| FEIS Home Page |
 |
||
| Figure 1. White spruce forest near the Alaska Range. Photo courtesy of L. B. Brubaker. |
AUTHORSHIP AND CITATION:
Abrahamson, Ilana. 2015. Picea glauca, white spruce.
In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: https://www.fs.usda.gov
/database/feis/plants/tree/picgla/all.html
[].
SUMMARY:
|
This review synthesizes the fire effects information and relevant ecology of white spruce in North America that was available in the scientific literature as of 2015. Details and documentation of source materials follow this summary. Introductory: The Introductory section discusses the taxonomy of white spruce, including hybrids and synonyms. Distribution and Occurrence: Although white spruce communities are widespread throughout the North American boreal region, the species is less prominent in the moist, eastern part of the continent and becomes increasingly prominent in the drier western and northwestern regions. Associated trees across much of its range include quaking aspen, paper birch, black spruce, and balsam poplar. White spruce typically occurs on riparian, upland, and treeline sites, although it occurs on a wide range of other sites as well. It grows best on well-drained soils but occurs on a variety of land forms and soil types, with many different plant associates. Botanical and Ecological Characteristics: White spruce crowns are usually densely foliated, and branches and needles are often retained low on the trunk. The arrangement of these vertically continuous branches may promote ignition and torching. White spruce stand structure ranges from very open to closed. Seed production is highly variable; mast years occur episodically, about every 10 to 12 years. Favorable seedbeds include mineral soil, thin organic soil, and rotten logs. White spruce establishment occurs throughout stand development, including early and late postfire succession. It is generally considered a mid- to late-successional species, but it occurs in all stages of boreal forest succession. Fire Effects and Management: Fire of any severity generally kills white spruce. After fire, white spruce typically establishes from seed from trees along fire edges or unburned trees within the burn area. Establishment depends on seed availability, seedbed conditions, fire characteristics, site and soil characteristics, and weather. White spruce abundance may be drastically reduced after fire because of high tree mortality and limited postfire recruitment. White spruce forests may be highly flammable, although less so than black spruce forests. Among white spruce sites, floodplain sites are less flammable than upland sites. Stands with more hardwoods are less flammable than those with a fewer hardwoods. Across white spruce's distribution, fires tend to be more frequent in drier western regions than in wetter eastern regions. White spruce tends to be limited to areas that burn infrequently; white spruce communities often have less frequent fire than other boreal forest types. The longest fire-rotation intervals in the western boreal region are likely in floodplain white spruce stands, where they may be about 300 years. Stands experience crown, surface, and ground fires, and nearly all fires in white spruce communities are stand-replacement. Across a landscape, white spruce communities often display a mosaic of unburned patches and burned patches with stand replacement. Most fires in white spruce stands are small, but large wildfires occurring in extreme fire years account for most acreage burned. Across Arctic and boreal regions, the area burned, fire behavior, and fire severity in white spruce-dominated communities will likely increase with global climate changes, although predicted future fire occurrence varies spatially throughout the region. Prescribed fire is often used to consume logging slash, improve seedbed conditions, and promote regeneration after white spruce stands are logged. However, white spruce regeneration is variable and often inadequate, because prescribed fires often fail to consume sufficient organic material to create large areas of suitable seedbed. Management Considerations: A wide variety of wildlife species use white spruce communities as habitat. Some animals prefer specific postfire successional stages. For example, moose, woodpeckers, and northern hawk owls use early successional stages, while caribou typically use late-seral woodlands. While most wildlife species avoid white spruce browse, it provides important winter forage for some species. Many wildlife species eat the seeds. Climate change is expected to have varied effects on white spruce distribution, growth, and establishment. The pattern, direction, and timing of change depends on local, landscape, and regional climatic and site conditions. Under a warmer climate, many studies suggest that white spruce may expand into areas formerly underlain by permafrost or beyond its current latitudinal and/or altitudinal extent, increase in density at the forest-tundra ecotone, or experience increased growth rates. Other studies suggest that many white spruce communities may be negatively affected by a warming and drying climate. |
Hybrids: White spruce hybridizes with others of its genus [135]. Natural hybrids where distributions of white spruce and other spruces overlap are:
White spruce and Engelmann spruce occur together over large areas in British Columbia, Montana, and Wyoming. White spruce predominates at lower elevations (<5,000 feet (1520 m)), and Engelmann spruce predominates at higher elevations (>6,000 feet (1830 m)). Western white spruce occurs where the 2 species overlap [305].
Lutz spruce occurs in northwestern British Columbia and in parts of Alaska where Sitka spruce and white spruce distributions overlap [305].
Hybrids between black and white spruce, sometimes called Rosendahl spruce, have been reported in Minnesota [252], British Columbia [346] and the forest-tundra treeline in central Canada [239,305].
See Appendix B for scientific names of plant taxa mentioned in this review and for links to available FEIS reviews.
SYNONYMS:
Abies canadensis Miller [135]
Picea alba (Aiton) Link [135]
Picea canadensis (Miller) Britton, Sterns, & Poggenburg [135,197,268]
Picea albertiana (S. Brown) [135]
Picea alba var. albertiana (S. Brown) Beissner [135]
Picea canadensis var. glauca (Moench) Sudworth [135,369]
Pinus alba Aiton [135]
Picea glauca var. albertiana (S. Brown) Sargent [135,197,369]
Picea glauca var. glauca [135,369]
Picea glauca var. porsildii Raup [135,197,369]
 |
| Figure 2. White spruce distribution. Map from USGS: 1971 USDA, Forest Service map provided by [401] |
White spruce is native to the United States and Canada [211,401]. It is primarily a boreal species occurring throughout much of Alaska and Canada, although it extends into the Great Lakes and the northeastern United States. Isolated populations occur in Montana, Wyoming, and South Dakota. White spruce becomes increasingly prominent in forest stands from the moist east to the drier west and northwest [356,358]. In the west, it is widespread in Alaska and all western Canadian provinces; white spruce is especially common in the interior of northern British Columbia [119].
States and provinces [409]:
United States: AK, CT, ID, MA, MD, ME, MI, MN, MT, NH, NY, PA, RI, SD, VT, WI, WY
Canada: AB, BC, LB, MB, NB, NF, NS, NT, NU, ON, PE, QC, SK, YT
Saint Pierre and Miquelon
SITE CHARACTERISTICS:
White spruce typically occurs in cold regions in riparian, upland, and treeline sites. It is the dominant tree species of the dry, usually upland North American boreal forest region [369]. It grows best on well-drained soils but occurs on a wide range of land forms and soil types, with many different associates, in various regional contexts [178,230].
Climate: White spruce grows in regions with long, cold winters and short, cool summers [3], but it can withstand large variations in temperature. In Alaska, Yukon, and Northwest Territories, the January temperature may average -20 °F (-29 °C), and throughout its range in Alaska and Canada the July temperature may average 55 °F (13 °C) [305]. At the northern extent of its distribution, climatic extremes may range from -54 °F (-65 °C) in January to 94 °F (34 °C) in July [305]. Precipitation generally increases from the northwest to the southeast of white spruce's distribution [179]. White spruce sites in Alaska and western Canada receive about 10 inches (250 mm) of precipitation annually, while sites in Nova Scotia and Newfoundland may average 50 inches (1,270 mm) [265,305].
Topography and elevation: White spruce typically grows on floodplains, upland slopes, and treeline sites [83,101,136,137,218], although it grows on a variety of landscape positions [349]. In Alaska and western Canada, lowland white spruce communities frequently occupy river terraces [137,188,303,356,403,415,418], while upland communities generally occupy warm, south-facing slopes [136,349,413,428,435]. In Alaska, white spruce commonly occurs on south-facing slopes within 5 miles (8 km) of major river valleys [137,435]. White spruce is often the dominant tree at altitudinal or arctic treeline [12,67,89,94,257,318,349,432]. In northeastern British Columbia, white spruce is associated with channels and concave slopes, which are generally richer and moister than ridges and convex slopes [9]. In Minnesota, at the southern end of its range, white spruce is often limited to lakeshore sites [143].
White spruce grows from sea level to nearly 7,000 feet (2,000 m) [184,305]. In Alaska, it reaches 3,000 feet (910 m) on the south slope of the Brooks Range [305]. In eastern forests, it grows from sea level to about 5,000 feet (1,520 m) [114]. In the Black Hills of South Dakota and Wyoming, white spruce occurs from about 5,700 to 6,700 feet (1,700 to 2,000 m) [184]. Although white spruce has a wide elevational range, it is often confined to stream bottoms and lower river benches [119].
 |
 |
|
| Figure 3. Floodplain habitat, Yukon Flats National Wildlife Refuge, Alaska | Figure 4. White spruce at treeline, Wolf Creek site, Yukon. Photo by Dr. Jill Johnstone | |
 |
||
| Figure 5. White spruce forest in Denali National Park and Preserve. Photo © 2005 Barbara Logan, dlogan@alaska.net | ||
Soils and soil moisture regimes: White spruce typically grows best on warm, moderately to well drained, upland or floodplain soils [119,136,137,418,425,435,457]. Although white spruce may grow in a range of moisture conditions [2,22,80,119,240,332,394], it rarely occurs where permafrost is close to the surface [136,435,472], and grows poorly in sites with stagnant water [305] or high water tables [119]. Trees are often stunted and scrubby when growing in stagnant water or where soils are very dry [394]. White spruce seedlings are less tolerant of cold or flooded soils than black spruce, Rocky Mountain lodgepole pine, and tamarack seedlings [458]; and white spruce trees are less tolerant of long periods of flooding than balsam fir and black spruce trees [6].
White spruce tolerates a range of fertility levels [305], but moderate fertility is necessary for good growth [394]. The most productive white spruce stands occur on deep fertile soils on floodplains where periodic flooding enriches the soil [119,303,356]. White spruce also grows in nutrient-poor soils such as in the open spruce-lichen woodlands in northern Quebec [292]. White spruce growth is more sensitive to nutrient deficiencies than associated species including black spruce, red spruce, and pines [394]. In the Lake States, white spruce has higher nutrient requirements than associated conifers (jack pine, red pine, eastern white pine) [305].
White spruce grows on both acidic and alkaline soils. Optimum pH values are likely between 4.7 and 7.0 or higher [305]. In Alaska, white spruce typically occurs on sites with higher pH than that of black spruce [349]. In interior Alaska, soil pH values ranged from 5.0 to 8.2 on white spruce-dominated floodplains [474], and mean pH was 5.4 on upland white spruce sites [417]. In the Black Hills of South Dakota and Wyoming, pH ranged from 5.4 to 7.3 in white spruce communities [184]. In northern Quebec, white spruce often occurs on highly acidic soils with pH ranging from 3.1 to 4.6 [245,292].
Organic layer depth varies in white spruce communities depending on local site characteristics, associated species, and time since fire. In warm, relatively dry upland white spruce stands in interior Alaska, the moss-organic layer may be only 0 to 4 inches (0-10 cm) deep, [434] (reviewed in [40]), while mature white spruce stands on Alaskan floodplains may have a continuous carpet of feather mosses 4 to 8 inches (10-20 cm) deep [413]. Organic layer thickness increases with time since fire. On interior Alaskan white spruce sites, the organic layer gradually thickens from almost nothing immediately after fire to ~5 inches (12 cm) deep in 150- to 200-year-old stands, and splendid feather moss may form an 8 to 10 inch (20-25 cm) deep carpet [137]. In mature white spruce-quaking aspen stands in west-central Alberta, the mean forest floor thickness ranged from 2.3 to 2.4 inches (5.8-6.1 cm) [253]. In mixed white spruce and black spruce-lichen woodlands in northern Quebec, the lichen mat was 2 to 4 inches (5-10 cm) thick in sites that had not burned in >100 years [292]. In Alaska, forest floor temperatures are lower and soil moisture is higher in black spruce forests than in white spruce forests because black spruce forests typically have thicker organic mats [414].
White spruce grows in all soil textures [119], often dominating in sandy or gravelly alluvial soil [67,203,400]. On the southern shore of Walker Lake, northern Alaska, white spruce dominated on river deposits and soils with at least 85% sand, and black spruce dominated on soils with 49% to 69% sand [67]. Although white spruce may have "exceptionally good development" on clay soils [305], seedlings may die if clay soils become water saturated and have insufficient aeration [145].
PLANT COMMUNITIES:
White spruce communities are widespread throughout the North American boreal region [119,318]. White spruce becomes increasingly prominent in forests from the moist east to the drier west and northwest [356,358]. Many factors contribute to plant species composition in white spruce communities, including climate, topography, drainage, presence and thickness of permafrost, fire history, and forest age [64,102,425].
See Appendix B for scientific names of taxa mentioned in this review and for links to available FEIS reviews.
White spruce grows in pure and mixed stands [114,119,137,178,318,425]. Associated trees across much of its range include quaking aspen, paper birch, black spruce, and balsam poplar. Balsam fir commonly occurs with white spruce from Saskatchewan eastward [230]. Rocky Mountain lodgepole pine, hereafter lodgepole pine, is a common associate in the northwest Cordilleran region (Alberta foothills, northern British Columbia, and the Yukon) [358]. Other trees that commonly occur with white spruce include red spruce, yellow birch, jack pine, and sugar maple in the east; and subalpine fir and Rocky Mountain Douglas-fir (hereafter, Douglas-fir) in the west [118,119,230,318,358,433,467].
Tall shrubs and low trees associated with white spruce vary across its range. In the northwest, willows are most frequent; in the central region, mountain alder, pin cherry, and chokecherry are most frequent; in the east, mountain apple, northern mountain ash, and beaked hazelnut are common. American green alder occurs throughout much of white spruce's range. Medium and low shrubs associated with white spruce across much of its range include highbush cranberry, swamp red currant, prickly rose, and red raspberry. Other low shrubs that are regionally common include: russet buffaloberry from Alaska to central Alberta; common juniper from northern British Columbia to Lake Winnipeg; Saskatoon serviceberry from the Northwest Territories to northwestern Ontario; limber honeysuckle from Great Slave Lake to Lake Michigan; and bush-honeysuckle from Lake Winnipeg to western Quebec [230].
Herbaceous plants and dwarf shrubs associated with white spruce across much of its range include fireweed, sidebells wintergreen, single delight, twinflower, naked miterwort, bunchberry, and lesser rattlesnake plantain. Several herbaceous plants and dwarf shrubs that commonly occur in white spruce stands have more regional distributions [230].
White spruce codominates with quaking aspen [118], paper birch [467], and black spruce [433] over large areas. The white spruce-aspen forest cover type occurs in all the western provinces of Canada, the Northwest Territories, and Alaska on upland sites [118]. The white spruce-paper birch forest cover type has a similar distribution but may also occur along rivers [467]. Both the white spruce-aspen and white spruce-paper birch communities frequently precede late-successional white spruce forest [118,467]. Black spruce-white spruce woodland and forest types occur in northwestern Alaska and extend eastward to the Hudson Bay in Canada [433]. These communities frequently occur in open stands at alpine treeline throughout interior Alaska and northwestern Canada.
For a list of plant communities in which white spruce may occur and information on associated fire regimes, enter “white spruce” in the FEIS home page under "Find Fire Regimes". More detailed descriptions of white spruce communities follow by region.
Alaska: White spruce dominates or codominates many Alaskan boreal landscapes [263,303,434,435]. It is widespread in south-central and interior Alaska, extends to the limits of tree growth along the Brooks Range [152,425], and is less common in coastal regions [118]. At the landscape level, Alaskan white spruce communities form mosaics with quaking aspen, paper birch, balsam poplar, black spruce, and mixed forest stands [416,462]. The distribution of vegetation types is determined by past wildfires, altitude, soil drainage, topography, presence or absence of permafrost, and climate [428,430]. The sharp boundaries between stands of quaking aspen or paper birch and white spruce indicate edges of fires [263]. White spruce generally occupies upland, warm, well-drained, permafrost-free sites [263,425,428,457] (see Site Characteristics), whereas black spruce generally occupies cold, poorly drained sites with shallow permafrost [263,457].
Alaskan white spruce communities typically occur in riparian, upland, and treeline sites [83]. Extensive riparian white spruce stands are highly productive [303,356,425] and occur along valley floors and river terraces with permafrost deep underground or no permafrost at all [83,415,425]. Upland white spruce generally occurs on well-drained, south-facing slopes less than 1,300 feet (400 m) above sea level and may have a deep permafrost layer [136,413]. Upland forests are productive, but less so than riparian forests [83]. White spruce dominates treeline stands at the forest-tundra ecotone [152]. These forests have low productivity and have widely spaced, slow-growing tress [83,152].
In Alaska, white spruce may occur in pure or mixed stands, but pure stands typically occur only in areas where fire is infrequent [263] or at elevational treeline [303]. Mixed stands, including white spruce-quaking aspen, white spruce-paper birch, and balsam poplar-white spruce communities, often represent intermediate successional stages that are later replaced by white spruce types [118,136,263,467]. White spruce-quaking aspen communities establish after fire on warm, well-drained, upland sites and are generally replaced by white spruce types after 100 years or more [118,136,263]. White spruce-paper birch stands typically occur on upland sites but also occur along rivers [263,467]. Balsam poplar-white spruce stands occur on floodplains before succeeding to white spruce [425] (see Successional Status).
Black spruce-white spruce forests and woodlands are common in interior, south-central, southwest, and northwest Alaska, especially near treeline [136,310]. In interior Alaska, these forests occur wherever the white spruce type and black spruce type overlap. This occurs most frequently on lower, southerly slopes where white spruce forests meet black spruce muskegs on valley bottoms and near treeline [136]. Permafrost is often present at 20- to 24-inch (50-60 cm) depths, but it may be absent. Many of these communities may be climax or successionally stable. Other stands, especially those in floodplains, may transition from white spruce to black spruce in response to increasing organic layer thickness, rising permafrost, and decreasing soil temperature and drainage (see Successional Status) [425].
White spruce occurs as a minor component in many communities where it is not dominant or codominant. It may occur in unproductive or poorly drained black spruce forests, paper birch forests and woodlands, alder shrublands, birch-willow shrublands, and mesic birch-ericaceous shrublands [425].
NatureServe [300,301] identifies the following white spruce forest and woodland types in Alaska.
Montana: White spruce and western white spruce occur in northwestern Montana in the northern Rocky Mountains where they associate with Douglas-fir, western larch, and lodgepole pine [166,331]. In western and central Montana, white spruce, western white spruce, or Engelmann spruce communities often dominate coniferous riparian sites. White spruce tends to occur at lower elevation sites, while Engelmann spruce dominates higher elevation sites; western white spruce occurs where the 2 spruces overlap [166]. Western white spruce occurs in seral stands of subalpine fir/queencup beadlily stands [331].
NatureServe [300,301] does not identify any white spruce types or associations in Montana, but the following western white spruce associations occur in Montana:
Northwestern Great Plains: White spruce has limited distribution in the Black Hills of South Dakota and Wyoming. White spruce stands occur at high elevations of the subalpine zone and in cool canyon bottoms [184]. In some areas, it is an overstory dominant, but it is often codominant with or subordinate to interior ponderosa pine (hereafter, ponderosa pine) [399]. White spruce is considered the climax species in some ponderosa pine and quaking aspen stands [184,372]. White spruce occurs rarely in the subalpine forests of Bighorn National Forest, Wyoming [286].
Two white spruce habitat types are identified in the Black Hills: white spruce/twinflower and white spruce/grouse whortleberry [184]. White spruce/twinflower typically occurs on northwest- to northeast-facing slopes. Ponderosa pine and quaking aspen are frequent seral species. The white spruce/grouse whortleberry habitat type generally occurs on cool and moist sites at somewhat higher elevations than other local forest habitat types [184].
NatureServe [300,301] identifies the following white spruce woodland type in the northwestern Great Plains:
Great Lakes: White spruce occurs in mixedwoods, swamps, bogs, stream borders, and on wooded dunes and gravelly shores in the Great Lakes region [438]. It often codominates with balsam fir in mixed forests [77,87,277]. However, white spruce may not be abundant in some balsam fir-white spruce-paper birch stands [52,77]. White spruce is an associate in the jack pine [271], balsam fir [141], black spruce [92], tamarack [205], paper birch [362], red pine [30], and black ash-American elm-red maple [359] forest cover types.
Balsam fir-white spruce forests occur in northern Minnesota [144,229]. In the Boundary Waters Canoe Area, northeastern Minnesota, white spruce is often confined to lakeshore and wetland refuges from fire [143]. It also occurs in upland communities and in intermediate-aged stands [162,307,309]. White spruce often grows in the understory of jack pine, black spruce-jack pine, red maple-quaking aspen-paper birch, eastern white pine, red pine, and northern white-cedar communities [307,309].
In northern Wisconsin, old stands of white spruce-balsam fir are associated with eastern white pine, red pine, and northern white-cedar. On inland mesic sites, shade-tolerant hardwoods gradually replace white spruce and balsam fir. Young stands of dense balsam fir and white spruce also occur under aging quaking aspen or paper birch stands [87]. White spruce occurs in eastern white pine, eastern hemlock, and sugar maple-American beech habitat types, where it typically has low cover [224].
In Isle Royale National Park, Michigan, balsam fir-paper birch-white spruce dominates late-successional communities. Although white spruce is less common than balsam fir and paper birch, occurring only sparingly in most places, it is most conspicuous because it is usually taller [77].
NatureServe [300,301] identifies the following white spruce forest type in the Great Lakes region:
Northeast: In northern New England and New York, white spruce occurs in many community types where it does not dominate. In the northeastern United States, white spruce is associated with red spruce, northern white-cedar, and jack pine [300,301,318]. In northern New England, white spruce occurs in both late-successional and second-growth forests and may be associated with paper birch, quaking aspen, balsam fir, red spruce, yellow birch, and sugar maple [318]. It occurs as a minor type on abandoned agricultural lands in northern New England [95,318]. In New York, white spruce occurs in Adirondack spruce-fir swamps and spruce flats, calcareous pavement barrens, and limestone woodlands [342]. In coastal Maine, white spruce is associated with red spruce and balsam fir [95,318]; pure stands of white spruce occur along the shore [95].
NatureServe [300,301] identifies 2 white spruce associations in the northeastern United States:
Western Canada: In British Columbia and Alberta, white spruce is widespread and occurs with black spruce, lodgepole pine, subalpine fir, Douglas-fir, quaking aspen, Engelmann spruce, and balsam poplar [80,285]. White spruce is especially important in the Boreal and Montane Forest regions in western Canada [357]. It occurs from lower elevations in the boreal mixedwoods through the subalpine zone [80,285]. In northern British Columbia, the low and middle elevation boreal forest is dominated by white spruce, black spruce, and lodgepole pine; the subalpine forest is dominated by white spruce and subalpine fir [285]. In the boreal mixedwoods of west-central Alberta, white spruce is most abundant in older stands on wetter sites; quaking aspen tends to dominate the drier, well-drained sites and white spruce occurs in the understory [80]. On a landscape scale, white spruce communities form mosaics with early-seral stands dominated by lodgepole pine and quaking aspen [332].
For more information on white spruce communities of western Canada, see these sources [2,107,231].
Where white spruce and Engelmann spruce occur in the same area, white spruce predominates in lower elevations in valley bottoms, whereas Engelmann spruce dominates higher elevations. Hybrids occur where the 2 species overlap [2,193,305].
In boreal forests of south-central and eastern Yukon, white spruce and black spruce are climax species on moderate- to well-drained sites, and black spruce is climax on poorly drained sites. However, most communities are dominated by lodgepole pine due to fire setting back succession. In southwestern Yukon, black spruce or mixed black and white spruce form the climax community due to the presence of permafrost. White spruce is also common at arctic treeline and alpine treeline in the west and north [313].
In the forest-tundra ecotone in the Northwest Territories, white spruce grows in an open parkland. White spruce dominates the well-drained, drier sites, while black spruce dominates the more mesic areas [270]. On the Mackenzie River Delta, late successional white spruce woodlands occur on the most elevated sites. Four distinct white spruce communities are recognized based on differences in site characteristics, understory species composition, and tree age. Moisture regimes range from xeric in the white spruce/lichen-crowberry association to hygric to poorly-drained in the white spruce-tamarack/sphagnum bog-type woodland [321]. In poorly drained bogs, white spruce occurs with [249] or without black spruce [321].
Old-growth riparian white spruce forests in western Canada are often restricted to terraces of major river valleys where flooding rarely occurs because terraces are elevated and well-drained. Fire is limited because moist floodplains, wide channels, oxbows, and low fuel loadings act as natural firebreaks. On wet, poorly-drained riparian sites, white spruce/field horsetail forests generally develop from balsam poplar/red-osier dogwood stands after 100 to 300 years. On mesic sites associated with lakes and sloughs, floodplain terraces, and steep wooded draws, white spruce/highbush cranberry develops from quaking aspen/highbush cranberry or balsam poplar/red-osier dogwood stands after 100 to 300 years. Although this is a major type at low- to mid-elevations in the Boreal Forest Natural Region, old-growth stands are rare because stands often burn before they reach advanced maturity [403].
NatureServe [300,301] identifies several white spruce forest and woodland types in western Canada:
NatureServe [300,301] identifies the following white spruce woody wetlands and riparian types in western Canada:
Central and eastern Canada: White spruce typically occupies approximately 10% of the canopy in the southern boreal mixedwood forests of central and eastern Canada (reviewed in [100,101,102]). However, white spruce is locally dominant in some stands on the Atlantic coast [315,318,320]. White spruce stands are also found at the altitudinal treeline of the highest plateaus; the southernmost subalpine white spruce stands occur on an extensive high plateau of the Gaspé Peninsula [100,101]. The central and eastern portions of the Boreal Forest Region [357] are characterized by black and white spruce, balsam fir, and jack pine with varying amounts of eastern white and red pine, yellow birch, sugar maple, black ash, and northern white-cedar in the east. The Great Lakes-St. Lawrence Forest Region [357] is characterized by eastern white and red pines, eastern hemlock, and yellow birch. These mixed forests often include several hardwood species including sugar maple, red maple, northern red oak, basswood, and white elm as well as boreal species including white and black spruce, balsam fir, jack pine, quaking aspen, balsam poplar, and paper birch. In the Acadian Forest Region (New Brunswick, Nova Scotia, Prince Edward Island) [357] of the Maritime provinces, where red spruce and associated balsam fir, yellow birch, and sugar maple are dominant, white spruce has increased importance since the 1900s due to widespread invasion of abandoned farmland.
In the boreal lowlands of the Saskatchewan River delta in east-central Saskatchewan, white spruce-hardwood forests are confined to raised alluvium levees with relatively dry moisture regimes and no peat. Associated hardwoods include balsam poplar and American elm. Associated ground cover species depend on the site but often include meadow horsetail, wild sarsaparilla, and bluejoint reedgrass [106]. In subarctic northeastern Saskatchewan, black spruce dominates forest and woodland communities and white spruce is uncommon [16]. In the dry grassland region of southwestern Saskatchewan, white spruce forests occur on the Cypress Hills plateau, typically on cool, moist sites [47,402]. Balsam poplar and quaking aspen are frequent associates. At higher elevations, white spruce transitions to lodgepole pine. In closed stands on relatively dry, well-drained soil, there is little ground cover, whereas moist sites contain tall and low shrubs, herbaceous plants, and mosses [47].
The boreal forest in northern and central Manitoba is characterized by conifers including black spruce, jack pine, balsam fir, white spruce, and tamarack. Mixed conifer-hardwood stands may include quaking aspen, paper birch, and balsam poplar. White spruce mixedwood communities occur late in succession on well-drained, moist soils. Other white spruce communities include white spruce mixedwood/feather moss and white spruce/balsam fir shrub [477]. In northern Manitoba, white spruce is confined to alluvial deposits and eskers (areas lacking peat). On the Hudson Bay Lowlands, pure white spruce stands have a sparse shrub strata and either an herbaceous understory or a moss-dwarf shrub understory [344].
In the Maritime provinces, white spruce occurs in pure stands, mixedwoods, and predominantly hardwood stands. Pure stands occur on abandoned farmland and along the coastline, especially in cleared or disturbed areas. On many sites, white spruce occurs in mixed conifer stands in valley bottoms and on steep slopes of narrow valleys. On upland slopes, rolling hills, and flats, white spruce occurs in mixedwoods with red spruce, balsam fir, sugar maple, yellow birch, and red maple. In the New Brunswick Highlands Ecoregion, balsam fir, white spruce, black spruce, paper birch, and eastern white pine occur on the well-drained slopes. Along the Atlantic coast, white spruce, black spruce, and balsam fir dominate the mostly open, windswept stands. On some sites, white spruce dominates the immediate coast, whereas black spruce and balsam fir are more abundant farther inland. White spruce also occurs with eastern hemlock and red pine in the Maritime provinces [261].
In central Newfoundland, white spruce is most often associated with moist balsam fir-paper birch forests that have a splendid feather moss ground cover. These forests are comprised of a dense balsam fir overstory and scattered paper birch and white spruce and occasional black spruce. This forest type typically occurs on middle and lower seepage slopes on rugged terrain and moist soils. Scattered white spruce also occurs in balsam fir/woodfern-clubmoss forests and alder swamps [91]. White spruce is rare in southeastern Labrador where the cool maritime climate enables the development of a thick bryophyte layer and deep organic humus [140].
NatureServe [300,301] identifies several white spruce forest and woodland types in central and eastern Canada:
Morphology: White spruce grows as a medium-sized tree or as a shrub. Trees typically average 80 feet (25 m) tall [194] but mature trees may exceed 100 feet (30 m) and 24 to 36 inches (60-90 cm) in diameter on favorable sites. The tallest individuals are more than 180 feet (55 m) tall [305]. In Alaska, white spruce is typically 40 to 70 feet (12-21 m) tall [435]. Prostrate and krummholz forms are common at or near treeline [18,93,152,272,369,396], where mature trees are often only 3 to 6 feet (1-2 m) tall [369,435], and sometimes only 4 to 8 inches (10-20 cm) tall [396].
The arrangement of vertically continuous branches may promote ignition and torching in white spruce [114,462,466]. White spruce trees typically have a straight bole with a broadly conical to narrow, almost linear crown and slightly drooping branches [135,194,197,435]. Trees in Alaska commonly have narrow, spire-like crowns [194,251], whereas in the northeastern United States, trees are narrow but not typically spire-like [151]. White spruce trees are typically much narrower than black spruce trees [406]. Crowns are usually densely foliated [129,356,394], and branches and needles are often retained low on the trunk [114,171,194,204,263] but sometimes are not [405]. Trees may shed their lower branches when growing in dense stands with low light [194]. The bole has thin smooth, scaly, or flaky bark, generally less than 8 mm thick [394]. Needles are short, ranging from 0.2 to 0.75 inch (5-18 mm) long [194,438]. The needles and bark are resinous [303], although less so than those of black spruce [405]. The pendulous cones are 1 to 2.4 inches (3-6 cm) long [197,435] and hang from the upper branches [314]. Seeds have a long, thin wing that is 2 to 3 times as long as the seed [435]. White spruce seeds are small (~0.001-0.003 gram) [82,471] but are larger and heavier than those of many associated boreal trees (e.g., paper birch, quaking aspen, black spruce, tamarack, alders, willows) [264,470].
 |
 |
|
| Figure 6. White spruce tree, Itasca State Park, Minnesota. Photo by Steven Katovich, USDA Forest Service, Bugwood.org | Figure 7. White spruce cones. Photo by Joseph O'Brien, USDA Forest Service, Bugwood.org |
White spruce has lateral, vertical, layered, and adventitious roots [56,439]. The root system tends to be shallow [129,194,413]. Most roots grow in the upper 6 to 12 inches (15-30 cm) of soil, in the organic-mineral soil interface or almost entirely in the organic mat, but taproots and sinker roots may reach 10 feet (3 m) deep (reviewed in [56,413]). Multilayered, secondary, and adventitious root systems occur on floodplains as a response to alluvial deposits and increases in humus and feather moss [201,439]. Adventitious roots generally establish on young trees and seedlings and extend laterally in the organic-mineral soil interface. Adventitious root development in alluvial deposits results in mature white spruce with strong lateral and sinker roots [439].
Stand structure: White spruce stand structure varies tremendously [90,300,301,435], ranging from open woodlands at treeline [300,301], on dry sites, and on high benches [171], to closed forests in lowland mixed stands [300,301]. White spruce trees growing in open woodlands tend to have broad crowns, short stature, and branches that extend to the ground [171]. In Alaska, open white spruce types generally have a vigorous understory, whereas closed stands may have few vascular plants and a deep, continuous moss cover [303]. On the northern Alaskan forest-tundra treeline, tree density and mean tree height decrease with increasing elevation [152].
White spruce tree density tends to decline with increasing stand age [263,303,426] or peak before stands senesce [17,404]. In the white spruce forest type in interior Alaska, white spruce densities are often high in young (20- to 25-year-old) stands with 2,000 to 3,000 trees/acre (4,900-7,400 trees/ha); whereas, in older, 160- to 180-year-old stands, density may range from 300 to 500 trees/acre (740-1,200 trees/ha) [263]. On the Tanana River floodplain in interior Alaska, mature white spruce density is highest (up to 800 trees/acre (2,000 trees/ha)) in 100-year-old, even-aged stands, and it declines as stands age, with 210 trees/acre (520 trees/ha) in 250-year-old, uneven-aged stands [426]. However, in interior Alaska, early seral stages (i.e., quaking aspen and paper birch stages) of white spruce types have fewer mature white spruce than older white spruce stands. In young quaking aspen and paper birch stands, white spruce may average 8 trees/acre (19 trees/ha) and 46 trees/acre (113 trees/ha), respectively. In the mature white spruce stage, white spruce may average 200 trees/acre (496 trees/ha) [136]. In 35 mixedwood stands in Saskatchewan that ranged from <1 to 201 years since fire, density of white spruce peaked 172 years after fire at about 570 stems/acre (1,410 stems/ha) and was about 240 trees/acre (600 trees/ha) in the oldest stands [17].
White spruce usually forms multi-aged stands comprised of trees that establish episodically [17,34,67,145,330,429], although even-aged stands that date back to the last fire sometimes occur [137,430,431,460].
White spruce generally has a moderate lifespan, although individuals at stressed sites such as latitudinal or elevational treeline may be long-lived. White spruce commonly lives 100 to 250 years [137,442], with older trees (250->300 years) occurring in areas that are protected from fire such as islands and river channels [56,358,469]. Treeline sites across its range may support white spruce >350 years old [93,221,319]. The oldest reported white spruce (nearly 1,000 years old) occurs above the Arctic Circle [149].
Raunkiaer [338]
life form:
Phanerophyte
SEASONAL DEVELOPMENT:
White spruce typically grows in areas with a >60-day growing season, although the growing season ranges from about 180 days in parts of Maine to 20 days in parts of Canada (reviewed in [304]). New growth begins in the spring [305]. Pollination occurs over a 3- to 5-day period in May, June, or July depending on the location and climate. In general, trees at northern and treeline locations are pollinated later than trees at southern and lower elevation sites (reviewed in [305,472]). Seed dispersal typically begins in August, peaks late-August through October, and continues throughout the winter (Table 1).
| Table 1. Regional phenology of white spruce | ||
| State or province | Event | Period |
| Alaska, interior | pollination | late May to early June [470] |
| seed dispersal begins | early Aug. [475] | |
| early Sept. [471] | ||
| mid-Sept. [468] | ||
| peak seed dispersal | early to mid-Sept. [475] | |
| Sept. [471] | ||
| British Columbia | reproductive buds differentiate & shoot growth ceases | mid- to late July |
| male buds become dormant | Oct. 1 | |
| female and vegetative buds become dormant | mid-Oct. (reviewed in [305]) | |
| seed dispersal begins | Aug. [109] | |
| peak seed dispersal | Sept. to Oct. [109] | |
| Manitoba | seeds disperse | early Aug. to late Sept. [440] |
| Minnesota | pollination | mid-May to early June [4] |
| seed dispersal begins | early Sept. to mid-Sept. [4] | |
| Quebec | seed dispersal begins | Sept. [352] |
| most seeds dispersed | Sept. to Oct. [352] | |
| Wisconsin | flowering | late May [87] |
| seeds ripen | Sept. [87] | |
| most seeds disperse | Sept. to winter [87] | |
| Yukon | seed dispersal begins | early- to mid-Sept. [14] |
White spruce seeds disperse when cones dry out and open in late summer and early fall, when the moisture content of the cones is about 28% [82]. In interior Alaska, Zasada and others [471] found that cone moisture content ranged from 25% to 80% shortly after seed dispersal began. Most seed is dispersed during the early dispersal season (i.e., typically late summer and fall), although some seeds are dispersed in the winter, spring, and early summer [109,352,356,385,457,464,472]. In Quebec [352], British Columbia [109], and interior Alaska [464], >50%, 70%, and 75% of the seeds are dispersed by the end of October, respectively. In Alaska, 75% to 90% of the seeds are dispersed within 3 to 4.5 months after initial cone opening [466,475].
Seeds released during the peak dispersal period generally have higher quality than seeds released outside of the peak period [109,148,352,464,468]. In the Bonanza Creek Experimental Forest, interior Alaska, seed viability decreased from late September (>70% viable) through March (~30% viable) [468]. In Quebec, white spruce seed viability was highest during peak seed dispersal (September-October) and gradually decreased through winter and spring, with minimum viability occurring in July and August [352]. Along the Tanana River, interior Alaska, where most seeds were not filled, >75% of the filled seed was released before mid-October [464].
White spruce seeds typically germinate during early summer, although germination may occur from mid-May through early August [305,356,471]. Generally, germination is 75% to 100% complete by early July (reviewed in [305]). Following a high seed production year in the Bonanza Creek Experimental Forest, germination patterns reflected soil moisture content. Germination peaked between late May and early June (after snowmelt) and again between late July and early August (after heavy rainfall) [471]. See Germination for additional information about germination requirements.
REGENERATION PROCESSES:
White spruce's primary method of reproduction is establishing from seed. Establishment occurs throughout stand development including early and late postfire succession. White spruce relies on annual seed production for regeneration [305], and seedling establishment often coincides with years of high seed production [328,351]. In addition to the information presented below, see Plant Response to Fire for information on regeneration of white spruce after fire.
Pollination and breeding system: White spruce is monoecious [305]. Some bisexual cones have been found in Alaska [471].
Cone and seed production: Seed production "in quantity" may begin once stands are at least 30 to 40 years old, although cones and seeds have been observed on planted trees as young as 4 years old [305,394,466]. Trees may not produce seeds until they are much older, especially when growing in "extreme" conditions [356]. At alpine treeline, western Northwest Territories, the youngest cone-bearing tree across 11 sites was 41 years old [396]. In Alaska, good cone crops generally occur on trees 45 to 170 years old or older [466,472]. White spruce generally produces seed at an older age than associated trees [263,473]; consequently shorter fire-return intervals tend to favor other tree species over white spruce.
Large, open-grown trees tend to have larger cone crops than smaller, shaded trees. Dominant and codominant trees typically produce heavier cone crops than intermediate and suppressed trees [440]. The percentage of cone-bearing trees typically increases with increasing average tree diameter in a stand [39], and seed production generally increases as basal area increases [155]. However, in quaking aspen-white spruce stands in Alberta, seed rain was not correlated with tree height (P=0.362), diameter at breast height (P=0.261), or total basal area (P=0.218) [385]. Trees growing in full sun may produce seed at shorter heights than shaded trees. In western Quebec, trees produced seeds once they reached 10 feet (3 m) tall when growing in full sun, 26 feet (8 m) tall when growing near the forest edge (somewhat shaded), and 46 feet (14 m) tall when growing farther from the forest edge (deeply shaded) [160].
White spruce seed production is highly variable from year to year [348,351,352,394,440,464,466,472,475]. Good seed crops tend to occur every 10 to 12 years [305,440,475], although white spruce may produce abundant seed every 2 to 6 years on favorable sites [305,394]. The time between mast years is greater at northern latitudes than southern latitudes [471]. Mast years are generally followed by years with low production [305]. Seed crop failures are not uncommon (e.g., [90,385,464,470]).
Annual white spruce seed production is influenced by climatic conditions. Several authors have theorized that large seed crops are associated with relatively warm, dry weather during the current and/or previous growing seasons (during reproductive bud differentiation [348,471,472] and/or pollen and cone maturation (reviewed in [473])). For instance, the excellent seed year of 1958 in interior Alaska was preceded by an exceptionally hot, dry summer [471]. The relationship between cone crop abundance and climate was tested in southwestern Yukon. Large white spruce cone crops were related to warm temperatures during the previous summer; however, there was no correlation between current-season temperature or precipitation and cone crop size [227]. A 25-year study from low- to high-elevation sites in interior Alaska suggested that seedfall is positively associated with summer precipitation and negatively associated with warmer summer temperatures in the 3 years before seedfall, except for the year prior to seedfall. However, seed viability showed the opposite response to warmer temperatures; it was positively associated with warmer summer temperatures and was especially high when the seedfall year was both warm and wet. This study suggests that a warm and dry early growing season the year before seedfall may trigger reproductive bud initiation, and the weather during the seedfall year affects the development and viability of those seeds [348]. Speculation and anecdotal observations suggest that mast years often coincide with or follow years with high fire activity (e.g., [93,466]), because the warm, dry conditions that promote mast years also promote fire activity.
Trees at high elevation or latitude may produce few cones and seeds, and these seeds typically have low viability. At latitudinal treeline, Northwest Territories, 1 year of cone collection indicated that mature white spruce trees had few cones/tree (mean=14±2(SD)), cones had few seeds (mean=15±8(SD)), and many trees had no cones. However, in the same year, white spruce at sites about 12 miles (20 km) south had an average of 395±211(SD) cones/tree [221]. During an excellent cone production year throughout much of Alaska, seed production failed in stands north of the Arctic Circle and in stands above 1,970 feet (600 m) because weather conditions prevented seed maturation. Flowering and cone development occurred 3 to 5 weeks later at high elevation sites than low elevation sites [471]. Individual trees near treeline may produce only 1 to 3 good seed crops in their lives due to climatic limitations [473]. When white spruce produces seed at high-elevation treeline sites, seeds tend to have low viability because seed development is restricted at low temperatures [348].
| Table 2. White spruce annual seedfall reported in a review by Gartner and others [148] | |
| Region | Total annual seedfall (seeds/m²) |
| Alaska | <100-4,000 |
| Alaska (Willow Island), August-May | 440-3,700 |
| Northeastern Alberta | 22-1,291 |
| Northeastern Alberta, before mid-October | 0-400 |
| Manitoba | <10-1,400 |
Seed predation, insects, and pathogens may reduce cone and seed production, making fewer seeds available for regeneration [48,90,148,191,326,439,470,472,475]. Red squirrels are the dominant predispersal seed predator; they cut and cache cones before the cones open [48,90,326]. In interior Alaska, where white spruce seed is their primary food, red squirrels harvested between 10% and 69% of cones during a "medium" cone production year [390]. One red squirrel may cache up to 16,000 cones per year [381]. In mixedwood forest in Alberta, red squirrels harvested 54.9% ± 2.1% of the cone crop over a 3 year period. Harvested cones (cached cones and squirrel-scattered seeds) are unlikely to contribute to regeneration because caches are not suitable seedbeds and seeds rapidly lose viability [48]. Deer mice and northern red-backed mice eat white spruce seeds after they are dispersed. These mice are active under the snow throughout the fall and winter and may eat large quantities of white spruce seed [439]. In Alaska, the spruce cone maggot often eats up to 50% of seeds per cone [191], and may eat 90% of seeds in low crop years [395]. In interior Alaska, inland spruce cone rust may cause 100% seed mortality [148,472].
Seed dispersal: Wind disperses white spruce's winged seeds [109,356,464]. The seeds are small and lightweight [82,356,471]. Most seeds fall within 2 tree heights, or 150 to 200 feet (45-60 m) from parent trees [430,436,466]. Seed density declines rapidly with distance from source trees [468], although seeds have been reported at more than 1,300 feet (400 m) from their source [465]. Seeds may disperse up to 330 feet (100 m) from the stand edge, although most seeds fall within the stand [109,464]. Late-dispersed seeds may be blown over crusted snow and ice [145,157,457]; in clearings, these seeds may be found at greater distances from their source than in forested areas [109,157]. Although dispersal over snow generally contributes little to total seed dispersal, in central Quebec, 30% to 50% of the white spruce seed crop falls on snow, facilitating dispersal distances greater than a few hundred meters (personal communication in [157]).
Seed dispersal distance is influenced by release height, intercepting canopy, and windspeed. White spruce seed dispersal through quaking aspen forests was studied by releasing artificial "seed" from different heights of a meteorological tower. Mean dispersal distance increased with height of release. Windspeed is affected by the forest canopy, and dispersal distances were consequently affected by canopy characteristics. Before quaking aspen leaf fall, most "seed" landed close to and in all directions around the tower. After leaf fall, the mean dispersal distance increased, with peak densities occurring 50 feet (15 m) downwind of the tower. However, actual white spruce seedlings were found much farther away from isolated white spruce seed trees within quaking aspen stands than the seed dispersal distances observed during the experiments. This suggests that most white spruce seed is released at much higher windspeeds than those observed during the experiments [384].
In general, seeds that fall closer to the parent tree are more likely to be viable than seeds that are dispersed farther away. In a mature white spruce stand bordering a clearcut in central British Columbia, seeds were estimated to be 48% sound within the first 330 feet (100 m) of the stand and 31% sound in the next 330 feet (100 m). However, seed density 990 feet (300 m) into the clearcut still exceeded about 300,000 seeds/acre (740,000 seeds/ha), suggesting adequate quantities of seed were available to regenerate that far into the clearcut [109]. On a floodplain island of the Tanana River, interior Alaska, few seeds dispersed beyond 390 feet (120 m) [464].
White spruce seed dispersal and seedling establishment may be limited within large fires [436]. White spruce seed in burned areas is typically dispersed from unburned trees within or adjacent to the burned sites, because white spruce trees are usually killed by fire and cones are not serotinous [156,263,466]. White spruce regeneration densities are typically highest along old fire edges and near unburned patches [145,156,457], because seed dispersal is greatest close to unburned source trees and stand edges [109,464]. See Seedling establishment and Plant response to fire for more information about seedling establishment after fire.
Water may disperse white spruce seed along floodplains. Water-dispersed seed may be deposited in shrub and balsam poplar stands by late summer floods. Occasionally, white spruce establishes and forms dense stands early in the floodplain successional sequence [303]. See Floodplain succession for more information about succession on Alaskan floodplains.
Seed banking: White spruce depends on annual seed production for regeneration [305,351]. White spruce does not have serotinous cones, and seeds do not persist in the soil. Because seed matures and falls within 1 year, there is no seed stored in trees [436].
Although white spruce does not have serotinous cones, crown-stored seed may occasionally be available after fire depending on fire timing, severity, and type [157,169,170,288,468]. Crown-stored seed is more likely to be available after fires in late summer—after seed is ripe, but before it is dispersed [288,466]. However, fires generally occur before white spruce seed is ripe; in Alaska, more than 86% of all fires occur before white spruce seed is mature [466]. One growing season after the August 1977 Bear Creek Fire in interior Alaska killed all the trees but left the canopy intact, white spruce seedlings were abundant (12,000 seedlings/acre (30,000 seedlings/ha)), which suggests that there were abundant crown-stored seeds even after the fire killed the trees [169,170]. In the Bonanza Creek Experimental Forest, fire-killed white spruce produced and dispersed viable seeds after an early season fire. The fire occurred at about the time of white spruce pollination, and female flowers were not affected by the severe ground fire. Trees that died by late summer due to severe burning of the roots and lower bole still produced large quantities of viable seeds (80% viable); trees with scorched or burned crowns did not produce seeds [468]. Simulation experiments suggest that white spruce seed contained in closed cones may survive heating by crown fire, and that approximately 12% of cones would contain viable seed after fire. However, the probability of a fire occurring when germinable seed is contained within cones (i.e., mid- to late-season fire) and coinciding with a mast year is low (perhaps 0.05) [288]. Crown-stored seed may explain why white spruce occasionally has high postfire recruitment [169,170], including in areas far from fire edges [288], and why even-aged white spruce stands occasionally develop after fire [137,430,431,460].
Soil seed banking does not appear important to white spruce, because seeds do not remain viable in the soil for long. Viability of seed in cones cached by red squirrels drops to nearly 0 after 1 to 2 years [305]. In quaking aspen mixedwood in Alberta — where white spruce comprised <20% of the basal area — no white spruce seed was found in the soil seed bank in unburned, lightly burned, or severely burned plots [242]. Seed bank samples near treeline in the Tuktoyaktuk region of northwest Canada yielded no germinable white spruce seed [283].
White spruce sometimes has "seedling banks" rather than soil seed banks [351,473], but seedlings would not survive fire. Seedling banks may establish after mast years when abundant seed germinates. Because mast years are episodic, seedling banks are replenished episodically and form a discontinuous age structure [351].
Germination: White spruce seeds are conditionally dormant [305] — that is, seeds that are dispersed in fall and winter do not germinate until conditions become favorable during late spring and summer [305,354,356,474] (see Seasonal development). Field studies from Alaska [321] and Manitoba [354] suggest that white spruce seeds germinate at mean temperatures of 50 to 57 °F (10-69 °C), and reviews report that optimum germination temperature ranges from 46 to 90 °F (8-32 °C) [71].
White spruce seed may remain viable for about 1 year [157], although viability drops steadily after seeds ripen [352,470]. Clean, dried seed may remain viable for up to 10 years in storage (reviewed in [15]).
In general, most white spruce seeds fail to germinate [61], and viability varies among years, stands, dispersal periods, and regions. High viability generally occurs in years with high seed production [466,472]; however, germination tests of the highly productive 1970 seed crop from 29 stands throughout Alaska found germination rates ranged from 0% to 85% [471]. Seed viability is typically highest during the peak dispersal period. In Quebec, seed viability was highest during highest seed rain in September and October, and gradually decreased through winter, spring, and the following summer [352]. In interior Alaska, seeds dispersed in September were 72% viable, whereas those dispersed in March were 29% viable [468]. Seed viability ranged from 6% to 82% in interior Alaska [466,475], 3% to 33% in northwestern Canada [283], 83% to ~95% in Manitoba [216], and 80% to 96% in Saskatchewan [61]. Seed viability may often be low in the northernmost limits of white spruce's range [283,470]. Near treeline in the Tuktoyaktuk region of northwest Canada, seed germinability ranged from 3% in isolated tree islands to 33% in forest-tundra. Seed bank samples yielded no germinable white spruce seed and there were few seedlings across the region [283].
Seedbed: When the seedbed is continually moist, white spruce can germinate on mineral soil, organic soil, rotten logs, and moss [148,457]; however, white spruce seedlings establish best on mineral or thin organic soils [61,71,103,124,206,336,385,439,468,471], which are typically made available when fire consumes organic horizons. In areas lacking exposed mineral soil or forest floor disturbance, white spruce seedlings often establish on rotting wood [17,39,61,63,243,378,439,472].
Additional seedbed characteristics that affect white spruce germination and establishment include soil structure, smothering by leaf litter, microsite topography, light and nutrient availability, and competing vegetation [103,148,263] (see Seedling establishment for more information on these topics).
Seedbeds with a stable moisture supply and moderate temperatures are critical for white spruce germination and establishment. Because white spruce germinants have short roots [61], and therefore require a stable moisture supply, mineral soil is a superior seedbed [206,263]. Other substrates, such as leaf litter, feather mosses, and deep organic mats tend to make poor seedbeds because they dry out easily [61,206,207,439], although organic mats may support germinants if they remain moist [39]. Warm seedbed temperatures are associated with higher germination and survival [103,148,336,378], although excessive soil temperatures (e.g., 104-122 °F (40-50 °C)) may prevent germination and kill seedlings [81,439,471].
Fire creates an ideal seedbed for white spruce by reducing the organic layer, exposing mineral soil, blackening the surface, raising soil temperatures, and removing competing vegetation [126,138,306,424,427,472]. Available soil moisture may also increase after fire [138,306]. Because consumption of organic layers is highly variable, suitable seedbeds with exposed mineral soil are patchy [148,158]. As litter and competing vegetation increase during postfire succession, favorable seedbed conditions deteriorate rapidly [148,336].
Severely burned sites with exposed mineral soil resulted in better white spruce establishment than unburned and lightly burned sites in Alaska and Canada [61,200,206]. On 5 sites in interior Alaska and the Yukon, plots burned in wildfire or prescribed fire were seeded with white spruce, black spruce, lodgepole pine, and quaking aspen within 2 years after fire. Seedling establishment was negatively associated with depth of the organic layer (P <0.025); most seedlings established on sites with <0.8 inch (2 cm) organic material. Although some establishment occurred on the intact organic layer (i.e., low-severity-burn plots), about 12 times as much seed by weight was needed to produce a 2-year-old seedling on these plots compared to plots that had complete combustion of the upper duff and partial to full consumption of lower duff (i.e., high-severity-burn plots). The authors attributed the higher establishment on mineral soil to its relatively stable moisture content compared to that of organic soils [206]. In southern boreal mixedwood forest in Saskatchewan, white spruce seeds sown 1 to 3 consecutive years after fire had higher cumulative survivorship (from sowing to the end of the first summer) on mineral soil and thin humus (0.4 to 2 inches (1-5 cm) deep) than on thick organic matter (6 to 8 inches (15-20 cm) deep) (P <0.05) (Table 3) [61]. In boreal mixedwoods in southeastern Manitoba, white spruce seedlings did not establish on mineral soil exposed by a severe spring fire until 3 growing seasons after the fire. White spruce seeds were sown in September immediately and 1 year after fire on scorched, lightly burned, and severely burned plots. Germination failed on severely burned seedbeds during the first year after fire. However, after 3 growing seasons white spruce seedling survival was higher on severely burned seedbeds than lightly burned (P ≤ 0.1) or scorched (P ≤ 0.05) seedbeds. The authors attributed higher survival on severely burned seedbeds to greater mineral soil and moisture availability than on lightly burned or scorched seedbeds [216].
| Table 3. Initial survivorship of white spruce seeds sown (seedlings/seeds sown) on burned seedbeds 1, 2, and 3 years after the Bittern Fire, Saskatchewan. Table modified from [61]. | |||
| Postfire year | Mineral soil | Thin humus | Thick organic material |
| 1 | 0.268 | 0.425 | 0.035 |
| 2 | 0.310 | 0.394 | 0.031 |
| 3 | 0.168 | 0.234 | 0.017 |
White spruce often depends on rotten logs for recruitment in sites lacking exposed mineral soil, such as those that have not burned in a long time [17,39,243,329,345,378], particularly moist sites [439]. Rotten logs may be superior seedbeds for white spruce because they have favorable light and nutrient conditions, stable moisture supply, less competition, less leaf litter accumulation, and allow better root growth (reviewed in [329]) than the litter- and duff-covered forest floor. After a spruce beetle outbreak on the Kenai Peninsula that killed most of the large trees without disturbing the forest floor, white spruce and Lutz spruce established on a variety of substrates but preferred heavily decayed logs (53%) and stumps (4%), which comprised only 2% of the plot area [39]. In eastern boreal mixedwoods in northwestern Quebec, white spruce preferentially established on logs. Seedling density was higher on logs than on the forest floor, and density was higher on decayed logs than on fresh logs [345].
Seedling establishment: White spruce often establishes after fire when seed and suitable seedbeds are available [72,328,329], although establishment later in succession is also common [17,34,67,83,145,148,329,463]. Establishment may be particularly high following episodic mast years [351], especially when masting and fire coincide [328]. Establishment rates are highly variable [148] and depend on several factors. Most importantly, adequate seed sources must be in close proximity to suitable seedbeds during favorable weather conditions. See Plant response to fire for additional information about regeneration of white spruce after fire.
Fire typically creates favorable seedbeds for white spruce, and white spruce seedlings often establish soon after fire [328]. Establishment also occurs episodically following mast years [328,351] and may be high when fires and masting coincide [327,328,330,336]. Studies in Alberta mixedwoods show mixed results. Over a 59-year period, white spruce densities were 2.5 times higher after mast-year fires than after fires in years of low cone production (P <0.001). In nonmast years, 53% of the stands had no postfire recruitment. Large cohorts did not occur when mast years occurred ≥4 years after fire, and very little regeneration occurred 7 to 20 years after fire [328]. After a mast-year fire where nearby seed sources were abundant, there was a complete lack of recruitment because a thick organic layer remained. White spruce seedling density 1 year after fire was negatively associated with organic layer depth and distance to seed source, and positively associated with seed source strength (P <0.01) [336]. A retrospective study of 5 fires in white spruce-dominated mixedwoods in central and northeastern Alberta, including 3 fires that occurred during mast years, found poor white spruce establishment. Fires occurred during the early summer before seed was ripe and likely resulted in poor organic matter consumption (not measured); very few seed trees remained on site or nearby [147].
While white spruce seedling establishment is often abundant after fire, many studies describe a more complex pattern of white spruce regeneration [17,34,67,145,330,429]. Out of 20 stands in Alberta boreal mixedwoods, 7 stands were dominated by initial postfire regeneration, 6 were dominated by delayed regeneration, and 7 had even mixtures of initial and delayed regeneration. Even when initial postfire regeneration is high following mast-year fires, delayed regeneration may constitute proportionally more of the total regeneration [329]. White spruce regularly established during the 75 years after fire in a Quebec southern boreal forest [34]. In boreal mixedwood forest, northwestern Quebec, white spruce established in 2 peaks after a stand replacement fire. The first peak occurred approximately 10 years after fire, and a second smaller peak occurred approximately 50 years after fire. The authors suggested that the first cohort was a likely seed source for the second cohort [145]. In black and white spruce woodlands of the central Brooks Range, Alaska, white spruce had a broad establishment period after fire without prominent peaks [67]. A chronosequence study of 35 stands in Saskatchewan southern boreal mixedwoods, which ranged from <1 to >200 years old found that white spruce seedlings established immediately after fire and recruitment continued at varying rates, peaking 50 years after fire at about 500 seedlings/acre (1,250 seedlings/ha). Seedling density was lowest between 110 and 125 years after fire. A second wave of recruitment began 127 years after fire and peaked 172 years after fire, at 622 seedlings/acre (1,537 seedlings/ha). The second peak in seedling recruitment may have resulted from the higher density of seed trees, increased light intensity due to gap formation, and increased availability of logs [17].
Most white spruce germinants die before they become established due to unfavorable seedbed and weather conditions, or smothering under leaf litter. Hot, dry summers tend to dry out the seedbed, especially on open sites (including recent burns) and sites with course-textured soil, feather mosses, and/or litter. Consequently, seedlings commonly die due to moisture stress or heat injury [123,148,178]. In interior Alaska, white spruce seedlings that germinated in May and June were most likely to die during their first summer, when conditions were hot and dry. Seedlings that germinated in July and August were most likely to die during their first winter. Most seedlings that survived the first summer and winter survived through the 5-year study period [471]. In 4 balsam fir-dominated stands in Quebec, white spruce seedling survival through their first winter ranged from 4% to 20% [351]. White spruce seedlings do not establish well in leaf litter, especially on hardwood sites, because the small seedlings get smothered and crushed under leaf litter and typically die [103,148,356,378,472]. It took 4 growing seasons before white spruce seedlings were large enough to avoid being smothered or crushed by leaves in an 80-year old paper birch stand in Alaska (reviewed in [472]). In spruce beetle-killed forests on the Kenai Peninsula, white and Lutz spruce seedling establishment was greater in plots that had <60% cover from litter of bluejoint reedgrass than in plots with greater cover (P = 0.04) [39]. Seedlings also die from frost, snowpress, flooding, browsing, and lack of resources due to competition (reviewed in [148]).
Interference from other species may reduce white spruce's rate of establishment, growth, and survival [58,73,75,122,139,147,148,356,374,441]. White spruce establishes more readily on recently disturbed sites if competition for light, moisture, and nutrients [58,356] is reduced [148,356,377]. After fire in Alaskan boreal forest, competition for nitrogen and carbon by early successional species inhibited white spruce establishment and growth (P ≤0.05). While bluejoint reedgrass appeared to be a stronger competitor than field horsetail, the authors suggested that apparent differences in competitive abilities were better explained by the temperature and moisture microenvironments that these species occupied [58]. In interior and south-central Alaska, growth of planted white spruce seedlings was greater in sites without interfering vegetation. White spruce seedlings were planted in untreated and "weed-free" (herbicide treatments that controlled interfering native vegetation) sites. Mean heights were 1.5 to 3.8 times greater and mean diameters were 2.0 to 3.8 times greater in the weed-free plots than those in untreated plots [73]. In Alaskan boreal forest, logging without disturbing the organic mat often leads to the establishment of bluejoint reedgrass, which may persist for 25 to 100 years, limiting the establishment of white spruce [75].
White spruce may establish on floodplains where deposited alluvium creates a suitable seedbed [62]. On floodplains in interior Alaska, white spruce often establishes in mid-succession under a canopy of balsam poplar; however, establishment may occur episodically after flooding during a good seed year [429].
Plant growth: Although white spruce is shade tolerant, seedlings require open conditions for optimal growth [124,225,305], and they grow fastest in sunny sites. In interior British Columbia, seedlings grown in 60% light were almost twice as tall as those grown in 20% light [124]. During their first growing season, seedlings typically grow 0.4 to 0.8 inches (1-2 cm), and roots may grow 0.8 to 4 inches (2-10 cm) deep [305]. In productive, upland sites in Alaska, the tallest seedlings were 1.2 to 1.6 inches (3-4 cm) tall and maximum root length was >4 inches (10 cm) by the end of the first growing season [470,471]. However, white spruce often grows very slowly in less favorable conditions. Seedlings (<5 feet (1.5 m) tall) in the understory of conifer and hardwood stands are often more than 40 years old [124,473]. Although initial white spruce growth is slow, growth often accelerates when the trees are mature [124,178].
White spruce seedling growth is typically slower than that of associated shrubs and hardwoods [59,356]. On the Tanana River floodplain in interior Alaska, seedlings of feltleaf willow, balsam poplar, and white spruce showed mean annual height growth of up 3.5, 4.7, and 0.8 inches/year (9, 12, and 2 cm/year), respectively (reviewed in [470]). Open grown white spruce seedlings may grow to 5 feet (1.5 m) in approximately 20 years, which is much slower than sprout growth of associated hardwoods [473].
White spruce seedlings that establish immediately after disturbance typically grow faster than those that establish later. In interior Alaska, "dominant" seedlings that germinated 1 year after seedbed clearing averaged 7.3 inches (18.5 cm) tall by the 5th growing season, whereas those that germinated 3 years after clearing were 2 inches (5 cm) tall; the same height as the regenerating mosses. While the later-germinating seedlings were younger, the authors suggested that the 1st seedling cohort would continue to dominate the site because the later cohort competed with mosses for moisture, light, and nutrients and thus grew more slowly [471]. After stand-replacing fires in interior Alaska, white spruce growth rates differed depending on when they established relative to hardwoods. When white spruce seedlings established at approximately the same time as quaking aspen and paper birch, they grew much faster than when they established ~25 years after the hardwoods (Figure 10) [463].
White spruce grows slowly when it occurs under poor site conditions. In Alaskan floodplain forests, white spruce growth is greatly reduced after 100 years. This reduction of growth may be due to cold soil temperatures caused by the insulating effect of the organic mat, which deepens in older stands (reviewed in [303]). In interior British Columbia, seedlings grew more slowly in litter than in mineral soil, although this effect was not significant until the seedling's 3rd growing season [124]. At treeline sites, where growing conditions are marginal, trees typically grow slowly and white spruce often forms shrub-like trees [40,305].
White spruce tree growth is influenced by climate. White spruce growth and temperature have positive relationships in many sites [12,18,393]; however, white spruce growth may be inhibited by moisture stress when warm temperatures are coupled with low precipitation [64]. For instance, in interior Alaska, white spruce trees often grow best in the coolest, wettest years [255]. See Climate change for more information on climate-growth relations and Fire regimes of Alaskan white spruce communities for information about climate-growth relations in Alaska.
Vegetative regeneration: Layering of white spruce occurs at some treeline sites in Alaska and Canada. Layering may be important for regeneration when sexual reproduction is limited due to harsh climatic conditions, such as at treeline sites [18,283,305,396,470,473]. Layering has been observed at the forest-tundra ecotone in southwestern Yukon [18], on north-facing treeline sites in the southwestern Yukon [93], at alpine treeline in the western Northwest Territories [396], at the northern extent of white spruce's range in the Northwest Territories [283], and on coastal sand dunes in Nova Scotia [173].
Overview and trends: Fire initiates succession throughout white spruce's range but is more prevalent in western than in eastern North America. In its eastern distribution (and on relatively wet sites in the west), insect outbreaks and subsequent gap succession may be more important than fire in initiating succession. See Regional studies for more information.
Fires in white spruce communities are often stand-replacing, and postfire succession generally progresses through herb, shrub, and hardwood stages before succeeding to white spruce [136,137,263,415,418,428,430]. The postfire successional sequence depends on numerous factors including fire characteristics (e.g., severity, timing, type), seed availability, seedbed conditions, site characteristics, weather, and prefire plant community composition [147,329,336,356,436]. See Plant response to fire for more information about how these factors affect recruitment and succession after fire.
White spruce is generally considered a mid- to late-successional species [79,172,177,192,244,375], but it occurs in all stages of boreal forest succession. White spruce often colonizes recently disturbed sites [169,170,328,329,336] (see Seedling establishment) but it is also shade tolerant [178], and can establish years or decades after disturbance [17,34,67,145,330,429]. White spruce seedlings often persist in the understory for extended periods before emerging to the canopy [263,463]. White spruce typically becomes dominant when early-seral trees, such as quaking aspen, paper birch, and lodgepole pine die off [79,137,192,418]. White spruce forests may be more persistent than other boreal forest types (e.g., lodgepole pine, quaking aspen, paper birch) because white spruce is more shade tolerant and longer-lived than these species, and because it can regenerate in the shade of mature forests [136,355].
White spruce regeneration and succession following stand-replacing fire can follow two routes: delayed regeneration or self-replacement. When regeneration after fire is delayed, white spruce typically establishes and grows beneath a canopy of hardwoods (paper birch and quaking aspen in upland stands, balsam poplar in riparian stands) before eventually succeeding to dominate in mature stands (reviewed in [83]). Most studies indicate that delayed regeneration typifies white spruce stand development [17,34,67,145,330,429]. White spruce generally does not replace itself [65], due to limited seed sources after fire and inadequate seedbed conditions [328,336]. Occasionally, white spruce establishes prolifically after fire along with the hardwoods. When this occurs, white spruce forms part of an even-aged stand [137,430,431,460] and succession towards a white spruce-dominated community is accelerated [463].
Successional trends in the North American boreal forest depend, in part, on differences in fire frequency. When fire is frequent, the same species that colonized the stand after fire may dominate until the next stand-replacing fire. This leads to the persistence of shade intolerant species such as jack pine, quaking aspen, and paper birch. In contrast, when fire is infrequent, stands eventually become dominated by shade tolerant species such as white spruce, balsam fir, and northern white-cedar. Succession on mesic eastern, central, and west-central North American boreal forests is summarized and reviewed by Brassard and Chen [46]:
Floodplain succession in Alaska and western Canada follows similar sequences. Generally, forbs and willows colonize new alluvium, followed by alders, balsam poplar, and white spruce [418,439]. However, floodplain succession is not fully predictable due to differences in disturbances, seed dispersal, seedling establishment, weather, and site characteristics [188,404,442]. Old growth riparian white spruce forests may persist for 200 years or more because natural firebreaks inhibit fires in large river valleys. Consequently, floodplain white spruce forest tends to have more old growth than boreal uplands [404]. On some sites, white spruce floodplain forests may be replaced by black spruce as the organic layers thicken, soil cools, and permafrost forms [413,415,426,428,429]. See Regional studies for further discussion.
Insects: Insect outbreaks—especially those of eastern spruce budworm and spruce beetles—affect forest structure and successional patterns in portions of white spruce's distribution. Both insects preferentially attack large, overstory host trees leaving the understory intact and able to emerge into the canopy.
 |
 |
|
| Figure 8. Eastern spruce budworm damage in white spruce. Photo by Steven Katovich, USDA, Forest Service, Bugwood.org | Figure 9. Spruce beetle kill of white spruce, Kenai Peninsula. Photo by William M. Ciesla, Forest Health Management International, Bugwood.org |
Eastern spruce budworm occurs in the eastern portion of white spruce's range; its distribution coincides with the range of balsam fir and spruce. Balsam fir is the preferred host species, but white spruce, black spruce, and red spruce are also attacked [46]. Unlike fire, which kills all or most conifers, eastern spruce budworm kills only some of the trees in a stand. Overstory hosts are killed or weakened, and smaller understory trees are spared [41,46,478]. Host trees die due to the chronic stress of intense defoliation, which often occurs over multiple years [365]. Between 1704 and 1950, eastern spruce budworm outbreaks occurred at 30- to 138-year intervals from western Ontario to eastern Quebec and Maine. In order for an outbreak to occur there must be extensive stands of mature balsam fir. Frequent fires limit balsam fir abundance, and, therefore, eastern spruce budworm epidemics. In drier regions where fire is more common, such as western Ontario, fewer old trees are available, and outbreaks are more limited in area. Consequently, outbreaks occur more frequently in the Atlantic region than in Ontario [37].
After an eastern spruce budworm outbreak kills overstory trees, understory trees typically emerge into the canopy [41,478]. Successional patterns depend on species composition before and after the outbreak [41]. While white spruce saplings may be available to grow into the canopy [41], balsam fir [41,478] or hardwoods [422] are more likely to replace the killed trees, and white spruce abundance may decrease after an outbreak [36,41]. In western Quebec mixed forests, an outbreak reduced white spruce from 44 trees/acre (108 trees/ha) to 24 trees/acre (36 trees/ha). There were only 30 saplings/acre (75 saplings/ha) of white spruce after the outbreak, while saplings of balsam fir were 80 times more abundant. White spruce was likely to be less common in the overstory after the outbreak than before [41].
Spruce beetle outbreaks occur in south-central and southwestern Alaska [32,39,190,373,454] and southwestern Yukon [32,185]. Spruce beetles do not normally kill all the trees in a stand, even during high-severity outbreaks [373]. Spruce beetles preferentially attack large-diameter mature spruce (white spruce, Lutz spruce, Sitka spruce, and rarely black spruce). Stands with high densities of mature white spruce are preferentially attacked, whereas stands with high densities of black spruce tend to be avoided (reviewed in [454]). Across south-central and southwestern Alaska, a 250-year record shows that the interval between outbreaks ranged from 10 to 165 years and averaged 48 years [373]. In white spruce and Lutz spruce forests on the Kenai Peninsula, spruce beetle outbreaks occurred every 50 years on average, whereas fires occurred approximately every 400 to 600 years [31]. This suggests that historically, spruce beetle outbreaks may have been a more important disturbance than fire in this region.
Vegetation change and succession following a spruce beetle outbreak vary by region due to differences in climate, soils, and competitive interactions among species [454]. On the Kenai Peninsula, vegetation change following recent spruce beetle outbreaks varied among geographic regions and forest type. On the southern Kenai Lowland, where white spruce was dominant, white spruce had high mortality (87% reduction in basal area of white spruce >5 inches (12.7 cm) DBH), and forests shifted toward early successional grasses and forbs. White spruce forests were converted to woodlands and herbaceous types due to expansion of bluejoint reedgrass populations and low densities of tree seedlings. In the Kenai Mountains, where stands were dominated by mountain hemlock and white spruce, white spruce had moderate mortality (46% reduction in basal area), and forests shifted towards a late-successional structure dominated by mountain hemlock. In another region of the Kenai Peninsula, where white spruce was a secondary species in mixed stands and had low mortality (28% reduction in basal area), no substantial shift in successional direction was detected. Over the entire study area, the authors speculated that 3% of the 115 plots had poor chance for forest regeneration due to abundant bluejoint reedgrass cover and lack of overstory trees. These areas are in the southern Kenai Lowlands [43]. On the Cook Inlet, a severe outbreak in the 1970s caused 65% mortality of white spruce >5 inches (12.7 cm) DBH. Paper birch became the dominant tree species in the residual stand (reviewed in [454]).
Gap succession: Insect outbreaks, mortality of individual trees, windthrow, and fungi can create gaps in communities where white spruce occurs. Gap dynamics are important in shaping forest structure, especially where fire-return intervals are long [46]; small-scale gap dynamics may shape succession in old growth stands in the east [278]. While succession throughout much of the boreal region is characterized by shade-tolerant species replacing shade-intolerant species, gaps allow early-successional species, such as hardwoods, to persist in late successional stands [422]. In northwestern Quebec mixedwoods, group tree mortality created small gaps in young (50-year-old) quaking aspen-dominated stands, and eastern spruce budworm created large gaps in old (234-year-old) balsam fir-dominated stands [222]. While understory white spruce may replace canopy trees [85,316,353] in small and large gaps, white spruce seedling abundance is generally insufficient for released seedlings and saplings to dominate mixedwood stands [85].
Regional studies: Examples of succession in white spruce communities follow by region.
Alaska: White spruce may be present in all stages of postfire succession in Alaska, although it generally does not regain dominance for over 100 years. White spruce often replaces hardwood (i.e., quaking aspen and paper birch) stands after approximately 100 to 150 years [137,262,264,415,418]; however, extensive fires may occur at about 100 to 150 year intervals in interior Alaska [136], precluding dominance by white spruce on many sites. Consequently, midseral communities dominated by quaking aspen or paper birch, or codominated by white spruce and hardwoods, are common and widespread throughout Alaska on relatively warm, upland sites [136,137,262]; and pure, old white spruce stands (i.e., stage 6, below) are less common than younger stands [136,137].
In the absence of subsequent fire, the most common postfire successional sequence on warm, well-drained white spruce sites in interior Alaska is characterized by white spruce gradually replacing herb, shrub, and hardwood stages [136,137,263,413,415,418,428,430,460]. Similar postfire successional patterns occur on the Kenai Peninsula in south-central Alaska [310]. Foote [137] describes 6 developmental stages in this postfire sequence:
White spruce may be absent or infrequent during the newly burned stage because seed may not be available during the first postfire year. Most white spruce seedlings establish during the first 30 years after fire (during the moss-herb and tall shrub-sapling stages) [137], although seedling establishment may peak again under older white spruce stands [136]. Although the dense tree phase is dominated by hardwoods, mature white spruce increases in density. White spruce gradually replaces hardwoods during the hardwood or mixed hardwood-spruce stage [137]. White spruce regains dominance by establishing and growing under the shade of other trees and living longer than earlier successional species [136].
A less common postfire successional sequence on white spruce sites may occur when white spruce seed is available after fire [137,169,170,262,418,430,431]. In this case, white spruce establishes with the hardwoods within the first few years after fire, and an even-aged white spruce stand may develop [137,430,431,460]. When white spruce establishes after hardwoods, its growth is suppressed by faster growing hardwood sprouts. However, when white spruce establishes along with hardwoods, its growth is less restricted and it reaches the canopy much faster (Figure 10) [463], which accelerates succession towards white spruce.
At arctic treeline, long fire-return intervals or absence of fire may lead to successional replacement of black spruce by white spruce. Following a fire in the early 1900s, black spruce had high recruitment for <30 years, while white spruce recruitment was consistently high after the fire and throughout the study period (about 100 years). By about 80 years after fire, black spruce established via layering, but white spruce establishment surpassed that of black spruce. After 100 years, white spruce seedling density was 14.6±16.9 seedlings/acre (90.3±41.7 seedlings/ha), and black spruce density was 8.7 ± 2.3 seedlings or clones/acre (21.6 ± 5.8 seedlings or clones/ha). This suggests that in the absence of fire, white spruce will become increasingly dominant in these stands [257].
Floodplain succession: Primary succession on floodplains of interior Alaska typically begins with a bare surface adjacent to the river and passes through several developmental stages as river terraces rise and flood frequency decreases. Succession finally stabilizes as mature white spruce or black spruce stands [413,415,418,426,428,429,430]. Van Cleve and Viereck [418] describe 8 successional stages that occur on the Tanana River floodplain:
Although white spruce can germinate on mineral soil of newly established siltbars [442], seedlings cannot survive repeated flooding, yearly sediment deposition, high erosion, or periods of drought commonly associated with early succession[13,303,429,430]. Consequently, white spruce often establishes during the shrub and balsam poplar stages and becomes dominant after balsam poplar matures and dies [418,426,429,442]. White spruce may live to be about 400 years old in late-successional floodplain forests [442].
Although succession often follows the general pattern described above, multiple trajectories are possible depending on landscape features, initial establishment, climate, and disturbance agents [188]. Occasionally, white spruce seeds germinate on mineral soil soon after flooding, and a dense, even-aged white spruce stand develops following the shrub stage [303,430]. This tends to occur following major channel shifts rather than during the gradual buildup of floodplain terraces, because shifted channels experience less frequent flooding [303]. Many authors suggest that black spruce replaces white spruce in floodplain forests as the organic layer thickens, soil cools, and permafrost forms [413,415,426,428,429]. However, a chronosequence study on the Tanana River floodplain showed no evidence that black spruce replaces white spruce successionally [188]. White spruce sites had no black spruce seedling or sapling recruitment, permafrost, or shifts in species composition that made them more similar to black spruce sites. Other recent studies suggest that the occurrence of black spruce stands on floodplain sites may be a function of site drainage and fire history [187,188,273]. Black spruce may be restricted to poorly drained back swamps, while white spruce forests dominate the well-drained meander belts [273].
Succession after fire on Alaskan floodplains follows patterns similar to those on uplands, with herbs, shrubs, and hardwoods initially replacing white spruce or black spruce. However, riparian white spruce stands may burn less frequently than upland stands because they are often protected by natural firebreaks (reviewed by [430]). For additional information about fire regimes in Alaskan floodplain communities, see Fire Regimes of Alaskan white spruce communities.
Northwestern Great Plains: In the Black Hills of South Dakota and Wyoming, white spruce is considered the climax species in some ponderosa pine and quaking aspen stands [184,372].
Great Lakes: White spruce is often considered a climax species in the Great Lakes region, although fires typically occur before forests reach climax conditions [177,244]. White spruce increases dominance with stand age [77,198,199] and may codominate late-successional communities [77]. Mature balsam fir-white spruce stands often experience eastern spruce budworm outbreaks that alter successional patterns [176].
In many areas of the Great Lakes region, balsam fir, white spruce, northern white-cedar, and paper birch dominate late-successional forests. In the absence of fire, earlier seral stands of red pine, eastern white pine, or jack pine often succeed to stands codominated by white spruce [77,177,244]. In the Boundary Waters Canoe Area, Minnesota, jack pine can persist in the overstory for 210 to 250 years, but these communities will likely succeed to balsam fir-spruce-northern white-cedar-birch or black spruce-feathermoss communities without fire. In red pine and eastern white pine stands that have not burned in a long time, balsam fir, white spruce, northern white-cedar, and paper birch establish and grow in the understory [177], where they are poised to recruit into the overstory as the pines die out. In Isle Royale National Park, basal area and relative density of white spruce increase with stand age, while shade-intolerant species (e.g., paper birch, quaking aspen, and jack pine) decrease [198,199].
In northeastern Wisconsin, white spruce occurs in the sapling layer in stands that were logged and burned more than 100 years prior. For ~80 years following logging and fire, paper birch and quaking aspen dominated the site. By ~100 years, red pine and eastern white pine regained dominance and balsam fir, sugar maple, and white spruce saplings dominated the understory. The composition of the sapling layer suggests that the pines will eventually succeed to balsam fir, sugar maple, and white spruce in absence of disturbance [383].
On upland mixedwood forests in Algonquin Provincial Park, Ontario, succession after fire generally proceeds from paper birch-quaking aspen to either balsam fir-white spruce, or from eastern white pine to shade tolerant hardwoods (mostly sugar maple), and finally to eastern hemlock. After fire, white spruce may establish with the faster growing hardwoods, but it typically does not codominate stands until after the hardwoods decline [275].
Northeast: In northern New England, white spruce occurs in many stages of succession. It is a pioneer species following blow-downs and other disturbances that create extensive openings. Along the Acadian coastline, white spruce often colonizes abandoned agricultural land and forms a distinct border [95]. While considered a climax species [318], it is less shade tolerant than associated eastern species (i.e., red spruce and balsam fir) [55,95]. White spruce may die off in closed stands, which favor dominance by red spruce [95].
Fire histories and consequent patterns of succession were not well documented for red spruce-white spruce forests of New England (as of 2015). It is likely that successional patterns of white spruce in New England are similar to those in the Acadian Forest Region, which is discussed in the Eastern Canada section below.Western Canada: White spruce occurs in all successional stages in western Canada, although its basal area typically peaks in mid- to late-successional stands [69]. In British Columbia and Alberta, white spruce tends to replace lodgepole pine and quaking aspen in the absence of fire [69,79,97,192,254,293,296]. After fire, white spruce or western white spruce may establish along with lodgepole pine; however, lodgepole pine dominates early seral stands because it has much higher stem densities and faster initial growth rates [69,79,192]. Lodgepole pines dominates until it dies out, from 70 to 250 years after fire [69,97,192]. Spruce (white and Engelmann) and subalpine fir become increasingly important as lodgepole pine declines [192]. In west-central British Columbia, western white spruce basal area peaked 201 to 250 years after fire when lodgepole pines died [69]. While lodgepole pine stands theoretically succeed to spruce-subalpine fir communities, they are often maintained indefinitely by recurring fires [79,332], especially on dry, west-facing slopes [293]. If subalpine fir-white spruce stands reach a decadent, late-successional stage (>230 years in west-central Alberta), white spruce abundance may decline while subalpine fir increases [97,293].
The rate of succession from lodgepole pine to spruce-subalpine fir depends on initial postfire stand composition and moisture conditions. If spruce (white or Engelmann) does not establish with lodgepole pine after fire, succession is slower than when spruce establishes immediately after fire [79]. In the Alberta foothills, the rate of successional replacement of lodgepole pine by white spruce or Engelmann spruce was associated with moisture conditions. Lodgepole pine had greater persistence in drier conditions [192], possibly because of recurring fires [293].
White spruce replaced quaking aspen before succeeding to balsam fir in a postfire chronosequence in Saskatchewan southern boreal mixedwood forest. Thirty-five stands were studied; they ranged from <1 to 201 years after fire. Mean density of white spruce increased with stand age, peaked 172 years after fire, and declined in stands 175 years old and older. White spruce replaced quaking aspen between 50 and 165 years after fire and dominated the canopy between 93 and 172 years after fire [17].
A chronosequence study in the northern boreal-cordilleran region, central Yukon, describes a postfire successional sequence on well-drained, low-gradient sites. The youngest stands (8-11 years old) were dominated by quaking aspen and willows. Quaking aspen, shrub, and herb cover peaked 50 to 70 years after fire while white spruce cover gradually increased. White spruce began to replace quaking aspen in 50- to 60-year-old stands, and white spruce cover equaled or exceeded that of quaking aspen 90 to 100 years after fire. The author speculated that white spruce replaced quaking aspen faster than it typically does in the southern boreal region because it established early after fire. Rather than white spruce seedlings and saplings filling gaps created by dead quaking aspen, white spruce "forced" the replacement of healthy quaking aspen because it was already established within and directly below the quaking aspen canopy. These stands differ from southern boreal forests in western Canada on similar well-drained sites because lodgepole pine and balsam poplar are infrequent and firs are absent from the northern boreal forests [391].
A study of mid- to late-successional boreal mixedwood stands (120-175 years old) in Riding Mountain National Park, Manitoba, indicates long-term persistence of hardwoods rather than successional replacement by white spruce. The persistence of hardwoods was due to a second cohort of quaking aspen and balsam poplar that established via root suckers after the initial cohort broke up (about 80-140 years after stand establishment). Consequently, 175-year-old stands consisted of a mixture of white spruce and hardwoods. The author suggests that the ability of hardwoods to recruit into late-successional stands indicates that boreal mixedwood communities (i.e., white spruce-hardwood) are self-sustaining and not a transitional phase leading to a white spruce community [248].
A preliminary comparison of burned and unburned subarctic woodland stands in the lower Mackenzie River Valley, Northwest Territories, suggests that without periodic fires, white and black spruce may be eliminated and woodlands converted to a tundra-like moss/lichen association. In sites where fire had not occurred for 150 years or more, there was a dense growth of lichens, few or no tree seedlings, an open, unhealthy spruce stand, and permafrost close to the soil surface. Recently burned areas were initially colonized by liverworts, followed by herbs, mosses and low ericaceous shrubs, and then by a relatively dense and vigorous spruce stand. Fires may be necessary to reduce dense lichen mats and expose mineral soil to enable spruce recruitment [389].
Primary succession on floodplains in western Canada is similar to that of Alaska. Generally, willows colonize new alluvium, and as alluvial deposits raise the ground level above the floodplain, alder, balsam poplar, and finally white spruce forests develop [439]. This pattern is variable since it is subject to stochastic events such as flooding and seed dispersal [404,442]. Old-growth riparian white spruce forests are restricted to terraces of major river valleys where primary succession has continued undisturbed for 200 years or more. Fires are often inhibited in large river valleys because of natural firebreaks such as meanders, oxbow lakes, and seepage sites. There is generally more old growth in boreal riparian white spruce forests than in boreal uplands [404]. In the Peace River Lowlands, Wood Buffalo National Park, succession from old-growth white spruce to black spruce has not occurred [404]. On the most elevated sites of the Mackenzie Delta, white spruce forests are dying out and being replaced by tundra vegetation in xeric sites, and by white spruce/bog woodlands in poorly-drained sites. White spruce seedling establishment is limited to early seral stages and stands that are flooded periodically, have moderately-closed canopies, and have a ground cover of herbs rather than feathermosses and lichens. The authors suggest that a decrease in flood frequency—and consequent poor seedbed conditions—may be responsible, at least in part, for poor white spruce regeneration on elevated sites [321].
Eastern Canada: Although fire occurs throughout eastern Canada, it is more frequent in drier western regions, such as Ontario, than in the moist, eastern Maritime provinces. The general successional sequence after fire in eastern Canadian mixed forests is initial dominance by hardwoods (i.e., paper birch, quaking aspen), followed by successional replacement with white spruce and balsam fir. Without subsequent fire, balsam fir often replaces white spruce. On subalpine sites in central Quebec, it may take between 370 and 480 years before white spruce stands succeed to balsam fir [101]. On some sites, northern white-cedar may replace both balsam fir and white spruce [316]. Where jack pine occurs, it typically dominates the early postfire environment; without fire, these forests succeed to balsam fir and white spruce [172].
Several studies describe postfire successional dynamics in mixedwood stands near Lake Duparquet, northwestern Quebec, where white spruce is an important forest component. Early-seral stands are dominated by shade-intolerant hardwoods (paper birch and quaking aspen) or jack pine. As the initial canopy dies out, midsuccessional stands become codominated by balsam fir and white spruce [33,172,316] (reviewed in [35]). On some sites, the oldest stands may be dominated by monospecific patches of northern white-cedar [316]. Several studies indicate that white spruce importance and basal area peak in midsuccessional stands (approximately 150 years after fire) [33,316] (reviewed in [35]). While white spruce may establish within 10 years after fire, it is typically suppressed and does not reach the canopy until midsuccession because it grows more slowly than the hardwoods [33]. In early succession, white spruce gradually becomes dominant as small gaps are created by the deaths of individual trees or small groups of trees [222,316]. In late succession, large gaps are caused by eastern spruce budworm [222]. After eastern spruce budworm outbreaks, white spruce abundance often declines. Most xeric stands become dominated by northern white-cedar and black spruce, while mesic sites become dominated by balsam fir and northern white-cedar [36].
Although quantitative studies of forest cover and succession in the Acadian Forest Region are rare, early descriptions by explorers, surveyors, and settlers suggest that white spruce is more abundant today than it was historically. This region was characterized by shade tolerant hardwoods, spruce-fir forest, and mixed types. Human activities such as land clearing have resulted in a shift from late-successional species including sugar maple, red spruce, eastern hemlock, yellow birch, northern white-cedar, and beech to earlier successional species including white spruce. Abandoned farmland was colonized by pioneering white spruce throughout the Maritime provinces (reviewed in [258]). According to Fernow (1912) (cited in [258]), white spruce only accounted for about 1% of the presettlement forest in Nova Scotia and did not naturally occur in pure stands like it does today. While white spruce often occurs on abandoned farmland in the Acadian Forest Region, it also occurs in old-growth and senescent balsam fir stands in nearby Atlantic provinces, including stands on the Gaspé Peninsula, Quebec [104], and on the Great Northern Peninsula, Newfoundland, where succession is characterized by small-scale gap dynamics [278].Effects on trees: Fire usually kills white spruce [186,262,472]. Lutz [262] reported that white spruce "is probably more susceptible to destruction by fire than any other tree in Alaska". White spruce is poorly adapted to survive fire because trees have thin bark and shallow roots [194,262,263,303,394]. Consequently, even low-intensity surface or ground fires usually kill white spruce [169,170]. On some sites, branches draped with lichens extend nearly to the ground [263,303], facilitating the spread of fire into the crowns. Because most of white spruce's roots occur in surface organic layers, slow-burning ground fires are particularly lethal. Ground fires may burn living roots as large as 8 to 9 inches (20-23 cm) in diameter [263].
Occasionally individual white spruce trees survive fire with scarring [67,174]. These rare fire-scarred trees typically occur along the edges of burned areas, where fire intensity was extremely low [174,262].
Effects on seeds: White spruce seeds and cones are easily destroyed by fire [5,263]. Most fires in boreal forests are high-intensity, stand-replacing fires [204] that occur before white spruce seeds are ripe [436,466]; consequently most white spruce seeds in crown-burned or scorched trees fail to ripen (e.g., [169,170]). However, crown-stored white spruce seed may occasionally survive fire even when the trees are killed [169,170,468] (see Seed banking). Simulation experiments suggest that germinable white spruce seed contained in closed cones may survive heating by crown fire, and that approximately 12% of cones would contain viable seed after fire [288].
Postfire regeneration strategy [386]:
Tree without a sprouting
root crown
Prostrate woody plant, stem growing in organic soil (only at some treeline sites)
Crown residual colonizer (on site, initial community) (uncommon)
Initial off-site colonizer (off site, initial community)
Secondary colonizer (on- or off-site seed sources)
Fire adaptations and plant response to fire:
Fire adaptations: White spruce is poorly adapted to survive fire compared to most associated trees [358], which makes its widespread distribution in fire-prone boreal forests perplexing. White spruce has many traits that favor its persistence under long fire-return intervals, such as requiring a long time to mature [263], variable seed production [138], short seed dispersal distance, thin bark, and non-serotinous cones (reviewed in [83]). Traits that enable white spruce to persist in boreal communities include the ability to establish on thin organic or mineral soil seedbeds after fire [61,329,336,356,468,472], years of high seed production, which sometimes coincide with fires [327,328,330,336,436], higher shade tolerance and longer lifespan than those of competing hardwoods [136], and the ability to establish in late-successional stands [17,34,67,145,330,429].
Rowe [358] compares the ability of boreal conifers to regenerate after fire based on life history traits (Table 4). White spruce ranks 4th out of 5 conifers.
Table 4. Relative ranking (1-5*, where 5 indicates best adaptation to fire) of characteristics of boreal conifers contributing to successful regeneration after fire. *Weighted up to twice the value of other characteristics, because it is the most important characteristic. Table modified from [358]. |
|||||
| Jack pine | Black spruce | Tamarack | White spruce | Balsam fir | |
| Seed retention on tree* | 10 | 6 | 2 | 3 | 1 |
| Earliness of seed production | 5 | 4 | 2 | 3 | 1 |
| Seed mobility (relative size) | 2 | 5 | 5 | 3 | 1 |
| Seedling frost hardiness | 5 | 3 | 4 | 2 | 1 |
| Seedling palatability to animals | 3 | 5 | 2 | 4 | 1 |
| Seedling growth rate | 5 | 2 | 4 | 3 | 1 |
| Seedling response to full exposure (opposite of shade tolerance) | 4 | 3 | 5 | 2 | 1 |
| Totals | 34 | 28 | 24 | 20 | 7 |
Plant response to fire: White spruce abundance may be drastically reduced after fire because of high tree mortality and limited postfire recruitment [54,147,178,308,356], although white spruce may establish after fire when adequate seed sources and seedbeds are available [169,170,328,329,336]. White spruce establishment after fire depends on seed availability, fire characteristics, seedbed conditions, site and soil characteristics, and weather.
After fire, white spruce typically establishes from seed from trees along fire edges or from unburned trees within the burn [146,156,174,178,262,430]. Seedling establishment is typically highest close to the unburned seed source [145]. In northwestern Quebec boreal mixedwood forest, the average density of white spruce after fire decreased from 280 trees/acre (693 trees/ha) near the edge of the burn (0-200 feet (0-60 m) from edge), to 21 trees/acre (51 trees/ha) in the interior of the burn (3,940-6,560 feet (1,201-2,000 m) from edge) [145]. Because white spruce typically has a short dispersal distance (<330 feet (100 m)) [430,436,466], small burn areas may be colonized entirely from trees along the edges, and large burns may be colonized only around the perimeter. White spruce does not typically recruit into the interior of large fires unless unburned white spruce remain within the burned area [430]. Models of postfire recruitment indicate that adequate regeneration of white spruce would be limited to about 230 feet (70 m) from the fire's edge when the external seed source had a 5 m²/ha basal area. In the interior of a burn (>2,600 feet (800 m) from a fire edge), about half of the white spruce recruits would establish from small residual stands [156]. White spruce may occasionally establish far from unburned seed sources via long-distance seed dispersal [457] (e.g., secondary dispersal over snow, up-drafts) or crown-stored seed in fire-killed trees [288].
Fire timing, type, and severity affect white spruce seed source availability and recruitment after fire. Early- to mid-season, stand-replacing crown fires, which commonly occur in boreal forests, typically destroy white spruce seeds (e.g., [147]). However, crown-stored seed may occasionally be available on fire-killed trees depending on fire timing and intensity [157,169,170,287,288,468] (see Seed banking). When ground or surface fires occur after seed is ripe (i.e., mid-to late-season fires), viable seed may be available in fire-killed trees [169,170]. Large amounts of viable seed were found on fire-killed trees with intact crowns after an early-season fire that did not consume the canopy in interior Alaska. Trees with scorched or burned crowns did not produce seeds [468]. Crown-stored seed may result in high postfire recruitment [169,170], even in the interior of the burned area [288]. Even-aged white spruce stands may develop from this type of postfire establishment [137,430,431,460].
After fire, white spruce seedlings typically establish best on sites where most or all of the organic soil is consumed, leaving only a thin organic layer or exposed mineral soil [61,329,336,356,468,472]. Severe fires that consume the organic layer not only create a suitable seed bed for white spruce establishment, but also kill the perennating buds of competing hardwoods, shrubs, and herbs, which often occur in the organic horizon [356]. However, most fires do not consume the entire forest floor; mosaic fires result in patches of exposed mineral soil intermixed with areas where organic layers are intact or partially consumed [148,158,159,263,290]. Within individual burns, exposed mineral soil is highly variable, ranging from 0 to 100%, but averages about 35% in boreal mixedwoods in Alberta and in Alaska [158,263]. Even less mineral soil may be exposed around the fire perimeter, where most white spruce seed is dispersed. On a large fire in Alberta boreal mixedwoods, soil exposure on the fire's edge averaged only 5%, whereas in the interior, it averaged 35% [158]. Spring fires typically consume less organic material and expose less mineral soil than summer fires; consequently less white spruce regeneration occurs after spring fires than summer fires [329].
White spruce seedling establishment may occur within 20 years after fire [108,145,328,468]— generally within a few years [328]— when seedbeds are most receptive [148,328,336,471]. Initial postfire establishment may be highest when fires and mast years coincide [327,328,330,336], particularly if the fires create suitable seedbed conditions. Some studies report delays in postfire recruitment (e.g., [34]). This is probably due to low seed availability at the time of the fire and in subsequent years, and/or inadequate seedbed conditions following fire [34]. Some retrospective studies that use tree rings in mature stands to estimate tree regeneration after fire suggest substantial delays in white spruce regeneration, but their methods may not precisely estimate tree ages (and postfire establishment year); consequently, these studies may overestimate delays in regeneration [327]. White spruce is not limited to initial postfire regeneration; it commonly establishes later in succession [17,34,67,145,330,429,463].
Because white spruce trees are easily killed by fire, fire-adapted trees such as quaking aspen, paper birch, jack pine, or black spruce commonly dominate after fire on sites previously dominated by white spruce [65,147,262]. Regeneration density after fire is related to prefire basal area for many boreal tree species (e.g., jack pine, quaking aspen, paper birch, black spruce), but generally not for white spruce, because it often regenerates poorly after fire regardless of prefire abundance [65,147].
Site characteristics, such as soil type and topographic position, may influence white spruce's response to fire. Sites with coarse-textured soils and sites on south-facing slopes dry out more rapidly and tend to burn more frequently than sites with fine soils and sites on north-facing slopes. On fire-prone sites, white spruce is often eliminated and replaced by species that have shorter reproductive cycles and serotinous or semiserotinous cones, such as jack pine and black spruce. Consequently, white spruce rarely dominates fire-prone sites [356].
White spruce seed production is typically poor at the forest-tundra ecotone, so little or no seed is available for postfire regeneration. White spruce establishment was limited after a "severe" wildfire in the black spruce-white spruce forest-tundra ecotone northeast of Inuvik, Northwest Territories and none of the white spruce that established in the first 5 years after the fire in the 0.74 acre (0.3 ha) study area survived. In the 22 years after fire, limited establishment occurred in clumps close to surviving white spruce. Establishment peaked every 3 or 4 years. The authors suggest that establishment was restricted due to a lack of viable seeds and a short seed dispersal distance [236]; site characteristics may have played a part as well.
See Regeneration Processes for further information, including information on seed production, seed dispersal, germination, establishment, and growth. Successional Status discusses general patterns of postfire succession in white spruce communities. Examples of white spruce response to fire by region follow.
Alaska: In Alaska, white spruce may colonize recently burned sites, but regeneration often occurs later in succession. Severe fires typically set back white spruce forests to communities dominated by seral species including paper birch and quaking aspen, especially when fires are large and all white spruce are killed [262]. It generally takes about 150 years before white spruce regains dominance after fire [137]. Pure white spruce stands may establish immediately after fire, but this is not common [137,262].
In burned white spruce-reindeer lichen woodlands in the central Brooks Range, few white spruce established within the first 10 years after fire, although seedling establishment was generally continuous during the next ~90 years. Seedling establishment may have been delayed due to unfavorable seedbed conditions after the fire. On 1 of the 4 white spruce plots, 75% of the white spruce trees present ~100 years after fire established in the first 25 years; this is similar to black spruce establishment patterns. On the rest of the plots, it took 50 to 80 years for 75% of white spruce to establish [67].
In early June of the 1st growing season after the Wickersham Dome Fire in interior Alaska, white spruce was seeded onto a variety of seedbeds in burned black spruce stands on the north and south slope. Seedbeds included mineral soil, ash, and charred mosses. Germination was best on mineral soil; no white spruce germinated on charred mosses. On the north slope, germination peaked between June 27 and July 11. Subsequent mortality was initially caused by something eating the cotyledons, and additional mortality was caused by damping off, flooding, and smothering by other vegetation after rains in August. On the south slope, germination peaked between July 11 and August 8 and subsequent mortality peaked 3 weeks later when conditions were dry. Survival of germinants to September 10 was 72% on the north slope and 77% on the south slope. Most surviving seedlings ranged from 0.6 to 1 inch (1.5-2.5 cm) tall [71].
After the low-intensity Bear Creek Fire in interior Alaska, white spruce had high establishment rates. The fire burned closed white spruce-balsam poplar stands and killed all the trees but left the canopy intact. White spruce seedlings were abundant 1 growing season after the fire, probably because seeds were mature at the time of the fire (August), and canopies and seeds were unharmed. A thin layer of fallen needles may have created a mulching effect over mineral soil which may have helped stabilize seedbed moisture conditions. White spruce regeneration was estimated at 12,000 seedlings/acre (30,000 seedlings/ha) [169,170].
After stand-replacing fires (~1915) in conifer-hardwood stands on upland, south-facing sites in interior Alaska, white spruce establishment differed among plant communities (2 quaking aspen types, 2 paper birch-quaking aspen types, and 1 white spruce-paper birch type), and subsequent height growth and stand development followed 2 distinct patterns. A chronosequence study used tree rings to estimate tree ages and establishment dates 46 to 141 years after fire. In quaking aspen community types, quaking aspen established soon after fire, and white spruce established about 25 years after fire (Figure 10a). In paper birch-quaking aspen and white spruce-paper birch community types, white spruce established rapidly and concurrently with the hardwoods and no establishment occurred afterward (Figure 10b). When white spruce established at around the same time as the hardwoods, it grew rapidly (Figure 10b); when white spruce established after the hardwoods, it grew more slowly (Figure 10a) [463].
| Figure 10. Tree growth and establishment patterns after stand-replacing fires (~1915) in (a) a quaking aspen community, and (b) a white spruce-paper birch community. Figure modified from Youngblood [463]. | |
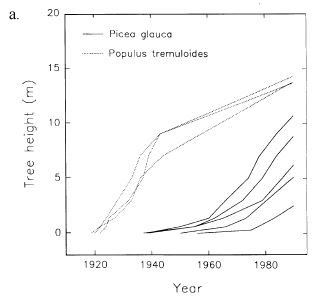 |
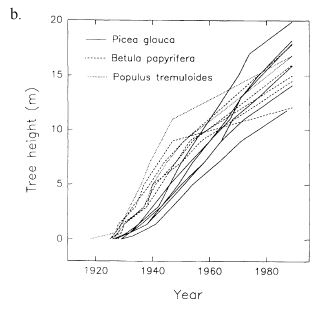 |
Great Lakes: White spruce response to fire is not well documented in the Great Lakes region. However, white spruce is probably eliminated or severely reduced following stand-replacement fires in boreal mixedwoods. Studies from the region suggest that white spruce does not readily establish immediately after fire [78,308], with some exceptions (e.g., [176]). After the Little Sioux Fire in northeastern Minnesota, white spruce was eliminated from jack pine and quaking aspen stands where it had been locally common in the understory of 3 of the 7 studied stands. No white spruce regeneration was reported in the 5-year study period [176,308]. Five years after a stand-replacing fire in a balsam fir-white spruce-paper birch forest on Isle Royale, Michigan, white spruce seedlings were rare [78]. In contrast, after a stand-replacing fire in mixedwood stands in the Boundary Waters Canoe Area, Minnesota, white spruce established in abundance along with black spruce, paper birch, white pine, jack pine, balsam fir, and northern white-cedar. According to this retrospective study, most trees, including white spruce, established within 5 years after the fire. The seed source was likely an unburned mature mixed forest across the river from the burn [176]. In the Great Lakes region, white spruce dominance increases with stand age [77,198,199], and white spruce is most likely to occur on sites that burn infrequently [176].
Western Canada: After stand-replacing fire in white spruce forest, hardwood and pine seedlings often establish and white spruce establishment fails. Ten to 20 years after 5 stand-replacing wildfires on mesic, upland sites in central and northeastern Alberta boreal mixedwoods, few white spruce trees remained and white spruce regeneration was poor. Prefire stands were dominated by quaking aspen, white spruce, or a mixture of both, and 3 of the 5 fires occurred during mast years. Nonetheless, 79% of the <14-year-old plots and 91% of the 20-year-old plots had no white spruce seedlings. Lodgepole pine and jack pine seedlings established in many stands, including those that were pure white spruce before fire, even though pines were not present in prefire stands. The spring and early summer fires may not have consumed sufficient organic material to create suitable seedbeds for white spruce. Because there were few white spruce trees left after any of the fires, the authors suggested that most white spruce seedlings established from seed sources >1,600 feet (500 m) away, or from trees that fell before the study sites were visited [147].
After the 1,846-acre (747-ha), early-June Aishihik Fire in south-central Yukon, white spruce regeneration was variable. Most stands were located on high terraces of the Aishihik River Valley; prefire communities were dominated by white spruce and/or quaking aspen. Where white spruce dominated the prefire stand, white spruce establishment after fire varied from about 1,100 to 6,300 seedlings/acre (2,700-15,600 seedlings/ha) 5 years after the fire. Where quaking aspen dominated the prefire stand, white spruce regeneration was virtually absent. In general, quaking aspen was the most pronounced species in most postfire plots; 3 years after fire it occurred in 100% of the plots. White spruce seedlings occurred in 44% of the plots by postfire year 3, and in 77% of the plots by postfire year 5. Quaking aspen suckers averaged about 5 feet (1.5 m) tall 5 years after fire, whereas white spruce seedlings averaged about 0.8 inch (2 cm) tall. This suggests that quaking aspen will initially dominate much of the regenerating Aishihik Fire. Table 5 shows the variation in white spruce and quaking aspen regeneration after the Aishihik Fire [312].
| Table 5. Tree regeneration 5 years after the Aishihik Fire, Yukon. Plots are 10 x 10 m. Table modified from Oswald and Brown [312]. | ||||||
| Plot | Prefire community | White spruce (seedlings) | Quaking aspen (suckers and seedlings) | White spruce (seedlings/ha) | Postfire exposed mineral soil (%) | Moisture regime |
| 1 | White spruce | 30 | 44 | 3,000 | 95 | subxeric |
| 2 | White spruce/quaking aspen | 44 | 141 | 4,400 | 95 | subxeric |
| 3 | White spruce | 156 | 37 | 15,600 | 100 | subhygric |
| 4 | Quaking aspen | 1 | 800 | 100 | 0 | mesic |
| 5 | White spruce/quaking aspen | 17 | 75 | 1,700 | 95 | mesic |
| 6 | White spruce | 27 | 1 | 2,700 | 95 | subxeric |
| 7 | Quaking aspen | 0 | 200 | 0 | 20 | submesic |
| 8 | White spruce/quaking aspen | 0 | 7 | 0 | 75 | mesic |
| 9 | Quaking aspen | 27 | 31 | 2,700 | 85 | mesic |
In northeastern Alberta, mixedwood boreal forests that are classified as "pure deciduous" often have a white spruce component. Emergent white spruce trees in these stands are much taller than the intermediate-age (≤70 years old) quaking aspen canopy (Figure 11). They are likely to be trees that survived previous fires because white spruce do not enter the canopy before 60 years and do not dominate the canopy until 100 to 120 years after fire. These white spruce are disproportionately large seed sources because of their size and position above the canopy. They have a strong influence on the distribution, abundance, and timing of white spruce recruitment immediately and for several decades after fire, when white spruce seedlings may establish under a mature aspen canopy [86].
 |
| Figure 11. Emergent white spruce within "pure" quaking aspen stands northwest of Peace River, Alberta. Photo by Ellen Macdonald, © Canadian Science Publishing or its licensors [86] |
Eastern Canada: White spruce is a common component of mixed stands in eastern Canada and is most important in mid- to late-successional stands. Like many other mixedwood sites in white spruce's distribution, early postfire succession in eastern Canada tends to be dominated by shade-intolerant species such as quaking aspen, paper birch, and jack pine, with few white spruce.
A study of 34 fires in 94 upland mixedwood stands in Ontario found that white spruce's relative composition in prefire stands was 14.9% greater than that in stands 5 to 18 years after fire (P<0.001). Stands that were dominated by white spruce before fire were dominated by quaking aspen after fire. White spruce seedling density was not related to its prefire basal area, while regenerating stem densities of quaking aspen, jack pine, balsam poplar, and black spruce were positively related to their prefire basal area [65].
A dendrochronological study of white spruce regeneration 69 years after a stand-replacing fire in boreal mixedwood forest, northwestern Quebec, found that initial regeneration was dominated by jack pine and quaking aspen. The fire left an 89-acre (36 ha) island of unburned forest within the interior of the fire. The basal area of the unburned island was dominated by white spruce, paper birch, and balsam fir, while the burned area was dominated by jack pine, quaking aspen, and paper birch. White spruce established in 2 waves after the fire. The first wave began in the first 5 postfire years and peaked in postfire year 20. The second, smaller wave began about 40 years after the fire and peaked about 10 years later. There was little white spruce recruitment between the 2 waves. The density of the initial white spruce cohort was negatively correlated with distance from unburned stands (R² = 0.37, P>0.0001). Average white spruce density decreased from 280 to 21 trees/acre (693-51 trees/ha) over a distance of 0 to 6,600 feet (0-2,000 m) from the unburned zone. The second cohort had slightly more seedlings closer to the unburned zone than farther away. The first cohort was probably a seed source for the second cohort [145].
One growing season after 4 prescribed fires in the Petawawa Research Forest, Ontario, white spruce had high rates of seedling establishment in stands that were dominated by red pine and eastern white pine, even though white spruce was a minor component of the stand. The "predominance" of white spruce seedlings 1 to 2 years after the fires was attributed to a "bumper crop" of white spruce seeds that year. About 280 to 24,700 white spruce seedlings/acre (690-61,000 seedlings/ha) established. Estimates of seedling abundance (white spruce and pines combined) were highest on severely burned sites [419]. For additional information about this study, see this Research Project Summary: Prescribed burning experiments in red and eastern white pine forests of eastern Ontario.
FUELS AND FIRE REGIMES:Fuels: White spruce trees and forests are highly flammable, although less so than black spruce [83]. White spruce needles and bark are resinous [303], but less so than black spruce [405]. Compared to black spruce, white spruce-dominated stands may be less dense and have a less flammable understory [266]. Nonetheless, large amounts of fuels accumulate in white spruce forests including woody fuels, flaky bark, feather mosses, and shrubs, making these forests highly susceptible to fire [114]. Among white spruce sites, floodplain sites are less flammable than upland sites because the forest floor typically remains moist, and the understory often contains alders and willows, which are not flammable during the growing season [45,358,449].
The structure of white spruce trees contributes to their flammability. The arrangement of vertically continuous branches may promote ignition and torching [114,462,466], especially when branches are retained low on the trunk [114,171,194,263]. The forest floor surrounding white spruce boles may be especially flammable because white spruce trees often have dense, narrow crowns, which shelters the forest floor from precipitation and contributes to the accumulation of needles [356].
It is generally accepted that conifer forests are more flammable and more likely to burn than hardwood forests in the boreal region [84,179], and that stands with more hardwoods are less flammable than stands with fewer hardwoods [111]. Hardwoods and hardwood stands have structural and fuel attributes that contribute to lower flammability than conifers. These attributes include low canopy bulk density, leaves with high moisture content, low concentrations of flammable resins and oils, discontinuity of fuels between the forest floor and tree crowns, high rates of decomposition for coarse woody debris, and relatively fire-retardant fine fuels and litter. Consequently, hardwood stands may limit the intensity and spread of large fires (reviewed in [111]). In Alberta boreal mixedwoods, more lightning-caused fires occurred in white spruce-dominated forests than in quaking aspen-dominated forests. On a landscape scale, forest type explained more variation in annual fire initiation than did weather indices, even during years with extreme fire weather. The authors suggest that differences in fuel characteristics (e.g., flammability, ladder fuels, duff characteristics) may account for the greater number of lightning fires in white spruce stands [226]. Fire occurrence is lower in the hardwood-dominated Great Lakes-St. Lawrence region than in adjacent boreal landscapes despite having similar trends in fire weather. The proportion of "deciduousness" may account for the difference in fire hazard between the 2 landscapes [111]. Paleoecological studies from Alaska support the idea that the flammability of black spruce may override climatic factors in regulating fire frequency [50,180,181,196,215].
Seasonal trends in foliar moisture content influence white spruce flammability and crowning potential. In central Alberta, new and old white spruce foliage was periodically sampled between early March and mid-September during 2 consecutive years. The moisture content of old foliage ranged from 83% to 127%; the moisture content of new foliage ranged from 146% to 480%. The moisture content of new foliage peaked soon after flushing and declined rapidly through the summer. The moisture content of old foliage was relatively stable except for when it fell to its lowest level, during the "spring dip", just before the new foliage flushed. By late summer, moisture contents of old and new foliage were similar [68]. In a similar study at Petawawa Forest Experiment Station, Ontario, the moisture content of conifer foliage (including white spruce) followed similar patterns. The moisture content of old conifer foliage fell to its lowest level (84% for white spruce) in May and early June—just before new foliage flushed—then gradually rose to its maximum (~115% for white spruce) in late summer. The moisture content of new conifer foliage was very high at flushing, decreased sharply, and gradually levelled out by late summer [420]. Because seasonal trends in live foliar moisture content and associated foliar chemistry influence flammability and fire behavior in conifers [208,420], conifers (including white spruce) may be most flammable during the "spring dip" in foliar moisture content.
Spruce beetles affect fuels in white spruce and Lutz spruce forests by creating snags and fallen logs. In the Resurrection Creek drainage on the Kenai Peninsula, high mortality of large Lutz spruce resulted in a substantial number of fallen dead trees. Over a 16-year spruce beetle outbreak, approximately 71 Lutz spruce/acre (176 Lutz spruce/ha) fell; most of these had been killed by spruce beetles. While most of the trees were killed during the first 10 years of the outbreak, only 7 trees/acre (19 trees/ha) fell during the first 10 years, and most fell during the last 6 years (64 trees/acre (157 trees/ha)) [190]. This resulted in an increase of woody fuels from a median of 8.88 tons/acre during the outbreak to 35.4 tons/acre 20 years after the outbreak began. About 65% of this woody fuel (by weight) was comprised of sound wood >3 inches (7.6 cm) in diameter [366].
A few studies quantify forest floor depths and fuel loads for white spruce types in Alaska. In interior Alaska, 100- to 200-year-old white spruce/highbush cranberry/field horsetail/splendid feather moss stands had a 3- to 6-inch (8-15 cm) deep organic layer and 8- to 10-inch (20-25 cm) deep carpet of splendid feather moss, while 120- to 200-year-old floodplain white spruce types had a 2-inch (5 cm) deep organic layer on average [137]. In the Bonanza Creek Experimental Forest, mean litter + humus depth was 4.4 inches (11.2 cm) in 62-year-old white spruce stands [25]. In >100-year-old upland white spruce stands in interior Alaska, small-diameter woody fuels averaged 6.2 T/ha, and large-diameter woody fuels averaged 20.9 T/ha. The organic layer in these stands is about 3 to 5 inches (8-12 cm) deep and is comprised of about 2 to 3 inches (6-7 cm) of green moss and 3 to 4 inches (7-9 cm) of brown moss. Compared to black spruce stands in interior Alaska, white spruce stands may have thinner organic layers, but more large-diameter woody debris because white spruce trees typically have larger boles than black spruce [136].
For information about stand structural dynamics and fuel accumulation in fire-initiated stands throughout the boreal region, see Brassard and Chen [46].
Fire Regimes: Of all disturbances in boreal forests, "fire is the most widespread, frequent, and pervasive in its influence" [240]. Consequently, white spruce's distribution and occurrence are highly influenced by fire. Lightning-caused fires burn the most area across the boreal region [83,105,204,212,358,388,405], although human-caused fires have become more numerous in recent decades [8,24,83,213,299,405]. Most fires occur during late-spring and summer, although fires may occur from April through October [388,430]. White spruce trees are typically killed by all types of fire (crown, surface, and ground) [137,169,170,273,333,358,445], although soil burn severity is highly variable and typically patchy [120,206,347,445]. Most fires are small, but large fires account for most of the area burned [23,83,297,358]. Across white spruce's distribution, fires tend to be more frequent in the drier, western range than in the wetter eastern range (reviewed in [46,178]). White spruce communities tend to have less frequent fire than adjacent forest types throughout the species' range [113,238,298,315,461].
Because white spruce is widely distributed and is associated with many plant communities, it occurs on sites that exhibit a range of fire regime characteristics. The following is a summary of fire regime characteristics in white spruce communities throughout North America. For a detailed synthesis of fire regime characteristics in Alaskan white spruce communities, see Fire regimes of Alaskan white spruce communities.
Ignition: Historically, lightning was the main source of ignition in North American boreal forests, including those dominated by white spruce [23,175,204,267]. Although human-ignited fires have outnumbered lightning-ignited fires throughout Alaska [8,24,83,213,405] and Canada [299,447] in recent decades, lightning-caused fires tend to be larger and account for most of the area burned in these boreal forests [83,105,204,212,358,388,405,447]. From 1956 to 1999, over 90% of the area burned in Alaska was ignited by lightning [175] and during the 1990s, 86% of the area burned in Canada was ignited by lightning [388].
Season: Most fires in white spruce ecosystems occur in late spring and summer. In boreal ecosystems of interior Alaska and Canada, the fire season begins in April and extends until September or October [388,430]. In Alaskan boreal forests, peak fire season occurs in June and July, coinciding with periods of high temperatures, frequent lightning, low humidity, and low precipitation [83,105,214,430]. However, fires may burn into August and September especially during "high fire years" (i.e., years where the area burned is >1.5 times the long-term average area burned) [214]. These late-running fire seasons are associated with higher severity fires (reviewed in [83]).
In the southern boreal forest, from central Alberta to northwestern Ontario, large areas burn in spring (April, May, and June) (reviewed in [202]). In boreal mixedwoods in Prince Albert National Park, central Saskatchewan, 97.7% of the fire scars, which spanned from 1831 to 1948, occurred in dormant or earlywood, indicating the dominance of a spring fire season. Between 1927 and 1945, about 90% of the area burned, burned in May. After 1945, about 55% of the area burned, burned in April, and about 95% of the area burned, burned in April through June [202].
Human-caused fires lengthen the fire season in Alaska and Canada [105,223,388]. Most early (April-May) and late season (September-October) fires are human-caused [105,388].
Type and severity: In the North American boreal forest, fires tend to be stand-replacing crown fires [172,178,204,451], although white spruce communities also experience stand-replacing surface and ground fires [147,298]. In general, white spruce stands experience less frequent crowning than black spruce stands because white spruce trees have fewer ladder fuels (higher canopy base height) and lower resin content than black spruce [137,298,405]. However, during extended dry periods, white spruce stands can burn with characteristics similar to those of black spruce [421], and closed white spruce forests often experience high intensity crown fires or severe, stand-replacing surface fires [179].
White spruce commonly occurs in mixedwood stands with varying proportions of hardwoods. Stands with a large component of hardwoods tend to be less flammable and have lower rates of fire spread than stands with few hardwoods, especially during the summer when hardwoods have leaves [204,298]. Similarly, stands with many hardwoods require higher rates of spread for crowning to occur than stands with few hardwoods [204]. Over a 36-year period in Alberta boreal mixedwood forests, hardwood stands had burn rates (i.e., burned area/total stand area/sample period (years)) less than one-third the rates of black spruce, white spruce, or pine [84].
Fires in white spruce stands tend to be stand-replacing [137,169,170,273,333,358,445] because of their thin bark, shallow roots, and exposed buds [194,204,263,394]. Low-intensity fires may kill white spruce [169,170], and they may have high severity. For example, in closed white spruce-balsam poplar stands in interior Alaska, the Bear Creek Fire killed all trees but left the canopy intact. "Many fine fuels (needles, leaves, small twigs) remain on shrubs and low tree branches, indicating this was not the result of an intense (hot) fire. Evidently the flame front passed through the area quickly, yet the fire continued to smolder in the dry duff, finally consuming it". In stands composed of approximately equal amounts of black and white spruce with 50% canopy closure, the same fire "burned both severely and intensely, crowning and killing all trees" [170].
Riparian white spruce stands may experience active crown fires during hot and dry weather. Fire behavior modeling of riparian, montane, >100-year-old white spruce stands in Banff National Park suggests that stands with high canopy bulk densities and/or low live crown base heights will support active crown fire under 90th percentile weather conditions. At 80th percentile weather conditions, stands were predicted to support surface or passive crown fire [311].
Some sites with white spruce experience mixed-severity fire regimes. In the Rocky Mountain foothills of west-central Alberta, mixed stands of lodgepole pine, white spruce, and black spruce were characterized by both high-severity fires and low-to-moderate-severity fires. Dendrochronological evidence indicated that high-severity fires (i.e., no surviving trees) initiated even-aged cohorts dominated by lodgepole pine. Subsequent, low-to-moderate-severity fires scarred, but did not kill, regenerating lodgepole pines. These fires gave rise to multiple cohorts of lodgepole pine, white spruce, black spruce, and subalpine fir [11]. A mixed-severity fire regime was also found in the North Fork of the Flathead Valley in Glacier National Park. Forests experienced both severe, stand-replacing fires and low-severity surface fires. Stands were dominated by western larch and lodgepole pine; western white spruce occurred within the study area and was considered a climax species [27].
In boreal forests, canopy mortality is often complete, while soil burn severity may be very patchy and highly variable [120,206,347,445]. Burn severity patterns are influenced by prefire organic layer depth and moisture content. Deeper and drier organic layers give rise to more intense surface fires and longer, deeper-burning ground fires, which result in more complete consumption of the organic layers [120]. Forest floor burn severity varied in white spruce stands after the Rosie Creek Fire (interior Alaska); stands lost between 5% and 76% of their prefire organic matter [417]. In mesic white spruce-quaking aspen stands in Alberta that burned during a single day of high-intensity fire that top-killed all of the sampled trees in the interior of the fire, the amount of forest floor combustion and exposed mineral soil (<0.8 inch (2 cm) humus) was highly variable. High variation in mineral soil exposure occurred both among and within stands; mineral soil exposure ranged from 0% to 100% among sample plots. Postfire organic layer depth averaged 40% less on burned sites compared to unburned sites, and exposed mineral soil occurred on approximately 35% of the burned area [158]. In the Gilles Creek Fire, interior Alaska, the depth of burn ranged from 0.3 to 11.9 inches (0.8-30.3 cm), with a mean burn depth of 7.6 inches (19.2 cm) in black spruce stands, and 4.5 inches (11.5 cm) in white spruce-quaking aspen stands. Prefire organic soil horizons were deeper in black spruce than white spruce-quaking aspen stands, but they were also wetter and less dense. This contributed to lower mean consumption of soil organic matter in black spruce stands (53%) than in white spruce-quaking aspen stands (66%) [347]. In southeastern Manitoba boreal mixedwood stands, the spring Black River Fire uniformly top-killed trees, but forest floor consumption was variable. Stands were codominated by quaking aspen, balsam fir, and white spruce. Severely burned plots had higher conifer basal area (38.2 m²/ha) and lower hardwood basal area (7.0 m²/ha) than scorched and lightly burned plots (P≤0.001). The author suggests that the difference may have occurred because the forest floor underneath the conifers was drier due to greater canopy interception, direct evaporation, and canopy transpiration [445].
Pattern and size: Fires typically do not burn uniformly in the boreal forest, which results in a mosaic of unburned, lightly burned, and severely burned areas (e.g., [10,120,337,417,449]). Topography, plant community composition, soil and fuel moisture, and weather influence burn patterns [10,84,358,449]. For instance, burn patterns may be more homogenous on flat landscapes than on hilly or mountainous landscapes [115], and differences in flammability among plant communities create different fire behavior [128,154]. Alaskan Monitoring Trends in Burn Severity data from 2004 (high fire year) and 2006 (low fire year) indicate that 20% and 66% of the area within fire perimeters did not burn, respectively, indicating the prevalence of mosaic fires in both high and low fire years [213].
Fire size varies greatly throughout white spruce's distribution. In Alaskan and Canadian boreal forests, most fires are small, but large fires account for most of the area burned [23,83,297,358,388]. In Alaska, approximately 60% to 80% of all fires are <12 acres (5 ha) [23], although large fires account for the most of the total area burned [23,105,130,213,214]. In Alberta and Saskatchewan, 98% of the fires account for less than l% of the area burned [297]. Large fires typically occur episodically because fire size is influenced by weather and climate patterns [1,26,83,116,131,213,297]. In severe fire years, individual fires in the Alaskan boreal forest tend to be large—often burning about 124,000 to >500,000 acres (50,000->200,000 ha); in contrast, in unusually wet years, the area burned may be negligible [121].
Frequency: White spruce is widely distributed and is associated with plant communities characterized fire regimes ranging from frequent, low-severity fires to infrequent, stand-replacing fires. White spruce communities do not occur where fire-return intervals are shorter than about 40 years because white spruce trees do not typically produce good cone crops until they are at least 45 years old (reviewed in [472]), and subsequent fires would kill regenerating trees before they reproduce. Fire history studies of white spruce stands are few, possibly because these stands occur within a broader mosaic of boreal forest types or because white spruce commonly occurs in mixed stands. White spruce communities typically have less frequent fire than other boreal forest types. Across the boreal region, fire-rotation intervals are generally shorter in the drier regions of western Canada and Alaska (50-100 years on average) than in the wetter areas of eastern Canada (average >200 years) (reviewed in [46,178]). The longest fire rotations in the western boreal region are likely in floodplain white spruce stands, where they may be ~300 years (reviewed in [178]). For a summary of reported fire-return intervals and fire-rotation intervals for white spruce communities in Alaska, see Table 2 in FEIS's Fire regimes of Alaskan white spruce communities synthesis.
Boreal white spruce forests tend to have less frequent fire than adjacent forest types in interior Alaska [113,298,461] and Canada [238,315]. A large-scale analysis of 371 stand ages in the approximately 89,000,000-acre (36,000,000 ha) Porcupine and Upper Yukon river drainages of eastern Alaska, found hardwood stands had the shortest fire-rotation interval (26 years), followed by black spruce (36 years), then white spruce (113 years); and fire-return intervals were estimated to be 30, 43, and 105 years for hardwood, black spruce, and white spruce stands, respectively [461]. Another fire history study of 27 sites in the same area found that the mean fire-return interval (MFRI) in white spruce/quaking aspen stands (82 years) was not significantly different than that in black spruce stands (67 years) [113]. An analysis of 166 sites in the 11,087,600-acre (448,700 ha) Wood Buffalo National Park, northern Alberta, found that mean fire-rotation intervals in jack pine (39 years, 95% CI: 29-56) and quaking aspen (39 years, 95% CI: 26-68) forests were significantly shorter than those in black spruce forests (78 years, 95% CI: 65-109) and white spruce forests (96 years, 95% CI: 71-142) (P≤ 0.01). Sites with longer fire-rotation intervals were closer to waterbreaks than sites with shorter fire-rotation intervals (P≤ 0.05). The author suggested that variations in soil type and mean distance to a waterbreak influence forest type and fire frequency, respectively, and that white spruce's frequent occurrence near waterbreaks may be influenced by the longer fire rotations on those sites [238]. In the James Bay region of Quebec, white spruce occurs in pure stands along the shore. It is replaced by black spruce 0.3 mile (0.5 km) from the shore, and jack pine occurs 13.7 miles (22 km) from the shore. Along this shore to inland gradient, fires become more frequent. No evidence of fire was observed in the white spruce coastal forest in the 250 years since the land rose above sea-level, whereas fire-rotation intervals were calculated at 495 years in the black spruce forest (2,563,395 acres (1,037,369 ha)) and 115 years in black spruce-jack pine forest (7,985,146 acres (3,231,474 ha)). White spruce frequency was greatest in areas with less frequent fire, and black spruce and jack pine frequency was greatest in areas with more frequent fire. The authors suggest that the exclusion of white spruce farther inland may be related to the shorter fire-rotation intervals on those sites [315].
Several authors suggest that white spruce is limited to areas that burn infrequently [100,238,315,337,356,436]. On Alaskan floodplains, white spruce is often found on islands or terraces close to the river where fires rarely burn. On the uplands, white spruce stands often occur in isolated stands surrounded by less flammable hardwoods, making them relatively protected by fire [436]. Quirk and Sykes [337] reported that upland white spruce stringers were unburned while nearby black spruce stands had burned several times. These white spruce stands occurred in depressions along rills or swales with diffuse springs. The authors concluded that these stands were less susceptible to fire than the surrounding forests due to higher soil moisture and sheltered topography. In its northern range, particularly at treeline, white spruce often occurs on sparsely vegetated, dry sites where fire spread is unlikely [356]. Rowe [356] suggested that near its southern distributional limit in Saskatchewan and Manitoba, white spruce is commonly scattered on the upper slopes and crests of stabilized dunes that may have escaped fires that burned through the denser hardwood vegetation of adjacent sites. In the black spruce zone of central Quebec, white spruce and balsam fir dominate isolated subalpine sites that have escaped fire for prolonged periods. These isolated stands may be remnants of the historical balsam fir zone (currently to the south), which was replaced by fire-prone black spruce after recurrent fires [100].
Regional studies: Regional trends in fire ignition, season, type and severity, pattern, and size are discussed below. Fire regimes in Canadian communities are not explicitly covered in this review except for when they inform adjacent communities in the United States. For instance, the fire regime in Kluane National Park, southwestern Yukon is discussed in the Fire regimes of Alaskan white spruce communities synthesis, and studies in southeastern Canada are included in the discussion on fire regimes in the northeastern United States. Research from Canada was also included in the descriptions of fire regime characteristics in white spruce communities, above (i.e., Ignition, Season, Type and severity, Frequency, Pattern and size, Climate change).
Alaska: This section is summarized from Fire regimes of Alaskan white spruce communities; see that synthesis for additional details and references. Historically, most fires in the Alaskan boreal forest were caused by lightning in June or July. Historical MFRIs in boreal white spruce communities typically range from about 80 to >250 years, and MFRIs in subboreal white spruce communities are longer. Floodplain, stringer, and treeline white spruce communities may have longer fire-return intervals than other boreal white spruce communities. Ground, surface, and crown fires can occur in white spruce communities, although crowning is generally less frequent than in Alaskan black spruce communities. Most fires are stand-replacing because white spruce is sensitive to fire. Most fires in the Alaskan boreal forest are small, but large fires account for most of the acreage burned.
Current fire regimes in white spruce communities may not differ much from historical regimes because most of the Alaskan boreal forest is sparsely populated and has little road access. Therefore, both human-caused ignitions and fire suppression efforts are limited. However, fire regimes in localized regions may have been influenced by human activity. Human-caused ignitions are increasingly common near settlements, but human-caused fires tend to be small because these areas are also where fires are actively suppressed. Climate change may lead to longer fire seasons, less effective moisture, and higher ignition rates and thus increase the area burned in Arctic and boreal regions. Climate change models predict varied effects on Alaskan white spruce communities; some communities may expand while others may decline. See the Fire regimes of Alaskan white spruce communities synthesis for more detailed information and documentation.
Montana: White spruce and western white spruce occur in the Rocky Mountains in northwestern Montana, where they are associated with Douglas-fir, western larch, and lodgepole pine [331]. A fire history study of 3 sites west of the Continental Divide in Glacier National Park described 2 different fire regimes: 1) a mixed-severity regime with both surface and stand-replacing fires occurring at 25- to 75-year mean intervals, and 2) a stand-replacing fire regime with fires occurring at 140- to 340-year mean intervals. While western larch-lodgepole pine forest dominated the sites, western white spruce occurred as a minor component throughout the study areas. In the dry sites of the North Fork of the Flathead Valley, western white spruce is considered a climax species along with Douglas-fir. These sites experienced both frequent, surface fires (MFRI 36, range 28-52 years) and infrequent stand-replacing fires (MFRI 141, range 79-203 years) [27].
Northwestern Great Plains: White spruce is limited to relatively high-elevation, moist sites in the northern Black Hills. The "northwestern Great Plains highland white spruce woodland" Biophysical Setting (BpS 2910480) is generally limited to sideslopes and depressions, and sometimes riparian zones [232]. Because ponderosa pine is dominant in this community, it influences the fire regime characteristics. Little information was available to adequately characterize the fire regime in this community as of 2015. Brown [49] compiled chronologies from over 1,000 trees collected at over 50 locations throughout the Black Hills. He estimated that prior to European settlement, surface fires had MFRIs ranging from 30 to 33 years at higher elevations (where white spruce may occur), and 10 to 13 years at lower elevations. It is unclear what role stand-replacing and mixed-severity fires played in this community. While higher elevation forests and north-facing slopes dominated by white spruce are thought to be more prone to stand-replacing fires than lower elevation ponderosa pine forests in the Black Hills [232], Brown was unsure that the synchronous tree establishment that occurred at multiple spatial scales was due to stand-replacing fire. He suggested that widespread tree mortality caused by severe drought may have opened the forest canopy, and subsequent wet conditions may have promoted abundant tree regeneration. In many stands where even-aged cohorts occurred, older trees were also present. This suggests that seedlings established under partially open stands during optimal climatic conditions [49].
Great Lakes: In the Great Lakes region, white spruce is often a minor component in the southern boreal mixedwood mosaic, where it is most prominent in late-successional stands [144,177]. Recurrent fires limit the abundance and distribution of white spruce on fire-prone sites. Where fires have not occurred in a long time, white spruce may occur in the overstory [144].
In the Boundary Waters Canoe Area (BWCA), northeastern Minnesota, presuppression era forests were comprised of a mixture of fire-adapted species including jack pine, black spruce, quaking aspen, paper birch, and red pine. Advance regeneration of shade-tolerant species including white spruce, balsam fir, and northern white-cedar was sparse (reviewed in [142]). Before fire suppression began in the early 1900s, the fire-rotation interval was estimated to be ~100 years for the BWCA (including all forest types). Periodic "major fire years" (i.e., fires that burned >64,000 acres (25,900 ha) or 6% of undisturbed forest) accounted for most of the area burned and occurred every 26 years, on average. Because white spruces probably occurred in the understory of pines, quaking aspen, and paper birch, they would have been "caught up in the same fire rotations" of both surface and crown fires. After fire suppression began in 1911, the fire-rotation interval lengthened to ~2,000 years and ladder fuels accumulated, facilitating the spread of surface fires into the canopy [177]. Landscape models predict that white spruce and balsam fir will dominate these forests if fire is excluded for extended periods (e.g., 300 years) [363].
Before European settlement, fire was a dominant factor in the establishment and maintenance of forest communities in Itasca State Park, Minnesota. Varying fire frequencies and patterns, soil types, and regeneration success created a mosaic of forest types with different stand ages and compositions. "Major" fires occurred every 10.3 years. Shade-intolerant, fire-adapted species including red pine, eastern white pine, jack pine, quaking aspen, and paper birch dominated forest stands. After fires were excluded (~1920), stands began to succeed to either northern hardwoods or balsam fir-white spruce. This study, published in 1973, reported that balsam fir-white spruce communities were present in small stands and also common in the understory of aging jack pine, red pine, and eastern white pine forests. Historically, this successional trend was arrested by periodic fires [144].
In the Pictured Rocks National Lakeshore, Michigan, white spruce is a minor species in mixedwood forests. The historical MFRI for surface fires in the pine-dominated forests is 21.8 years [259], which is too frequent for white spruce stands to persist.
The Great Lakes-St. Lawrence-Acadian forests, where white spruce is often a minor component, had several distinct fire regimes. In the north, stand-replacing crown fires were typical. In western xeric sites, red and eastern white pine forests experienced both moderate-severity surface fires at 20- to 40-year intervals, and more severe fires that killed much of the stand at 15- to 200-year intervals. Farther east on mesic sites, eastern white pine forest experienced stand-replacing crown fires and severe surface fires at around 200- to 300-year intervals. Balsam fir, white spruce, and northern white-cedar often established during long intervals between fires, but these species rarely replaced eastern white pine before fire returned (reviewed in [178]).
Northeast: Little information was available on fire regimes of white spruce communities in the northeastern United States as of 2015. Along the northern New England and Canadian Maritime coast where white spruce is dominant or codominant, cool temperatures and frequent fogs create mesic conditions. Consequently, fires are likely infrequent. In northeastern Maine, land survey records from 1793 to 1827 and representative undisturbed stands indicate that 88% of the region was covered by late-successional forest and that shade-intolerant species were of minor importance. The author speculates that if crown fires occurred as frequently as every 100 years, at least half of the study area would have been dominated by pioneering birch-quaking aspen stands. He estimates that the fire-rotation interval is >806 years [260]. Elsewhere in the northeast, white spruce is a minor component in mixed forests. LANDFIRE models of the Acadian low-elevation spruce-fir-hardwood forest—where white spruce may occur—predict a MFRI of ~1,100 years for stand-replacing fires (e.g., BpS 6313730) [233].
The following paragraphs describe fire regime characteristics in southeastern Canada; similar patterns may exist in nearby northeastern states.
Fire is infrequent in mixedwoods and northern hardwood forests in the Timiskaming region, Quebec. White spruce is often subdominant in these forests. A study in western Quebec, north of western New York, found that more than 60% of the 443,100-acre (179,300 ha) study area remained unburned over the 413-year study period. For the entire study area, the presettlement fire-rotation interval was 909 years (95% CI: 489-1,690), the settlement period fire-rotation interval was 153 years (95% CI: 108-218), the post-settlement fire-rotation interval was 5,081 years (95% CI: 3,593-20,318), and the fire-rotation interval for the entire study period was 494 years (95% CI: 373-694) [112]. A 617,800-acre (250,000 ha) study in the same area also found relatively long fire-rotation intervals, which dipped during European settlement. For the entire study area, the presettlement fire-rotation interval was 262 years (95% CI: 163-422) and during the settlement period (1890-1948), the fire-rotation interval was 96 years (95% CI: 73-126). From 1950 to 1998, there were no large fires, and fewer than 12,400 acres (5,000 ha) burned in the entire study area. In parts of the study area where humans may have had less of an influence, fire-rotation intervals were estimated at 188 years for the entire study period and 314 years before 1890 [161].
In the lower St. Lawrence region of eastern Quebec, bordering Maine, historical survey data indicate that presettlement forests were dominated by fire-intolerant, late-successional species including balsam fir, white spruce, northern white-cedar, and sugar maple. Fire-adapted species such as quaking aspen and pines were infrequent throughout the study area. The dominance of late-successional species in presettlement forests suggests that fires were infrequent. Clearcutting and human-caused fires increased greatly after 1900, resulting in substantial shifts in species composition [117].
In the Gaspesian mixedwood forest, northeast of Maine, fires are generally very infrequent, but occasional large fires occur during dry periods. In a 1,601,000-acre (648,000 ha) study area, the fire-rotation interval increased from 89 to 176 years (P≤0.0001) since the end of the Little Ice Age (LIA) (~1850). This lengthening of the fire cycle may be due to the predominance of warmer, more humid air masses replacing the predominantly cold, dry air masses of the LIA. While fires are infrequent, dry conditions enable fires to occur. A dry, warm period in the early 1920s led to extensive fires in 1924. Since 1925, the mean and maximum fire weather index (a measure of fire risk) decreased, and the corresponding fire-rotation interval lengthened to 650 years. The authors suggest a fire-rotation interval between 170 and 250 years from 1850 to present, based on archival data (1920-2003) and survival analysis (1850-2003). This study did not find any significant differences in fire-rotation intervals between the 2 bioclimatic regions (eastern balsam fir-yellow birch and eastern balsam fir-white birch), or among different physiographic features (i.e., valleys, lowlands, or highlands) [241].
Climate change effects on fire regimes: In recent decades, fires have been burning over increasingly larger areas in the boreal region [150], and this trend is expected to continue throughout the century. Projected climate-change scenarios predict that North American boreal forests and their fire regimes will be dramatically altered (reviewed in [447]). Warmer temperatures, altered precipitation, and increasingly severe fire weather will directly and indirectly alter fire regimes [99,132,335,447]. Although the response of fire regimes to climate change is complex, across Arctic and boreal regions, the area burned, fire intensity, and fire severity will likely increase as fire seasons lengthen, effective moisture declines, and ignition rates increase [99,133,180,274,279,408] (reviewed in [132,335,447]). Across Canada, results from global climate models suggest that the number of fires will increase 75% to 140% from baseline (1975 to 1995) by 2100 [459] (reviewed in [335]). However, predictions of future fire occurrence vary spatially throughout the boreal forest [44,397]. Boulanger and others [44] predict heterogeneous responses of fire regimes to climate change across Canada. The largest increase in fire activity is predicted to occur in northwestern and central Canada. By 2071 to 2100, the predicted high fire occurrence and area burned in parts of this region may far exceed their historical range of variability. In sites where area burned, fire frequency, intensity, or severity is expected to increase, coniferous forests (including white spruce forests) may convert to early successional hardwood forests [28,98,215,360]. For instance, in quaking aspen-white spruce forests in Prince Albert National Park, shorter simulated fire-rotation intervals resulted in higher quaking aspen stem densities and lower white spruce stem densities than 1975 to 1990 densities [98]. As coniferous forest converts to hardwood, subsequent fire occurrence, frequency, and intensity could decrease due to the lower flammability of hardwoods, which could offset the increased likelihood of fire [28,98,215,397].
See Fire regimes of Alaskan white spruce communities (separate synthesis) and Climate change and fire (below) for more information this topic.
Find additional fire regime information for plant communities in which white spruce may occur by entering ?white spruce? in the FEIS home page under "Find Fire Regimes", or see the Fire Regime Table for information on fire regimes of "potential natural" vegetation communities in which white spruce may occur.
FIRE MANAGEMENT CONSIDERATIONS:
Prescribed fire after logging: Prescribed fire is often used to consume logging slash, improve seedbed conditions, and promote regeneration after white spruce stands are logged (e.g., [19,163,220,284,325,407,465,476]). White spruce regeneration is variable and often inadequate after logging, in part because logging may not reduce thick organic layers or expose mineral soil [127,148,439,465]. Because white spruce typically establishes best on mineral or thin organic soils [61,71,103,124,206,336,385,439,468,472], prescribed fire may be used to expose mineral soil. In 2 white spruce floodplain forests in interior Alaska, prescribed broadcast burning following logging reduced small-diameter, downed woody fuels by 67% and 81%, and organic horizon depth by 43% and 55%. However, only 13% and 8% of the surface was exposed mineral soil after fire (desired exposed soil was 30%-40%). This was probably because of the high duff moisture content (130%-150%) at the time of burning. While seedbed conditions were somewhat improved by these experimental fires, the authors recommend mechanical site preparation or burning under drier duff conditions to increase mineral soil exposure [476].
Prescribed fire prior to planting white spruce may promote growth by reducing competition and warming the underlying soil [19,284]. In north-central interior British Columbia, white spruce seedlings grew best on severely burned sites. White spruce seedlings were planted on previously harvested sites where prescribed fire left a mosaic of severely burned, lightly burned, and unburned patches. On the severely burned patches, competing vegetation was greatly reduced and most of the organic horizon was removed, whereas on the unburned patches, competing vegetation was dense and averaged 32 inches (80 cm) tall. Competing vegetation and organic matter were barely affected on the lightly burned patches. Two years after planting, seedling growth was highest on severely burned patches (11 inches (28 cm)), followed by lightly burned patches (7.8 inches (20 cm)), then unburned sites (5.5 inches (14 cm)). Seedling survival was significantly higher on lightly burned (100%) and severely burned (83%) patches than on unburned (46%) patches [284].
While prescribed fires typically reduce logging slash fuels and improve seedbed conditions in small patches, regeneration of white spruce by seed is often inadequate because prescribed fires fail to consume sufficient organic material [127,407]). Additional seedbed treatments may be necessary to promote regeneration (reviewed in [472]).
For additional information about prescribed burning on sites that were clearcut in British Columbia see the following Research Papers:
For information about white spruce seedling establishment on experimental prescribed burns in red and eastern white pine forests see the following Research Project Summary:
Prescribed fire for habitat enhancement: Prescribed fire is used to enhance wildlife habitat on white spruce and Lutz spruce sites on the Kenai Peninsula [42,443,452]. Even though these forests probably had very long fire-return intervals historically (MFRI=~515 years) [31], managers were concerned because young birch-willow-quaking aspen habitat and hardwood stands were succeeding to white spruce and mountain hemlock, and moose numbers were declining. In an effort to restore winter range moose habitat and increase moose browse, the Chugach National Forest experimentally burned 12 sites. Sites were burned in May and June before greenup, with and without prior slashing. Burning increased browse production from an average of 9 pounds/acre to 37 pounds/acre by 3 years after fire, and moose used these areas "heavily" during the winter; however, browse quality increased for only 1 year after burning. On sites that were slashed prior to burning, fire intensity, tree top-kill, fuel consumption, hardwood sprouting, and hardwood seedling establishment were generally higher than on sites that were not slashed. Sites that were not slashed had patchier burns. Both burning treatments resulted in low duff reduction (13% on slashed sites, 6% on unslashed site). To generate higher fire intensities on sites that are not slashed, the author suggests burning during the fall [452].
Spruce beetle: There is concern that the extensive tree mortality and associated high fuel loads created by the massive spruce beetle outbreak of the 1990s in south-central Alaska will increase the risk of wildfire, especially in the wildland-urban interface areas around Anchorage and on the Kenai Peninsula [153,190,350,367]. While spruce beetles are within their native range, and outbreaks historically occurred relatively frequently (every ~50 years on average) [31,373], spruce beetle populations in the 1990s outbreak were "unprecedented" (reviewed in [350]). Since the outbreak, surface fuel loads increased as needles, branches, and snags fell to the forest floor. Between 1987 and 2000, fuel heights, fine fuels, and sound large fuels increased in beetle-killed white spruce stands (P=0.05). In unharvested white spruce stands, sound 1000-hour fuels increased by 3.02 tons/acre (P=0.05). In harvested beetle-killed white spruce stands, small fuels (10-hour and 100-hour) increased more than in unharvested stands (P=0.05) [367]. Fires that occur in beetle-killed areas are perceived to be intense and difficult to suppress, and result in "undesirable" conditions where spruce forests are replaced by grasses and shrubs due to a lack of seed source [350].
Dendrological and soil charcoal evidence indicated no association between fire activity and the relatively frequent spruce beetle outbreaks over the past ~2500 years [31]. However, Berg and Anderson [31] caution that the "trend of warmer summers coupled with an increasing human population and associated sources of ignitions may create a greater risk in all fuel types than was present during the time period covered by our study". The human-caused, 2014 Funny River Fire grew uncharacteristically large (195,858 acres (79,260 ha)) in beetle-killed forest on the Kenai Peninsula [7] (Figures 12, 13).
Because spruce beetles typically colonize stressed or dying spruce (e.g., windthrown, fire damaged, logged) (reviewed in [454]), a warmer, drier climate or increased fire frequency may result in increased susceptibility to spruce beetle outbreaks.
For additional information about the effects of spruce beetle outbreaks, see Insects.
 |
 |
|
| Figure 12. Funny River Fire (2014) burning on the Kenai Peninsula. The brown-red area outside of the fire perimeter may be beetle-killed trees. Photo courtesy of Jesse Allen, NASA Earth Observatory. | Figure 13. Immediately after the Funny River Fire (2014) on the Kenai Peninsula. Photo courtesy of the Office of the Governor. |
Eastern spruce budworm: Fire exclusion and climate change may affect eastern spruce budworm dynamics in central and eastern Canada and the northeastern United States. Since fire suppression began in eastern Canada (1920), eastern spruce budworm outbreaks have occurred at shorter intervals, are more widespread, and result in more mortality, especially of white spruce. Fire suppression coupled with logging of eastern white pine resulted in more continuous balsam fir-spruce stands and less of a mosaic comprised of early successional, non-susceptible species (reviewed in [280]). With climate change, wetter conditions are predicted to lengthen the fire-return interval of southeastern Canadian boreal forests [447]. Consequently, more extensive conifer forest may be available to support more extensive insect outbreaks (reviewed in [437]), including eastern spruce budworm.
Fuel loading and fire hazard following eastern spruce budworm attacks vary regionally over time. Eastern spruce budworm-killed trees create dead ladder fuels, which can support fast-moving crown fires [387]; however, live spruce and balsam fir are also highly flammable ladder fuels [392]. Conventional thought is that insect-caused tree mortality enhances fire potential (reviewed in [134,280]); however, this trend may only be supported at short time scales (i.e., <10 years) [134,387]. Over longer time scales, spruce budworm outbreaks may lessen fire risk [392]. In eastern spruce budworm-killed stands in central Ontario, experimental fires were conducted in the spring and summer up to 5 years after trees died. Stands were dominated by dead balsam fir; other trees included eastern white pine, jack pine, white spruce, and birches. Spring fires conducted before the understory vegetation flushed "exhibited spectacular behavior", with crown fire rates-of-spread as high as 269 feet/min (82 m/min). The fires "behaved explosively" regardless of whether the dead crowns were intact or on the ground. Summer fires that were ignited a few years after the trees died failed to spread, even under severe burning conditions. The open tree canopy resulted in a moist, green understory, which reduced fire spread. Fire potential was highest 5 to 8 years after trees died, when surface fuel loads peaked. By 4 to 5 years after the trees died, there were enough woody surface fuels to enable fires to spread in the summer [387].
Following the major eastern spruce budworm epidemic in the 1970s on the Cape Breton Highlands, Nova Scotia, there was little surface fuel accumulation. Balsam fir comprised 90% of the forest and white spruce and paper birch comprised the rest. Dead fuels decomposed rapidly due to the moist climate, and fires were not sustained in budworm-killed stands. Two late spring fires that occurred before understory plants leafed out originated in open fields and spread towards budworm-killed stands. Because there was little surface fuel in the budworm-killed stands, the fires stopped at the stand edge. Only live, young balsam fir trees growing along the edge of the stand were consumed [322]. In the Boundary Waters Canoe Area, simulation models of both presettlement era forests and contemporary forests indicated that area burned and fire severity during outbreak decades were similar to those of non-outbreak decades. Simulated eastern spruce budworm disturbance lengthened the fire-return intervals in both time periods (mean =229.1±6.3 (SE) years) relative to fire-only treatments (mean=199.2±3.1 (SE) years). The authors conclude that periodic eastern spruce budworm outbreaks reduce ladder fuels, which may partially mitigate future fire risk over the long term [392].
Very frequent fires: Repeated fires that occur in short intervals prevent white spruce from regenerating. In central Saskatchewan, agricultural clearance fires in the early 1900s escaped into southern boreal mixedwoods in and adjacent to Prince Albert National Park. Between 1883 and 1942, 81% of the forests burned in 2 or more escaped crown fires. Stands that experienced multiple short-interval fires shifted composition from mainly white spruce to mainly quaking aspen. Since 1883, sample points dominated by white spruce forest types have decreased from 41% to 19%, while sample points dominated by quaking aspen have increased from 29% to 49%. Sample points dominated by jack pine also increased from 8% to 21% at the expense of white spruce-paper birch. In stands that were logged and then burned in escaped fires, white spruce decreased even more than in unlogged stands. Since 1883, logged white spruce forest types decreased from 56% of the sample points to 9%, while quaking aspen-dominated forests increased from 25% to 75%. Because the fires burned in 15- to 20-year intervals, any white spruce that regenerated after the first fires would not have had sufficient time to produce seed before subsequent fires. Consequently white spruce regeneration was limited and populations were substantially reduced [450].
Climate change and fire: Climate change has the potential to affect white spruce distribution, abundance, and growth both directly and by altering fire regimes.
Increases in fire extent, frequency, and severity could facilitate a shift from coniferous forests to early successional hardwood forest [28,215,274,360]. In interior Alaska, the ALFRESCO model indicates that climate-driven changes in the fire regime are already occurring and will continue over the next 30 years. Hardwood stands have already replaced black and white spruce stands at many sites. By 2020, the replacement of spruce by hardwoods is predicted to slow because hardwood cover will be twice that of spruce. Additionally, once the fire-return interval drops below a threshold of 60 to 80 years, the model predicts that spruce will be increasingly excluded because they will not have enough time to replace faster growing hardwoods before subsequent fires. The future forest predicted by the ALFRSCO model may be similar to the poplar-dominated parkland of the early Holocene and the present-day boreal mixedwoods of south-central Canada, which grow under slightly warmer and drier conditions than forests in interior Alaska [274].
Logistic regression simulation models indicate that climate change (temperature and/or precipitation), fire frequency, and especially their interactions will affect the distribution of white spruce in interior Alaska in future years. With a warming climate and without increasing precipitation, white spruce distribution is predicted to decline; the drier the climate, the faster the decline. However, if precipitation increases as temperature increases (predicted increase 1.8-9 °F (1-5 °C)), white spruce distribution could expand to roughly twice its current distribution, peaking at 3.6 °F (2 °C) warming. With even greater increases in temperature and precipitation, the pattern is reversed: Under the Hadley CM2 model, which predicts increases in average growing season temperature of 5 °F (2.8 °C) and precipitation (+6 %) for interior Alaska by 2100, the landscape is predicted to consist mostly of black spruce, some hardwoods, and very little white spruce at intermediate elevations, and tundra at high elevations. Under this scenario, white spruce forest is predicted to decrease from 9% to 2% cover in interior Alaska [57].
With an unchanging climate, a lengthening or shortening of the fire-return interval by 30% is predicted to result in slight increases and decreases in white spruce distribution, respectively. However, increases or decreases in the fire-return interval accompanied by either a wetter or drier climate could lead to substantial declines in white spruce distribution. While these models are based on empirical data, they lack regional-scale (remotely sensed) validation data that distinguish black spruce from white spruce. In addition, it is unclear how processes such as seed dispersal, establishment, changes in soil conditions, and thawing permafrost are captured by the models [57].
See Climate change in Other Management Considerations and Fire regimes of Alaskan white spruce communities for more information on this topic.FEDERAL LEGAL STATUS:
None
OTHER STATUS:
Information on state- and province-level protection status of plants in the United States and Canada is available at NatureServe.
IMPORTANCE TO WILDLIFE AND LIVESTOCK:
Many wildlife species use white spruce communities. Mammals using white spruce communities as habitat include red squirrels [14,48,169,217], snowshoe hares [13,169], American marten [269,423], voles (northern red-backed voles, meadow voles, yellow-cheeked voles) [269,423], moose [289,324,412,443,452], American black bear [368], and caribou [237,370,380]. A variety of bird species use white spruce communities [76,182,295,364,398], including woodpeckers [195,295,398] and sharp-shinned hawks [70]. Many birds nest or forage in white spruce trees (reviewed in [364]).
Many wildlife species are adapted to particular successional stages in white spruce communities. For example, moose [246,410], black backed-woodpeckers [195,364], other woodpeckers [195,364], and northern hawk owls [165,364] use early postfire stages. Caribou use late-seral, open lichen woodlands dominated by white spruce as winter habitat in northeastern Alaska. In these woodlands, fires reduce available lichens in the short-term, which are the principal winter forage [237].
In interior Alaska, all white spruce postfire successional stages have important food sources for browse animals. In the earliest stages (1-5 years after fire), young trees (e.g., quaking aspen and paper birch) are most heavily browsed; in early-to midseral stages (6-50 years), willows are most heavily browsed; in late-successional white spruce stands (>50 years), non-willow shrubs are most heavily browsed [136]. In general, the greatest variety of wildlife occurs during the tall shrub-sapling stage (6-25 years), when plentiful forage, cover, and denning/nesting sites are available [410].
See Appendix C for links to FEIS reviews available for animal species mentioned in this section.
Wildlife population trends after fire: On white spruce sites on the Kenai Peninsula, moose populations are largest during early postfire succession [246,310,324,368,382,443,452,453]. After fire, willows, quaking aspen, and paper birch provide winter browse on white spruce sites. Browse production and density are generally high from about 7 to 30 years after fire and peak 15 years after fire [310,382,453]. Moose populations increase and are maintained from about 5 to 25 years after fire, sometimes much longer, as long as adequate forage is available; this is especially true in wintering areas [382,452]. Mature white spruce stands (>100 years old) lack enough willow to maintain moose herds, and paper birch browse is too tall (reviewed in [443]). Although mature white spruce stands provide less browse than early seral stands, mature stands comprised of paper birch, white spruce, and quaking aspen may provide year-round escape cover and winter refugia from deep snow. These stands may also provide alternate food sources such as mountain cranberry, which can be of considerable importance [246].
On the Kenai Peninsula, American black bears, hereafter 'bears', use mixed spruce (white and black)-hardwood sites in early- mid- and late-successional postfire stages. Bear density was similar in recent (13- to 18-year-old) and intermediate-aged (35- to 40-year-old) burns. However, bears in the recent burn had superior growth and reproduction, likely because they ate 4 times more moose calves than bears in the intermediate-aged burn. The recent burn was excellent moose habitat and had twice as many moose as the intermediate-aged burn. Bears living in both the recent and intermediate-aged burns migrated to old-growth stands each summer to eat devil's-club [368].
On the Bear Creek Fire, interior Alaska, American martens used burned (7-8 years after fire) and unburned white spruce habitat, because both provided food and winter cover. Most American marten observations were in unburned white spruce, a habitat that the author speculates has greater value for cover than for food. However, the burned white spruce forest had excellent cover and was used for resting and hunting. While northern red-backed voles (stable American marten food) were most abundant in unburned forest, tundra voles and meadow voles (preferred American marten foods) were most common in burned forest. The author concluded that fires may benefit American martens because fires create and maintain heterogeneous habitats [423].
Population dynamics of gray wolves were minimally affected after a 208,800-acre (84,500 ha) wildfire burned white spruce and black spruce forest and tundra communities in northwestern Alaska. Fire severity ranged from unburned to high severity. Gray wolves used the burned area more than expected during the summer of the fire and the following summer, but less than expected during the 2 subsequent winters. The authors suggest that lower use of the burned area during winter was due to shifts in caribou distribution, possibly caused by the fire. Gray wolf use of the burned area resembled prefire use 3 years after the fire [20].
Black-backed and three-toed woodpeckers occupy recently burned white spruce forests. Black-backed woodpeckers are extremely rare and three-toed woodpeckers have low population densities in interior Alaska; however, after the Rosie Creek Fire, both woodpeckers were common on the perimeter of burned mature white spruce stands. Black-backed woodpeckers were common in burned white spruce stands for 2 years after fire, rare 3 years after fire, and absent by the 4th postfire year. Black-backed woodpeckers fed almost exclusively on larval wood-boring beetles on moderately to heavily burned trees. These insects occurred on dying trees for only 2 to 3 years after the fire. Three-toed woodpeckers were common to abundant the 2nd winter after the fire and much less common to rare by the 3rd winter after fire. Three-toed woodpeckers primarily fed on bark beetle larvae on lightly to moderately burned trees. By the 3rd postfire year, bark beetle populations had declined [295]. In east-central Alberta, black-backed woodpeckers occurred in white spruce stands that burned 2 years prior; the nearest black-backed woodpeckers detected in unburned forest were in old white spruce stands 46 to 93 miles (75-150 km) from the fire. Three-toed woodpeckers were not detected in mature stands [195]. In mixedwood stands in north-central Alberta, bird communities were compared among stands that were either burned or logged 1 to 28 years prior. Black-backed and three-toed woodpeckers occurred only in stands that burned 1 year prior; they did not occur in older burned forests or forests that were logged [183].
Bird surveys in the Kluane Ranges, Yukon Territory, showed little difference in density, species richness, or species composition across 6 lowland (i.e., not subalpine or tundra) communities spanning several successional stages, although some species were more abundant in specific successional stages. A few species (darkeyed junco, Swainson's thrush, yellow-rumped warbler) were abundant in every seral stage. Wilson's warblers and American robins were less common in mature white spruce forests than in earlier stages of succession. In mature white spruce forests, 16 species were found. Darkeyed juncos were most abundant, followed by yellow-rumped warblers and boreal chickadees; these 3 species made up 55% of the total bird density in white spruce forests. The few differences in species composition along the successional sequence were mainly due to more aerial insect feeders (Bohemian waxwing, alder flycatcher, western wood pewee, and olive-sided flycatcher) on burned areas than elsewhere [398].
Literature reviews and meta-analyses of bird community composition after fire or harvest indicate differences among disturbance types, seral stages, and forest types in boreal forests of western North America. Bird communities present immediately after harvest differed from those present after fire, but these differences disappeared with stand age. After fire, communities are dominated by birds that nest in cavities of snags and/or forage on beetles that occur in snags. Both logged and burned sites were dominated by relatively few bird species 31 to 75 years after disturbance. Some birds that use old forests were present at this time, and then bird species richness increased 76 to 125 years after disturbance; however, bird community composition differed among quaking aspen, mixedwood, and white spruce forests. Most of the bird species common in white spruce forests >76 years old were also present in mixedwood forests, especially mixedwoods >125 years old. As mixedwood stands aged and became increasingly dominated by white spruce, many bird species that nest and forage in large quaking aspen trees became less common. Species reviewed are available in Schieck and Song [364].
Palatability and nutritional value: Wild ungulates and livestock rarely browse white spruce [167,209,228,317,453]. Moose occasionally eat white spruce [247,340,341], but it is generally avoided [340]. During the winter, caribou occasionally eat the needles and branches of small white spruce saplings [380]. Because white spruce is rarely browsed, tree species composition may shift in favor of white spruce under heavy browsing pressure [209,282,317,334]. An extreme example of this occurred in Isle Royale National Park, Michigan, where heavy moose browsing resulted in a "spruce moose savanna" [317].
White spruce is important browse for some birds and small mammals. Snowshoe hares browse white spruce throughout much of its range (reviewed in [168]). In some areas, white spruce is a preferred food, and in other areas, it is avoided (reviewed in [379]). In feeding trials on the Kluane research base, Yukon Territory, mature white spruce twigs were a consistently preferred food (ranked 2 of 10), while juvenile white spruce twigs were rarely eaten [379]. Snowshoe hares eat the resinous buds and new growth of seedlings and young trees, which may cause extensive damage and mortality [13,439]. In interior Alaska, white spruce needles are an important food source for spruce grouse in the late fall and winter [448], although white and black spruce needles are eaten less than needles of other conifers, and saplings <14 years old are avoided [51]. White spruce is more palatable to spruce grouse than black spruce (reviewed in [51]). In interior Alaska, red squirrels eat white spruce buds when the seeds are not available [48].
Numerous birds (reviewed in [148]) and mammals eat white spruce seed. It is a primary food for red squirrels, which harvest and cache white spruce cones and eat the seeds [48,90,326,381,390]. In some years, seed predation by red squirrels may substantially reduce regeneration [390] (see Cone and seed production). Deer mice, northern red-backed voles, meadow voles, and shrews eat white spruce seed after it is dispersed [439,470].
Cover value: White spruce provides good cover for moose, white-tailed deer, and ruffed grouse. In south-central Alaskan boreal floodplains, moose rest in shaded, mature white spruce forest during sunny, spring days even though forage may not be available [74]. On Isle Royale, moose used balsam fir-white spruce habitat during a period of deep snow, probably because snow was more shallow under the dense canopy than elsewhere on the Island [228]. In the Black Hills, where white spruce occurs at high elevations and on cool slopes and valley bottoms, white spruce habitat may provide important thermal and hiding cover for white-tailed deer in the summer and fall (reviewed in [376]). In southwestern Alberta, ruffed grouse preferentially select drumming sites with young white spruce cover [38]. Along the Tuchodi River, British Columbia, elk rarely use mature white spruce forest for forage or for resting [323].
In interior Alaska, sharp-shinned hawks nest in white spruce trees that occur within a matrix of hardwood trees [70].
VALUE FOR REHABILITATION OF DISTURBED SITES:
White spruce is useful for long-term revegetation of coal mine overburden. In Alberta, it is considered one of the best conifers for this purpose. Information about planted white spruce survival on reclaimed sites is available [446]. Western white spruce established on abandoned coal mine sites in the Rocky Mountain foothills, west-central Alberta [361]. White spruce has also established on coal mine overburden in south-central Alaska. This site was part of a reclamation project where the overburden (clay content 42-44%) was redeposited on the mined area and graded, scarified, seeded with graminoids and forbs, and fertilized; white spruce was not included in the seed mix [125]. White spruce has also colonized abandoned borrow pits (5-37 years after disturbance) in tundra regions of northwestern Canada, although white spruce cover was very low (0.01-1.04%) [219].
White spruce often colonizes abandoned agricultural fields in Maritime Canada and New England [95,305].
OTHER USES:
White spruce is one of the most important commercial species in North American boreal forests [305] and is considered the most important commercial species in Alaska [291,444]. White spruce wood is light-weight, straight grained, and resilient. It is used primarily for pulpwood and as lumber [96,305,314]. The best timber often occurs on well-drained soils in river bottoms [291,428,444].
Historically, white spruce bark was used to cover dwellings and for smoking hides, roots were used for lashing in baskets and canoes, boughs were used for bedding, and pitch was used in medicines [189,305]. For additional information about Native American uses for white spruce, see the University of Michigan's database of Native American Enthnobotany.
OTHER MANAGEMENT CONSIDERATIONS:
Logging: Regeneration of white spruce after logging is often inadequate. Methods to improve regeneration include seedbed preparation via prescribed fire or scarification. For information about regeneration after prescribed fire, see Prescribed fire after logging. Gartner and others [148] review methods that promote white spruce regeneration after harvesting. To prepare the seedbed, the authors recommend scarification during or after harvest and leaving nurse logs. In addition to seedbed preparation, ensuring an adequate seed supply is critical to white spruce regeneration. The authors recommend maintaining at least 2 mature white spruce trees/acre (5 trees/ha) within cut areas or leaving dense stands on edges of cutovers to ensure pollination and even seed distribution.
Numerous studies are available regarding post-logging regeneration and succession in white spruce stands (e.g., [53,122,200,276,339,404,439]). Refer to these sources for additional information.
Salvage logging after fire alters bird community assemblages. The effects of salvage logging were examined in mixedwood (quaking aspen and white spruce) and jack pine stands after the Hawk Fire in west-central Saskatchewan. Sites were sampled 3 years after fire and 2 or 3 years after salvage logging. In all stands combined, burned forests supported a distinct species assemblage of songbirds relative to unburned forests (P=0.001), and salvage logging altered this community. In mixedwood stands, bird communities in burned stands did not differ significantly from those in unburned stands, but they did differ significantly from bird communities in salvaged stands (P=0.001). Salvaged sites had fewer detections of resident species, canopy and cavity nesters, and insectivores than burned sites. "Species less sensitive to salvage logging tended to be habitat generalists, omnivores, and species that nest on the ground or in shrubs" [294].
Spruce beetle: Between 1990 and 2000, a spruce beetle outbreak infested 2.94 million acres (1.19 million ha) of spruce (mostly white spruce, Sitka spruce, and Lutz spruce) forests in south-central Alaska. The severity of the outbreak was considered historically unprecedented. In many stands, more than 90% of the spruce >4.3 inches (11 cm) diameter at breast height were killed. This outbreak was related to the relatively high densities of large and aging spruce across the region and to a warming trend. The warm conditions increased spruce susceptibility to beetle attack and reduced the life cycle of many spruce beetles from 2 years to 1 year. Consequently, the number of beetles increased in a given year (reviewed in [454]). Werner and others [454] review the biology and ecology of spruce beetles, host susceptibility and resistance, effects on forest stands, management strategies, and impacts to non-timber resources in south-central Alaska. See Successional Status and Fire Management Considerations for additional information on this topic.
Eastern spruce budworm: Eastern spruce budworm occurs in the eastern portion of white spruce's range. While balsam fir is the preferred host species, white spruce is also attacked [46]. Host trees die due to chronic stress of intense defoliation, which often occurs over multiple years [365]. After an eastern spruce budworm outbreak kills overstory trees, understory trees typically emerge to the canopy [41,478] creating uneven-aged stands. See Successional Status and Fire Management Considerations for additional information on this topic.
Other damaging agents: White spruce is susceptible to numerous needle and bud rusts, root diseases, fungi, and insects that result in defoliation, reduced growth and vigor, or death. These diseases usually remain at low levels and are localized. Eastern dwarf mistletoe rarely kills white spruce [305]. White spruce is prone to windthrow along stand edges and in thinned stands on shallow or poorly drained soils where root systems are shallow [302,305,411]; however, windthrow is not typically a major cause of white spruce mortality [371]. White spruce may be less prone to windthrow-caused mortality than associated early successional species [343].
Climate change: Climate change is expected to have varied effects on white spruce distribution, growth, and establishment. The pattern, direction, and timing of change depends on local, landscape, and regional climatic and site conditions.
Several studies suggest that under a warmer climate, white spruce may expand into areas formerly underlain by permafrost or beyond its current latitudinal or altitudinal extent [93,218,272,319,349,393,457]. White spruce may also increase in density (infill) at the forest-tundra ecotone [18,93,272,396] or experience increased growth rates [12,18,393]. However, the response of white spruce to warming conditions varies within and among sites and depends on local site conditions [93,218,221,272,319].
 |
| Figure 14. Treeline expansion in Denali National Park and Preserve, Alaska. Photos by Fred Dean (top) and Carl Roland (bottom), "Exploring land cover change through repeat photography". |
While white spruce growth is positively related to temperature in many sites [12,18,218,393], this relationship may have weakened with the rapidly warming temperatures of the late 20th century [12,88,89,272,455]. Studies in northern Canada and Alaska indicate both positive and negative growth responses of white spruce to climate warming [210,221,455,456]. These differences in growth response may be related to moisture stress, with negative responders growing in drier conditions or on sites with excessive evaporative demand [29,89,221,455,456]. White spruce trees may have positive growth responses to warming temperatures until they reach a physiological threshold above which water stress becomes limiting and growth declines rapidly [88,255,281,456]. At treeline sites in interior Alaska, the temperature threshold at which white spruce growth declines was estimated to be 51.8 to 53.6 °F (11-12 °C) mean July temperature [456]; at elevational treeline in the Yukon Territory, the temperature threshold was estimated to be 52.3 °F (11.3 °C) mean July-August temperature [88]. Modeling suggests that white spruce is limited by moisture once the average growing season temperature increases by 2 °C [57].
Other studies suggest that many white spruce trees and communities may be negatively affected by a warming and drying climate [21,57,60,66,143,210,255,256,281,456]. A synthesis of information on white spruce growth across 25 sites in interior Alaska showed only 5 sites with consistently positive growth responses to warming temperatures; 19 sites had consistently negative responses, and 1 site had a mixed response. Four out of the 5 sites with positive responses were located in the coolest, wettest regions of the Brooks and Alaska mountain ranges. This suggests that white spruce growth may benefit from warmer temperatures only in the coolest, wettest areas, while it is unlikely to benefit in most other areas [281]. In the Boundary Waters Canoe Area Wilderness, Minnesota, climate warming is predicted to cause boreal forests to shift to savanna and/or temperate hardwood forest over the next century. Paleological and historical evidence indicates that this area is particularly sensitive to climate change and has seen many species shifts in recent millennia: The warm and dry climate from 8,000 to 5,000 years BP resulted in plant species migrating to the northeast and a high abundance of oaks and grasses in the Boundary Waters, and then the cooler climate of the last 3,000 years resulted in boreal returning to the area and replacing the oaks and grasses. Based on climate-distribution relationships, species at the southern limits of their range in the Boundary Waters, such as white spruce, are predicted to decline or disappear under both a warm/dry and a warm/wet climate scenario [143].
See Fire Management Considerations and Fire regimes of Alaskan white spruce communities for further discussions on this topic.
| Fire regime information on vegetation communities in which white spruce may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [235], which were developed by local experts using available literature, local data, and/or expert estimates. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||||
|
|||||||||
| Northern and Central Rockies | |||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||
| Northern and Central Rockies Forested | |||||||||
| Douglas-fir (warm mesic interior) | Replacement | 28% | 170 | 80 | 400 | ||||
| Mixed | 72% | 65 | 50 | 250 | |||||
| Grand fir-Douglas-fir-western larch mix | Replacement | 29% | 150 | 100 | 200 | ||||
| Mixed | 71% | 60 | 3 | 75 | |||||
| Lodgepole pine, lower subalpine | Replacement | 73% | 170 | 50 | 200 | ||||
| Mixed | 27% | 450 | 40 | 500 | |||||
| Ponderosa pine (Black Hills, high elevation) | Replacement | 12% | 300 | ||||||
| Mixed | 18% | 200 | |||||||
| Surface or low | 71% | 50 | |||||||
| Western larch-lodgepole pine-Douglas-fir | Replacement | 33% | 200 | 50 | 250 | ||||
| Mixed | 67% | 100 | 20 | 140 | |||||
| Great Lakes | |||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||
| Conifer lowland (embedded in fire-prone ecosystem) | Replacement | 45% | 120 | 90 | 220 | ||||
| Mixed | 55% | 100 | |||||||
| Conifer lowland (embedded in fire-resistant ecosystem) | Replacement | 36% | 540 | 220 | >1,000 | ||||
| Mixed | 64% | 300 | |||||||
| Great Lakes pine forest, eastern white pine-eastern hemlock (frequent fire) | Replacement | 52% | 260 | ||||||
| Mixed | 12% | >1,000 | |||||||
| Surface or low | 35% | 385 | |||||||
| Great Lakes pine forest, jack pine | Replacement | 67% | 50 | ||||||
| Mixed | 23% | 143 | |||||||
| Surface or low | 10% | 333 | |||||||
| Great Lakes spruce-fir | Replacement | 100% | 85 | 50 | 200 | ||||
| Minnesota spruce-fir (adjacent to Lake Superior and Drift and Lake Plain) | Replacement | 21% | 300 | ||||||
| Surface or low | 79% | 80 | |||||||
| Northern hardwood-eastern hemlock forest (Great Lakes) | Replacement | 99% | >1,000 | ||||||
| Red pine-eastern white pine (less frequent fire) | Replacement | 30% | 166 | ||||||
| Mixed | 47% | 105 | |||||||
| Surface or low | 23% | 220 | |||||||
| Northeast | |||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||
| Northeast Forested | |||||||||
| Northern hardwoods-spruce | Replacement | 100% | >1,000 | 400 | >1,000 | ||||
| Northeast spruce-fir forest | Replacement | 100% | 265 | 150 | 300 | ||||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [164,234]. |
| Common name | Scientific name |
| Lichens | |
| beard lichens | Usnea spp. |
| Bryophytes | |
| feather mosses | Hylocomiaceae |
| sphagnum | Sphagnum spp. |
| splendid feather moss | Hylocomium splendens |
| Ferns and fern allies | |
| clubmoss | Lycopodium spp. |
| field horsetail | Equisetum arvense |
| meadow horsetail | Equisetum pratense |
| woodfern | Dryopteris spp. |
| Forbs | |
| American skunkcabbage | Lysichiton americanus |
| lesser rattlesnake plantain | Goodyera repens |
| naked miterwort | Mitella nuda |
| queencup beadlily | Clintonia uniflora |
| Rocky Mountain groundsel | Packera streptanthifolia |
| sidebells wintergreen | Orthilia secunda |
| single delight | Moneses uniflora |
| twinflower | Linnaea borealis |
| wild sarsaparilla | Aralia nudicaulis |
| Graminoids | |
| bluejoint reedgrass | Calamagrostis canadensis |
| grasses | Poaceae |
| softleaf sedge | Carex disperma |
| Shrubs | |
| alders | Alnus spp. |
| American green alder | Alnus viridis subsp. crispa |
| beaked hazelnut | Corylus cornuta |
| bunchberry | Cornus canadensis |
| bush-honeysuckle | Diervilla lonicera |
| chokecherry | Prunus virginiana |
| common juniper | Juniperus communis |
| devil's-club | Oplopanax horridus |
| eastern dwarf mistletoe | Arceuthobium pusillum |
| ericaceous shrubs | ericaceous shrubs |
| grouse whortleberry | Vaccinium scoparium |
| highbush cranberry | Viburnum edule |
| limber honeysuckle | Lonicera dioica |
| menziesia | Menziesia ferruginea |
| mountain alder | Alnus viridis subsp. crispa |
| mountain apple | Acer spicatum |
| mountain cranberry | Vaccinium vitis-idaea |
| northern mountain ash | Sorbus decora |
| pin cherry | Prunus pensylvanica |
| prickly rose | Rosa acicularis |
| red-osier dogwood | Cornus sericea |
| red raspberry | Rubus idaeus |
| russet buffaloberry | Shepherdia canadensis |
| Saskatoon serviceberry | Amelanchier alnifolia |
| swamp red currant | Ribes triste |
| willows | Salix spp. |
| Trees | |
| American beech | Fagus grandifolia |
| American elm | Ulmus americana |
| balsam fir | Abies balsamea |
| balsam poplar | Populus balsamifera subsp. balsamifera |
| basswood | Tilia americana |
| birches | Betula spp. |
| black ash | Fraxinus nigra |
| black spruce | Picea mariana |
| eastern hemlock | Tsuga canadensis |
| eastern white pine | Pinus strobus |
| Engelmann spruce | Picea engelmannii |
| interior ponderosa pine | Pinus ponderosa var. scopulorum |
| jack pine | Pinus banksiana |
| Lutz spruce | Picea × lutzii |
| mountain hemlock | Tsuga mertensiana |
| northern red oak | Quercus rubra |
| northern white-cedar | Thuja occidentalis |
| oaks | Quercus spp. |
| paper birch | Betula papyrifera |
| quaking aspen | Populus tremuloides |
| red maple | Acer rubrum |
| red spruce | Picea rubens |
| Rocky Mountain Douglas-fir | Pseudotsuga menziesii var. glauca |
| Rocky Mountain lodgepole pine | Pinus contorta var. latifolia |
| Sitka spruce | Picea sitchensis |
| spruces | Picea spp. |
| subalpine fir | Abies lasiocarpa |
| sugar maple | Acer saccharum |
| tamarack | Larix laricina |
| white spruce | Picea glauca |
| yellow birch | Betula alleghaniensis |
| western hemlock | Tsuga heterophylla |
| western redcedar | Thuja plicata |
| white elm | Ulmus americana |
| Common name | Scientific name |
| Mammals | |
| American black bear | Ursus americanus |
| American marten | Martes americana |
| caribou | Rangifer tarandus |
| deer mouse | Peromyscus maniculatus |
| elk | Cervus elaphus |
| gray wolf | Canis lupus |
| meadow vole | Microtus pennsylvanicus |
| moose | Alces americanus |
| northern red-backed vole | Myodes rutilus |
| red squirrel | Tamiasciurus hudsonicus |
| snowshoe hare | Lepus americanus |
| white-tailed deer | Odocoileus virginianus |
| Birds | |
| black-backed woodpecker | Picoides arcticus |
| ruffed grouse | Bonasa umbellus |
| sharp-shinned hawk | Accipiter striatus |
1. Abatzoglou, John T.; Kolden, Crystal A. 2011. Relative importance of weather and climate on wildfire growth in interior Alaska. International Journal of Wildland Fire. 20(4): 479-486. [83124]
2. Achuff, Peter L. 1989. Old-growth forests of the Canadian Rocky Mountain national parks. Natural Areas Journal. 9(1): 12-26. [7442]
3. Achuff, Peter L.; La Roi, George H. 1977. Picea-Abies forests in the highlands of northern Alberta. Vegetatio. 33(2/3): 127-146. [83873]
4. Ahlgren, C. E. 1957. Phenological observations of nineteen native tree species in northeastern Minnesota. Ecology. 38(4): 622-628. [74]
5. Ahlgren, C. E. 1974. Effects of fires on temperate forests: north central United States. In: Kozlowski, T. T.; Ahlgren, C. E., eds. Fire and ecosystems. New York: Academic Press: 195-223. [7198]
6. Ahlgren, Clifford E.; Hansen, Henry L. 1957. Some effects of temporary flooding on coniferous trees. Forestry. 55(9): 647-650. [2924]
7. Alaska Division of Forestry. 2014. Funny River Fire update, [Online]. In: Alaska Wildland Fire Information. Anchorage, AK: Alaska Division of Forestry (Producer). Available: http://akfireinfo.com/2014/06/23/funny-river-fire-update-13/ [2015, June 3]. [88289]
8. Alaska Fire Science Consortium. [2012]. Research summary: Alaska climate change adaptation series--wildfires, [Online]. In: Library--Newsletters, fact sheets and summaries. Fairbanks, AK: Alaska Fire Science Consortium (Producer). Available: http://www.frames.gov/files/7913/4764/4448/CES_Wildfire_and_Climate_Summary.pdf [2015, June 3]. [86239]
9. Albani, Marco; Andison, David W.; Kimmins, J. P. (Hamish). 2005. Boreal mixedwood species composition in relationship to topography and white spruce seed dispersal constraint. Forest Ecology and Management. 209(3): 167-180. [52852]
10. Allen, Jennifer L.; Sorbel, Brian. 2008. Assessing the differenced Normalized Burn Ratio's ability to map burn severity in the boreal forest and tundra ecosystems of Alaska's national parks. International Journal of Wildland Fire. 17(4): 463-475. [86059]
11. Amoroso, Mariano M.; Daniels, Lori D.; Bataineh, Mohammad; Andison, David W. 2011. Evidence of mixed-severity fires in the foothills of the Rocky Mountains of west-central Alberta, Canada. Forest Ecology and Management. 262(12): 2240-2249. [84144]
12. Andreu-Hayles, Laia; D'Arrigo, Rosanne; Anchukaitis, Kevin J.; Beck, Pieter S. A.; Frank, David; Goetz, Scott. 2011. Varying boreal forest response to Arctic environmental change at the Firth River, Alaska. Environmental Research Letters. 6(4): doi:10.1088/1748-9326/6/4/049502. [88779]
13. Angell, Amy C.; Kielland, Knut. 2009. Establishment and growth of white spruce on a boreal forest floodplain: interactions between microclimate and mammalian herbivory. Forest Ecology and Management. 258(11): 2475-2480. [77484]
14. Archibald, Devan W.; McAdam, Andrew G.; Boutin, Stan; Fletcher, Quinn E.; Humphries, Murray M. 2012. Within-season synchrony of a masting conifer enhances seed escape. The American Naturalist. 179(4): 536-544. [87389]
15. Archibold, O. W. 1989. Seed banks and vegetation processes in coniferous forests. In: Leck, Mary Allessio; Parker, V. Thomas; Simpson, Robert L., eds. Ecology of soil seed banks. San Diego, CA: Academic Press: 107-122. [60861]
16. Argus, George W. 1966. Botanical investigations in northeastern Saskatchewan: the subarctic Patterson-Hasbala Lakes region. Canadian Field-Naturalist. 80(3): 119-143. [8406]
17. Awada, Tala; Henebry, Geoffrey M.; Redmann, Robert E.; Sulistiyowati, Hari. 2004. Picea glauca dynamics and spatial pattern of seedlings regeneration along a chronosequence in the mixedwood section of the boreal forest. Annals of Forest Science. 61(8): 789-794. [55431]
18. Ayotte, Nicole. 2002. White spruce dynamics in the forest-tundra ecotone, the southwest Yukon Territory. Ottawa, Ontario: University of Ottawa. Thesis. 116 p. [88362]
19. Ballard, T. M.; Hawkes, B. C. 1989. Effects of burning and mechanical site preparation on growth and nutrition of planted white spruce. Information Report BC-X-309. Victoria, BC: Forestry Canada, Pacific and Yukon Region, Pacific Forestry Centre. 19 p. [6818]
20. Ballard, Warren B.; Krausman, Paul R.; Boe, Sue; Cunningham, Stan; Whitlaw, Heather A. 2000. Short-term response of gray wolves, Canis lupus, to wildfire in northwestern Alaska. The Canadian-Field Naturalist. 114(2): 241-247. [46985]
21. Barber, Valerie A.; Juday, Glenn Patrick; Finney, Bruce P. 2000. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature. 405: 668-673. [88299]
22. Barbour, Michael G.; Billings, William Dwight, eds. 1988. North American terrestrial vegetation. Cambridge, New York: Cambridge University Press. 434 p. [13876]
23. Barney, R. J.; Stocks, B. J. 1983. Fire frequencies during the suppression period. In: Wein, Ross W.; MacLean, David A., eds. The role of fire in northern circumpolar ecosystems. Scope 18. New York: John Wiley & Sons: 45-61. [12079]
24. Barney, Richard J. 1969. Interior Alaska wildfires: 1956-1965. Juneau, AK: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station, Institute of Northern Forestry. 47 p. [20191]
25. Barney, Richard J.; Bevins, Collin D.; Bradshaw, Larry S. 1981. Forest floor fuel loads, depths, and bulk densities in four interior Alaskan cover types. Res. Note INT-304. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 7 p. [8182]
26. Barrett, Carolyn M. 2013. Responses of boreal fire regimes to climatic and land-cover changes: perspectives from multiple spatiotemporal scales. Urbana, IL: University of Illinois at Urbana-Champaign. 107 p. Dissertation. [88028]
27. Barrett, Stephen W.; Arno, Stephen F.; Key, Carl H. 1991. Fire regimes of western larch - lodgepole pine forests in Glacier National Park, Montana. Canadian Journal of Forest Research. 21(12): 1711-1720. [17290]
28. Beck, Pieter S. A.; Goetz, Scott J.; Mack, Michelle C.; Alexander, Heather D.; Jin, Yufang; Randerson, James T.; Loranty, M. M. 2011. The impacts and implications of an intensifying fire regime on Alaskan boreal forest composition and albedo. Global Change Biology. 17(9): 2853-2866. [86612]
29. Beck, Pieter S. A.; Juday, Glenn P.; Alix, Clair; Barber, Valerie A.; Winslow, Stephen E.; Sousa, Emily E.; Heiser, Patricia; Herriges, James D.; Goetz, Scott J. 2011. Changes in forest productivity across Alaska consistent with biome shift. Ecology Letters. 14(4): 373-379. [88297]
30. Benzie, John W. 1980. Red pine. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 24-25. [49886]
31. Berg, Edward E.; Anderson, R. Scott. 2006. Fire history of white and Lutz spruce forests on the Kenai Peninsula, Alaska, over the last two millennia as determined from soil charcoal. Forest Ecology and Management. 227(3): 275-283. [62654]
32. Berg, Edward E.; David, Henry J.; Fastie, Christopher L.; De Volder, Andrew D.; Matsuoka, Steven M. 2006. Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: relationship to summer temperatures and regional differences in disturbance regimes. Forest Ecology and Management. 227(3): 219-232. [88098]
33. Bergeron, Yves. 2000. Species and stand dynamics in the mixed woods of Quebec's southern boreal forest. Ecology. 81(6): 1500-1516. [37017]
34. Bergeron, Yves; Charron, Danielle. 1994. Postfire stand dynamics in a southern boreal forest (Quebec): a dendroecological approach. Ecoscience. 1(2): 173-184. [26053]
35. Bergeron, Yves; Denneler, Bernhard; Charron, Danielle; Girardin, Martin-Philippe. 2002. Using dendrochronology to reconstruct disturbance and forest dynamics around Lake Duparquet, northwestern Quebec. Dendrochronologia. 20(1-2): 175-189. [54317]
36. Bergeron, Yves; Dubuc, Michelle. 1989. Succession in the southern part of the Canadian boreal forest. Vegetatio. 79(1-2): 51-63. [5042]
37. Blais, J. R. 1968. Regional variation in susceptibility of eastern North American forests to budworm attack based on history of outbreaks. Forestry Chronicle. 44(3): 17-23. [16530]
38. Boag, D. A.; Sumanik, K. M. 1969. Characteristics of drumming sites selected by ruffed grouse in Alberta. The Journal of Wildlife Management. 33(3): 621-628. [15648]
39. Boggs, Keith; Sturdy, Michelle; Rinella, Daniel J.; Rinella, Matthew J. 2008. White spruce regeneration following a major spruce beetle outbreak in forests on the Kenai Peninsula, Alaska. Forest Ecology and Management. 255(10): 3571-3579. [70917]
40. Bonan, Gordon B.; Shugart, Herman H. 1989. Environmental factors and ecological processes in boreal forests. Annual Review of Ecology and Systematics. 20: 1-28. [14170]
41. Bouchard, Mathieu; Kneeshaw, Daniel; Bergeron, Yves. 2005. Mortality and stand renewal patterns following the last spruce budworm outbreak in mixed forests of western Quebec. Forest Ecology and Management. 204(2/3): 297-313. [55411]
42. Boucher, Tina V. 2003. Vegetation response to prescribed fire in the Kenai Mountains, Alaska. Res. Pap. PNW-RP-554. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 59 p. [48392]
43. Boucher, Tina V.; Mead, Bert R. 2006. Vegetation change and forest regeneration on the Kenai Peninsula, Alaska following a spruce beetle outbreak, 1987-2000. Forest Ecology and Management. 227(3): 233-246. [66316]
44. Boulanger, Yan; Gauthier, Sylvie; Burton, Philip J. 2014. A refinement of models projecting future Canadian fire regimes using homogenous fire regime zones. Canadian Journal of Forest Research. 44(4): 365-376. [88004]
45. Bourgeau-Chavez, Laura L.; Alexander, Martin E.; Stocks, Brian J.; Kasischke, Eric S. 2000. Distribution of forest ecosystems and the role of fire in the North American boreal region. In: Caldwell, M. M.; Heldmaier, G.; Lange, O. L.; Mooney, H. A.; Schulze, E. -D.; Sommer, U., eds. Fire, climate change, and carbon cycling in the boreal forest. Ecological Studies, Vol. 138. New York: Springer-Verlag: 111-130. [86481]
46. Brassard, Brian W.; Chen, Han Y. H. 2006. Stand structural dynamics of North American boreal forests. Critical Reviews in Plant Sciences. 25(2): 115-137. [66679]
47. Breitung, August J. 1954. A botanical survey of the Cypress Hills. Canadian Field-Naturalist. 68: 55-92. [6262]
48. Brink, C. Holden; Dean, Frederick C. 1966. Spruce seed as a food of red squirrels and flying squirrels in interior Alaska. The Journal of Wildlife Management. 30(3): 503-512. [13253]
49. Brown, Peter Mark. 2003. Fire, climate, and forest structure in ponderosa pine forests of the Black Hills. Fort Collins, CO: Colorado State University. 103 p. Dissertation. [88621]
50. Brubaker, Linda B.; Higuera, Philip E.; Rupp, T. Scott; Olson, Mark A.; Anderson, Patricia M.; Hu, Feng Sheng. 2009. Linking sediment-charcoal records and ecological modeling to understand causes of fire-regime change in boreal forests. Ecology. 90(7): 1788-1801. [85385]
51. Bryant, John P.; Kuropat, Peggy J. 1980. Selection of winter forage by subarctic browsing vertebrates: the role of plant chemistry. Annual Review of Ecology and Systematics. 11: 261-285. [88166]
52. Buell, Murray F.; Niering, William A. 1957. Fir-spruce-birch forest in northern Minnesota. Ecology. 38(4): 602-610. [14172]
53. Bulmer, C.; Schmidt, M. G.; Kishchuk, B.; Preston, C. 1998. Impacts of blading and burning site preparation on soil properties and site productivity in the sub-boreal spruce zone of central British Columbia. Information Report BC-X-377. Victoria, BC: Canadian Forest Service, Pacific Forestry Centre, Effects of Forestry Practices Network. 33 p. [29307]
54. Burn, C. R. 1998. The response (1958-1997) of permafrost and near-surface ground temperatures to forest fire, Takhini River valley, southern Yukon Territory. Canadian Journal of Earth Science. 55(2): 184-199. [88255]
55. Burns, Russell M.; Honkala, Barbara H., tech. coords. 1990. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service. 675 p. [13362]
56. Burns, Russell M.; Honkala, Barbara H. 1990. Summary of tree characteristics. In: Burns, Russell M.; Honkala, Barbara H., tech. coords. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 646-649. [13424]
57. Calef, Monika P.; McGuire, A. David; Epstein, Howard E.; Rupp, T. Scott; Shugart, Herman H. 2005. Analysis of vegetation distribution in interior Alaska and sensitivity to climate change using a logistic regression approach. Journal of Biogeography. 32(5): 863-878. [85883]
58. Cater, Timothy C.; Chapin, F. Stuart, III. 2000. Differential effects of competition or microenvironment on boreal tree seedling establishment after fire. Ecology. 81(4): 1086-1099. [35929]
59. Chapin, F. S., III. 1986. Controls over growth and nutrient use by taiga forest trees. In: Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Vol. 57. New York: Springer-Verlag: 96-111. [86303]
60. Chapin, F. S., III; McGuire, A. D.; Ruess, R. W.; Hollingsworth, T. N.; Mack, M. C.; Johnstone, J. F.; Kasischke, E. S.; Euskirchen, E. S.; Jones, J. B.; Jorgenson, M. T.; Kielland, K.; Kofinas, G. P.; Turetsky, M. R.; Yarie, J.; Lloyd, A. H.; Taylor, D. 2010. Resilience of Alaska's boreal forest to climatic change. Canadian Journal of Forest Research. 40(7): 1360-1370. [82445]
61. Charron, I.; Greene, D. F. 2002. Post-wildfire seedbeds and tree establishment in the southern mixedwood boreal forest. Canadian Journal of Forest Research. 32(9): 1607-1615. [43075]
62. Charron, I.; Johnson, E. A. 2006. The importance of fires and floods on tree ages along mountainous gravel-bed streams. Ecological Applications. 16(5): 1757-1770. [64733]
63. Charron, Isabelle. 1998. Sexual recruitment of trees following fire in the southern mixedwood boreal forest of Canada. Montreal, Quebec: Concordia University. 109 p. Thesis. [88365]
64. Chavardes, Raphael D.; Daniels, Lori D.; Waeber, Patrick O.; Innes, John L.; Nitschke, Craig R. 2012. Did the 1976-77 switch in the Pacific Decadal Oscillation make white spruce in the southwest Yukon more susceptible to spruce bark beetle? The Forestry Chronicle. 88(5): 513-518. [86466]
65. Chen, Han Y. H.; Vasiliauskas, Stan; Kayahara, Gordan J.; Ilisson, Triin. 2009. Wildfire promotes broadleaves and species mixture in boreal forest. Forest Ecology and Management. 257(1): 343-350. [81471]
66. Chhin, Sophan; Wang, G. Geoff. 2002. Spatial and temporal pattern of white spruce regeneration within mixed-grass prairie in the Spruce Woods Provincial Park of Manitoba. Journal of Biogeography. 29(7): 903-912. [63603]
67. Christiansen, Janet S. 1988. A spruce-lichen woodland in northern Alaska: post-fire regeneration and community dynamics. Seattle, WA: University of Washington. 94 p. Thesis. [85885]
68. Chrosciewicz, Z. 1986. Foliar moisture content variations in four coniferous tree species of central Alberta. Canadian Journal of Forest Research. 16(1): 157-162. [45795]
69. Clark, Donald F.; Antos, Joseph A.; Bradfield, Gary E. 2003. Succession in sub-boreal forests of west-central British Columbia. Journal of Vegetation Science. 14(5): 721-732. [48408]
70. Clarke, Ronald G. 1982. Nest site selection by sharp-shinned hawks in interior Alaska. In: Ladd, Wilbur N.; Schempf, Philip F., eds. Raptor management and biology in Alaska and western Canada: Proceedings of a symposium and workshop; 1981 February 17-20; Anchorage, AK. FWS/AK/PROC-82. Anchorage, AK: U.S. Department of the Interior, Fish and Wildlife Service, Alaska Regional Office: 155-162. [25659]
71. Clautice, Stephan Fitzgerald. 1974. Spruce and birch germination on different seedbeds and aspects after fire in interior Alaska. Fairbanks, AK: University of Alaska. 94 p. Thesis. [22284]
72. Cogbill, Charles V. 1985. Dynamics of the boreal forests of the Laurentian Highlands, Canada. Canadian Journal of Forest Research. 15(1): 252-261. [19928]
73. Cole, Elizabeth; Youngblood, Andrew; Newton, Michael. 2003. Effects of competing vegetation on juvenile white spruce (Picea glauca (Moench) Voss) growth in Alaska. Annals of Forest Science. 60(7): 573-583. [53302]
74. Collins, William B.; Helm, D. J. 1997. Moose, Alces alces, habitat relative to riparian succession in the boreal forest, Susitna River, Alaska. The Canadian Field-Naturalist. 111(4): 567-574. [78570]
75. Collins, William B.; Schwartz, Charles C. 1998. Logging in Alaska's boreal forest: creation of grasslands or enhancement of moose habitat. Alces. 34(2): 355-374. [39752]
76. Cooper, David Jonathan. 1983. Arctic-alpine tundra ecosystems of the Arrigetch Creek Valley, Central Brooks Range, Alaska. Bolder, CO: University of Colorado. 827 p. [+ tables and figures]. Dissertation. [88142]
77. Cooper, William S. 1913. The climax forest of Isle Royale, Lake Superior, and its development. I. Botanical Gazette. 55(1): 1-44. [11537]
78. Cooper, William S. 1928. Seventeen years of successional change upon Isle Royale, Lake Superior. Ecology. 9(1): 1-5. [7297]
79. Cormack, R. G. H. 1953. A survey of coniferous forest succession in the eastern Rockies. The Forestry Chronicle. 29(3): 218-232. [16458]
80. Corns, I. G. W.; Annas, R. M. 1986. Field guide to forest ecosystems of west-central Alberta. Edmonton, AB: Natural Resources Canada, Canadian Forestry Service, Northern Forestry Centre. 251 p. [8998]
81. Coyea, M. R.; Lieffers, V. J.; Woodard, P. M. 1989. Factors affecting white spruce (Picea glauca [Moench] Voss) seed germination on burned forest litter. In: MacIver, D. C.; Auld, H.; Whitewood, R., eds. Proceedings of the 10th conference on fire and forest meteorology; 1989 April 17-21; Ottawa, ON. Chalk River, ON: Forestry Canada, Petawawa National Forestry Institute; Downsview, ON: Atmospheric Environment Service: 122-125. [13523]
82. Cram, W. H.; Worden, H. A. 1957. Maturity of white spruce cones and seed. Forest Science. 3(3): 263-269. [88419]
83. Cronan, James; McKenzie, Donald; Olson, Diana. [n.d.]. Fire regimes of the Alaskan boreal forest. Draft manuscript. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 67 p. (+ figures, tables, and appendices). In cooperation with: Seattle, WA: University of Washington, School of Forest Resources; New Haven, CT: Yale School of Forestry and Environmental Studies; Moscow, ID: University of Idaho; Fairbanks, AK: U.S. Department of the Interior, Bureau of Land Management, Alaska Fire Service. Available online: http://www.frames.gov/documents/alaska/fire_history/fire_regimes_alaskan_boreal_forest_draft_gtr.zip [2015, May 8]. [85879]
84. Cumming, S. G. 2001. Forest type and wildfire in the Alberta boreal mixedwood: what do fires burn? Ecological Applications. 11(1): 97-110. [39417]
85. Cumming, S. G.; Schmiegelow, F. K. A.; Burton, P. J. 2000. Gap dynamics in boreal aspen stands: Is the forest older than we think? Ecological Applications. 10(3): 744-759. [85419]
86. Cumming, Steve; Trindade, Mariana; Greene, David; Macdonald, S. Ellen. 2009. Canopy and emergent white spruce in "pure" broadleaf stands: frequency, predictive models, and ecological importance. Canadian Journal of Forest Research. 39(10): 1997-2004. [88364]
87. Curtis, John T. 1959. Boreal forest. In: The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press: 243-257. [60525]
88. D'Arrigo, Rosanne D.; Kaufmann, Robert K.; Davi, Nicole; Jacoby, Gordon C.; Laskowski, Cheryl; Myneni, Ranga B.; Cherubini, Paolo. 2004. Thresholds for warming-induced growth decline at elevational tree line in the Yukon Territory, Canada. Global Biogeochemical Cycles. 18(3): doi:10.1029/2004GB002249. [88783]
89. D'Arrigo, Rosanne; Jacoby, Gordon; Buckley, Brendan; Sakulich, John; Frank, David; Wilson, Rob; Curtis, Ashley; Anchukaitis, Kevin. 2009. Tree growth and inferred temperature variability at the North American Arctic treeline. Global and Planetary Change. 65(1-2): 71-82. [88782]
90. Dale, Mark R. T.; Francis, Shawn; Krebs, Charles J.; Nams, Vilis O. 2001. Ecosystem Dynamics of the Boreal Forest. In: Krebs, Charles J.; Boutin, Stan; Boonstra, Rudy. Trees. New York: Oxford University Press: 116-137. [87652]
91. Damman, A. W. H. 1964. Some forest types of central Newfoundland and their relation to environmental factors. Forest Science Monograph 8. Washington, DC: Society of American Foresters. 62 p. [14281]
92. Damman, A. W. H.; Johnston, William F. 1980. Black spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 11-14. [49853]
93. Danby, Ryan K.; Hik, David S. 2007. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. Journal of Ecology. 95(2): 352-363. [88366]
94. Daubenmire, Rexford. 1953. Notes on the vegetation of forested regions of the far northern Rockies and Alaska. Northwest Science. 27(4): 125-138. [10816]
95. Davis, Ronald B. 1966. Spruce-fir forests of the coast of Maine. Ecological Monographs. 36(2): 79-94. [8228]
96. Day, R. J.; Harvey, E. M. 1981. Forest dynamics in boreal mixedwood. In: Whitney, R. D.; McClain, K. M., compilers. Boreal mixedwood symposium. Proceedings of a symposium; 1980 September 16-18; Thunder Bay, ON. COJFRC Symposium Proceedings O-P-9. Sault Ste. Marie, ON: Environment Canada, Canadian Forestry Service, Great Lakes Forestry Research Centre: 29-41. [14204]
97. Day, Robert J. 1972. Stand structure, succession, and use of southern Alberta's Rocky Mountain forest. Ecology. 53(3): 472-478. [12976]
98. de Groot, W. J.; Bothwell, P. M.; Carlsson, D. H.; Logan, K. A. 2003. Simulating the effects of future fire regimes on western Canadian boreal forests. Journal of Vegetation Science. 14(3): 355-364. [50270]
99. de Groot, William J.; Flannigan, Michael D.; Cantin, Alan S. 2013. Climate change impacts on future boreal fire regimes. Forest Ecology and Management. 294: 35-44. [86909]
100. de Lafontaine Guillaume; Payette, Serge. 2010. The origin and dynamics of subalpine white spruce and balsam fir stands in boreal eastern North America. Ecosystems. 13(6): 932-947. [88369]
101. de Lafontaine, Guillaume; Payette, Serge. 2012. How climate and fire disturbances influence contrasted dynamics of Picea glauca ecotones at alpine tree lines in Atlantic and continental eastern North America. In: Myster, R. W., ed. Ecotones between forest and grassland. New York, NY: Springer: 299-312. [87587]
102. de Lafontaine, Guillaume; Payette, Serge. 2012. Long-term fire and forest history of subalpine balsam fir (Abies balsamea) and white spruce (Picea glauca) stands in eastern Canada inferred from soil charcoal analysis. Holocene. 22(2): 191-201. [85756]
103. DeLong, H. B.; Lieffers, V. J.; Blenis, P. V. 1997. Microsite effects on first-year establishment and overwinter survival of white spruce in aspen-dominated boreal mixedwoods. Canadian Journal of Forest Research. 27(9): 1452-1457. [88452]
104. Desponts, Mireille; Brunet, Genevieve; Belanger, Louis; Bouchard, Mathieu. 2004. The eastern boreal old-growth balsam fir forest: a distinct ecosystem. Canadian Journal of Botany. 82(6): 830-849. [50267]
105. DeWilde, La'ona; Chapin, F. Stuart, III. 2006. Human impacts on the fire regime of interior Alaska: interactions among fuels, ignition sources, and fire suppression. Ecosystems. 9(8): 1342-1353. [69884]
106. Dirschl, H. J.; Coupland, R. T. 1972. Vegetation patterns and site relationships in the Saskatchewan River delta. Canadian Journal of Botany. 50(3): 647-675. [7449]
107. Dirschl, Herman J.; Dabbs, Don L.; Gentle, Garry C. 1974. Landscape classification and plant successional trends in the Peace-Athabasca Delta. Canadian Wildlife Service Report Series 30. Ottawa, ON: Canadian Wildlife Service. 33 p. [6177]
108. Dix, R. L.; Swan, J. M. A. 1971. The roles of disturbance and succession in upland forest at Candle Lake, Saskatchewan. Canadian Journal of Botany. 49(5): 657-676. [12808]
109. Dobbs, R. C. 1976. White spruce seed dispersal in central British Columbia. The Forestry Chronicle. 52(2): 225-228. [87601]
110. Dorn, Robert D. 1977. Flora of the Black Hills. Cheyenne, WY: Robert D. Dorn and Jane L. Dorn. 377 p. [820]
111. Drever, C. Ronnie; Drever, Mak C.; Messier, Christian; Bergeron, Yves; Flannigan, Mike. 2008. Fire and the relative roles of weather, climate and landscape characteristics in the Great Laske-St. Lawrence forest of Canada. Journal of Vegetation Science. 19(1): 57-66. [74742]
112. Drever, C. Ronnie; Messier, Christian; Bergeron, Yves; Doyon, Frederik. 2006. Fire and canopy species composition in the Great Lakes-St. Lawrence forest of Temiscamingue, Quebec. Forest Ecology and Management. 231(1/3): 27-37. [65022]
113. Drury, S. A.; Grissom, P. J. 2008. Fire history and fire management implications in the Yukon Flats National Wildlife Refuge, interior Alaska. Forest Ecology and Management. 256(3): 304-312. [71131]
114. Duchesne, Luc C.; Hawkes, Brad C. 2000. Fire in northern ecosystems. In: Brown, James K.; Smith, Jane Kapler, eds. Wildland fire in ecosystems: effects of fire on flora. Gen. Tech. Rep. RMRS-GTR-42-vol. 2. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 35-51. [36982]
115. Duffy, Paul A.; Epting, Justin; Graham, Jonathan M.; Rupp, T. Scott; McGuire, A. David. 2007. Analysis of Alaskan burn severity patterns using remotely sensed data. International Journal of Wildland Fire. 16(3): 277-284. [88140]
116. Duffy, Paul A.; Walsh, John E.; Graham, Jonathan M.; Mann, Daniel H.; Rupp, T. Scott. 2005. Impacts of large-scale atmospheric-ocean variability on Alaskan fire season severity. Ecological Applications. 15(4): 1317-1330. [54538]
117. Dupuis, S.; Arseneault, D.; Sirois, L. 2011. Change from pre-settlement to present-day forest composition reconstructed from early land survey records in eastern Quebec, Canada. Journal of Vegetation Science. 22(3): 564-575. [82550]
118. Dyrness, C. T. 1980. White spruce-aspen. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 82. [50013]
119. Dyrness, C. T. 1980. White spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 81. [50012]
120. Dyrness, C. T.; Norum, Rodney A. 1983. The effects of experimental fires on black spruce forest floors in interior Alaska. Canadian Journal of Forest Research. 13(5): 879-893. [7299]
121. Dyrness, C. T.; Viereck, L. A.; Van Cleve, K. 1986. Fire in taiga communities of interior Alaska. In: Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Ecological Studies 57. New York: Springer-Verlag: 74-86. [3881]
122. Eis, S. 1981. Effect of vegetative competition on regeneration of white spruce. Canadian Journal of Forest Research. 11(1): 1-8. [10104]
123. Eis, Slavoj. 1965. Development of white spruce and alpine fir seedlings on cut-over areas in the central interior of British Columbia. Forestry Chronicle. 41(4): 419-431. [45904]
124. Eis, Slavoj. 1967. Establishment and early development of white spruce in the interior of British Columbia. Forestry Chronicle. 43(2): 174-177. [45905]
125. Elliott, Charles L.; McKendrick, Jay D.; Helm, D. 1987. Plant biomass, cover, and survival of species used for stripmine reclamation in south-central Alaska, U.S.A. Arctic and Alpine Research. 19(4): 572-577. [6116]
126. Endean, F. 1972. Soil temperature, seedling growth and white spruce regeneration. In: McMinn, R. G., ed. White spruce: the ecology of a northern resource; proceedings of a symposium; 1971 June 21; Edmonton, AB. Information Report NOR-X-40. Edmonton, AB: Canadian Forestry Service, Department of the Environment, Northern Forest Research Center: 15-20. [86723]
127. Endean, F.; Johnston, W. D. 1974. Prescribed fire and regeneration on clearcut spruce-fir sites in the foothills of Alberta. Inf. Rep. NOR-X-126. Edmonton, AB: Environment Canada, Forestry Service, Northern Forest Research Centre. 33 p. [12987]
128. Epting, Justin; Verbyla, David. 2005. Landscape-level interactions of prefire vegetation, burn severity, and postfire vegetation over a 16-year period in interior Alaska. Canadian Journal of Forest Research. 35(6): 1367-1377. [55816]
129. Farrar, John Laird. 1995. Trees of the northern United States and Canada. Ames, IA: Blackwell Publishing. 502 p. [60614]
130. Fauria, Marc Macias; Johnson, E. A. 2008. Climate and wildfires in the North American boreal forest. Philosophical Transactions of the Royal Society. 363(1501): 2317-2329. [74741]
131. Fauria, Marc Macias; Johnson, Edward A. 2006. Large-scale climatic patterns control large lightning fire occurrence in Canada and Alaska forest regions. Journal of Geophysical Research. 111(G4): doi:10.1029/2006JG000181. [85427]
132. Flannigan, Mike D.; Krawchuk, Meg A.; de Groot, William J.; Wotton, B. Mike; Gowman, Lynn M. 2009. Implications of changing climate for global wildland fire. International Journal of Wildland Fire. 18(5): 483-507. [78110]
133. Flannigan, Mike; Cantin, Alan S.; de Groot, William J.; Wotton, Mike; Newbery, Alison; Gowman, Lynn M. 2013. Global wildland fire season severity in the 21st century. Forest Ecology and Management. 294: 54-61. [88853]
134. Fleming, Richard A.; Candau, Jean-Noel; McAlpine, Rob S. 2002. Landscape-scale analysis on interactions between insect defoliation and forest fire in central Canada. Climatic Change. 55(1-2): 251-272. [45066]
135. Flora of North America Editorial Committee, eds. 2015. Flora of North America north of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
136. Foote, Joan. 1976. Classification, description, and dynamics of plant communities following fire in the taiga of interior Alaska. Final report: "Fire effects study"; BLM Contract No. 53500-CT2-244. Juneau, AK: U.S. Department of the Interior, Bureau of Land Management. 211 p. [36000]
137. Foote, M. Joan. 1983. Classification, description, and dynamics of plant communities after fire in the taiga of interior Alaska. Res. Pap. PNW-307. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 108 p. [7080]
138. Foote, M. Joan. 1995. The role of fire in the boreal forest of interior Alaska. In: Managing forests to meet peoples' needs: Proceedings of the 1994 Society of American Foresters/Canadian Institute of Forestry annual convention; 1994 September 18-22; Anchorage, AK. Bethesda, MD: Society of American Foresters: 179-184. [85352]
139. Foster, Cathy. 2002. White spruce regeneration thirty-nine years post-fire in the boreal mixedwoods of Duck Mountain, Manitoba. Winnipeg, MB: University of Manitoba, Department of Botany. 188 p. Thesis. [67327]
140. Foster, David R. 1984. Phytosociological description of the forest vegetation of southeastern Labrador. Canadian Journal of Botany. 62(5): 899-906. [15356]
141. Frank, Robert M.; Majcen, Zoran; Gagnon, Gilles. 1980. Balsam fir--Forest Cover Type 5. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 10-11. [49851]
142. Frelich, Lee E.; Reich, Peter B. 1995. Spatial patterns and succession in a Minnesota southern-boreal forest. Ecological Monographs. 63(3): 325-346. [26533]
143. Frelich, Lee E.; Reich, Peter B. 2009. Wilderness conservation in an era of global warming and invasive species: a case study from Minnesota's Boundary Waters Canoe Area Wilderness. Natural Areas Journal. 29(4): 385-393. [77775]
144. Frissell, Sidney S., Jr. 1973. The importance of fire as a natural ecological factor in Itasca State Park, Minnesota. Quaternary Research. 3(3): 397-407. [12988]
145. Galipeau, Christine; Kneeshaw, Daniel; Bergeron, Yves. 1997. White spruce and balsam fir colonization of a site in the southeastern boreal forest as observed 68 years after fire. Canadian Journal of Forest Research. 27(2): 139-147. [29242]
146. Garet, Jerome; Pothier, David; Bouchard, Mathieu. 2009. Predicting the long-term yield trajectory of black spruce stands using time since fire. Forest Ecology and Management. 257(10): 2189-2197. [74286]
147. Gartner, Stefanie M.; Bokalo, Mike; Macdonald, S. Ellen; Stadt, Ken. 2014. Variation in post-wildfire regeneration of boreal mixedwood forests: underlying factors and implications for natural disturbance-based management. New Forests. 45(2): 215-234. [88407]
148. Gartner, Stefanie M.; Lieffers, Victor J.; Macdonald, S. Ellen. 2011. Ecology and management of natural regeneration of white spruce in the boreal forest. Environmental Reviews. 19: 461-478. [87595]
149. Giddings, J. L. 1962. Development of tree-ring dating as an archeological aid. In: Kozlowski, Theodore T., ed. Tree growth. NY: Ronald Press Company: 119-132. [87722]
150. Gillett, N. P.; Weaver, A. J.; Zwiers, F. W.; Flannigan, M. D. 2004. Detecting the effect of climate change on Canadian forest fires. Geophysical Research Letters. 31(18): doi:10.1029/2004GL020876. [88625]
151. Gleason, Henry A.; Cronquist, Arthur. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd ed. New York: New York Botanical Garden. 910 p. [20329]
152. Goldstein, Guillermo Herman. 1981. Ecophysiological and demographic studies of white spruce (Picea glauca) (Moench) Voss at treeline in the central Brooks Range. Seattle, WA: University of Washington. 193 p. Dissertation. [86061]
153. Goodman, Lilly F.; Hungate, Bruce A. 2006. Managing forests infested by spruce beetles in south-central Alaska: effects on nitrogen availability, understory biomass, and spruce regeneration. Forest Ecology and Management. 227(3): 267-274. [88368]
154. Gracz, Michael. 2014. (Email to Ilana Abrahamson). August 21. Regarding fire in white spruce communities in the Kenai National Wildlife Refuge. Soldotna, AK: Kenai Watershed Forum. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [88213]
155. Greene, D. F.; Johnson, E. A. 1994. Estimating the mean annual seed production of trees. Ecology. 75(3): 642-647. [51823]
156. Greene, D. F.; Johnson, E. A. 2000. Tree recruitment from burn edges. Canadian Journal of Forest Research. 30(8): 1264-1274. [38974]
157. Greene, D. F.; Zasada, J. C.; Sirois, L.; Kneeshaw, D.; Morin, H.; Charron, I.; Simard, M.-J. 1999. A review of the regeneration dynamics of North American boreal forest tree species. Canadian Journal of Forest Research. 29(6): 824-839. [40703]
158. Greene, David F.; Macdonald, S. Ellen; Cumming, Steve; Swift, Lynn. 2005. Seedbed variation from the interior through the edge of a large wildfire in Alberta. Canadian Journal of Forest Research. 35(7): 1640-1647. [61213]
159. Greene, David F.; Macdonald, S. Ellen; Haeussler, Sybille; Domenicano, Susy; Noel, Josee; Jayen, Karelle; Charron, Isaelle; Gauthier, Sylvie; Hunt, Simon; Gielau, E. Trent; Bergeron, Yves; Swift, Lynn. 2007. The reduction of organic-layer depth by wildfire in the North American boreal forest and its effect on tree recruitment by seed. Canadian Journal of Forest Research. 37(6): 1012-1023. [69785]
160. Greene, David, F.; Messier, Christian; Asselin, Hugo; Fortin, Marie-Josee. 2002. The effect of light availability and basal area on cone production in Abies balsamea and Picea glauca. Canadian Journal of Botany. 80(4): 370-377. [87597]
161. Grenier, Daniel J.; Bergeron, Yves; Kneeshaw, Daniel; Gauthier, Sylvie. 2005. Fire frequency for the transitional mixedwood forest of Timiskaming, Quebec, Canada. Canadian Journal of Forest Research. 35(3): 656-666. [61484]
162. Grigal, D. F.; Ohmann, Lewis F. 1975. Classification, description, and dynamics of upland plant communities within a Minnesota wilderness area. Ecological Monographs. 45(4): 389-407. [61235]
163. Hamilton, Evelyn H. 2006. Fire effects and post-burn vegetation development in the sub-boreal spruce zone: Mackenzie (Windy Point) site. Technical Report 033. Victoria, BC: Ministry of Forests and Range, Forest Science Program (Producer). 19 p. Available online: http://www.for.gov.bc.ca/hfd/pubs/Docs/Tr/Tr033.pdf [2015, June 24]. [64177]
164. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org. [81749]
165. Hannah, Kevin C.; Hoyt, Jeff S. 2004. Northern hawk owls and recent burns: does burn age matter? The Condor. 106(2): 420-423. [50287]
166. Hansen, Paul L.; Chadde, Steve W.; Pfister, Robert D. 1988. Riparian dominance types of Montana. Misc. Publ. No. 49. Missoula, MT: University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station. 411 p. [5660]
167. Hansen, Paul L.; Pfister, Robert D.; Boggs, Keith; Cook, Bradley J.; Joy, John; Hinckley, Dan K. 1995. Classification and management of Montana's riparian and wetland sites. Misc. Publ. No. 54. Missoula, MT: The University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station. 646 p. [24768]
168. Hansen, R. M.; Flinders, J. T. 1969. Food habits of North American hares. Range Science Department Scientific Series No. 1. Fort Collins, CO: Colorado State University. 17 p. [63965]
169. Hanson, Bill. 1978. First year results of the Bear Creek Burn: Fire effects studies. Anchorage, AK: U.S. Department of the Interior, Bureau of Land Management, Anchorage District Office. 70 p. [+ appendices]. [86573]
170. Hanson, William A. 1979. Preliminary results of the Bear Creek fire effects studies. Proposed open file report. Anchorage, AK: U.S. Department of the Interior, Bureau of Land Management, Anchorage District Office. 83 p. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [6400]
171. Hardy, Charles E.; Franks, James W. 1963. Forest fires in Alaska. Res. Pap. INT-5. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 163 p. [21540]
172. Harvey, Brian D.; Leduc, Alain; Gauthier, Sylvie; Bergeron, Yves. 2002. Stand-landscape integration in natural disturbance-based management of the southern boreal forest. Forest Ecology and Management. 155: 369-385. [40707]
173. Harvey, Leroy H. 1919. A coniferous sand dune in Cape Breton Island. Botanical Gazette. 67(5): 417-426. [63542]
174. Hawkes, Brad C. 1983. Fire history and ecology of forest ecosystems in Kluane National Park. In: Wein, Ross Wallace; Riewe, Roderick R.; Methven, Ian R., eds. Resources and dynamics of the boreal zone: Proceedings of a conference; 1982 August; Thunder Bay, ON. Ottawa, ON: Association of Canadian Universities for Northern Studies: 266-280. [7444]
175. Hayasaka, Hiroshi; Lynch, Mary. 2003. Probability of lightning-ignited forest fires in Alaska. In: Galley, Krista E. M.; Klinger, Robert C.; Sugihara, Neil G., eds. Proceedings of fire conference 2000: the 1st national congress on fire ecology, prevention, and management; 2000 November 27-December 1; San Diego, CA. Miscellaneous Publication No. 13. Tallahassee, FL: Tall Timbers Research Station (abstract): 225-226. [51400]
176. Heinselman, Miron L. 1973. Fire in the virgin forests of the Boundary Waters Canoe Area, Minnesota. Quaternary Research. 3(3): 329-382. [282]
177. Heinselman, Miron L. 1973. Restoring fire to the canoe country. Naturalist. 24(4): 21-31. [15810]
178. Heinselman, Miron L. 1981. Fire and succession in the conifer forests of northern North America. In: West, Darrell C.; Shugart, Herman H.; Botkin, Daniel B., eds. Forest succession: concepts and applications. New York: Springer-Verlag: 374-405. [29237]
179. Heinselman, Miron L. 1981. Fire intensity and frequency as factors in the distribution and structure of northern ecosystems. In: Mooney, H. A.; Bonnicksen, T. M.; Christensen, N. L.; Lotan, J. E.; Reiners, W. A., tech. coords. Fire regimes and ecosystem properties: Proceedings of the conference; 1978 December 11-15; Honolulu, HI. Gen. Tech. Rep. WO-26. Washington, DC: U.S. Department of Agriculture, Forest Service: 7-57. [4390]
180. Higuera, Philip E.; Brubaker, Linda B.; Anderson, Patricia M.; Hu, Feng Sheng; Brown, Thomas A. 2009. Vegetation mediated the impacts of postglacial climate change on fire regimes in the south-central Brooks Range, Alaska. Ecological Monographs. 79(2): 201-219. [74641]
181. Higuera, Philip Edward. 2006. Late glacial and Holocene fire history in the southcentral Brooks Range, Alaska: direct and indirect impacts of climatic change on fire regimes. Seattle, WA: University of Washington. 175 p. Dissertation. [85572]
182. Hobson, Keith A.; Bayne, Erin. 2000. Effects of forest fragmentation by agriculture on avian communities in the southern boreal mixed woods of western Canada. The Wilson Bulletin. 112(3): 373-387. [64349]
183. Hobson, Keith A.; Schieck, Jim. 1999. Changes in bird communities in boreal mixedwood forest: harvest and wildfire effects over 30 years. Ecological Applications. 9(3): 849-863. [36010]
184. Hoffman, George R.; Alexander, Robert R. 1987. Forest vegetation of the Black Hills National Forest of South Dakota and Wyoming: a habitat type classification. Res. Pap. RM-276. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 48 p. [1181]
185. Hogg, E. H. (Ted); Wein, Ross W. 2005. Impacts of drought on forest growth and regeneration following fire in southwestern Yukon, Canada. Canadian Journal of Forest Research. 35(9): 2141-2150. [61538]
186. Holliday, N. J. 1984. Carabid beetles (Coleoptera: Carabidae) from a burned spruce forest (Picea spp.). The Canadian Entomologist. 116(7): 919-922. [8337]
187. Hollingsworth, T. N.; Walker, M. D.; Chapin, F. S., III; Parsons, A. L. 2006. Scale-dependent environmental controls over species composition in Alaskan black spruce communities. Canadian Journal of Forest Research. 36(7): 1781-1796. [85777]
188. Hollingsworth, Teresa N.; Lloyd, Andrea H.; Nossov, Dana R.; Ruess, Roger W.; Charlton, Brian A.; Kielland, Knut. 2010. Twenty-five years of vegetation change along a putative successional chronosequence on the Tanana River, Alaska. Canadian Journal of Forest Research. 40(7): 1273-1287. [83520]
189. Holloway, Patricia S.; Alexander, Ginny. 1990. Ethnobotany of the Fort Yukon region, Alaska. Economic Botany. 44(2): 214-225. [13625]
190. Holsten, Edward H.; Werner, Richard A.; Develice, Robert L. 1995. Effects of a spruce beetle (Coleoptera: Scolytidae) outbreak and fire on Lutz spruce in Alaska. Environmental Entomology. 24(6): 1539-1547. [26580]
191. Holsten, Edward; Hennon, Paul; Trummer, Lori; Kruse, James; Schultz, Mark; Lundquist, John. 2008. Insects and diseases of Alaskan forests. R10-TP-140. Anchorage, AK: U.S. Department of Agriculture, Forest Service, Alaska Region, State and Private Forestry. 245 p. [88438]
192. Horton, K. W. 1956. The ecology of lodgepole pine in Alberta and its role in forest succession. Tech. Note No. 45. Ottawa, Canada: Department of Northern Affairs and National Resources, Forestry Branch, Forest Research Division. 29 p. [13734]
193. Horton, K. W. 1959. Characteristics of subalpine spruce in Alberta. Tech. Note No. 76. Ottawa, Canada: Department of Northern Affairs and National Resources, Forestry Branch, Forest Research Division. 20 p. [14279]
194. Hosie, R. C. 1969. Native trees of Canada. 7th ed. Ottawa, ON: Canadian Forestry Service, Department of Fisheries and Forestry. 380 p. [3375]
195. Hoyt, Jeff S.; Hannon, Susan J. 2002. Habitat associations of black-backed and three-toed woodpeckers in the boreal forest of Alberta. Canadian Journal of Forest Research. 32(10): 1881-1888. [43215]
196. Hu, Feng Sheng; Brubaker, Linda B.; Gavin, Daniel G.; Higuera, Philip E.; Lynch, Jason A.; Rupp, T. Scott; Tinner, Willy. 2006. How climate and vegetation influence the fire regime of the Alaskan boreal biome: the Holocene perspective. Mitigation and Adaptation Strategies for Global Change. 11(4): 829-846. [66359]
197. Hulten, Eric. 1968. Flora of Alaska and neighboring territories. Stanford, CA: Stanford University Press. 1008 p. [13403]
198. Janke, Robert A. 1979. Moose-forest-fire ecology in Isle Royale National Park. In: Linn, Robert M, ed. Proceedings of the First Conference on Scientific Research in the National Parks. Volume 2; 1976 November 9-12; New Orleans, LA. National Park Service Trans. and Proceedings Series No. 5. Washington, DC: U.S. Department of the Interior, National Park Service; American Institute of Biological Sciences: 1243-1251. [8396]
199. Janke, Robert A.; Lowther, John L. 1980. Post-fire succession in the boreal forest type of Isle Royale National Park. In: Proceedings, 2nd conference on scientific research in the National Parks; 1979 November 26-30; San Francisco, CA. Volume 7: Ecosystem Studies/Interdisciplinary Studies. Washington, DC: U.S. Department of the Interior, National Park Service; American Institute of Biological Sciences: 99-135. [19929]
200. Jarvis, J. M. 1966. Seeding white spruce, black spruce, and jack pine on burned seedbeds in Manitoba. Rep. No. 1166. Ottawa, Canada: Canadian Forestry Service. 8 p. [14278]
201. Jeffrey, W. W. 1959. White Spruce rooting modifications on the fluvial deposits of the lower Peace River. The Forestry Chronicle. 25(4): 304-311. [87720]
202. Johnson, E. A.; Miyanishi, K.; O'Brien, N. 1999. Long-term reconstruction of the fire season in the mixedwood boreal forest of western Canada. Canadian Journal of Botany. 77(8): 1185-1188. [33061]
203. Johnson, E. A.; Rowe, J. S. 1977. Fire and vegetation change in the western subarctic. ALUR 75-76-61. Ottawa, ON: Arctic Land Use Research Program, Northern Natural Resources and Environment Branch, Department of Indian Affairs and Northern Development. 58 p. [69456]
204. Johnson, Edward A. 1992. Fire and vegetation dynamics: Studies from the North American boreal forest. Cambridge Studies in Ecology. Cambridge, UK: Cambridge University Press. 129 p. [19950]
205. Johnston, William F. 1980. Tamarck--Forest Cover Type 38. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 15-16. [49857]
206. Johnstone, Jill F.; Chapin, F. Stuart, III. 2006. Effects of soil burn severity on post-fire tree recruitment in boreal forest. Ecosystems. 9(1): 14-31. [62101]
207. Johnstone, Jill; Hollingsworth,Teresa; Chapin, Terry. 2007. Workbook of potential successional trajectories in burned stands of black spruce in interior Alaska [Draft]. Joint Fire Science Project No. 05-1-2-06. Fairbanks, AK: University of Alaska Fairbanks; Portland. OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Boreal Ecology Cooperative Research Unit. 16 p. [87182]
208. Jolly, W. Matt; Hadlow, Ann M.; Huguet, Kathleen. 2014. De-coupling seasonal changes in water content and dry matter to predict live conifer foliar moisture content. International Journal of Wildland Fire. 23(4): 480-489. [88471]
209. Jordan, Peter A.; McLaren, Brian E.; Sell, Scott M. 2000. A summary of research on moose and related ecological topics at Isle Royale, U.S.A. Alces. 36: 233-267. [78879]
210. Juday, Glenn P.; Barber, Valerie; Duffy, Paul; Linderholm, Hans; Rupp, Scott; Sparrow, Steve; Vaganov, Eugene; Yarie, John; Berg, Edward; D'Arrigo, Rosanne; Eggertsson, Olafur; Furyaev, V. V.; Hogg, Edward H.; Huttunen, Satu; Jacoby, Gordon; Kaplunov, V. 2005. Forests, land management, and agriculture. In: Symon, Carolyn; Arris, Lelani; Heal, Bill eds. Arctic Climate Impact Assessment. New York: Cambridge University Press: 781-862. [87960]
211. Kartesz, J. T.; The Biota of North America Program (BONAP). 2015. Taxonomic Data Center, [Online]. Chapel Hill, NC: The Biota of North America Program (Producer). Available online: bonap.org. [maps generated from Kartesz, J. T. 2010. Floristic synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]. [84789]
212. Kasischke, Eric S.; Turetsky, Merritt R. 2006. Recent changes in the fire regime across the North American boreal region--spatial and temporal patterns of burning across Canada and Alaska. Geophysical Research Letters. 33(9): doi:10.1029/2006GL025677. [85446]
213. Kasischke, Eric S.; Verbyla, David L.; Rupp, T. Scott; McGuire, A. David; Murphy, Karen A.; Jandt, Randi; Barnes, Jennifer L.; Hoy, Elizabeth E.; Duffy, Paul A.; Calef, Monida; Turetsky, Merritt R. 2010. Alaska's changing fire regime--implications for the vulnerability of its boreal forests. Canadian Journal of Forest Research. 40(7): 1313-1324. [82442]
214. Kasischke, Eric S.; Williams, David; Barry, Donald. 2002. Analysis of the patterns of large fires in the boreal forest region of Alaska. International Journal of Wildland Fire. 11(2): 131-144. [43088]
215. Kelly, Ryan; Chipman, Melissa L.; Higuera, Philip E.; Stefanova, Ivanka; Brubaker, Linda B.; Hu, Feng Sheng. 2013. Recent burning of boreal forests exceeds fire regime limits of the past 10,000 years. Proceedings of the National Academy of Sciences. 110(32): 13055-13060. [88344]
216. Kemball, Kevin J.; Wang, G. Geoff; Westwood, A. Richard. 2006. Are mineral soils exposed by severe wildfire better seedbeds for conifer regeneration? Canadian Journal of Forest Research. 36(8): 1943-1950. [66364]
217. Kemp, Gerald A.; Keith, Lloyd B. 1970. Dynamics and regulation of red squirrel (Tamiasciurus hudsonicus) populations. Ecology. 51(5): 763-779. [25260]
218. Kennedy, Chris. 2011. Dendroclimatology of Picea glauca at tree line in northern Labrador, Canada. St, John's, NL: Memorial University of Newfoundland. 99 p. Thesis. [87593]
219. Kershaw, G. Peter; Kershaw, Linda J. 1987. Successful plant colonizers on disturbances in tundra areas of northwestern Canada. Arctic and Alpine Research. 19(4): 451-460. [6115]
220. Kiil, A. D. 1971. Prescribed fire effects in subalpine spruce-fir slash. Inf. Rep. NOR-X-2. Edmonton, AB: Canadian Forestry Service, Northern Forest Research Centre. 30 p. [13000]
221. King, Gregory M. 2009. Factors influencing the growth of white spruce (Picea glauca) in the Mackenzie Delta, NT. Ottawa, ON: Carleton University. 146 p. Thesis. [88409]
222. Kneeshaw, Daniel D.; Bergeron, Yves. 1998. Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology. 79(3): 783-794. [28526]
223. Komarek, E. V., Sr. 1971. Principles of fire ecology and fire management in relation to the Alaskan environment. In: Slaughter, C. W.; Barney, Richard J.; Hansen, G. M., eds. Fire in the northern environment--a symposium: Proceedings; 1971 April 13-14; Fairbanks, AK. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Range and Experiment Station: 3-22. [15712]
224. Kotar, John; Kovach, Joseph A.; Locey, Craig T. 1988. Field guide to forest habitat types of northern Wisconsin. Madison, WI: University of Wisconsin, Department of Forestry; Wisconsin Department of Natural Resources. 217 p. [11510]
225. Krasny, M. E.; Vogt, K. A.; Zasada, J. C. 1984. Root and shoot biomass and mycorrhizal development of white spruce seedlings naturally regenerating in interior Alaskan floodplain communities. Canadian Journal of Forestry. 14(4): 554-558. [88145]
226. Krawchuk, M. A.; Cumming, S. G.; Flannigan, M. D.; Wein, R. W. 2006. Biotic and abiotic regulation of lightning fire initiation in the mixedwood boreal forest. Ecology. 87(2): 458-468. [61824]
227. Krebs, C. J.; LaMontagne, J. M.; Kenney, A. J.; Boutin, S. 2012. Climatic determinants of white spruce cone crops in the boreal forest of southwestern Yukon. Botany. 90(2): 113-119. [88264]
228. Krefting, Laurits W. 1974. The ecology of the Isle Royale moose with special reference to the habitat. Technical Bulletin 297--1974: Forestry Series 15. Minneapolis, MN: University of Minnesota, Agricultural Experiment Station. 75 p. [8678]
229. Kurmis, Vilis; Webb, Sara L.; Merriam, Lawrence C., Jr. 1986. Plant communities of Voyageurs National Park, Minnesota, U.S.A. Canadian Journal of Botany. 64(3): 531-540. [16088]
230. La Roi, George H. 1967. Ecological studies in the boreal spruce-fir forests of the North American taiga. I. Analysis of the vascular flora. Ecological Monographs. 37(3): 229-253. [8864]
231. La Roi, George H. 1992. Classification and ordination of southern boreal forests from the Hondo - Slave Lake area of central Alberta. Canadian Journal of Botany. 70(3): 614-628. [18702]
232. LANDFIRE Biophysical Settings. 2009. Biophysical setting 2910480: Northwestern Great Plains highland white spruce woodland. In: LANDFIRE Biophysical Setting Model: Map zone 29, [Online]. In: Vegetation Dynamics Models. In: LANDFIRE. Washington, DC: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory; U.S. Geological Survey; Arlington, VA: The Nature Conservancy (Producers). Available: http://www.landfire.gov/national_veg_models_op2.php [2015, June 8]. [88620]
233. LANDFIRE Biophysical Settings. 2009. Biophysical setting 6313730: Acadian low-elevation spruce-fir-hardwood forest. In: LANDFIRE Biophysical Setting Model: Map zone 63, [Online]. In: Vegetation Dynamics Models. In: LANDFIRE. Washington, DC: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory; U.S. Geological Survey; Arlington, VA: The Nature Conservancy (Producers). Available: http://www.landfire.gov/national_veg_models_op2.php [2015, June 8]. [88622]
234. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1). Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior. 72 p. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [66741]
235. LANDFIRE Rapid Assessment. 2007. Rapid assessment potential natural vegetation groups (PNVGs): associated vegetation descriptions and geographic distributions. Washington, DC: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; Arlington, VA: The Nature Conservancy. 84 p. [66533]
236. Landhausser, Simon M.; Wein, Ross W. 1993. Postfire vegetation recovery and tree establishment at the Arctic treeline: climate-change--vegetation response hypotheses. Journal of Ecology. 81(4): 665-672. [22741]
237. LaPerriere, Arther Joseph Louis, III. 1976. Feasibility of caribou winter habitat analysis using satellite data. Fairbanks, AK: University of Alaska. 167 p. Dissertation. [88119]
238. Larsen, C. P. S. 1997. Spatial and temporal variations in boreal forest fire frequency in northern Alberta. Journal of Biogeography. 24(5): 663-673. [29316]
239. Larsen, James A. 1965. The vegetation of Ennadai Lake area, N. W. T.: studies in subarctic and Arctic bioclimatology. Ecological Monographs. 35(1): 37-59. [87756]
240. Larsen, James A. 1980. Boreal communities and ecosystems: local variation. In: Larsen, James A., ed. The boreal ecosystem. New York: Academic Press: 281-350. [64914]
241. Lauzon, Eve; Kneeshaw, Daniel; Bergeron, Yves. 2007. Reconstruction of fire history (1680-2003) in Gaspesian mixedwood boreal forests of eastern Canada. Forest Ecology and Management. 244(1/3): 41-49. [68082]
242. Lee, Philip. 2004. The impact of burn intensity from wildfires on seed and vegetative banks, and emergent understory in aspen-dominated boreal forests. Canadian Journal of Botany. 82(10): 1468-1480. [51462]
243. Lee, Philip; Sturgess, Kelly. 2001. The effects of logs, stumps, and root throws on understory communities within 28-year-old aspen-dominated boreal forests. Canadian Journal of Botany. 79(8): 905-916. [38852]
244. Lee, Shun Ching. 1924. Factors controlling forest succession at Lake Itasca, Minnesota. Botanical Gazette. 78(2): 129-174. [41396]
245. Legare, Sonia; Bergeron, Yves; Leduc, Alain; Pare, David. 2001. Comparison of the understory vegetation in boreal forest types of southwest Quebec. Canadian Journal of Botany. 79(9): 1019-1027. [38854]
246. LeResche, R. E.; Bishop, R. H.; Coady, J. W. 1974. Distribution and habitats of moose in Alaska. Le Naturaliste Canadien. 101: 143-178. [15190]
247. LeResche, Robert E.; Davis, James L. 1973. Importance of nonbrowse foods to moose on the Kenai Peninsula, Alaska. The Journal of Wildlife Management. 37(3): 279-287. [13123]
248. Levac, Joshua. 2012. Long-term stand dynamics of the boreal mixed-wood forests of west-central Manitoba. Winnipeg, MB, University of Manitoba. 158 p. Thesis. [87588]
249. Lindsey, Alton A. 1953. Notes of some plant communities in the northern Mackenzie Basin, Canada. Botanical Gazette. 115(1): 44-55. [62728]
250. Little, Elbert L., Jr. 1953. A natural hybrid spruce in Alaska. Journal of Forestry. 51(10): 745-747. [87733]
251. Little, Elbert L., Jr. 1979. Checklist of United States trees (native and naturalized). Agric. Handb. 541. Washington, DC: U.S. Department of Agriculture, Forest Service. 375 p. [2952]
252. Little, Elbert L., Jr.; Pauley, Scott S. 1958. A natural hybrid between black and white spruce in Minnesota. The American Midland Naturalist. 60(1): 202-211. [85786]
253. Little, T. I.; Pluth, D. J.; Corns, I. G. W.; Gilmore, D. W. 2002. Post-fire forest floor development along toposequences of white spruce - trembling aspen mixedwood communities in west-central Alberta. Canadian Journal of Forest Research. 32(5): 892-902. [43234]
254. Lloret, Francisco; Zedler, Paul H. 2009. The effect of forest fire on vegetation. In: Cerda, Artemi; Robishaud, Peter R., eds. Fire effects on soils and restoration strategies. Volume 5: Land Reconstruction and Management. Enfield, NH: Science Publishers: 257-295. [81446]
255. Lloyd, Andrea H.; Duffy, Paul A.; Mann, Daniel H. 2013. Nonlinear responses of white spruce growth to climate variability in interior Alaska. Canadian Journal of Forest Research. 43(4): 331-343. [88296]
256. Lloyd, Andrea H.; Fastie, Christopher L. 2002. Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Climate Change. 52(4): 481-509. [88348]
257. Lloyd, Andrea H.; Wilson, Alexis E.; Fastie, Christopher L.; Landis, R. Matthew. 2005. Population dynamics of black spruce and white spruce near the arctic tree line in the southern Brooks Range, Alaska. Canadian Journal of Forest Research. 35(9): 2073-2081. [62408]
258. Loo, J.; Ives, N. 2003. The Acadian forest: historical condition and human impacts. The Forestry Chronicle. 79(3): 462-474. [45560]
259. Loope, Walter L. 1991. Interrelationships of fire history, land use history, and landscape pattern within Pictured Rocks National Seashore, Michigan. The Canadian Field-Naturalist. 105(1): 18-28. [5950]
260. Lorimer, Craig G. 1977. The presettlement forest and natural disturbance cycle of northeastern Maine. Ecology. 58: 139-148. [9711]
261. Loucks, O. L. 1959. A forest classification for the Maritime Provinces. In: Proceedings, Nova Scotian Institute on Science. 25(2): 86-167. [15408]
262. Lutz, H. J. 1953. The effects of forest fires on the vegetation of interior Alaska. Station Paper No. 1. Juneau, AK: U.S. Department of Agriculture, Forest Service, Alaska Forest Research Center. 36 p. [7076]
263. Lutz, H. J. 1956. Ecological effects of forest fires in the interior of Alaska. Tech. Bull. No. 1133. Washington, DC: U.S. Department of Agriculture, Forest Service. 121 p. [7653]
264. Lutz, H. J. 1956. Forest succession following fires in the Alaska interior. In: Society of American Foresters, Proceedings of symposium: 57-59. [16525]
265. Lutz, H. J. 1960. Fire as an ecological factor in the boreal forest of Alaska. Journal of Forestry. 58(6): 454-460. [16603]
266. Lynch, Jason A.; Hollis, Jeremy L.; Hu, Feng Sheng. 2004. Climatic and landscape controls of the boreal forest fire regime: Holocene records from Alaska. Journal of Ecology. 92(3): 477-489. [48477]
267. Lynch, Jason Anthony. 2001. Fire history of boreal forests: implications for past climate change. Durham, NC: Duke University. 175 p. Dissertation. [85587]
268. Magee, Dennis W.; Ahles, Harry E. 2007. Flora of the Northeast: A manual of the vascular flora of New England and adjacent New York. 2nd ed. Amherst, MA: University of Massachusetts Press. 1214 p. [74293]
269. Magoun, Audrey J.; Vernam, Donald J. 1986. An evaluation of the Bear Creek burn as marten (Martes americana) habitat in interior Alaska. Final Report: Special Project Cooperative Agreement AK-950-CAH-0. Fairbanks, AK: U.S. Department of the Interior; Alaska Deppartment of Fish and Game. 58 p. [76025]
270. Maini, J. S. 1966. Phytoecological study of sylvotundra at Small Tree Lake, N.W.T. Arctic. 19(3): 220-243. [8259]
271. Majcen, Zoran; Gagnon, Gilles; Benzie, John. 1980. Jack pine. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 8-9. [49850]
272. Mamet, Steven D.; Kershaw, G. Peter. 2012. Subarctic and alpine tree line dynamics during the last 400 years in north-western and central Canada. Journal of Biogeography. 39(5): 855-868. [88370]
273. Mann, Daniel H.; Fastie, Christopher L.; Rowland, Erika L.; Bigelow, Nancy H. 1995. Spruce succession, disturbance, and geomorphology on the Tanana River floodplain, Alaska. Ecoscience. 2(2): 184-199. [26577]
274. Mann, Daniel H.; Rupp, T. Scott; Olson, Mark A.; Duffy, Paul A. 2012. Is Alaska's boreal forest now crossing a major ecological threshold? Arctic, Antarctic, and Alpine Research. 44(3): 319-331. [88371]
275. Martin, N. D. 1959. An analysis of forest succession in Algonquin Park, Ontario. Ecological Monographs. 29(3): 187-218. [19930]
276. Martin-DeMoor, Jonathan; Lieffers, Victor J.; Macdonald, S. Ellen. 2010. Natural regeneration of white spruce in aspen-dominated boreal mixedwoods following harvesting. Canadian Journal of Forest Research. 40(3): 585-594. [81901]
277. Maycock, Paul F. 1961. The spruce-fir forests of the Keweenaw Peninsula, northern Michigan. Ecology. 42(2): 357-365. [62688]
278. McCarthy, John W.; Weetman, Gordon. 2006. Age and size structure of gap-dynamic, old-growth boreal forest stands in Newfoundland. Silva Fennica. 40(2): 209-230. [64046]
279. McCoy, V. M.; Burn, C. R. 2005. Potential alteration by climate change of the forest-fire regime in the boreal forest of central Yukon Territory. Arctic. 58(3): 276-285. [61211]
280. McCullough, Deborah G.; Werner, Richard A.; Neumann, David. 1998. Fire and insects in northern and boreal forest ecosystems of North America. Annual Review of Entomology. 43: 107-127. [30374]
281. McGuire, A. David; Ruess, Roger W.; Lloyd, A.; Yarie, J.; Clein, Joy S.; Juday, Glenn P. 2010. Vulnerability of white spruce tree growth in interior Alaska in response to climate variability: dendrochronological, demographic, and experimental perspectives. Canadian Journal of Forest Research. 40(7): 1197-1209. [88294]
282. McInnes, Pamela F.; Naiman, Robert J.; Pastor, John; Cohen, Yosef. 1992. Effects of moose browsing on vegetation and litter of the boreal forest, Isle Royale, Michigan, USA. Ecology. 73(6): 2059-2075. [18427]
283. McLeod, T. Katherine. 2002. The ecology of Picea glauca (Moench) Voss at its range limits in northwest Canada. Vancouver, BC: The University of British Columbia. 198 p. Dissertation. [88411]
284. McMinn, R. G. 1982. Ecology of site preparation to improve performance of planted white spruce in northern latitudes. In: Murray, Mayo, ed. Proceedings, Forest regeneration at high latitudes: experiences from northern British Columbia; 1981 August 29 - September 1; Prince George, BC. Misc. Report No. 82-1. Fairbanks, AK: University of Alaska, School of Agriculture and Land Resources; Portland, OR: U.S. Dept. of Agriculture, Forest Service., Pacific Northwest Forestry and Range Experiment Station: 25-32. [7940]
285. Meidinger, Del; Pojar, Jim. 1991. Ecosystems of British Columbia. Special Report Series 6. Victoria, BC: British Columbia Ministry of Forests. 330 p. [80264]
286. Meyer, Carolyn B.; Knight, Dennis H.; Dillon, Gregory K. 2005. Historic range of variability for the upland vegetation in the Bighorn National Forest, Wyoming. Gen. Tech. Rep. RMRS-GTR-140. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 94 p. [60766]
287. Michaletz, S. T.; Johnson, E. A.; Meil, W. E.; Greene, D. F. 2012. Timing of fire relative to seed development controls availability of non-serotinous aerial seed banks. Biogeosciences. 9(11): 16705-16751. [87801]
288. Michaletz, S. T.; Johnson, E. A.; Mell, W. E.; Greene, D. F. 2013. Timing for fire relative to seed development may enable non-serotinous species to recolonize from the aerial seed banks of fire-killed trees. Biogeosciences. 10(7): 5061-5078. [87583]
289. Miquelle, Dale G.; Van Ballenberghe, Victor. 1989. Impact of bark stripping by moose on aspen-spruce communities. The Journal of Wildlife Management. 53(3): 577-586. [8911]
290. Miyanishi, K.; Johnson, E. A. 2002. Process and patterns of duff consumption in the mixedwood boreal forest. Canadian Journal of Forest Research. 32(7): 1285-1295. [42238]
291. Montgomery, James M. 1976. Forest harvest, residue treatment, reforestation and protection of water quality. EPA 910/9-76-020. Washington, DC: U.S. Environmental Protection Agency, Region 10. 273 p. [16264]
292. Moore, T. R. 1980. The nutrient status of subarctic woodland soils. Arctic and Alpine Research. 12(2): 147-160. [51702]
293. Morgantini, Luigi E.; Kansas, John L. 2003. Differentiating mature and old-growth forests in the upper foothills and subalpine subregions of west-central Alberta. The Forestry Chronicle. 79(3): 602-612. [46038]
294. Morissette, J. L.; Cobb, T. P.; Brigham, R. M.; James, P. C. 2002. The response of boreal forest songbird communities to fire and post-fire harvesting. Canadian Journal of Forest Research. 32(12): 2169-2183. [43889]
295. Murphy, Edward C.; Lehnhausen, William A. 1998. Density and foraging ecology of woodpeckers following a stand replacement fire. The Journal of Wildlife Management. 62(4): 1359-1372. [30131]
296. Nadeau, L. B.; Corns, I. G. W. 2002. Post-fire vegetation of the montane natural subregion of Jasper National Park. Forest Ecology and Management. 163: 165-183. [41546]
297. Nash, C. H.; Johnson, E. A. 1996. Synoptic climatology of lightning-caused forest fires in subalpine and boreal forests. Canadian Journal of Forest Research. 26(10): 1859-1874. [26984]
298. National Wildfire Coordinating Group. 1992. Fire behavior field reference guide. Boise, ID: National Wildfire Coordinating Group. 87 p. [88097]
299. Natural Resources Canada. 2014. Fire behaviour, [Online]. In: Forests, forest topics, fire. Natural Resources Canada (Producer) Available: http://www.nrcan.gc.ca/forests/fire/13145 [2015 June 12]. [88612]
300. NatureServe. 2013. International Ecological Classification Standard: Terrestrial Ecological Classifications of the Untied States and Canada. In: NatureServe Central Databases. Arlington, VA, (Producer). 1530 p. Available: http://explorer.natureserve.org/servlet/NatureServe?init=Ecol [2015, July 14]. [89169]
301. NatureServe. 2015. International ecological classification standard: terrestrial ecological classifications, [Online]. In: NatureServe Central Databases. Arlington, VA: NatureServe (Producer). Available: http://explorer.natureserve.org/servlet/NatureServe?init=Ecol [2015, May 1]. [87721]
302. Navratil, S.; Brace, L. G.; Sauder, E. A.; Lux, S. 1994. Silvicultural and harvesting options to favor immature white spruce and aspen regeneration in boreal mixedwoods. Information Report NOR-X-337. Edmonton, AB: Natural Resources Canada, Canadian Forest Service, Northwest Region, Northern Forestry Centre. 78 p. [24465]
303. Neiland, Bonita J.; Viereck, Leslie A. 1977. Forest types and ecosystems. In: North American forest lands at latitudes north of 60 degrees: Proceedings of a symposium; 1977 September 19-22; Fairbanks, AK. Fairbanks, AK: University of Alaska, Fairbanks: 109-136. [19933]
304. Nienstaedt, Hans. 1965. White spruce (Picea glauca (Moench) Voss). In: Fowells, H. A., comp. Silvics of forest trees of the United States. Agriculture Handbook 271. Washington, DC: U.S. Department of Agriculture, Forest Service, Division of Timber Management Research: 318-327. [88421]
305. Nienstaedt, Hans; Zasada, John C. 1990. Picea glauca (Moench) Voss white spruce. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service, Division of Timber Management Research: 204-226. [13385]
306. O'Neill, Katherine P.; Kasischke, Eric S.; Richter, Daniel D. 2002. Environmental controls on soil CO2 flux following fire in black spruce, white spruce, and aspen stands of interior Alaska. Canadian Journal of Forest Research. 32(9): 1525-1541. [43116]
307. Ohmann, Lewis F.; Cushwa, Charles T.; Lake, Roger E.; Beer, James R.; Brander, Robert B. 1973. Wilderness ecology: the upland plant communities, woody browse production, and small mammals of two adjacent 33-year-old wildfire areas in northeastern Minnesota. Gen. Tech. Rep. NC-7. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 30 p. [82873]
308. Ohmann, Lewis F.; Grigal, David F. 1979. Early revegetation and nutrient dynamics following the 1971 Little Sioux Forest Fire in northeastern Minnesota. Forest Science Monograph 21. Bethesda, MD: Society of American Foresters. 80 p. [6992]
309. Ohmann, Lewis F.; Ream, Robert R. 1971. Wilderness ecology: virgin plant communities of the Boundary Waters Canoe Area. Res. Pap. NC-63. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 55 p. [9271]
310. Oldemeyer, John L.; Regelin, Wayne L. 1987. Forest succession, habitat management, and moose on the Kenai National Wildlife Refuge. Swedish Wildlife Research. Supplement 1(Part 1): 163-179. [79205]
311. Osiowy, Robert James. 2012. Fuel load and fire behaviour in montane riparian white spruce forests of Banff National Park; strategies for community protection and ecosystem sustainability. Victoria, BC: Royal Roads University. 74 p. Thesis. [88414]
312. Oswald, E. T.; Brown, B. N. 1990. Vegetation establishment during 5 years following wildfire in northern British Columbia and southern Yukon Territory. Information Report BC-X-320. Victoria, BC: Forestry Canada, Pacific and Yukon Region, Pacific Forestry Centre. 46 p. [16934]
313. Oswald, E. T.; Senyk, J. P. 1977. Ecoregions of Yukon Territory. Victoria, BC: Canadian Forestry Service, Pacific Forest Research Centre. 115 p. [86568]
314. Parish, Roberta; Thomson, Sandra. 1948. White spruce. In: Tree book: learning to recognize trees of British Columbia. Canada-British Columbia Partnership Agreement on Forest Resouce Development: FRDA II. Victoria, BC: Canadian Forest Service: 60-64. [88746]
315. Parisien, Marc-Andre; Sirois, Luc. 2003. Distribution and dynamics of tree species across a fire frequency gradient in the James Bay region of Quebec. Canadian Journal of Forest Research. 33(2): 243-256. [44221]
316. Park, Andrew; Kneeshaw, Daniel; Bergeron, Yves; Leduc, Alain. 2005. Spatial relationships and tree species associations across a 236-year boreal mixedwood chronosequence. Canadian Journal of Forest Research. 35(3): 750-761. [64109]
317. Pastor, John; Naiman, Robert J.; Dewey, Bradley; McInnes, Pamela. 1988. Moose, microbes, and the boreal forest. BioScience. 38(11): 770-777. [10228]
318. Payette, Serge. 1980. White spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 15. [49856]
319. Payette, Serge. 2007. Contrasted dynamics of northern Labrador tree lines caused by climate change and migrational lag. Ecology. 88(3): 770-780. [88373]
320. Payette, Serge; Boudreau, Stephane; Morneau, Claude; Pitre, Nadia. 2004. Long-term interactions between migratory caribou, wildfires and Nunavik hunters inferred from tree rings. Ambio. 33(8): 482-486. [61106]
321. Pearce, C. M.; McLennan, D.; Cordes, L. D. 1988. The evolution and maintenance of white spruce woodlands on the Mackenzie Delta, N. W. T., Canada. Holarctic Ecology. 11(4): 248-258. [10472]
322. Pech, Gyula. 1993. Fire hazard in budworm-killed balsam fir stands on Cape Breton Highlands. Forestry Chronicle. 69(2): 178-186. [21397]
323. Peck, V. Ross; Peek, James M. 1991. Elk, Cervus elaphus, habitat use related to prescribed fire, Tuchodi River, British Columbia. The Canadian Field-Naturalist. 105(3): 354-362. [18204]
324. Peek, James M. 2007. Habitat relationships. In: Franzmann, Albert W.; Schwartz, Charles C.; McCabe, Richard E., eds. Ecology and management of the North American moose. 2nd ed. Boulder, CO: University Press of Colorado: 351-376. [79104]
325. Peltzer, Duane A.; Bast, Marcy L.; Wilson, Scott D.; Gerry, Ann K. 2000. Plant diversity and tree responses following contrasting disturbances in boreal forest. Forest Ecology and Management. 127(1-3): 191-203. [48541]
326. Peters, Susan; Boutin, Stan; Macdonald, Ellen. 2003. Pre-dispersal seed predation of white spruce cones in logged boreal mixedwood forest. Canadian Journal of Forest Research. 33(1): 33-40. [43905]
327. Peters, Vernon S.; Macdonald, S. Ellen; Dale, Mark R. T. 2002. Aging discrepancies of white spruce affect the interpretation of age structure in boreal mixedwoods. Canadian Journal of Forest Research. 32(8): 1496-1501. [47485]
328. Peters, Vernon S.; MacDonald, S. Ellen; Dale, Mark R. T. 2005. The interaction between masting and fire is key to white spruce regeneration. Ecology. 86(7): 1744-1750. [55520]
329. Peters, Vernon S.; Macdonald, S. Ellen; Dale, Mark R. T. 2006. Patterns of initial versus delayed regeneration of white spruce in boreal mixedwood succession. Canadian Journal of Forest Research. 36(6): 1597-1609. [63797]
330. Peters, Vernon Scott. 2003. Keystone processes affect succession in boreal mixedwoods - the relationship between masting in white spruce and fire history. Edmonton, AB: University of Alberta. 203 p. Dissertation. [88478]
331. Pfister, Robert D.; Kovalchik, Bernard L.; Arno, Stephen F.; Presby, Richard C. 1977. Forest habitat types of Montana. Gen. Tech. Rep. INT-34. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 174 p. [1878]
332. Pojar, J.; Trowbridge, R.; Coates, D. 1984. Ecosystem classification and interpretation of the sub-boreal spruce zone, Prince Rupert forest region, British Columbia. Land Management Report No. 17. Victoria, BC: Province of British Columbia, Ministry of Forests. 319 p. [6929]
333. Potkin, Michele. 1997. Fire history disturbance study of the Kenai Peninsula mountainous portion of the Chugach National Forest. Unpublished report on file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 75 p. [87954]
334. Potvin, Francois; Beaupre, Pierre; Laprise, Gaetan. 2003. The eradication of balsam fir stands by white-tailed deer on Anticosti Island, Quebec: a 150-year process. Ecoscience. 10(4): 487-495. [88737]
335. Price, David T.; Alfaro, R. I.; Brown, K. J.; Flannigan, M. D.; Fleming, R. A.; Hogg, E. H.; Girardin, M. P.; Lakusta, T.; Johnston, M.; McKenney, D. W.; Pedlar, J. H.; Stratton, T.; Sturrock, R. N.; Thompson, I. D.; Trofymow, J. A.; Venier, L. A. 2013. Anticipating the consequences of climate change for Canada's boreal forest ecosystems. Environmental Review. 21(4): 322-365. [88624]
336. Purdy, Brett G.; Macdonald, S. Ellen; Dale, Mark R. T. 2002. The regeneration niche of white spruce following fire in the mixed-wood boreal forest. Silva Fennica. 36(1): 289-306. [46072]
337. Quirk, William A.; Sykes, Dwane J. 1971. White spruce stringers in a fire-patterned landscape in interior Alaska. In: Slaughter, C. W.; Barney, Richard J.; Hansen, G. M., eds. Fire in the northern environment--a symposium: Proceedings; 1971 April 13-14; Fairbanks, AK. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Range and Experiment Station: 179-197. [15728]
338. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford, England: Clarendon Press. 632 p. [2843]
339. Rees, Daniel C.; Juday, Glenn Patrick. 2002. Plant species diversity on logged versus burned sites in central Alaska. Forest Ecology and Management. 155(1-3): 291-302. [40745]
340. Regelin, Wayne L.; Schwartz, Charles C.; Franzmann, Albert W. 1987. Effects of forest succession on nutritional dynamics of moose forage. In: Viltrevy: Swedish wildlife research: Proceedings, 2nd international moose symposium. Supplement 1--Part 1: 247-264. [79488]
341. Renecker, Lyle A.; Schwartz, Charles C. 2007. Food habits and feeding behavior. In: Franzmann, Albert W.; Schwartz, Charles C.; McCabe, Richard E., eds. Ecology and management of the North American moose. 2nd ed. Boulder, CO: University Press of Colorado: 403-440. [79106]
342. Reschke, Carol. 1990. Ecological communities of New York State. Latham, NY: New York State Department of Environmental Conservation, Natural Heritage Program. 96 p. [21441]
343. Rich, Roy L.; Frelich, Lee E.; Reich, Peter B. 2007. Wind-throw mortality in the southern boreal forest: effects of species, diameter and stand age. Journal of Ecology. 95(6): 1261-1273. [85833]
344. Ritchie, J. C. 1960. The vegetation of northern Manitoba. Canadian Journal of Botany. 38(5): 769-788. [55687]
345. Robert, Emilie; Brais, Suzanne; Harvey, Brian D.; Greene, David. 2012. Seedling establishment and survival on decaying logs in boreal mixedwood stands following a mast year. Canadian Journal of Forest Research. 42(8): 1446-1455. [87531]
346. Roche, L. 1969. A genecological study of the genus Picea in British Columbia. New Phytologist. 68(2): 505-554. [87755]
347. Rogers, B. M.; Veraverbeke, S.; Azzari, G.; Czimczik, C. I.; Holden, S. R.; Mouteva, G. O.; Sedano, F.; Treseder, K. K.; Randerson, J. T. 2014. Quantifying fire-wide carbon emissions in interior Alaska using field measurements and Landsat imagery. Journal of Geophysical Research: Biogeosciences. 19(8): 1608-1629. [88345]
348. Roland, Carl A.; Schmidt, Joshua H.; Johnstone, Jill F. 2014. Climate sensitivity of reproduction in a mast-seeding boreal conifer across its distributional range from lowland to treeline forests. Oecologia. 174(3): 665-677. [89369]
349. Roland, Carl A.; Schmidt, Joshua H.; Nicklen, E. Fleur. 2013. Landscape-scale patterns in tree occupancy and abundance in subarctic Alaska. Ecological Monographs. 83(1): 19-48. [88094]
350. Ross, Darrell W.; Daterman, Gary E.; Boughton, Jerry L.; Quigley, Thomas M. 2001. Forest health restoration in south-central Alaska: a problem analysis. Gen. Tech. Rep. PNW-GTR-523. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 38 p. [87689]
351. Rossi, Sergio; Morin, Hubert; Gionest, Francois; Laprise, Danielle. 2012. Episodic recruitment of the seedling banks in balsam fir and white spruce. American Journal of Botany. 99(12): 1942-1950. [87585]
352. Rossi, Sergio; Morin, Hubert; Laprise, Danielle; Gionest, Francois. 2012. Testing masting mechanisms of boreal forest species at different stand densities. Oikos. 121(5): 665-674. [87584]
353. Rossi, Sergio; Tremblay, Marie-Josee; Morin, Hubert; Levasseur, Valerie. 2009. Stand structure and dynamics of Picea mariana on the northern border of the natural closed boreal forest in Quebec, Canada. Canadian Journal of Forest Research. 39(12): 2307-2318. [82196]
354. Rowe, J. S. 1953. Delayed germination of white spruce seed on burned ground. Project MS-159. Silvicultural Leaflet No. 84. Ottawa, ON: Canadian Department of Resources and Development, Forestry Branch, Division of Forest Research. 3 p. [41818]
355. Rowe, J. S. 1961. Critique of some vegetational concepts as applied to forests of northwestern Alberta. Canadian Journal of Botany. 39(5): 1007-1017. [6468]
356. Rowe, J. S. 1971. Spruce and fire in northwest Canada and Alaska. In: Proceedings, annual Tall Timbers fire ecology conference; 1970 August 20-21; Fredericton, NB, Canada. No. 10. Tallahassee, FL: Tall Timbers Research Station: 245-254. [12895]
357. Rowe, J. S. 1972. Forest regions of Canada. Publication No. 1300. Ottawa,ON: Canadian Forestry Service, Department of the Environment. 172 p. [88103]
358. Rowe, J. S.; Scotter, G. W. 1973. Fire in the boreal forest. Quaternary Research. 3(3): 444-464. [72]
359. Rudolf, Paul O. 1980. Black ash-American elm-red maple--Forest Cover Type 39. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 37-38. [49904]
360. Rupp, T. Scott; Chapin, F. Stuart, III; Starfield, Anthony M. 2000. Response of subarctic vegetation to transient climatic change on the Seward Peninsula in north-west Alaska. Global Change Biology. 6(5): 541-555. [85482]
361. Russell, W. B. 1985. Vascular flora of abandoned coal-mined land, Rocky Mountain Foothills, Alberta. The Canadian Field-Naturalist. 99(4): 503-516. [10461]
362. Safford, L. O. 1980. Paper birch. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 18. [49860]
363. Scheller, Robert M.; Mladenoff, David J.; Crow, Thomas R.; Sickley, Theodore A. 2005. Simulating the effects of fire reintroduction versus continued fire absence on forest composition and landscape structure in the Boundary Water Canoe Area, northern Minnesota, U.S.A. Ecosystems. 8(4): 396-411. [54788]
364. Schieck, Jim; Song, Samantha J. 2006. Changes in bird communities throughout succession following fire and harvest in boreal forests of western North America: literature review and meta-analyses. Canadian Journal of Forest Research. 36(5): 1299-1318. [64262]
365. Schmalholz, Martin; Hylander, Kristoffer; Frego, Katherine. 2011. Bryophyte species richness and composition in young forests regenerated after clear-cut logging versus after wildfire and spruce budworm outbreak. Biodiversity and Conservation. 20(12): 2575-2596. [86098]
366. Schulz, Bethany. 1995. Changes over time in fuel-loading associated with spruce beetle-impacted stands of the Kenai Peninsula, Alaska. Tech. Rep. R10-TO-53. Anchorage, AK: U.S. Department of Agriculture, Forest Service, Forest Health Management. 17 p. [88608]
367. Schulz, Bethany. 2003. Changes in downed and dead woody material following a spruce beetle outbreak on the Kenai Peninsula, Alaska. Res. Pap. PNW-RP-559. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 9 p. [48566]
368. Schwartz, Charles C.; Franzmann, Albert W. 1991. Interrelationship of black bears to moose and forest succession in the northern coniferous forest. Wildlife Monographs, No. 113. Washington, DC: The Wildlife Society. 58 p. [67724]
369. Scoggan, H. J. 1978. The flora of Canada. Part 2: Pteridophyta, Gymnospermae, Monocotyledoneae. National Museum of Natural Sciences: Publications in Botany, No. 7(2). Ottawa, ON: National Museums of Canada. 545 p. [75494]
370. Scotter, George W. 1971. Fire, vegetation, soil, and barren-ground caribou relations in northern Canada. In: Slaughter, C. W.; Barney, Richard J.; Hansen, G. M., eds. Fire in the northern environment--a symposium: Proceedings; 1971 April 13-14; Fairbanks, AK. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Range and Experiment Station: 209-230. [15730]
371. Senecal, Dominic; Kneeshaw, Daniel; Messier, Christian. 2004. Temporal, spatial, and structural patterns of adult trembling aspen and white spruce mortality in Quebec's boreal forest. Canadian Journal of Forest Research. 34(2): 396-404. [60713]
372. Severson, Kieth E.; Thilenius, John F. 1976. Classification of quaking aspen stands in the Black Hills and Bear Lodge Mountains. Res. Pap. RM-166. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 24 p. [2111]
373. Sherriff, R. L.; Berg, E. E.; Miller, A. E. 2011. Climate variability and spruce beetle (Dendroctonus rufipennis) outbreaks in south-central and southwest Alaska. Ecology. 92(7): 1459-1470. [87589]
374. Shirley, Hardy L. 1945. Reproduction of upland conifers in the Lake States as affected by root competition and light. The American Midland Naturalist. 33(3): 537-612. [10367]
375. Shorohava, Ekaterina; Kneeshaw, Daniel; Kuuluvainen, Timo; Gauthier, Sylvie. 2011. Variability and dynamics of old-growth forests in the circumboreal zone: implications for conservation, restoration and management. Silva Fennica. 45(5): 785-806. [84907]
376. Sieg, Carolyn Hull; Severson, Kieth E. 1996. Managing habitats for white-tailed deer: Black Hills and Bear Lodge Mountains of South Dakota and Wyoming. Gen. Tech. Rep. RM-GTR-274. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 24 p. [86436]
377. Simard, Marie-Josee; Bergeron, Yves; Sirois, Luc. 1998. Conifer seedling recruitment in a southeastern Canadian boreal forest: the importance of substrate. Journal of Vegetation Science. 9(4): 575-582. [88480]
378. Simard, Marie-Josee; Bergeron, Yves; Sirois, Luc. 2003. Substrate and litterfall effects on conifer seedling survivorship in southern boreal stands of Canada. Canadian Journal of Forest Research. 33(4): 672-681. [44602]
379. Sinclair, A. R. E.; Smith, J. N. M. 1984. Do plant secondary compounds determine feeding preferences of snowshoe hares? Oecologia. 61(3): 403-410. [88175]
380. Skoog, Ronald Oliver. 1968. Ecology of the caribou (Rangifer tarandus granti) in Alaska. Berkeley, CA: University of California, Berkeley. 699 p. Dissertation. [37914]
381. Smith, Michael C. 1968. Red squirrel responses to spruce cone failure in interior Alaska. The Journal of Wildlife Management. 32(2): 305-317. [87599]
382. Spencer, David L.; Hakala, John B. 1964. Moose and fire on the Kenai. In: Proceedings, 3rd annual Tall Timbers fire ecology conference; 1964 April 9-10; Tallahassee, FL. Tallahassee, FL: Tall Timbers Research Station: 10-33. [5970]
383. Stearns, Forest; Likens, Gene E. 2002. One hundred years of recovery of a pine forest in northern Wisconsin. American Midland Naturalist. 148(1): 2-19. [43566]
384. Stewart, James D.; Hogg, Edward H.; Hurdle, Patrick A.; Stadt, Kenneth J.; Tollestrup, Peter; Lieffers, Victor J. 1998. Dispersal of white spruce seed in mature aspen stands. Canadian Journal of Botany. 76(2): 181-188. [28637]
385. Stewart, James D.; Landhausser, Simon M.; Stadt, Kenneth J.; Lieffers, Victor J. 2000. Regeneration of white spruce under aspen conopies: seeding, planting, and site preparation. Western Journal of Applied Forestry. 15(4): 177-182. [36429]
386. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
387. Stocks, B. J. 1987. Fire potential in the spruce budworm-damaged forests of Ontario. The Forestry Chronicle. 63(1): 8-14. [12369]
388. Stocks, B. J.; Mason, J. A.; Todd, J. B.; Bosch, E. M.; Wotton, B. M.; Amiro, B. D.; Flannigan, M. D.; Hirsch, K. G.; Logan, K. A.; Martell, D. L.; Skinner, W. R. 2003. Large forest fires in Canada, 1959-1997. Journal of Geophysical Research. 107(D1): doi:10.1029/2001JD000484. [88611]
389. Strang, R. M. 1973. Succession in unburned subarctic woodlands. Canadian Journal of Forest Research. 3(1): 140-143. [7889]
390. Streubel, Donald Paul. 1968. Food storing and related behavior of red squirrels (Tamiasciurus hudsonicus) in interior Alaska. Fairbanks, AK: University of Alaska. 56 p. Thesis. [88437]
391. Strong, W. L. 2009. Populus tremuloides Michx. postfire stand dynamics in the northern boreal-cordilleran ecoclimatic region of central Yukon Territory, Canada. Forest Ecology and Management. 258(7): 1110-1120. [77258]
392. Sturtevant, Brian R.; Miranda, Brian R.; Shinneman, Douglas J.; Gustafson, Eric J.; Wolter, Peter T. 2012. Comparing modern and presettlement forest dynamics of a subboreal wilderness: does spruce budworm enhance fire risk? Ecological Applications. 22(4): 1278-1296. [85842]
393. Suarez, Frank; Binkely, Dan; Kaye, Margot W. 1999. Expansion of forest stands into tundra in the Noatak National Preserve, northwest Alaska. Ecoscience. 6(3): 465-470. [38930]
394. Sutton, R. F. 1969. Silvics of white spruce. Forestry Branch Publ. No. 1250. Ottawa, ON: Department of Fisheries and Forestry. 57 p. [13676]
395. Sweeney, Jon; Quiring, Dan T. 1998. Oviposition site selection and intraspecific competition influence larval survival and pupal weight of Strobilomyia neanthracina (Diptera: Anthomyiidae) in white spruce. Ecoscience. 5(4): 454-462. [87600]
396. Szeicz, Julian M.; MacDonald, Glen M. 1995. Recent white spruce dynamics at the subarctic alpine treeline of north-western Canada. Journal of Ecology. 83(5): 873-885. [25938]
397. Terrier, Aurelie; Girardin, Martin P.; Perie, Catherine; Legendre, Pierre; Bergeron, Yves. 2013. Potential changes in forest composition could reduce impacts of climate change on boreal wildfires. Ecological Applications. 23(1): 21-35. [88406]
398. Theberge, John B. 1976. Bird populations in the Kluane Mountains, southwest Yukon, with special reference to vegetation and fire. Canadian Journal of Zoology. 54(8): 1346-1356. [41684]
399. Thilenius, John F. 1972. Classification of deer habitat in the ponderosa pine forest of the Black Hills, South Dakota. Res. Pap. RM-91. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 28 p. [2317]
400. Thomas, D. C.; Barry, S. J.; Alaie, G. 1996. Fire - caribou - winter range relationships in northern Canada. Proceedings, 2nd international arctic ungulate conference; August 13-17; Fairbanks, AK. In: Rangifer. 16(2): 57-67. [28961]
401. Thompson, Robert S.; Anderson, Katherine H.; Bartlein, Patrick J. 1999. Digital representations of tree species range maps from "Atlas of United States trees" by Elbert L. Little, Jr. (and other publications), [Online]. In: Atlas of relations between climatic parameters and distributions of important trees and shrubs in North America. Denver, CO: U.S. Geological Survey, Information Services (Producer). Available: esp.cr.usgs.gov/data/little/ [2015, May 12]. [82831]
402. Thompson, William H.; Hansen, Paul H. 2001. Classification and management of riparian and wetland sites of the Saskatchewan prairie ecozone and parts of adjacent subregions. Regina, SK: Saskatchewan Wetland Conservation Corporation. 298 p. [82588]
403. Thompson, William H.; Hansen, Paul L. 2003. Classification and management of riparian and wetland sites of Alberta's Parkland Natural Region and Dry Mixedwood Natural Subregion. Cows and Fish Report No. 020. Lethbridge, AB: Alberta Riparian Habitat Management Program, Cows and Fish. 340 p. [82586]
404. Timoney, Kevin P.; Peterson, George; Wein, Ross. 1997. Vegetation development of boreal riparian plant communities after fire, flooding, and logging, Peace River, Canada. Forest Ecology and Management. 93(1-2): 101-120. [27427]
405. Todd, Susan K.; Jewkes, Holly Ann. 2006. Wildland fire in Alaska: a history of organized fire suppression and management in the last frontier. Bulletin No. 114. Fairbanks, AK: University of Alaska Fairbanks, Agricultural and Forestry Experiment Station. 63 p. [85899]
406. Treter, U. 1995. Fire-induced succession of lichen-spruce woodland in central Labrador-Ungava, Canada. Phytocoenologia. 25(2): 161-183. [66303]
407. Tucker, R. E.; Jarvis, J. M. 1967. Prescribed burning in a white spruce--trembling aspen stand in Manitoba. Woodlands Review. July: 2-4. [3641]
408. Tymstra, Cordy; Flannigan, Mike D.; Armitage, Owen B.; Logan, Kimberley. 2007. Impact of climate change on area burned in Alberta's boreal forest. International Journal of Wildland Fire. 16(2): 153-160. [85900]
409. U.S. Department of Agriculture, Natural Resources Conservation Service. 2015. PLANTS Database, [Online]. Available: https://plants.usda.gov /. [34262]
410. U.S. Department of the Interior. 1982. Alaska Interagency Fire Management Plan: Tanana/Minchumina Planning Area. Environmental Assessment: Final. Anchorage, AK: U.S. Department of the Interior. 148 p. [21538]
411. Urban, S. T.; Lieffers, V. J.; Macdonald, S. E. 1994. Release in radial growth in the trunk and structural roots of white spruce as measured by dendrochronology. Canadian Journal of Forest Research. 24(8): 1550-1556. [24460]
412. Van Ballenberghe, Victor. 1992. Behavioral adaptations of moose to treeline habitats in subarctic Alaska. Alces. Supplement 1: 193-206. [78811]
413. Van Cleve, K.; Chapin, F. S., III; Dyrness, C. T.; Viereck, L. A. 1991. Element cycling in taiga forests: state-factor control. Bioscience. 41(2): 78-88. [13192]
414. Van Cleve, K.; Dyrness, C. T.; Viereck, L. A.; Fox, J.; Chapin, F. S., III; Oechel, W. 1983. Taiga ecosystems in interior Alaska. BioScience. 33(1): 39-44. [7884]
415. Van Cleve, K.; Viereck, L. A.; Dyrness, C. T. 1996. State factor control of soils and forest succession along the Tanana River in interior Alaska, U.S.A. Arctic and Alpine Research. 28(3): 388-400. [65672]
416. Van Cleve, Keith; Dyrness, C. T. 1983. Introduction and overview of a multidisciplinary research project: the structure and function of a black spruce (Picea mariana) forest in relation to other fire-affected taiga ecosystems. Canadian Journal of Forest Research. 13(5): 695-702. [7883]
417. Van Cleve, Keith; Dyrness, C. Theodore. 1985. The effect of the Rosie Creek Fire on soil fertility. In: Juday, Glenn P.; Dyrness, Theodore C., eds. Early results of the Rosie Creek Fire Research Project, 1984. Misc. Pub. 85-2. Fairbanks, AK: University of Alaska, Agriculture and Forest Experiment Station: 7-11. [88105]
418. Van Cleve, Keith; Viereck, Leslie A. 1981. Forest succession in relation to nutrient cycling in the boreal forest of Alaska. In: Fire and succession in conifer forests of North America. New York: Springer-Verlag: 185-211. [50633]
419. Van Wagner, C. E. 1963. Prescribed burning experiments: Red and white pine. Publ. No. 1020. Ottawa, ON: Department of Forestry, Forest Research Branch. 27 p. [13642]
420. Van Wagner, C. E. 1967. Seasonal variation in moisture content of eastern Canadian tree foliage and the possible effect on crown fires. Departmental Publ. No. 1204. Ottawa, ON: Department of Forestry and Rural Development, Forestry Branch. 15 p. [15404]
421. Vanderlinden, Larry A. 1996. Applying stand replacement prescribed fire in Alaska. In: Hardy, Colin C.; Arno, Stephen F., eds. The use of fire in forest restoration: A general session at the annual meeting of the Society for Ecological Restoration; 1995 September 14-16; Seattle, WA. Gen. Tech. Rep. INT-GTR-341. U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 78-80. [28684]
422. Vepakomma, Udayalakshmi; Kneeshaw, Daniel; St-Onge, Benoit. 2010. Interactions of multiple disturbances in shaping boreal forest dynamics: a spatially explicit analysis using multi-temporal lidar data and high-resolution imagery. Journal of Ecology. 98(3): 526-539. [81374]
423. Vernam, Donald J. 1987. Marten habitat use in the Bear Creek Burn, Alaska. Fairbanks, AK: University of Alaska. 72 p. Thesis. [76940]
424. Viereck, L. A.; Dyrness, C. T., tech. eds. 1979. Ecological effects of the Wickersham Dome fire near Fairbanks, Alaska. Gen. Tech. Rep. PNW-90. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 71 p. [6392]
425. Viereck, L. A.; Dyrness, C. T.; Batten, A. R.; Wenzlick, K. J. 1992. The Alaska vegetation classification. Gen. Tech. Rep. PNW-GTR-286. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 278 p. [2431]
426. Viereck, L. A.; Dyrness, C. T.; Foote, M. J. 1993. An overview of the vegetation and soils of the floodplain ecosystems of the Tanana River, interior Alaska. Canadian Journal of Forest Research. 23(5): 889-898. [21887]
427. Viereck, L. A.; Foote, M. J. 1979. Abiotic factors: the soil--Effect of burning on soil temperature. In: Viereck, L. A.; Dyrness, C. T., tech. eds. Ecological effects of the Wickersham Dome fire near Fairbanks, Alaska. Gen. Tech. Rep. PNW-90. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: 14-17. [85956]
428. Viereck, L. A.; Van Cleve, K.; Dyrness, C. T. 1986. Forest ecosystem distribution in the taiga environment. In: Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Vol. 57. New York: Springer-Verlag: 22-43. [85917]
429. Viereck, Leslie A. 1970. Forest succession and soil development adjacent to the Chena River in interior Alaska. Arctic and Alpine Research. 2(1): 1-26. [12466]
430. Viereck, Leslie A. 1973. Wildfire in the taiga of Alaska. Quaternary Research. 3(3): 465-495. [7247]
431. Viereck, Leslie A. 1975. Forest ecology of the Alaska taiga. In: Proceedings of the circumpolar conference on northern ecology; 1975 September 15-18; Ottawa, ON. Fairbanks, AK: U.S. Forest Service, Department of Agriculture, Pacific Northwest Forest and Range Experiment Station. Supplement: 22 p. [7315]
432. Viereck, Leslie A. 1979. Characteristics of treeline plant communities in Alaska. Holarctic Ecology. 2(4): 228-238. [8251]
433. Viereck, Leslie A. 1980. Black spruce-white spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 84-85. [50019]
434. Viereck, Leslie A.; Dyrness, C. T.; Van Cleve, Keith; Foote, M. Joan. 1983. Vegetation, soils, and forest productivity in selected forest types in interior Alaska. Canadian Journal of Forest Research. 13(5): 703-720. [88791]
435. Viereck, Leslie A.; Little, Elbert L., Jr. 1972. Alaska trees and shrubs. Agric. Handb. 410. Washington, DC: U.S. Department of Agriculture, Forest Service. 265 p. [6884]
436. Viereck, Leslie A.; Schandelmeier, Linda A. 1980. Effects of fire in Alaska and adjacent Canada: a literature review. BLM-Alaska Tech. Rep. 6, BLM/AK/TR-80/06. Anchorage, AK: U.S. Department of the Interior, Bureau of Land Management, Alaska State Office. 124 p. [28862]
437. Volney, W. Jan A.; Hirsch, Kelvin G. 2005. Disturbing forest disturbances. The Forestry Chronicle. 81(5): 662-668. [60328]
438. Voss, Edward G. 1972. Michigan flora. Part I: Gymnosperms and monocots. Bulletin 55. Bloomfield Hills, MI: Cranbrook Institute of Science; Ann Arbor, MI: University of Michigan Herbarium. 488 p. [11471]
439. Wagg, J. W. Bruce. 1964. White spruce regeneration on the Peace and Slave River lowlands. Publ. No. 1069. Ottawa, ON: Canadian Department of Forestry, Forest Research Branch. 35 p. [12998]
440. Waldron, R. M. 1965. Cone production and seedfall in a mature white spruce stand. The Forestry Chronicle. 41(3): 314-329. [87596]
441. Walker, Lawrence R.; Chapin, F. Stuart, III. 1986. Physiological controls over seedling growth in primary succession on an Alaskan floodplain. Ecology. 67(6): 1508-1523. [82085]
442. Walker, Lawrence R.; Zasada, John C.; Chapin, F. Stuart, III. 1986. The role of life history processes in primary succession on an Alaskan floodplain. Ecology. 67(5): 1243-1253. [9072]
443. Walker, Robert. 1979. 1979 progress report on the Chugach moose-fire program. In: Hoefs, M.; Russell, D., eds. Wildlife and wildfire: Proceedings of workshop; 1979 November 27-28; Whitehorse, YT. Whitehorse, YT: Yukon Wildlife Branch: 66-122. [14075]
444. Wallingford, D. E.; Packee, E. C. 1988. Alaska's interior forest. In: Samoil, J. K., ed. Management and utilization of northern mixedwoods: Proceedings of a symposium; 1988 April 11-14; Edmonton, AB. Inf. Rep. NOR-X-296. Edmonton, AB: Canadian Forestry Service, Northern Forestrty Centre: 41-49. [13046]
445. Wang, G. G. 2002. Fire severity in relation to canopy composition within burned boreal mixedwood stands. Forest Ecology and Management. 163: 85-92. [41625]
446. Watson, L. E.; Parker, R. W.; Polster, D. F. 1980. Manual of plant species suitability for reclamation in Alberta. Vol. 2: Forbs, shrubs and trees. RRTAC 80-5. Edmonton, AB: Land Conservation and Reclamation Council. 537 p. [8855]
447. Weber, M. G.; Flannigan, M. D. 1997. Canadian boreal forest ecosystem structure and function in a changing climate: impact on fire regimes. Environmental Review. 5(3-4): 145-166. [50485]
448. Weeden, Robert B. 1965. Grouse and ptarmigan in Alaska: Their ecology and management. Federal Aid in Wildlife Restoration Project Report. Vol. V: Project W-6-R-5, Work Plan I. Juneau, AK: Alaska Department of Fish and Game. 110 p. [43851]
449. Wein, R. W. 1975. Vegetation recovery in Arctic tundra and forest-tundra after fire, ALUR Rep. 74-75-62. Ottawa, ON: Department of Indian Affairs and Northern Development, Arctic Land Use Research Program. 62 p. [12990]
450. Weir, J. M. H.; Johnson, E. A. 1998. Effects of escaped settlement fires and logging on forest composition in the mixedwood boreal forest. Canadian Journal of Forest Research. 28(3): 459-467. [29651]
451. Weir, J. M. H.; Johnson, E. A.; Miyanishi, K. 2000. Fire frequency and the spatial age mosaic of the mixed-wood boreal forest in western Canada. Ecological Applications. 10(4): 1162-1177. [85845]
452. Weixelman, D. A. 1987. Prescribed burning for moose habitat improvements: Chugach National Forest--1987 progress report. R10-MB-38. Anchorage, AK: U.S. Department of Agriculture, Forest Service, Alaska Region. 25 p. [79876]
453. Weixelman, David A.; Bowyer, R. Terry; Van Ballenberghe, Victor. 1998. Diet selection by Alaskan moose during winter: effects of fire and forest succession. In: Ballard, W. B.; Rodgers, A. R. J., eds. Proceedings, 33rd North American moose conference and workshop/4th international moose symposium; 1997 May 17-23; Fairbanks, AK. In: Alces. 34(1): 213-238. [30325]
454. Werner, Richard A.; Holsten, Edward H.; Matsuoka, Steven M.; Burnside, Roger E. 2006. Spruce beetles and forest ecosystems in south-central Alaska: a review of 30 years of research. Forest Ecology and Management. 227(3): 195-206. [88102]
455. Wilmking, M.; D'Arrigo, R.; Jacoby, G. C.; Juday, G. P. 2005. Increased temperature sensitivity and divergent growth trends in circumpolar boreal forests. Geophysical Research Letters. 32(15): doi:10.1029/2005GL023331. [88781]
456. Wilmking, Martin; Juday, Glenn P.; Barber, Valerie A.; Zald, Harold S. J. 2004. Recent climate warming forces contrasting growth responses to white spruce at treeline in Alaska through temperature thresholds. Global Change Biology. 10(10): 1724-1736. [88160]
457. Wirth, C.; Lichstein, J. W.; Dushoff, J.; Chen, A.; Chapin, F. S., III. 2008. White spruce meets black spruce: dispersal, postfire establishment, and growth in a warming climate. Ecological Monographs. 78(4): 489-505. [73344]
458. Wolken, Jane M.; Landhausser, Simon M.; Lieffers, Victor J.; Silins, Uldis. 2011. Seedling growth and water use of boreal conifers across different temperatures and near-flooded soil conditions. Canadian Journal of Forest Research. 41(12): 2292-2300. [85307]
459. Wotton, B. M.; Nock, C. A.; Flannigan, M. D. 2010. Forest fire occurrence and climate change in Canada. International Journal of Wildland Fire. 19(3): 253-271. [82368]
460. Yarie, J.; Viereck, L.; Van Cleve, K.; Dryness, C. T. 1988. The chronosequence as an aid to understanding the long-term consequences of management activities. In: Dyck, W. J.; Mees, C. A., eds. Research strategies for long-term productivity: Proceedings, IEA/BE A3 workshop; 1988 August; Seattle, WA. IEA/BE A3 Report No. 8. Rotorua, New Zealand: Forest Research Institute: 25-38. [17745]
461. Yarie, John. 1981. Forest fire cycles and life tables: a case study from interior Alaska. Canadian Journal of Forest Research. 11(3): 554-562. [12276]
462. Youngblood, Andrew. 1993. Community type classification of forest vegetation in young, mixed stands, interior Alaska. Res. Pap. PNW-RP-458. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 42 p. [22029]
463. Youngblood, Andrew. 1995. Development patterns in young conifer-hardwood forests of interior Alaska. Journal of Vegetation Science. 6(2): 229-236. [25923]
464. Youngblood, Andrew; Max, Timothy A. 1992. Dispersal of white spruce seed on Willow Island in interior Alaska. Res. Pap. PNW-RP-443. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 17 p. [19656]
465. Zasada, J. 1986. Natural regeneration of trees and tall shrubs on forest sites in interior Alaska. In: Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Vol. 57. New York: Springer-Verlag: 44-73. [86265]
466. Zasada, John C. 1971. Natural regeneration of interior Alaska forests - seed, seedbed, and vegetative reproduction considerations. In: Slaughter, C. W.; Barney, Richard J.; Hansen, G. M., eds. Fire in the northern environment--a symposium: Proceedings; 1971 April 13-14; Fairbanks, AK. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Range and Experiment Station: 231-246. [15731]
467. Zasada, John C. 1980. White spruce-paper birch. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 82. [50014]
468. Zasada, John C. 1985. Production, dispersal, and germination of white spruce and paper birch and first-year seedling establishment after the Rosie Creek Fire. In: Juday, Glenn P.; Dyrness, Theodore C., eds. Early results of the Rosie Creek Fire Research Project, 1984. Misc. Pub. 85-2. Fairbanks, AK: University of Alaska, Agriculture and Forest Experiment Station: 34-37. [50942]
469. Zasada, John C. 1985. Site classification and regeneration practices on floodplain sites in interior Alaska. In: Murray, Mayo, ed. Forest classification at high latitudes as an aid to regeneration; 1983 August 15-17; Fairbanks, AK. Gen. Tech. Rep. PNW-177. Fairbanks, AK: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station; University of Alaska Fairbanks, School of Agriculture and Land Resources Management: 35-39. [87725]
470. Zasada, John C.; Foote, M. Joan; Deneke, Frederick J.; Parkerson, Robert H. 1978. Case history of an excellent white spruce cone and seed crop in interior Alaska: cone and seed production, germination, and seedling survival. Gen. Tech. Rep. PNW-65. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 53 p. [50982]
471. Zasada, John C.; Gregory, Robert A. 1969. Regeneration of white spruce with reference to interior Alaska: a literature review. Res. Pap. PNW-79. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 37 p. [87648]
472. Zasada, John C.; Sharik, Terry L.; Nygren, Markku. 1992. The reproductive process in boreal forest trees. In: Shugart, H. H.; Leemans, R.; Bonan, G., eds. A systems analysis of the global boreal. A systems analysis of the global boreal forest. Cambridge, New York: Cambridge University Press: 85-125. [86611]
473. Zasada, John C.; Van Cleve, Keith; Werner, Richard A.; McQueen, John A.; Nyland, Edo. 1978. Forest biology and management in high-latitude North American forests. In: North American forest lands at latitudes north of 60 degrees: Proceedings of a symposium; 1977 September 19-22; Fairbanks, AK. Fairbanks, AK: U.S. Department of Agriculture, Alaska Region: 137-195. [13613]
474. Zasada, John C.; Viereck, Leslie A. 1970. White spruce cone and seed production in interior Alaska, 1957-68. Research Note PNW-129. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 11 p. [85375]
475. Zasada, John C. [n.d.]. Taiga community structure as influenced by patterns of succession, [Draft]. Fairbanks, AK: U.S. Department of Agriculture, Forest Service, Institute of Northern Forestry. 119 p. [87170]
476. Zasada, John; Norum, Rodney. 1986. Prescribed burning white spruce slash in interior Alaska. Northern Journal of Applied Forestry. 3(1): 16-18. [7881]
477. Zoladeski, C. A.; Delorme, R. J.; Wickware, G. M.; Corns, I. G. W.; Allan, D. T. 1998. Forest ecosystem toposequences in Manitoba. Special Report 12. Edmonton, AB: Natural Resources Canada, Canadian Forest Service, Northern Forestry Centre. 63 p. [35768]
478. Zoladeski, Christopher A.; Maycock, Paul F. 1990. Dynamics of the boreal forest in northwest Ontario. The American Midland Naturalist. 124(2): 289-300. [13496]