranges, poison-ivies commonly occur in wetlands [23,170,208]. They often occur in seasonally or intermittently flooded areas (e.g., [58,89,118,216]) as well as areas such as marshes and swamps that are flooded for long periods (e.g., [198,216]). On these sites, poison-ivies often occur on elevated microsites such as on hummocks or tree bases [73,174]. Eastern poison-ivy on north-central Florida pondcypress heads decreased in importance with increased flooding depth; most eastern poison-ivy plants were found on large, elevated peat mats around the bases of pondcypress trees [169]. In Piatt County, Illinois, poison-ivies were the most frequently occurring woody species on flood-prone sites in silver maple (Acer saccharinum) streamside forests; these sites were inundated more than 20% of the time during the historical record. However, poison-ivies also occurred in upland white oak (Quercus alba) forests that were never flooded [22]. On wet meadows and other riparian prairie communities along the Middle Loup and Loup rivers in central Nebraska, poison-ivies increased with increased groundwater depth [172].
Poison-ivies appear tolerant of flooding [73]. Observations at the Montezuma National Wildlife Refuge, New York, suggested that eastern poison-ivy density declined 1 year after controlled flooding of 2 red maple-green ash (Acer rubrum-Fraxinus pennsylvanica) wetland reservoirs. Two years after flooding, eastern poison-ivy density had increased but remained below that on control sites. Eighteen years after flooding, density on flooded sites exceeded that on the controls [53]. A bottomland hardwood forest along the Mississippi River in Louisiana was sampled before and after a 105-day flood. Submerged eastern poison-ivy plants were top-killed by the flooding, while those with leaves above water remained green. The lower portion of the stem sprouted "vigorously" after the flood water receded. Forty-three days after the flood, eastern poison-ivy cover was greater than that before the flood, although the difference was not statistically significant [183].
Poison-ivies appear intolerant of drought. Western poison-ivy in the prairies of eastern Nebraska, western Iowa, and Kansas was injured or killed by the severe drought of the 1930s [271]. In Tennessee, the number of eastern poison-ivy growing points was reduced by half during a severe drought in the 1980s [68]. In New Jersey, eastern poison-ivy cover varied in response to drought among 6 old fields. In one field, it was reduced by 58%, but in another field it increased 3%. Overall, eastern poison-ivy cover in the old fields declined during the drought. It returned to predrought levels in 2 years. The authors considered eastern poison-ivy a "drought-susceptible" species [284].
Salinity: Poison-ivies are tolerant of mildly saline water [60,62,73,136] and soils [130] and can tolerate light to moderate salt spray [94,173,216].
Climate: Poison-ivies' wide distributions suggest they are adapted to a wide range of climates [171]. They grow in semiarid [115,205,261], humid [213,269,272], subtropical, and tropical [269] regions of the United States. In coastal Maine, poison-ivies occurred in a perhumid climate with localized fog [165]. Poison-ivies occur in regions of the United States and Canada with average annual temperatures ranging from 39 °F (4 °C) (southern Quebec) [265] to 72 °F (22 °C) (central Florida) [1] and average annual rainfall ranging from 15.7 inches (400 mm) (southeastern Arizona) [211] to 61.9 inches (1572 mm) (southern Florida) [62]. The average number of frost-free days ranges from 0 days (southern Florida) [62] to 111 days (South Dakota) [205]. Poison-ivies appear intolerant of extreme cold. In Canada, sections of horizontal rootstocks and vertical stems of poison-ivies were often winter-killed [171].
Lianas, such as eastern poison-ivy, are hypothesized to benefit from warmer temperatures and elevated carbon dioxide levels predicted by global climate change [152,221]. Under experimentally elevated levels of atmospheric carbon dioxide, eastern poison-ivy increased photosynthesis, water-use efficiency, growth, and population biomass during 5 growing seasons. Plants exposed to elevated carbon dioxide also produced more urushiol. These results suggested that under elevated levels of carbon dioxide, eastern poison-ivy may grow larger and become more noxious [166]. However, in mixed-hardwood forests in Wisconsin, it decreased in abundance during 45 years despite increased atmospheric carbon dioxide levels and increased mean winter temperatures of 4.3 °F (2.4 °C). This suggested that eastern poison-ivy was limited by factors other than carbon dioxide levels and low winter temperatures, such as light availability [152]. See Schnitzer and others [221] and Mohan and others [167] for more information on possible effects of climate change on eastern poison-ivy.
Plant communities: Poison-ivies occur in a variety of plant communities from barrier-island sand dunes to subalpine sites [49,83,285]. They occur primarily in wetlands, floodplains, bottomlands, and riparian communities throughout their ranges, but they also occur frequently in upland hardwood, mixed hardwood-conifer, and conifer forests and woodlands (e.g., [49,71,83,174,233,250]). In forests, they often occur in canopy gaps and on edges (see Shade tolerance). They also occur in prairies and other grasslands (e.g., [3,21,111,113,130,134,172,204,240]) as well as on rocky fields, talus slopes, and cliffs [83,171]. See the Fire Regime Table for a list of plant communities in which poison-ivies may occur and information on the fire regimes associated with those communities.
In the West, western poison-ivy most often occurs, but is seldom predominant, in aspen and cottonwood (Populus spp.), ash, maple, and birch (Betula spp.) gallery forests and floodplain communities (e.g., [27,86,102,105,178,198]). Among shrublands, it is most common in western snowberry (Symphoricarpos occidentalis) [44,104,259], red-osier dogwood (Cornus sericea) [102,198], chokecherry (Prunus virginiana) [103,105,105,198], silver buffaloberry (Shepherdia argentea) [103,105], and willow (Salix spp.) [102,103,105] communities. Along the Little Missouri River in North Dakota, western poison-ivy was abundant in green ash/western snowberry communities [104], and it was common in eastern cottonwood/Rocky Mountain juniper (Populus deltoides/Juniperus scopulorum) communities [85]. In southern Idaho, western poison-ivy occurred with the greatest mean cover in quaking aspen (P. tremuloides)/red-osier dogwood communities, with less cover in red-osier dogwood, Utah juniper (J. osteosperma)/red-osier dogwood, black cottonwood (P. balsamifera subsp. trichocarpa), and willow communities [102]. In Montana, western poison-ivy had the greatest cover in eastern cottonwood/western snowberry, boxelder/chokecherry, and Rocky Mountain juniper/red-osier dogwood communities. It also occurred in a silver buffaloberry community where animal trampling had created gaps in the canopy [103,105]. In Alberta, western poison-ivy was the 4th most abundant shrub in eastern cottonwood/western snowberry communities on alluvial river bars, and it occurred in silver buffaloberry communities on alluvial floodplain terraces [259].
Poison-ivies are common and occasionally important or predominant in upland forests, particularly those with oak, hickory, maple, eastern redcedar (Juniperus virginiana), and pines (e.g., Virginia pine (Pinus virginiana), loblolly pine (P. taeda), shortleaf pine (P. echinata), and eastern white pine (P. strobus)) (e.g., [54,174,177,190,228,254,270]). They were among the most abundant species in Virginia pine forests in Virginia [190,254] and eastern redcedar woodlands in Pennsylvania, New Jersey, and Virginia [190,195]. Poison-ivies are especially common in upland oak forests and woodlands [138,174,190]. Bur oak (Q. macrocarpa), northern pin oak, black oak (Q. velutina), white oak, and post oak (Q. stellata) are typical overstory dominants [54,110,117,138,149,174,194]. In Indiana, eastern poison-ivy was a dominant species in a black oak/Carolina rose (Rosa carolina)-eastern poison-ivy savanna on an open valley site and in a black oak/eastern poison-ivy/woodland sunflower (Helianthus divaricatus) savanna on a south-facing slope [149]. In central Minnesota, western poison-ivy was dominant in a northern pin oak-bur oak/bluegrass (Poa spp.) community and a northern pin oak-bur oak/big bluestem savanna [54]. In Wisconsin, poison-ivies were understory dominants in a white oak-northern pin oak/leadplant community and were of secondary importance in a northern pin oak/wintergreen-New Jersey tea (Gaultheria procumbens-Ceanothus americanus) community and an eastern white pine/hog peanut (Amphicarpa bracteata) community [138]. Western poison-ivy dominated a bur oak-western poison-ivy grove in Manitoba [194].
In the West, western poison-ivy occasionally occurs in uplands, but it is rarely important or predominant (e.g., [70,85,103,117,148]). On upper slopes in North Dakota, it was abundant in quaking aspen/water birch communities [104], and it was less abundant in quaking aspen/chokecherry and quaking aspen/paper birch (Betula papyrifera) communities on upper slopes [85]. Western poison-ivy is a minor species in upland ponderosa pine (Pinus ponderosa) forests in Nevada [198], Montana [103,105], and Wyoming [117] and in Rocky Mountain juniper and Utah juniper woodlands in Idaho [102], Montana [103,105], and North Dakota [85]. In Fort Bayard, New Mexico, western poison-ivy was a dominant understory shrub in a Gambel oak (Q. gambelii)/western poison-ivy/bottlebrush squirreltail-mutton grass-pinyon ricegrass (Elymus elymoides subsp. hordeoides-Poa fendleriana-Piptochaetium fimbriatum) community type [162].
Shoreland communities: Along the Gulf and Atlantic coasts, poison-ivies are common in maritime hammocks and on sand dunes along shores and barrier islands [16,16,49,60,62,71,136,285]. When western poison-ivy occurs on sand dunes, it is usually missing from adjacent dune forests [83], whereas eastern poison-ivy is frequently reported in these forests [94,151]. Eastern poison-ivy was one of the most abundant species in the American holly (Ilex opaca) forest alliance on lee sides of sand dunes along the Atlantic coast from New Jersey to Massachusetts [136]. On sand dunes from Delaware north to central Maine, it was one of the most abundant species in the northern bayberry-beach plum (Prunus maritima) shrubland alliance on protected sand dunes. In coastal areas from southern New Hampshire to New Jersey, it was one of the most abundant species in the chokecherry-Canadian serviceberry-oak (Prunus serotina-Amelanchier canadensis-Quercus spp.) shrubland alliance on sheltered back dunes, bluffs, and interior coastal areas. It was a dominant species in the cat greenbrier (Smilax glauca)-eastern poison-ivy vine-shrubland alliance on sand dunes in New England south to Maryland [136,257].
Wetlands: Eastern poison-ivy is common and often important or predominant in a variety of wetlands including forested swamps, scrub-shrub wetlands, and tidal and nontidal marshes [71,137]. It was the 3rd most common understory plant in pondcypress heads in north-central Florida [169], and it was a common understory species in baldcypress swamps in Florida [62,169], Georgia [219], North Carolina [175,216], and Illinois [58]. On barrier islands along the North Carolina coast, eastern poison-ivy dominated red maple-swamp tupelo (Nyssa sylvatica var. biflora)/eastern poison-ivy maritime swamp forest [216]. In New York, it was a characteristic species in silver maple-ash swamps and was of secondary importance in freshwater tidal swamps dominated by ash, red maple, slippery elm (U. rubra), and American hornbeam (Carpinus caroliniana) [210]. Poison-ivies were among the most abundant species in a silky dogwood (Cornus amomum) palustrine shrubland in the Delaware Water Gap [195]. Eastern poison-ivy dominated a wax-myrtle (Myrica cerifera)-eastern poison-ivy/sand cordgrass (Spartina bakeri) tidal shrub swamp in Virginia [191], and it was common in the common reed tidal salt marsh herbaceous alliance along the Atlantic coast [136]. It was an important species in a common cattail (Typha latifolia) community in North Carolina [7]. Western poison-ivy was codominant with common reed (Phragmites australis) in riparian communities in Idaho [128].
Grasslands: Poison-ivies are common, but rarely important or predominant, in many grasslands [3,21,107,111,114,130,201]. They occurred in indiangrass (Sorghastrum nutans) tallgrass prairie in Oklahoma [3], in big bluestem-indiangrass-little bluestem (Schizachyrium scoparium var. scoparium) tallgrass prairie in northeastern Nebraska [111], and in big bluestem-prairie dropseed (Andropogon gerardii-Sporobolus heterolepis) prairie in southwestern Minnesota [21]. In North Dakota, western poison-ivy occurred in a little bluestem-creeping juniper (Juniperus horizontalis) community on rocky soils, a shrubby cinquefoil (Dasiphora fruticosa spp. floribunda)-little bluestem community on steep, upland slopes, and a big bluestem community in moist depressions [114]. It was a common species on prairie sandreed-needle-and-thread grass (Calamovilfa longifolia-Hesperostipa comata) sand dunes of the Niobrara Valley Preserve, Nebraska [107]. In southeastern Montana, western poison-ivy occurred on uplands in xeric western wheatgrass/blue grama (Pascopyrum smithii/Bouteloua gracilis) grasslands [130].
Rocky outcrops, talus slopes, and cliffs: Poison-ivies are common and often important or predominant on rocky outcrops, talus slopes, and cliffs (e.g., [116,174,176,195,247,258]). They were dominant in the poison-ivy-granite gooseberry-pawpaw (Ribes curvatum-Asimina triloba) shrub-vineland association on scree fields and talus slopes in the Ouachita Mountains, Oklahoma [116]. Eastern poison-ivy dominated the sparsely covered eastern poison-ivy/Cossatot Mountain leafcup (Polymnia cossatotensis) vegetation type on steep, unstable talus slopes on Tom, Blaylock, and Peter mountains, Arkansas [174]. On dry cliffs of Pisgah National Forest, North Carolina, eastern poison-ivy dominated the eastern poison-ivy/American alumroot (Heuchera americana) herbaceous vegetation association [176]. In Acadia National Park, Maine, western poison-ivy was a characteristic species of the rock polypody-Appalachian polypody (Polypodium virginianum-P. appalachianum) vegetation community on open talus slopes [154].Form and architecture: Eastern poison-ivy is a rhizomatous plant that may be an erect, low shrub or a high-climbing or trailing liana that climbs via aerial roots [40,87,92,124,137,204,234,244,283]. Western poison-ivy is a rhizomatous, erect, low shrub that has no aerial roots, so it does not climb [47,87,92,109,133,156,214,234,266,268]. Eastern poison-ivy plants are much branched and woody throughout (see Image 1), whereas western poison-ivy plants are sparsely branched and woody only about 2 to 48 inches (5-122 cm) from the base (see Image 2) [47,87,92,156,214,223,266,275,275]. The stems of poison-ivies are thin-barked [32]. When a shrub, eastern poison-ivy may be 2 to 10 feet (0.5-3.0 m) tall [60,283]. When climbing, the main stem may be >160 feet (50 m) long [240,283] and up to 6 inches (15 cm) in diameter [60,88,234,240,266,283]. Western poison-ivy is typically <3 feet (1 m) tall but may be up to 10 feet (3 m) tall [47,83,87,92,109,214,234,245]. In Utah, western poison-ivy plants are rarely >1 foot (0.3 m) tall [275]. Aboveground stems of both species arise from much-branched rhizomes [83].
Leaves: Poison-ivies have alternate, deciduous, compound leaves. Each leaf has 3 leaflets [47,87,202,204,214,234,244,275]. Leaflets of both species vary greatly in shape and size [88,266]. Eastern poison-ivy leaflets are 2 to 8 inches (5-20 cm) long and 1 to 6 inches (2-16 cm) wide [87,88,204,240,244,283]. Western poison-ivy leaflets are 1 to 6 inches (2-15 cm) long and 1 to 4 inches (2-10 cm) wide [47,234,266,275].
Reproductive structures: Poison-ivy flowers have 5 petals and occur in loose clusters from the axils of the leaves [234,266]. The inflorescence of eastern poison-ivy usually has more than 25 flowers, whereas the inflorescence of western poison-ivy usually has fewer than 25 flowers [47,83,87]. Poison-ivy fruit is a small, dry, round drupe [87,92,202,266,275]. Drupes occur in dense, erect or ascending "grape-like" clusters [109,240]. Each drupe is 3 to 7 mm in diameter [47,87,88,92,204,234,240,245,266,275] and has a single, hard, 3- to 4-mm diameter seed [240,245].
Rhizomes and roots: Poison-ivies have creeping, underground rhizomes [47,87,92,109,156,245,283]. Rhizomes may be long. They may occur at the surface or be deep in the soil [144], although they tend to be shallow, extending only about 4 to 6 inches (10-15 cm) deep [108]. Poison-ivies have fibrous roots that grow from the rhizomes; the roots may be up to 12 feet (3.7 m) deep [188,261].
Eastern poison-ivy has abundant adventitious, aerial roots that adhere to supports [87,124,204,244,266]. Aerial roots are produced when a stem contacts a substrate [124,283].
Stand and age class structure: As a result of an extensive network of rhizomes, poison-ivies frequently form dense thickets [83,92,92,124,223,234]. These thickets may represent a single clone or several individuals [47,83,87]. Eastern poison-ivy density ranged from <0.1 stem/ha in white oak-red maple stands in northeastern Pennsylvania [57] to more than 290,000 stems/ha in logged loblolly pine stands in Texas [171]. In sandhills of northeastern Colorado, western poison-ivy occurred in patches from many square feet up to 1 acre (0.4 ha) in sandhill muhly (Muhlenbergia pungens) communities, especially on eastern slopes [206]. Poison-ivies may inhibit the growth of other vegetation [68]. See Impacts for more information.
 |
| Dense eastern poison-ivy in a clearcut on the Bessey Ranger District, Nebraska. Photo courtesy of the USDA Forest Service-Region 2-Rocky Mountain Region Archive, Bugwood.org. |
The longevity of poison-ivy plants was unknown as of this writing (2012). Gillis [84] reported that a 4.7-inch (12 cm) diameter eastern poison-ivy stem had 38 growth rings.
Raunkiaer [207] life form:The timing of flowering and fruiting is related to latitude, with flowering dates progressively later toward the north [84]. In Canada, flower buds of poison-ivies, which form on new growth in late summer and early fall of the previous year, open in late May or early June [171]. According to Lakela [144], flowering occurs when leaves are about half open [144]. Mulligan and Junkins [171] stated that flowering does not occur until leaves are fully expanded. Generally, eastern poison-ivy flowers from March to July [40,168,244,281] and fruits from July to January [124,156,204,229,234,283]. Western poison-ivy flowers from April to June [19,47,55,92,133,245] and fruits from July to November (Table 1) [156,234]. On the Great Plains, western poison-ivy plants sometimes bloom twice in one season; first in May or June and again in August or September [92]. Eastern poison-ivy plants in southeastern Arizona may have 2 periods of maximum flowering, 1 in April and May and 1 in mid-August to early September [84]. In Canada, peak flowering of poison-ivies occurs in June, but some additional flowering occurs sporadically until early fall [171]. The length of time required for fruits to ripen varies by site. In southern Florida, eastern poison-ivy fruits ripen in 3 months; in northern Florida fruits ripen in 4 months; and in southern New England fruits ripen in 2 months [84]. Poison-ivy fruits often persist over winter and into early spring [92,140,171,234,240].
| Table 1. General flowering dates for poison-ivies throughout their ranges in North America | |||
| Species | Location | Flowering period | |
| Eastern poison-ivy | Arkansas | throughout | May-July [124] |
| Florida | Florida panhandle | March-April [40] | |
| northern Florida | April-May [229] | ||
| Illinois | throughout | May-July [168] | |
| Mississippi | throughout | April-October [63] | |
| Missouri | throughout | May-July [283] | |
| North and South Carolina | throughout | late April-May [204] | |
| Texas | central | mid-April to May [55] | |
| West Virginia | throughout | May-July [244] | |
| East | Gulf and Atlantic coasts from Texas north to southern Nova Scotia | March-June [60] | |
| Great Plains | north-central | late May [240] | |
| Northeast | northeastern and north-central United States and adjacent Canada | May-July [87] | |
| Southeast | Blue Ridge Mountains | May-June [281] | |
| Southwest | southeastern Arizona and the Sierra Madre Occidental, Mexico | March-June [83] | |
| Ontario | throughout | June-early July [234] | |
| Western poison-ivy | Arizona | throughout | April-September [133] |
| Colorado | throughout | May-June [55] | |
| Kansas | throughout | May-June [19] | |
| North Dakota | throughout | May-July [55,241] | |
| Utah | throughout | June-July [55] | |
| Wyoming | throughout | June-September [55] | |
| Great Plains | throughout | May-June [92,245], sometimes again August-September [92] | |
| Intermountain region | throughout | May-June [47] | |
| Ontario | throughout | June-early July [234] | |
Pollination and breeding system: Poison-ivies are dioecious [88,124,171]. Their flowers are not specialized for any particular pollinator type [224]. They are visited and pollinated by ants, bees, beetles, butterflies, flies, true bugs, and wasps [84,171,224]. Ants, bees, and wasps appear to be the most important pollinators [224].
Seed production: Because male and female flowers are on separate plants, not all poison-ivy plants bear fruit [215]. In sand dunes along Lake Erie, 47% of plants bore seeds, with an average of 131 seeds/shoot [285].
Poison-ivy fruit and seed production is often high. An eastern poison-ivy plant with a 3.5-inch (9 cm) diameter main stem had a 4.3-foot (1.3 m) long branch with 6,130 flowers [84]. In September in Maryland, before birds began harvesting seeds, poison-ivies had an average of 315 fruits/plant [52]. In sand dunes along Lake Erie, they had 2,165 fruits/m² in April [285]. In Manitoba, western poison-ivy plants averaged 26 fruits/plant in January and early February; 49 days later there was only an average of 1 fruit/plant because most were consumed by birds and squirrels [194].
Some researchers reported low seed production in poison-ivies. In hardwood forest on the Pisgah National Forest, North Carolina, poison-ivies never fruited in 5 years [93]. Although poison-ivies were abundant in both logged and unlogged shortleaf pine-mixed hardwood stands in west-central Arkansas and east-central Oklahoma, they produced few fruits [196].
Poison-ivies first produce seed at 3 years old [81].
Seed dispersal: Seeds of poison-ivies are dispersed by birds and mammals and sometimes by water [161,220]. Fruits are eaten and dispersed by numerous birds and mammals during fall, winter, and early spring, and the hard seeds pass through their digestive tracts in viable condition (e.g., [55,171,193,234]). For more information on this topic, see Importance to Wildlife and Livestock. Unconsumed fruits may be retained on the plant through winter and deposited beneath the parent plant in spring.
Seed banking: Germination tests from soils suggest that poison-ivies may form a persistent seed bank [13,24,25,46,67,157]. Because seeds retained on the plant over winter are dormant, poison-ivies also have a temporary aboveground seed reserve [285].
Many researchers have reported poison-ivies in seed banks. In a maple bottomland swamp in western New York, where mean eastern poison-ivy cover was 3%, seedling emergence tests in a greenhouse indicated a mean density of 0.1 seed/120 cm² in the upper 2 inches (5 cm) of soil in April [25]. Using soil samples collected from eastern white pine, red pine (Pinus rubra), and Virginia pine plantations in southern Ohio, seedling emergence tests in a greenhouse indicated a mean density of 117 poison-ivy seeds/m² in the upper 4 inches (10 cm) of mineral soil [13]. In an old field (11 years since cultivation) in Prince George's County, Maryland, seedling emergence tests in a greenhouse indicated a mean volume of 11 poison-ivy seeds/1,000 cm³ in the upper 3 inches (8 cm) of soil in late November and early December [24]. In an upland oak-pine forest in New Jersey, seedling emergence tests in a greenhouse indicated a mean density of 10 poison-ivy seeds/m² in the upper 4 inches (10 cm) of soil collected in June and July [157]. In eastern Tennessee, 1,626 eastern poison-ivy seedlings/ha germinated from seeds in topsoil the first growing season after the topsoil was removed from mixed-hardwood stands and spread over surface mine soils [67].
In contrast, other researchers reported that seeds of poison-ivies either failed to germinate from or were sparse in soil samples despite abundance in the standing vegetation (e.g., [41,56,211]). Leicht-Young and others [149], observing that eastern poison-ivy was dominant in the aboveground vegetation in a black oak savanna but did not germinate from soil samples in a greenhouse, concluded that eastern poison-ivy does not commonly form a seed bank. Eastern poison-ivy was the most common understory plant in a midsuccessional sweetgum-loblolly pine-red maple forest in Virginia, with an average of 41% cover. However, seedling emergence tests in a greenhouse indicated a mean density of only 1 seedling/m² in the upper 3 inches (8 cm) of soil in November [177]. Although western poison-ivy relative abundance was 16% in the Garden Canyon floodplain in the Huachuca Mountains of Arizona, no western poison-ivy seedlings germinated in the laboratory from samples taken from the upper 2 inches (5 cm) of soil in March [211]. In a loblolly pine plantation in North Carolina, eastern poison-ivy was present in the aboveground vegetation, but no seedlings emerged during greenhouse germination tests from samples taken from the upper 4 inches (10 cm) of soil in August and May [41].
Germination: Poison-ivy seeds have a dormant embryo and a hard endocarp that inhibits germination. In a series of germination tests of eastern poison-ivy seeds, only seeds from Florida and Bermuda germinated without cold stratification to break dormancy [84].
Seed viability and germination rates may be high. In sand dunes along Lake Erie, all fruits collected from poison-ivies had viable seeds [285]. Mean germination reported in the literature ranged from 0% to 96% [84,139,218]. Seeds of poison-ivies remain viable for at least 6 years in the laboratory [84].
Typically, seeds of poison-ivies must be scarified and/or cold-stratified for long periods (3-4 months) for germination to occur [218]. In the laboratory, ≤1% of seeds of poison-ivies collected in December and February and planted in the laboratory germinated without scarification or stratification. Seeds scarified with sulfuric acid had better germination (63%) than unscarified seeds. Seeds collected in February and stratified had the best germination (90%-96%). The authors suggested that seeds picked in February had the highest germination rates because they experienced a longer period of cold stratification in the field prior to collection compared to seeds picked in December. Scarification of seeds prior to stratification did not improve germination (66%-72%) [218]. Germination of stratified eastern poison-ivy seeds from 14 locations throughout the species' range varied from 4% to 82%. Germination was 0% to 3% for western poison-ivy seeds from 5 locations. It was unclear why rates of western poison-ivy seed germination were so much lower than those of eastern poison-ivy [84].
In nature, poison-ivy seeds may be scarified by passing through an animal's digestive tract [171]. Western poison-ivy seeds that had passed through sharp-tailed grouse digestive tracts gave 86% germination after warm, then cold stratification [139]. However, no information on controls was provided in this study. In a bur oak-western poison-ivy grove in Manitoba, western poison-ivy seeds extracted from ruffed grouse feces and those taken directly from plants in February had similar germination rates [194].
Germination of poison-ivies in the wild may be high. In South Carolina, seedfall of eastern poison-ivy was positively associated with the number of germinants during 2 years (P<0.01), indicating that seedfall from the previous year strongly contributed to that germination [131].
Seedling establishment and plant growth: As of this writing (2012), little information was available regarding seedling establishment and growth of poison-ivies. The available information suggests that, while poison-ivies are able to persist for decades in shaded areas, best survival and growth are obtained in moderate to high light (see Successional Status). Once established, survival of poison-ivies may be high and their growth rapid. In a maple-green ash-American elm bottomland swamp in western New York, first-year eastern poison-ivy seedlings averaged 2.3 inches (5.9 cm) tall in summer [25]. Mean diameter growth of eastern poison-ivy stems ranged from 0.03 to 0.09 inch (0.8-2.2 mm)/year during the 12 years after Hurricane Hugo. Plants grew more rapidly, though not significantly so, on trees that suffered severe branch loss. Eastern poison-ivy mortality rates ranged from 5% to 12% during posthurricane years 1 to 12 [5]. Eastern poison-ivy was one of the earliest woody species to invade old fields in southeastern Ontario; it appeared within 3 years of agricultural abandonment. It colonized the old fields by seeds and by movements of "clonal fronts" from surrounding communities [48].
Vegetative regeneration: Poison-ivies sprout from root crowns and rhizomes [83,144,171,262]. Plants may reproduce vegetatively in their 1st growing season. In Canada, poison-ivy plants in their 1st or 2nd growing seasons may produce rhizomes from the base of the primary vertical shoot. Rhizomes have buds that produce secondary vertical stems similar to the primary vertical stem; they usually also produce adventitious roots just below each bud. The secondary vertical stems produce further rhizomes, resulting in a large interconnected clone with many vertical stems and rhizomes above or beneath the ground [171]. A poison-ivy seedling that germinated on 25 April had its first rhizomes by August, and it had 2 well-developed rhizomes and 2 secondary vertical stems by September (Muenscher and Kingsbury 1964 cited in [171]). The horizontal spread of poison-ivies may be slow and is rarely more than 4 inches (10 cm)/year [171,215]. On some sites, rhizomes may extend up to 7 feet (2 m) beyond the parent plant [262]. According to Gillis [84], poison-ivies rarely establish from plant fragments.
SUCCESSIONAL STATUS:
Poison-ivies tolerate both sun and shade. They are generally rated as moderately
shade tolerant
[140,143]. They are common from the first stages of plant succession to late succession [97], being most common in early to midsuccession (see
Successional stage).
Poison-ivies commonly occur on disturbed sites such as floodplains, sand dunes, and talus slopes (e.g., [60,83,95,156,156,204,268,283]) and often increase after disturbances that open the canopy, such as fire, windstorms, forest pathogen outbreaks, and logging. Because of poison-ivies' affinity for forest edges and canopy gaps, researchers suggested poison-ivies are likely to increase with forest fragmentation [34,120].
Shade tolerance: Poison-ivies occur in sunny to shaded sites. They are most abundant in moderately shady sites (e.g., [8,29,47,83,87,109,238]). Gillis [83] stated that western poison-ivy is seldom found in closed-canopy forests because it is likely to be shaded out. Eastern poison-ivy may be better able persist in closed-canopy forests than western poison-ivy because of its ability to climb into the forest canopy and access light [143]. Ladwig and Meiners [143] suggested that eastern poison-ivy employs a "sit-and-wait" strategy in late succession by persisting at low cover in the forest canopy until treefall gaps and other canopy-opening disturbances allow it to spread. In Minnesota, frequency of poison-ivies in northern pin oak-bur oak woodlands was about 15% when the canopy was 8% closed. Their frequency peaked at 55% when the canopy was 22% closed. As canopy closure increased, frequency of poison-ivies declined steadily. When the canopy was 75% closed, their frequency was as low as their frequency in the most open sites (15%). They persisted at very low frequency (2%) even when the canopy was 100% closed [197]. In maple-beech (Fagus spp.) "climax" forest in northern Minnesota, western poison-ivy was "suppressed and scattered" in dense shade but more abundant in areas with more light [238]. In southeastern Ohio, eastern poison-ivy frequency was greater in 2nd-growth oak forest than in old-growth forest, apparently due to relatively greater light availability in 2nd-growth oak forest [184]. Poison-ivies showed an affinity for open microsites in a 73-year-old northern mixed-mesophytic hardwood forest in Ithaca, New York [78]. Eastern poison-ivy decreased as canopy closure increased during 15 years in an old-growth oak-hickory forest in southwestern Illinois. The authors concluded that eastern poison-ivy was "intolerant of heavy shade" [225]. In contrast, its importance value increased 2.3-fold in a mixed pine-hardwood baygall in west-central Louisiana during 15 years. Because its abundance increased as the canopy closed, eastern poison-ivy was described as shade tolerant [6].
Abundance of poison-ivies is often higher on forest edges than in forest interiors (e.g., [34,74,120,152]). Londre and Schnitzer [152] hypothesized that the high abundance of eastern poison-ivy at forest edges may be due to increased temperature, decreased relative humidity, increased wind turbulence, and trellis availability near forest edges. Eastern poison-ivy may also colonize forest edges rapidly because of seed dispersal by avian frugivores, which generally spend more time consuming fruits and depositing seeds along edges than within interiors of temperate forests [152]. Hardin [106] observed that encroachment of poison-ivies into a southeastern Ohio prairie occurred mostly under overhanging tree limbs. Poison-ivies were among the most abundant species on oak forest edges on the Shawnee National Forest, Illinois. They were abundant up to 164 feet (50 m) from the forest edge into the forest interior but declined at greater distances [120]. In the Roanoke River basin, North Carolina, eastern poison-ivy occurred within 66 feet (20 m) of the edge of mixed-hardwood forests. It penetrated deeper into the forest on south-facing edges than north-facing edges [74]. In a hardwood forest in Massachusetts, trees near the edge of the forest supported more eastern poison-ivy stems than those in the forest interior, presumably because of greater light availability [34]. In Wisconsin, eastern poison-ivy density was 2 times greater at the forest edge than in the forest interior (P<0.05). Abundance decreased sharply at 16 feet (5 m) from the edge. Edge sites had the highest light availability and the lowest canopy density (P≤0.05 for both variables) [152]. A 50-year study examined eastern poison-ivy population expansion into an abandoned field in the Hutcheson Memorial Forest, New Jersey. Population expansion in years 10 to 25 was greatest near the edge of the bordering old-growth forest and decreased with increasing distance into the field. By years 30 to 40, the population near the edge of the old-growth forest was declining [143].
Poison-ivies often establish in forest gaps (e.g., [9,90,239]). In Madison County, New York, they were described as "gap species" in northern whitecedar-balsam fir (Thuja occidentalis-Abies balsamea) forest because they had a higher importance value in medium (650-910 feet ² (60-85 m²)) and large (1,290-2,050 feet² (120-190 m²)) canopy gaps than in closed-canopy forest (P<0.05) [9]. Eastern poison-ivy was an important species in old-growth shortleaf pine forest in Missouri, where canopy gaps (≤6 years old) constituted 4% of the total area and gap size averaged 2,260 feet² (210 m²) [239]. In contrast, in New London, Connecticut, frequency of poison-ivies in an eastern hemlock (Tsuga canadensis)-mixed hardwood forest did not change substantially (10%-17%) over 45 years despite tree mortality and opening of the forest overstory due to hemlock woolly adelgid [90].
Logging creates openings in the forest canopy that often benefit poison-ivies. On the Nantahala National Forest, North Carolina, poison-ivies were present in a northern red oak/flame azalea (Rhododendron calendulaceum) forest after hurricane windthrow and posthurricane logging had created 0.25- to 0.50-acre (0.1-0.2 ha) openings in the canopy; however, they were absent from an adjacent unlogged forest [64]. In the Tennessee National Wildlife Refuge, thinned oak-hickory stands had greater mean eastern poison-ivy cover than unthinned stands 4 years after logging [256]. Mean eastern poison-ivy density was higher in southern pine beetle-infested and logged loblolly pine stands (290,080 stems/ha) than in undisturbed stands (16,950 stems/ha) in Turkey Hill Wilderness, Texas (P<0.05) [171]. In contrast, mean eastern poison-ivy density was similar between southern pine beetle-infested and logged loblolly pine stands (24,720 stems/ha) and undisturbed stands (123,775 stems/ha) in Indian Mounds Wilderness, Texas [43].
Poison-ivies often increase substantially after windstorms that open the forest canopy. On Long Pine Key, Florida, eastern poison-ivy and other lianas and vines grew rapidly in maritime hammocks within 1 month after Hurricane Andrew, apparently in response to the canopy opening [153]. Eastern poison-ivy was "prolific" the first growing season after Hurricane Hugo removed much of the overstory of an old-growth maritime forest on Bull Island, South Carolina [231]. In a mixed-hardwood forest in Ottawa, Ontario, eastern poison-ivy seedlings were "more abundant" during the 4 years following an ice storm than before the storm [51]. Near Franklin, North Carolina, frequency, density, and cover of poison-ivies increased from 1 to 3 years after salvage logging of a high-elevation hickory-black oak-yellow-poplar (Liriodendron tulipifera) stand damaged by Hurricane Opal. Poison-ivies were not present in undisturbed stands [64].
The effect of windstorms on abundance of poison-ivies is influenced by the amount of canopy removed, although the effect is unclear. A study in Minnesota found that poison-ivies increased most in the most severely hurricane-damaged areas [189], whereas a study in South Carolina reported that eastern poison-ivy increased most in less severely damaged areas [5]. In Cedar Creek National Historical Park, Minnesota, eastern poison-ivy frequency in an eastern white pine forest increased 19% fourteen years after a windstorm caused high tree mortality, but in a northern pin oak forest, eastern poison-ivy frequency increased 14% during postdisturbance years 1 to 7, then declined to predisturbance levels. The authors suggested that eastern poison-ivy increased more in the eastern white pine forest because the storm damaged that forest more than it damaged the northern pin oak forest [189]. In an old-growth bottomland hardwood forest in Congaree National Park, South Carolina, eastern poison-ivy response to damage from Hurricane Hugo varied with damage severity. In the most severely damaged areas, density decreased 55% in the 1st year after the hurricane and remained low through year 5. Eight years after the hurricane, however, eastern poison-ivy density had nearly doubled, and by posthurricane year 12, it exceeded that before the hurricane. In areas with less severe damage, eastern poison-ivy density decreased initially, but only slightly. By year 5, density exceeded that before the hurricane, and it continued to increase through the end of the study in year 12 [5].
Successional stage: Poison-ivies occur in every stage of succession. However, they often increase after disturbance, and they are typically most common in early to midsuccession. They often persist into later stages of succession, but in lesser abundance [83,86]. In late-successional forests, they often persist in canopy gaps or along forest edges (see Shade tolerance). Because of its ability to access light in the forest canopy, eastern poison-ivy appears more common in late succession than western poison-ivy.
Although sometimes common in early succession, poison-ivies typically reach peak abundance during midsuccession. Studies of old-field succession reported that poison-ivies were often absent immediately after agricultural abandonment but increased soon after. For example, eastern poison-ivy was one of the earliest woody species to invade old fields in southeastern Ontario; it was observed within 3 years of abandonment [48]. Cover of poison-ivies typically peaks in midsuccession, from about 20 years to 60 years after abandonment, then declines in late succession, when poison-ivies often persist in forest gaps or along forest edges (e.g., [18,48,69,100,121,143,200,203,264]).
| Table 2. Observations of poison-ivy abundance in old fields after agricultural abandonment | |||||
| Species | Location | Observations | |||
| eastern poison-ivy | southeastern Connecticut | present in trace amounts 9 years after abandonment | 28 years after abandonment, when the old field was a thicket of trees and shrubs 13-20 feet (4-6 m) tall, cover was 3% | cover remained similar (2%-4%) 38 and 47 years after abandonment [69] | |
| New Jersey | present 1 year after abandonment | 4% cover 15 years after abandonment, when old fields were open and "park-like" and eastern redcedars were 7-8 feet (2-2.5 m) tall | 8% cover 40 years after abandonment | 30% cover 60 years after abandonment, when old fields were eastern redcedar groves with a thicket understory [18] | |
| present within 7 years of abandonment | cover peaked 22 years after abandonment (15%), when young trees began to provide support | cover decreased to about 5% by year 50 [143] | |||
| southwestern Ohio | absent 2 and 10 years after abandonment | common 50 years after abandonment | present only in disturbed areas 90 and >200 years after abandonment [264] | ||
| central Tennessee | absent from herb-dominated old fields, 1-12 years after abandonment | present in shrub-herb thickets and young forests, 8-20 years after abandonment | peak cover in elm and hackberry (Celtis spp.) forests, the oldest fields studied, 25 years after abandonment [203] | ||
| poison-ivies | central New Jersey | absent from old fields in years 1-6 after abandonment | present in low abundance 7 years after abandonment | cover increased steadily until 20 years after abandonment when the study ended [200] | |
| southeastern Indiana | absent 1 and 2 years after abandonment | present 3 and 10 years after abandonment [121] | |||
Poison-ivies are common in infrequently to frequently disturbed floodplains and lakeshores [83]. On floodplain sites along the Little Missouri River in North Dakota, highest western poison-ivy cover (18%) occurred on the most recent alluvial deposits in eastern cottonwood communities. Its cover gradually declined as the overstory canopy closed. In a "climax" green ash/western snowberry community, its cover was only 4% [86]. In contrast, in South Dakota, eastern poison-ivy occurred in 35-year-old and 50-year-old eastern cottonwood stands in floodplains along the Missouri River but not in 10-, 14-, or 23-year-old stands [280]. Along the Yellowstone River in Wyoming, western poison-ivy was absent from seedling and sapling eastern cottonwood stands and had <1% cover in pole eastern cottonwood stands, but it had 12% cover in mature eastern cottonwood stands [27].
Poison-ivies often occur in postfire successional communities. For more information on this topic, see Plant response to fire.The effect of fire on poison-ivy seeds in the seed bank was unclear as of this writing (2012). Prescribed fire was applied to 2 oak stands near Russellville, Arkansas, in February, and seeds were germinated in a greenhouse from soils taken from the upper 2 inches (5.0 cm) of soil immediately prior to and after the fire. In soils from one stand, the density of eastern poison-ivy germinants was 76% less in burned than in unburned soils. In the other stand, the density of germinants was 300% more in burned than in unburned soils [222].
Postfire regeneration strategy [242]:
Prostrate woody plant, stem growing in organic soil
Surface rhizome and a
chamaephytic root crown
in organic soil or on soil surface
Small shrub, adventitious buds and a sprouting root crown
Rhizomatous low woody plant, rhizome in organic soil
Rhizomatous shrub, rhizome in soil
Ground residual colonizer (on site, initial community)
Crown residual colonizer (on site, initial community) (for eastern poison-ivy)
Initial off-site colonizer (off site, initial community)
Secondary colonizer (on- or off-site seed sources)
Fire adaptations and plant response to fire:
Fire adaptations: Poison-ivies are moderately shade tolerant, but they also grow in full sunlight. They frequently establish after disturbances that open the canopy, such as fire, flood scour, and windthrow. They are common from early to late succession (see Successional Status) [97]. After fire, poison-ivies reproduce vegetatively via surviving perennating buds located along much-branched rhizomes (see Vegetative regeneration) [26,68]. Climbing eastern poison-ivy plants on tree hosts may retain their seeds in tree canopies until the following spring, allowing postfire seedling establishment from crown-stored seeds (see Seed dispersal). Seeds of poison-ivies also have the potential for long distance dispersal via animals and water and, because seeds are dormant (see Germination) and potentially long-lived, they may persist in the seed bank for many years. Seeds of poison-ivies require scarification for successful germination, a condition that may result from fire.Plant response to fire: Poison-ivies sprout from the root crown and/or from rhizomes after top-kill by fire [68]. Poison-ivies may also establish from the soil seed bank. A study in Arkansas found that at least some eastern poison-ivy seeds in the soil survived a prescribed fire [222], indicating the potential for on-site postfire establishment. Poison-ivies may also establish from off-site animal- or water-dispersed seed after fire. Eastern poison-ivy may establish from seed dispersed from lianas in tree crowns.
Occurrence of poison-ivies after wild and prescribed fires has been reported in a variety of plant communities. Poison-ivies have variable responses to fire; patterns with regard to season, frequency, and severity of fire are not evident, so studies are listed below according to fire response.
Many studies reported that poison-ivies increased soon after fire or within the first growing season [10,11,39,68,110,126]:
| Table 3. Studies reporting increases in abundance of poison-ivies soon after fire | ||||
| Species | Location | Plant Community | Fire description | Observations |
| eastern poison-ivy | south-central Tennessee | oak-hickory forest | July prescribed fire | increased on burned plots and decreased on control plots 1 year after fire compared to prefire levels (Table 5) [68] |
| east-central Mississippi | loblolly pine-oak forest | early spring prescribed fire; forest had been burned 7 other times during the previous 12 years | increased after fire and was most abundant during postfire year 4, when the study ended [126] | |
| north-central Florida | longleaf pine-turkey oak (Pinus palustris-Quercus laevis) forest | January prescribed fire | "far greater" in abundance in burned areas than in unburned areas 3 months after fire [11] | |
| western poison-ivy | southeastern Ontario | xeric northern whitecedar-quaking aspen-balsam fir woodland | severe June wildfire | cover averaged 5.3% and frequency averaged 13.6% 100 days after fire [39] |
| poison-ivies | southwestern Illinois | post oak/little bluestem barrens | low-severity, March prescribed fire | absent before fire but present 7 months later [110] |
| northeastern Illinois | closed-canopy oak woodland | fall prescribed fire | greater importance the 1st growing season after fire than before fire [10] | |
Some studies reported that poison-ivies decreased after fire, exhibited little or no change after fire, or that fire had mixed effects on poison-ivies [3,122,142,185,186,212,251,267]:
| Table 4. Studies reporting that poison-ivies decreased after fire, exhibited little or no change after fire, or that fire had mixed effects on poison-ivies | ||||
| Species | Location | Plant Community | Fire description | Observations |
| eastern poison-ivy | northeastern Illinois | white oak savanna | spring and fall prescribed fires applied 1 to 4 times in 7 years | 7 years after fires, density was >3 times lower than before fires; at a nearby unburned control site, density was >4 times lower [142] |
| central North Carolina | loblolly pine forest | mixed-severity wildfire | density was higher on burned than unburned plots 9 years after fire but mean frequency was similar between treatments [186]; 20 years after fire, density had declined by ≥50% [185] | |
| poison-ivies | northwestern Wisconsin | upland jack pine (Pinus banksiana)-northern pin oak savanna | March and April prescribed fires; sites were burned 1-4 times in approximately 13 years | frequency was similar on burned and unburned control sites sampled 2-17 months after fires [267] |
| Illinois | oak-hickory barrens | 2 mixed-severity prescribed fires; the 1st fire was applied in late November, the 2nd fire in mid-March 5 years later | cover of poison-ivies was similar before and 1 growing season after fires [251] | |
| east-central Illinois | 20-year-old herb-dominated old field with scattered trees and shrubs | 2 successive March prescribed fires; the 1st fire was high-severity, the 2nd fire was low-severity | frequency was similar before and 2 years after fires [122] | |
| Oklahoma | indiangrass tallgrass prairie | a summer prescribed fire and a "hotter" March prescribed fire | not detected 1 year after fires [3] | |
| east-central Texas | shortleaf pine-sweetgum-loblolly pine forest | low-severity, February and March prescribed fires | cover similar before and 1 year after fires [212] | |
Although poison-ivies often increase after fire, the effect may be short term. In a southern Appalachian Virginia pine-shortleaf pine-scarlet oak (Quercus coccinea)-white oak forest, eastern poison-ivy occurred with 5.0% frequency and 0.2% cover 1 growing season after a March prescribed fire. The 2nd growing season, both measures had declined [65]. Two studies in the Black Hills, South Dakota, found that western poison-ivy increased after fire in the short term. The 1st growing season after an April low-severity (<5% top-kill of trees) prescribed fire in bur oak woodlands, western poison-ivy density was higher in burned (3.1 stems/m²) than unburned (0.8 stem/m²) plots (P=0.05). The 2nd growing season after fire, however, its density was similar in burned and unburned plots, which were both similar to prefire levels [227]. In a ponderosa pine-grassland ecotone, western poison-ivy densities were unchanged during the first 2 years after low-severity, April and May prescribed fires [26].
Other researchers reported increased abundance of poison-ivies after fire in the long term. In a 53-year study of postfire forest succession in northern Michigan, poison-ivies had the greatest frequency 38 and 51 years after fire [217].
The effect of fire on poison-ivies depends in part on the plant communities involved. To reduce fuels after Hurricane Hugo, prescribed fire was applied to loblolly pine stands and nonnative Chinese tallow (Triadica sebifera) stands. Burning was conducted in winter and was of low severity, with most of the duff layer remaining unburned. Two years after the prescribed fire, eastern poison-ivy density in loblolly pine stands was similar in burned (22,500 stems/ha) and unburned (25,000 stems/ha) stands. In Chinese tallow stands, however, eastern poison-ivy density was much higher in burned (30,001 stems/ha) than unburned (4,169 stems/ha) stands [231].
Prescribed fire is often used in conjunction with logging. Several studies reported greater abundance of poison-ivies on logged and burned sites than unburned (logged or unlogged) sites [33,125,226], but other studies reported lesser abundance on logged and burned sites than other sites [160,199]. On the Bankhead National Forest, Alabama, liana and vine cover—mostly eastern poison-ivy—on logged stands burned under prescription in fall or spring was twice (range: 11-15%) that of a logged but unburned stand (6%). The plant community was an upland oak-mixed hardwood forest [125]. At the Fort Benning Military Base, Georgia, midstories of longleaf pine-loblolly pine stands were masticated and burned under prescription in either December, May, or July. Eastern poison-ivy abundance in postfire year 1 was greater on treated than on control plots [33]. In southeastern Ontario, frequency of poison-ivies decreased on logged eastern white pine-paper birch-red pine forests 1 month after July prescribed fire, but it also increased on uncut and unburned controls. However, the difference was not significant, and the author concluded that logging and prescribed fire had no effect on frequency or biomass of poison-ivies [226]. In southeastern Virginia and northeastern North Carolina, eastern poison-ivy occurred in neither in an Atlantic white-cedar (Chamaecyparis thyoides) peat swamp that was burned in a wildfire 2 years previously nor an area that was logged and then burned. It was abundant, however, in an untreated area [160]. Three years after treatment in Clemson Experimental Forest, South Carolina, eastern poison-ivy increased on thinned plots and plots burned in a moderately severe April prescribed fire, but it declined on plots that were thinned and burned in a low-severity fire 1 year later. The authors suggested that eastern poison-ivy declined on the thinned and burned plots because of the repeated disturbance [199].
Fire timing, frequency, and severity may affect the response of poison-ivies to fire.
Fire timing: Fire season may affect poison-ivies' response to fire. The number of eastern poison-ivy growing points (defined as any foliage- and root-bearing horizontal stem section >3 inches (8 cm) long) in oak-hickory forest increased more on fall-burned plots than winter-burned plots 1 year after prescribed fires in Chickamauga and Chattanooga National Military Park. The authors suggested that a decrease in the number of eastern poison-ivy growing points on control plots was probably due to summer drought, while the increase in eastern poison-ivy growing points on burned plots was the result of postfire sprouting from surviving stems and underground reproductive structures. Although growing points increased, total biomass was apparently reduced [68].
| Table 5. Mean number of eastern poison-ivy growing points/m² in a control and 2 treatments in Chickamauga and Chattanooga National Military Park, Tennessee [68] | |||
| Treatment | Count |
% change* | |
| Pretreatment | Posttreatment | ||
| Control | 10.6 | 5.3 | -49.8 a |
| Fall prescribed fire | 26.7 | 38 | +42.6 b |
| Winter prescribed fire | 12.0 | 12.5 | +4.6 c |
| *Values having different postscripts were significantly different at P=0.05. | |||
Fire frequency: Poison-ivies occur on sites where fire is frequent and on sites where fire is infrequent. For example, in logged loblolly pine-shortleaf pine stands in southeastern Arkansas, cover of eastern poison-ivy was similar in stands with a variety of burn histories: 1 year after a fourth 3-year burn cycle (2.3% cover); 4 years after a second 6-year burn cycle (3.4% cover); 1 year after a second 9-year burn cycle (1.3% cover); and in an untreated control stand (1.2% cover). Stands were burned under prescription in December, January, or February [37]. Eastern poison-ivy was common in shortleaf pine stands in Arkansas that had been burned 1 to 5 times in 15 years [236,237]. Poison-ivies occurred in white oak-post oak barrens in east-central Illinois, 1 and 2 years after fall prescribed fire, and at a site burned under prescription 3 times in 10 years [159]. In Missouri, eastern poison-ivy was present in oak-hickory flatwoods burned annually in spring for 8 years [192].
Poison-ivies are often abundant after repeated annual or biennial fires. In a northern pin oak community in east-central Minnesota, poison-ivies were more frequent (12.7%) on a site that was burned prior to leaf-out every spring for 13 years than on an adjacent unburned control site (5.0%) [277]. Low-severity May prescribed fires were applied to 2 eastern white pine-red pine stands in Michigan. Cover of poison-ivies was greater on a stand 2 years after a single fire (1.4%) and a stand 1 year after a second biennial fire (1.3%) than on unburned control plots (0.7%) [180]. For more information, see the Research Project Summary of this study.
Poison-ivies are often common in communities burned at >5-year intervals. At the Teft Savanna Nature Preserve in Indiana, density of poison-ivies in white oak barrens did not change during 20 years despite mixed-severity spring prescribed fire used at an average of every 5.7 years [99]. In a southern Appalachian oak-hickory forest, Holzmueller and others [119] compared understory diversity and composition among oak-hickory stands in the Great Smoky Mountains that were burned 1 to 3 times during 20 years. Poison-ivies were most indicative of stands that were burned 2 times in 20 years (P=0.04) [119]. In Wisconsin, frequency of poison-ivies in a northern pin oak savanna subjected to 2 prescribed fires in 15 years was higher than in adjacent unburned savanna; they apparently established in burned sites after fire opened the canopy [20]. In northern Florida, 2nd-growth loblolly pine-shortleaf pine woodlands were burned under prescription during 75 years at frequencies ranging from 1 to 13 times in 13 years. However, eastern poison-ivy was less abundant on these burned plots than on plots that had not been burned during the 75 years (P<0.02) [163]. At the Cedar Creek Ecosystem Science Reserve, western poison-ivy was a dominant plant in a pin oak-bur oak/big bluestem savanna burned 3 times during 37 years [54].
Other researchers reported that abundance of poison-ivies was reduced on sites burned frequently (every 1 to 4 years) [113] or that poison-ivies were less abundant on sites burned frequently than on sites burned at intervals of 5 years or more [111,141,163]. In DuPage County, Illinois, mean cover of poison-ivies was lower in a northern red oak-white oak-bur oak forest subjected to annual low- to moderate-severity prescribed fire during fall or spring for 17 years (0.05%) than in unburned controls (1.8%) (see the Research Paper by Bowles and others) [31]. Annual spring prescribed fire for 3 years in a big bluestem-prairie dropseed prairie in southwestern Minnesota reduced western poison-ivy cover in the short term. Prior to the fires, western poison-ivy cover was 1.6%. The summer immediately following the 1st prescribed fire, its cover was 0.1%. The following spring, its cover increased slightly to 0.4%. After 2 annual prescribed fires, its cover was less than half of prefire levels (0.7%) [21]. In Escambia Experimental Forest in Alabama, eastern poison-ivy was absent from longleaf pine stands burned biennially in winter, spring, or summer during 22 years, but it had 1.2% cover in unburned control stands [141]. Poison-ivies decreased 81% in a tallgrass prairie in southeastern Michigan that was burned at 1- to 3-year-intervals in April or November over 16 years [113]. In big bluestem-indiangrass-little bluestem prairie in northeastern Nebraska, poison-ivies were absent on sites either burned under prescription annually in April or burned every 4 years in April. However, they were frequent (33%) on sites burned once in 18 years. Prior to the study, all sites were burned every 2 to 3 years, and poison-ivies were absent from all sites [111]. In a south-central Illinois oak-hickory forest, the number of plants of poison-ivies declined 31% after 4 prescribed fires during 8 years. Fires occurred in February and March [252].
Fire severity: Poison-ivies are reported after fire severities ranging from low to high. In Ontario, they were dominant understory plants in a quaking aspen stand 20 years after a severe wildfire [273]. In a Rocky Mountain juniper community in the Little Missouri Badlands, North Dakota, that sustained different levels of fire damage, western poison-ivy had the highest frequency (33%) in stands with upper crown damage. It had 20% frequency in stands with surface damage and in stands with lower crown damage [205].
FUELS AND FIRE REGIMES: Fuels: Eastern poison-ivy may form fuel ladders. Fuel ladders formed by climbing eastern poison-ivy stems were reduced 1 year after a July prescribed fire in Chickamauga and Chattanooga National Military Park compared to prefire levels. Although all fine fuels present in these ladders were consumed by flare-ups, some large main stems remained. However, nearly all of the remaining stems were dead because the fire consumed them up to about 3 feet (1 m) from the ground, thus severing them from their roots. The authors concluded that because large ladder fuels were separated from the ground, the prescribed fire reduced the possibility of future surface fires becoming crown fires [68].Although other fuel characteristics for poison-ivies were unknown as of this writing (2012), they are likely to differ substantially between eastern poison-ivy, which climbs and is woody throughout, and western poison-ivy, which does not climb and is woody only about 2 to 48 inches (5-122 cm) from the base (see Botanical description).
Fire regimes: Poison-ivies occur in a variety of plant communities in the United States and are probably adapted to a wide range of fire regimes. They occur in communities with short (e.g., pine rockland, southern tallgrass prairie, and longleaf pine/bluestem (Andropogon spp.) prairie) to long (e.g., mixed-mesophytic hardwood, southern floodplain, and northern hardwood) fire-return intervals and in areas with mostly surface fire regimes (e.g. pine rockland, interior Highlands oak-hickory-pine, Appalachian oak forest (dry-mesic), and ponderosa pine), mixed fire regimes (e.g., pocosin and riparian (Wyoming)), and stand-replacement fire regimes (e.g., southern tidal brackish to freshwater marsh, Gambel oak, and mosaic of bluestem prairie and oak-hickory). See the Fire Regime Table for further information on fire regimes of vegetation communities in which poison-ivies may occur. Find further fire regime information for the plant communities in which these species may occur by entering the species' names in the FEIS home page under "Find Fire Regimes".
FIRE MANAGEMENT CONSIDERATIONS:Poison-ivies often occur in canopy openings (see Successional Status), so small, patchy fires may benefit poison-ivies by providing openings that allow them to spread vegetatively or establish from on- or off-site seed sources. Although poison-ivies often increase after prescribed fire, they may also decrease after fire, depending in part of fire timing, frequency, and severity (see Plant response to fire). Viable poison-ivy seeds have been found in the soil seed bank of some forest communities after fire (see Immediate fire effect on plant) [222]. Thus, poison-ivies may establish from the seed bank after prescribed fire. Prescribed fire may temporarily reduce poison-ivy seed production. However, on the Noxubee National Wildlife Refuge, eastern poison-ivy fruit was abundant 4 years after plants were top-killed by a March prescribed fire in loblolly pine-oak forest. The forest had been burned 7 other times during the previous 12 years [126].
Wildlife: At least 75 species of birds, particularly gallinaceous birds such as wild turkeys, northern bobwhites, ruffed grouse, and sharp-tailed grouse, eat the fruits and seeds of poison-ivies (e.g., [17,109,124,139,147,171,187,245,279]). Many mammals—including bears, mule deer, white-tailed deer, moose, foxes, woodchucks, muskrats, rabbits, squirrels, woodrats, and mice—consume the leaves, stems, and fruits of poison-ivies (e.g., [97,124,181,187,194,209,249,255]). In southern Indiana, eastern poison-ivy was 1 of the 7 most important plants consumed year-round by white-tailed deer. White-tailed deer ate the leaves with greater frequency in summer (81%) than in spring (67%) [235]. Poison-ivy fruits may be particularly important during winter [17,81] or during poor mast years [61,97] when other fruits are unavailable. In Maryland, ripe eastern poison-ivy fruits were available on plants in September, but birds did not begin harvesting them until late October [52].
Livestock: According to a fact sheet, livestock typically browse poison-ivies only sparsely [262]. However, heavy livestock browsing of poison-ivies may occur and sometimes reduces abundance of poison-ivies locally. See Control for more information on this topic.
Palatability and nutritional value: Wildlife and livestock can browse poison-ivies without ill effects to the animals [42,109,171,262]. Western poison-ivy palatability is generally low to good. It is "poor" to "good" forage for small mammals and birds; poor forage for pronghorn, elk, mule deer, and white-tailed deer; and poor to fair forage for cattle, domestic sheep, and horses. It is rated poor in both energy and protein value [55].
Cover value: According to Dittberner and Olson [55], western poison-ivy cover value for wildlife is "poor" to "fair". However, western poison-ivy was the most common understory plant in a riparian eastern cottonwood/willow community in Scotts Bluff National Monument, Nebraska, and this community provided important cover for more than 80 species of birds, mammals, reptiles, and amphibians [45].
VALUE FOR REHABILITATION OF DISTURBED SITES:Poison-ivies are often considered "weeds" throughout much of their distribution [49,171]. They may be deleterious to other plant species by forming dense mats or tangles that exclude other plants [49,68,153]. Climbing eastern poison-ivy stems may negatively affect forest tree species by direct physical suppression, shading, or via competition with roots for water and nutrients [276]. In eastern cottonwood stands along the Missouri River, eastern poison-ivy, Virginia creeper (Parthenocissus quinquefolia), and frost grape (Vitis vulpina) density was so great and the mass of the lianas was so heavy that shrubby understory species were often bent or broken [280]. Buron and others [34] speculated that the heavy weight of eastern poison-ivy stems on trees may cause tree falls. In South Carolina bottomland hardwood forest, small trees with eastern poison-ivy and other lianas suffered disproportionately greater damage from Hurricane Hugo than small trees with no lianas, and trees of all sizes that supported ≥3 lianas were more likely to be damaged [4]. On Hog Island, Virginia, a dense thicket of eastern poison-ivy, wax-myrtle, and red raspberry (Rubus idaeus) appeared to limit seedling establishment of loblolly pine due to shading [129]. Whigham [276] removed eastern poison-ivy and other lianas from the trunk, branches, and ground of an old field (40 years since cultivation) in Maryland. Complete removal of lianas increased sweetgum growth in each of 4 study years (P<0.001), but removal that left belowground plant parts intact did not increase growth. He concluded that sweetgum growth increase was due to a reduction in belowground competition [276].
Eastern poison-ivy is a mechanical parasite that depends upon trees to provide support as it grows from the forest floor to better lit areas in the forest canopy [253]. It is unable to grow on small branches or twigs [5,253] and thus requires large hosts [143]. In old-growth mesophytic forest of northern Alabama, where 38% of trees >4 inches (10 cm) DBH supported eastern poison-ivy stems, eastern poison-ivy stems were 1.5 times the expected abundance on trees >24 inches (60 cm) DBH [253]. In 2nd-growth hardwood forests in Massachusetts, 48% of trees >4 inches DBH had lianas, mostly eastern poison-ivy, growing on them, and large trees (>10 inches (25 cm) DBH) had more lianas than small trees [34]. In 2nd-growth hardwood forest in New Jersey, eastern poison-ivy was more likely to colonize large trees than small trees (P<0.001). Because eastern poison-ivy was abundant on the site before canopy closure, it may have climbed some of the earliest trees to establish; thus, it was unknown whether colonization of the trees was more closely associated with tree age or size [143].
 |
| Eastern poison-ivy stems with aerial roots. Photo courtesy of the Ohio State Weed Lab Archive, The Ohio State University, Bugwood.org. |
Eastern poison-ivy prefers to colonize some trees more than others. In hardwood forests in Massachusetts, most eastern poison-ivy grew on shagbark hickory (Carya ovata) and pignut (C. glabra) hickory, apparently because the shaggy bark of these trees made it easier for stems to adhere [34]. In bottomland hardwood forests in South Carolina, sweetgum was more likely to host eastern poison-ivy than other trees [4]. In hardwood forest in New Jersey, eastern poison-ivy had the highest probability of colonizing eastern redcedar and oaks and the least probability of colonizing black walnut (Juglans nigra), an allelopathic species [143]. In old-growth mesophytic forest in Alabama, eastern poison-ivy was more abundant than expected on shagbark hickory and northern red oak and less abundant than expected on sugar maple and 2 allelopathic species: black walnut and sassafras (Sassafras albidum). The authors suggested that eastern poison-ivy grew most successfully on the least allelopathic hardwood species [253]. In contrast, in old fields (5 to >50 years since cultivation) in Tennessee, eastern poison-ivy abundance was greater beneath canopies of allelopathic sassafras than outside of sassafras canopies [77].
Control: Because poison-ivies may cause debilitating rashes in humans, many means of control were recommended in reviewed literature. Some sources indicated that poison-ivies can be controlled by repeated cultivation [108,171,187]. In southern Illinois, cover of poison-ivies decreased 1 year after disking of cherrybark oak (Quercus pagoda) and post oak-cherrybark oak bottomlands (P≤0.05) [150]. However, activities that disturb poison-ivies, such as hand-pulling, may stimulate growth of plants from fragments left in the ground. Management guidelines suggest that small infestations of poison-ivies may be eradicated by carefully digging out plants; however, all stems and roots must be removed for this technique to be effective [35,108]. Herbicides may control poison-ivies (e.g., [68,164]). However, while herbicides may be effective in gaining initial control of newly established plants or areas of dense vegetation, they are rarely a complete or long-term solution to weed management [36].
Poison-ivies can be partially controlled by livestock browsing, particularly by domestic goats and cattle, but there is often a resurgence of growth after browsing stops [35,66,108,262]. Poison-ivies decreased steadily throughout 4 grazing seasons in domestic goat- and cattle-grazed pastures in Waynesville, North Carolina (P<0.05) [155]. In green ash communities in the North Dakota Badlands, western poison-ivy cover was greater in lightly and moderately cattle-browsed sites (3.1%-3.2%) than in heavily browsed sites (0.6%) [182]. In northeastern Ohio, poison-ivies were absent from a grazed sugar maple woodland but present in a sugar maple woodland ungrazed for 10 years [50]. In contrast, in a riparian community in southeastern Kansas, cover of poison-ivies over 2 years was statistically similar on cattle-grazed sites (0.47%) and on sites where cattle were excluded (1.04%) [121].
Wildlife browsing may reduce local abundance of poison-ivies. In the Sunken Forest, a maritime American holly forest in New York, poison-ivies in the understory decreased from 1,214 stems/ha to zero during 35 years, even though overstory structure and composition changed minimally during this time. However, in an American holly forest at Sandy Hook, New Jersey, density of poison-ivies remained the same (range: 139-256 stems/ha) during 14 years. The difference in density of poison-ivies between the 2 sites was attributed to higher white-tailed deer populations in the Sunken Forest than in Sandy Hook [72]. In central Ohio, stems of poison-ivies were 3 times more numerous in white-tailed deer exclosure plots than in browsed plots (P<0.05) [14]. In Clark County, Virginia, mean eastern poison-ivy cover was higher (3.5%) in plots where white-tailed deer were excluded than in browsed plots (1.6%) 4 years after an old field (10 years since cultivation) was sprayed with herbicides and disked [30]. In contrast, abundance of poison-ivies did not differ between sites with low and high white-tailed deer densities in northeastern Nebraska [96].| Fire regime information on vegetation communities in which eastern and western poison-ivy may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [146], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||||||||||||
|
|||||||||||||||||
| Pacific Northwest | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Pacific Northwest Grassland | |||||||||||||||||
| Marsh | Replacement | 74% | 7 | ||||||||||||||
| Mixed | 26% | 20 | |||||||||||||||
| Pacific Northwest Woodland | |||||||||||||||||
| Oregon white oak-ponderosa pine | Replacement | 16% | 125 | 100 | 300 | ||||||||||||
| Mixed | 2% | 900 | 50 | ||||||||||||||
| Surface or low | 81% | 25 | 5 | 30 | |||||||||||||
| Pine savannah (ultramafic) | Replacement | 7% | 200 | 100 | 300 | ||||||||||||
| Surface or low | 93% | 15 | 10 | 20 | |||||||||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||||||||
| Mixed | 17% | 60 | |||||||||||||||
| Surface or low | 78% | 13 | |||||||||||||||
| Pacific Northwest Forested | |||||||||||||||||
| Ponderosa pine (xeric) | Replacement | 37% | 130 | ||||||||||||||
| Mixed | 48% | 100 | |||||||||||||||
| Surface or low | 16% | 300 | |||||||||||||||
| Dry ponderosa pine (mesic) | Replacement | 5% | 125 | ||||||||||||||
| Mixed | 13% | 50 | |||||||||||||||
| Surface or low | 82% | 8 | |||||||||||||||
| Mixed conifer (eastside dry) | Replacement | 14% | 115 | 70 | 200 | ||||||||||||
| Mixed | 21% | 75 | 70 | 175 | |||||||||||||
| Surface or low | 64% | 25 | 20 | 25 | |||||||||||||
| Mixed conifer (eastside mesic) | Replacement | 35% | 200 | ||||||||||||||
| Mixed | 47% | 150 | |||||||||||||||
| Surface or low | 18% | 400 | |||||||||||||||
| Southwest | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Southwest Grassland | |||||||||||||||||
| Shortgrass prairie | Replacement | 87% | 12 | 2 | 35 | ||||||||||||
| Mixed | 13% | 80 | |||||||||||||||
| Shortgrass prairie with shrubs | Replacement | 80% | 15 | 2 | 35 | ||||||||||||
| Mixed | 20% | 60 | |||||||||||||||
| Shortgrass prairie with trees | Replacement | 80% | 15 | 2 | 35 | ||||||||||||
| Mixed | 20% | 60 | |||||||||||||||
| Plains mesa grassland | Replacement | 81% | 20 | 3 | 30 | ||||||||||||
| Mixed | 19% | 85 | 3 | 150 | |||||||||||||
| Plains mesa grassland with shrubs or trees | Replacement | 76% | 20 | ||||||||||||||
| Mixed | 24% | 65 | |||||||||||||||
| Montane and subalpine grasslands | Replacement | 55% | 18 | 10 | 100 | ||||||||||||
| Surface or low | 45% | 22 | |||||||||||||||
| Montane and subalpine grasslands with shrubs or trees | Replacement | 30% | 70 | 10 | 100 | ||||||||||||
| Surface or low | 70% | 30 | |||||||||||||||
| Southwest Shrubland | |||||||||||||||||
| Gambel oak | Replacement | 75% | 50 | ||||||||||||||
| Mixed | 25% | 150 | |||||||||||||||
| Southwest Woodland | |||||||||||||||||
| Mesquite bosques | Replacement | 32% | 135 | ||||||||||||||
| Mixed | 67% | 65 | |||||||||||||||
| Madrean oak-conifer woodland | Replacement | 16% | 65 | 25 | |||||||||||||
| Mixed | 8% | 140 | 5 | ||||||||||||||
| Surface or low | 76% | 14 | 1 | 20 | |||||||||||||
| Pinyon-juniper (mixed fire regime) | Replacement | 29% | 430 | ||||||||||||||
| Mixed | 65% | 192 | |||||||||||||||
| Surface or low | 6% | >1,000 | |||||||||||||||
| Pinyon-juniper (rare replacement fire regime) | Replacement | 76% | 526 | ||||||||||||||
| Mixed | 20% | >1,000 | |||||||||||||||
| Surface or low | 4% | >1,000 | |||||||||||||||
| Ponderosa pine/grassland (Southwest) | Replacement | 3% | 300 | ||||||||||||||
| Surface or low | 97% | 10 | |||||||||||||||
| Southwest Forested | |||||||||||||||||
| Riparian forest with conifers | Replacement | 100% | 435 | 300 | 550 | ||||||||||||
| Riparian deciduous woodland | Replacement | 50% | 110 | 15 | 200 | ||||||||||||
| Mixed | 20% | 275 | 25 | ||||||||||||||
| Surface or low | 30% | 180 | 10 | ||||||||||||||
| Ponderosa pine-Gambel oak (southern Rockies and Southwest) | Replacement | 8% | 300 | ||||||||||||||
| Surface or low | 92% | 25 | 10 | 30 | |||||||||||||
| Ponderosa pine-Douglas-fir (southern Rockies) | Replacement | 15% | 460 | ||||||||||||||
| Mixed | 43% | 160 | |||||||||||||||
| Surface or low | 43% | 160 | |||||||||||||||
| Southwest mixed conifer (warm, dry with aspen) | Replacement | 7% | 300 | ||||||||||||||
| Mixed | 13% | 150 | 80 | 200 | |||||||||||||
| Surface or low | 80% | 25 | 2 | 70 | |||||||||||||
| Southwest mixed conifer (cool, moist with aspen) | Replacement | 29% | 200 | 80 | 200 | ||||||||||||
| Mixed | 35% | 165 | 35 | ||||||||||||||
| Surface or low | 36% | 160 | 10 | ||||||||||||||
| Aspen with spruce-fir | Replacement | 38% | 75 | 40 | 90 | ||||||||||||
| Mixed | 38% | 75 | 40 | ||||||||||||||
| Surface or low | 23% | 125 | 30 | 250 | |||||||||||||
| Stable aspen without conifers | Replacement | 81% | 150 | 50 | 300 | ||||||||||||
| Surface or low | 19% | 650 | 600 | >1,000 | |||||||||||||
| Great Basin | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Great Basin Grassland | |||||||||||||||||
| Great Basin grassland | Replacement | 33% | 75 | 40 | 110 | ||||||||||||
| Mixed | 67% | 37 | 20 | 54 | |||||||||||||
| Mountain meadow (mesic to dry) | Replacement | 66% | 31 | 15 | 45 | ||||||||||||
| Mixed | 34% | 59 | 30 | 90 | |||||||||||||
| Great Basin Shrubland | |||||||||||||||||
| Gambel oak | Replacement | 75% | 50 | ||||||||||||||
| Mixed | 25% | 150 | |||||||||||||||
| Mountain shrubland with trees | Replacement | 22% | 105 | 100 | 200 | ||||||||||||
| Mixed | 78% | 29 | 25 | 100 | |||||||||||||
| Great Basin Woodland | |||||||||||||||||
| Juniper and pinyon-juniper steppe woodland | Replacement | 20% | 333 | 100 | >1,000 | ||||||||||||
| Mixed | 31% | 217 | 100 | >1,000 | |||||||||||||
| Surface or low | 49% | 135 | 100 | ||||||||||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||||||||
| Mixed | 17% | 60 | |||||||||||||||
| Surface or low | 78% | 13 | |||||||||||||||
| Great Basin Forested | |||||||||||||||||
| Interior ponderosa pine | Replacement | 5% | 161 | 800 | |||||||||||||
| Mixed | 10% | 80 | 50 | 80 | |||||||||||||
| Surface or low | 86% | 9 | 8 | 10 | |||||||||||||
| Ponderosa pine-Douglas-fir | Replacement | 10% | 250 | >1,000 | |||||||||||||
| Mixed | 51% | 50 | 50 | 130 | |||||||||||||
| Surface or low | 39% | 65 | 15 | ||||||||||||||
| Great Basin Douglas-fir (dry) | Replacement | 12% | 90 | 600 | |||||||||||||
| Mixed | 14% | 76 | 45 | ||||||||||||||
| Surface or low | 75% | 14 | 10 | 50 | |||||||||||||
| Aspen with conifer (low to midelevations) | Replacement | 53% | 61 | 20 | |||||||||||||
| Mixed | 24% | 137 | 10 | ||||||||||||||
| Surface or low | 23% | 143 | 10 | ||||||||||||||
| Douglas-fir (warm mesic interior) | Replacement | 28% | 170 | 80 | 400 | ||||||||||||
| Mixed | 72% | 65 | 50 | 250 | |||||||||||||
| Stable aspen-cottonwood, no conifers | Replacement | 31% | 96 | 50 | 300 | ||||||||||||
| Surface or low | 69% | 44 | 20 | 60 | |||||||||||||
| Stable aspen without conifers | Replacement | 81% | 150 | 50 | 300 | ||||||||||||
| Surface or low | 19% | 650 | 600 | >1,000 | |||||||||||||
| Northern and Central Rockies | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Northern and Central Rockies Grassland | |||||||||||||||||
| Northern prairie grassland | Replacement | 55% | 22 | 2 | 40 | ||||||||||||
| Mixed | 45% | 27 | 10 | 50 | |||||||||||||
| Mountain grassland | Replacement | 60% | 20 | 10 | |||||||||||||
| Mixed | 40% | 30 | |||||||||||||||
| Northern and Central Rockies Shrubland | |||||||||||||||||
| Riparian (Wyoming) | Mixed | 100% | 100 | 25 | 500 | ||||||||||||
| Mountain shrub, nonsagebrush | Replacement | 80% | 100 | 20 | 150 | ||||||||||||
| Mixed | 20% | 400 | |||||||||||||||
| Mountain big sagebrush steppe and shrubland | Replacement | 100% | 70 | 30 | 200 | ||||||||||||
| Northern and Central Rockies Woodland | |||||||||||||||||
| Ancient juniper | Replacement | 100% | 750 | 200 | >1,000 | ||||||||||||
| Northern and Central Rockies Forested | |||||||||||||||||
| Ponderosa pine (Northern Great Plains) | Replacement | 5% | 300 | ||||||||||||||
| Mixed | 20% | 75 | |||||||||||||||
| Surface or low | 75% | 20 | 10 | 40 | |||||||||||||
| Ponderosa pine (Northern and Central Rockies) | Replacement | 4% | 300 | 100 | >1,000 | ||||||||||||
| Mixed | 19% | 60 | 50 | 200 | |||||||||||||
| Surface or low | 77% | 15 | 3 | 30 | |||||||||||||
| Ponderosa pine (Black Hills, low elevation) | Replacement | 7% | 300 | 200 | 400 | ||||||||||||
| Mixed | 21% | 100 | 50 | 400 | |||||||||||||
| Surface or low | 71% | 30 | 5 | 50 | |||||||||||||
| Ponderosa pine-Douglas-fir | Replacement | 10% | 250 | >1,000 | |||||||||||||
| Mixed | 51% | 50 | 50 | 130 | |||||||||||||
| Surface or low | 39% | 65 | 15 | ||||||||||||||
| Northern Great Plains | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Northern Plains Grassland | |||||||||||||||||
| Nebraska Sandhills prairie | Replacement | 58% | 11 | 2 | 20 | ||||||||||||
| Mixed | 32% | 20 | |||||||||||||||
| Surface or low | 10% | 67 | |||||||||||||||
| Northern mixed-grass prairie | Replacement | 67% | 15 | 8 | 25 | ||||||||||||
| Mixed | 33% | 30 | 15 | 35 | |||||||||||||
| Southern mixed-grass prairie | Replacement | 100% | 9 | 1 | 10 | ||||||||||||
| Central tallgrass prairie | Replacement | 75% | 5 | 3 | 5 | ||||||||||||
| Mixed | 11% | 34 | 1 | 100 | |||||||||||||
| Surface or low | 13% | 28 | 1 | 50 | |||||||||||||
| Northern tallgrass prairie | Replacement | 90% | 6.5 | 1 | 25 | ||||||||||||
| Mixed | 9% | 63 | |||||||||||||||
| Surface or low | 2% | 303 | |||||||||||||||
| Southern tallgrass prairie (East) | Replacement | 96% | 4 | 1 | 10 | ||||||||||||
| Mixed | 1% | 277 | |||||||||||||||
| Surface or low | 3% | 135 | |||||||||||||||
| Oak savanna | Replacement | 7% | 44 | ||||||||||||||
| Mixed | 17% | 18 | |||||||||||||||
| Surface or low | 76% | 4 | |||||||||||||||
| Northern Plains Woodland | |||||||||||||||||
| Oak woodland | Replacement | 2% | 450 | ||||||||||||||
| Surface or low | 98% | 7.5 | |||||||||||||||
| Northern Great Plains wooded draws and ravines | Replacement | 38% | 45 | 30 | 100 | ||||||||||||
| Mixed | 18% | 94 | |||||||||||||||
| Surface or low | 43% | 40 | 10 | ||||||||||||||
| Great Plains floodplain | Replacement | 100% | 500 | ||||||||||||||
| Great Lakes | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Great Lakes Grassland | |||||||||||||||||
| Mosaic of bluestem prairie and oak-hickory | Replacement | 79% | 5 | 1 | 8 | ||||||||||||
| Mixed | 2% | 260 | |||||||||||||||
| Surface or low | 20% | 2 | 33 | ||||||||||||||
| Great Lakes Woodland | |||||||||||||||||
| Great Lakes pine barrens | Replacement | 8% | 41 | 10 | 80 | ||||||||||||
| Mixed | 9% | 36 | 10 | 80 | |||||||||||||
| Surface or low | 83% | 4 | 1 | 20 | |||||||||||||
| Jack pine-open lands (frequent fire-return interval) | Replacement | 83% | 26 | 10 | 100 | ||||||||||||
| Mixed | 17% | 125 | 10 | ||||||||||||||
| Northern oak savanna | Replacement | 4% | 110 | 50 | 500 | ||||||||||||
| Mixed | 9% | 50 | 15 | 150 | |||||||||||||
| Surface or low | 87% | 5 | 1 | 20 | |||||||||||||
| Great Lakes Forested | |||||||||||||||||
| Northern hardwood maple-beech-eastern hemlock | Replacement | 60% | >1,000 | ||||||||||||||
| Mixed | 40% | >1,000 | |||||||||||||||
| Conifer lowland (embedded in fire-prone ecosystem) | Replacement | 45% | 120 | 90 | 220 | ||||||||||||
| Mixed | 55% | 100 | |||||||||||||||
| Conifer lowland (embedded in fire-resistant ecosystem) | Replacement | 36% | 540 | 220 | >1,000 | ||||||||||||
| Mixed | 64% | 300 | |||||||||||||||
| Great Lakes floodplain forest | Mixed | 7% | 833 | ||||||||||||||
| Surface or low | 93% | 61 | |||||||||||||||
| Great Lakes spruce-fir | Replacement | 100% | 85 | 50 | 200 | ||||||||||||
| Minnesota spruce-fir (adjacent to Lake Superior and Drift and Lake Plain) | Replacement | 21% | 300 | ||||||||||||||
| Surface or low | 79% | 80 | |||||||||||||||
| Great Lakes pine forest, jack pine | Replacement | 67% | 50 | ||||||||||||||
| Mixed | 23% | 143 | |||||||||||||||
| Surface or low | 10% | 333 | |||||||||||||||
| Maple-basswood | Replacement | 33% | >1,000 | ||||||||||||||
| Surface or low | 67% | 500 | |||||||||||||||
| Maple-basswood mesic hardwood forest (Great Lakes) | Replacement | 100% | >1,000 | >1,000 | >1,000 | ||||||||||||
| Maple-basswood-oak-aspen | Replacement | 4% | 769 | ||||||||||||||
| Mixed | 7% | 476 | |||||||||||||||
| Surface or low | 89% | 35 | |||||||||||||||
| Northern hardwood-eastern hemlock forest (Great Lakes) | Replacement | 99% | >1,000 | ||||||||||||||
| Oak-hickory | Replacement | 13% | 66 | 1 | |||||||||||||
| Mixed | 11% | 77 | 5 | ||||||||||||||
| Surface or low | 76% | 11 | 2 | 25 | |||||||||||||
| Pine-oak | Replacement | 19% | 357 | ||||||||||||||
| Surface or low | 81% | 85 | |||||||||||||||
| Red pine-eastern white pine (frequent fire) | Replacement | 38% | 56 | ||||||||||||||
| Mixed | 36% | 60 | |||||||||||||||
| Surface or low | 26% | 84 | |||||||||||||||
| Red pine-eastern white pine (less frequent fire) | Replacement | 30% | 166 | ||||||||||||||
| Mixed | 47% | 105 | |||||||||||||||
| Surface or low | 23% | 220 | |||||||||||||||
| Great Lakes pine forest, eastern white pine-eastern hemlock (frequent fire) | Replacement | 52% | 260 | ||||||||||||||
| Mixed | 12% | >1,000 | |||||||||||||||
| Surface or low | 35% | 385 | |||||||||||||||
| Eastern white pine-eastern hemlock | Replacement | 54% | 370 | ||||||||||||||
| Mixed | 12% | >1,000 | |||||||||||||||
| Surface or low | 34% | 588 | |||||||||||||||
| Northeast | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Northeast Grassland | |||||||||||||||||
| Northern coastal marsh | Replacement | 97% | 7 | 2 | 50 | ||||||||||||
| Mixed | 3% | 265 | 20 | ||||||||||||||
| Northeast Woodland | |||||||||||||||||
| Eastern woodland mosaic | Replacement | 2% | 200 | 100 | 300 | ||||||||||||
| Mixed | 9% | 40 | 20 | 60 | |||||||||||||
| Surface or low | 89% | 4 | 1 | 7 | |||||||||||||
| Rocky outcrop pine (Northeast) | Replacement | 16% | 128 | ||||||||||||||
| Mixed | 32% | 65 | |||||||||||||||
| Surface or low | 52% | 40 | |||||||||||||||
| Pine barrens | Replacement | 10% | 78 | ||||||||||||||
| Mixed | 25% | 32 | |||||||||||||||
| Surface or low | 65% | 12 | |||||||||||||||
| Oak-pine (eastern dry-xeric) | Replacement | 4% | 185 | ||||||||||||||
| Mixed | 7% | 110 | |||||||||||||||
| Surface or low | 90% | 8 | |||||||||||||||
| Northeast Forested | |||||||||||||||||
| Northern hardwoods (Northeast) | Replacement | 39% | >1,000 | ||||||||||||||
| Mixed | 61% | 650 | |||||||||||||||
| Eastern white pine-northern hardwood | Replacement | 72% | 475 | ||||||||||||||
| Surface or low | 28% | >1,000 | |||||||||||||||
| Northern hardwoods-eastern hemlock | Replacement | 50% | >1,000 | ||||||||||||||
| Surface or low | 50% | >1,000 | |||||||||||||||
| Northern hardwoods-spruce | Replacement | 100% | >1,000 | 400 | >1,000 | ||||||||||||
| Appalachian oak forest (dry-mesic) | Replacement | 2% | 625 | 500 | >1,000 | ||||||||||||
| Mixed | 6% | 250 | 200 | 500 | |||||||||||||
| Surface or low | 92% | 15 | 7 | 26 | |||||||||||||
| Beech-maple | Replacement | 100% | >1,000 | ||||||||||||||
| South-central US | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| South-central US Grassland | |||||||||||||||||
| Southern shortgrass or mixed-grass prairie | Replacement | 100% | 8 | 1 | 10 | ||||||||||||
| Southern tallgrass prairie | Replacement | 91% | 5 | ||||||||||||||
| Mixed | 9% | 50 | |||||||||||||||
| Oak savanna | Replacement | 3% | 100 | 5 | 110 | ||||||||||||
| Mixed | 5% | 60 | 5 | 250 | |||||||||||||
| Surface or low | 93% | 3 | 1 | 4 | |||||||||||||
| South-central US Shrubland | |||||||||||||||||
| Shinnery oak-mixed grass | Replacement | 96% | 7 | ||||||||||||||
| Mixed | 4% | 150 | |||||||||||||||
| Shinnery oak-tallgrass | Replacement | 93% | 7 | ||||||||||||||
| Mixed | 7% | 100 | |||||||||||||||
| South-central US Woodland | |||||||||||||||||
| Oak-hickory savanna (East Texas) | Replacement | 1% | 227 | ||||||||||||||
| Surface or low | 99% | 3.2 | |||||||||||||||
| Interior Highlands dry oak/bluestem woodland and glade | Replacement | 16% | 25 | 10 | 100 | ||||||||||||
| Mixed | 4% | 100 | 10 | ||||||||||||||
| Surface or low | 80% | 5 | 2 | 7 | |||||||||||||
| Oak woodland-shrubland-grassland mosaic | Replacement | 11% | 50 | ||||||||||||||
| Mixed | 56% | 10 | |||||||||||||||
| Surface or low | 33% | 17 | |||||||||||||||
| Interior Highlands oak-hickory-pine | Replacement | 3% | 150 | 100 | 300 | ||||||||||||
| Surface or low | 97% | 4 | 2 | 10 | |||||||||||||
| Pine bluestem | Replacement | 4% | 100 | ||||||||||||||
| Surface or low | 96% | 4 | |||||||||||||||
| South-central US Forested | |||||||||||||||||
| Interior Highlands dry-mesic forest and woodland | Replacement | 7% | 250 | 50 | 300 | ||||||||||||
| Mixed | 18% | 90 | 20 | 150 | |||||||||||||
| Surface or low | 75% | 22 | 5 | 35 | |||||||||||||
| Gulf Coastal Plain pine flatwoods | Replacement | 2% | 190 | ||||||||||||||
| Mixed | 3% | 170 | |||||||||||||||
| Surface or low | 95% | 5 | |||||||||||||||
| West Gulf Coastal plain pine (uplands + flatwoods) | Replacement | 4% | 100 | 50 | 200 | ||||||||||||
| Mixed | 4% | 100 | 50 | ||||||||||||||
| Surface or low | 93% | 4 | 4 | 10 | |||||||||||||
| West Gulf Coastal Plain pine-hardwood woodland or forest upland | Replacement | 3% | 100 | 20 | 200 | ||||||||||||
| Mixed | 3% | 100 | 25 | ||||||||||||||
| Surface or low | 94% | 3 | 3 | 5 | |||||||||||||
| Southern floodplain | Replacement | 42% | 140 | ||||||||||||||
| Surface or low | 58% | 100 | |||||||||||||||
| Southern floodplain (rare fire) | Replacement | 42% | >1,000 | ||||||||||||||
| Surface or low | 58% | 714 | |||||||||||||||
| Cross Timbers | Replacement | 3% | 170 | ||||||||||||||
| Mixed | 2% | 250 | |||||||||||||||
| Surface or low | 94% | 6 | |||||||||||||||
| Southern Appalachians | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Southern Appalachians Grassland | |||||||||||||||||
| Bluestem-oak barrens | Replacement | 46% | 15 | ||||||||||||||
| Mixed | 10% | 69 | |||||||||||||||
| Surface or low | 44% | 16 | |||||||||||||||
| Eastern prairie-woodland mosaic | Replacement | 50% | 10 | ||||||||||||||
| Mixed | 1% | 900 | |||||||||||||||
| Surface or low | 50% | 10 | |||||||||||||||
| Southern Appalachians Woodland | |||||||||||||||||
| Appalachian shortleaf pine | Replacement | 4% | 125 | ||||||||||||||
| Mixed | 4% | 155 | |||||||||||||||
| Surface or low | 92% | 6 | |||||||||||||||
| Table Mountain-pitch pine | Replacement | 5% | 100 | ||||||||||||||
| Mixed | 3% | 160 | |||||||||||||||
| Surface or low | 92% | 5 | |||||||||||||||
| Oak-ash woodland | Replacement | 23% | 119 | ||||||||||||||
| Mixed | 28% | 95 | |||||||||||||||
| Surface or low | 49% | 55 | |||||||||||||||
| Southern Appalachians Forested | |||||||||||||||||
| Bottomland hardwood forest | Replacement | 25% | 435 | 200 | >1,000 | ||||||||||||
| Mixed | 24% | 455 | 150 | 500 | |||||||||||||
| Surface or low | 51% | 210 | 50 | 250 | |||||||||||||
| Mixed-mesophytic hardwood | Replacement | 11% | 665 | ||||||||||||||
| Mixed | 10% | 715 | |||||||||||||||
| Surface or low | 79% | 90 | |||||||||||||||
| Appalachian oak-hickory-pine | Replacement | 3% | 180 | 30 | 500 | ||||||||||||
| Mixed | 8% | 65 | 15 | 150 | |||||||||||||
| Surface or low | 89% | 6 | 3 | 10 | |||||||||||||
| Eastern hemlock-eastern white pine-hardwood | Replacement | 17% | >1,000 | 500 | >1,000 | ||||||||||||
| Surface or low | 83% | 210 | 100 | >1,000 | |||||||||||||
| Red pine-eastern white pine (frequent fire) | Replacement | 38% | 56 | ||||||||||||||
| Mixed | 36% | 60 | |||||||||||||||
| Surface or low | 26% | 84 | |||||||||||||||
| Eastern white pine-northern hardwood | Replacement | 72% | 475 | ||||||||||||||
| Surface or low | 28% | >1,000 | |||||||||||||||
| Oak (eastern dry-xeric) | Replacement | 6% | 128 | 50 | 100 | ||||||||||||
| Mixed | 16% | 50 | 20 | 30 | |||||||||||||
| Surface or low | 78% | 10 | 1 | 10 | |||||||||||||
| Appalachian Virginia pine | Replacement | 20% | 110 | 25 | 125 | ||||||||||||
| Mixed | 15% | 145 | |||||||||||||||
| Surface or low | 64% | 35 | 10 | 40 | |||||||||||||
| Appalachian oak forest (dry-mesic) | Replacement | 6% | 220 | ||||||||||||||
| Mixed | 15% | 90 | |||||||||||||||
| Surface or low | 79% | 17 | |||||||||||||||
| Southeast | |||||||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||||||
| Southeast Grassland | |||||||||||||||||
| Southeast Gulf Coastal Plain Blackland prairie and woodland | Replacement | 22% | 7 | ||||||||||||||
| Mixed | 78% | 2.2 | |||||||||||||||
| Floodplain marsh | Replacement | 100% | 4 | 3 | 30 | ||||||||||||
| Palmetto prairie | Replacement | 87% | 2 | 1 | 4 | ||||||||||||
| Mixed | 4% | 40 | |||||||||||||||
| Surface or low | 9% | 20 | |||||||||||||||
| Pondcypress savanna | Replacement | 17% | 120 | ||||||||||||||
| Mixed | 27% | 75 | |||||||||||||||
| Surface or low | 57% | 35 | |||||||||||||||
| Southern tidal brackish to freshwater marsh | Replacement | 100% | 5 | ||||||||||||||
| Gulf Coast wet pine savanna | Replacement | 2% | 165 | 10 | 500 | ||||||||||||
| Mixed | 1% | 500 | |||||||||||||||
| Surface or low | 98% | 3 | 1 | 10 | |||||||||||||
| Southeast Shrubland | |||||||||||||||||
| Pocosin | Replacement | 1% | >1,000 | 30 | >1,000 | ||||||||||||
| Mixed | 99% | 12 | 3 | 20 | |||||||||||||
| Southeast Woodland | |||||||||||||||||
| Longleaf pine/bluestem | Replacement | 3% | 130 | ||||||||||||||
| Surface or low | 97% | 4 | 1 | 5 | |||||||||||||
| Longleaf pine (mesic uplands) | Replacement | 3% | 110 | 40 | 200 | ||||||||||||
| Surface or low | 97% | 3 | 1 | 5 | |||||||||||||
| Longleaf pine-Sandhills prairie | Replacement | 3% | 130 | 25 | 500 | ||||||||||||
| Surface or low | 97% | 4 | 1 | 10 | |||||||||||||
| Pine rocklands | Mixed | 1% | 330 | ||||||||||||||
| Surface or low | 99% | 3 | 1 | 5 | |||||||||||||
| Pond pine | Replacement | 64% | 7 | 5 | 500 | ||||||||||||
| Mixed | 25% | 18 | 8 | 150 | |||||||||||||
| Surface or low | 10% | 43 | 2 | 50 | |||||||||||||
| South Florida slash pine flatwoods | Replacement | 6% | 50 | 50 | 90 | ||||||||||||
| Surface or low | 94% | 3 | 1 | 6 | |||||||||||||
| Atlantic wet pine savanna | Replacement | 4% | 100 | ||||||||||||||
| Mixed | 2% | 175 | |||||||||||||||
| Surface or low | 94% | 4 | |||||||||||||||
| Southeast Forested | |||||||||||||||||
| Sand pine scrub | Replacement | 90% | 45 | 10 | 100 | ||||||||||||
| Mixed | 10% | 400 | 60 | ||||||||||||||
| Coastal Plain pine-oak-hickory | Replacement | 4% | 200 | ||||||||||||||
| Mixed | 7% | 100 | |||||||||||||||
| Surface or low | 89% | 8 | |||||||||||||||
| Atlantic white-cedar forest | Replacement | 34% | 200 | 25 | 350 | ||||||||||||
| Mixed | 8% | 900 | 20 | 900 | |||||||||||||
| Surface or low | 59% | 115 | 10 | 500 | |||||||||||||
| Maritime forest | Replacement | 18% | 40 | 500 | |||||||||||||
| Mixed | 2% | 310 | 100 | 500 | |||||||||||||
| Surface or low | 80% | 9 | 3 | 50 | |||||||||||||
| Mesic-dry flatwoods | Replacement | 3% | 65 | 5 | 150 | ||||||||||||
| Surface or low | 97% | 2 | 1 | 8 | |||||||||||||
| Loess bluff and plain forest | Replacement | 7% | 476 | ||||||||||||||
| Mixed | 9% | 385 | |||||||||||||||
| Surface or low | 85% | 39 | |||||||||||||||
| Southern floodplain | Replacement | 7% | 900 | ||||||||||||||
| Surface or low | 93% | 63 | |||||||||||||||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [101,145]. |
|||||||||||||||||
1. Abrahamson, Warren G.; Johnson, Ann F.; Layne, James N.; Peroni, Paricia A. 1984. Vegetation of the Archbold Biological Station, Florida: an example of the Southern Lake Wales Ridge. Florida Scientist. 47(4): 209-250. [20272]
2. Adams, Dwight E.; Anderson, Roger C. 1980. Species response to a moisture gradient in central Illinois forests. American Journal of Botany. 67(3): 381-392. [13295]
3. Adams, Dwight E.; Anderson, Roger C.; Collins, Scott L. 1982. Differential response of woody and herbaceous species to summer and winter burning in an Oklahoma grassland. The Southwestern Naturalist. 27(1): 55-61. [6282]
4. Allen, Bruce P.; Pauley, Eric F.; Sharitz, Rebecca R. 1997. Hurricane impacts on liana populations in an old-growth southeastern bottomland forest. Journal of the Torrey Botanical Society. 124(1): 34-42. [84053]
5. Allen, Bruce P.; Sharitz, Rebecca R.; Goebel, P. Charles. 2005. Twelve years post-hurricane liana dynamics in an old-growth southeastern floodplain forest. Forest Ecology and Management. 218(1-3): 259-269. [55795]
6. Allen, Charles M.; Pate, John; Thames, Sara; Trichell, Spencer; Ezell, Lacy. 2004. Changes in baygall vegetation from 1986 to 2001 at Fort Polk in west central Louisiana. Sida. 21(1): 419-427. [83787]
7. Allen, Peter H. 1958. A tidewater swamp forest and succession after clearcutting. Durham, NC: Duke University. 48 p. Thesis. [42218]
8. Andersen, Berniece A.; Holmgren, Arthur H. [1976]. Mountain plants of northeastern Utah. Circular 319. Logan, UT: Utah State University, Extension Services. 148 p. [312]
9. Anderson, Kimberly L.; Leopold, Donald J. 2002. The role of canopy gaps in maintaining vascular plant diversity at a forested wetland in New York State. Journal of the Torrey Botanical Society. 129(3): 238-250. [44695]
10. Apfelbaum, Steven I.; Haney, Alan W. 1990. Management of degraded oak savanna remnants in the Upper Midwest: preliminary results from three years of study. In: Hughes, H. Glenn; Bonnicksen, Thomas M., eds. Restoration `89: the new management challenge: Proceedings, 1st annual meeting of the Society for Ecological Restoration; 1989 January 16-20; Oakland, CA. Madison, WI: The University of Wisconsin Arboretum, Society for Ecological Restoration: 280-291. [14705]
11. Arata, Andrew A. 1959. Effects of burning on vegetation and rodent populations in a longleaf pine turkey oak association in north central Florida. Journal of the Florida Academy of Sciences. 22(2): 94-104. [12260]
12. Archambault, Louis; Barnes, Burton V.; Witter, John A. 1989. Ecological species groups of oak ecosystems of southeastern Michigan. Forest Science. 35(4): 1058-1074. [9768]
13. Artigas, Francisco J.; Boerner, Ralph E. J. 1989. Advance regeneration and seed banking of woody plants in Ohio pine plantations: implications for landscape change. Landscape Ecology. 2(3): 139-150. [13633]
14. Asnani, Kashmira M.; Klips, Robert A.; Curtis, Peter S. 2006. Regeneration of woodland vegetation after deer browsing in Sharon Woods Metro Park, Franklin County, Ohio. Ohio Journal of Science. 106(3): 86-92. [66351]
15. Association for Biodiversity Information. 2001. International classification of ecological communities: Terrestrial vegetation of the United States--Talladega and Tuskegee National Forests. Final Report. Arlington, VA: Association for Biodiversity Information; Durham, NC: ABI-South Community Ecology Group. 168 p. Available online: http://www.natureserve.org/library/tallatusk.pdf [2011, February 16]. [79605]
16. Au, Shu-fun. 1974. Vegetation and ecological processes on Shackleford Bank, North Carolina. National Park Service Scientific Monograph Series No. 6. Washington, DC: U.S. Department of the Interior, National Park Service. 86 p. [16101]
17. Baird, John W. 1980. The selection and use of fruit by birds in an eastern forest. The Wilson Bulletin. 92(1): 63-73. [10004]
18. Bard, Gily E. 1952. Secondary succession on the Piedmont of New Jersey. Ecological Monographs. 22(3): 195-215. [4777]
19. Bare, Janet E. 1979. Wildflowers and weeds of Kansas. Lawrence, KS: The Regents Press of Kansas. 509 p. [3801]
20. Beck, Alan M.; Vogl, Richard J. 1972. The effects of spring burning on rodent populations in a brush prairie savanna. Journal of Mammalogy. 53(2): 336-346. [84049]
21. Becker, Donald A. 1989. Five years of annual prairie burns. In: Bragg, Thomas A.; Stubbendieck, James, eds. Prairie pioneers: ecology, history and culture: Proceedings, 11th North American prairie conference; 1988 August 7-11; Lincoln, NE. Lincoln, NE: University of Nebraska: 163-168. [14037]
22. Bell, David T. 1974. Studies on the ecology of a streamside forest: composition and distribution of vegetation beneath the tree canopy. Bulletin of the Torrey Botanical Club. 101(1): 14-20. [81213]
23. Best, G. Ronnie; Segal, Debra S.; Wolfe, Charlotte. 1990. Soil-vegetation correlations in selected wetlands and uplands of north-central Florida. Biol. Rep. 90(9). Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service. 51 p. [18161]
24. Bigwood, Douglas W.; Inouye, David W. 1988. Spatial pattern analysis of seed banks: an improved method and optimized sampling. Ecology. 69(2): 497-507. [84095]
25. Blood, Laura E.; Pitoniak, Hilary J.; Titus, Jonathan H. 2010. Seed bank of a bottomland swamp in western New York. Castanea. 75(1): 19-38. [80474]
26. Bock, Jane H.; Bock, Carl E. 1984. Effects of fires on woody vegetation in the pine-grassland ecotone of the southern Black Hills. The American Midland Naturalist. 112(1): 35-42. [477]
27. Boggs, Keith; Weaver, T. 1992. Response of riparian shrubs to declining water availability. In: Clary, Warren P.; McArthur, E. Durant; Bedunah, Don; Wambolt, Carl L., comps. Proceedings--symposium on ecology and management of riparian shrub communities; 1991 May 29-31; Sun Valley, ID. Gen. Tech. Rep. INT-289. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 48-51. [19094]
28. Bonner, Franklin T.; Karrfalt, Robert P., eds. 2008. Woody plant seed manual. Agric. Handbook 727. Washington, DC: U.S. Department of Agriculture, Forest Service. 1223 p. [70515]
29. Bowers, Janice E.; McLaughlin, Steven P. 1987. Flora and vegetation of the Rincon Mountains, Pima County, Arizona. Desert Plants. 8(2): 50-94. [495]
30. Bowers, Michael A. 1993. Influence of herbivorous mammals on an old-field plant community: years 1--4 after disturbance. Oikos. 67(1): 129-141. [22626]
31. Bowles, Marlin L.; Jacobs, Karel A.; Mengler, Jeffrey L. 2007. Long-term changes in an oak forest's woody understory and herb layer with repeated burning. Journal of the Torrey Botanical Society. 134(2): 223-237. [69792]
32. Bragg, Don C. 2002. Reference conditions for old-growth pine forests in the Upper West Gulf Coast Plain. Journal of the Torrey Botanical Society. 129(4): 261-288. [44664]
33. Brockway, Dale G.; Outcalt, Kenneth W.; Estes, Becky L.; Rummer, Robert B. 2009. Vegetation response to midstorey mulching and prescribed burning for wildfire hazard reduction and longleaf pine (Pinus palustris Mill.) ecosystem restoration. Forestry. 82(3): 299-314. [75412]
34. Buron, Jennifer; Lavigne, Danielle; Grote, Kristine; Takis, Rebecca; Sholes, Owen. 1998. Association of vines and trees in second-growth forest. Northeastern Naturalist. 5(4): 359-362. [72919]
35. Burrill, L. C.; Callihan, R. H.; Parker, R. 1994. Poison oak and poison ivy: Rhus diversiloba T. & G. and Rhus radicans L. PNW 108. Corvallis, OR: Oregon State University Extension Service; Pullman, WA: Washington State University Cooperative Extension; Moscow, ID: University of Idaho Cooperative Extension System. 4 p. Available online: http://hdl.handle.net/1957/15869 [2012, January 3]. [83720]
36. Bussan, Alvin J.; Dyer, William E. 1999. Herbicides and rangeland. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 116-132. [35716]
37. Cain, Michael D.; Wigley, T. Bently; Reed, Derik J. 1998. Prescribed fire effects on structure in uneven-aged stands of loblolly and shortleaf pines. Wildlife Society Bulletin. 26(2): 209-218. [30045]
38. Carter, Jack L. 1997. Trees and shrubs of New Mexico. Boulder, CO: Johnson Books. 534 p. [72647]
39. Catling, Paul M.; Sinclair, Adrianne; Cuddy, Don. 2001. Vascular plants of a successional alvar burn 100 days after a severe fire and their mechanisms of re-establishment. The Canadian Field-Naturalist. 115(2): 214-222. [45889]
40. Clewell, Andre F. 1985. Guide to the vascular plants of the Florida Panhandle. Tallahassee, FL: Florida State University Press. 605 p. [13124]
41. Cohen, Susan; Braham, Richard; Sanchez, Felipe. 2004. Seed bank viability in disturbed longleaf pine sites. Restoration Ecology. 12(4): 503-515. [55811]
42. Coile, Nancy C. 1996. Poison-ivy (Toxicodendron radicans (L.) Kuntze) and its relatives in Florida. Botany Circular No. 31. Gainesville, FL: Florida Department of Agriculture and Consumer Services, Division of Plant Industry. 6 p. Available online: http://www.uflib.ufl.edu/ufdc/?b=UF00002314 [2012, January 3]. [83723]
43. Coleman, T. W.; Clarke, Stephen R.; Meeker, James R.; Rieske, L. K. 2008. Forest composition following overstory mortality from southern pine beetle and associated treatments. Canadian Journal of Forest Research. 38(6): 1406-1418. [83608]
44. Cooper, Stephen V.; Jean, Catherine. 2001. Wildfire succession in plant communities natural to the Alkali Creek vicinity, Charles M. Russell National Wildlife Refuge, Montana. Unpublished report prepared for the U.S. Fish and Wildlife Service: USFWS Agreement Number 60181-0-J206. Helena, MT: Montana Natural Heritage Program. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 32 p. [70496]
45. Cox, Mike K.; Franklin, William L. 1989. Terrestrial vertebrates of Scotts Bluff National Monument, Nebraska. The Great Basin Naturalist. 49(4): 597-613. [11004]
46. Crawford, Edward R.; Young, Donald R. 1998. Spatial/temporal variations in shrub thicket soil seed banks on an Atlantic Coast Barrier Island. American Journal of Botany. 85(12): 1739-1744. [83330]
47. Cronquist, Arthur; Holmgren, Noel H.; Holmgren, Patricia K. 1997. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Vol. 3, Part A: Subclass Rosidae (except Fabales). New York: The New York Botanical Garden. 446 p. [28652]
48. Crowder, A.; Harmsen, R. 1998. Notes on forest succession in old fields in southeastern Ontario: the woody species. The Canadian Field-Naturalist. 112(3): 410-418. [35971]
49. Curtis, John T. 1959. The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press. 657 p. [7116]
50. Dambach, Charles A. 1944. A ten-year ecological study of adjoining grazed and ungrazed woodlands in northeastern Ohio. Ecological Monographs. 14(3): 255-270. [84088]
51. Darwin, Angela T.; Ladd, David; Galdins, Robert; Contreras, Thomas A.; Fahrig, Lenore. 2004. Response of forest understory vegetation to a major ice storm. Journal of the Torrey Botanical Society. 131(1): 45-52. [79965]
52. Davidar, Priya; Morton, Eugene S. 1986. The relationship between fruit crop sizes and fruit removal rates by birds. Ecology. 67(1): 262-265. [20743]
53. Deller, Amy S.; Baldassarre, Guy A. 1998. Effects of flooding on the forest community in a greentree reservoir 18 years after flood cessation. Wetlands. 18(1): 90-99. [49447]
54. Dickie, Ian A.; Schnitzer, S. A.; Reich, P. B.; Hobbie, S. E. 2007. Is oak establishment in old-fields and savanna openings context dependent? Journal of Ecology. 95(2): 309-320. [84033]
55. Diggs, George M., Jr.; Lipscomb, Barney L.; O'Kennon, Robert J. 1999. Illustrated flora of north-central Texas. Sida Botanical Miscellany, No. 16. Fort Worth, TX: Botanical Research Institute of Texas. 1626 p. [35698]
56. Dobberpuhl, J. 1980. Seed banks of forest soils in east Tennessee. Knoxville, TN: University of Tennessee. 219 p. Thesis. [46755]
57. Donahue, William H. 1954. Some plant communities in the Anthracite Region of northeastern Pennsylvania. The American Midland Naturalist. 51(1): 203-231. [64481]
58. Dorge, Carol L.; Mitsch, William J.; Wiemhoff, John R. 1984. Cypress wetlands in southern Illinois. In: Ewel, Katherine Carter; Odum, Howard T., eds. Cypress swamps. Gainesville, FL: University of Florida Press: 393-404. [14861]
59. Dorn, Robert D. 1988. Vascular plants of Wyoming. Cheyenne, WY: Mountain West Publishing. 340 p. [6129]
60. Duncan, Wilbur H.; Duncan, Marion B. 1987. The Smithsonian guide to seaside plants of the Gulf and Atlantic coasts from Louisiana to Massachusetts, exclusive of lower peninsular Florida. Washington, DC: Smithsonian Institution Press. 409 p. [12906]
61. Edwards, John W.; Guynn, David C., Jr.; Loeb, Susan C. 1993. Seasonal mast availability of wildlife in the Piedmont region of Georgia. Res. Pap. SE-287. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southeastern Forest Experiment Station. 13 p. [23041]
62. Egler, Frank E. 1952. Southeast saline Everglades vegetation, Florida, and its management. Vegetatio. 3(4/5): 213-265. [11479]
63. Eleuterius, Lionel N.; Caldwell, John D. 1984. Flowering phenology of tidal marsh plants in Mississippi. Castanea. 49(4): 172-179. [84044]
64. Elliott, Katherine J.; Hitchcock, Stephanie L.; Krueger, Lisa. 2002. Vegetation response to large scale disturbance in a southern Appalachian forest: Hurricane Opal and salvage logging. Journal of the Torrey Botanical Society. 129(1): 48-59. [42033]
65. Elliott, Katherine J.; Vose, James M. 2005. Effects of understory prescribed burning on shortleaf pine (Pinus echinata Mill.)/mixed-hardwood forests. Journal of the Torrey Botanical Society. 132(2): 236-251. [60637]
66. Evans, James E. 1983. Literature review of management practices for smooth sumac (Rhus glabra), poison ivy (Rhus radicans), and other sumac species. Natural Areas Journal. 3(1): 16-26. [6248]
67. Farmer, Robert E., Jr.; Cunningham, Maureen; Barnhill, Mary Ann. 1982. First-year development of plant communities originating from forest topsoils placed on southern Appalachian minesoils. Journal of Applied Ecology. 19(1): 283-294. [84096]
68. Faulkner, Jerry L.; Clebsch, Edward E. C.; Sanders, William L. 1989. Use of prescribed burning for managing natural and historic resources in Chickamauga and Chattanooga National Military Park, U.S.A. Environmental Management. 13(5): 603-612. [13020]
69. Fike, Jean; Niering, William A. 1999. Four decades of old field vegetation development and the role of Celastrus orbiculatus in the northeastern United States. Journal of Vegetation Science. 10(4): 483-492. [37337]
70. Fitzhugh, E. Lee; Moir, William H.; Ludwig, John A.; Ronco, Frank, Jr. 1987. Forest habitat types in the Apache, Gila, and part of the Cibola National Forests, Arizona and New Mexico. Gen. Tech. Rep. RM-145. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 116 p. [4206]
71. Florida Natural Areas Inventory. 1990. Guide to the natural communities of Florida, [Online]. In: Species and communities. Tallahassee, FL: Florida Natural Areas Inventory; Florida State University (Producer). Available: http://www.fnai.org/PDF/Natural_Communities_Guide.pdf [2012, January 12]. [74325]
72. Forrester, Jodi A.; Leopold, Donald J.; Art, Henry W. 2007. Disturbance history and mortality patterns in a rare Atlantic barrier island maritime holly forest. Natural Areas Journal. 27(2): 169-182. [67193]
73. Francis, John K. 2004. Toxicodendron radicans. In: Francis, John K., ed. Wildland shrubs of the United States and its territories: thamnic descriptions: volume 1. Gen. Tech. Rep. IITF-GTR-26. San Juan, PR: U.S. Department of Agriculture, Forest Service, International Institute of Tropical Forestry; Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 769-771. [52270]
74. Fraver, Shawn. 1994. Vegetation responses along edge-to-interior gradients in the mixed hardwood forests of the Roanoke River Basin, North Carolina. Conservation Biology. 8(3): 822-832. [50702]
75. Freeman, C. E.; Dick-Peddie, W. A. 1970. Woody riparian vegetation in the Black and Sacramento Mountain ranges, southern New Mexico. The Southwestern Naturalist. 15(2): 145-164. [6470]
76. Funderbuck, David O.; Skeen, James N. 1976. Spring phenology in a mature Piedmont forest. Castanea. 41(1): 20-30. [71755]
77. Gant, Robert E.; Clebsch, E. C. 1975. The allelopathic influences of Sassafras albidum in old-field succession in Tennessee. Ecology. 56(3): 604-615. [21919]
78. Gauch, Hugh G., Jr.; Stone, Earl L. 1979. Vegetation and soil pattern in a mesophytic forest at Ithaca, New York. The American Midland Naturalist. 102(2): 332-345. [84040]
79. Gibson, Earl S. 1963. Vascular flora of Crawford County, Kansas. Transactions of the Kansas Academy of Science. 66(4): 685-726. [75346]
80. Gilliam, Frank S.; Christensen, Norman L. 1986. Herb-layer response to burning in pine flatwoods of the lower Coastal Plain of South Carolina. Bulletin of the Torrey Botanical Club. 113(1): 42-45. [4419]
81. Gillis, William T. 1971. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). [Part 1]. Rhodora. 73(793): 72-159. [84120]
82. Gillis, William T. 1971. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). [Part 2]. Rhodora. 73(794): 161-237. [84121]
83. Gillis, William T. 1971. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). [Part 3]. Rhodora. 73(795): 370-443. [84123]
84. Gillis, William T. 1971. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). [Part 4]. Rhodora. 73(796): 465-540. [84122]
85. Girard, Michele M.; Goetz, Harold; Bjugstad, Ardell J. 1989. Native woodland habitat types of southwestern North Dakota. Res. Pap. RM-281. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 36 p. [6319]
86. Girard, Michele Marie. 1985. Native woodland ecology and habitat classification of southwestern North Dakota. Fargo, ND: North Dakota State University. 314 p. Dissertation. [1025]
87. Gleason, Henry A.; Cronquist, Arthur. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd ed. New York: New York Botanical Garden. 910 p. [20329]
88. Godfrey, Robert K. 1988. Trees, shrubs, and woody vines of northern Florida and adjacent Georgia and Alabama. Athens, GA: The University of Georgia Press. 734 p. [10239]
89. Goslee, S. C.; Brooks, R. P.; Cole, C. A. 1997. Plants as indicators of wetland water source. Plant Ecology. 131(2): 199-206. [63946]
90. Goslee, Sarah C.; Niering, William A.; Urban, Dean L.; Christensen, Norman L. 2005. Influence of environment, history and vegetative interactions on stand dynamics in a Connecticut forest. The Journal of the Torrey Botanical Society. 132(3): 471-482. [84034]
91. Graves, James H.; Monk, Carl D. 1985. A comparison of soils and vegetation over marble and schist along tributaries to Panther Creek, Stephens County, Georgia. Castanea. 50(3): 146-163. [84099]
92. Great Plains Flora Association. 1986. Flora of the Great Plains. Lawrence, KS: University Press of Kansas. 1392 p. [1603]
93. Greenberg, Cathryn H.; Levey, Douglas J.; Loftis, David L. 2007. Fruit production in mature and recently regenerated forests of the Appalachians. The Journal of Wildlife Management. 71(2): 321-335. [66983]
94. Griffiths, Megan E. 2006. Salt spray and edaphic factors maintain dwarf stature and community composition in coastal sandplain heathlands. Plant Ecology. 186(1): 69-86. [84036]
95. Grimshaw, Susan; Bradley, Ted R. 1973. The vascular flora of Great Falls National Park, Fairfax County, Virginia. Castanea. 38(3): 229-261. [71699]
96. Gubanyi, Joseph A.; Savidge, Julie A.; Hygnstrom, Scott E.; VerCauteren, Kurt C.; Garabrandt, Gary W.; Korte, Seth P. 2008. Deer impact on vegetation in natural areas in southeastern Nebraska. Natural Areas Journal. 28(2): 121-129. [83337]
97. Halls, Lowell K., ed. 1977. Southern fruit-producing woody plants used by wildlife. Gen. Tech. Rep. SO-16. New Orleans, LA: U.S. Department of Agriculture, Forest Service, Southern Region; Southern Forest Experiment Station; Southeastern Area, State and Private Forestry. 235 p. [23521]
98. Hamilton, Ernest S.; Limbird, Arthur. 1982. Selective occurrence of arborescent species on soils in a drainage toposequence, Ottawa County, Ohio. Ohio Journal of Science. 82(5): 282-292. [4343]
99. Haney, Alan; Bowles, Marlin; Apfelbaum, Steven; Lain, Emily; Post, Tom. 2008. Gradient analysis of an eastern sand savanna's woody vegetation, and its long-term responses to restored fire processes. Forest Ecology and Management. 256(8): 1560-1571. [72686]
100. Hanks, Jess Paul. 1971. Secondary succession and soils on the Inner Coastal Plain of New Jersey. Bulletin of the Torrey Botanical Club. 98(6): 315-321. [80551]
101. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org/niftt/released/FRCC_Guidebook_2010_final.pdf. [81749]
102. Hansen, Paul L.; Hall, James B. 2002. Classification and management of USDI Bureau of Land Management's riparian and wetland sites in eastern and southern Idaho. Corvallis, MT: Bitterroot Restoration. 304 p. [82582]
103. Hansen, Paul L.; Hoffman, George R. 1988. The vegetation of the Grand River/Cedar River, Sioux, and Ashland Districts of the Custer National Forest: a habitat type classification. Gen. Tech. Rep. RM-157. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 68 p. [771]
104. Hansen, Paul L.; Hoffman, George R.; Bjugstad, Ardell J. 1984. The vegetation of Theodore Roosevelt National Park, North Dakota: a habitat type classification. Gen. Tech. Rep. RM-113. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 35 p. [1077]
105. Hansen, Paul L.; Pfister, Robert D.; Boggs, Keith; Cook, Bradley J.; Joy, John; Hinckley, Dan K. 1995. Classification and management of Montana's riparian and wetland sites. Miscellaneous Publication No. 54. Missoula, MT: The University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station. 646 p. [24768]
106. Hardin, E. Dennis. 1988. Succession in Buffalo Beats Prairie and surrounding forest. Bulletin of the Torrey Botanical Club. 115(1): 13-24. [4414]
107. Harrison, A. Tyrone. 1980. The Niobrara Valley Preserve: its biogeographic importance and description of its biotic communities. Unpublished report to the Nature Conservancy. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 116 p. [5736]
108. Haws, Pete. 2000. Poison oak and ivy management. Journal of Pesticide Reform. 20(4): 10-11. [83730]
109. Hayes, Doris W.; Garrison, George A. 1960. Key to important woody plants of eastern Oregon and Washington. Agric. Handb. 148. Washington, DC: U.S. Department of Agriculture, Forest Service. 227 p. [1109]
110. Heikens, Alice Long; West, K. Andrew; Robertson, Philip A. 1994. Short-term response of chert and shale barrens vegetation to fire in southwestern Illinois. Castanea. 59(3): 274-285. [27228]
111. Heisler, Jana L.; Briggs, John M.; Knapp, Alan K. 2003. Long-term patterns of shrub expansion in a C4-dominated grassland: fire frequency and the dynamics of shrub cover and abundance. American Journal of Botany. 90(3): 423-428. [44631]
112. Helm, A. C.; Nicholas, N. S.; Zedaker, S. M.; Young, S. T. 1991. Maritime forests on Bull Island, Cape Romain, South Carolina. Bulletin of the Torrey Botanical Club. 118(2): 170-175. [15686]
113. Heslinga, Justin L., Grese, Robert E. 2010. Assessing plant community changes over sixteen years of restoration in a remnant Michigan tallgrass Prairie. The American Midland Naturalist. 164(2): 322-336. [81403]
114. Hirsch, Kathie Jean. 1985. Habitat classification of grasslands and shrublands of southwestern North Dakota. Fargo, ND: North Dakota State University. 281 p. Dissertation. [40326]
115. Hladek, Kenneth Lee. 1971. Growth characteristics and utilization of buffaloberry (Shepherdia argentea Nutt.) in the Little Missouri River Badlands of southwestern North Dakota. Fargo, ND: North Dakota State University of Agriculture and Applied Science. 115 p. Dissertation. [12120]
116. Hoagland, Bruce. 2000. The vegetation of Oklahoma: a classification for landscape mapping and conservation planning. The Southwestern Naturalist. 45(4): 385-420. [41226]
117. Hoffman, George R.; Alexander, Robert R. 1987. Forest vegetation of the Black Hills National Forest of South Dakota and Wyoming: a habitat type classification. Res. Pap. RM-276. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 48 p. [1181]
118. Holland, Marjorie M.; Burk, C. John. 1990. The marsh vegetation of three Connecticut River oxbows: a ten-year comparison. Rhodora. 92(871): 166-204. [14521]
119. Holzmueller, Eric J.; Jose, Shibu; Jenkins, Michael A. 2009. The response of understory species composition, diversity, and seedling regeneration to repeated burning in southern Appalachian oak-hickory forests. Natural Areas Journal. 29(3): 255-262. [81582]
120. Honu, Yohanes A. K.; Gibson, David J. 2008. Patterns of invasion: trends in abundance of understory vegetation, seed rain, and seed bank from forest edge to interior. Natural Areas Journal. 28(23): 228-239. [79161]
121. Hoover, David E.; Gipson, Philip S.; Pontius, Jeffrey S.; Hynek, Alan E. 2001. Short-term effects of cattle exclusion on riparian vegetation in southeastern Kansas. Transactions of the Kansas Academy of Science. 104(3/4): 212-222. [82467]
122. Hruska, Mary C.; Ebinger, John E. 1995. Monitoring a savanna restoration in east-central Illinois. Transactions of the Illinois State Academy of Science. 88(3 and 4): 109-117. [41436]
123. Huebner, Cynthia D.; Randolf, J. C.; Parker, G. R. 1995. Environmental factors affecting understory diversity in second-growth deciduous forests. The American Midland Naturalist. 134(1): 155-165. [84067]
124. Hunter, Carl G. 1989. Trees, shrubs, and vines of Arkansas. Little Rock, AR: The Ozark Society Foundation. 207 p. [21266]
125. Huntley, Jimmy C.; McGee, Charles E. 1981. Timber and wildlife implications of fire in young upland hardwoods. In: Barnett, James P., ed. Proceedings, 1st biennial southern silvicultural research conference; 1980 November 6-7; Atlanta, GA. Gen. Tech. Rep. SO-34. New Orleans, LA: U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station: 56-66. [12080]
126. Hurst, George A. 1978. Effects of controlled burning on wild turkey poult food habits. Proceedings, Annual Conference of Southeastern Association of Fish and Wildlife Agencies. 32: 30-37. [14648]
127. Hutchinson, Todd F.; Boerner, Ralph E. J.; Iverson, Louis R.; Sutherland, Steve; Sutherland, Elaine Kennedy. 1999. Landscape patterns of understory composition and richness across a moisture and nitrogen mineralization gradient in Ohio (U.S.A.) Quercus forests. Plant Ecology. 144(2): 177-189. [84100]
128. Jankovsky-Jones, Mabel; Rust, Steven K.; Moseley, Robert K. 1999. Riparian reference areas in Idaho: a catalog of plant associations and conservation sites. Gen. Tech. Rep. RMRS-GTR-20. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 141 p. [29900]
129. Johnson, Stephen R.; Young, Donald R. 1993. Factors contributing to the decline of Pinus taeda on a Virginia barrier island. Bulletin of the Torrey Botanical Club. 120(4): 431-438. [23602]
130. Johnston, Barry C. 1987. Plant associations of Region 2: Potential plant communities of Wyoming, South Dakota, Nebraska, Colorado, and Kansas. 4th ed. R2-ECOL-87-2. Lakewood, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Region. 429 p. [54304]
131. Jones, Robert H.; Sharitz, Rebecca R.; Dixon, Philip M.; Segal, Debra S.; Schneider, Rebecca L. 1994. Woody plant regeneration in four floodplain forests. Ecological Monographs. 64(3): 345-367. [23445]
132. Jones, Stanley D.; Wipff, Joseph K.; Montgomery, Paul M. 1997. Vascular plants of Texas. Austin, TX: University of Texas Press. 404 p. [28762]
133. Kearney, Thomas H.; Peebles, Robert H.; Howell, John Thomas; McClintock, Elizabeth. 1960. Arizona flora. 2nd ed. Berkeley, CA: University of California Press. 1085 p. [6563]
134. Khan, Nancy R.; Block, Timothy A.; Rhoads, Ann F. 2008. Vascular flora and community assemblages of Evansburg State Park, Montgomery County, Pennsylvania. Journal of the Torrey Botanical Society. 135(3): 438-458. [72478]
135. Klinkenberg, Brian, ed. 2010. E-Flora BC: Electronic atlas of the plants of British Columbia, [Online]. Vancouver, BC: University of British Columbia, Department of Geography, Lab for Advanced Spatial Analysis (Producer). Available: www.eflora.bc.ca [2012, January 3]. [54933]
136. Klopfer, Scott D.; Olivero, Adele; Sneddon, Lesley. 2002. Final report of the NPS vegetation mapping project at Fire Island National Seashore. Blacksburg, VA: Conservation Management Institute, GIS & Remote Sensing Division, Virginia Tech, College of Natural Resources. 193 p. [79626]
137. Klotz, Larry H. 1986. The vascular flora of Wallops Island and Wallops Mainland, Virginia. Castanea. 51(4): 306-326. [78279]
138. Kotar, John; Kovach, Joseph A.; Locey, Craig T. 1988. Field guide to forest habitat types of northern Wisconsin. Madison, WI: University of Wisconsin, Department of Forestry; Wisconsin Department of Natural Resources. 217 p. [11510]
139. Krefting, Laurits W.; Roe, Eugene I. 1949. The role of some birds and mammals in seed germination. Ecological Monographs. 19(3): 269-286. [8847]
140. Kudish, Michael. 1992. Adirondack upland flora: an ecological perspective. Saranac, NY: The Chauncy Press. 320 p. [19376]
141. Kush, John S.; Meldahl, Ralph S.; Boyer, William D. 2000. Understory plant community response to season of burn in natural longleaf pine forests. In: Moser, W. Keith; Moser, Cynthia F., eds. Fire and forest ecology: innovative silviculture and vegetation management: Proceedings of the 21st Tall Timbers fire ecology conference: an international symposium; 1998 April 14-16; Tallahassee, FL. No. 21. Tallahassee, FL: Tall Timbers Research: 32-39. [37606]
142. Laatsch, Janeen R.; Anderson, Roger C. 2000. An evaluation of oak woodland management in northeastern Illinois, USA. Natural Areas Journal. 20(3): 211-220. [36106]
143. Ladwig, Laura M.; Meiners, Scott J. 2010. Spatiotemporal dynamics of lianas during 50 years of succession to temperate forest. Ecology. 91(3): 671-680. [80628]
144. Lakela, O. 1965. A flora of northeastern Minnesota. Minneapolis, MN: University of Minnesota Press. 541 p. [18142]
145. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: http://www.landfire.gov/downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
146. LANDFIRE Rapid Assessment. 2007. Rapid assessment reference condition models, [Online]. In: LANDFIRE. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; The Nature Conservancy (Producers). Available: http://www.landfire.gov/models_EW.php [2008, April 18] [66533]
147. Laudenslager, Scott L.; Flake, Lester D. 1987. Fall food habits of wild turkeys in south central South Dakota. Prairie Naturalist. 19(1): 37-40. [251]
148. Lauver, Chris L.; Kindscher, Kelly; Faber-Langendoen, Don; Schneider, Rick. 1999. A classification of the natural vegetation of Kansas. The Southwestern Naturalist. 44(4): 421-443. [38847]
149. Leicht-Young, Stacey A.; Pavlovic, Noel B.; Grundel, Ralph; Frohnapple, Krystalynn J. 2009. A comparison of seed banks across a sand dune successional gradient at Lake Michigan dunes (Indiana, USA). Plant Ecology. 202(2): 299-308. [84042]
150. Lhotka, John M.; Zaczek, James J. 2003. Soil scarification effects on oak reproduction in two mixed-oak bottomland stands of southern Illinois. Southern Journal of Applied Forestry. 27(3): 164-171. [45557]
151. Lichter, John. 1998. Primary succession and forest development on coastal Lake Michigan sand dunes. Ecological Monographs. 68(4): 487-510. [29313]
152. Londre, Ronald A.; Schnitzer, Stefan A. 2006. The distribution of lianas and their change in abundance in temperate forests over the past 45 years. Ecology. 87(12): 297-2978. [81997]
153. Loope, Lloyd; Duever, Michael; Herndon, Alan; Snyder, James; Jansen, Deborah. 1994. Hurricane impact on uplands and freshwater swamp forest. Bioscience. 44(4): 238-246. [81132]
154. Lubinski, Sara; Hop, Kevin; Gawler, Susan. 2003. U.S. Geological Survey-National Park Service Vegetation Mapping Program: Acadia National Park, Maine. Final report. [Revised edition]. La Crosse, WI: U.S. Department of the Interior, U.S. Geological Survey, Upper Midwest Environmental Studies Center. 50 p. [+ appendices]. Available online: http://biology.usgs.gov/npsveg/acad/acadrpt.pdf [2012, January 12]. [79619]
155. Luginbuhl, J-M.; Harvey, T. E.; Green, J. T., Jr.; Poore, M. H.; Mueller, J. P. 1999. Use of goats as biological agents for the renovation of pastures in the Appalachian region of the United States. Agroforestry Systems. 44(2-3): 241-252. [41447]
156. Magee, Dennis W.; Ahles, Harry E. 2007. Flora of the Northeast: A manual of the vascular flora of New England and adjacent New York. 2nd ed. Amherst, MA: University of Massachusetts Press. 1214 p. [74293]
157. Matlack, Glenn R.; Good, Ralph E. 1990. Spatial heterogeneity in the soil seed bank of a mature coastal plain forest. Bulletin of the Torrey Botanical Club. 117(2): 143-152. [22905]
158. Maycock, P. F.; Curtis, J. T. 1960. The phytosociology of boreal conifer-hardwood forests of the Great Lakes region. Ecological Monographs. 30(1): 1-36. [62820]
159. McClain, William E.; Edgin, Bobby R.; Esker, Terry L.; Ebinger, John E. 2007. Two closed-canopy barren plant communities in east-central Illinois. Northeastern Naturalist. 14(1): 35-50. [84091]
160. McKinley, Carol E.; Day, Frank P., Jr. 1979. Herbaceous production in cut-burned, uncut-burned, and control areas of a Chamaecyparis thyoides (L.) BSP (Cupressaceae) stand in the Great Dismal Swamp. Bulletin of the Torrey Botanical Club. 106(1): 20-28. [41796]
161. McNair, James B. 1923. Rhus dermatitis. Chicago, IL: University of Chicago Press. 298 p. [19527]
162. Medina, Alvin L. 1987. Woodland communities and soils of Fort Bayard, southwestern New Mexico. Journal of the Arizona-Nevada Academy of Science. 21(2): 99-112. [3978]
163. Mehlman, David W. 1992. Effects of fire on plant community composition of north Florida second growth pineland. The Journal of the Torrey Botanical Society. 119(4): 376-383. [84038]
164. Miller, James H.; Boyd, Robert S.; Edwards, M. Boyd. 1999. Floristic diversity, stand structure, and composition 11 years after herbicide site preparation. Canadian Journal of Forest Research. 29(7): 1073-1083. [38475]
165. Milne, Bruce T.; Forman, Richard T. 1986. Peninsulas in Maine: woody plant diversity, distance, and environmental patterns. Ecology. 67(4): 967-974. [4557]
166. Mohan, Jacqueline E.; Ziska, Lewis H.; Schlesinger, William H.; Thomas, Richard B.; Sicher, Richard C.; George, Kate; Clark, James S. 2006. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proceedings of the National Academy of Sciences. 103(24): 9086-9089. [84050]
167. Mohan, Jacqueline E.; Ziska, Lewis H.; Thomas, Richard B.; Sischer, Richard C.; George, Kate; Clark, James S.; Schlesinger, William H. 2008. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2: reply. Ecology. 89(2): 585-587. [84103]
168. Mohlenbrock, Robert H. 1986. Guide to the vascular flora of Illinois. [Revised edition]. Carbondale, IL: Southern Illinois University Press. 507 p. [17383]
169. Monk, Carl D.; Brown, Timothy W. 1965. Ecological consideration of cypress heads in north-central Florida. The American Midland Naturalist. 74(1): 126-140. [10848]
170. Muldavin, Esteban; Durkin, Paula; Bradley, Mike; Stuever, Mary; Mehlhop, Patricia. 2000. Handbook of wetland vegetation communities of New Mexico. Volume 1: classification and community descriptions. Albuquerque, NM: University of New Mexico, Biology Department; New Mexico Natural Heritage Program. 172 p. [+ appendices]. [45517]
171. Mulligan, Gerald A.; Junkins, Bonny E. 1977. The biology of Canadian weeds. 23. Rhus radicans L. Canadian Journal of Plant Science. 57(2): 515-523. [83869]
172. Nagel, Harold G.; Rothenberger, Steven. 1999. Response of wetland plants to groundwater depth on the Middle Loup River, Nebraska. In: Springer, J. T., ed. The central Nebraska Loess Hills prairie: Proceedings of the 16th North American prairie conference; 1998 July 26-29; Kearney, NE. No. 16. Kearney, NE: University of Nebraska: 216-225. [46834]
173. NatureServe. 2002. International classification of ecological communities: Terrestrial ecological vegetation of the United States, National Forests in Florida final report. Atlanta, GA; Biological Conservation Datasystem. 237 p. [79607]
174. NatureServe. 2004. International ecological classification standard: terrestrial ecological classifications--National Forests of Arkansas (Ouchita, Ozark, St. Francis) final report. NatureServe Central Databases. Arlington, VA: NatureServe; Durham, NC: NatureServe Ecology South. 196 p. Available online: http://www.natureserve.org/library/arNF.pdf [2011, September 8]. [79606]
175. NatureServe. 2004. International ecological classification standard: terrestrial ecological classifications. Croatan National Forest final report. NatureServe Central Databases. Arlington, VA: NatureServe; Durham, NC: NatureServe Ecology South. 105 p. Available: http://www.natureserve.org/library/croatanNF.pdf [2012, January 12]. [79629]
176. NatureServe. 2004. International ecological classification standard: terrestrial ecology classifications, Nantahala-Pisgah National Forests final report. Arlington, VA: NatureServe. 195 p. Available online: http://www.natureserve.org/library/nantPisgNF.pdf [2011, September 7]. [79631]
177. Naumann, Julie C.; Young, Donald R. 2007. Relationship between community structure and seed bank to describe successional dynamics of an Atlantic Coast maritime forest. Journal of the Torrey Botanical Society. 134(1): 89-98. [68945]
178. Nelson, Jack Raymond. 1961. Composition and structure of the principal woody vegetation types in the North Dakota Badlands. Fargo, ND: North Dakota State University. 195 p. Thesis. [161]
179. Nessel, John K.; Bayley, Suzanne E. 1984. Distribution and dynamics of organic matter and phosphorus in a sewage- enriched cypress swamp. In: Ewel, Katherine Carter; Odum, Howard T., eds. Cypress swamps. Gainesville, FL: University of Florida Press: 262-278. [14851]
180. Neumann, David D.; Dickmann, Donald I. 2001. Surface burning in a mature stand of Pinus resinosa and Pinus strobus in Michigan: effects on understory vegetation. International Journal of Wildland Fire. 10(1): 91-101. [40201]
181. Nixon, Charles M.; McClain, Milford W.; Russell, Kenneth R. 1970. Deer food habits and range characteristics in Ohio. The Journal of Wildlife Management. 34(4): 870-886. [16398]
182. Noble, Daniel L.; Winokur, Robert P., eds. 1984. Wooded draws: characteristics and values for the Northern Great Plains: Symposium proceedings; 1984 June 12-13; Rapid City, South Dakota. Great Plains Agricultural Council Publication No. 111. Rapid City, SD: South Dakota School of Mines and Technology; 1984. 52 p. [1762]
183. Noble, Robert E.; Murphy, Patrick K. 1975. Short term effects of prolonged backwater flooding on understory vegetation. Castanea. 40(3): 228-238. [84069]
184. Olivero, Adele M.; Hix, David M. 1998. Influence of aspect and stand age on ground flora of southeastern Ohio forest ecosystems. Plant Ecology. 139(2): 177-187. [84039]
185. Oosting, H. J.; Livingston, R. B. 1964. A resurvey of a loblolly pine community twenty-nine years after ground and crown fire. Bulletin of the Torrey Botanical Club. 91(5): 387-395. [39020]
186. Oosting, Henry J. 1944. The comparative effect of surface and crown fire on the composition of a loblolly pine community. Ecology. 25(1): 61-69. [9919]
187. Pacific Northwest Extension Service. 1983. Poison oak and ivy. PNW 108. Corvallis, OR: Pacific Northwest Extention Service. 4 p. [6613]
188. Palmer, M. W.; Rusch, G. M. 2001. How fast is the carousel? Direct indices of species mobility with examples from an Oklahoma grassland. Journal of Vegetation Ecology. 12(3): 305-318. [43386]
189. Palmer, Michael W.; McAlister, Suzanne D.; Arevalo, Jose Ramon; DeCoster, James K. 2000. Changes in the understory during 14 years following catastrophic windthrow in two Minnesota forests. Journal of Vegetation Science. 11(6): 841-854. [42541]
190. Patterson, Karen D. 2008. Vegetation classification and mapping at Appomattox Court House National Historical Park, Virginia. Technical Report NPS/NER/NRTR--2008/125. Philadelphia, PA: U.S. Department of the Interior, National Park Service, Northeast Region. 261 p. [78026]
191. Patterson, Karen D. 2008. Vegetation classification and mapping at Colonial National Historical Park, Virginia. Technical Report NPS/NER/NRTR--2008/129. Philadelphia, PA: U.S. Department of the Interior, National Park Service, Northeast Region. 369 p. [79670]
192. Paulsell, Lee K. 1957. Effects of burning on Ozark hardwood timberlands. Res. Bull. 640. Columbia, MO: University of Missouri, College of Agriculture, Agricultural Experiment Station. 24 p. [11885]
193. Pendleton, Rosemary L.; Pendleton, Burton K.; Harper, Kimball T. 1989. Breeding systems of woody plant species in Utah. In: Wallace, Arthur; McArthur, E. Durant; Haferkamp, Marshall R., comps. Proceedings--symposium on shrub ecophysiology and biotechnology; 1987 June 30 - July 2; Logan, UT. Gen. Tech. Rep. INT-256. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 5-22. [5918]
194. Penner, Rodney; Moodie G. Eric E.; Staniforth, Richard J. 1999. The dispersal of fruits and seeds of poison-ivy, Toxicodendron radicans, by ruffed grouse, Bonasa umbellus, and squirrels, Tamiasciurus hudsonicus and Sciurus carolinensis. The Canadian Field-Naturalist. 113(4): 616-620. [78447]
195. Perles, Stephanie J.; Podniesinski, Gregory S.; Eastman, E.; Sneddon, Lesley A.; Gawler, Sue C. 2007. Classification and mapping of vegetation and fire fuel models at Delaware Water Gap National Recreation Area: Volume 2 of 2--Appendix G, [Online]. Technical Report NPS/NER/NRTR--2007/076. Philadelphia, PA: U.S. Department of the Interior, National Park Service, Northeast Region, Natural Resource Stewardship and Science (Producer). 400 p. Available: http://www.nps.gov/nero/science/FINAL/DEWA_veg_map/DEWA_veg_map.htm [2010, March 3]. [79090]
196. Perry, Roger W.; Thill, Ronald E.; Peitz, David G.; Tappe, Philip A. 1999. Effects of different silvicultural systems on initial soft mast production. Wildlife Society Bulletin. 27(4): 915-923. [39046]
197. Peterson, David Wassell. 1998. Fire effects on oak savanna and woodland vegetation in Minnesota. Minneapolis, MN: University of Minnesota. 130 p. Dissertation. [82453]
198. Peterson, Eric B. 2008. International vegetation classification alliances and associations occurring in Nevada with proposed additions. Carson City, NV: Nevada Natural Heritage Program. 347 p. Available online: http://heritage.nv.gov/reports/ivclist.pdf [2011, July 18]. [77864]
199. Phillips, R. J.; Waldrop, T. A. 2008. Changes in vegetation structure and composition in response to fuel reduction treatments in the South Carolina Piedmont. Forest Ecology and Management. 255(8-9): 3107-3116. [74333]
200. Pickett, S. T. A. 1982. Population patterns through twenty years of oldfield succession. Vegetatio. 49(1): 45-59. [80602]
201. Podniesinski, Gregory S.; Sneddon, Lesley A.; Lundgren, Julie; Devine, Hugh; Slocumb, Bill; Koch, Frank. 2005. Vegetation classification and mapping of Valley Forge National Historical Park. Technical Report NPS/NER/NRTR--2005/028. Philadelphia, PA: U.S. Department of the Interior, National Park Service, Northeast Region. 115 p. Available online: http://biology.usgs.gov/npsveg/vafo/vaforpt.pdf [2012, January 12]. [79639]
202. Powell, A. Michael. 1988. Trees and shrubs of Trans-Pecos Texas: Including Big Bend and Guadalupe Mountains National Parks. Big Bend National Park, TX: Big Bend Natural History Association. 536 p. [6130]
203. Quarterman, Elsie. 1957. Early plant succession on abandoned cropland in the central basin of Tennessee. Ecology. 38(2): 300-309. [77156]
204. Radford, Albert E.; Ahles, Harry E.; Bell, C. Ritchie. 1968. Manual of the vascular flora of the Carolinas. Chapel Hill, NC: The University of North Carolina Press. 1183 p. [7606]
205. Ralston, Robert Dean. 1960. The structure and ecology of the north slope juniper stands of the Little Missouri Badlands. Salt Lake City, UT: University of Utah. 85 p. Thesis. [192]
206. Ramaley, Francis. 1939. Sand-hill vegetation of northeastern Colorado. Ecological Monographs. 9(1): 1-51. [5546]
207. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
208. Reed, Porter B., Jr. 1986. 1986 wetland plant list, Montana. St. Petersburg, FL: U.S. Department of the Interior, Fish and Wildlife Service, National Wetlands Inventory. 26 p. [8381]
209. Renecker, Lyle A.; Schwartz, Charles C. 2007. Food habits and feeding behavior. In: Franzmann, Albert W.; Schwartz, Charles C.; McCabe, Richard E., eds. Ecology and management of the North American moose. 2nd ed. Boulder, CO: University Press of Colorado: 403-440. [79106]
210. Reschke, Carol. 1990. Ecological communities of New York State. Latham, NY: New York State Department of Environmental Conservation, Natural Heritage Program. 96 p. [21441]
211. Richter, Rebecca; Stromberg, Juliet C. 2005. Soil seed banks of two montane riparian areas: implications for restoration. Biodiversity and Conservation. 14(4): 993-1016. [60044]
212. Rideout, Sandra; Oswald, Brian P. 2002. Effects of prescribed burning on vegetation and fuel loading in three East Texas state parks. Texas Journal of Science. 54(3): 211-226. [43757]
213. Robertson, Philip A.; Weaver, George T.; Cavanaugh, James A. 1978. Vegetation and tree species patterns near the northern terminus of the southern floodplain forest. Ecological Monographs. 48(3): 249-267. [10381]
214. Roland, A. E.; Smith, E. C. 1969. The flora of Nova Scotia. Halifax, NS: Nova Scotia Museum. 746 p. [13158]
215. Royer, France; Dickinson, Richard. 1999. Weeds of the northern U.S. and Canada: a guide for identification. Edmonton, AB: The University of Alberta Press; Renton, WA: Lone Pine Publishing. 434 p. [52727]
216. Schafale, Michael P.; Weakley, Alan S. 1990. Classification of the natural communities of North Carolina: 3rd approximation. Raleigh, NC: Department of Environment, Health, and Natural Resources, Division of Parks and Recreation, North Carolina Natural Heritage Program. 325 p. Available online: http://www.ncnhp.org/Images/Other%20Publications/class.pdf [2011, August 29]. [41937]
217. Scheiner, Samuel M.; Teeri, James A. 1981. A 53-year record of forest succession following fire in northern lower Michigan. Michigan Botanist. 20(1): 3-14. [5022]
218. Schiff, Nathan M.; Connor, Kristina F.; Devall, Margaret S. 2004. Germination conditions for poison ivy. In: Connor, Kristina F., ed. Proceedings of the 12th biennial southern silvicultural research conference; 2003 February 24-28; Biloxi, MS. Gen. Tech. Rep. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station Service: 531-532. [83729]
219. Schlesinger, William H. 1978. On the relative dominance of shrubs in Okefenokee Swamp. The American Naturalist. 112(987): 949-954. [15360]
220. Schneider, Rebecca L.; Sharitz, Rebecca R. 1988. Hydrochory and regeneration in a bald cypress-water tupelo swamp forest. Ecology. 69(4): 1055-1063. [84097]
221. Schnitzer, Stefan A.; Londre, Ronald A.; Klironomos, John; Reich, Peter B. 2008. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2: comment. Ecology. 89(2): 581-585. [84102]
222. Schuler, Jamie L.; Liechty, Hal O. 2008. Seed bank emergence following prescribed burning in the Ozark Highlands. In: Jacobs, Douglass F.; Michler, Charles H., eds. Proceedings; 16th central hardwood forest conference; 2008 April 8-9; West Lafayette, IN. Gen. Tech. Rep. NRS-P-24. Newton Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station: 516-524. [81555]
223. Scoggan, H. J. 1978. The flora of Canada. Part 3: Dicotyledoneae (Saururaceae to Violaceae). National Museum of Natural Sciences: Publications in Botany, No. 7(3). Ottawa: National Museums of Canada. 1115 p. [75493]
224. Senchina, David S.; Summerville, Keith S. 2007. Great diversity of insect floral associates may partially explain ecological success of poison ivy (Toxicodendron radicans subsp. negundo [Greene] Gillis, Anacardiaceae). The Great Lakes Entomologist. 40(3/4): 120-128. [83728]
225. Shotola, Steven J.; Weaver, G. T.; Robertson, P. A.; Ashby, W. C. 1992. Sugar maple invasion of an old-growth oak-hickory forest in southwestern Illinois. The American Midland Naturalist. 127(1): 125-138. [84041]
226. Sidhu, S. S. 1973. Early effects of burning and logging in pine-mixedwoods. I. Frequency and biomass of minor vegetation. Information Report PS-X-46. Chalk River, ON: Canadian Forestry Service, Petawawa Forest Experiment Station. 47 p. [7901]
227. Sieg, Carolyn Hull; Wright, Henry A. 1996. The role of prescribed burning in regenerating Quercus macrocarpa and associated woody plants in stringer woodlands in the Black Hills, South Dakota. International Journal of Wildland Fire. 6(1): 21-29. [26769]
228. Singhurst, Jason R.; Cathy, James C.; Prochaska, Dale; Haucke, Hayden; Kroh, Glenn C.; Holmes, Walter C. 2003. The vascular flora of Gus Engeling Wildlife Management Area, Anderson County, Texas. Southeastern Naturalist. 2(3): 347-368. [76708]
229. Skeate, Stewart T. 1987. Interactions between birds and fruits in a northern Florida hammock community. Ecology. 68(2): 297-309. [84043]
230. Small, Christine J.; McCarthy, Brian C. 2001. Vascular flora of the Waterloo Wildlife Research Station, Athens County, Ohio. Castanea. 66(4): 363-382. [71703]
231. Smith, G. F.; Nicholas, N. S.; Zedaker, S. M. 1997. Succession dynamics in a maritime forest following Hurricane Hugo and fuel reduction burns. Forest Ecology and Management. 95(3): 275-283. [28017]
232. Smith, Jane Kapler; Zouhar, Kristin; Sutherland, Steve; Brooks, Matthew L. 2008. Fire and nonnative invasive plants--introduction. In: Zouhar, Kristin; Smith, Jane Kapler; Sutherland, Steve; Brooks, Matthew L., eds. Wildland fire in ecosystems: fire and nonnative invasive plants. Gen. Tech. Rep. RMRS-GTR-42-vol. 6. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 1-6. [70898]
233. Smith, Kimberly G.; Mlodinow, Michael; Self, Janet S.; Haggerty, Thomas M.; Hocut, Tamara R. 2004. Birds of upland oak forests in the Arkansas Ozarks: present community structure and potential impacts of burning, borers, and forestry practices. In: Spetich, Marin A., ed. Upland oak ecology symposium: history, current conditions, and sustainability: Proceedings; 2002 October 7-10; Fayetteville, AR. Gen. Tech. Rep. SRS-73. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station: 243-252. [60870]
234. Soper, James H.; Heimburger, Margaret L. 1982. Shrubs of Ontario. Life Sciences Miscellaneous Publications. Toronto, ON: Royal Ontario Museum. 495 p. [12907]
235. Sotala, Dennis J.; Kirkpatrick, Charles M. 1973. Foods of white-tailed deer, Odocoileus virginianus, in Martin County, Indiana. The American Midland Naturalist. 89(2): 281-286. [15056]
236. Sparks, Jeffrey C.; Masters, Ronald E.; Engle, David M.; Palmer, Michael W.; Bukenhofer, George A. 1998. Effects of late growing-season and late dormant-season prescribed fire on herbaceous vegetation in restored pine-grassland communities. Journal of Vegetation Science. 9(1): 133-142. [28995]
237. Sparks, Jeffrey C.; Masters, Ronald E.; Engle, David M.; Payton, Mark E.; Bukenhofer, George A. 1999. Influence of fire season and fire behavior on woody plants in red-cockaded woodpecker clusters. Wildlife Society Bulletin. 27(1): 124-133. [41831]
238. Stallard, Harvey. 1929. Secondary succession in the climax forest formations of northern Minnesota. Ecology. 10(4): 476-547. [3808]
239. Stambaugh, Michael C.; Muzika, Rose-Marie; Guyette, Richard P. 2002. Disturbance characteristics and overstory composition of old-growth shortleaf pine (Pinus echinata Mill.) forest in the Ozark Highlands, Missouri, USA. Natural Areas Journal. 22(2): 108-119. [46104]
240. Stephens, H. A. 1973. Woody plants of the north Central Plains. Lawrence, KS: The University Press of Kansas. 530 p. [3804]
241. Stevens, O. A. 1956. Flowering dates of weeds in North Dakota. North Dakota Agricultural Experiment Station Bimonthly Bulletin. 18(6): 209-213. [5168]
242. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in Northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
243. Stieber, Michael T. 1971. The vascular flora of Anne Arundel County, Maryland: an annotated checklist. Castanea. 36(4): 263-312. [75361]
244. Strausbaugh, P. D.; Core, Earl L. 1977. Flora of West Virginia. 2nd ed. Morgantown, WV: Seneca Books. 1079 p. [23213]
245. Stubbendieck, James; Coffin, Mitchell J.; Landholt, L. M. 2003. Weeds of the Great Plains. 3rd ed. Lincoln, NE: Nebraska Department of Agriculture, Bureau of Plant Industry. 605 p. In cooperation with: University of Nebraska, Lincoln. [50776]
246. Stuever, Mary C.; Hayden, John S. 1996. Plant associations (habitat types) of the forests and woodlands of Arizona and New Mexico. Final report: Contract R3-95-27. Placitas, NM: Seldom Seen Expeditions. 520 p. [28868]
247. Suiter, Dale W.; Evans, Dan K. 1999. Vascular flora and rare species of New River Gorge National River, West Virginia. Castanea. 64(1): 23-49. [71705]
248. Sutherland, Steve; Hutchinson, Todd F.; Windus, Jennifer L. 2003. Understory vegetation. In: Sutherland, Elaine Kennedy; Hutchinson, Todd F., eds. Characteristics of mixed-oak forest ecosystems in southern Ohio prior to the reintroduction of fire. Gen. Tech. Rep. NE-299. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northeastern Research Station: 67-83. [43587]
249. Swihart, Robert K. 1990. Common components of orchard ground cover selected as food by captive woodchucks. The Journal of Wildlife Management. 54(3): 412-417. [80420]
250. Taft, John B. 2003. Composition and structure of an old-growth floodplain forest of the lower Kaskaskia River. In: Van Sambeek, J. W.; Dawson, J. O.; Ponder, F., Jr.; Loewenstein, E. F.; Fralish, J. S., eds. Proceedings, 13th central hardwood forest conference; 2002 April 1-3; Urbana, IL. Gen. Tech. Rep. NC-234. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Research Station: 146-158. [51222]
251. Taft, John B. 2003. Fire effects on community structure, composition, and diversity in a dry sandstone barrens. Journal of the Torrey Botanical Society. 130(3): 170-192. [52070]
252. Taft, John B. 2005. Fire effects on structure, composition, and diversity in a south-central Illinois flatwoods remnant. Castanea. 70(4): 298-313. [61618]
253. Talley, Sharon M.; Lawton, Robert O.; Setzer, William N. 1996. Host preferences of Rhus radicans (Anacardiaceae) in a southern deciduous hardwood forest. Ecology. 77(4): 1271-1276. [84045]
254. Taverna, Kristin. 2008. Vegetation classification and mapping at Fredericksburg and Spotsylvania National Military Park. Technical Report NPS/NER/NRTR--2008/126. Philadelphia, PA: U.S. Department of the Interior, National Park Service, Northeast Region. 277 p. [79671]
255. Terrel, Ted L. 1972. The swamp rabbit (Sylvilagus aquaticus) in Indiana. The American Midland Naturalist. 87(2): 283-295. [63017]
256. Thatcher, Benjamin Stephen. 2007. Evaluation of forest management to improve breeding habitat for songbirds in oak-hickory forests at Tennessee National Wildlife Refuge. Knoxville, TN: University of Tennessee. 256 p. Dissertation. [84098]
257. The Nature Conservancy. 1995. USGS-NPS vegetation mapping program: Vegetation classification of Assateague Island National Seashore. Arlington, VA: The Nature Conservancy. Variously paginated. [79668]
258. Thompson, Ralph L.; Fleming, Chris A. 2004. Vascular flora and plant communities of the John B. Stephenson Memorial Forest State Nature Preserve (Anglin Falls Ravine), Rockcastle County, Kentucky. Castanea. 69(2): 125-138. [71702]
259. Thompson, William H.; Hansen, Paul L. 2002. Classification and management of riparian and wetland sites of the Alberta Grassland Natural Region and adjacent subregions. Cows and Fish Report No. 018. Lethbridge, AB: Alberta Riparian Habitat Management Program, Cows and Fish. 416 p. [82587]
260. Thomson, Paul M.; Anderson, Roger C. 1976. An ecological investigation of the Oakwood Bottoms Greentree Reservoir in Illinois. In: Fralish, James S.; Weaver, George T.; Schlesinger, Richard C., eds. Central hardwood forest conference: Proceedings of a meeting; 1976 October 17-19; Carbondale, IL. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Research Station: 45-64. [3812]
261. Tolstead, W. L. 1942. Vegetation of the northern part of Cherry County, Nebraska. Ecological Monographs. 12(3): 255-292. [4470]
262. U.S. Department of Agriculture, Forest Service. 1937. Range plant handbook. Washington, DC: U.S. Department of Agriculture, Forest Service 532 p. [2387]
263. U.S. Department of Agriculture, Natural Resources Conservation Service. 2012. PLANTS Database, [Online]. Available: http://plants.usda.gov/. [34262]
264. Vankat, John L.; Snyder, Gary W. 1991. Floristics of a chronosequence corresponding to old field--deciduous forest succession in southwestern Ohio. I. Undisturbed vegetation. Bulletin of the Torrey Botanical Club. 118(4): 365-376. [18758]
265. Vincent, Gilles; Bergeron, Yves; Meilleur, Alain. 1986. Plant community pattern analysis: a cartographic approach applied in the Lac des Deux-Montagnes area (Quebec). Canadian Journal of Botany. 64(2): 326-335. [16948]
266. Vines, Robert A. 1960. Trees, shrubs, and woody vines of the Southwest. Austin, TX: University of Texas Press. 1104 p. [7707]
267. Vogl, Richard John. 1961. The effects of fire on some upland vegetation types. Madison, WI: University of Wisconsin. 154 p. Dissertation. [52282]
268. Voss, Edward G. 1985. Michigan flora. Part II. Dicots (Saururaceae--Cornaceae). Bulletin 59. Bloomfield Hills, MI: Cranbrook Institute of Science; Ann Arbor, MI: University of Michigan Herbarium. 724 p. [11472]
269. Wade, Dale D.; Langdon, O. Gordon. 1990. Sabal palmetto (Walt.) Lodd. ex J. A. & J. H. Schult. cabbage palmetto. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Vol. 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 762-767. [13806]
270. Weaver, George T.; Ashby, William C. 1971. Composition and structure of an old-growth forest remnant in unglaciated southwestern Illinois. The American Midland Naturalist. 86(1): 46-56. [84104]
271. Weaver, J. E.; Albertson, F. W. 1936. Effects of the great drought on the prairies of Iowa, Nebraska, and Kansas. Ecology. 17(4): 567-639. [84101]
272. Weber, M. G. 1990. Response of immature aspen ecosystems to cutting and burning in relation to vernal leaf-flush. Forest Ecology and Management. 31(1-2): 15-33. [10373]
273. Weber, M. G. 1991. Aspen management options using fire or cutting. Information Report PI-X-100. Chalk River, ON: Forestry Canada, Petawawa National Forestry Institute. 11 p. [17250]
274. Welsh, S. L.; Atwood, N. D.; Goodrich, S.; Neese, E.; Thorne, K. H.; Albee, Beverly. 1981. Preliminary index of Utah vascular plant names. The Great Basin Naturalist. 41(1): 1-108. [11499]
275. Welsh, Stanley L.; Atwood, N. Duane; Goodrich, Sherel; Higgins, Larry C., eds. 1987. A Utah flora. The Great Basin Naturalist Memoir No. 9. Provo, UT: Brigham Young University. 894 p. [2944]
276. Whigham, Dennis. 1984. The influence of vines on the growth of Liquidambar styraciflua L. (sweetgum). Canadian Journal of Forest Research. 14(1): 37-39. [15865]
277. White, Alan S. 1983. The effects of thirteen years of annual prescribed burning on a Quercus ellipsoidalis community in Minnesota. Ecology. 64(5): 1081-1085. [3518]
278. Wiggins, Ira L. 1980. Flora of Baja California. Stanford, CA: Stanford University Press. 1025 p. [21993]
279. Williamson, Penelope. 1971. Feeding ecology of the red-eyed vireo (Vireo olivaceous) and associated foliage-gleaning birds. Ecological Monographs. 41(2): 129-152. [8103]
280. Wilson, Roger E. 1970. Succession in stands of Populus deltoides along the Missouri River in southeastern South Dakota. The American Midland Naturalist. 83(2): 330-342. [25441]
281. Wofford, B. Eugene. 1989. Guide to the vascular plants of the Blue Ridge. Athens, GA: The University of Georgia Press. 384 p. [12908]
282. Wunderlin, R. P.; Hansen, B. F. 2008. Atlas of Florida vascular plants, [Online]. In: PlantAtlas.org. Tampa, FL: University of South Florida, Institute for Systematic Botany (Producer). Available: http://www.florida.plantatlas.usf.edu/ [2009, October 15]. [54934]
283. Yatskievych, George. 1999. Steyermark's flora of Missouri. Vol. 2. [Revised edition]. Jefferson City, MO: The Missouri Department of Conservation. 1181 p. In cooperation with: The Missouri Botanical Garden Press. [83141]
284. Yurkonis, Kathryn A.; Meiners, Scott J. 2006. Drought impacts and recovery are driven by local variation in species turnover. Plant Ecology. 184(2): 325-336. [80706]
285. Zhang, Jianhua; Maun, M. Anwar. 1994. Potential for seed bank formation in seven Great Lakes sand dune species. American Journal of Botany. 81(4): 387-394. [23033]