| FEIS Home Page |
 |
|
| Water birch growing along Huntington Creek in Huntington County, Utah. Photo © Tony Frates, Intermountain Region Herbarium Network |
AUTHORSHIP AND CITATION:
Gucker, Corey. 2012. Betula occidentalis.
In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: https://www.fs.usda.gov
/database/feis/plants/tree/betocc/all.html
[].
FEIS ABBREVIATION:
BETOCC
COMMON NAMES:
water birch
mountain birch
river birch
western birch
TAXONOMY:
The scientific name of water birch is Betula occidentalis Hook.
(Betulaceae) [31,61].
Hybrids: Water birch hybridizes with other western North American birch species, resulting in the following hybrids:
Eastwood's birch (Betula × eastwoodae) Sarg., a water birch × bog birch (Betula glandulosa) hybrid [23,117]
Northwestern paper birch (Betula × utahensis) Britton, a water birch
× paper birch (Betula papyrifera) hybrid [23,31,102,117,140]
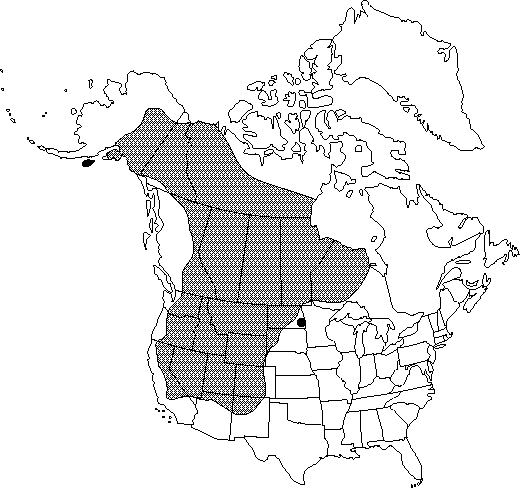 |
| North American distribution for water birch. Map courtesy of the Flora of North America Association. 2012, 21 May. |
Water birch occurs primarily in western North America. Populations are especially common throughout the Rocky Mountains and reach as far east as northwestern Ontario [31]. Water birch does not occur east of 82° W in Canada [123].
Distribution of water birch is much more sporadic than the map above suggests. Populations of water birch are spotty throughout the Intermountain West, but more continuous in the aspen parklands of central Canada [140] and more frequent in the northern and central Cordillera [68]. In the Pacific Northwest, water birch is generally confined to areas east of the Coast Ranges in British Columbia and the Cascade Range in Oregon and Washington [4,49]. It is considered the most common birch in eastern Washington, eastern Oregon, and Idaho [46,102]. Water birch occurs primarily within the prairie region in Saskatchewan [10] and western Montana [8]. In Colorado, populations are scattered in the mountains [44]. Water birch is the only native birch in Utah, New Mexico, and Arizona [58,79]. It occurs throughout Utah [139] but is most common along streams connecting mountain and desert regions [73], and in Tooele and Juab counties it is restricted to the Deep Creek Mountains [27]. In Nevada, water birch is most common in mountain ranges in the central part of the state [62]. In California, water birch populations are widely disjunct. Populations occur in the Sierra Nevada in Mono, Inyo, Tulare, Kings, and Fresno counties and in north-central California [36,114]. Water birch populations are most abundant on the eastern side of the Sierra Nevada and only occasional at high-elevation sites within the Mojave Desert [36].
States and provinces (as of 2012 [131]):
United States: AK, AZ, CA, CO, ID, MT, ND, NE, NM, NV, OR, SD, UT, WA, WY
Canada: AB, BC, MB, NT, NU, ON, SK, YT
The western North American distributions for water birch hybrids (Eastwood's birch and Northwestern paper birch) are described by Dugle [23].
SITE CHARACTERISTICS AND PLANT COMMUNITIES:
Site characteristics: Water birch is primarily a riparian species and occurs near waterways or other moist sites such as wet swales, marshes, ravines, bogs, or moist open woods [4,21,31,35,139]. Water birch is common along streams in steep areas of the Intermountain West. In the Sawtooth National Forest of south-central Idaho, the water birch/red-osier dogwood (Cornus sericea) association occurred along high-gradient streams with coarse-textured soils [56]. In the mountains, valleys, and foothills of Montana, water birch-dominated community types occur along high-gradient, fast-moving streams at low- to mid-elevation sites. Water birch communities were particularly common along the upper Clark Fork and lower Blackfoot rivers and other high-gradient streams in southwestern Montana and along the Rocky Mountain Front [42]. Carlson and others [12] found water birch in narrow bands of riparian vegetation along streams described as erosional fluvial canyons, characterized by high-gradient, high-velocity, low- to mid-order streams in confined, v-shaped canyons in the Intermountain region. In Nevada and eastern California, water birch community types were most common along large, steep streams with aerated flows [83,84].
Soil and moisture: Coarse-textured, moist to wet soils are most common in water birch habitats [127]. The water birch community type in Montana occurs on loamy to sandy soils with abundant gravel and water tables near the soil surface throughout summer. Coarse-textured soils provide for the rapid movement of highly aerated ground water. Water birch is generally absent from heavy clay soils [40,42]. Low-statured water birch is abundant in the water birch-red-osier dogwood community type on coarse-loamy soils along streams in New Mexico [96]. In British Columbia, water birch was most frequent at wet to very wet sites [68]. When undiverted (natural flow) and diverted (low flow) reaches of Bishop Creek in eastern Sierra Nevada were compared, adult and juvenile water birch experienced greater water stress on diverted than undiverted reaches. On diverted reaches, water birch leaves were significantly (P<0.05) smaller and thicker and total leaf area was lower than on undiverted reaches [122]. Although water birch typically occurs on moist sites, populations are occasional on dry sites [54]. On Twin Lakes Hill near Churchill, Manitoba, researchers found a single water birch clump, which might represent its furthest northeast distribution. The clump occupies a very dry, acidic site. At this site, water birch failed to flower in 2 of the 3 years of observation (see Seed production) [118].
Water birch was often associated with alkaline soil or water conditions, but the northeastern outlier clump of water birch growing near Churchill, Manitoba, occurred where the soil pH was 4.4 to 4.8, which was much more acidic than what was typical for the area (7.8-8.8 pH) [118]. In British Columbia, water birch was most frequent at nutrient-rich sites with high levels of calcium and magnesium [68]. In central and southern Idaho, water birch dominated riparian vegetation along several alkaline streams and springs, which included portions of Summit Creek, a spring-fed, highly alkaline stream between the Little Lost and Lemhi mountains; Formation Springs Preserve, a hardwater spring with high levels of calcium and magnesium north of Soda Springs; and Thousand Springs Ranch Preserve, an alkaline water-fed spring southeast of Hagerman [105].
Reviews suggest that water birch can tolerate flooding for "most of one growing season" [134,135], but other studies suggest that abundance and growth may be greater at sites flooded for shorter periods. Along the Red Deer River in Alberta, presence of water birch was greatest in the understory of young cottonwood (Populus spp.) and mature shrub communities. Young cottonwood stands flooded about once every 10 years, but mature shrub communities at the upper elevations of the floodplain had not flooded in 80 years [15]. In Utah, water birch occurs in riparian areas where the stream gradient is high enough to allow for soil drainage [6]. In vegetation along an 83-mile (134 km) section of the San Miguel River in southwestern Colorado, relative abundance of water birch peaked at sites with inundation recurring every 2.2 to 4.6 years [33]. In Nevada, water birch community types were common in narrow steep-sided valleys along streams where the water table was often within 3 feet (1 m) of the soil surface [83]. The water birch-red-osier dogwood community type in the Rocky Mountains of northern and central New Mexico frequently floods and may be temporarily flooded early in the growing season. Hydric conditions are typical in the top 20 inches (50 cm) of the soil surface [96].
Climate: Within its riparian habitats, water birch tolerates a considerable range of climatic conditions. When radial tree growth, climate, and stream flow variations were evaluated along Bishop Creek in the eastern Sierra Nevada, researchers concluded that water birch was well adapted to a wide range of variations in climate and water availability [20]. Although common to semiarid climates in the West [4], water birch is not particularly drought tolerant [87]. In California, water birch is relatively frequent in the Klamath Mountains and on the eastern slope of the southern Sierra Nevada. These areas receive more summer precipitation than most of California [51].
Harsh climatic conditions are typical in high-elevation semiarid valleys of central Idaho, southwestern Montana, and western Wyoming, where water birch occurs. Hard freezes are possible even in midsummer [4]. Harsh conditions also occur in the Blue Mountain region of Oregon, where water birch occurs on steep slopes, at low-elevation sites where summers are hot and dry [98]. A water birch assemblage is common along perennial streams in parts of the Tsegi Canyon in Navajo County, Arizona, where the climate is arid, winters are cold, and summers are hot. Daily average temperatures range from 93 to 100 °F (34-38 °C) in July and -9 to 55 °F (-23 to 13 °C) in winter. The average frost-free season lasts 155 days, and average precipitation levels based on a 17-year period, range from 7 to 19 inches (170-480 mm) [50]. In British Columbia, water birch was most frequent in the subalpine boreal climate type, with cold, snowy weather and no distinct dry season. The driest month generally received more than 1.2 inches (30 mm) of precipitation, and the average monthly temperature exceeded 50 °F (10 °C) in fewer than 4 months of the year [68].
Elevation: Throughout its North American range, water birch occurs at elevations of 300 to 10,000 feet (100-3,000 m) [31]. Elevation ranges are generally higher at southern than northern locations. Water birch occupies moist sites up to 10,000 feet (3,000 m) in the Colorado Rockies and up to 5,500 feet (1,700 m) in the Canadian Rockies [23]. Ranges from 1,000 to 6,000 feet (300-2,000 m) were reported in the Pacific Northwest and the northern Rocky Mountain regions of the United States [135].
| Local elevation ranges for water birch reported in the United States | |
| Arizona | 3,400-8,000 feet (1,000-2,400 m) [9,64,79] |
| California | 2,000-8,200 feet (600-2,500 m) [48,114] |
| Colorado | 5,000-9,000 feet (1,500-2,700 m) [44,65] |
| Great Basin | 5,000-8,000 feet (1,500-2,400 m) [73] |
| Montana | 3,000-6,000 feet (900-1,800 m) for water birch-dominated community type [42] |
| Nevada | 5,000-10,000 feet (1,500-3,000 m) [62] |
| New Mexico | 5,000-8,000 feet (1,500-2,400 m) [13,79,86] |
| Utah | 4,000-8,810 feet (1,220-2,690 m) [58,139] |
Plant communities: Water birch is most common in riparian forests, woodlands, or shrublands immediately surrounding waterways or other wet sites and often occurs with cottonwoods, willows (Salix spp.), alders (Alnus spp.), or red-osier dogwood [26,40,50,66,112,137], although dense pure thickets of water birch can also occur [54,83,97,116]. Similar water birch communities were described in widely distributed riparian areas, but upland communities associated with water birch riparian habitats, even within a fairly small area, often occurred in a diversity of ecosystems, such as: moist spruce-fir (Picea-Abies spp.) forests, dry ponderosa pine (Pinus ponderosa) forests, semiarid singleleaf pinyon-Utah juniper (P. monophylla-Juniperus osteosperma) woodlands, semiarid shrublands, and dry grasslands [17,73,80,83]. Although most commonly described in riparian vegetation, water birch was also described as a component of acidic, mesic, tundra shrublands in northwestern Canada [129]; in quaking aspen (Populus tremuloides) stands on the eastern slopes of the Sierra Nevada [128]; in successional Douglas-fir (Pseudotsuga menziesii) and white fir forests at 7,500 to 10,000 feet (2,300-3,000 m) in northern, central, and eastern Arizona [81]; and in white pine (Pinus strobus), western redcedar (Thuja plicata), hemlock (Tsuga spp.), and lowland white fir (A. concolor) forests in northern Idaho after fire or other canopy-opening disturbances [74].
The same water birch communities or habitat types were recognized in many different regions:
Upland vegetation types associated with riparian habitats supporting water birch varied widely regardless of region:
Pacific Northwest: In the Pacific Northwest, water birch occurs in riparian areas within a range of habitats from dry bluebunch wheatgrass (Pseudoroegneria spicata) grasslands [80] to cool, moist, climax spruce-fir forests [16].
Great Basin: Riparian habitats with water birch are found within upland habitats ranging from semiarid mountain big sagebrush (Artemisia tridentata subsp. vaseyana) shrublands [83] to fir forests [73].
Southern Rocky Mountains: Water birch riparian habitats occur from desert [21] to montane zones [85,112] in the southern Rocky Mountain region.
 |
 |
|
| Photos © Tom DeGomez, University of Arizona, Bugwood.org | ||
GENERAL BOTANICAL CHARACTERISTICS:
Aboveground description: Water birch is native to North America and grows as a deciduous small tree (<33 feet (10 m) tall and 12 inches (30 cm) in diameter) or large shrub (<20 feet (6 m) tall) throughout most of its range [9,48,58,123,132]. It has reached large tree size in the Puget Sound area of Washington (130 feet (40 m) tall) [11] and in the Sawtooth National Forest of Idaho. The particularly large specimen in Idaho was 50 feet (15 m) tall with a 7-foot (2 m) crown, and a 29-inch (74 cm) DBH [102]. In Canada, the large shrub form is most common [54], and in the western portion of the northern Great Plains and in Utah, water birch is a smaller shrub (10-20 feet (3-6 m) tall) [125,139]. As a tree, water birch typically produces clumps of curved or leaning trunks with broad open crowns with ascending to drooping branches [4,54]. As mature trees produce new sprouts from the base of the trunk, a "massive clump" of 100 or more various-aged stems is possible [73]. As a shrub, water birch typically grows in thickets of spreading branches and broad open crowns [23,54,132]. The bark on water birch stems or trunks is thin, red-brown to nearly black in color, and very rarely peels [54,132]. Northwestern paper birch (water birch × paper birch hybrid) in eastern Washington grows as a small tree with pinkish bark. In this area, water birch grows as a multi-stemmed shrub with brownish-red bark, and paper birch grows as a moderately large tree with white bark [67].
Water birch leaves are alternate, simple, and nearly oval shaped. Leaves are thin and firm, measure up to 5 inches (13 cm) long and are slightly less wide. Leaves are most broad just below the middle. Usually, leaf margins are sharply double serrate with 2 distinct tooth sizes; leaf tips are blunt to pointed [11,23,54,123]. Water birch flowers occur in catkins with closely overlapping scales. Female catkins are mostly erect, solitary, and 0.6 inch (1.5 cm) long. Male catkins are pendent, occur in clusters of 2 or more, and are about 0.4 to 1.2 inches (1-3 cm) longer than female catkins [70,123,139]. Water birch produces single-seeded nutlets in samaras [8,23,70,123].
Belowground description: Water birch produces a dense, spreading, finely branched root system, but because it grows where water tables are high, it is generally only shallowly rooted and susceptible to windthrow [73,91,127].
Raunkiaer [106]
life form:
Phanerophyte
SEASONAL DEVELOPMENT:
Water birch flowers are generally produced by late spring and appear before leaves
[31,62,79,123,125]. Fruits are typical in August, and seeds disperse from fall through the following spring [35,60,91]. Although seeds may disperse as early as late summer, these seeds are rarely viable [60].
REGENERATION PROCESSES:
Water birch regenerates sexually by seed and asexually by basal sprouting. While information is available on water birch seed production, germination, and seedling establishment (see below), it is sparse and fails to identify the field conditions necessary for successful water birch reproduction from seed. Vegetative sprouts are common following aboveground damage or top-kill [42,120] but are also produced in the absence of disturbance. Massive clumps are possible [73].
Pollination and breeding system: Water birch produces unisex flowers [60,70], but both male and female flowers occur on the same plant [70]. Selfing is highly unlikely but not impossible for water birch (Williams 2000 cited in [140]). Pollen dispersal distances of up to 370 miles (600 km) have been reported for birches (Betula spp.) (Wallin and others 1991 cited in [140]).
Seed production: Birch species are generally said to have regular and abundant seed production (review by [60]). Very abundant seed production was reported for water birch in cottonwood forests on the South Fork of the Snake River [87]. However, the quality and germination capacity of seed produced by members of the Betulacea family is often very low according to a review by Jones and others [59].
Although birch species typically flower at young age (10 to 15 years) (review by [60]), site conditions or isolation may limit water birch's capacity for seed production. A single outlying clump of water birch growing on a very dry site near Churchill, Manitoba, produced seed in 1 of 3 years of observation [118].
Seed dispersal: Seeds produced by species of the Betulacea family are very small and light (1,500-2,500 seeds/g). Long-distance dispersal by wind or water is possible (reviews by [59,91]). Dispersal distances of several kilometers have been reported (Holm 1994 cited in [140]). Sweet birch (B. lenta) seeds were blown on crusty snow a distance of up to 262 feet (80 m) from the parent plant (review by [60]).
Field studies indicate that primary and secondary dispersal of seeds can be facilitated by water (hydrochory). Water birch seeds were recovered from traps along and within rivers in the southern Rocky Mountains of Colorado. Water birch was a dominant species along both rivers sampled and was the most abundant seed collected in traps along the rivers (34% of all seeds trapped). The abundance of water birch seed in hydrochoric samples peaked in early June and late August. Researchers reported that the June distribution in hydrochoric samples reflected remobilization and secondary dispersal of seeds initially deposited on floodplains in the previous year, and August distribution reflected wind dispersal of the current year's seed [89].
Experiments showed that water birch seeds may be transported long distances by water, and dispersal distances could vary with modification of the hydrologic regime. Of nearly 3,000 water birch seeds released within a 66-foot (20 m) long experimental flume, less than 26% were deposited. Seed deposition was concentrated in zones with low flow velocity and strong flow recirculation (eddies, flow expansions, point bars, pool margins, and slackwaters). Flumes were designed to mimic natural and modified hydrologic regimes. The "descending" regime represented natural snow melt for high-latitude mountain rivers; the "stepped" regime represented the flows below dams with periods of heavy flow and periods of rapid drawdown; the "ascending" regime represented flows below dams at agricultural storage reservoirs with increased flows during snow and rain events and in middle to late growing seasons. More than 98% of seeds were flushed completely through the ascending flume, suggesting that long-distance seed dispersal might occur when ascending flows coincided with water birch's seed release. Seed trapping in the stepped flume was about 11 times that in the ascending flume. Significantly more seeds were deposited in areas of slow flow than in areas of rapid flow (cut banks, flow constrictions, islands, straight margins) (P<0.001) [88].
Seed banking: Birch species produce short-lived seeds that rarely remain viable for more than 2 to 3 years, according to a review by Karrfalt [60].
Germination: Reviews [60,91] and controlled studies [24,43,59] suggest that germination of water birch seeds can be stimulated by exposure to cold temperatures, light, and mineral soil. Cool stratification at 36 to 37 °F (2-3 °C) for 30 to 90 days has been recommended to break seed dormancy and improve germination [24,43,60,91]. Germination is best when seeds are only lightly covered with soil (about 3 mm). On consistently moist soils, seeds may not need to be covered [24,60,91]. Monsen and others [91] indicate that water birch seed germination is favored on exposed mineral soil. For water birch seeds collected from Taos County, New Mexico, average germination ranged from 5% to 19% and was generally improved with stratification [59].
Seedling establishment and plant growth: In the available literature (2012), very little was reported about water birch seedling establishment and growth. With respect to shading and the establishment and growth of water birch seedlings, studies report conflicting information. A review reports that 2- to 3-month-old water birch seedlings may benefit from shading [91], and in a survey of bottomland hardwood forests in western Montana, water birch seedling densities were greatest along tributaries with low light and little mineral soil. Sapling densities were greatest in the youngest stands sampled [32]. However, in a greenhouse study, survival of water birch seedlings was significantly lower in heavy shade than in light shade (P=0.01). Greenhouse shading conditions were similar to those found along Norway maple (Acer platanoides)-invaded portions of lower Rattlesnake Creek in western Montana. Along heavily invaded reaches, canopy cover of Norway maple averaged 76% and photosynthetically active radiation was just 5% of that along uninvaded reaches of the creek. Survival of water birch seedlings was 55% lower when grown at PAR levels similar to invaded reaches than at PAR levels similar to uninvaded reaches [107].
Plant growth: Water birch grows rapidly and is considered short-lived, but neither growth rates nor life expectancy were reported in the literature as of 2012 [91,127]. A water birch clump growing near Churchill, Manitoba, and thought to represent the furthest northeastern specimen, was estimated to be at least 100 years old and may have been 200 years old [118].
Vegetative regeneration: Water birch often forms clumps by sprouting from the base of the trunk [44], and one source reports root sprouting [22]. Sprouts can develop following top-kill [42,120] or in the absence of aboveground damage [73], but relationships between new stem production and plant age, site conditions, and disturbance regimes were poorly described in the available literature (2012). As water birch matures, new stems develop from the base of the trunk, and a "massive clump" of 100 or more various-aged stems is possible [73]. However, vegetative regeneration was described as "moderate" in cottonwood forests on the South Fork of the Snake River [87]. At Brule Lake in central Alberta, water birch survived burial by sand deposited by the Athabasca River. Sprouting from adventitious roots was reported when upper branches remained unburied [22].
SUCCESSIONAL STATUS:
Available successional studies (as of 2012) do not indicate that water birch is restricted to any particular stage of forest or floodplain development. Although most common along streams and in canopy openings, studies suggest that water birch is at least moderately shade tolerant. At a high-elevation site in southeastern Wyoming, maximum stomatal conductance was only 10% lower for water birch in sunlight levels of less than 100 W/m² than in sunlight levels of 750 W/m² [142]. In cottonwood stands on the South Fork of the Snake River in Idaho, water birch's shade tolerance was described as moderate [87].
Water birch occurs in early-, mid-, and late-seral forest and woodland communities. In a study of floristic and structural changes monitored for the first 40 years after timber harvests in western hemlock (Tsuga heterophylla) habitat types in northern Idaho, researchers indicated that water birch was vulnerable to successional replacement in the very early successional stages (within 8 years of logging) [144]. In North Dakota's Theodore Roosevelt National Park, the quaking aspen/water birch habitat type on upper northwest- to east-facing slopes is a topographic climax type, which represents a self-perpetuating community in a unique microclimate created by certain soil and slope configurations [41]. In the eastern Rocky Mountains of Alberta, water birch is described along streams and in low places within the climax spruce-fir forests [16]. In Boulder County, Colorado, water birch is scattered in canyon bottoms within the narrowleaf cottonwood-Scouler willow (Salix scouleriana) formation, which is almost constantly in flux. The researcher reported that the community "lacks continuity" with "characteristic species only occasionally exercising control" [143].
In succession of floodplains or wetlands, water birch may occur at any successional stage but generally seems most common in mid-seral communities. In the ponderosa pine zone in Utah, the 1st tree establishing on streamside floodplains is narrowleaf cottonwood, and water birch is "a close 2nd". In the pinyon zone, water birch and boxelder (Acer negundo) replace narrowleaf cottonwood as streamside shade levels increase [21]. On floodplains in western Montana, cottonwoods and narrowleaf willow are common pioneers on fresh alluvial deposits. A dense thicket of narrowleaf willow is overtopped in about 15 years by the cottonwoods. As cottonwoods mature, stands thin and additional species such as water birch, snowberry (Symphoricarpos spp.), red-osier dogwood, gray alder, and conifers establish. After about 100 years in the absence of substantial flooding, stable conifer climax communities develop [40]. Water birch was mentioned only after fire or in canopy openings in densely shaded white pine, cedar, hemlock, and lowland white fir forests in northern Idaho [74]. The water birch-dominated community type, which occurs along high-gradient, fast-moving streams at low- to mid-elevations of western Montana, represents an early- to mid-seral stage in succession. At some sites, it is replaced by a Rocky Mountain juniper (Juniperus scopulorum)/red-osier dogwood type. At other sites, the water birch type may dominate following disturbance in spruce/field horsetail community types. Heavy grazing may cause the water birch community type to transition to a willow community type [42]. On alluvial flats and the edges of moist meadows in the Rocky Mountains, water birch, gray alder, and willows replace cottonwoods over time in the absence of disturbances. The time frame to replacement was not reported [104]. In the Black Hills of South Dakota, the water birch-willow-cottonwood vegetation type is considered the preclimax phase to montane forests at the upper reaches of mountain streams and to deciduous forests at the lower reaches [47]. In gallery forests along the Snake River in Hells Canyon, Idaho, white alder (Alnus rhombifolia) dominated unstable, low-elevation sites while water birch dominated higher elevations where stream disturbances were less recent [90]. After evaluating 7 bogs in Whatcom County, Washington, a researcher concluded that water birch was most commonly associated with late-seral bogs or bogs that were drained, burned, and/or pastured. Numerous water birch seedlings occurred in the late-seral Dickey bog, which had burned "a few years ago", and at Ferndale bog, which was drained and cleared of surrounding forest vegetation. At Dalstrom bog, which was drained and used as a pasture, there were dense thickets of young water birch 4 to 8 feet (1.2-2.4 m) tall [110].
Water birch can regenerate quickly following aboveground damage and has been reported in early succession after fire and logging. In the Schell Creek Range of eastern Nevada, sites dominated by white fir and a low tree layer of water birch were dominated by water birch after fire. Time since fire was not reported. Along McCoy Creek in the Humboldt National Forest, water birch sprouts were observed 4 to 5 years after fire [84]. When burned areas within the Bitterroot Mountains were surveyed, water birch was found in communities representing the 2nd postfire successional stage, which typically occurs 2 to 3 years after the herbaceous-dominated stage [75]. Density of water birch increased after clearcutting mature white spruce (Picea glauca) forest stands in west-central Alberta. In mature, undisturbed stands there were 7 water birch stems/acre. Water birch did not occur in the 1st postlogging year on mechanically scarified or unscarified sites. Six years after logging, though, water birch stem density was 27 stems/acre on scarified and 54 stems/acre on unscarified sites. Seventeen years after logging, there were 86 water birch stems/acre, regardless of scarification [124].
Immediate fire effect on plant: Survival, top-kill [77], and mortality [95] are possible effects of fire on water birch. Survival is likely restricted to low-severity fires. Top-kill is typical following low- to moderate-severity fires (Dwire and Kauffman 2003 cited in [109]). Mortality can occur following severe fires [95].
Postfire regeneration strategy [126]:
Tree with adventitious buds, a sprouting root crown, sobols, and/or root sprouts
Tall shrub, adventitious buds
and/or a sprouting root crown
Initial off-site colonizer (off site, initial community)
Secondary colonizer (on- or off-site seed sources)
Fire adaptations and plant response to fire:
Fire adaptations: Several sources report that water birch is generally top-killed and sprouts from the base following fire [42,84,120]. However, few fire studies provide details on sprouting potential as it relates to prefire stem size or stem age, prefire and postfire site conditions, or severity of fire effects.
Water birch is also said to germinate well on mineral soil (review by [91]), and "numerous" water birch seedlings occurred on a bog site burned "a few years ago" [110]. Because the water birch seed bank is likely short-lived and seed dispersal can occur over long distances, off-site seed is the likely source for postfire seedling establishment. However, additional studies are needed to rule out or determine the contribution from on-site seed sources.
Plant response to fire: Water birch survival, sprouting, seedling establishment, and mortality have all been reported in the handful of fire studies available from water birch habitats (as of 2012). Although the likelihood of survival and postfire sprouting appears to decrease with increasing fire severity, more information is needed to determine how survival, top-kill, and mortality of water birch relate to prefire maturity, prefire site conditions, fire frequency, and fire severity.
Several studies report survival and sprouting of water birch after fire. In northern Idaho's Fish Creek area, 1 of 3 water birch shrubs marked before a March prescribed fire had some surviving top-growth when the burned area was evaluated in August. The 2 other shrubs were completely top-killed. All shrubs produced basal sprouts after the fire and had more basal sprouts after than before the fire. Number of water birch basal sprouts on the site was 3.3 before the fire and 31.3 after the fire. Sprouts averaged 2 feet (0.6 m) taller after the fire [77]. In their survey of the vegetation in Humboldt County, researchers reported rapid growth and development of water birch sprouts after fire. Sprouts were noted along McCoy Creek 4 to 5 years after fire [84]. No water birch mortality was reported within a year of an early spring, low-severity surface fire in western British Columbia. Shrubs and saplings in the burned area were scorched but none were killed. Water birch was, however, infected by Cytospora spp. after the fire [19].
In a plains cottonwood (Populus deltoides subsp. monilifera) stand along the Red Deer River in Alberta, about half the water birch were killed in a severe fire. The fire burned on 15 August and was described as a "major fire event in terms of its intensity". "Significant vegetation changes" were predicted. Air temperature and relative humidity at the time of the fire were 76.6 °F (24.8 °C) and 38%, respectively. In areas with thick shrub undergrowth, flame heights reached 18 feet (5.5 m) and burn scars on trees reached 30 feet (9 m) [95].
Water birch's persistence in repeatedly burned and long unburned sites was rarely discussed in the available literature (2012), but one study suggests that it may persist under a regime of frequent fire, and another suggests that it is most abundant in early succession. A single water birch clump was described in an area that researchers noted had burned frequently, but neither the fire frequency nor the time between fires was reported [118]. A postfire chronosequence study (4-75 years since fire) of coniferous forests in central British Columbia suggests that abundance of water birch generally decreases with time since fire. In a spruce-balsam fir (Abies balsamifera) stand, density of water birch averaged 160 trees/acre in stands that averaged 8 years old. Water birch density was considerably lower (30 trees/acre) in stands that averaged 43 years old. In cedar-spruce stands, density of water birch water averaged 487 trees/acre in stands that averaged 7 years old. Water birch density was considerably lower (204 trees/acre) in stands that averaged 28 years old [34].
Fuels: The fuel characteristics of water birch were not specifically described in the available literature (2012), but general information reported from riparian areas may apply to water birch and its habitats. Characteristics of the fuels in riparian areas have been used to explain the potential fire behavior in riparian communities, for which fire history studies are rare. Few studies have investigated the relationships between fire regimes in riparian communities that occur in uplands dominated by semiarid shrublands or grasslands. Most riparian fire regime information comes from areas where riparian communities are dominated and surrounded by conifers (review by [25]). In his review of fire regimes associated with riparian vegetation, Williamson [141] reported that it is often assumed that fuel moisture is high in riparian habitats, reducing the likelihood of carrying a low-severity fire. Because of this, fires are generally considered to burn less frequently in riparian areas than in adjacent uplands. However, the dense, complex, multi-layered structure of fuels in riparian vegetation suggests these areas might be susceptible to high-severity crown fires in certain weather conditions, and Williamson’s results were consistent with this observation. Using modeling and field measurements, he investigated the fuel and fire behavior relationships for riparian and upland vegetation in 3 forest series in the Blue Mountains of Oregon. Ten-hour fuel moisture contents were not significantly different for conifers growing closest to the stream and those growing in upland forests. The fuel moisture contents of shrubs and herbs were significantly greater in riparian than upland sites within Douglas-fir and grand fir (Abies grandis) forests, but not in subalpine fir (A. lasiocarpa) forests. Basal area, stand density, and canopy foliage weight were significantly greater in riparian than upland areas. In modeling simulations, nearly all stands sampled were at risk for the vertical spread of fire into the crowns. The potential for crown fire was similar for riparian and upland areas [141].
The following table provides a framework that could be useful in predicting or understanding fire behavior and spread in riparian areas in western United States. This table was taken in its entirety from a review by Dwire and Kauffman [25].
| Fuel characteristic, topography, and microclimate factors associated with riparian areas and their potential relationship to fire behavior and fire effects [25] | ||
| Fire risk factor | Riparian characteristic | Fire effect |
| Fuel loads | High fuel loads due to high net primary productivity; accumulation of fuels due to long fire-return intervals | High fuel loads can increase vulnerability to fire in drought conditions, and influence fire severity, intensity, and return intervals |
| Fuel moisture content | High fuel moisture content due to proximity to water, shallow water tables, and dense shade | Fuel loads may remain too moist for sustained fire spread late into the fire season |
| Fuel continuity | Active channels, gravel bars, and wet meadows may function as natural fuel breaks | Breaks in fuel continuity can prevent or slow the spread of fire |
| Topographic position | Canyon bottoms; lowest points on the landscape | High fuel moisture, high relative humidity, and few lightning strikes may decrease fire frequency and severity; more human-caused ignitions may increase fire frequency |
| Microclimate | Topography, presence of water, and dense shade can create cooler, moister conditions | High relative humidity and cool temperatures may lessen fire intensity and rate of spread |
Fire regimes: For this review, the discussion of fire regimes in riparian areas is restricted to regions within water birch's range but includes studies that did not mention water birch in the study area description. Because water birch sometimes occurred within a very narrow band of vegetation immediately adjacent to streams [21] and contributed little to the relative community abundance [5], its absence from study area descriptions does not necessarily mean absence from the study area. Additionally, the general scarcity of fire history studies in riparian areas and the potential applicability of these riparian studies to water birch habitats makes a broader fire regime discussion necessary.
Fire history studies of riparian areas are rare. This may be because fires were historically rare in riparian areas, fires in riparian areas burned at such low intensity that trees were not scarred, or fires were so patchy that evidence of their occurrence was missed [119]. "There are few data with which to evaluate the flammability of riparian zones" because site conditions allow for rapid postfire succession, or fires are infrequent or of very low severity in riparian areas (review by [1]).
In the cottonwood-dominated floodplain along the South Fork of the Snake River in southern Idaho, fire was considered a minor disturbance. Only about 2% of the riparian forest showed evidence of fire through scars or mortality. The largest fire was an estimated 7 acres (3 ha), and the evidence for most fires was relatively recent—from the last 30 years [87]. In the upper Frijoles Canyon in New Mexico's Jemez Mountains, the riparian mixed-conifer forest had a mean fire-return interval of 6.8 to 18.9 years, and the last major fire occurred about 5 years prior to the study. The researcher indicated that the fire history for the canyon was "more closely related to the fire regimes of adjacent uplands than to the idiosyncrasies of the canyon itself" [2].
A review by Olson [99] suggests that fire-return intervals are generally assumed to be longer in riparian areas than adjacent uplands, but the presence and extent of these differences varies with stream size and forest type. Upland and riparian fire-return interval differences are thought to be larger for sites along large streams than small streams. The fire-return interval differences are thought to be smaller when cool, moist riparian forests are surrounded by large areas of dry, warm forests than when surrounded by large areas of cool, moist forests. However, few of these assumptions are based on actual observations [99].
A fire study in Oregon was designed to better understand the relationships between fire frequency in upland and riparian forests as influenced by stream size and forest type. Riparian vegetation in 3 forest types was sampled as close to the stream as possible. When data from all forest types were combined, fire-return intervals between 1650 and 1900 did not differ significantly in riparian and upland sites, but the fire-return interval for riparian areas within dry forest types (12 yrs) was significantly shorter than that for riparian areas within mesic forest types (19 yrs) (P=0.01). In ponderosa pine and Douglas-fir forests in the southeastern Blue Mountains and grand fir and Douglas-fir forests in the Elkhorn Mountains, fire-return intervals were not significantly different between riparian and upland sites, and fire-return intervals were similar for riparian and upland sites at large- and small-sized streams. In western hemlock and Pacific silver fir forests, the fire-return intervals for riparian and upland sites at large streams were 35 and 27 years (P=0.13) and at small streams were 39 and 36 years, respectively (P=0.80). The researcher indicated that forest composition was an important influence on fire-return interval differences between riparian and upland sites; however, the species composition of the canopy and understory were not described in detail [99].
Fire studies in the Klamath Mountains of northern California indicated that fire-return intervals were longer but more variable in riparian areas than adjacent uplands. The median fire-return interval for riparian areas was generally twice that of adjacent upland areas; however, the range of fire-return intervals was not different for riparian and upland areas. Researchers indicated that riparian vegetation along perennial streams was an effective barrier to the spread of low- and some moderate-severity fires and influenced burn patterns and landscape heterogeneity [119,120]. At the Shasta-Trinity Divide site within the Klamath Mountains, the fire history of riparian and upland areas was determined from fire scars at 5 previously logged sites. Stumps were used because the scars on most living riparian trees had healed and were difficult to pick out in the riparian zone. The researcher indicated that the study area experienced pronounced drought periods each year, with conditions severe enough to support fire ignition and spread in both the riparian and upland areas. Riparian areas, however, recorded fewer fires than the coniferous uplands. Along perennial streams, the median fire-return interval in the riparian area was at least twice that of adjacent uplands. At the upper reaches of the watershed, however, where riparian areas were smaller and occurred along intermittent streams, fire frequencies for riparian and upland sites were similar. The range of fire-return intervals was similar for all riparian and upland sites, suggesting the fire-return variability was greater for riparian than upland sites. Several ideas were suggested as reasons for fewer fires recorded in riparian areas: fire intensity was reduced by the moister fuel conditions in the riparian areas and these low-intensity fires failed to produce scars; fires burned more heterogeneously in riparian than upland areas and as a result fewer trees were scarred; and/or fires did not burn into the riparian areas [119].
While higher fuel moisture contents of some of the vegetation within riparian areas suggests that fire severity would be low in the riparian zone, this is not always the case. In northeastern California, researchers speculated that presettlement fire-return intervals were longer in riparian areas than in adjacent uplands but that fire severity was moderate to high in the riparian areas [109]. Along Little French Creek in the Payette National Forest of Idaho, a fire burned much more severely in the riparian zone than in adjacent uplands. The riparian zone included a multilayered stand structure with a substantial amount of dead Engelmann spruce (Picea engelmannii), which burned in a crown fire. Upland sites dominated by widely spaced lodgepole pine with a grouse whortleberry (Vaccinium scoparium) understory had little coarse woody debris and burned in a surface fire (personal observation cited in [1]).
See the Fire Regime Table for further information on fire regimes of vegetation communities in which water birch may occur. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
FIRE MANAGEMENT CONSIDERATIONS:
The use of prescribed fire to manage water birch specifically was not discussed in the available literature (2012). Periodic low- to moderate-severity fires in riparian areas would not likely affect the survival and persistence of water birch (see Plant response to fire). In Wind Cave National Park, South Dakota, prescribed fire was used to discourage the establishment and spread of ponderosa pine in riparian vegetation. Prescribed fire was considered effective and its continued use was suggested, but prescription details were lacking [82].
Fire in riparian areas can lead to increases in stream temperatures, which suggests that fire timing and its potential effect on aquatic organisms should be considered when planning prescribed fire in the riparian zone. In Idaho's Payette National Forest, water temperatures were compared for 1st and 2nd order streams (3-10 feet (1-3 m) wide) in burned and unburned catchments. The wildfire burned in September and stream temperatures were monitored for a year, beginning about 10 months after the fire. In late summer and early fall, the daily maximum temperatures were greater for burned 62.4 °F (16.9 °C) than unburned 57.2 °F (14 °C) streams. The greatest difference between burned and unburned streams was captured in the difference between daily maximum and minimum temperatures, which was 2 to 3 times greater for burned than unburned streams. Researchers reported that a 3.6 to 5.4 °F (2-3 °C) increase in stream temperature can result in changes in the size at maturity, timing of emergence, and sex ratios for aquatic organisms [111].
FEDERAL LEGAL STATUS:
None
OTHER STATUS:
Information on state- and province-level protection status of plants in the United States and Canada is available at NatureServe.
IMPORTANCE TO WILDLIFE AND LIVESTOCK:
Utilization of water birch as forage, nesting, or cover by wildlife and livestock is rarely extensive, but this may have more to do with water birch's narrow distribution or scarcity within a study area than with deliberate avoidance. However, American beavers reportedly used water birch as damming material only when preferred trees were not available [37].
Big game: Although not often reported as important big game forage, water birch was rated as highly important winter forage for moose in British Columbia [7] and received low to moderate use by mule deer in the West. Moose in Alberta and Utah utilized water birch forage, and in the mountains of southwestern Alberta where high winds minimize snow accumulations, aspen, willow, and water birch shrublands were considered important moose habitat (review by [108]). A review reports that mule deer use of water birch in the Rocky Mountains was minimal to low in the winter and minimal to moderate in the summer [69]. Water birch did not occur in elk, bighorn sheep, or cattle fecal samples collected on winter ranges in north-central Montana, but it made up 0.4% of mule deer fecal samples from a site with 0.5% canopy cover of water birch [63]. Captive mule deer in northern Utah consumed moderate quantities of water birch in mid-summer [121]. On mule deer winter range in northern Inyo County, California, water birch made up 3.7% by volume and 20% by frequency of February diets. Use of water birch was less in January and March and little to none in December and April [76].
Birds: Water birch provides important bird habitat [40]. In narrowleaf cottonwood riparian forests along the South Fork of southeastern Idaho's Snake River, black-capped chickadees, mourning doves, and yellow-breasted chats were positively associated with microhabitats characterized by a subcanopy of water birch and other small trees. The strongest positive relationship was for yellow-breasted chats [113]. In the Toiyabe Mountain Range in central Nevada, warbling vireos were frequent in water birch-dominated vegetation, and downy woodpeckers and lazuli buntings were common in highly degraded water birch vegetation. Degree of degradation was assigned based on seral stage, characteristics of dominant plants, and soil temperature [136]. Least flycatchers nest in water birch riparian habitats in Montana (Fletcher unpublished information [30]). Of 26 black-headed grosbeak nests found in riparian areas of Colorado State University's San Juan Basin Research Center in La Plata County, 10 occurred in water birch trees [101]. In Nevada, hummingbirds and red-naped sapsuckers have been observed feeding on water birch sap (Hall 1938 cited in [37]).
Livestock: Water birch is considered good sheep and goat forage in the Southwest [64,132] and perhaps elsewhere [18]. Cattle seldom browsed water birch in cottonwood forests on the South Fork of the Snake River, even in areas with heavy grazing pressure [87]. Dense water birch thickets were generally avoided by all classes of livestock in Montana [40].
Palatability and nutritional value: Palatability ratings for water birch range from poor to very good [18,42], and some report that water birch has little forage value [93]. Use and palatability may differ by livestock class and/or region. In Montana, water birch was fairly palatable to sheep but had poor palatability for cattle. Energy and protein values were rated moderate, and water birch was only lightly browsed unless more palatable species were unavailable [42]. On some California ranges, however, water birch is considered fairly important browse. It is often more palatable than alders. Browse ratings were good to fair for deer, fair for goats, fair to poor for cattle and sheep, and poor to useless for horses [114].
Cover value: Dense water birch thickets provide excellent thermal and hiding cover for various wildlife species [40,42]. Because it often overhangs streams, water birch provides important shade for fisheries [40,58]. Linear water birch stands provide protective travel corridors for wildlife. Water birch stands are considered fair elk and good white-tailed deer, mule deer, small mammal, and bird cover [42].
VALUE FOR REHABILITATION OF DISTURBED SITES:
OTHER MANAGEMENT CONSIDERATIONS:
When researchers modeled the effects of future predicted changes in climate on tree species in British Columbia, they found that the amount of new habitat suited for water birch growth would exceed the amount of suitable habitat lost at the 2025, 2055, and 2085 time periods. However, if water birch failed to colonize the newly suitable habitats, its frequency in British Columbia could decrease by as much as 19% by 2085 [38].
| Fire regime information on vegetation communities in which water birch may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [72], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||||||
|
|||||||||||
| Pacific Northwest | |||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||
| Pacific Northwest Grassland | |||||||||||
| Alpine and subalpine meadows and grasslands | Replacement | 68% | 350 | 200 | 500 | ||||||
| Mixed | 32% | 750 | 500 | >1,000 | |||||||
| Bluebunch wheatgrass | Replacement | 47% | 18 | 5 | 20 | ||||||
| Mixed | 53% | 16 | 5 | 20 | |||||||
| Idaho fescue grasslands | Replacement | 76% | 40 | ||||||||
| Mixed | 24% | 125 | |||||||||
| Marsh | Replacement | 74% | 7 | ||||||||
| Mixed | 26% | 20 | |||||||||
| Pacific Northwest Shrubland | |||||||||||
| Low sagebrush | Replacement | 41% | 180 | ||||||||
| Mixed | 59% | 125 | |||||||||
| Mountain big sagebrush (cool sagebrush) | Replacement | 100% | 20 | 10 | 40 | ||||||
| Wyoming big sagebrush semidesert | Replacement | 86% | 200 | 30 | 200 | ||||||
| Mixed | 9% | >1,000 | 20 | ||||||||
| Surface or low | 5% | >1,000 | 20 | ||||||||
| Wyoming big sagebrush steppe | Replacement | 89% | 92 | 30 | 120 | ||||||
| Mixed | 11% | 714 | 120 | ||||||||
| Pacific Northwest Woodland | |||||||||||
| Oregon white oak-ponderosa pine | Replacement | 16% | 125 | 100 | 300 | ||||||
| Mixed | 2% | 900 | 50 | ||||||||
| Surface or low | 81% | 25 | 5 | 30 | |||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||
| Mixed | 17% | 60 | |||||||||
| Surface or low | 78% | 13 | |||||||||
| Subalpine woodland | Replacement | 21% | 300 | 200 | 400 | ||||||
| Mixed | 79% | 80 | 35 | 120 | |||||||
| Western juniper (pumice) | Replacement | 33% | >1,000 | ||||||||
| Mixed | 67% | 500 | |||||||||
| Pacific Northwest Forested | |||||||||||
| California mixed evergreen (northern California and southern Oregon) | Replacement | 6% | 150 | 100 | 200 | ||||||
| Mixed | 29% | 33 | 15 | 50 | |||||||
| Surface or low | 64% | 15 | 5 | 30 | |||||||
| Douglas-fir (Willamette Valley foothills) | Replacement | 18% | 150 | 100 | 400 | ||||||
| Mixed | 29% | 90 | 40 | 150 | |||||||
| Surface or low | 53% | 50 | 20 | 80 | |||||||
| Lodgepole pine (pumice soils) | Replacement | 78% | 125 | 65 | 200 | ||||||
| Mixed | 22% | 450 | 45 | 85 | |||||||
| Mixed conifer (eastside dry) | Replacement | 14% | 115 | 70 | 200 | ||||||
| Mixed | 21% | 75 | 70 | 175 | |||||||
| Surface or low | 64% | 25 | 20 | 25 | |||||||
| Mixed conifer (eastside mesic) | Replacement | 35% | 200 | ||||||||
| Mixed | 47% | 150 | |||||||||
| Surface or low | 18% | 400 | |||||||||
| Ponderosa pine (xeric) | Replacement | 37% | 130 | ||||||||
| Mixed | 48% | 100 | |||||||||
| Surface or low | 16% | 300 | |||||||||
| Ponderosa pine, dry (mesic) | Replacement | 5% | 125 | ||||||||
| Mixed | 13% | 50 | |||||||||
| Surface or low | 82% | 8 | |||||||||
| Spruce-fir | Replacement | 84% | 135 | 80 | 270 | ||||||
| Mixed | 16% | 700 | 285 | >1,000 | |||||||
| Subalpine fir | Replacement | 81% | 185 | 150 | 300 | ||||||
| Mixed | 19% | 800 | 500 | >1,000 | |||||||
| California | |||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||
| California Grassland | |||||||||||
| Herbaceous wetland | Replacement | 70% | 15 | ||||||||
| Mixed | 30% | 35 | |||||||||
| Wet mountain meadow-lodgepole pine (subalpine) | Replacement | 21% | 100 | ||||||||
| Mixed | 10% | 200 | |||||||||
| Surface or low | 69% | 30 | |||||||||
| California Woodland | |||||||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||
| Mixed | 17% | 60 | |||||||||
| Surface or low | 78% | 13 | |||||||||
| California Forested | |||||||||||
| Aspen with conifer | Replacement | 24% | 155 | 50 | 300 | ||||||
| Mixed | 15% | 240 | |||||||||
| Surface or low | 61% | 60 | |||||||||
| California mixed evergreen | Replacement | 10% | 140 | 65 | 700 | ||||||
| Mixed | 58% | 25 | 10 | 33 | |||||||
| Surface or low | 32% | 45 | 7 | ||||||||
| Coast redwood | Replacement | 2% | ≥1,000 | ||||||||
| Surface or low | 98% | 20 | |||||||||
| Interior white fir (northeastern California) | Replacement | 47% | 145 | ||||||||
| Mixed | 32% | 210 | |||||||||
| Surface or low | 21% | 325 | |||||||||
| Mixed conifer (north slopes) | Replacement | 5% | 250 | ||||||||
| Mixed | 7% | 200 | |||||||||
| Surface or low | 88% | 15 | 10 | 40 | |||||||
| Mixed conifer (south slopes) | Replacement | 4% | 200 | ||||||||
| Mixed | 16% | 50 | |||||||||
| Surface or low | 80% | 10 | |||||||||
| Sierra Nevada lodgepole pine (cold wet upper montane) | Replacement | 23% | 150 | 37 | 764 | ||||||
| Mixed | 70% | 50 | |||||||||
| Surface or low | 7% | 500 | |||||||||
| Sierra Nevada lodgepole pine (dry subalpine) | Replacement | 11% | 250 | 31 | 500 | ||||||
| Mixed | 45% | 60 | 31 | 350 | |||||||
| Surface or low | 45% | 60 | 9 | 350 | |||||||
| Southwest | |||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||
| Southwest Grassland | |||||||||||
| Desert grassland | Replacement | 85% | 12 | ||||||||
| Surface or low | 15% | 67 | |||||||||
| Desert grassland with shrubs and trees | Replacement | 85% | 12 | ||||||||
| Mixed | 15% | 70 | |||||||||
| Montane and subalpine grasslands | Replacement | 55% | 18 | 10 | 100 | ||||||
| Surface or low | 45% | 22 | |||||||||
| Montane and subalpine grasslands with shrubs or trees | Replacement | 30% | 70 | 10 | 100 | ||||||
| Surface or low | 70% | 30 | |||||||||
| Plains mesa grassland | Replacement | 81% | 20 | 3 | 30 | ||||||
| Mixed | 19% | 85 | 3 | 150 | |||||||
| Plains mesa grassland with shrubs or trees | Replacement | 76% | 20 | ||||||||
| Mixed | 24% | 65 | |||||||||
| Southwest Shrubland | |||||||||||
| Gambel oak | Replacement | 75% | 50 | ||||||||
| Mixed | 25% | 150 | |||||||||
| Low sagebrush shrubland | Replacement | 100% | 125 | 60 | 150 | ||||||
| Mountain-mahogany shrubland | Replacement | 73% | 75 | ||||||||
| Mixed | 27% | 200 | |||||||||
| Mountain sagebrush (cool sagebrush) | Replacement | 75% | 100 | ||||||||
| Mixed | 25% | 300 | |||||||||
| Southwest Woodland | |||||||||||
| Pinyon-juniper (mixed fire regime) | Replacement | 29% | 430 | ||||||||
| Mixed | 65% | 192 | |||||||||
| Surface or low | 6% | >1,000 | |||||||||
| Pinyon-juniper (rare replacement fire regime) | Replacement | 76% | 526 | ||||||||
| Mixed | 20% | >1,000 | |||||||||
| Surface or low | 4% | >1,000 | |||||||||
| Ponderosa pine/grassland (Southwest) | Replacement | 3% | 300 | ||||||||
| Surface or low | 97% | 10 | |||||||||
| Riparian deciduous woodland | Replacement | 50% | 110 | 15 | 200 | ||||||
| Mixed | 20% | 275 | 25 | ||||||||
| Surface or low | 30% | 180 | 10 | ||||||||
| Southwest Forested | |||||||||||
| Aspen, stable without conifers | Replacement | 81% | 150 | 50 | 300 | ||||||
| Surface or low | 19% | 650 | 600 | >1,000 | |||||||
| Aspen with spruce-fir | Replacement | 38% | 75 | 40 | 90 | ||||||
| Mixed | 38% | 75 | 40 | ||||||||
| Surface or low | 23% | 125 | 30 | 250 | |||||||
| Lodgepole pine (Central Rocky Mountains, infrequent fire) | Replacement | 82% | 300 | 250 | 500 | ||||||
| Surface or low | 18% | >1,000 | >1,000 | >1,000 | |||||||
| Ponderosa pine-Douglas-fir (southern Rockies) | Replacement | 15% | 460 | ||||||||
| Mixed | 43% | 160 | |||||||||
| Surface or low | 43% | 160 | |||||||||
| Ponderosa pine-Gambel oak (southern Rockies and Southwest) | Replacement | 8% | 300 | ||||||||
| Surface or low | 92% | 25 | 10 | 30 | |||||||
| Riparian forest with conifers | Replacement | 100% | 435 | 300 | 550 | ||||||
| Southwest mixed conifer (cool, moist with aspen) | Replacement | 29% | 200 | 80 | 200 | ||||||
| Mixed | 35% | 165 | 35 | ||||||||
| Surface or low | 36% | 160 | 10 | ||||||||
| Southwest mixed conifer (warm, dry with aspen) | Replacement | 7% | 300 | ||||||||
| Mixed | 13% | 150 | 80 | 200 | |||||||
| Surface or low | 80% | 25 | 2 | 70 | |||||||
| Spruce-fir | Replacement | 96% | 210 | 150 | |||||||
| Mixed | 4% | >1,000 | 35 | >1,000 | |||||||
| Great Basin | |||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||
| Great Basin Grassland | |||||||||||
| Great Basin grassland | Replacement | 33% | 75 | 40 | 110 | ||||||
| Mixed | 67% | 37 | 20 | 54 | |||||||
| Mountain meadow (mesic to dry) | Replacement | 66% | 31 | 15 | 45 | ||||||
| Mixed | 34% | 59 | 30 | 90 | |||||||
| Great Basin Shrubland | |||||||||||
| Basin big sagebrush | Replacement | 80% | 50 | 10 | 100 | ||||||
| Mixed | 20% | 200 | 50 | 300 | |||||||
| Curlleaf mountain-mahogany | Replacement | 31% | 250 | 100 | 500 | ||||||
| Mixed | 37% | 212 | 50 | ||||||||
| Surface or low | 31% | 250 | 50 | ||||||||
| Gambel oak | Replacement | 75% | 50 | ||||||||
| Mixed | 25% | 150 | |||||||||
| Mountain big sagebrush | Replacement | 100% | 48 | 15 | 100 | ||||||
| Mountain big sagebrush (cool sagebrush) | Replacement | 75% | 100 | ||||||||
| Mixed | 25% | 300 | |||||||||
| Mountain big sagebrush with conifers | Replacement | 100% | 49 | 15 | 100 | ||||||
| Mountain shrubland with trees | Replacement | 22% | 105 | 100 | 200 | ||||||
| Mixed | 78% | 29 | 25 | 100 | |||||||
| Wyoming big sagebrush semidesert | Replacement | 86% | 200 | 30 | 200 | ||||||
| Mixed | 9% | >1,000 | 20 | >1,000 | |||||||
| Surface or low | 5% | >1,000 | 20 | >1,000 | |||||||
| Wyoming big sagebrush semidesert with trees | Replacement | 84% | 137 | 30 | 200 | ||||||
| Mixed | 11% | >1,000 | 20 | >1,000 | |||||||
| Surface or low | 5% | >1,000 | 20 | >1,000 | |||||||
| Wyoming big sagebrush steppe | Replacement | 89% | 92 | 30 | 120 | ||||||
| Mixed | 11% | 714 | 120 | ||||||||
| Great Basin Woodland | |||||||||||
| Juniper and pinyon-juniper steppe woodland | Replacement | 20% | 333 | 100 | >1,000 | ||||||
| Mixed | 31% | 217 | 100 | >1,000 | |||||||
| Surface or low | 49% | 135 | 100 | ||||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||||||
| Mixed | 17% | 60 | |||||||||
| Surface or low | 78% | 13 | |||||||||
| Great Basin Forested | |||||||||||
| Aspen with conifer (low to midelevations) | Replacement | 53% | 61 | 20 | |||||||
| Mixed | 24% | 137 | 10 | ||||||||
| Surface or low | 23% | 143 | 10 | ||||||||
| Aspen with conifer (high elevations) | Replacement | 47% | 76 | 40 | |||||||
| Mixed | 18% | 196 | 10 | ||||||||
| Surface or low | 35% | 100 | 10 | ||||||||
| Aspen-cottonwood, stable aspen without conifers | Replacement | 31% | 96 | 50 | 300 | ||||||
| Surface or low | 69% | 44 | 20 | 60 | |||||||
| Aspen, stable without conifers | Replacement | 81% | 150 | 50 | 300 | ||||||
| Surface or low | 19% | 650 | 600 | >1,000 | |||||||
| Aspen with spruce-fir | Replacement | 38% | 75 | 40 | 90 | ||||||
| Mixed | 38% | 75 | 40 | ||||||||
| Surface or low | 23% | 125 | 30 | 250 | |||||||
| Douglas-fir (Great Basin, dry) | Replacement | 12% | 90 | 600 | |||||||
| Mixed | 14% | 76 | 45 | ||||||||
| Surface or low | 75% | 14 | 10 | 50 | |||||||
| Douglas-fir (interior, warm mesic) | Replacement | 28% | 170 | 80 | 400 | ||||||
| Mixed | 72% | 65 | 50 | 250 | |||||||
| Ponderosa pine-Douglas-fir | Replacement | 10% | 250 | >1,000 | |||||||
| Mixed | 51% | 50 | 50 | 130 | |||||||
| Surface or low | 39% | 65 | 15 | ||||||||
| Ponderosa pine, interior | Replacement | 5% | 161 | 800 | |||||||
| Mixed | 10% | 80 | 50 | 80 | |||||||
| Surface or low | 86% | 9 | 8 | 10 | |||||||
| Spruce-fir-pine (subalpine) | Replacement | 98% | 217 | 75 | 300 | ||||||
| Mixed | 2% | >1,000 | |||||||||
| Northern and Central Rockies | |||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||
| Northern and Central Rockies Grassland | |||||||||||
| Mountain grassland | Replacement | 60% | 20 | 10 | |||||||
| Mixed | 40% | 30 | |||||||||
| Northern prairie grassland | Replacement | 55% | 22 | 2 | 40 | ||||||
| Mixed | 45% | 27 | 10 | 50 | |||||||
| Northern and Central Rockies Shrubland | |||||||||||
| Basin big sagebrush | Replacement | 60% | 100 | 10 | 150 | ||||||
| Mixed | 40% | 150 | |||||||||
| Riparian (Wyoming) | Mixed | 100% | 100 | 25 | 500 | ||||||
| Low sagebrush shrubland | Replacement | 100% | 125 | 60 | 150 | ||||||
| Mountain big sagebrush steppe and shrubland | Replacement | 100% | 70 | 30 | 200 | ||||||
| Mountain shrub, nonsagebrush | Replacement | 80% | 100 | 20 | 150 | ||||||
| Mixed | 20% | 400 | |||||||||
| Salt desert shrub | Replacement | 50% | >1,000 | 500 | >1,000 | ||||||
| Mixed | 50% | >1,000 | 500 | >1,000 | |||||||
| Wyoming big sagebrush | Replacement | 63% | 145 | 80 | 240 | ||||||
| Mixed | 37% | 250 | |||||||||
| Northern and Central Rockies Woodland | |||||||||||
| Ancient juniper | Replacement | 100% | 750 | 200 | >1,000 | ||||||
| Northern and Central Rockies Forested | |||||||||||
| Douglas-fir (cold) | Replacement | 31% | 145 | 75 | 250 | ||||||
| Mixed | 69% | 65 | 35 | 150 | |||||||
| Douglas-fir (warm mesic interior) | Replacement | 28% | 170 | 80 | 400 | ||||||
| Mixed | 72% | 65 | 50 | 250 | |||||||
| Douglas-fir (xeric interior) | Replacement | 12% | 165 | 100 | 300 | ||||||
| Mixed | 19% | 100 | 30 | 100 | |||||||
| Surface or low | 69% | 28 | 15 | 40 | |||||||
| Grand fir-Douglas-fir-western larch mix | Replacement | 29% | 150 | 100 | 200 | ||||||
| Mixed | 71% | 60 | 3 | 75 | |||||||
| Grand fir-lodgepole pine-western larch-Douglas-fir | Replacement | 31% | 220 | 50 | 250 | ||||||
| Mixed | 69% | 100 | 35 | 150 | |||||||
| Lodgepole pine, lower subalpine | Replacement | 73% | 170 | 50 | 200 | ||||||
| Mixed | 27% | 450 | 40 | 500 | |||||||
| Lodgepole pine, persistent | Replacement | 89% | 450 | 300 | 600 | ||||||
| Mixed | 11% | >1,000 | |||||||||
| Lower subalpine (Wyoming and Central Rockies) | Replacement | 100% | 175 | 30 | 300 | ||||||
| Mixed-conifer upland western redcedar-western hemlock | Replacement | 67% | 225 | 150 | 300 | ||||||
| Mixed | 33% | 450 | 35 | 500 | |||||||
| Ponderosa pine (Black Hills, low elevation) | Replacement | 7% | 300 | 200 | 400 | ||||||
| Mixed | 21% | 100 | 50 | 400 | |||||||
| Surface or low | 71% | 30 | 5 | 50 | |||||||
| Ponderosa pine (Black Hills, high elevation) | Replacement | 12% | 300 | ||||||||
| Mixed | 18% | 200 | |||||||||
| Surface or low | 71% | 50 | |||||||||
| Ponderosa pine (Northern and Central Rockies) | Replacement | 4% | 300 | 100 | >1,000 | ||||||
| Mixed | 19% | 60 | 50 | 200 | |||||||
| Surface or low | 77% | 15 | 3 | 30 | |||||||
| Ponderosa pine (Northern Great Plains) | Replacement | 5% | 300 | ||||||||
| Mixed | 20% | 75 | |||||||||
| Surface or low | 75% | 20 | 10 | 40 | |||||||
| Ponderosa pine-Douglas-fir | Replacement | 10% | 250 | >1,000 | |||||||
| Mixed | 51% | 50 | 50 | 130 | |||||||
| Surface or low | 39% | 65 | 15 | ||||||||
| Upper subalpine spruce-fir (Central Rockies) | Replacement | 100% | 300 | 100 | 600 | ||||||
| Western redcedar | Replacement | 87% | 385 | 75 | >1,000 | ||||||
| Mixed | 13% | >1,000 | 25 | ||||||||
| Northern Great Plains | |||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||
| Northern Plains Grassland | |||||||||||
| Northern mixed-grass prairie | Replacement | 67% | 15 | 8 | 25 | ||||||
| Mixed | 33% | 30 | 15 | 35 | |||||||
| Northern Plains Woodland | |||||||||||
| Great Plains floodplain | Replacement | 100% | 500 | ||||||||
| Northern Great Plains wooded draws and ravines | Replacement | 38% | 45 | 30 | 100 | ||||||
| Mixed | 18% | 94 | |||||||||
| Surface or low | 43% | 40 | 10 | ||||||||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [39,71]. |
|||||||||||
1. Agee, James K. 1998. The landscape ecology of western forest fire regimes. Northwest Science. 72(17): 24-34. [85053]
2. Allen, Craig Daniel. 1989. Changes in the landscape of the Jemez Mountains, New Mexico. Berkeley, CA: University of California. 346 p. Dissertation. [42116]
3. Anderson, J. P. 1959. Flora of Alaska and adjacent parts of Canada. Ames, IA: Iowa State University Press. 543 p. [9928]
4. Arno, Stephen F.; Hammerly, Ramona P. 1977. Northwest trees. Seattle, WA: The Mountaineers. 222 p. [4208]
5. Baker, William L. 1989. Classification of the riparian vegetation of the montane and subalpine zones in western Colorado. The Great Basin Naturalist. 49(2): 214-228. [7985]
6. Banner, Roger E. 1992. Vegetation types of Utah. Journal of Range Management. 14(2): 109-114. [20298]
7. Blower, Dan. 1982. Key winter forage plants for B.C. ungulates. Victoria, BC: British Columbia Ministry of the Environment, Terrestrial Studies Branch. 57 p. [17065]
8. Booth, W. E.; Wright, J. C. 1962. Flora of Montana: Part II--Dicotyledons. [Revised]. Bozeman, MT: Montana State College, Department of Botany and Bacteriology. 280 p. [47286]
9. Brasher, Jeffrey W. 2001. Betulaceae birch family. Journal of the Arizona-Nevada Academy of Science. 33(1): 1-8. [85036]
10. Breitung, August J. 1957. Annotated catalogue of the vascular flora of Saskatchewan. The American Midland Naturalist. 58(1): 1-72. [85037]
11. Butler, Bertram T. 1909. The western American birches. Bulletin of the Torrey Botanical Club. 36(8): 421-440. [66188]
12. Carlson, Jack R.; Conaway, Gary L.; Gibbs, Jacy L.; Hoag, J. Chris. 1992. Design criteria for revegetation in riparian zones of the Intermountain area. In: Clary, Warren P.; McArthur, E. Durant; Bedunah, Don; Wambolt, Carl L., compilers. Proceedings--symposium on ecology and management of riparian shrub communities; 1991 May 29-31; Sun Valley, ID. Gen. Tech. Rep. INT-289. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 145-150. [19109]
13. Carter, Jack L. 1997. Trees and shrubs of New Mexico. Boulder, CO: Johnson Books. 534 p. [72647]
14. Collins, Ellen I. 1984. Preliminary classification of Wyoming plant communities. Cheyenne, WY: Wyoming Natural Heritage Program; The Nature Conservancy. 42 p. [661]
15. Cordes, L. D.; Hughes, F. M. R.; Getty, M. 1997. Factors affecting the regeneration and distribution of riparian woodlands along a northern prairie river: the Red Deer River, Alberta, Canada. Journal of Biogeography. 24(5): 675-695. [85038]
16. Cormack, R. G. H. 1953. A survey of coniferous forest succession in the eastern Rockies. The Forestry Chronicle. 29(3): 218-232. [16458]
17. Crawford, Rex C.; Kagan, Jimmy. 2001. 25. Eastside riparian-wetlands. In: Chappell, Christopher B.; Crawford, Rex C.; Barrett, Charley; Kagan, Jimmy; Johnson, David H.; O'Mealy, Mikell; Green, Greg A.; Ferguson, Howard L.; Edge, W. Daniel; Greda, Eva L.; O'Neil, Thomas A. Wildlife habitats: descriptions, status, trends, and system dynamics. In: Johnson, David H.; O'Neil, Thomas A., managing directors. Wildlife-habitat relationships in Oregon and Washington. Corvallis, OR: Oregon State University Press: 98-100. [67918]
18. Dayton, William A. 1931. Important western browse plants. Misc. Publ. No. 101. Washington, DC: U.S. Department of Agriculture. 214 p. [768]
19. Dearness, John; Hansbrough, J. R. 1934. Cytospora infection following fire injury in western British Columbia. Canadian Journal of Research. 10(1): 125-128. [33069]
20. Disalvo, Angela C.; Hart, Stephen C. 2002. Climatic and stream-flow controls on tree growth in a western montane riparian forest. Environmental Management. 30(5): 678-691. [85039]
21. Dixon, Helen. 1935. Ecological studies on the high plateaus of Utah. Botanical Gazette. 97(2): 272-320. [15672]
22. Dowding, Eleanor S. 1929. The vegetation of Alberta: III. The sandhill areas of central Alberta with particular reference to the ecology of Arceuthobium americanum Nutt. Journal of Ecology. 17(1): 82-105. [63560]
23. Dugle, Janet R. 1966. A taxonomic study of western Canadian species in the genus Betula. Canadian Journal of Botany. 44(7): 929-1007. [66573]
24. Dumroese, R. Kasten; Hutton, Kathy M.; Wenny, David L. 1997. Propagating woody riparian plants in nurseries. In: Landis, Thomas D.; Thompson, Jan R., tech. coords. National proceedings: forest and conservation nursery associations--1997; Regeneration, reforestation, restoration: The seedling is the key; 1997 August 11-14; Bemidji, MN; 1997 August 19-21; Boise, ID. Gen. Tech. Rep. PNW-GTR-419. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 71-76. [29119]
25. Dwire, Kathleen A.; Kauffman, J. Boone. 2003. Fire and riparian ecosystems in landscapes of the western USA. In: Young, Michael K.; Gresswell, Robert E.; Luce, Charles H., eds. Selected papers from an international symposium on effects of wildland fire on aquatic ecosystems in the western USA; 2002 April 22-24; Boise, ID. In: Forest Ecology and Management. Special Issue: The effects of wildland fire on aquatic ecosystems in the western USA. 178(1-2): 61-74. [44923]
26. Ehleringer, James R.; Arnow, Lois A.; Arnow, Ted; McNulty, Irving B.; Negus, Norman C. 1992. Red Butte Canyon Research Natural Area: history, flora, geology, climate, and ecology. The Great Basin Naturalist. 52(2): 95-121. [19687]
27. Erdman, Kimball S. 1961. Distribution of the native trees of Utah. Brigham Young University Science Bulletin: Biological Series. 11(3): 1-34. [35781]
28. Fechner, Gilbert H. 1980. Blue spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 95-96. [50031]
29. Fechner, Gilbert H. 1990. Picea pungens Engelm. blue spruce. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 238-249. [13387]
30. Fletcher, Robert J., Jr. 2009. Does attraction to conspecifics explain the patch-size effect? An experimental test. Oikos. 118(8): 1139-1147. [77001]
31. Flora of North America Editorial Committee, eds. 2012. Flora of North America north of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
32. Foote, Geoffrey G. 1965. Phytosociology of the bottomland hardwood forests in western Montana. Missoula, MT: University of Montana. 140 p. Thesis. [17369]
33. Friedman, Jonathan M.; Auble, Gregor T.; Andrews, Edmund D.; Kittel, Gwen; Madole, Richard F.; Griffin, Eleanor R.; Allred, Tyler M. 2006. Transverse and longitudinal variation in woody riparian vegetation along a montane river. Western North American Naturalist. 66(1): 78-91. [62273]
34. Garman, E. H. 1929. Natural reproduction following fires in central British Columbia. The Forestry Chronicle. 5(3): 28-44. [20224]
35. Great Plains Flora Association. 1986. Flora of the Great Plains. Lawrence, KS: University Press of Kansas. 1392 p. [1603]
36. Griffin, James R.; Critchfield, William B. 1972. The distribution of forest trees in California. Res. Pap. PSW-82. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 118 p. [1041]
37. Gullion, Gordon W. 1964. Wildlife uses of Nevada plants. Contributions toward a flora of Nevada: No. 49. CR-24-64. Beltsville, MD: U.S. Department of Agriculture, Agricultural Research Service, Crops Research Division; Washington, DC: U.S. National Arboretum, Herbarium. 170 p. [6729]
38. Hamann, Andreas; Wang, Tongli. 2006. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology. 87(11): 2773-2786. [85040]
39. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org/niftt/released/FRCC_Guidebook_2010_final.pdf. [81749]
40. Hansen, Paul L.; Chadde, Steve W.; Pfister, Robert D. 1988. Riparian dominance types of Montana. Misc. Publ. No. 49. Missoula, MT: University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station. 411 p. [5660]
41. Hansen, Paul L.; Hoffman, George R.; Bjugstad, Ardell J. 1984. The vegetation of Theodore Roosevelt National Park, North Dakota: a habitat type classification. Gen. Tech. Rep. RM-113. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 35 p. [1077]
42. Hansen, Paul L.; Pfister, Robert D.; Boggs, Keith; Cook, Bradley J.; Joy, John; Hinckley, Dan K. 1995. Classification and management of Montana's riparian and wetland sites. Miscellaneous Publication No. 54. Missoula, MT: The University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station. 646 p. [24768]
43. Harlan, Annita; Dennis, Arthur E. 1976. A preliminary plant geography of Canyon de Chelly National Monument. Journal of the Arizona Academy of Science. 11(2): 69-78. [75981]
44. Harrington, H. D. 1964. Manual of the plants of Colorado. 2nd ed. Chicago, IL: The Swallow Press. 666 p. [6851]
45. Harris, Richard R. 1989. Riparian communities of the Sierra Nevada and their environmental relationships. In: Abell, Dana L., tech. coord. Proceedings of the California riparian systems conference: Protection, management, and restoration for the 1990's; 1988 September 22-24; Davis, CA. Gen. Tech. Rep. PSW-110. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 393-398. [13768]
46. Hayes, Doris W.; Garrison, George A. 1960. Key to important woody plants of eastern Oregon and Washington. Agric. Handb. 148. Washington, DC: U.S. Department of Agriculture, Forest Service. 227 p. [1109]
47. Hayward, Herman E. 1928. Studies of plants in the Black Hills of South Dakota. Botanical Gazette. 85(4): 353-412. [1110]
48. Hickman, James C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p. [21992]
49. Hitchcock, C. Leo; Cronquist, Arthur. 1973. Flora of the Pacific Northwest. Seattle, WA: University of Washington Press. 730 p. [1168]
50. Holiday, Susan. 2000. A floristic study of Tsegi Canyon, Arizona. Madrono. 47(1): 29-42. [38998]
51. Holstein, Glen. 1984. California riparian forests: deciduous islands in an evergreen sea. In: Warner, Richard E.; Hendrix, Kathleen M., eds. California riparian systems: Ecology, conservation, and productive management: Proceedings of a conference; 1981 September 17-19; Davis, CA. Berkeley, CA: University of California Press: 2-22. [5830]
52. Horner, Michael A. 2001. Vascular flora of the Glass Mountain region, Mono County, California. Aliso. 20(2): 75-105. [53374]
53. Horton, Howard, ed./comp. 1989. Interagency forage and conservation planting guide for Utah. Extension Circular 433. Logan, UT: Utah State University, Cooperative Extension Service. 67 p. [12231]
54. Hosie, R. C. 1969. Native trees of Canada. 7th ed. Ottawa, ON: Canadian Forestry Service, Department of Fisheries and Forestry. 380 p. [3375]
55. Hough, Walter. 1898. Environmental interrelations in Arizona. American Anthropologist. 11(5): 133-155. [85041]
56. Hudak, Howard G.; Ketcheson, Gary L. 1992. Willow community types as influenced by valley bottom and stream types. In: Clary, Warren P.; McArthur, E. Durant; Bedunah, Don; Wambolt, Carl L., compilers. Proceedings--symposium on ecology and management of riparian shrub communities; 1991 May 29-31; Sun Valley, ID. Gen. Tech. Rep. INT-289. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 16-17. [19089]
57. Jankovsky-Jones, Mabel; Rust, Steven K.; Moseley, Robert K. 1999. Riparian reference areas in Idaho: a catalog of plant associations and conservation sites. Gen. Tech. Rep. RMRS-GTR-20. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 141 p. [29900]
58. Johnson, Carl M. 1970. Common native trees of Utah. Special Report 22. Logan, UT: Utah State University, College of Natural Resources, Agricultural Experiment Station. 109 p. [9785]
59. Jones, Cindy L.; Harrington, John T.; Dreesen, David R. 2002. Refinement and stratification of thinleaf alder and water birch seeds from New Mexico. Native Plants Journal. 3(2): 142-150. [85042]
60. Karrfalt, Robert P. 2008. Betula L.: birch. In: Bonner, Franklin T.; Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 303-312. [67844]
61. Kartesz, John T. 1999. A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. 1st ed. In: Kartesz, John T.; Meacham, Christopher A. Synthesis of the North American flora (Windows Version 1.0), [CD-ROM]. Chapel Hill, NC: North Carolina Botanical Garden (Producer). In cooperation with: The Nature Conservancy; U.S. Department of Agriculture, Natural Resources Conservation Service; U.S. Department of the Interior, Fish and Wildlife Service. [36715]
62. Kartesz, John Thomas. 1988. A flora of Nevada. Reno, NV: University of Nevada. 1729 p. Dissertation. [In 2 volumes]. [42426]
63. Kasworm, Wayne F.; Irby, Lynn R.; Ihsle Pac, Helga B. 1984. Diets of ungulates using winter ranges in northcentral Montana. Journal of Range Management. 37(1): 67-71. [63610]
64. Kearney, Thomas H.; Peebles, Robert H.; Howell, John Thomas; McClintock, Elizabeth. 1960. Arizona flora. 2nd ed. Berkeley, CA: University of California Press. 1085 p. [6563]
65. Kelly, George W. 1970. A guide to the woody plants of Colorado. Boulder, CO: Pruett Publishing. 180 p. [6379]
66. Knight, Dennis H.; Jones, George P.; Akashi, Yoshiko; Myers, Richard W. 1987. Vegetation ecology in the Bighorn Canyon National Recreation Area: Wyoming and Montana. Final Report. Laramie, WY: University of Wyoming; National Park Service Research Center. 114 p. [12498]
67. Kovalchik, Bernard L.; Clausnitzer, Rodrick R. 2004. Classification and management of aquatic, riparian, and wetland sites on the national forests of eastern Washington: series description. Gen. Tech. Rep. PNW-GTR-593. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 354 p. [53329]
68. Krajina, V. J.; Klinka, K.; Worrall, J. 1982. Distribution and ecological characteristics of trees and shrubs of British Columbia. Vancouver, BC: University of British Columbia. 131 p. [6728]
69. Kufeld, Roland C.; Wallmo, O. C.; Feddema, Charles. 1973. Foods of the Rocky Mountain mule deer. Res. Pap. RM-111. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 31 p. [1387]
70. Lackschewitz, Klaus. 1991. Vascular plants of west-central Montana--identification guidebook. Gen. Tech. Rep. INT-227. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 648 p. [13798]
71. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: http://www.landfire.gov/downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
72. LANDFIRE Rapid Assessment. 2007. Rapid assessment reference condition models, [Online]. In: LANDFIRE. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; The Nature Conservancy (Producers). Available: http://www.landfire.gov/models_EW.php [2008, April 18] [66533]
73. Lanner, Ronald M. 1983. Trees of the Great Basin: A natural history. Reno, NV: University of Nevada Press. 215 p. [1401]
74. Larsen, J. A. 1923. Association of trees, shrubs, and other vegetation in the northern Idaho forests. Ecology. 4(1): 63-67. [60168]
75. Larsen, J. A. 1929. Fires and forest succession in the Bitterroot Mountains of northern Idaho. Ecology. 10(1): 67-76. [6990]
76. Leach, Howard R. 1956. Food habits of the Great Basin deer herds of California. California Fish and Game. 38: 243-308. [3502]
77. Leege, Thomas A.; Hickey, William O. 1966. Lochsa elk study. Job Completion Report: Big game surveys and investigations. Federal Aid to Wildlife Restoration Project: W-85-R-17. Job No. 8: July 1, 1965 to June 30, 1966. Boise, ID: State of Idaho Fish and Game Department. 22 p. [16759]
78. Lindsey, Alton A. 1953. Notes of some plant communities in the northern Mackenzie Basin, Canada. Botanical Gazette. 115(1): 44-55. [62728]
79. Little, Elbert L., Jr. 1950. Southwestern trees: A guide to the native species of New Mexico and Arizona. Agric. Handb. No. 9. Washington, DC: U.S. Department of Agriculture, Forest Service. 109 p. [20317]
80. Lloyd, D.; Angove, K.; Hope, G.; Thompson, C. 1990. A guide to site identification and interpretation for the Kamloops Forest Region. Land Management Handbook No. 23. Victoria, BC: British Columbia Ministry of Forests, Research Branch. 399 p. [37061]
81. Lowe, Charles H. 1964. Arizona's natural environment: Landscapes and habitats. Tucson, AZ: The University of Arizona Press. 136 p. [20736]
82. Magruder, T. L. 1985. Wind Cave's riparian habitats. South Dakota Conservation Digest. 52(3): 20-23. [13752]
83. Manning, Mary E.; Padgett, Wayne G. 1989. Preliminary riparian community type classification for Nevada. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Region. Preliminary draft. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 135 p. [11531]
84. Manning, Mary E.; Padgett, Wayne G. 1995. Riparian community type classification for Humboldt and Toiyabe National Forests, Nevada and eastern California. R4-Ecol-95-01. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Region. 306 p. [42196]
85. Marr, John W. 1961. Ecosystems of the east slope of the Front Range in Colorado. University of Colorado Studies, Series in Biology: No. 8. Boulder, CO: University of Colorado Press. 134 p. [5724]
86. Martin, William C.; Hutchins, Charles R. 1981. A flora of New Mexico. Volume 2. Germany: J. Cramer. 2589 p. [37176]
87. Merigliano, Michael F. 1996. Ecology and management of the South Fork Snake River cottonwood forest. Tech. Bull. 96-9. Boise, ID: U.S. Department ot the Interior, Bureau of Land Management, Idaho State Office. 79 p. [27350]
88. Merritt, David M.; Wohl, Ellen E. 2002. Processes governing hydrochory along rivers: hydraulics, hydrology, and dispersal phenology. Ecological Applications. 12(4): 1071-1087. [43078]
89. Merritt, David M.; Wohl, Ellen E. 2006. Plant dispersal along rivers fragmented by dams. River Research and Applications. 22(1): 1-26. [61821]
90. Miller, Thomas B.; Johnson, Frederic D. 1986. Sampling and data analyses of narrow, variable-width gallery forests over environmental gradients. Tropical Ecology. 27: 132-142. [12310]
91. Monsen, Stephen B.; Stevens, Richard; Shaw, Nancy L. 2004. Shrubs of other families. In: Monsen, Stephen B.; Stevens, Richard; Shaw, Nancy L., comps. Restoring western ranges and wildlands. Gen. Tech. Rep. RMRS-GTR-136-vol-2. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 598-698. [52846]
92. Mooney, H. A. 1973. Plant communities and vegetation. In: Lloyd, Robert M.; Mitchell, Richard S., eds. A flora of the White Mountains: California and Nevada. Berkeley, CA: University of California Press: 7-17. [77475]
93. Morris, Melvin S.; Schmautz, Jack E.; Stickney, Peter F. 1962. Winter field key to the native shrubs of Montana. Bulletin No. 23. Missoula, MT: Montana State University, Montana Forest and Conservation Experiment Station. 70 p. [17063]
94. Moseley, Robert K. 1998. Riparian and wetland community inventory of 14 reference areas in southwestern Idaho. Technical Bulletin No. 98-5. Boise, Idaho: U.S. Department of the Interior, Bureau of Land Management, Boise State Office. 52 p. [75569]
95. Mowat, Catherine. 1990. Fire effects study for Quail Flats fire, Dinosaur Provincial Park. Calgary, AB: Alberta Recreation, Parks and Wildlife Foundation, Dinosaur National Park. 37 p. [+ appendices]. On file with: U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Fire Sciences Laboratory, Missoula, MT. [17454]
96. Muldavin, Esteban; Durkin, Paula; Bradley, Mike; Stuever, Mary; Mehlhop, Patricia. 2000. Handbook of wetland vegetation communities of New Mexico. Volume 1: classification and community descriptions. Albuquerque, NM: University of New Mexico, Biology Department; New Mexico Natural Heritage Program. 172 p. [+ appendices]. [45517]
97. Nevada Department of Conservation and Natural Resources, Nevada Natural Heritage Program. 2003. National vegetation classification for Nevada, [Online]. Carson City, NV: Nevada Department of Conservation and Natural Resources, Nevada Natural Heritage Program (Producer). 15 p. Available: http://heritage.nv.gov/ecology/nv_nvc.htm [2005, November 3]. [55021]
98. Ohmann, Janet L.; Spies, Thomas A. 1998. Regional gradient analysis and spatial pattern of woody plant communities of Oregon forests. Ecological Monographs. 68(2): 151-182. [62828]
99. Olson, Diana L. 2000. Fire in riparian zones: a comparison of historical fire occurrence in riparian and upslope forests in the Blue Mountains and southern Cascades of Oregon. Seattle, WA: University of Washington. 274 p. Thesis. [46928]
100. Olson, R. A.; Gerhart, W. A. 1982. A physical and biological characterization of riparian habitat and its importance to wildlife in Wyoming. Cheyenne, WY: Wyoming Game and Fish Department. 188 p. [6755]
101. Ortega, Catherine P.; Ortega, Joseph C. 2003. Comparison of black-headed grosbeaks nesting in riparian and gambel oak pastures in southwestern Colorado. The Southwestern Naturalist. 48(3): 383-388. [46060]
102. Patterson, Patricia A.; Neiman, Kenneth E.; Tonn, Jonalea. 1985. Field guide to forest plants of northern Idaho. Gen. Tech. Rep. INT-180. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 246 p. [1839]
103. Peek, James M. 1963. Appraisal of a moose range in southwestern Montana. Journal of Range Management. 16(5): 227-231. [16489]
104. Peet, Robert K. 1988. Forests of the Rocky Mountains. In: Barbour, Michael G.; Billings, William Dwight, eds. North American terrestrial vegetation. New York: Cambridge University Press: 63-101. [6714]
105. Rabe, Fred W.; Elzinga, Caryl; Breckenridge, Roy. 1994. Classification of meandering glide and spring stream natural areas in Idaho. Natural Areas Journal. 14(3): 188-202. [23961]
106. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
107. Reinhart, Kurt O.; Gurnee, Julie; Tirado, Reyes; Callaway, Ragan M. 2006. Invasion through quantitative effects: intense shade drives native decline and invasive success. Ecological Applications. 16(5): 1821-1831. [65277]
108. Renecker, Lyle A.; Schwartz, Charles C. 2007. Food habits and feeding behavior. In: Franzmann, Albert W.; Schwartz, Charles C.; McCabe, Richard E., eds. Ecology and management of the North American moose. 2nd ed. Boulder, CO: University Press of Colorado: 403-440. [79106]
109. Riegel, Gregg M.; Miller, Richard F.; Skinner, Carl N.; Smith, Sydney E. 2006. Northeastern Plateaus bioregion. In: Sugihara, Neil G.; van Wagtendonk, Jan W.; Shaffer, Kevin E.; Fites-Kaufman, Joann; Thode, Andrea E., eds. Fire in California's ecosystems. Berkeley, CA: University of California Press: 225-263. [65541]
110. Rigg, George B. 1922. Birch succession in sphagnum bogs. Journal of Forestry. 20(8): 848-850. [85045]
111. Royer, Todd V.; Minshall, G. Wayne. 1997. Temperature patterns in small streams following wildfire. Archiv fur Hydrobiologie. 140(2): 237-242. [30305]
112. Rydberg, P. A. 1920. Phytogeographical notes on the Rocky Mountain region. IX. Wooded formations of the montane zone of the Southern Rockies. Bulletin of the Torrey Botanical Club. 47(10): 441-454. [64247]
113. Saab, Victoria. 1999. Importance of spatial scale to habitat use by breeding birds in riparian forests: a hierarchical analysis. Ecological Applications. 9(1): 135-151. [85047]
114. Sampson, Arthur W.; Jespersen, Beryl S. 1963. California range brushlands and browse plants. Berkeley, CA: University of California, Division of Agricultural Sciences; California Agricultural Experiment Station, Extension Service. 162 p. [3240]
115. Sargent, Charles S. 1901. New or little known North American trees. III. Botanical Gazette. 31(4): 217-240. [85048]
116. Schneider, Rick E.; Faber-Langendoen, Don; Crawford, Rex C.; Weakley, Alan S. 1997. The status of biodiversity in the Great Plains: Great Plains vegetation classification--Supplemental document 1. [Cooperative Agreement # X 007803-01-3]. In: Ostlie, Wayne R.; Schneider, Rick E.; Aldrich, Janette Marie; Faust, Thomas M.; McKim, Robert L. B.; Chaplin, Stephen J., comps. The status of biodiversity in the Great Plains. Arlington, VA: The Nature Conservancy, Great Plains Program. 75 p. Available online: http://conserveonline.org/docs/2005/02/greatplains_vegclass_97.pdf [2011, September 8]. [62020]
117. Scoggan, H. J. 1978. The flora of Canada. Part 3: Dicotyledoneae (Saururaceae to Violaceae). National Museum of Natural Sciences: Publications in Botany, No. 7(3). Ottawa: National Museums of Canada. 1115 p. [75493]
118. Scott, Peter A., Staniforth, Richard J.; Fayle, David C. F. 1992. Re-examination of a water birch, Betula occidentalis, outlier of the northwestern Hudson Bay lowlands. The Canadian Field-Naturalist. 106(3): 348-351. [85049]
119. Skinner, Carl N. 2003. A tree-ring based fire history of riparian reserves in the Klamath Mountains. In: Faber, Phyllis M., ed. California riparian systems: processes and floodplains management, ecology and restoration. Riparian habitat and floodplains conference proceedings; 2001 March 12-15; Sacramento, CA. Sacramento, CA: Riparian Habitat Joint Venture: 116-119. [85054]
120. Skinner, Carl N.; Taylor, Alan H.; Agee, James K. 2006. Klamath Mountains bioregion. In: Sugihara, Neil G.; van Wagtendonk, Jan W.; Shaffer, Kevin E.; Fites-Kaufman, Joann; Thode, Andrea E., eds. Fire in California's ecosystems. Berkeley, CA: University of California Press: 170-194. [65539]
121. Smith, Arthur D. 1953. Consumption of native forage species by captive mule deer during summer. Journal of Range Management. 6(1): 30-37. [2161]
122. Smith, Stanley D.; Wellington, A. Bruce. 1991. Functional responses of riparian vegetation to streamflow diversion in the eastern Sierra Nevada. Ecological Applications. 1(1): 89-97. [39201]
123. Soper, James H.; Heimburger, Margaret L. 1982. Shrubs of Ontario. Life Sciences Miscellaneous Publications. Toronto, ON: Royal Ontario Museum. 495 p. [12907]
124. Stelfox, J. G.; Lynch, G. M.; McGillis, J. R. 1976. Effects of clearcut logging on wild ungulates in the central Albertan foothills. The Forestry Chronicle. 52(2): 65-70. [13506]
125. Stephens, H. A. 1973. Woody plants of the north Central Plains. Lawrence, KS: The University Press of Kansas. 530 p. [3804]
126. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in Northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
127. Sutton, Richard F.; Johnson, Craig W. 1974. Landscape plants from Utah's mountains. EC-368. Logan, UT: Utah State University, Cooperative Extension Service. 135 p. [49]
128. Thorne, Robert F. 1982. The desert and other transmontane plant communities of southern California. Aliso. 10(2): 219-257. [3768]
129. Timoney, Kevin P.; La Roi, George H.; Zoltai, Stephen C.; Robinson, Anne L. 1993. Vegetation communities and plant distributions and their relationships with parent materials in the forest-tundra of northwestern Canada. Ecography. 16(2): 174-188. [23007]
130. Turner, Nancy Chapman; Bell, Marcus A. M. 1971. The ethnobotany of the Coast Salish Indians of Vancouver Island. Economic Botany. 25(3): 63-104. [21014]
131. U.S. Department of Agriculture, Natural Resources Conservation Service. 2012. PLANTS Database, [Online]. Available: https://plants.usda.gov /. [34262]
132. Vines, Robert A. 1960. Trees, shrubs, and woody vines of the Southwest. Austin, TX: University of Texas Press. 1104 p. [7707]
133. Walford, Gillian; Jones, George; Fertig, Walt; Mellman-Brown, Sabine; Houston, Kent E. 2001. Riparian and wetland plant community types of the Shoshone National Forest. Gen. Tech. Rep. RMRS-GTR-85. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Cody, WY: U.S. Department of Agriculture, Natural Resources Conservation Service, Cody Conservation District. 122 p. [40599]
134. Walters, M. Alice; Teskey, Robert O.; Hinckley, Thomas M. 1980. Impact of water level changes on woody riparian and wetland communities. Volume 7: Mediterranean region, western arid and semi-arid region. FWS/OBS-78/93. Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service, Biological Services Program. 84 p. [52899]
135. Walters, M. Alice; Teskey, Robert O.; Hinckley, Thomas M. 1980. Impact of water level changes on woody riparian and wetland communities. Volume 8. Pacific Northwest and Rocky Mountain regions. Final report FWS/OBS 78/94. Kearneysville, WV: U.S. Department of Interior, Fish and Wildlife Service, Biological Services Program. 47 p. [85052]
136. Warkentin, Ian G.; Reed, J. Michael. 1999. Effects of habitat type and degradation on avian species richness in Great Basin riparian habitats. The Great Basin Naturalist. 59(3): 205-212. [85050]
137. Wasser, C. H.; Hess, Karl. 1982. The habitat types of Region 2--U.S. Forest Service: a synthesis. Final report: Cooperative Agreement No. 16-845-CA. Lakewood, CO: U.S. Department of Agriculture, Forest Service, Region 2. 140 p. [5594]
138. Weber, William A. 1987. Colorado flora: western slope. Boulder, CO: Colorado Associated University Press. 530 p. [7706]
139. Welsh, Stanley L.; Atwood, N. Duane; Goodrich, Sherel; Higgins, Larry C., eds. 1987. A Utah flora. The Great Basin Naturalist Memoir No. 9. Provo, UT: Brigham Young University. 894 p. [2944]
140. Williams, J. H., Jr.; Arnold, M. L. 2001. Sources of genetic structure in the woody perennial Betula occidentalis. International Journal of Plant Sciences. 162(5): 1097-1109. [85051]
141. Williamson, Nathan Michael. 1999. Crown fuel characteristics, stand structure, and fire hazard in riparian forests of the Blue Mountains, Oregon. University of Washington. 98 p. Thesis. [85055]
142. Young, Donald R.; Burke, Ingrid C.; Knight, Dennis H. 1985. Water relations of high-elevation phreatophytes in Wyoming. The American Midland Naturalist. 114(2): 384-392. [4968]
143. Young, Robert T. 1907. The forest formations of Boulder County, Colorado. Botanical Gazette. 44(5): 321-352. [64439]
144. Zack, Arthur C.; Morgan, Penelope. 1994. Early succession on two hemlock habitat types in northern Idaho. In: Baumgartner, David M.; Lotan, James E.; Tonn, Jonalea R., compilers. Interior cedar-hemlock-white pine forests: ecology and management: Symposium proceedings; 1993 March 2-4; Spokane, WA. Pullman, WA: Washington State University, Department of Natural Resources: 71-84. [25792]