| FEIS Home Page |
 |
|
| Photo © Joy Viola, Northeastern University, Bugwood.org. |
AUTHORSHIP AND CITATION:
Gucker, Corey L. 2012. Cornus canadensis.
In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: https://www.fs.usda.gov/database/feis/plants/forb/corcan/all.html
[].
FEIS ABBREVIATION:
CORCAN
COMMON NAMES:
bunchberry dogwood
bunchberry
Canadian dwarf cornel
dwarf dogwood
TAXONOMY:
The scientific name of bunchberry dogwood is Cornus canadensis L. (Cornaceae) [95,138,299]. Bunchberry dogwood belongs to the Arctocrania subgenus or the dwarf cornels group [70,74,205].
Bunchberry dogwood hybridizes with Lapland cornel (C. suecica) to produce Cornus × intermedia (Farr) Calder & Roy L. Taylor [251,261]. Bunchberry dogwood also hybridizes with western cordilleran bunchberry dogwood (Cornus unalaschkensis) [70]. According to Murrell [205], western cordilleran bunchberry dogwood resulted from a past hybridization event in the Pacific Northwest between bunchberry dogwood and Lapland cornel.
SYNONYMS:
Chamaepericlymenum canadense (L.) Asch. and Graebn. [305]
LIFE FORM:
Forb-shrub
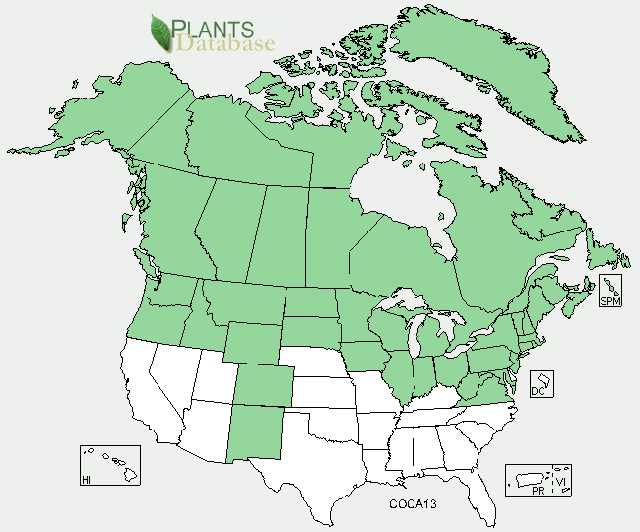 |
| North American distribution of bunchberry dogwood. Map courtesy of USDA, NRCS. 2011. The PLANTS Database. National Plant Data Team, Greensboro, NC. 2011, 16 November. |
Bunchberry dogwood is a widely distributed, partially circumboreal species [151]. In North America, it occurs throughout Canada, Alaska, and other northern US latitudes [298,299]. Bunchberry dogwood is much less common and often restricted to cool, moist, and/or high-elevation sites in its southern US range [29,52,74,198,272,299]. As of 1934, the southernmost distribution of bunchberry dogwood in the eastern United States was thought to be about 38° 35' in an upper elevation site in the Appalachians [309]. In the 1960s, however, Stevens [268] discovered a disjunct bunchberry dogwood population farther south in the Blue Ridge Mountains of Albemarle County, Virginia.
States and provinces (as of 2011) [290]:
United States: AK, CO, CT, IA, ID, IL, IN, MA, MD, ME, MI, MN, MT, ND, NH, NJ, NM, NY, OH, OR, PA, RI, SD, VA, VT, WA, WI, WV, WY
Canada: AB, BC, LB, MB, NB, NF, NS, NT, NU, ON, PE, QC, SK, YT
SITE CHARACTERISTICS AND PLANT COMMUNITIES:
Site characteristics: Bunchberry dogwood typically occurs in coniferous, deciduous, and mixed forests, but can also be found in heathlands, barrens, hummocks, bogs, meadows, and thickets [93,95,151,178,222,241,298]. Bunchberry dogwood habitats are typically cool and moist (see Plant communities) and occur from about sea level to 5,000 feet (1,500 m) [145,166,222] or higher [50]. Soils in bunchberry dogwood habitats often have a relatively thick organic surface horizon [120,150,168], and bunchberry dogwood is sometimes associated with decaying wood [162,261,286]; however, bunchberry dogwood tolerates a range of soil types and moisture and nutrient conditions (see Soils).
Throughout its range, bunchberry dogwood occupies a variety of habitats. Researchers described a wide ecological amplitude for bunchberry dogwood in the sub-boreal spruce zone in British Columbia [300]. In west-central Alberta, bunchberry dogwood occupied sites ranging from wet to dry, poor to rich, and from 1,600 feet (500 m) to nearly 6,600 feet (2,000 m) in elevation in the Boreal Mixedwood, Lower and Upper Boreal Cordillera, and Subalpine ecoregions [50]. In the Adirondack Uplands of New York, bunchberry dogwood was "prolific" in well-drained, mixedwood forest sites receiving full sun to partial shade but also occurred in poorly drained spruce (Picea spp.) and fir (Abies spp.) forests with dense shade [145]. Bunchberry dogwood habitats and site characteristics are also described in Plant communities.
Climate: In northern North America, bunchberry dogwood predominantly occupies continental climates that are cold and moist. Average temperatures in the coldest month are below 30 °F (0 °C) and in the warmest month generally exceed 50 °F (10 °C). Monthly precipitation can average 1 inch (30 mm) or more in any season. In bunchberry dogwood habitats along the West Coast, temperature ranges are similar to those of continental climates, but precipitation in the summer months typically averages less than 1 inch (30 mm) [217]. After evaluating southeastern habitats within and outside bunchberry dogwood's range, one researcher suggests that bunchberry dogwood is restricted to northern habitats because of its failure to establish on sites where summer soil temperatures exceed 65 °F (18 °C) [309].
Microclimate preferences and tolerances reported from a portion of bunchberry dogwood's western range suggest that bunchberry dogwood abundance may be greater on warmer sites in its northern range and cooler sites in its southern range. Bunchberry dogwood constancy was high in boreal forests of central and northern Alberta, but its cover was significantly greater at central than northern latitudes (P<0.001) [276]. In the central portion of the Cascade Range in Washington, bunchberry dogwood occupied a greater range of habitats on the west than the east side. Because of the rain shadow effect, western forests experience cooler temperatures, higher snow packs, and lower evapotranspiration rates during the growing season. In the winter, western forests are warmer, experience less diurnal temperature fluctuations, and have higher humidity levels than eastern forests [61]. In coniferous forests in the central Cascade Range of Oregon, bunchberry dogwood was most important in stands with the coldest environments [318].
Bunchberry dogwood may tolerate a smaller range of temperature extremes on exposed sites. In northern Idaho, sharp changes in temperature exposure may have caused the "disappear(ance)" of bunchberry dogwood after western white pine (Pinus monticola) stands were clearcut. Nighttime temperatures were 10 °F (6 °C) cooler and daytime temperatures were 10 °F (6 °C) warmer in clearcuts than in uncut forests. Minimum and maximum soil temperatures fluctuated 4 to 5 °F (2-3 °C) in clearcuts and just 1 °F (0.5 °C) in forests. The time between clearcutting and bunchberry dogwood's mortality was not reported [157].
Elevation: From the few areas for which bunchberry dogwood's elevational range was reported, it appears that ranges are similar (from about sea level to 5,000 feet (1,500 m)) in the Pacific Northwest [166,222] and the Northeast [145]. In west-central Alberta, bunchberry dogwood occupied sites ranging from 1,600 feet (500 m) to nearly 6,600 feet (2,000 m) [50]. In western Oregon and Washington, bunchberry dogwood was most common at intermediate elevations (2,000-3,500 feet (600-1,000 m)) [265,286]. In the southern Rocky Mountains, bunchberry dogwood was restricted to high-elevation sites (7,500 to 11,000 feet (2,300-3,400 m)) [116,186].
Soils: Bunchberry dogwood tolerates a variety of soil textures and a range of moisture and nutrient regimes. In west-central Alberta, bunchberry dogwood occupied sites ranging from wet to dry and poor to rich [50]. A study of the environmental and phytosociological conditions in deciduous, coniferous, and mixed forests in northwestern New Brunswick reported wide nutrient and moisture tolerances for bunchberry dogwood [171]. On Newfoundland islands, bunchberry dogwood was common on upland and lowland sites where soils were well-drained to very poorly drained [235]. In Gros Morne National Park, Newfoundland, bunchberry dogwood was associated with characteristics found in forested areas, which included limited bare ground, low light availability, and soils with moderate to high moisture levels and carbon to nitrogen ratios, and low pH, magnesium, calcium, and potassium levels [242]. A growing guide reported that bunchberry dogwood growth was best in moist but well drained soils that were rich in humus and ranged from very to slightly acidic. Bunchberry dogwood also grew in sandy soils when moisture was not limited [263].
Bunchberry dogwood occupies sites with a range of moisture regimes. In the Adirondack Uplands of New York, bunchberry dogwood was more prolific in mixedwood forests on well-drained soils than in spruce and fir forests on poorly drained soils [145]. In the boreal mixedwoods region of north-central Alberta, bunchberry dogwood was most common in the dry to mesic part of the moisture gradient but occurred on sites ranging from xeromesic to hygromesic [148]. In the Great Lakes states, bunchberry dogwood was associated with strongly leached, sandy soils, and poorly drained, mineral soils [312]. Bunchberry dogwood occurred across the range of dry to wet moisture conditions in 102 boreal conifer-hardwood stands in the northern Great Lakes region. Frequency of bunchberry dogwood averaged 61% in dry, 24% in dry-mesic, 24% in mesic, 31% in wet-mesic, and 55% in wet stands [188]. In white spruce-balsam fir (Picea glauca-Abies balsamea) stands on the Keweenaw Peninsula in northern Michigan, bunchberry dogwood frequency averaged 15% \in dry-mesic, 53% in mesic, and 30% in wet-mesic stands [189]. In southwestern Manitoba, bunchberry dogwood occurred primarily on hummocks and relatively dry microsites in black spruce (P. mariana) swamp peatlands [168].
Bunchberry dogwood is tolerant of nutrient poor soils but is not restricted to them. In the boreal mixedwoods region of north-central Alberta, it was most common in nutrient-poor sands, but also occurred in mesotrophic to eutrophic, fine-textured and clay-rich soils [148]. In New Brunswick, bunchberry dogwood was often dominant or distinctive in balsam fir forests and red spruce (P. rubens) forests on dry soils with poor to moderately poor nutrient levels [28]. In western Quebec's Lake Abitibi region, the presence of bunchberry dogwood often represented nutrient-poor and/or xeric sites [22]. In peatlands in the Bas-Saint-Laurent region of southeastern Quebec, bunchberry dogwood was abundant at the edge of peatlands bordering agricultural fields, where conditions were minerotrophic [150]. In wetland ecosystems in northern Lower Michigan, bunchberry dogwood occurred almost exclusively in low-light, forest-dominated wetlands with saturated, slightly acidic to neutral, and relatively rich soils [319].
Bunchberry dogwood tolerates a range of pH, but is most often described on slightly to very acidic sites (e.g., [242,319]). In the Lake Agassiz Peatlands Natural Area, Minnesota, bunchberry dogwood was more plentiful in rich swamp forests where soil pH ranged from 6 to 6.5, than in poor swamp forests where soil pH ranged from 4.5 to 6 [120]. In New York's Adirondack Uplands, bunchberry dogwood grew in soils with a pH range of 3.75 to 5.0 [145]. On the Apostle Islands of northern Wisconsin, bunchberry dogwood was frequent in pine (Pinus spp.) and wet balsam fir-paper birch (Betula papyrifera) stands where soil pH ranged from 4.2 to 4.8 [20].
Bunchberry dogwood grows on both organic and mineral soils but is often described on organic substrates, such as “raw humus” in western Montana [151], coarse woody peat soils in the Great Lakes states [312], peatlands in the Bas-Saint-Laurent region of southeastern Quebec [150], and black spruce swamp peatlands in the southern boreal region of Manitoba where the peat depth averaged 35 inches (90 cm) [168]. In the Lake Agassiz Peatlands Natural Area, bunchberry dogwood occurred on peat soils in rich and poor swamp forests [120]. In Berkshire County, Massachusetts, bunchberry dogwood occurred in acidic conifer swamps on a thin, peaty surface layer underlain with shallow, rocky mineral soil [304]. In the taiga of interior Alaska, the quaking aspen-black spruce/bunchberry dogwood community type occurred on well drained soils with shallow organic layers (about 5 inches (12 cm)) [80].
Coarse woody debris: Bunchberry dogwood is often associated with woody material and found growing on and through tree trunks, stumps, and fallen logs [84,222,261]. In cool, moderately moist coniferous forests in western Oregon, bunchberry dogwood was common on thick duff or rotted logs [286]. In young, quaking aspen-dominated, boreal forests near Slave Lake, Alberta, bunchberry dogwood associated more with logs and stumps classified as decay class 4 or greater than with forest floor. Decay classes ranged from 1 to 7, and larger numbers were associated with increased percentages of air space or softwood and decreased hardness [162].
Plant communities: Throughout northern North America, bunchberry dogwood occurs in coniferous, deciduous, and mixed forests [128,240,261]. It is particularly widespread in the understory of spruce and fir forests [17,147,297]. Bunchberry dogwood occurred in 88% of 34 white spruce-fir stands and in 96% of 26 black spruce stands distributed from central Alaska to Newfoundland. Sampled stands occurred at sites ranging from 450 to 4,300 feet (140-1,300 m) in elevation [147].
Bunchberry dogwood is more commonly associated with conifers than hardwoods [19,240,299]. When mixed forests were surveyed from northern Wisconsin to Nova Scotia, bunchberry dogwood was more common beneath eastern hemlock (Tsuga canadensis) than hardwoods [240]. In a large area of northwestern Quebec that included pure quaking aspen, mixed forests, and old-growth northern whitecedar (Thuja occidentalis) forests, bunchberry dogwood was associated with low light levels and conifer dominance [19]. However, when researchers surveyed 231 black spruce and quaking aspen stands in northern British Columbia, frequency of bunchberry dogwood was nearly equal in black spruce (79%) and quaking aspen (74%) stands [226], and in the Caribou-Poker Creeks Watershed in interior Alaska, bunchberry dogwood was more common in quaking aspen-paper birch stands than black spruce stands [287]. In the Anthracite Region of northeastern Pennsylvania, bunchberry dogwood was most common in the ecotone between white oak-red maple and eastern hemlock-black spruce communities [64].
Bunchberry dogwood's often greater occurrence or abundance in coniferous than deciduous forest types likely relates more to succession than to intrinsic properties of the coniferous or deciduous species. In many of the coniferous forest types where bunchberry dogwood is a common or predominant understory species, deciduous species such as alder (Alnus spp.), willow (Salix spp.), birch (Betula spp.), or quaking aspen dominate following fire or other stand-replacing disturbances [80,81,82,231,292]. Although bunchberry dogwood cover can be high in young, deciduous stands (<50 years old) [82,257], it is rarely described as a dominant in these stands. Bunchberry dogwood is often considered a dominant in mature, coniferous stands but may be less abundant in the early seral stages of conifer stands. Because of the sparse understory in heavily shaded, late-seral forests, bunchberry dogwood's dominance may reflect a lack of other understory species more than its absolute abundance or cover.
Bunchberry dogwood is commonly recognized as an understory dominant in habitat and community classifications throughout its range [52,80,148,192,221,233,236,283,285]. Because bunchberry dogwood is rarely restricted to particular moisture conditions or soil types [167,221,300,315], it is less commonly an indicator species [105,163]. Generally, bunchberry dogwood is likely to occur in the understory of cool, temperate and boreal forest types. However, at specific sites or within smaller geographic areas, bunchberry dogwood may be more closely associated with particular overstory species and/or site conditions. The discussion below presents community and environmental relationships reported by local studies. This discussion illustrates the range of species and site charactertistics associated with bunchberry dogwood, but is not a definitive description of bunchberry dogwood habitat, since these studies represent only a small fraction of the community types in which bunchberry dogwood is important.
Western North America: Bunchberry dogwood is common in montane forests dominated by western hemlock (Tsuga heterophylla), western redcedar (Thuja plicata), and lodgepole pine (Pinus contorta) and in subalpine forests dominated by spruce, fir, and hemlock (Tsuga spp.) [218].
Bunchberry dogwood is a common understory species in the following forest cover types recognized by the Society of American Foresters in western North America:
Alaska, Yukon Territory, and Pacific Northwest: Bunchberry dogwood is a typical understory species in coniferous forests of Alaska and northwestern North America. In coastal areas of Alaska, common overstory species include black spruce, white spruce, Sitka spruce (Picea sitchensis), mountain hemlock (Tsuga mertensiana), and western hemlock [48,56,233,289]. In a survey of 129 spruce-dominated forest plots on the Kenai Peninsula, bunchberry dogwood occurred in 105 plots [233]. When bogs, forests, and forest-bog ecotones were compared in the southeast Alaska panhandle, bunchberry dogwood was chiefly a forest species, but bunchberry dogwood × Lapland cornel hybrids were common in the bogs and ecotones [207]. In the taiga of interior Alaska, the quaking aspen-black spruce/bunchberry dogwood community type was common on sites burned 60 to 70 years earlier. Stands were typical of warm sites on well-drained soils with shallow organic layers (about 5 inches (12 cm)) [80]. On well-drained uplands in southwestern Yukon Territory and neighboring northern British Columbia, bunchberry dogwood was the principal understory species in forb-rich white spruce forests [56]. Along the Alaska Highway in Yukon Territory, bunchberry dogwood occurred in spruce and lodgepole pine forest types [214]. In western Alaska, bunchberry dogwood was sometimes common in northern rough fescue (Festuca altaica) grasslands [114].
Coniferous forests are the most typical bunchberry dogwood habitats in the Pacific Northwest, and associated overstory species include many of those mentioned for Alaska and the Yukon Territory but also include Engelmann spruce (Picea emgelmannii), subalpine fir (Abies lasiocarpa), Pacific silver fir, Douglas-fir (Pseudotsuga menziesii), and Alaska-cedar (Chamaecyparis nootkatensis) [88,119,123,143,264,273]. Bunchberry dogwood was a dominant understory species in the following communities:
Bunchberry dogwood occupies a wide range of edaphic conditions and forest types in British Columbia. Researchers described a very wide ecological amplitude for bunchberry dogwood in the sub-boreal spruce biogeoclimatic zone in British Columbia [300], and in northern British Columbia, bunchberry dogwood occurred in nearly all moisture and nutrient regimes within the boreal spruce, sub-boreal spruce, northern Engelmann spruce-subalpine fir, and sub-boreal pine-spruce biogeoclimatic zones [21]. Bunchberry dogwood was considered "virtually ubiquitous" in the Engelmann spruce-subalpine fir zone in northwestern British Columbia [221,315]. In the Kamloops Forest region, bunchberry dogwood occupied habitats ranging from dry to xeric montane spruce forests to moist and very wet interior western red cedar-western hemlock forests [167]; however, in mature, high-elevation forests in the Engelmann spruce-subalpine fir zone, bunchberry dogwood was common on moist or wet sites and only occasional on dry or very dry sites [62]. On the south-central coast of British Columbia, bunchberry dogwood was most important in ecotone or transitional forests between coastal fringe forests and inland peatland forests. Transitional forests were dominated by western hemlock and were drier than the inland peatland forests dominated by lodgepole pine [153].
In Washington and Oregon, bunchberry dogwood was often described in cool, moist forests. The western hemlock/Alaska blueberry/bunchberry dogwood association occurred in the cooler part of the western hemlock zone where moisture conditions were moderately high [285]. Bunchberry dogwood was an indicator of moist, cool sites in the western hemlock zone of the Mt Hood National Forest [105]. In Oregon's western Cascade Range, bunchberry dogwood was common in old-growth stands dominated by western hemlock, Pacific silver fir, or Alaska-cedar that were generally found on moist, cool sites [66].
Alberta, Manitoba, Idaho, and Montana: Canopy associates within bunchberry dogwood habitats in the northern Rockies and northern Plains regions of North America are very similar to those already mentioned. Bunchberry dogwood was recognized as a dominant in the following communities:
In the highlands of northern Alberta, bunchberry dogwood occurred in all 30 surveyed spruce-fir stands but had the greatest cover (up to 32%) in the white spruce-balsam fir/bunchberry dogwood-twinflower community type [1]. In lowland areas of north-central Alberta, bunchberry dogwood was the diagnostic understory species in the oligo-mesotrophic jack pine/bunchberry dogwood community type on relatively dry, low-nutrient sands. It also occurred in white spruce-quaking aspen community types on moister, more nutrient rich, fine-textured soils [148]. On Duck Mountain in southwestern Manitoba, bunchberry dogwood occurred primarily on hummocks and drier areas in black spruce swamp peatlands where the peat depth ranged from 16 to 79 inches (40-200 cm) [168]. In early-seral, shrub-dominated communities that established after logging and/or fire in the western redcedar-western hemlock zone of northern Idaho, bunchberry dogwood was more abundant on granitic than quartzite soils and was more frequent on north- than south-facing aspects [202]. In the Bear Paw Mountains of north-central Montana, the Douglas-fir/bunchberry dogwood forest type is considered the wettest of the Douglas-fir forests [236].
Eastern North America: In eastern North America, bunchberry dogwood occurs in coniferous, cold deciduous, and mixed forest types. Overstory associates in these forests typically include black spruce, red spruce, balsam fir, northern whitecedar, jack pine, eastern white pine (P. strobus), quaking aspen, or paper birch [54,55,133,137,187,283,320].
Bunchberry dogwood is a common understory species in the following forest cover types recognized by the Society of American Foresters in eastern North America:
Eastern Canada: Bunchberry dogwood occurs in forests [235], bogs [223], woodlands [82], and blueberry (Vaccinium spp.) crops [103]. In New Brunswick, bunchberry dogwood was often dominant or distinctive in balsam fir forests and red spruce forests on dry soils with poor to moderately poor nutrient levels [28]. Bunchberry dogwood had the highest indicator value in jack pine stands when the understory vegetation was compared in quaking aspen, paper birch, jack pine, or white spruce-balsam fir forest plots in southwestern Quebec (P<0.0001). Light levels were similar among the forest types [163]. In southeastern Labrador, bunchberry dogwood occurred in vegetation types ranging from early-seral paper birch to late-seral fir and spruce. Extensive carpets of bunchberry dogwood were described in paper birch stands, and scattered bunchberry dogwood was described in fir and spruce stands [84]. On Newfoundland islands, bunchberry dogwood was common but had low cover in tamarack (Larix laricina) forests on upland and lowland sites where soils were well-drained to very poorly drained [235].
Great Lakes region: Although bunchberry dogwood occurs in a variety of coniferous, deciduous, and mixed forest types [320], it is generally more common in coniferous and mixed forests than in deciduous forests. In the Boundary Waters Canoe Area, bunchberry dogwood occurred in upland forest types dominated by black spruce, balsam fir, eastern whitecedar, jack pine, red pine (Pinus resinosa), red maple (Acer rubrum), or quaking aspen. Cover of bunchberry dogwood was greatest (3.8-4.5%) in balsam fir-paper birch and black spruce-feather moss stands and least (0.2-0.5%) in jack pine-oak and red maple-quaking aspen-paper birch stands [97]. In Michigan, bunchberry dogwood was more frequent in northern boreal forest than in southern deciduous forest [189]. Based on his comprehensive study of Michigan flora, Voss [299] reported that bunchberry dogwood often occurred in coniferous forests, mixed forests, swamps, and all but the driest jack pine forests, but rarely occurred in deciduous woodlands [299]. Bunchberry dogwood occurred in nearly all forest types in Isle Royale National Park, except for the exclusively deciduous red maple-birch forest. Cover was greatest in paper birch-quaking aspen-white spruce and black spruce-northern whitecedar forest types [113]. When vegetation of Isle Royale National Park was classified and mapped in 1999, bunchberry dogwood was considered a distinguishing species in the rare red maple-ash (Fraxinus spp.)-paper birch/bunchberry dogwood forest type; however, it was not listed among the most abundant or characteristic understory species for the type. Bunchberry dogwood was listed among the characteristic or most abundant understory species in the following forest types:
In the Great Lakes region, bunchberry dogwood occurs on sites that include a range of edaphic conditions, but bunchberry dogwood appears to be most common in coniferous stands on sites with mesic moisture regimes. In the Lake Agassiz Peatlands Natural Area, bunchberry dogwood was plentiful but had low cover in rich swamp forests and was sparse with very low cover in poor swamp forests. Eastern whitecedar dominated the rich forests on very wet sites where soil pH ranged from 6 to 6.5, and peat depths were 1 to 6 feet (1.8 m). Stunted tamarack was the usual dominant in poor forests on normally saturated sites where soil pH ranged from 4.5 to 6, and peat layers measured 10 to 25 feet (3-8 m) thick [120]. On the Apostle Islands of northern Wisconsin, bunchberry dogwood was frequent in pine and wet balsam fir-paper birch stands. In these stands, light levels were high, soil pH ranged from 4.2 to 4.8, and moisture was limited [20]. In a survey of lowland forests in northern Wisconsin, bunchberry dogwood's presence was highest in eastern whitecedar-dominated stands that occurred along streams or around springs and lakes with non-stagnant water [39]. In white spruce-balsam fir stands on the Keweenaw Peninsula of northern Michigan, bunchberry dogwood frequency averaged 15% in dry-mesic, 53% in mesic, and 30% in wet-mesic stands [189].
New England: Bunchberry dogwood was described in coniferous forests, mixed forests, and alpine communities in New England. In Berkshire County, Massachusetts, bunchberry dogwood occurred in mesic northern conifer forests and acidic conifer swamps. Swamp soil had a thin peaty surface layer underlain with shallow rocky mineral soil [304]. Bunchberry dogwood was also reported in a rare dwarf pitch pine (Pinus rigida) community on Mt Everett in Berkshire County. Harsh edaphic conditions, including shallow, rocky soils, and frequent ice storms were common in the dwarf pitch pine community [201]. Along transects from a stream bank to the center of a bog in Vermont, bunchberry dogwood was found within 200 feet (50 m) of the stream but not in the bog. The height and density of black spruce, soil pH, and soil nutrient levels decreased from the stream to bog center. Soil surface water and the water table increased from the stream to bog center [31]. In the central Green Mountains of Vermont, bunchberry dogwood was restricted to the red pine/American mountain-ash/bluebead (Sorbus americana/Clintonia borealis) forest type on mesic infertile sites. Bunchberry dogwood did not occur in hardwood or eastern hemlock forest types [260]. In the Presidential Range in New Hampshire, bunchberry dogwood occurred in alpine vegetation that included dwarf shrublands dominated by blueberries and bog Labrador tea (Ledum groenlandicum) and snow bank communities adjacent to krummholz vegetation at and above timberline [24]. In the Adirondack Uplands of New York, bunchberry dogwood was "prolific" in well-drained, mixedwood forests receiving full sun to part shade but also occurred in poorly drained, spruce and fir forests with dense shade [145].
Central Appalachians: At its southernmost distribution in the eastern United States, Albemarle County, Virginia, bunchberry dogwood was found beneath clumps of paper birch at 2,700 feet (820 m) on a north-facing slope, which was drier than northern bunchberry dogwood habitats [268].
For further fire regime information for the plant communities in which this species may occur, enter the species name in the FEIS home page under "Find Fire Regimes". Also see the Fire Regime Table below. |
|
| Photo © Dave Powell, USDA Forest Service, Bugwood.org. |
GENERAL BOTANICAL CHARACTERISTICS:
Botanical description: This description covers characteristics that may be relevant to fire ecology and is not meant for identification. Keys for identification are available (e.g., [11,27,124,272,299]). For a description of bunchberry dogwood × Lapland cornel hybrids, see Hultén [128].
Aboveground description: Bunchberry dogwood is a low-growing, mostly herbaceous perennial that often forms patches or clumps from extensive, creeping rhizomes [124,151,261]. Stems are slender, typically less than 10 inches (25 cm) tall, and somewhat woody at the base [95,222]. Leaves are firm and occur in false whorls of 4 to 7 near the top of stems [95,124,261]. Whorls of 6 leaves are typical of flowering stems; whorls of 4 are typical of sterile stems [169,262,299]. Morphology may be affected by site conditions. In Oneida County, Wisconsin, leaves and shoots of bunchberry dogwood were larger and thicker in open-canopy than woodland habitats [14], but in Nova Scotia, leaf thickness, stem thickness, and plant height differences were not consistently correlated with coastal barren, inland forest, or intermediate habitats from 2 sites. However at one site, leaves were thicker at the coastal barrens and inland forest than at the intermediate habitat [159]. Individual bunchberry dogwood flowers are very small and occur in a terminal cyme surrounded by 4 showy, petal-like bracts [124,261]. There are typically 10 to 25 flowers per inflorescence [18,95]. Fruits are clusters of small (5-8 mm), 2-seeded, berry-like drupes [124,261,298].
Belowground description: Bunchberry dogwood grows laterally along rhizomes, which can grow up to 12 inches (30 cm) a year [103]. Along the shoreline of Douglas Lake in Cheboygan County, Michigan, bunchberry dogwood plantlets occurred 7 feet (2 m) apart on a single rhizome [262]. In the same county, primary bunchberry dogwood rhizomes excavated near Reese's Swamp were slender (0.2-1 mm in diameter), with a bark-like appearance and texture. The rhizomes were fine but strong [169].
Most bunchberry dogwood rhizomes and roots occur 1.6 to 5 inches (4-13 cm) deep. In the Douglas-fir forest zone of southern interior British Columbia, bunchberry dogwood roots and rhizomes occurred 2 to 5 inches (5-13 cm) below the mineral soil surface [191]. On sandy sites in central Alberta, maximum bunchberry dogwood rooting depths were 3.5 inches (9 cm) and 5 inches (13 cm) below the ground surface in black spruce and jack pine stands, respectively [275]. Along the shoreline of Douglas Lake in Cheboygan County, bunchberry dogwood rhizomes occurred beneath more than 4 inches (10 cm) of sand [262]. From a stream bank in Cheboygan, bunchberry dogwood rhizomes were removed from a maximum depth of 11.8 inches (30 cm) [169]. At the Acadia Forest Experiment Station in New Brunswick, the average depth of bunchberry dogwood rhizomes ranged from 1.6 to 5 inches (4-13 cm). Rhizome depth was evaluated at a variety of sites, which included 3- to 16-year-old clearcuts and undisturbed areas in spruce, balsam fir, hardwood, and mixed forest types. Rhizomes were typically found in the mineral soil layer. Depth of the rhizomes did not appear related to forest type or past disturbance [78]. See Vegetative regeneration for additional descriptions of bunchberry dogwood rhizomes.
Raunkiaer [228]
life form:
Hemicryptophyte
SEASONAL DEVELOPMENT:
Throughout its range, bunchberry dogwood flowering is most typical from May to July [186,241,261,272]. However, flowering may be delayed on cold, exposed sites [299]. In the Adirondack Uplands of New York, the earliest bunchberry dogwood flowering date was 26 May, and the latest was 19 October [145].
Bunchberry dogwood fruits are common near the end of summer but can occur anytime from July to October [145,178,261,299]. In the Canadian Arctic, bunchberry dogwood produced both flowers and fruits within 6 to 7 weeks [103].
The percentage of nonstructural carbohydrates in bunchberry dogwood rhizomes increased from spring to fall in Nova Scotia. The dry weight of nonstructural carbohydrates was 5% to 10% for rhizomes collected in the spring, a little more than 15% in the summer, and a little less than 20% in the fall [76].
REGENERATION PROCESSES:Bunchberry dogwood regenerates by seed and rhizomes. Sprouting from rhizomes is its primary regeneration method following top-kill [37,173]. Clonal growth is important to bunchberry dogwood's long-term persistence in densely shaded, late-seral forests where flowering is rare [9,14,94].
Pollination and breeding system: Bunchberry dogwood produces perfect flowers [11,251] that are self incompatible and insect pollinated [103]. Self fertilization of bunchberry dogwood flowers is prevented by protandry. Bees and flies commonly visit bunchberry dogwood flowers, and an extensive list of insect visitors was recorded by Lovell [172]. In central New Brunswick, the number of insect visits to bunchberry dogwood flowers was "relatively high" among the 12 understory species observed in the area. When flowers were bagged to exclude insect visitors, virtually no bunchberry dogwood fruit was set. In controlled experiments, 21.5% of cross-pollinated flowers set fruit, 10.7% of open-pollinated flowers set fruit, but no self-pollinated flowers set fruit. Low fruit set for cross-pollinated flowers suggested that bunchberry dogwood aborts fruits as resources become limited [18]. Wind pollination of bunchberry dogwood flowers was suspected in Isle Royale National Park, after researchers protected flowers from insects and found that 3 of 9 protected inflorescences produced seed. Seed production was greater for unprotected inflorescences [310].
Bunchberry dogwood flowers are equipped with an appendage that explosively releases pollen when touched [70,172,222]. Researchers recorded and studied explosive pollen release from bunchberry dogwood plants collected from relatively undisturbed forest habitat in Isle Royale National Park. When the flower appendage was touched, pollen was launched straight upward at high speeds. Bunchberry dogwood pollen was carried over 8.7 inches (22 cm) in a room with minor air currents. Researchers calculated that any wind speed greater than 0.4 feet (0.12 m)/second was sufficient to transport bunchberry dogwood pollen. The speed of pollen release was sufficient to lodge pollen into an insect's hairs, and in the field, insects that triggered flower explosions were coated with pollen [310].
| Seed production: Studies often report poor seed production by bunchberry dogwood; however, seed production may increase with increased light availability. Weather conditions and flowering date can also affect seed production. While the age at which wild-growing bunchberry dogwood plants produce flowers and fruits was not reported in the available literature (as of 2011), in a nursery setting, bunchberry dogwood plants grown from seed took 5 years to produce flowers [73]. |  |
| Photo © Joy Viola, Northeastern University, Bugwood.org. |
Several studies suggest that bunchberry dogwood seed production is greater in high-light than low-light environments. In the Chugach National Forest on the Kenai Peninsula, bunchberry dogwood fruit production increased with tree morality from spruce beetle infestations. Regression analyses revealed that the number of fruits produced decreased by a factor of 0.84 with every 10% increase in canopy cover. When data were pooled from all study plots regardless of beetle infestation levels, bunchberry dogwood fruit production was greatest (35.3 berries/m²) in white spruce stands and least (10.6 berries/m²) in mountain hemlock stands [278]. In Itasca State Park, Minnesota, bunchberry dogwood was typically found in a vegetative state in densely shaded areas of jack pine-white spruce-balsam fir forests. Only 3.1% of plants were found in a flowering or fruiting state where light intensity averaged 16% of full sun [94]. At the Enterprise Radiation Site in Oneida County, Wisconsin, bunchberry dogwood flowers were rare in a woodland, but up to 15.6% of bunchberry dogwood shoots were flowering at an open-canopy site. However, fruit set was low at all sites, and seed viability was very low [14]. Over 4 growing seasons in the Sitka spruce-western hemlock zone of southeastern Alaska, seed production by understory species was compared in clearcut, second-growth, and old-growth stands. Cornus spp. fruit production in clearcuts was 400 times the production in old-growth stands. No fruit was produced by any understory species in areas where solar transmissivity was 3% or less [9].
Abundance and size of bunchberry dogwood fruits can vary with seasonal conditions and time of year. During wet, cool conditions in central New Brunswick, duration of bunchberry dogwood flower production was reduced, and late-developing flowers had low fruit set. During the 1st growing season in the spruce-fir study area, conditions were warm and dry, and bunchberry dogwood flowered for 26 days; in the 2nd growing season, conditions were cool and wet, and bunchberry dogwood flowered for 18 days. The number of days from the 1st open flowers to peak flowering was 6 days shorter in the cool, wet growing season. From a total of 207 bunchberry dogwood plants, 5,221 flower buds were produced. Nearly 87% of flowers opened and almost 11% produced mature fruit. Fruit set was best (almost 20%) for flowers produced at the initial or early flowering periods; fruit set was less (nearly 9%) for flowers produced at peak flowering time. Fruit set was poor to nonexistent (≤1.6%) for flowers produced near or at the end of the flowering period [122]. In Kings County, Nova Scotia, bunchberry dogwood fruits were sampled from 25 shoots at 2 times in a single growing season. Fruits sampled at the end of July averaged 11.8 per inflorescence and 0.077 g/fruit; fruits sampled in mid-September averaged 9.2 per inflorescence and 0.149 g/fruit [103]. It was unclear if sampled shoots were protected and whether or not the reduction in fruits/inflorescence reflects both herbivory and maturation events.
In the southernmost bunchberry dogwood population, a relatively dry site in Albemarle County, Virginia, plants produced no fruits in 5 years of observation. About 10% of plants produced flowers but none developed into fruits. The author did not speculate about the prevailing cause of fruit failure [268].
Seed dispersal: Bunchberry dogwood fruits are consumed by many mammals and birds [69,248,299], and bunchberry dogwood seeds have been recovered from the feces of many species [58,86,129]. By early fall in central New Brunswick, 41% of bunchberry dogwood fruits were gone, 5% occurred beneath the parent plant, and the rest remained on plant (17% were whole, 33% were rotten, 4% were shriveled). The percentage of fruits removed from unprotected plants was 46.1% and from protected plants was 8.2% (P<0.001) [122]. In Newfoundland, researchers monitored the fate of bunchberry dogwood fruits and seeds for 3 fall seasons. Removal of fruits averaged 53%, damage by invertebrates averaged 23%, and fungal or microbe infection averaged 18%. Three percent of fruits were shriveled but remained on the plant, 2% of fruits were firm and remained on the plant, and 1% of fruits fell beneath the plant. Almost 6% of bunchberries were bitten off at the stem, suggesting removal by small mammals. Slugs were the major invertebrate feeders. Frugivores preferred intact, fresh berries; 92% of fruits removed were intact, and most fruits were taken within 5 weeks of ripening. American robins and white-throated sparrows fed on bunchberry dogwood fruits, and intact bunchberry dogwood seeds were recovered from ruffed grouse droppings. Field observations and exclusion experiments suggested that migratory birds were the primary bunchberry dogwood fruit predators or dispersers in Newfoundland [33]. Another study reported that white-throated sparrows may crush bunchberry dogwood seeds and may therefore be considered predators or poor dispersers (Thompson and Willson 1979 cited in [33]).
Seed banking: Several studies report that bunchberry dogwood seed is dormant upon maturity and requires cold stratification for germination. Bunchberry dogwood seed can remain viable in the soil seed bank for at least 3 years [282]. Several studies indicate that viable bunchberry dogwood seed occurs in the soil, but the quantity varies from no seed [7,89,266] up to 242 bunchberry dogwood seeds/ha [7]. Seed bank density does not seem to be associated with aboveground frequency (e.g., [6,7,266]). Differences among studies may be due to differences in seed production, which can be highly variable due to low light or late flowering; differences in seed predation (see Seed dispersal and Importance to wildlife); or differences in methodology used to determine seed bank composition [30].
In field studies conducted near Juneau, Alaska, emergence of bunchberry dogwood seedlings was monitored for seeds that were buried 0.4 inch (1 cm) below the forest floor in fine-mesh packets. Year 1 emergence ranged from 47% to 80%, year 2 emergence ranged from 18% to 53%, and year 3 emergence ranged from 1% to 16%. When ungerminated seeds were collected after a year of burial in the field, germination in the laboratory averaged 85% [282].
Seed bank studies in northeastern Minnesota suggested that bunchberry dogwood seed bank density may not correspond to the aboveground frequency of bunchberry dogwood. Only 4 bunchberry dogwood seeds/ha were extracted from soil samples collected in undisturbed jack pine stands where the aboveground frequency of bunchberry dogwood averaged 97%. Soil samples included the top 1 inch (2.5 cm) of mineral soil and surface litter layers [6]. In the Boundary Waters Canoe Area, no bunchberry dogwood seeds were extracted from soil samples collected from pine stands where the average aboveground frequency of bunchberry dogwood ranged from 27% to 67%. However, in a balsam fir stand where the aboveground frequency of bunchberry dogwood was only 3%, 242 bunchberry dogwood seeds/ha were extracted. Soil samples included the top 1 inch (2.5 cm) of mineral soil and the surface litter layers [7]. Samples of the top 1.2 inches (3 cm) of soil were taken from subalpine and low alpine white spruce forests in southern Quebec in early September. At both sites, aboveground cover of bunchberry dogwood averaged about 10% and 22 to 28 bunchberry dogwood seeds/m² were recovered [200].
Estimates of bunchberry dogwood seed bank density were low to none when researchers used the seedling emergence method [115,266], which may underestimate seed bank density [30]. When seedling emergence and seed extraction methods were compared for soil samples from a recently clearcut, mixed-deciduous forest in southern Ontario, no bunchberry dogwood seedlings emerged from soil samples in the greenhouse, but researchers successfully extracted almost 70 bunchberry dogwood seeds/m² from the soil. All soil samples were cold stratified then dried, and all roots and plant debris were removed from the samples [30].
Because the seedling emergence method may underestimate the density of bunchberry dogwood seed in the soil, the following studies may not reflect the true size of bunchberry dogwood seed banks. Bunchberry dogwood did not emerge from cold-stratified soil samples from disturbed or undisturbed Douglas-fir forests in south-central British Columbia. The frequency of bunchberry dogwood was about 15% in 2 of 8 sample sites [266]. Just 10 bunchberry dogwood seedlings/m² emerged from soil samples collected from old-growth, mixed-conifer forests in the HJ Andrews Experimental Forest in Oregon, where the aboveground cover of bunchberry dogwood averaged 9.4% [115]. In quaking aspen stands in northeastern Alberta, a small number of bunchberry dogwood seedlings (1.9/m²) emerged from unburned soil samples, but no seedlings emerged from soil collected from lightly or severely burned plots. Within about 2 years of burning, aboveground bunchberry dogwood cover averaged 3.2% on unburned, 7.6% on lightly burned, and 5.6% on severely burned plots [161].
The bunchberry dogwood seed bank may be limited to the upper soil layers. In jack pine- and white spruce-dominated stands in central Alberta, bunchberry dogwood seedlings did not emerge from mineral soil samples, but 22 seedlings/m² emerged from soil samples of the organic layer from a white spruce stand. Aboveground abundance of bunchberry dogwood was not reported [89].
Germination: Bunchberry dogwood seed germinates best when a cold stratification period is followed by alternating temperatures [63] and light [282].
Several controlled studies report that bunchberry dogwood seed fails to germinate without cold stratification [2,190,210]. Maximum germination of bunchberry dogwood seed collected from Kamloops, British Columbia, was 38% after stratification [190]. Maximum germination was 2% when bunchberry dogwood seed was not exposed to cold temperatures or was dried before cold temperature exposure. When seeds overwintered outdoors, germination was 28% [2]. Bunchberry dogwood seeds collected in August from the Bonanza Creek Experimental Forest in central Alaska germinated best when cold stratified and subsequently exposed to alternating cool and warm temperatures 43 to 81 °F (6-27 °C). Germination was significantly less (P<0.05) for cold stratified seeds exposed to constant temperatures and for seeds exposed to warm and cold pretreatments before exposure to either constant or alternating germination temperatures [63].
Field and laboratory studies indicate that bunchberry dogwood germination typically occurs in the spring and is best for unburied seed. In central Alaska, germination of bunchberry dogwood seed was much better for uncovered than buried seeds. Most seed germinated in June, and all seed germinated by the end of July. Seeds sown in late August did not germinate in the fall [63]. For bunchberry dogwood seed collected near Juneau, Alaska, germination in the laboratory was 61% to 87% after cold stratification. In the field, the average germination of uncovered seed was 78%. Seeds buried by 0.4 inch (1 cm) of litter and/or duff material germinated poorly (1-8%). Bunchberry dogwood seedlings emerged over a 3-year period for 2 seeding trials [282]. See Seed banking for details.
Bunchberry dogwood seedling emergence is possible on sunny, shady, burned, or unburned sites. Emergence of bunchberry dogwood was greater in old-growth (22%) than in young (12%) western hemlock-Sitka spruce stands near Juneau, Alaska. The percentage of light and canopy openings was greater in old-growth than young stands [282]. When seedling emergence was evaluated in the greenhouse in soil samples that were experimentally exposed to shady, sunny, disturbed, undisturbed, burned, and unburned conditions, bunchberry dogwood emergence was low but did not appear sensitive to any one condition. Two bunchberry dogwood seedlings emerged from a 1,560 cm³ soil sample that was collected and kept as an undisturbed block of forest soil, put under shade cloth, and burned with a propane torch. Two seedlings also emerged from a soil block that was thoroughly mixed, exposed to full sun, and not burned. Soil blocks were collected in late April from old-growth Douglas-fir stands in Oregon's HJ Andrews Experimental Forest [130].
Seedling establishment and plant growth: Bunchberry dogwood seedling establishment and plant growth were not well documented in the available literature (as of 2011). Factors that are favorable or unfavorable to bunchberry dogwood seedling establishment are unknown or have gone unreported. In southeastern Alaska, bunchberry dogwood seedling survival averaged 13% in 3 to 4 years after experimental seeding in western hemlock-Sitka spruce stands [282]. After surveying numerous bunchberry dogwood habitats in Nova Scotia for 2 summers, researchers found no bunchberry dogwood seedlings. One of the summers was very dry [103].
Vegetative regeneration: Bunchberry dogwood clones can be extensive, but the size of clones and abundance of nodes and sprouts along rhizomes vary by site and are likely greater in high-light environments. Three large bunchberry dogwood clones were excavated as completely as possible from a stream bank near Reese's Swamp in Cheboygan County, Michigan. The greatest total length of rhizomes for an individual clone was 13.8 feet (4.2 m), and the greatest depth from which rhizomes were recovered was 11.8 inches (30 cm) [169]. Bunchberry dogwood rhizome length, annual growth, and number of nodes were greater in clearcut stands than in young and old-growth stands in southeastern Alaska. Old-growth stands were dominated by western hemlock and Sitka spruce; young stands were clearcut 40 years earlier, and clearcut stands were 4- to 6-years old. The longest living bunchberry dogwood rhizome found was 172 inches (436 cm) and was estimated to be 36 years old. The longest internode length found was 30 inches (75 cm) and occurred in an area where the rhizome grew through decayed logs [282]. Bunchberry dogwood rhizomes are also described in Belowground description.
| Average characteristics of bunchberry dogwood clones in old-growth, young, and clearcut stands in the western hemlock-Sitka spruce zone in southeastern Alaska [282] | |||
| Stand type* | Old-growth | Young | Clearcut |
| Total rhizome length (cm) | 165 | 15 | 309 |
| Annual rhizome growth (cm) | 9 | 4 | 131 |
| Nodes/clone (number) | 9 | not measured | 20 |
| *Percentage of open canopy: old-growth 2.8-6.4%; young 0.6-2.3%; clearcut 100%. | |||
Bunchberry dogwood rhizomes may sprout when light availability increases [14] or aboveground stems are buried [12] or killed (see Plant response to fire). After the eruption of Mount St Helens, bunchberry dogwood stems grew through 3.5 inches (9 cm) of ash [12]. In Oneida County, Wisconsin, density of bunchberry dogwood stems increased from 30 to 94 shoots/m² within a year after canopy removal [14].
Research suggests that bunchberry dogwood's dispersal potential from rhizome pieces may be limited. In southeastern Canada, 3-foot (1 m) long sections of bunchberry dogwood rhizomes were dug from forest sites and transplanted within an hour into a common garden. Rhizome sections were planted at a depth equal to that from which they were excavated at the forest sites. Five months after transplanting, bunchberry dogwood regrowth was poor from transplanted rhizomes [79].
SUCCESSIONAL STATUS:
Bunchberry dogwood tolerates the range of conditions present from early to late forest succession [57,103,104,241]. bunchberry dogwood abundance can be greater in open-canopy than closed-canopy habitats, but it still persists in dense forest shade. If the canopy is removed but soil is left intact, bunchberry dogwood "exists indefinitely" as the new canopy develops [103]. Bunchberry dogwood cover can be similar in young and mature forests, but it is more commonly described as a dominant understory species in mature than young forests. This may reflect the rarity of other understory species in mature or old-growth stands rather than an abundance of bunchberry dogwood.
Shade relationships: Bunchberry dogwood grows in habitats ranging from full sun to nearly complete shade [145,151,177,303]. Studies report each of the following conditions: greater abundance of bunchberry dogwood in closed-canopy than open-canopy sites [16,19,51], greater abundance of bunchberry dogwood in open-canopy than closed-canopy sites [8,59,277], and similar abundance in open-canopy and closed-canopy sites [208,224,269]. There was no discernable pattern to bunchberry dogwood's abundance as related to light availability, but large, open sites devoid of grasses and shrubs that could shade a low-growing species such as bunchberry dogwood were rarely described.
The following studies reported that the abundance of bunchberry dogwood was greatest in sites with low light conditions. In western white pine stands in northern Idaho, bunchberry dogwood was restricted to shady sites that occurred beneath tree canopies and "disappear(ed)" after clearcutting [157,158]. Cover of bunchberry dogwood was greater in mature (120-year old) lodgepole pine stands than in stands clearcut 6 to 12 years earlier in the Lower Foothills of Alberta [51]. In west-central Alberta, bunchberry dogwood cover increased as forest canopy cover increased. Average cover was significantly lower in early-seral (2.5%) than mid-seral (7.6%) stands (P<0.001); forest canopy was less than 30% in early-seral stands and greater than 30% in mid-seral stands [16]. In the Chippewa National Forest in north-central Minnesota, the greatest frequency of bunchberry dogwood occurred in red pine forest plots receiving less than 20% of full light [255]. When overstory and understory patterns were studied over a large area of northwestern Quebec, which included quaking aspen, mixed deciduous-conifer forests, and old-growth northern whitecedar forests, researchers found that bunchberry dogwood was associated with low light levels and conifer canopies [19].
Bunchberry dogwood abundance was greater in open-canopy than closed-canopy forests in many studies. Bunchberry dogwood flower and seed crops were "heavy" on logging roads scraped of vegetation [263]. In the Chugach National Forest on the Kenai Peninsula of Alaska, bunchberry dogwood cover increased as overstory canopy cover reached 50% then decreased as canopy cover reached 100% [277]. In Sitka spruce-western hemlock forests in southeastern Alaska, bunchberry dogwood cover was significantly less (P<0.01) beneath the canopy than in canopy gaps [8]. Bunchberry dogwood biomass was significantly greater (P=0.03) in boreal mixedwood forest plots where all erect shrubs were removed than in plots where shrubs were retained. The amount of diffuse, direct, and total incoming light at 20 inches (50 cm) above ground was significantly lower in control than removal plots (P<0.0017) [38]. In early-seral, shrub-dominated communities regenerating after logging and/or fire in the western redcedar-western hemlock zone in northern Idaho, frequency of bunchberry dogwood was greatest in stands with 26% to 55% tree canopy cover and least on sites with 56% to 100% canopy cover [202]. In southern boreal forests along Lake Duparquet in northwestern Quebec, bunchberry dogwood cover was typically greater after experimental canopy gaps were created. Bunchberry dogwood abundance was compared before and up to 4 years after gap creation [59]. In red spruce forests near Liverpool, Nova Scotia, abundance of bunchberry dogwood was high in semi-open clearcut forests and in mature forests with large canopy gaps [199]. Bunchberry dogwood was considered "prolific" in full sun to partial shade mixedwood forests but also occurred in spruce and fir forests with dense shade in the Adirondack Uplands of New York [145].
In several other studies there was little to no difference in bunchberry dogwood abundance related to degree of shading. Cover and frequency of bunchberry dogwood were nearly identical in harvested and canopy tree retention sites in a mixed-conifer forest on the west slope of the Cascade Range in southwestern Washington. Percentage of open sky was 17% in retention and 45% in harvest areas. Cover of logging slash was 10% in retention and 66% in harvest areas [208]. In the Willamette National Forest, Oregon, bunchberry dogwood cover was 20.6% beneath Douglas-fir canopies and 26.9% in canopy openings [269]. Cover of bunchberry dogwood was not significantly different in a tornado blow down area and an adjacent undisturbed site in mixed-conifer-northern hardwood forests in the Boundary Waters Canoe Wilderness Area. Researchers evaluated the sites 1, 2, 3, and 4 years following the tornado [224].
Disturbance tolerance: Bunchberry dogwood is rarely killed by forest disturbances, but abundance of bunchberry dogwood is often lower on disturbed than undisturbed sites, which may relate to the disturbance itself or changes in the mircoenvironment resulting from the disturbance. In the Harvard Forest in Petersham, Massachusetts, bunchberry dogwood frequency (8%) had not recovered to pre-hurricane levels (18%) within 53 years of a hurricane [175]. In mixed-conifer forests impacted by the eruption of Mount St Helens, bunchberry dogwood frequency was greater in scorched areas (33%), where trees were killed but remained standing, than in blow down areas (15%), where trees were killed and snapped or uprooted [193]. Bunchberry dogwood was associated with the least disturbed sites when restoration sites and naturally recovering sites were compared in the industrially impacted area of Sudbury, Ontario. Forests were damaged by logging, mining, and smelting operations that began in the 1800s [229].
Abundance of bunchberry dogwood can be reduced by logging. Bunchberry dogwood cover was negatively correlated (r = -0.650) with mechanical site treatments, when cleared and mechanically treated boreal and sub-boreal sites were visited 10 years after logging. Severity of the treatments ranged from low to high severity and included disk trenching, plowing, rotoclearing, and/or windrow burning [100]. In the Cascade Range in Oregon and Washington, bunchberry dogwood occurred in young, mature, and old-growth Douglas-fir forests. However, its development was best in old-growth stands, and it was considered sensitive to disturbance based on the evaluation of experimental stands that were logged, clearcut, and/or slash burned [104]. When disturbed and undisturbed western redcedar-western hemlock stands were compared in northern Idaho, average bunchberry dogwood cover was greatest (12%) in 100-year-old, undisturbed stands. Average bunchberry dogwood cover was much lower (<1-4%) in stands clearcut, thinned, or burned 30 years earlier [131]. Bunchberry dogwood cover decreased on both undisturbed and clearcut plots in a mixed coniferous-deciduous forest in southeastern New Brunswick. Average cover of bunchberry dogwood had decreased by about 1% on the undisturbed and clearcut sites when evaluated in the second posttreatment year. On a site that was clearcut, scarified, and planted with the jack pine seedlings, average bunchberry dogwood cover increased 0.4% from pretreatment levels [237].
Bunchberry dogwood is likely to persist following disturbance. Cover of bunchberry dogwood was similar in unlogged and salvage logged portions of a severely wind-damaged black spruce-jack pine forest in Minnesota's Superior National Forest. Two years after the microburst and 1 year after salvage logging, bunchberry dogwood cover was 5.2% in unlogged and 6.5% in salvage-logged areas. A year later, bunchberry dogwood cover was 1.3% in unlogged and 3.2% in salvage-logged areas [152]. On caribou summer range in northern Quebec and Labrador, bunchberry dogwood cover was not significantly different between grazed (1.8%) and ungrazed (1.0%) areas [180].
Forest succession: Generally bunchberry dogwood can occur in all stages of forest succession. Bunchberry dogwood occurred in nearly all of 121 plots surveyed throughout the entire range of black spruce in British Columbia. Forest age ranged from 37 to about 185 years old and included a wide range of soil moisture, nutrient, and aeration conditions [142]. Two successional trends were identified in the boreal forest zone of west-central Alberta, and bunchberry dogwood occurred in nearly all successional forest types, including communities transitioning from lodgepole pine and quaking aspen to white spruce on moist, low-elevation uplands, and communities transitioning from lodgepole pine and black spruce to spruce-subalpine fir forests on moist but well-drained, high-elevation uplands [49]. In Montana's Glacier National Park, bunchberry dogwood was more frequent in pioneer and early-seral lodgepole pine forests than in late-seral and climax western hemlock and western redcedar forests [98], but it was considered common in climax western redcedar and grand fir (Abies grandis) stands over 300 years old in Idaho's Selway-Bitterroot Wilderness [99]. In Isle Royale National Park, bunchberry dogwood occurs in the early succession of rocky sites [44] and in climax balsam fir-paper birch-white spruce forests [45]. In a region south of James Bay in Ontario and Quebec, researchers surveyed 197 boreal forest stands and found bunchberry dogwood throughout the studied stand types. Abundance of bunchberry dogwood was similar along the full spectrum of successional stages identified [35].
In forested wetland or riparian sites, bunchberry dogwood may be restricted to later seral stages. Along the Chena River near Fairbanks, Alaska, bunchberry dogwood occurred in older floodplain terraces and upper elevations. Bunchberry dogwood did not occur in 15-year-old willow (Salix spp.) or 50-year-old balsam poplar (Populus balsamifera) stands, but did occur in 120-year-old white spruce, 220-year-old white spruce-black spruce, and climax black spruce/sphagnum (Sphagnum spp.) stands. Soil froze quicker, deeper, and reached lower temperatures in the early-seral deciduous stands than in late-seral conifer stands [296]. Xerosere and hydrosere successions occur in Algonquin Park, Ontario, where the climatic climax species is eastern hemlock. Xerosere succession involves transitioning from pioneer woodlands dominated by red maple, quaking aspen, paper birch, or jack pine to white pine or balsam fir-white spruce forests, then to eastern hemlock forest. Bunchberry dogwood occurs in nearly all forest types along the xerosere. In hydrosere succession, which involves transitioning from bog vegetation to black spruce and balsam fir forests and then to eastern hemlock forest, bunchberry dogwood is restricted to later successional stages [185].
Fire-related forest succession: Generally bunchberry dogwood can be present in recently burned as well as long unburned forests throughout its range. Studies suggest, however, that bunchberry dogwood abundance is lower in early postfire succession than later successional stages.
Bunchberry dogwood typically survives fire. Bunchberry dogwood frequency was relatively consistent in fire-origin lodgepole pine stands less than 100 years old in Banff and Jasper National Parks in Alberta [149]. In southeastern Manitoba, bunchberry dogwood occurred in severely burned 5-year-old stands and forests over 90 years old [247]. In northern Lower Michigan, bunchberry dogwood was described as a relic species. It occurred in mature pine, fir, and spruce stands, and after fire, it occurred in early-seral quaking aspen forests colonizing the burned area [92]. On Isle Royale, bunchberry dogwood "thrive(s) better than ever after the destruction of forest cover" by fires consuming only small amounts of the humus layer [46]. Bunchberry dogwood occurred on all burned sites in the boreal region of northwestern Ontario that experienced "intense, fast-spreading" wildfires 1, 4, 14, and 45 years earlier [196]. In coniferous and mixedwood forests of southwestern Nova Scotia, bunchberry dogwood was present in nearly all 1- to 40-year-old burned stands [183]. See the section on Early postfire succession for more information.
Bunchberry dogwood can occur on burned sites 1 to 400+ years old, but abundance of bunchberry dogwood is often lower in the early than the mid- or late stages of forest regeneration. In central Alaska, bunchberry dogwood was considered a late-seral dominant in burned white spruce forests. Researchers surveyed stands from the initiation phase (1-5 years since fire) through the mature hardwood phase (75-95 years since fire) [231]. In the spruce region of interior British Columbia, bunchberry dogwood's frequency index was 8 in stands burned 4 to 22 years prior and 10 in stands burned 37 to 75 years prior. A value of 10 indicated the most uniform distribution [91]. In black spruce forests in northern Saskatchewan, bunchberry dogwood was a dominant in stands burned 11 to more than 30 years prior but not in stands burned less than 10 years prior [253]. In black spruce or jack pine boreal forest stands in northern Quebec, bunchberry dogwood was more common on sites burned 16 to 110 years prior than sites burned less than 10 years prior [81]. In sub-boreal forests of west-central British Columbia, bunchberry dogwood had the highest cover of all herbaceous forest species in all but the youngest stands, although the absolute cover of bunchberry dogwood was greatest in these young stands. Early-seral forests were dominated by lodgepole pine, which rarely lived beyond 200 years. Subalpine fir and Engelmann spruce established in young stands and increased in abundance as forests aged [43].
| Average cover of bunchberry dogwood along a postfire chronosequence in sub-boreal British Columbia [43] | |||||||||
| Forest age (yrs) | 0-50 | 51-100 | 101-150 | 151-200 | 201-250 | 251-300 | 301-350 | 351-400 | 400+ |
| Bunchberry dogwood cover (%) | 11.0 | 4.5 | 6.9 | 10.0 | 3.8 | 1.5 | 4.5 | 3.3 | 7.3 |
Although it is common to find more bunchberry dogwood in the mid- and late stages than in the early stages of a postfire chronosequence, there are exceptions to this trend. Along a postfire chronosequence spanning 80 years in burned jack pine-black spruce forests in northeastern Minnesota, cover was lowest (5%) in 1-year-old burned plots, ranged from 9% to 12% in 2- to 5-year-old burned plots, 12% to 16% in 10- to 20-year-old burned plots, and 8% to 10% in 30- to 80-year old burned plots [3]. In northeastern New Brunswick, there were no consistent differences in bunchberry dogwood abundance with time since fire in jack pine and black spruce stands [176]. Bunchberry dogwood was most abundant in 2-year-old and 140-year-old forest plots in open black spruce stands in western Labrador, compared to 18-, 40-, and 80-year-old stands [257]. In southeastern Labrador, the frequency of bunchberry dogwood was 100% in paper birch stands burned 6 to 100 years prior. Frequency of bunchberry dogwood ranged from 40% to 95% in black spruce or balsam forests unburned for more than 100 years [82].
Bunchberry dogwood often dominated 50- to 200-year-old stands when long postfire chronosequences were evaluated. In white spruce forests in central Alaska, bunchberry dogwood and field horsetail (Equisetum arvense) typically replaced bluejoint reedgrass (Calamagrostis canadensis) and fireweed (Chamerion angustifolium) as the dominant understory species in hardwood stands 51 to 100 years after fire. Bunchberry dogwood persists 100 to 250 years after fire in white spruce-hardwood and mature white spruce/moss stands. The changes occurring once forests age beyond 200 years are relatively unknown because white spruce forests of that age are rare in Alaska's interior [292]. In Isle Royale National Park, the relative cover of bunchberry dogwood was greater in older stands that developed after stand-replacing fire. Bunchberry dogwood was unimportant in 40- and 99-year old stands. Relative cover was 4.3%, 8.9%, and 8.3% in 117-year-old, 170-year-old, and 223-year-old stands, respectively. As stand age increased, the relative densities of balsam fir and white spruce increased and paper birch and quaking aspen decreased [132]. Bunchberry dogwood cover was greatest, although still low, in 167- and 174-year-old stands in a chronosequence spanning 26 to 230 years since stand-replacing fire in southern boreal forests along Lake Duparquet in northwestern Quebec [60].
Fire adaptations and plant response to fire:
Fire adaptations: Bunchberry dogwood survives most fires by sprouting from rhizomes. Postfire seedling establishment on burned sites was not described in the available literature (2011). However, bunchberry dogwood seeds may have survived wildfire in a mixed forest stand in northern Saskatchewan where bunchberry dogwood was present in the prefire community. Burned soil was collected from plots that burned at severities ranging from light surface fire to crown fire. Fourteen bunchberry dogwood stems emerged. Emergents were not identified as developing from remnant vegetative material, as were other emerging species, so they may have developed from seed [13]. Bunchberry dogwood seedlings failed to emerge from soil collected immediately after a spring wildfire in quaking aspen stands in northeastern Alberta. A small number of bunchberry dogwood seedlings (1.9/m²) did emerge from unburned soil, but none emerged from soil collected from lightly or severely burned plots. Note, however, that seedling emergence studies may underestimate bunchberry dogwood seed bank density (see Seed banking) [161]. Bunchberry dogwood sprouted after a prescribed fire in white pine-mixed hardwood stands in Strafford County, New Hampshire, but no bunchberry dogwood seedlings were observed [37].
Rhizome characteristics: Excavation studies from widely separated regions report that bunchberry dogwood rhizomes typically occur about 1.6 to 5 inches (4-13 cm) below the duff or mineral soil surface. For details, see Belowground description.
Bunchberry dogwood rhizomes may be killed by temperatures of 100 °F (38 °C) or above, depending on duration of exposure [77,169]. Bunchberry dogwood rhizomes collected from an average depth of 3 inches (8 cm) in the Acadian forest type in Nova Scotia that were heated to 113 °F (45 °C) for 5 minutes produced more shoots than unheated rhizomes or rhizomes heated to higher temperatures. Rhizomes heated to 122 °F (50 °C) for 5 minutes also sprouted, but those heated to 131 °F (55 °C) and 140 °F (60 °C) were killed. Rhizomes collected in the fall produced more sprouts after heat treatments than those collected in the spring or summer [77]. This finding may relate to seasonal differences in the levels of total nonstructural carbohydrates, which are greatest in the fall (see Seasonal Development). Bunchberry dogwood sprouted after small controlled fires in the Acadian forest conducted in spring, summer, and fall. Temperatures at 0.8 inch (2 cm) into the duff layer reached 131 °F (55 °C) for at least 5 minutes during these fires. In this area, bunchberry dogwood rhizomes were found an average of 3 inches (8 cm) below the litter layer and typically grew into mineral soil [79]. Heat was the suspected cause of ramet death after bunchberry dogwood clones were excavated from a stream bank in Cheboygan County, Michigan, and transplanted to different environments. All ramets planted outdoors beneath shade cloth or in full sun survived, but all ramets planted in the greenhouse died within 2 weeks of transplanting. Greenhouse temperatures exceeded 100 °F (38 °C) on full-sun days [169].
Plant response to fire: Bunchberry dogwood sprouts are common within months of burning, regardless of fire season [37], and increased flower and fruit production have been reported on burned sites ([85], personal observation cited in [280]). Bunchberry dogwood may not survive severe fires that produce long-duration soil heating or short-duration temperature spikes in the soil [77,169]. Increases in bunchberry dogwood abundance are common after fire; sometimes these increases are immediate [161,243], and other times they are delayed [206,294]. Bunchberry dogwood sprouts were "vigorous" after spring and fall prescribed surface fires in white pine-mixed hardwood stands in Strafford County, New Hampshire. Fires did not burn layers beneath the surface litter. Bunchberry dogwood failed to flower in the first postfire growing season [37]. Bunchberry dogwood cover was greater on 5-year-old burned (15%) than unburned (3%) sites in a mature mixedwood boreal forest near Prince Albert National Park, central Saskatchewan. The wildfire burned understory vegetation but did not penetrate deeply into the organic layer [219]. On Isle Royale, bunchberry dogwood was reported on burned sites where the organic layer was not entirely consumed in a balsam fir-paper birch-white spruce stand. The fire burned into the peat layer and killed most trees, but the report did not clearly indicate whether or not bunchberry dogwood was restricted to less severely burned areas [47]. Researchers observed bunchberry dogwood sprouts 2 months after an August wildfire in 200- to 300-year-old red pine and white pine stands. Based on anecdotal observations, the fire was severe, burned into the canopy, consumed crown foliage, and burned through swamps, marshes, and lowland black spruce stands that often serve as fire breaks. Complete consumption of the organic soil layer was reported in some places [173].
Fire severity: High-severity fires that consume a substantial portion of the duff and litter layers may kill bunchberry dogwood plants. Information in the literature is difficult to interpret in regard to fire severity, however, because the studies do not often describe fire severity as it applies to strictly low-growing understory species like bunchberry dogwood. In boreal forests, the degree of forest floor consumption by fire can vary with the degree of smoldering that occurs after the fire front has passed, and depth of burn is often unrelated to the intensity of the fire front [301]. For example, in northern conifer forests of the Boundary Waters Canoe Area, an intense crown fire, during a dry spring when wind speeds were high, burned heavy fuel accumulations but rarely consumed the entire duff layer. The following summer, buncherry was common in burned areas [26].
Several studies report postfire occurrence of bunchberry dogwood after what they variously describe as high-severity fires. Bunchberry dogwood covered large areas 3 to 4 years after a "very severe" summer forest fire near Rangeley Lake, western Maine [259]. Bunchberry dogwood was present within a year of a "very severe" fire in a mixedwood forest in Queen's County, Nova Scotia. The fire crossed the study area 3 times before being extinguished [182]. Bunchberry dogwood was widespread within 16 months of a crown fire in a white spruce stand in the Agassiz Provincial Forest in Manitoba. The May 1981 fire top-killed all vegetation and consumed the peat layer in many sites within the burned area. No living vegetation was observed in the burned area throughout the remainder of 1981 [126]. Bunchberry dogwood occurred within 2 years of an August fire in black spruce forest near Yellowknife, Northwest Territories. The fire was considered "quite severe"; there was persistent smoldering, and "much" mineral soil was exposed [267]. Ten to 11 years after a severe wildfire, which killed nearly all trees in mixed-conifer forest in the Oregon Coast Range, bunchberry dogwood was more frequent in adjacent unburned (14%) than severely burned (8%) forest areas [206].
On some sites, bunchberry dogwood abundance may be reduced by high-severity crown fires. When the composition and abundance of understory vegetation were compared in 36 deciduous, coniferous, and mixed forest sites that burned lightly, moderately, or severely in an early June wildfire in the northern Clay Belt Region of Quebec, bunchberry dogwood was considered an indicator of moderately burned, mixed forests. Lightly burned sites had less than 25% tree mortality, moderately burned sites had 25% to 75% tree mortality, and severely burned sites had more than 75% tree mortality [225]. Bunchberry dogwood was described as an understory dominant before a "high-intensity crown fire" in a jack pine-black spruce stand in the Northwest Territories but was not among the understory dominants described in the 2nd or 4th postfire years [125]. Bunchberry dogwood responded differently after a late June wildfire in two community types at Wickersham Dome in interior Alaska: black spruce and quaking aspen. The fire consumed the majority of tree crowns and blackened 90% of the understory. In black spruce stands, bunchberry dogwood cover and frequency were not much different between unburned and "heavily" burned plots by the 2nd postfire growing season, and they were greater on burned than unburned plots by the 4th postfire growing season. In quaking aspen, however, bunchberry dogwood cover was 3.5% to nearly 5% lower on burned than unburned plots in the first 4 postfire growing seasons [295].
Bunchberry dogwood abundance was also greater on low-severity than high-severity burns when severity was measured in the ground layer [161,302]. Cover of bunchberry dogwood was greater on low-severity burned (7.56%) than high-severity burned (5.65%) and unburned (3.16%) patches 2 years after a spring wildfire in quaking aspen stands in northeastern Alberta. No bunchberry dogwood seedlings emerged from low-severity or high-severity burned soils collected immediately after the fire, although seedlings did emerge from unburned soil. Vegetative sprouts emerged from high-severity burned (0.10 sprouts/m²), low-severity burned (0.17 sprouts/m²), and unburned (0.17 sprouts/m²) soil samples. The low-severity fire killed all aboveground plant parts, partially oxidized small and medium-sized downed wood, and consumed 0.8 inch (2 cm) of the organic soil layer. The high-severity fire consumed all aboveground vegetation, oxidized woody material over 8 inches (20 cm) in diameter, and consumed 2 to 4 inches (6-10 cm) of the organic soil layer [161]. When burned plots were evaluated in the first 4 years after a May wildfire in quaking aspen-mixed conifer stands in southeastern Manitoba, average bunchberry dogwood cover and frequency were greatest on low-severity burned plots (6.9% and 73%), least on high-severity burned plots (0.9% and 26%), and intermediate on scorched plots (4.3% and 60%) that burned at the lowest severity. The fire was stand replacing, and degree of forest floor consumption was used to classify fire severity. On scorched plots, litter was only partly burned. On low-severity burned plots, litter was burned, but duff consumption was limited. On high-severity burned plots, litter was entirely consumed, and duff was partly consumed [301,302].
Bunchberry dogwood recovered to prefire frequencies sooner on low- than high-severity burns in jack pine, spruce-fir, and quaking aspen forest types in Minnesota's Superior National Forest. Time since fire varied from 4 months to 5 years. On severely burned plots, all foliage, small branches (≥0.5 inch (1.3 cm) in diameter), litter, and duff were consumed, and mineral soil was exposed. On lightly burned plots, only loose litter was consumed, and crown scorch was slight. Bunchberry dogwood frequency averaged 90% to 93% on 2 unburned sites, 67% on a 2-year-old, severely burned site, and 97% on the 5-year-old, severely burned site. Frequency averaged 97% to 100% on 4-month old, lightly burned plots [4].
In a beetle-attacked Lutz spruce (Picea × lutzii) stand in the Chugach National Forest in south-central Alaska, bunchberry dogwood was less abundant 7 years after a June prescribed fire than 4 years before the fire, but it also decreased on unburned plots. Most living and beetle-killed trees were still standing at the time of the fire. All aboveground overstory and understory vegetation was top-killed in the fire, and mineral soil was exposed in spots [127].
| Frequency and cover of bunchberry dogwood on burned and unburned plots evaluated before and after a prescribed fire in Alaska [127] | ||||
| Time of evaluation | Burned | Unburned | ||
| Frequency | Cover | Frequency | Cover | |
| Before fire (4 yrs) | 59 | 15 | 54 | 8 |
| After fire (7 yrs) | 47 | 9 | 23 | 6 |
Fire season: The few studies that evaluated the effects of different fire seasons in bunchberry dogwood habitats suggest that growing-season fires may impact bunchberry dogwood populations more severely than dormant-season fires. In mixed conifer-hardwood forests in Minnesota's Superior National Forest, the frequency of bunchberry dogwood on summer-burned plots did not reach that of unburned plots by the 11th postfire year, but on spring-burned plots the frequency of bunchberry dogwood exceeded unburned levels by the 3rd postfire year. The spring fire burned in late April when the ground was cold and moist and winds were strong and burned little, if any, of the duff layer. The summer fire burned in July, and although it was described as "hot", there was little to no duff consumption [144]. In jack pine stands in northeastern Minnesota, bunchberry dogwood occurred in the first postfire growing season after a spring wildfire (14 May) but not after a summer wildfire (27 July). However, prefire comparisons were lacking, so it is unclear whether or not the summer fire killed bunchberry dogwood plants. Surface-fire severity was greater in stands burned in the summer where virtually all litter and duff was consumed. The spring fire consumed primarily the litter layer. Both fires burned during dry, windy (23-49 feet (7-15 m)/s) conditions and resulted in "intense" crowning. Average bunchberry dogwood biomass ranged from less than 0.1 to 4.1 g/m² and density ranged from 0.2 to 44.8 stems/m² in spring-burned stands. Bunchberry dogwood was not reported in either of the 2 summer-burned stands [213]. Bunchberry dogwood importance was greater after fire than before fire in the first postfire growing season on fall-burned, spring-burned, and unburned plots in white pine and white pine-mixed hardwood stands in eastern New Hampshire. Increases were greatest in fall-burned plots and relatively small in spring-burned and unburned stands. Prescribed fires were low severity, "relatively cool, surface headfires". Average litter consumption for fall and spring fires was similar among stands: In burned mixed stands, 1.4 inches (3.5 cm) of litter was consumed and 1 inch (2.5 cm) remained; in burned white pine stands, 1 inch (2.5 cm) of litter was consumed and 0.6 inch (1.5 cm) remained [243].
Early postfire succession: The following studies present information on early postfire succession (primarily the first 10 postfire years). For longer postfire succession studies, see Fire-related forest succession.
Bunchberry dogwood is generally present in recently burned sites, but it may take several years to reach prefire or unburned abundance levels. Bunchberry dogwood appeared within 11 months of a wildfire in a balsam fir stand in northwestern Newfoundland, and within 10 years of the fire, formed an "almost complete carpet" in the understory layer. The wildfire lightly charred the surface layer [294]. Bunchberry dogwood frequency generally increased with each successive postfire sampling in the 3 years following a summer fire (1989) in black spruce/lichen and jack pine/lichen forests in northern Quebec's Ecomiak Lake area. In the 2nd postfire year, bunchberry dogwood frequency was evaluated multiple times over the growing season. Frequency was greatest in the last summer (August) sampling period [258].
| Frequency (%) of bunchberry dogwood in the first 3 postfire growing seasons after a summer fire (1989) in northern Quebec [258] | ||||||
| Sampling date | July 1990 | June 1991 | July 1991 | August 1991 | July 1992 | July 1993 |
| Black spruce/lichen | 0.6 | <0.5 | <0.5 | 0.9 | 2.1 | 3.1 |
| Jack pine/lichen | 1.6 | 1.1 | 2.8 | 4.4 | 7.2 | 7.9 |
Cover of bunchberry dogwood typically peaked in the 2nd or 3rd postfire year after spring and summer fires in spruce-willow-birch vegetation in northern British Columbia and southern Yukon Territory. Cover and frequency declined in the 3rd or 4th postfire year, but bunchberry dogwood persisted as stands reached maturity [215]. Findings were similar when burned barrens vegetation in Newfoundland was evaluated 0.5 to 37 years after fire [220].
| Frequency and cover of bunchberry dogwood on burned sites as time since fire increased in western Canada [220] | ||||||
| Time since fire (years) | 0.5-1 | 2-5 | 6-9 | 10-19 | 20-36 | 37 |
| Frequency (%) | 64 | 92 | 67 | 71 | 86 | 70 |
| Cover (%) | 3 | 13 | 6 | 6 | 6 | 3 |
Effects of multiple fires: Two studies that evaluated twice-burned bunchberry dogwood habitats suggest that frequent fire may reduce bunchberry dogwood abundance. Bunchberry dogwood density and frequency were reduced 2 months after a June prescribed fire in a red and white pine stand at Ontario's Petawawa Forest Experimental Station. Bunchberry dogwood abundance was further reduced after a 2nd June fire the following year. Bunchberry dogwood density, frequency, and biomass remained below prefire levels 14 months after the 2nd fire. Prescribed fires were described as "gentle" and consumed all vegetation less than 12 inches (30 cm) tall. Fires burned into the litter but not into the duff. The 1st fire consumed 21% of the total available fuel and the 2nd fire consumed 5% of total available fuel. Fire intensities were similar, about 18 kcal/m/s [197]:
| Density, frequency, and biomass of bunchberry dogwood before and after 1 and 2 fires in pine stands in Ontario [197] | ||||
| Time since fire(s) | Prefire | 2 months after 1st fire | 2 months after 2nd fire | 14 months after 2nd fire |
| Density (stems/ha) | 1,602 | 247 | 129 | 301 |
| Frequency (%) | 39 | 18 | 9 | 15 |
| Biomass (kg/ha) | 35.2 | ----* | ---- | 3.9 |
| * Not measured. | ||||
Bunchberry dogwood was more abundant in unburned quaking aspen stands than in stands burned twice in 5 years in central Alberta, while abundance was similar on unburned stands and stands burned only once in the same period. Bunchberry dogwood cover and frequency in the twice-burned stand were about 7% and 8% lower, respectively, than in unburned stands. The 1st prescribed fire occurred in fall 1972, produced an average depth of burn of 0.6 inch (1.5 cm) and a fireline intensity of 236 kW/m. The 2nd prescribed fire occurred in spring 1978, produced an average depth of burn of 1.4 inch (3.5 cm) and a fireline intensity of 4,392 kW/m, which is characteristic of a high-intensity surface fire [227]. See the Summary of this fire study by Quintilio and others for additional details.
Logged and burned sites: Bunchberry dogwood often persists after logging and burning [5,40,102] and may increase [102,117] unless the fire is severe [5,67]. Bunchberry dogwood occurred on cut and burned jack pine and black spruce stands in Manitoba and Saskatchewan that ranged from 2 to 5 years since the last disturbance [40,41,42]. In old-growth western hemlock-Sitka spruce forest in southeast Alaska, bunchberry dogwood cover was 5% greater on 7-year-old logged-and-burned than on logged-and-unburned sites. Winter logging removed saplings, pole-sized trees, snags, and left a moderate amount of slash. The "light" burn occurred in July after 6 days without rain [117]. Bunchberry dogwood density was about 70 stems/transect greater and cover was about 1% greater than predisturbance levels after clearcutting and 2 consecutive fires in a 25-year-old balsam fir-red pine woodlot in southwestern New Brunswick. Frequency of bunchberry dogwood, however, was about 7% lower than predisturbance levels on the cut and twice burned site [102]. In jack pine stands in Minnesota's Superior National Forest, bunchberry dogwood frequency increased on logged-and-burned sites when soil surface temperatures during slash burning were less than 900 °F (480 °C) but decreased when burning produced soil surface temperatures above 900 °F (480 °C). At the site of the cooler burn, the predisturbance frequency of bunchberry dogwood was 87%. In the first 3 postdisturbance years, bunchberry dogwood frequency was 90% to 100%. At the site of the hotter burn, the predisturbance frequency of bunchberry dogwood was 90%. In the 1st postdisturbance year, bunchberry dogwood frequency was 7%, and in the 2nd postdisturbance year was 63% [5]. Bunchberry dogwood was not present in the 1st or 2nd years after clearcutting and fire in the floodplain white spruce forest type on Willow Island near Fairbanks, Alaska. Prior to the disturbances, bunchberry dogwood cover was 5.7% and frequency was 90%. The fire was considered severe; researchers reported ash and mineral soil but no charred material on the burned site [67].
On logged-and-burned sites, it is common for bunchberry dogwood abundance to increase as time since disturbance increases. However, the length of time for increases varies. In clearcut and slash-burned old-growth Douglas-fir forest in Oregon's HJ Andrews Experimental Forest, frequency of bunchberry dogwood generally increased rapidly in the first 4 to 5 postfire years but increased more slowly thereafter [314]. On northern Vancouver Island, the above- and belowground bunchberry dogwood biomass increased as time since fire increased from 2 to 8 years in clearcut-and-burned areas. Aboveground bunchberry dogwood biomass averaged 2 lbs (1 kg)/ha on 2-year-old, 57 lbs (26 kg)/ha on 4-year-old, and 895 lbs (406 kg)/ha on 8-year-old clearcut-and-burned plots. Biomass was significantly greater on 8-year-old than 2- or 4-year-old sites (P<0.05). Fine root biomass and small rhizome biomass increases were large between the 4th and 8th postdisturbance years [195].
Cover of bunchberry dogwood exceeded prefire levels 3 to 5 years after clearcutting and burning in subalpine fir and western white spruce (Engelmann spruce × white spruce) forest sites in British Columbia. Sites were clearcut in the winter and burned the following summer. Bunchberry dogwood cover increased with time since disturbance up to the 5th or 10th postfire year [108,109]:
| Average cover (%) of bunchberry dogwood after fire in clearcut forest sites in British Columbia [108] | ||||||
| Site as described by burn characteristics | Time since disturbance (years) | |||||
Before fire, |
1 | 2 | 3 | 5 | 10 | |
| Fairly low severity; 28.5% woody fuel consumption; average depth of burn: 1 cm [108] | 8.00 | 3.0 | 7.5 | 10.2 | 11.7 | 2.2 |
| Moderate severity; 47.7% woody fuel consumption; average depth of burn: 3.8 cm [109] | 10.0 | 2.2 | 4.3 | 8.0 | 12.5 | 15.0 |
| Moderate severity; 58.6% woody fuel consumption; average depth of burn: 1.8 cm [109] | present (no cover data) |
1.5 | 2.2 | 3.0 | 4.8 | 6.2 |
Comparing canopy-removal disturbances: When studies compared burned, logged, and logged-and-burned sites, there were no clear trends in bunchberry dogwood abundance. Three studies reported greater bunchberry dogwood cover on burned than logged or logged-and-burned sites [101,139,146], and 3 reported lower bunchberry dogwood cover on burned than logged or logged-and-burned sites [106,135,216]. In another study, bunchberry dogwood cover was greater on burned (21%) than logged-and-burned (17%) stands about 4 years after disturbance in jack pine-black spruce stands in the Superior National Forest in Minnesota; and greater on logged-and-burned (9%) than burned (2%) stands about 14 years after disturbance in jack pine-black spruce stands in Quetico Provincial Park, Ontario [211].
Bunchberry dogwood was significantly (P<0.05) more abundant in burned than clearcut quaking aspen stands in the southern boreal region of Ontario. Researchers determined that prior to the disturbances physiographic, soil, and stand characteristics were not significantly different between burned and clearcut stands at the site, stand, or landscape scales. Sites were visited 3 years after disturbances. Logging occurred in summer or winter of 1996 or 1997, and wildfires burned as crown fires in late May or early June 1997 [101].
Cover of bunchberry dogwood was greater on burned than logged stands in boreal mixedwoods in southeastern Manitoba but was greatest in stands impacted by spruce budworm. Sites were disturbed 10 to 15 years prior to the study. Bunchberry dogwood cover averaged 6.3% in stands severely impacted by spruce budworm, 2.9% in burned stands, and 1.1% in logged stands. Researchers thought that gradual increases in light by canopy removal from budworm may have promoted increases in established understory species, whereas rapid increases in light from immediate canopy removal may have favored increases and establishment of fast-growing, shade-intolerant species [139].
In midboreal mixedwood stands burned by wildfire in Alberta, bunchberry dogwood cover was significantly (P≤0.03) greater on sites that were not salvage logged than on sites that were salvage logged. In early postdisturbance succession, density of deciduous saplings was greater and litter cover and downed wood were less on burned than burned-and-logged sites. In mid-seral stands, organic matter depths and soil moisture were greater and woody stem densities were less on burned than burned-and-logged sites [146].
| Average cover and frequency of bunchberry dogwood on burned and burned-and-salvage logged sites 2 and 34 years after a wildfire in midboreal mixedwood stands in Alberta [146] | ||
| Burned | Burned and logged | |
| Early-seral stands (2 years since fire) | ||
| Cover (%) | 6.7 | 3.6 |
| Frequency (%) | 100 | 93 |
| Mid-seral stands (34 years since fire) | ||
| Cover (%) | 5.7 | 3.5 |
| Frequency (%) | 100 | 85 |
Bunchberry dogwood cover was much lower before than 2 to 11 years after prescribed fire in a clearcut Engelmann spruce-subalpine fir stand in interior British Columbia. The fire was considered severe. Average total woody fuel consumption was 53%, and the average depth of burn was 1.2 inches (3.1 cm). Stands were clearcut in winter 1987-88, vegetation was measured in the 1988 growing season, and the prescribed fire burned in mid-September 1989. Bunchberry dogwood cover averaged 6.1% before the fire in the clearcut area, and 11%, 20.5%, and 27.9% in the 2nd, 5th, and 11th postfire years, respectively [106]. Findings were similar 2 to 10 years after slash burning in a clearcut area in the very wet and cool zone of the sub-boreal forest in northern British Columbia. Bunchberry dogwood cover was less than 1% after winter clearcutting operations, but 5 years after slash burning, bunchberry dogwood cover increased to 5.4% and 8.2% in logged areas where the forest floor remained intact and on skid roads where mineral soil was exposed, respectively. Slash burning occurred in mid-August following clearcutting in the previous winter. Average woody fuel consumption was 32%. Average forest floor consumption was 17%, and the average depth of burn was about 0.8 inch (2 cm) [107]. For additional details on these studies, see the original research papers by Hamilton (2006a and 2006b).
Relative bunchberry dogwood cover was greatest on clearcut sites when researchers compared undisturbed, clearcut, burned, and clearcut-and-burned black spruce stands in northwestern Ontario. Relative cover of bunchberry dogwood was less than 1% in 60- to 70-year-old, undisturbed forest, 1% in wildfire-burned forest, and 13% in clearcut forest. Bunchberry dogwood was absent from clearcut-and-burned stands. Surface fuel was more abundant and the wildfire much more intense in the clearcut (71,000 kW/m calculated) than in uncut stands (21,000 kW/m). The wildfire burned on 12 June when the temperature was 88 °F (31 °C), relative humidity was 52%, and wind speeds were 11 km/h [135]. Bunchberry dogwood density was highest on winter-logged sites when control, spring-logged, winter-logged, and winter-logged-and-burned sites were compared in the spruce-fir zone at the University of Minnesota's Cloquet Forestry Center. Density of bunchberry dogwood in the 1st and 2nd years after spring logging and in the 1st year after burning the winter-logged site was significantly (P<0.05) lower than bunchberry dogwood density on control and winter-logged sites [216].
No differences in bunchberry dogwood frequency were found between undisturbed, clearcut, and burned portions of black spruce forest in central Quebec, but bunchberry dogwood frequency increased with time since disturbance in burned-and-logged stands. Frequency of bunchberry dogwood was about 60% less in areas burned 2 years earlier than in areas burned 14 years earlier and in areas logged 5 years earlier than in areas logged 16 years earlier [209].
Fuels: Bunchberry dogwood fuel characteristics were not reported in the available literature (2011). In boreal forests, bunchberry dogwood is probably unimportant as a fuel that drives or affects fire behavior.
In northeastern Minnesota, bunchberry dogwood plants growing in mature forests averaged 270% moisture content from 24 June to 24 July and 237% from 25 July to 26 August. The area experienced a late summer drought. The moisture content of bunchberry dogwood was moderate compared to the other 20 understory species evaluated [170].
Fire regimes: Bunchberry dogwood occurs in forests with varied presettlement fire regimes, including those characterized predominantly by stand-replacement fires with long fire-return intervals, such as Sitka spruce-western hemlock forests; those characterized by stand-replacement fires with relatively short fire-return intervals, such as spruce-fir and jack pine forests; and those characterized by surface fire regimes such as mixed conifer forests. In the boreal forests of North America, bunchberry dogwood typically persists regardless of fire cycle length [244]. The Fire Regime Table provides information on fire regimes of plant communties in which bunchberry dogwood occurs. For further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
Western North America: In the various forest types that provide bunchberry dogwood habitat in the western half of North America, the average fire-return interval can range from 20 to more than 1,000 years. In western Montana, bunchberry dogwood occurred in moist lower subalpine, grand fir, western redcedar, and western hemlock forest types, where fires are generally infrequent but severe [75]. In the cedar-hemlock zone of northern Idaho, average fire frequency can range from 25 to more than 500 years. Early-seral forests are more likely to have low-intensity surface fires at shorter intervals compared to late-seral or climax forests, which are more likely to have stand-replacement fires at longer intervals [254]. See the Fire Regime Table for more information on fire regimes for communities in which bunchberry dogwood may occur.
Fire regime information for plant communities in Canada and Alaska where bunchberry dogwood is common are not described in the Fire Regime Table. Fire history was studied on many sites in the Mackenzie Valley, Northwest Territories. Lightning was the most common cause of fire, and lightning fires were common from June to August. Fire size and annual area burned were typically large. The fire regime in a site dominated by mixedwood and pine forests was characterized by surface fires at intervals of 20 to 30 years. Fires became less frequent after about 1925. At a site dominated by black spruce forests, the average fire-return interval was estimated at 80 to 100 years. Nearly all of the study area dominated by black spruce forests burned in the last 180 years except for some high-elevation and floodplain sites. Throughout the Valley, size of the area burned increased northward, but fire frequency did not. Researchers reported that for their record of fires in the Valley from about the mid 1850s to 1973 "there are years with scarcely any fires and years when all hell breaks loose" [245]. Based on pollen and charcoal records, researchers found that 12 large fires burned in the boreal white spruce forests around Rainbow Lake, northeastern Alberta during the past 840 years. The interval between fires ranged from 30 to 130 years, and the average fire-return interval was 69 years [156].
Eastern North America: The average presettlement fire-return interval in forest types that provide bunchberry dogwood habitat in eastern North America also ranges from 20 to more than 1,000 years. A 1000-year charcoal record from the Lake of the Clouds in the Boundary Waters Canoe Area indicated that on average, the area's pine- and spruce-dominated forests burned every 70 to 80 years. The range of time between fires, however, ranged from 20 to more than 100 years. There was no evidence that fire frequency had changed much in this area with European settlement, over the past 300 years [280]. However, this was not the case throughout bunchberry dogwood's range (see Fire regime changes). In an upland jack pine forest in the boreal region of northwestern Ontario, fire scars recorded 10 major fires from 1848 to 1967 in the Sachigo Hills study area. The shortest period between fires was 5 years and the longest was 30 years [174]. In southern boreal forests along Lake Duparquet in northwestern Quebec, the fire cycle is 100 years (Bergeron 1991 cited in [60]). Quaking aspen, white spruce, and balsam fir are typical in early-seral forests and may persist 200 years, but at about 100 to 150 years after fire, balsam fir and northern white cedar increasingly dominate the overstory [60].
In the studies that described fire behavior in bunchberry dogwood habitats in the eastern half of Canada, characteristics ranged from small, patchy fires in boreal forests of interior Labrador [83] to surface fires and active crown fires in jack pine stands in Ontario [271]. From 1870 to 1980, 80 fires burned 21% of the 18,700-mi² (48,500 km²) boreal forest region of interior Labrador. Because the interior region is largely uninhabited wilderness, nearly all fires were ignited by lightning and burned without human interference. In the study area, the fire cycle was slightly greater than 500 years. Growing-season precipitation was lowest during the year in which the largest area burned. Most fires were small and patchy. Fires were more common in open regenerating forests than in mature unburned forests. It was common for only the fringes of dense, mature forests to burn. Although fires occurred from early June to late October, ignitions peaked in late June and early July. Fall fires were often extinguished quickly by rain or snow [83]. Researchers monitored fire behavior during 12 experimental fires in mature jack pine stands with a black spruce understory near Kenshoe Lake, Ontario. Surface fuel loads were moderate in the stands. Fires occurred between late May and early June, when it had been 1 to 15 days since last rain. At the time of the fires, air temperatures ranged from 60 to 85 °F (10-29 °C), relative humidity was 30% to 48%, and wind speeds were 2 to 18 miles (3-29 km)/hour. Fire behavior included surface fires with slow or moderate spread rates and a low frontal fire intensity (<500 kW/m), surface fires with intermittent torching and crowning and moderate intensity (500-3000 kW/m), and active, high-intensity, crown fires (>4000 kW/m). Depth of burn ranged from 0.7 to 2 inches (1.8-5.1 cm) and was greatest for fires that burned after the longest period without rain [271].
Fire regime changes: Fire regimes in many bunchberry dogwood habitats have been altered by human settlement, development, and active fire suppression. Reduced fire frequency during the past century was reported in bunchberry dogwood habitats in Canada [23], Washington, Oregon [72], and Minnesota [121], but changes are likely not limited to these areas.
When information from literature reviews, contemporary databases, and modeling was used to compare historical (after 1714), current (1959-1999), and predicted future fire frequencies in Canadian boreal forests, researchers found that current fire frequencies were significantly lower than historical. Climate changes and improved fire protection were suggested as the reasons for reduced fire frequency from historic to current times. Historical fire information came from 18 study areas throughout Canada's boreal forest region, and the current fire frequency was estimated from a large Canadian fire database. With modeling, researchers predicted that the relationship between future fire activity and potential changes in climate would vary regionally. Increased temperatures were associated with predictions of increased fire frequency. However, in areas where increased temperatures were combined with increased frequency and amount of precipitation, increased moisture was predicted to moderate the effects of temperature increases so that fire frequency would be unchanged or even decreased [23].
Fire history studies completed in the 1970s in wilderness areas in the Cascade Range of Washington and Oregon suggest that fire frequency decreased between 1910 and 1969. The Pasayten Wilderness in north-central Washington is about 400,000 acres (160,000 ha) and supports a subalpine fir climax forest type. Between 1910 and 1969, the Wilderness averaged 4.5 lightning and 0.6 human-caused fires/year and burned a little less than 100,000 acres. The largest area burned by large fires was from 1920 to 1929, and no area burned in large fires from 1960 to 1969. The last lightning fire that burned more than 1,000 acres (400 ha) occurred in the 1950s. The Mt Jefferson Wilderness in west-central Oregon is about 100,000 acres (40,000 ha) and mostly occurs at elevations over 5,000 feet (1,500 m). Climax forest types are the true fir-mountain hemlock and western hemlock-western redcedar types. Between 1910 and 1969, Mt Jefferson averaged 3 lightning and 1.4 human-caused fires/year and less than 8,000 acres (3,000 ha) burned. All Mt Jefferson fires larger than 40 acres (16 ha) occurred from 1911 to 1924 [72].
From 1542 to 1972 in the Boundary Waters Canoe Area (BWCA), the average time between fire years was 6.1 years, and the average time between large fire years (>100 square miles burned) was 48 years. The natural fire rotation was about 100 years for the BWCA, which is about 800,000 acres (300,000 ha). The time between fires decreased from the presettlement (1727-1868) to settlement period (1868-1910) and increased from the settlement to suppression period (1911-1972). The average fire-return interval in pine stands where at least some trees survived was 36 years but ranged from 5 to 100 years. Large upland ridge sites with jack pine, black spruce, aspen, or birch stand burned most frequently or intensely. Swamps, valleys, ravines, and the east, northeast, north, and southeast sides of large lakes and streams burned least frequently or intensely. In these areas, white pine, red pine, white spruce, and northern white cedar stands were typical [121].
FIRE MANAGEMENT CONSIDERATIONS:
FEDERAL LEGAL STATUS:
None
OTHER STATUS:
Information on state- and province-level protection status of plants in the United States and
Canada is available at
NatureServe.
IMPORTANCE TO WILDLIFE AND LIVESTOCK:
Bunchberry dogwood can be an important forage species for caribou, moose, elk, and deer [15,53,140,249,298]. Bunchberry dogwood fruits are utilized by bears, small mammals, and many bird species [181,250,299]. Bunchberry dogwood does not appear to be a food source for cattle [140].
Ungulates: A variety of large ungulates in northern North America utilize bunchberry dogwood. Caribou dig through snow to feed on bunchberry dogwood in the winter in British Columbia (review in [141]). On the Slate Islands of Lake Superior in Ontario, caribou frequently feed on bunchberry dogwood in the spring [53]. Two out of 20 rumens from caribou taken from the Northwest Territories, northern Saskatchewan, and northern Manitoba had bunchberry dogwood, and weight of bunchberry dogwood was only a trace of the contents recovered [252]. Reviews report that bunchberry dogwood is eaten by moose [15,232]. When researchers observed feeding by 3 tame moose on Kenai Peninsula, bunchberry dogwood was occasionally eaten from May to October [165]. On Brunette Island, Newfoundland, 15% of the bites taken by a tame moose over 50 minutes were from bunchberry dogwood and Lapland cornel [34]. In eastern Maine, bunchberry dogwood was early winter moose forage [160]. A review reports that the average percentage of bunchberry dogwood in the diets of elk in British Columbia and Washington was greatest in the summer (5.4%) and fall (3.9%). Bunchberry dogwood was much less common in winter (0.5%) and spring (1.4%) diets [134]. In northern Idaho, elk and white-tailed deer fed on bunchberry dogwood more in grand fir than western redcedar forests. In grand fir forests, the composition of early fall diets for elk and white-tailed deer averaged 7.4% and 8.7% bunchberry dogwood, respectively. Bunchberry dogwood was not a part of mid-summer elk diets and made up only 3.6% of white-tailed deer diets in mid-summer [140].
Deer use of bunchberry dogwood can be extensive. Bunchberry dogwood is considered highly preferred and is highly digestible by mule deer in British Columbia and Alaska [212]. In western hemlock forests on warm winter-use areas of the Tongass National Forest, Alaska, severe browsing by mule deer can result in the "virtual elimination" of bunchberry dogwood from the understory [184]. Mule deer feces collected from Admiralty Island in southeastern Alaska were 33%, 26%, 14%, and 19% bunchberry dogwood in the winter, spring, summer, and fall, respectively [111]. In the Upper Swan Valley of northwestern Montana, bunchberry dogwood was an important component of white-tailed deer diets in the fall [203,204]. On a reclaimed mine site in west-central Alberta, white-tailed deer and mule deer feces contained 31.2% bunchberry dogwood, but no bunchberry dogwood was recovered from moose feces collected from the same area [239]. bunchberry dogwood made up at least 4% of the rumen contents from 14 white-tailed deer killed in northeastern Minnesota [308]. On Anticosti Island, Quebec, bunchberry dogwood was significantly (P<0.05) associated with fenced areas of a clearcut located 79 to 3,020 feet (24-919 m) from the edge of a balsam fir-spruce forest. White-tailed deer and snowshoe hares were excluded by fencing. Immediately after cutting, bunchberry dogwood cover averaged 2.9% in fenced and 1.1% in unfenced areas; 8 years after cutting, cover averaged 9.8% in fenced and 1.6% in unfenced areas [36].
Bear: Bunchberry dogwood fruits are eaten by black bears from Alaska and Oregon to Newfoundland. Use may be greatest in the late summer or fall. On the Kenai Peninsula, bunchberry dogwood frequency and volume in fall black bear diets were 5.9% and 37.5%, respectively, in a northern coniferous forest that had burned 10 to 15 years earlier. Bunchberry dogwood was not found in spring or summer black bear diets or in scat collected on a less recently burned forest area [250]. In fecal samples collected in northeastern Oregon, bunchberry dogwood fruits made up 4% or less of the volume of black bear diets [32]. In black bear scat collected from spring through fall in Gros Morne National Park and Terra Nova National Park in Newfoundland, the frequency of bunchberry dogwood ranged from 0% to 15.7%. Scat was collected for 3 to 4 years. Frequency of bunchberry dogwood was greater in late-summer and fall collections than spring-summer collections, and in one year, no bunchberry dogwood was recovered in any season [58].
Small mammals: Chipmunks, martens, cottontails, and snowshoe hares feed on bunchberry dogwood stems and fruits [284,299,306,313]. In Michigan, chipmunks presented bunchberry dogwood fruits fed on them "eagerly" (Gorchov personal communication cited in [262]). Bunchberry dogwood was not recovered from all marten scat collected over a 6-year period in northwestern Glacier National Park, but a high of 3.6% bunchberry dogwood was recovered from one collection [306]. Marten scat collected from Algonquin Park, Ontario, occasionally contained bunchberry dogwood seeds; however, when a released marten was observed feeding, it ignored ripe bunchberries [86]. In western Massachusetts, damage to bunchberry dogwood was rated as severe after 6 years of eastern cottontail feeding [281]. In the winter near Syracuse, New York, eastern cottontails fed "sparingly" on bunchberry dogwood [284]. Based on the analysis of stomach contents, the diet of snowshoe hares near Fairbanks, Alaska, was 1.8% bunchberry dogwood in April, 2.6% in May, and 1% in the fall [313].
Birds: Bunchberry dogwood is utilized by song and game birds. Bunchberry dogwood fruits are eaten by thrushes, veeries, vireos, and grouse [181]. In Alaska, the occurrence of bunchberry dogwood flowers in the crops of spruce grouse was 4.3% in the spring, and the occurrence of bunchberry dogwood fruits was 3.9% in September and 9.3% in October [69]. From ruffed grouse droppings collected from the University of Idaho's Experimental Forest in Latah County, researchers determined that utilization of bunchberry dogwood seeds averaged 13% in the last half of July, 27% in the first half of August, and 29% in the last half of August. Availability of bunchberry dogwood averaged 23% [129]. Ruffed grouse fed on bunchberry dogwood in the spring in the boreal forest region of northern Minnesota [279], and sharp-tailed grouse fed on bunchberry dogwood fruits in the winter in Quebec [248]. Other researchers report that bunchberry dogwood is often found in the vegetation surrounding American woodcock nests [194].
Palatability and nutritional value: Palatability information was generally unavailable as of 2011. After reviewing the available literature, Strong and Gates [274] suggested that the preference rating for bunchberry dogwood was low for elk and low to moderate for moose.
Digestibility and nutritional content of bunchberry dogwood vary with season and site conditions. In Sitka spruce-western hemlock forests in southeastern Alaska, the 48-hour digestibility of bunchberry dogwood averaged 65.9%, and bunchberry dogwood was most digestible in the winter [110]. Based on captive feeding trials for black bears and grizzly bears, researchers determined that the dry matter digestibility of bunchberry dogwood fruits was 45.6% [307]. In southeastern Alaska, the chemical composition of bunchberry dogwood leaves varied significantly with stand age and season (P<0.001). Generally, nitrogen and potassium levels were greater in older than younger stands and decreased from spring to fall. Nonstructural carbohydrate and astringency levels increased with increasing stand age [293]. Studies from the same region reported that bunchberry dogwood from an old-growth forest had significantly greater nitrogen content and digestibility than bunchberry dogwood from scrub stands (Schoen and Kirchhoff 1984 cited in [249]) and that protein content of bunchberry dogwood leaves was 27% greater from a forest site than an adjacent clearcut (personal observation cited in [234]). The average crude protein content of bunchberry dogwood pulp was 4.3% and of seed was 10% from fruits collected in Idaho, Washington, Montana, and/or Alaska [307]. Bunchberry dogwood fruits collected from northern Ontario had fat, protein, and soluble carbohydrate levels intermediate among the 16 other species evaluated [291]. On Admiralty Island, Alaska, bunchberry dogwood was considered an important and nutritious winter food for mule deer [249]. For a comparison of the seasonal composition of bunchberry dogwood in southeastern Alaska, see Hanley and McKendrick [110], and for a comparison of the chemical composition of bunchberry dogwood in New Hampshire, Wisconsin, and Canada, see Siccama and others [256].
VALUE FOR REHABILITATION OF DISTURBED SITES:
No information is available on this topic.
OTHER USES:
Although edible, bunchberry dogwood fruits are nearly flavorless [68,145]. They have been important in the diets of Eskimos of the Northern Bering Sea and Arctic regions of Alaska [10], Indians of the Nuxalk Nation in British Columbia [164], and Coast Salish Indians on Vancouver Island [288]. Bunchberry dogwood leaves have been smoked as a tobacco substitute. A tea from bunchberry dogwood roots has been used to treat colic in infants. In ancient Scotland, bunchberry dogwood was referred to as the "plant of gluttony" because it was thought to increase the appetite [238]. The Hoh and Quileute Indians of the Olympic peninsula used bunchberry dogwood fruits in ceremonies, bunchberry dogwood bark in medicinal teas, and bunchberry dogwood leaves as an intoxicating smoke [230].
OTHER MANAGEMENT CONSIDERATIONS:
Experimental findings suggest that bunchberry dogwood may improve conditions in areas impacted by acid rain. When 4 boreal forest understory species were compared, bunchberry dogwood leaves neutralized experimental acid rain treatments most [90].
| Fire regime information on vegetation communities in which bunchberry dogwood may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [155], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||||||||
|
|||||||||||||
| Pacific Northwest | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Pacific Northwest Forested | |||||||||||||
| Sitka spruce-western hemlock | Replacement | 100% | 700 | 300 | >1,000 | ||||||||
| Douglas-fir (Willamette Valley foothills) | Replacement | 18% | 150 | 100 | 400 | ||||||||
| Mixed | 29% | 90 | 40 | 150 | |||||||||
| Surface or low | 53% | 50 | 20 | 80 | |||||||||
| Douglas-fir-western hemlock (dry mesic) | Replacement | 25% | 300 | 250 | 500 | ||||||||
| Mixed | 75% | 100 | 50 | 150 | |||||||||
| Douglas-fir-western hemlock (wet mesic) | Replacement | 71% | 400 | ||||||||||
| Mixed | 29% | >1,000 | |||||||||||
| Mountain hemlock | Replacement | 93% | 750 | 500 | >1,000 | ||||||||
| Mixed | 7% | >1,000 | |||||||||||
| Pacific silver fir (low elevation) | Replacement | 46% | 350 | 100 | 800 | ||||||||
| Mixed | 54% | 300 | 100 | 400 | |||||||||
| Pacific silver fir (high elevation) | Replacement | 69% | 500 | ||||||||||
| Mixed | 31% | >1,000 | |||||||||||
| Subalpine fir | Replacement | 81% | 185 | 150 | 300 | ||||||||
| Mixed | 19% | 800 | 500 | >1,000 | |||||||||
| Mixed conifer (eastside dry) | Replacement | 14% | 115 | 70 | 200 | ||||||||
| Mixed | 21% | 75 | 70 | 175 | |||||||||
| Surface or low | 64% | 25 | 20 | 25 | |||||||||
| Spruce-fir | Replacement | 84% | 135 | 80 | 270 | ||||||||
| Mixed | 16% | 700 | 285 | >1,000 | |||||||||
| California | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| California Forested | |||||||||||||
| Aspen with conifer | Replacement | 24% | 155 | 50 | 300 | ||||||||
| Mixed | 15% | 240 | |||||||||||
| Surface or low | 61% | 60 | |||||||||||
| Sierra Nevada lodgepole pine (cold wet upper montane) | Replacement | 23% | 150 | 37 | 764 | ||||||||
| Mixed | 70% | 50 | |||||||||||
| Surface or low | 7% | 500 | |||||||||||
| Southwest | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Southwest Forested | |||||||||||||
| Riparian forest with conifers | Replacement | 100% | 435 | 300 | 550 | ||||||||
| Aspen with spruce-fir | Replacement | 38% | 75 | 40 | 90 | ||||||||
| Mixed | 38% | 75 | 40 | ||||||||||
| Surface or low | 23% | 125 | 30 | 250 | |||||||||
| Spruce-fir | Replacement | 96% | 210 | 150 | |||||||||
| Mixed | 4% | >1,000 | 35 | >1,000 | |||||||||
| Northern and Central Rockies | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northern and Central Rockies Forested | |||||||||||||
| Western redcedar | Replacement | 87% | 385 | 75 | >1,000 | ||||||||
| Mixed | 13% | >1,000 | 25 | ||||||||||
| Douglas-fir (warm mesic interior) | Replacement | 28% | 170 | 80 | 400 | ||||||||
| Mixed | 72% | 65 | 50 | 250 | |||||||||
| Douglas-fir (cold) | Replacement | 31% | 145 | 75 | 250 | ||||||||
| Mixed | 69% | 65 | 35 | 150 | |||||||||
| Mixed conifer-upland western redcedar-western hemlock | Replacement | 67% | 225 | 150 | 300 | ||||||||
| Mixed | 33% | 450 | 35 | 500 | |||||||||
| Persistent lodgepole pine | Replacement | 89% | 450 | 300 | 600 | ||||||||
| Mixed | 11% | >1,000 | |||||||||||
| Lower subalpine lodgepole pine | Replacement | 73% | 170 | 50 | 200 | ||||||||
| Mixed | 27% | 450 | 40 | 500 | |||||||||
| Lower subalpine (Wyoming and Central Rockies) | Replacement | 100% | 175 | 30 | 300 | ||||||||
| Upper subalpine spruce-fir (Central Rockies) | Replacement | 100% | 300 | 100 | 600 | ||||||||
| Northern Great Plains | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northern Plains Woodland | |||||||||||||
| Northern Great Plains wooded draws and ravines | Replacement | 38% | 45 | 30 | 100 | ||||||||
| Mixed | 18% | 94 | |||||||||||
| Surface or low | 43% | 40 | 10 | ||||||||||
| Great Lakes | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Great Lakes Woodland | |||||||||||||
| Jack pine-open lands (frequent fire-return interval) | Replacement | 83% | 26 | 10 | 100 | ||||||||
| Mixed | 17% | 125 | 10 | ||||||||||
| Great Lakes Forested | |||||||||||||
| Conifer lowland (embedded in fire-prone ecosystem) | Replacement | 45% | 120 | 90 | 220 | ||||||||
| Mixed | 55% | 100 | |||||||||||
| Conifer lowland (embedded in fire-resistant ecosystem) | Replacement | 36% | 540 | 220 | >1,000 | ||||||||
| Mixed | 64% | 300 | |||||||||||
| Great Lakes spruce-fir | Replacement | 100% | 85 | 50 | 200 | ||||||||
| Minnesota spruce-fir (adjacent to Lake Superior and Drift and Lake Plain) | Replacement | 21% | 300 | ||||||||||
| Surface or low | 79% | 80 | |||||||||||
| Great Lakes pine forest, jack pine | Replacement | 67% | 50 | ||||||||||
| Mixed | 23% | 143 | |||||||||||
| Surface or low | 10% | 333 | |||||||||||
| Northern hardwood-eastern hemlock forest (Great Lakes) | Replacement | 99% | >1,000 | ||||||||||
| Pine-oak | Replacement | 19% | 357 | ||||||||||
| Surface or low | 81% | 85 | |||||||||||
| Red pine-eastern white pine (frequent fire) | Replacement | 38% | 56 | ||||||||||
| Mixed | 36% | 60 | |||||||||||
| Surface or low | 26% | 84 | |||||||||||
| Red pine-eastern white pine (less frequent fire) | Replacement | 30% | 166 | ||||||||||
| Mixed | 47% | 105 | |||||||||||
| Surface or low | 23% | 220 | |||||||||||
| Great Lakes pine forest, eastern white pine-eastern hemlock (frequent fire) | Replacement | 52% | 260 | ||||||||||
| Mixed | 12% | >1,000 | |||||||||||
| Surface or low | 35% | 385 | |||||||||||
| Eastern white pine-eastern hemlock | Replacement | 54% | 370 | ||||||||||
| Mixed | 12% | >1,000 | |||||||||||
| Surface or low | 34% | 588 | |||||||||||
| Northeast | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northeast Woodland | |||||||||||||
| Rocky outcrop pine (Northeast) | Replacement | 16% | 128 | ||||||||||
| Mixed | 32% | 65 | |||||||||||
| Surface or low | 52% | 40 | |||||||||||
| Northeast Forested | |||||||||||||
| Eastern white pine-northern hardwood | Replacement | 72% | 475 | ||||||||||
| Surface or low | 28% | >1,000 | |||||||||||
| Northern hardwoods-spruce | Replacement | 100% | >1,000 | 400 | >1,000 | ||||||||
| Northeast spruce-fir forest | Replacement | 100% | 265 | 150 | 300 | ||||||||
| Southeastern red spruce-Fraser fir | Replacement | 100% | 500 | 300 | >1,000 | ||||||||
| Southern Appalachians | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Southern Appalachians Forested | |||||||||||||
| Eastern hemlock-eastern white pine-hardwood | Replacement | 17% | >1,000 | 500 | >1,000 | ||||||||
| Surface or low | 83% | 210 | 100 | >1,000 | |||||||||
| Eastern white pine-northern hardwood | Replacement | 72% | 475 | ||||||||||
| Surface or low | 28% | >1,000 | |||||||||||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [112,154]. |
|||||||||||||
1. Achuff, Peter L.; La Roi, George H. 1977. Picea-Abies forests in the highlands of northern Alberta. Vegetatio. 33(2/3): 127-146. [83873]
2. Adams, John. 1927. The germination of the seeds of some plants with fleshy fruits. American Journal of Botany. 14(8): 415-428. [48174]
3. Ahlgren, C. E. 1974. Effects of fires on temperate forests: north central United States. In: Kozlowski, T. T.; Ahlgren, C. E., eds. Fire and ecosystems. New York: Academic Press: 195-223. [7198]
4. Ahlgren, Clifford E. 1960. Some effects of fire on reproduction and growth of vegetation in northeastern Minnesota. Ecology. 41(3): 431-445. [207]
5. Ahlgren, Clifford E. 1966. Small mammals and reforestation following prescribed burning. Journal of Forestry. 64(9): 614-618. [206]
6. Ahlgren, Clifford E. 1979. Buried seed in prescribe-burned jack pine forest soils, northeastern Minnesota. Minnesota Forestry Research Notes. No. 272. St. Paul, MN: University of Minnesota, College of Forestry. 3 p. [65922]
7. Ahlgren, Clifford E. 1979. Buried seed in the forest floor of the Boundary Waters Canoe Area. Minnesota Forestry Research Notes No. 271. St. Paul, MN: University of Minnesota, College of Forestry. 4 p. [3459]
8. Alaback, Paul B. 1984. Plant succession following logging in the Sitka spruce-western hemlock forests of southeast Alaska. Gen. Tech. Rep. PNW-173. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 26 p. [7849]
9. Alaback, Paul B.; Tappeiner, John C., II. 1986. Seed and fruit production of forest understory plants in coastal Alaska. In: Proceedings, 67th annual meeting of the Pacific Division, American Association for the Advancement of Science and the 1986 meeting of the arctic science conference (Arctic Division, American Association for the Advancement of Science); 1986 June 8-13; Vancouver, BC. Volume 5, part 1. [San Francisco, CA]: Pacific Division, American Association for the Advancement of Science: 20. Abstract. [83874]
10. Anderson, J. P. 1939. Plants used by the Eskimo of the Northern Bering Sea and Arctic regions of Alaska. American Journal of Botany. 26(9): 714-716. [55594]
11. Anderson, J. P. 1959. Flora of Alaska and adjacent parts of Canada. Ames, IA: Iowa State University Press. 543 p. [9928]
12. Antos, Joseph A.; Zobel, Donald B. 1985. Plant form, developmental plasticity and survival following burial by volcanic tephra. Canadian Journal of Botany. 63(12): 2083-2090. [12553]
13. Archibold, O. W. 1979. Buried viable propagules as a factor in postfire regeneration in northern Saskatchewan. Canadian Journal of Botany. 57(1): 54-58. [5934]
14. Armentano, Thomas Vincent. 1973. Population ecology and response to stress of Aster macrophyllus and Cornus canadensis. Chapel Hill, NC: University of North Carolina at Chapel Hill. 211 p. Dissertation. [83945]
15. Ayotte, Jeremy B.; Parker, Katherine L.; Arocena, Joselito M.; Gillingham, Michael P. 2006. Chemical composition of lick soils: functions of soil ingestion by four ungulate species. Journal of Mammalogy. 87(5): 878-888. [78499]
16. Bainbridge, E. L.; Strong, W. L. 2005. Pinus contorta understory vegetation dynamics following clearcutting in west-central Alberta, Canada. Forest Ecology and Management. 213(1-3): 133-150. [54341]
17. Barbour, Michael G.; Burk, Jack H.; Pitts, Wanna D. 1980. Major vegetation types of North America. In: Terrestrial plant ecology. Menlo Park, CA: The Benjamin/Cummings Publishing Company: 486-583. [45729]
18. Barrett, Spencer C.; Helenurm, Kaius. 1987. The reproductive biology of boreal forest herbs. I. Breeding systems and pollination. Canadian Journal of Botany. 65(10): 2036-2046. [6624]
19. Bartemucci, Paula; Messier, Christian; Canham, Charles D. 2006. Overstory influences on light attenuation patterns and understory plant community diversity and composition in southern boreal forests of Quebec. Canadian Journal of Forest Research. 36(9): 2065-2079. [65316]
20. Beals, E. W.; Cottam, Grant. 1960. The forest vegetation of the Apostle Islands, Wisconsin. Ecology. 41(4): 743-751. [62783]
21. Beaudry, Leisbet; Coupe, Ray; Delong, Craig; Pojar, Jim. 1999. Plant indicator guide for northern British Columbia: boreal, sub-boreal, and subalpine biogeoclimatic zones (BWBS, SBS, SBPS, and northern ESSF). Victoria, BC: British Columbia Ministry of Forests, Forestry Division Sciences Branch. 134 p. [70419]
22. Bergeron, Yves; Bouchard, Andre. 1983. Use of ecological groups in analysis and classification of plant communities in a section of western Quebec. Vegetatio. 56(1): 45-63. [83875]
23. Bergeron, Yves; Flannigan, Mike; Gauthier, Sylvie; Leduc, Alain; Lefort, Patrick. 2004. Past, current, and future fire frequency in the Canadian boreal forest: implications for sustainable forest management. Ambio. 33(6): 356-360. [83877]
24. Bliss, L. C. 1963. Alpine plant communities of the Presidential Range, New Hampshire. Ecology. 44(4): 678-697. [66539]
25. Blum, Barton M.; Frank, Robert M.; Gordon, Alan G. 1980. Red spruce-yellow birch. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 21-22. [49879]
26. Books, David J.; Heinselman, Miron L.; Ohmann, Lewis F. 1971. Revegetation research on the Little Sioux Burn. Naturalist. 22: 12-21. [3856]
27. Booth, W. E.; Wright, J. C. 1962. Flora of Montana: Part II--Dicotyledons.[Revised]. Bozeman, MT: Montana State College, Department of Botany and Bacteriology. 280 p. [47286]
28. Bowling, Colin; Zelazny, Vincent. 1992. Forest site classification in New Brunswick. Forestry Chronicle. 68(1): 34-41. [19241]
29. Braun, E. Lucy. 1989. The woody plants of Ohio. Columbus, OH: Ohio State University Press. 362 p. [12914]
30. Brown, Doug. 1992. Estimating the composition of a forest seed bank: a comparison of the seed extraction and seedling emergence methods. Canadian Journal of Botany. 70(8): 1603-1612. [69376]
31. Bubier, Jill L. 1991. Patterns of Picea mariana (black spruce) growth and raised bog development in Victory Basin, Vermont. Bulletin of the Torrey Botanical Club. 118(4): 399-411. [66067]
32. Bull, Evelyn L.; Torgersen, Torolf R.; Wertz, Tara L. 2001. The importance of vegetation, insects, and neonate ungulates in black bear diet in northeastern Oregon. Northwest Science. 75(3): 244-253. [67680]
33. Burger, A. E. 1987. Fruiting and frugivory of Cornus canadensis in boreal forest in Newfoundland. Oikos. 49(1): 3-10. [8930]
34. Butler, C. E. 1986. Summer food utilization and observations of a tame moose Alces alces. The Canadian Field-Naturalist. 100: 85-88. [8871]
35. Carleton, T. J.; Maycock, P. F. 1980. Vegetation of the boreal forests south of James Bay: non-centered component analysis of the vascular flora. Ecology. 61(5): 1199-1212. [14734]
36. Casabon, Christine; Pothier, David. 2008. Impact of deer browsing on plant communities in cutover sites on Anticosti Island. Ecoscience. 15(3): 389-397. [83878]
37. Chapman, Rachel Ross; Crow, Garrett E. 1981. Application of Raunkiaer's life form system to plant species survival after fire. Bulletin of the Torrey Botanical Club. 108(4): 472-478. [617]
38. Chavez, Virginia; Macdonald, S. Ellen. 2010. Understory species interactions in mature boreal mixedwood forests. Botany. 88(10): 912-922. [81400]
39. Christensen, E. M.; Clausen, J. J. (Jones); Curtis, J. T. 1959. Phytosociology of the lowland forests of northern Wisconsin. The American Midland Naturalist. 62(1): 232-247. [49627]
40. Chrosciewicz, Z. 1976. Burning for black spruce regeneration on a lowland cutover site in southeastern Manitoba. Canadian Journal of Forest Research. 6(2): 179-186. [7280]
41. Chrosciewicz, Z. 1983. Jack pine regeneration following postcut burning and seeding in central Saskatchewan. Information Report NOR-X-253. Edmonton, AB: Environment Canada, Canadian Forestry Service, Northern Forest Research Centre. 11 p. [16916]
42. Chrosciewicz, Z. 1988. Forest regeneration on burned, planted, and seeded clear-cuts in central Saskatchewan. Information Report NOR-X-293. Edmonton, AB: Canadian Forestry Service, Northern Forestry Centre. 16 p. [16697]
43. Clark, Donald F.; Antos, Joseph A.; Bradfield, Gary E. 2003. Succession in sub-boreal forests of west-central British Columbia. Journal of Vegetation Science. 14(5): 721-732. [48408]
44. Cooper, William S. 1913. The climax forest of Isle Royale, Lake Superior, and its development. I. Botanical Gazette. 55(1): 1-44. [11537]
45. Cooper, William S. 1913. The climax forest of Isle Royale, Lake Superior, and its development. II. Botanical Gazette. 55(2): 115-140. [11538]
46. Cooper, William S. 1913. The climax forest of Isle Royale, Lake Superior, and its development. III. Botanical Gazette. 55(3): 189-235. [11539]
47. Cooper, William S. 1928. Seventeen years of successional change upon Isle Royale, Lake Superior. Ecology. 9(1): 1-5. [7297]
48. Cooper, William S. 1942. Vegetation of the Prince William Sound region, Alaska; with a brief excursion into post-Pleistocene climatic history. Ecological Monographs. 12(1): 1-22. [62718]
49. Corns, I. G. W. 1983. Forest community types of west-central Alberta in relation to selected environmental factors. Canadian Journal of Forest Research. 13(5): 995-1010. [691]
50. Corns, I. G. W.; Annas, R. M. 1986. Field guide to forest ecosystems of west-central Alberta. Edmonton, AB: Natural Resources Canada, Canadian Forestry Service, Northern Forestry Centre. 251 p. [8998]
51. Corns, Ian G.; La Roi, George H. 1976. A comparison of mature with recently clear-cut and scarified lodgepole pine forests in the Lower Foothills of Alberta. Canadian Journal of Forest Research. 6(1): 20-32. [34970]
52. Cox, Donald D. 2002. Wetlands as ecosystems. In: A naturalist's guide to wetland plants: An ecology for eastern North America. Syracuse, NY: Syracuse University Press: 1-26. [69691]
53. Cringan, Alexander Thom. 1957. History, food habits and range requirements of the woodland caribou of continental North America. In: Transactions, 22nd North American wildlife conference; 1957 March 4-6; Washington, DC. Washington, DC: Wildlife Management Institute: 485-501. [15651]
54. Curtis, John T. 1959. Boreal forest. In: The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press: 243-257. [60525]
55. Curtis, John T. 1959. Northern forests-xeric. In: The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press: 202-220. [60523]
56. Daubenmire, Rexford. 1953. Notes on the vegetation of forested regions of the far northern Rockies and Alaska. Northwest Science. 27(4): 125-138. [10816]
57. Daubenmire, Rexford. 1969. Structure and ecology of coniferous forests of the northern Rocky Mountains. In: Taber, Richard D., ed. Coniferous forests of the northern Rocky Mountains: Proceedings of the 1968 symposium; 1968 September 17-20; Missoula, MT. Missoula, MT: University of Montana Foundation, Center for Natural Resources: 25-41. [7539]
58. Day, Susan Marie. 1997. Aspects of Newfoundland black bear (Ursus americanus hamiltoni) food habits and habitat use in human-influenced environments. Wolfville, NS: Acadia University. 107 p. Thesis. [83946]
59. De Grandpre, Louis; Bergeron, Yves. 1997. Diversity and stability of understory communities following disturbance in the southern boreal forest. Journal of Ecology. 85(6): 777-784. [28596]
60. De Grandpre, Louis; Gagnon, Daniel; Bergeron, Yves. 1993. Changes in the understory of Canadian southern boreal forest after fire. Journal of Vegetation Science. 4(6): 803-810. [23019]
61. del Moral, Roger; Watson, Alan F. 1978. Gradient structure of forest vegetation in the central Washington Cascades. Vegetatio. 38(1): 29-48. [8800]
62. DeLong, Craig; Meidinger, Del. 2003. Ecological variability of high elevation forests in central British Columbia. The Forestry Chronicle. 79(2): 259-262. [44770]
63. Densmore, Roseann Van Essen. 1979. Aspects of the seed ecology of woody plants of the Alaskan taiga and tundra. Durham, NC: Duke University. 285 p. Dissertation. [70495]
64. Donahue, William H. 1954. Some plant communities in the Anthracite Region of northeastern Pennsylvania. The American Midland Naturalist. 51(1): 203-231. [64481]
65. Dyrness, C. T. 1980. White spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 81. [50012]
66. Dyrness, C. T.; Franklin, J. F.; Moir, W. H. 1974. A preliminary classification of forest communities in the central portion of the western Cascades in Oregon. Bulletin No. 4. Seattle, WA: University of Washington, Ecosystem Analysis Studies, Coniferous Forest Biome. 123 p. [8480]
67. Dyrness, C. T.; Viereck, L. A.; Foote, M. J.; Zasada, J. C. 1988. The effect on vegetation and soil temperature of logging flood-plain white spruce. Res. Pap. PNW-RP-392. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 45 p. [7471]
68. Elias, Thomas S.; Dykeman, Peter A. 1982. Field guide to North American edible wild plants. New York: Outdoor Life Books. 286 p. [21103]
69. Ellison, Laurence. 1966. Seasonal foods and chemical analysis of winter diet of Alaskan spruce grouse. The Journal of Wildlife Management. 30(4): 729-735. [9735]
70. Eyde, Richard H. 1988. Comprehending Cornus: puzzles and progress in the systematics of the dogwoods. Botanical Review. 54(3): 233-351. [6144]
71. Eyre, F. H.; Gagnon, Gilles. 1980. Aspen. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 16-17. [49858]
72. Fahnestock, George Reeder. 1977. Interactions of forest fire, flora, and fuels in two Cascade Range wilderness areas. Seattle, WA: University of Washington. 179 p. Dissertation. [10431]
73. Feng, Chun-Miao; Qu, Rongda; Zhou, Li-Li; Xie, De-Yu; Xiang, Qiu-Yun (Jenny). 2009. Shoot regeneration of dwarf dogwood (Cornus canadensis L.) and morphological characterization of the regenerated plants. Plant Cell, Tissue and Organ Culture. 97(1): 27-37. [83880]
74. Ferguson, I. K. 1966. The Cornaceae in the southeastern United States. Journal of the Arnold Arboretum. 47: 106-116. [7616]
75. Fischer, William C.; Bradley, Anne F. 1987. Fire ecology of western Montana forest habitat types. Gen. Tech. Rep. INT-223. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 95 p. [633]
76. Flinn, Marguerite A.; Fisher, Sharon E.; Martin, Earl V.; Blum, Ilya E. 1985. Seasonal variation in the nonstructural carbohydrate composition of rhizomes of forest understory species. Proceedings of the Nova Scotian Institute of Science. 35: 91-97. [10488]
77. Flinn, Marguerite A.; Pringle, Joan K. 1983. Heat tolerance of rhizomes of several understory species. Canadian Journal of Botany. 61(2): 452-457. [8444]
78. Flinn, Marguerite A.; Wein, Ross W. 1977. Depth of underground plant organs and theoretical survival during fire. Canadian Journal of Botany. 55(19): 2550-2554. [6362]
79. Flinn, Marguerite Adele. 1980. Heat penetration and early postfire regeneration of some understory species in the Acadian forest. Halifax, NB: University of New Brunswick. 87 p. Thesis. [9876]
80. Foote, M. Joan. 1983. Classification, description, and dynamics of plant communities after fire in the taiga of interior Alaska. Res. Pap. PNW-307. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 108 p. [7080]
81. Fortin, Marie-Josee; Payette, Serge; Marineau, Kim. 1999. Spatial vegetation diversity index along a postfire successional gradient in the northern boreal forest. Ecoscience. 6(2): 204-213. [36002]
82. Foster, D. R.; King, G. A. 1986. Vegetation pattern and diversity in southeastern Labrador, Canada: Betula papyrifera (birch) forest development in relation to fire history and physiography. Journal of Ecology. 74(2): 465-483. [14651]
83. Foster, David R. 1983. The history and pattern of fire in the boreal forest of southeastern Labrador. Canadian Journal of Botany. 61: 2459-2471. [9683]
84. Foster, David R. 1984. Phytosociological description of the forest vegetation of southeastern Labrador. Canadian Journal of Botany. 62: 899-906. [15356]
85. Foster, David R. 1985. Vegetation development following fire in Picea mariana (black spruce)-Pleurozium forests of south-eastern Labrador, Canada. Journal of Ecology. 73(2): 517-534. [7222]
86. Francis, George Reid. 1958. Ecological studies of marten, Martes americana, in Algonquin Park, Ontario. Vancouver, BC: University of British Columbia. 74 p. [+ appendices]. Thesis. [76922]
87. Franklin, Jerry F. 1980. Coastal true fir-hemlock. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 103-104. [50041]
88. Franklin, Jerry F.; Dyrness, C. T. 1973. Natural vegetation of Oregon and Washington. Gen. Tech. Rep. PNW-8. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 417 p. [961]
89. Fyles, James W. 1989. Seed bank populations in upland coniferous forests in central Alberta. Canadian Journal of Botany. 67: 274-278. [6388]
90. Gaber, B. A.; Hutchinson, T. C. 1988. The neutralization of acid rain by the leaves of four boreal forest species. Canadian Journal of Botany. 66(9): 1877-1882. [8872]
91. Garman, E. H. 1929. Natural reproduction following fires in central British Columbia. The Forestry Chronicle. 5(3): 28-44. [20224]
92. Gates, Frank C. 1930. Aspen association in northern Lower Michigan. Botanical Gazette. 40(3): 233-259. [16933]
93. Gleason, Henry A.; Cronquist, Arthur. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd ed. New York: New York Botanical Garden. 910 p. [20329]
94. Good, Norma Frauendorf. 1963. Reproduction and productivity patterns in a pine-spruce-fir community in Itasca Park, Minnesota. Bulletin of the Torrey Botanical Club. 90(5): 287-292. [61333]
95. Great Plains Flora Association. 1986. Flora of the Great Plains. Lawrence, KS: University Press of Kansas. 1392 p. [1603]
96. Griffin, Ralph H. 1980. Red spruce-balsam fir. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 19-20. [49862]
97. Grigal, D. F.; Ohmann, Lewis F. 1975. Classification, description, and dynamics of upland plant communities within a Minnesota wilderness area. Ecological Monographs. 45(4): 389-407. [61235]
98. Habeck, James R. 1968. Forest succession in the Glacier Park cedar-hemlock forests. Ecology. 49(5): 872-880. [6479]
99. Habeck, James R. 1978. A study of climax western redcedar (Thuja plicata Donn.) forest communities in the Selway-Bitterroot Wilderness, Idaho. Northwest Science. 52(1): 67-76. [7354]
100. Haeussler, Sybille; Bedford, Lorne; Boateng, Jacob O.; MacKinnon, Andy. 1999. Plant community responses to mechanical site preparation in northern interior British Columbia. Canadian Journal of Forest Research. 29(7): 1084-1100. [38978]
101. Haeussler, Sybille; Bergeron, Yves. 2004. Range of variability in boreal aspen plant communities after wildfire and clear-cutting. Canadian Journal of Forest Research. 34(2): 274-288. [48445]
102. Hall, I. V. 1955. Floristic changes following the cutting and burning of a woodlot for blueberry production. Canadian Journal of Agricultural Science. 35: 143-152. [9012]
103. Hall, Ivan V.; Sibley, Jack D. 1976. The biology of Canadian weeds. 20. Cornus canadensis L. Canadian Journal of Plant Science. 56(4): 885-892. [83881]
104. Halpern, Charles B.; Spies, Thomas A. 1995. Plant species diversity in natural and managed forests of the Pacific Northwest. Ecological Applications. 5(4): 913-934. [62677]
105. Halverson, Nancy M.; Topik, Christopher; Van Vickle, Robert. 1986. Plant association and management guide for the western hemlock zone: Mt. Hood National Forest. R6-ECOL-232A. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 111 p. [1068]
106. Hamilton, E. H.; Peterson, L. D. 2006. Succession after slashburning in an Engelmann spruce-subalpine fir subzone variant: West Twin site. Tech. Rep. No. 028. Victoria, BC: British Columbia Ministry of Forests and range, Forest Science Program. 21 p. [64622]
107. Hamilton, E. 2006. Vegetation development and fire effects at the Walker Creek site: comparison of forest floor and mineral soil plots. Technical Report No. 026. Victoria, BC: British Columbia Ministry of Forests and Range, Forest Science Program. 28 p. [64621]
108. Hamilton, Evelyn H. 2006. Vegetation response, fire effects, and tree growth after slashburning in the Engelmann spruce-subalpine fir zone: Goat River Site. Technical Report No. 037. Kamloops, BC: British Columbia Ministry of Forests and Range, Research Branch, Forest Science Program. 26 p. [66358]
109. Hamilton, Evelyn H. 2007. Post-fire vegetation development and fire effects in the SBS zone: Haggen Creek, Francis Lake, Genevieve Lake, Brink, and Indianpoint sites. Technical Report 041. Victoria, BC: Ministry of Forests and Range, Forest Science Program. 74 p. [71203]
110. Hanley, Thomas A.; McKendrick, Jay D. 1983. Seasonal changes in chemical composition and nutritive values of native forages in a spruce-hemlock forests, southeastern Alaska. Res. Pap. PNW-312. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 41 p. [8770]
111. Hanley, Thomas A.; Robbins, Charles T.; Spalinger, Donald E. 1989. Forest habitats and the nutritional ecology of Sitka black-tailed deer: a research synthesis with implications for forest management. Gen. Tech. Rep. PNW-GTR-230. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 52 p. [7509]
112. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org/niftt/released/FRCC_Guidebook_2010_final.pdf. [81749]
113. Hansen, Henry L.; Krefting, Laurits W.; Kurmis, Vilis. 1973. The forest of Isle Royale in relation to fire history and wildlife. Technical Bulletin 294/Forestry Series 13. Minneapolis, MN: University of Minnesota, Agricultural Experiment Station. 44 p. [8120]
114. Hanson, Herbert C. 1951. Characteristics of some grassland, marsh, and other plant communities in western Alaska. Ecological Monographs. 21(4): 317-378. [62710]
115. Harmon, Janice M.; Franklin, Jerry F. 1995. Seed rain and seed bank of third- and fifth-order streams on the western slope of the Cascade Range. Res. Pap. PNW-RP-480. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 27 p. [25915]
116. Harrington, H. D. 1964. Manual of the plants of Colorado. 2nd ed. Chicago, IL: The Swallow Press. 666 p. [6851]
117. Harris, A. S. 1966. Effects of slash burning on conifer regeneration in southeast Alaska. Research Note NOR-18. Juneau, AK: U.S. Department of Agriculture, Forest Service, Northern Forest Experiment Station. 6 p. [7304]
118. Harris, A. S. 1980. Western redcedar-western hemlock. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 104-105. [50042]
119. Harris, A. S. 1990. Chamaecyparis nootkatensis (D. Don) Spach Alaska-cedar. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 97-102. [13373]
120. Heinselman, M. L. 1970. Landscape evolution, peatland types and the environment in the Lake Agassiz Peatlands Natural Area, Minnesota. Ecological Monographs. 40(2): 235-261. [8378]
121. Heinselman, Miron L. 1973. Fire in the virgin forests of the Boundary Waters Canoe Area, Minnesota. Quaternary Research. 3(3): 329-382. [282]
122. Helenurm, Kaius; Barrett, Spencer C. H. 1987. The reproductive biology of boreal forest herbs. II. Phenology of flowering and fruiting. Canadian Journal of Botany. 65(10): 2047-2056. [6623]
123. Hines, William Wester. 1971. Plant communities in the old-growth forests of north coastal Oregon. Corvallis, OR: Oregon State University. 146 p. Thesis. [10399]
124. Hitchcock, C. Leo; Cronquist, Arthur. 1973. Flora of the Pacific Northwest. Seattle, WA: University of Washington Press. 730 p. [1168]
125. Hobbs, Michael W.; Alexander, Martin E.; Weber, Michael G. 2004. Understory vegetative response following high-intensity crown fires in jack pine-black spruce stands. In: Engstrom, R. Todd; Galley, Krista E. M.; de Groot, William J., eds. Fire in temperate, boreal, and montane ecosystems: Proceedings of the 22nd Tall Timbers fire ecology conference: an international symposium; 2001 October 15-18; Kananaskis Village, AB. No. 22. Tallahassee, FL: Tall Timbers Research: 202. Abstract. [52323]
126. Holliday, N. J. 1984. Carabid beetles (Coleoptera: Carabidae) from a burned spruce forest (Picea spp.). The Canadian Entomologist. 116: 919-922. [8337]
127. Holsten, Edward H.; Werner, Richard A.; Develice, Robert L. 1995. Effects of a spruce beetle (Coleoptera: Scolytidae) outbreak and fire on Lutz spruce in Alaska. Environmental Entomology. 24(6): 1539-1547. [26580]
128. Hulten, Eric. 1968. Flora of Alaska and neighboring territories. Stanford, CA: Stanford University Press. 1008 p. [13403]
129. Hungerford, Kenneth E. 1957. Evaluating ruffed grouse foods for habitat improvement. Transactions, 22nd North American Wildlife Conference. 22: 380-395. [15905]
130. Ingersoll, Cheryl A.; Wilson, Mark V. 1990. Buried propagules in an old-growth forest and their response to experimental disturbances. Canadian Journal of Botany. 68(5): 1156-1162. [11767]
131. Irwin, Larry L. 1976. Effects of intensive silviculture on big game forage sources in northern Idaho. In: Hieb, S., ed. Proceedings, elk-logging roads symposium; [1975 December 16-17]; [Moscow, ID]. Moscow, ID: University of Idaho: 135-142. [16146]
132. Janke, Robert A.; Lowther, John L. 1980. Post-fire succession in the boreal forest type of Isle Royale National Park. In: Proceedings, 2nd conference on scientific research in the National Parks; 1979 November 26-30; San Francisco, CA. Volume 7: Ecosystem Studies/Interdisciplinary Studies. Washington, DC: U.S. Department of the Interior, National Park Service; The American Institute of Biological Sciences: 99-135. [19929]
133. Janssen, C. R. 1967. A floristic study of forests and bog vegetation, northwestern Minnesota. Ecology. 48(5): 751-765. [49684]
134. Jenkins, Kurt J.; Starkey, Edward E. 1991. Food habits of Roosevelt elk. Rangelands. 13(6): 261-265. [17351]
135. Johnston, M. H.; Elliott, J. A. 1996. Impacts of logging and wildfire on an upland black spruce community in northwestern Ontario. Environmental Monitoring and Assessment. 39(1-3): 283-297. [28557]
136. Johnston, William F. 1980. Northern white-cedar. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 23-24. [49885]
137. Johnston, William F. 1990. Thuja occidentalis L. northern white-cedar. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 580-589. [13418]
138. Kartesz, John T. 1999. A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. 1st ed. In: Kartesz, John T.; Meacham, Christopher A. Synthesis of the North American flora (Windows Version 1.0), [CD-ROM]. Chapel Hill, NC: North Carolina Botanical Garden (Producer). In cooperation with: The Nature Conservancy; U.S. Department of Agriculture, Natural Resources Conservation Service; U.S. Department of the Interior, Fish and Wildlife Service. [36715]
139. Kemball, Kevin J.; Wang, G. Geoff; Dang, Qing-Lai. 2005. Response of understory plant community of boreal mixedwood stands to fire, logging, and spruce budworm outbreak. Canadian Journal of Botany. 83(12): 1550-1560. [63193]
140. Kingery, James L.; Mosley, Jeffrey C.; Bordwell, Kirsten C. 1996. Dietary overlap among cattle and cervids in northern Idaho forests. Journal of Range Management. 49(1): 8-15. [26611]
141. Kinley, Trevor A.; Bergenske, John; Davies, Julie-Anne; Quinn, David. 2003. Characteristics of early-winter caribou, Rangifer tarandus caribou, feeding sites in the southern Purcell Mountains, British Columbia. The Canadian Field-Naturalist. 117(3): 352-359. [65798]
142. Klinka, K.; Krestov, P. V.; Chourmouzis, C. 2002. Classification and ecology of the mid-seral Picea mariana forests of British Columbia. Applied Vegetation Science. 5(2): 227-235. [47096]
143. Kovalchik, Bernard L.; Clausnitzer, Rodrick R. 2004. Classification and management of aquatic, riparian, and wetland sites on the national forests of eastern Washington: series description. Gen. Tech. Rep. PNW-GTR-593. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 354 p. [53329]
144. Krefting, Laurits W.; Ahlgren, Clifford E. 1974. Small mammals and vegetation changes after fire in a mixed conifer-hardwood forest. Ecology. 55(6): 1391-1398. [9874]
145. Kudish, Michael. 1992. Adirondack upland flora: an ecological perspective. Saranac, NY: The Chauncy Press. 320 p. [19376]
146. Kurulok, Stephanie E.; Macdonald, S. Ellen. 2007. Impacts of postfire salvage logging on understory plant communities of the boreal mixedwood forest 2 and 34 years after disturbance. Canadian Journal of Forest Research. 37(12): 2637-2651. [70545]
147. La Roi, George H. 1967. Ecological studies in the boreal spruce-fir forests of the North American taiga. I. Analysis of the vascular flora. Ecological Monographs. 37(3): 229-253. [8864]
148. La Roi, George H. 1992. Classification and ordination of southern boreal forests from the Hondo - Slave Lake area of central Alberta. Canadian Journal of Botany. 70(3): 614-628. [18702]
149. La Roi, George H.; Hnatiuk, Roger J. 1980. The Pinus contorta forests of Banff and Jasper National Parks: a study in comparative synecology and syntaxonomy. Ecological Monographs. 50(1): 1-29. [8347]
150. Lachance, Daniel; Lavoie, Claude. 2004. Vegetation of Sphagnum bogs in highly disturbed landscapes: relative influence of abiotic and anthropogenic factors. Applied Vegetation Science. 7(2): 183-192. [83927]
151. Lackschewitz, Klaus. 1991. Vascular plants of west-central Montana--identification guidebook. Gen. Tech. Rep. INT-227. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 648 p. [13798]
152. Lain, Emily J.; Haney, Alan; Burris, John M.; Burton, Julia. 2008. Response of vegetation and birds to severe wind disturbance and salvage logging in a southern boreal forest. Forest Ecology and Management. 256(5): 863-871. [71530]
153. Lamb, Eric G; Megill, William. 2003. The shoreline fringe forest and adjacent peatlands of the southern central British Columbia coast. The Canadian Field-Naturalist. 117(2): 209-217. [83884]
154. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: https://www.landfire.gov /downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
155. LANDFIRE Rapid Assessment. 2007. Rapid assessment reference condition models, [Online]. In: LANDFIRE. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; The Nature Conservancy (Producers). Available: https://www.landfire.gov /models_EW.php [2008, April 18] [66533]
156. Larsen, C. P. S.; MacDonald, G. M. 1998. An 840-year record of fire and vegetation in a boreal white spruce forest. Ecology. 79(1): 106-118. [28521]
157. Larsen, J. A. 1922. Effect of removal of the virgin white pine stand upon the physical factors of site. Ecology. 3(4): 302-305. [12935]
158. Larsen, J. A. 1940. Site factor variations and responses in temporary forest types in northern Idaho. Ecological Monographs. 10(1): 1-54. [12933]
159. Lau, Jennifer H. T. 2009. Phenotypic and genotypic differentiation of plant populations between coastal barrens and forests in Nova Scotia, Canada. Halifax, NS: Saint Mary's University. 85 p. Thesis. [83947]
160. Lautenschlager, R. A.; Crawford, H. S.; Stokes, M. R.; Stone, T. L. 1997. Forest disturbance type differentially affects seasonal moose forage. Alces. 33: 49-73. [78861]
161. Lee, Philip. 2004. The impact of burn intensity from wildfires on seed and vegetative banks, and emergent understory in aspen-dominated boreal forests. Canadian Journal of Botany. 82(10): 1468-1480. [51462]
162. Lee, Philip; Sturgess, Kelly. 2001. The effects of logs, stumps, and root throws on understory communities within 28-year-old aspen-dominated boreal forests. Canadian Journal of Botany. 79(8): 905-916. [38852]
163. Legare, Sonia; Bergeron, Yves; Leduc, Alain; Pare, David. 2001. Comparison of the understory vegetation in boreal forest types of southwest Quebec. Canadian Journal of Botany. 79(9): 1019-1027. [38854]
164. Lepofsky, Dana; Turner, Nancy J.; Kuhnlein, Harriet V. 1985. Determining the availability of traditional wild plant foods: an example of Nuxalk foods, Bella Coola, British Columbia. Ecology of Food and Nutrition. 16: 223-241. [7002]
165. LeResche, Robert E.; Davis, James L. 1973. Importance of nonbrowse foods to moose on the Kenai Peninsula, Alaska. The Journal of Wildlife Management. 37(3): 279-287. [13123]
166. Lesher, Robin D.; Henderson, Jan A. 1989. Indicator species of the Olympic National Forest. R6-ECOL-TP003-88. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 79 p. [15376]
167. Lloyd, D.; Angove, K.; Hope, G.; Thompson, C. 1990. A guide to site identification and interpretation for the Kamloops Forest Region. Land Management Handbook No. 23. Victoria, BC: British Columbia Ministry of Forests, Research Branch. 399 p. [37061]
168. Locky, David A.; Bayley, Suzanne E.; Vitt, Dale H. 2005. The vegetational ecology of black spruce swamps, fens, and bogs in southern boreal Manitoba, Canada. Wetlands. 25(3): 564-582. [67524]
169. Long-Robinson, Tammy M. 1990. A study of the clonal behavior of Cornus canadensis. Biology 556, Boreal Flora. Pellston, MI: University of Michigan, Biological Station. 18 p. [83948]
170. Loomis, Robert M.; Roussopoulos, Peter J.; Blank, Richard W. 1979. Summer moisture contents of understory vegetation in northeastern Minnesota. Res. Pap. NC-179. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 7 p. [14330]
171. Loucks, O. L. 1962. Ordinating forest communities by means of environmental scalars and phytosociological indices. Ecological Monographs. 32(2): 137-166. [19824]
172. Lovell, John H. 1898. The insect-visitors of flowers. Bulletin of the Torrey Botanical Club. 25(7): 382-390. [83926]
173. Lynham, T. J.; Curran, T. R. 1998. Vegetation recovery after wildfire in old-growth red and white pine. Frontline: Forestry Research Applications/Technical Note No. 100. Sault Ste. Marie, ON: Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre. 4 p. [30685]
174. Lynham, Timothy J.; Stocks, B. J. 1991. The natural fire regime of an unprotected section of the boreal forest in Canada. In: Proceedings, 17th Tall Timbers fire ecology conference; 1989 May 18-21; Tallahassee, FL. Tallahassee, FL: Tall Timbers Research Station: 99-109. [17602]
175. Mabry, Cathy; Korsgren, Tobe. 1998. A permanent plot study of vegetation and vegetation-site factors fifty-three years following disturbance in central New England, U.S.A. Ecoscience. 5(2): 232-240. [45981]
176. MacLean, David A.; Wein, Ross W. 1977. Changes in understory vegetation with increasing stand age in New Brunswick forests: species composition, cover, biomass, and nutrients. Canadian Journal of Botany. 55: 2818-2831. [10106]
177. MacQuarrie, Kate; Lacroix, Christian. 2003. The upland hardwood component of Prince Edward Island's remnant Acadian forest: determination of depth of edge and patterns of exotic invasion. Canadian Journal of Botany. 81(11): 1113-1128. [47131]
178. Magee, Dennis W.; Ahles, Harry E. 2007. Flora of the Northeast: A manual of the vascular flora of New England and adjacent New York. 2nd ed. Amherst, MA: University of Massachusetts Press. 1214 p. [74293]
179. Majcen, Zoran; Gagnon, Gilles; Benzie, John. 1980. Jack pine. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 8-9. [49850]
180. Manseau, M.; Huot, J.; Crete, M. 1996. Effects of summer grazing by caribou on composition and productivity of vegetation: community and landscape level. Journal of Ecology. 84(4): 503-513. [26980]
181. Martin, Alexander C.; Zim, Herbert S.; Nelson, Arnold L. 1951. American wildlife and plants. New York: McGraw-Hill. 500 p. [4021]
182. Martin, J. Lynton. 1955. Observations on the origin and early development of a plant community following a forest fire. The Forestry Chronicle. 31(2): 154-161. [11363]
183. Martin, J. Lynton. 1956. An ecological survey of burned-over forest land in southwestern Nova Scotia. The Forestry Chronicle. 32(3): 313-336. [8932]
184. Martin, Jon R.; Trull, Susan J.; Brady, Ward W.; West, Randolph A.; Downs, Jim M. 1995. Forest plant association management guide: Chatham Area, Tongass National Forest. R10-TP-57. Juneau, AK: U.S. Department of Agriculture, Forest Service, Alaska Region. Variously paginated. [67100]
185. Martin, N. D. 1959. An analysis of forest succession in Algonquin Park, Ontario. Ecological Monographs. 29(3): 187-218. [19930]
186. Martin, William C.; Hutchins, Charles R. 1981. A flora of New Mexico. Volume 2. Germany: J. Cramer. 2589 p. [37176]
187. Maycock, P. F. 1956. Composition of an upland conifer community in Ontario. Ecology. 37(4): 846-848. [80300]
188. Maycock, P. F.; Curtis, J. T. 1960. The phytosociology of boreal conifer-hardwood forests of the Great Lakes region. Ecological Monographs. 30(1): 1-36. [62820]
189. Maycock, Paul F. 1961. The spruce-fir forests of the Keweenaw Peninsula, northern Michigan. Ecology. 42(2): 357-365. [62688]
190. McLean, Alastair. 1967. Germination of forest range species from southern British Columbia. Journal of Range Management. 6(5): 321-322. [83928]
191. McLean, Alastair. 1968. Fire resistance of forest species as influenced by root systems. Journal of Range Management. 22(2): 120-122. [1621]
192. McLean, Alastair. 1970. Plant communities of the Similkameen Valley, British Columbia. Ecological Monographs. 40(4): 403-424. [1620]
193. Means, Joseph E.; McKee, W. Arthur; Moir, William H.; Franklin, Jerry F. 1982. Natural revegetation of the northeastern portion of the devastated area. In: Keller, S. A, C.; ed. Mount St. Helens: one year later: Proceedings of a symposium; 1981 May 17-18; Cheney, WA. Cheney, WA: Eastern Washington University Press: 93-103. [5977]
194. Mendall, Howard L.; Aldous, Clarence M. 1943. The ecology and management of the American woodcock. Orono, ME: Maine Cooperative Wildlife Research Unit. 201 p. [73807]
195. Messier, Christian; Kimmins, James P. 1991. Above- and below-ground vegetation recovery in recently clearcut and burned sites dominated by Gaultheria shallon in coastal British Columbia. Forest Ecology and Management. 46(3-4): 275-294. [17206]
196. Methven, I. R.; Van Wagner, C. E.; Stocks, B. J. 1975. The vegetation of four burned areas in northwestern Ontario. Inf. Rep. PS-X-60. Chalk River, ON: Canadian Forestry Service, Petawawa Forest Experiment Station. 10 p. [13114]
197. Methven, Ian R. 1973. Fire, succession and community structure in a red and white pine stand. Information Report PS-X-43. Chalk River, ON: Environment Canada, Forestry Service, Petawawa Forest Experiment Station. 18 p. [18601]
198. Mohlenbrock, Robert H. 1986. Guide to the vascular flora of Illinois. [Revised edition]. Carbondale, IL: Southern Illinois University Press. 507 p. [17383]
199. Moola, F. M.; Vasseur, L. 2004. Recovery of late-seral vascular plants in a chronosequence of post-clearcut forest stands in coastal Nova Scotia, Canada. Plant Ecology. 172(2): 183-197. [83930]
200. Morin, Hubert; Payette, Serge. 1988. Buried seed populations in the montane, subalpine, and alpine belts of Mont Jacques-Cartier, Quebec. Canadian Journal of Botany. 66(1): 101-107. [6376]
201. Motzkin, Glenn; Orwig, David A.; Foster, David R. 2002. Vegetation and disturbance history of a rare dwarf pitch pine community in western New England. Journal of Biogeography. 29(10-11): 1455-1467. [46053]
202. Mueggler, Walter F. 1965. Ecology of seral shrub communities in the cedar-hemlock zone of northern Idaho. Ecological Monographs. 35(2): 165-185. [4016]
203. Mundinger, John D. 1978. Population ecology and habitat relationships of white-tailed deer in coniferous forest habitat of northwestern Montana. Montana deer studies: Job progress report 1977-1978. Helena, MT: Montana Department of Fish and Game. 74 p. [21525]
204. Mundinger, John G. 1979. Population ecology and habitat relationships of white-tailed deer in coniferous forest habitat of northwestern Montana. Montana deer studies: Job progress report 1978-1979. Helena, MT: Montana Department of Fish and Game. 65 p. [21526]
205. Murrell, Zack E. 1994. Dwarf dogwoods: intermediacy and the morphological landscape. Systematic Botany. 19(4): 539-556. [83931]
206. Neiland, Bonita J. 1958. Forest and adjacent burn in the Tillamook Burn area of northwestern Oregon. Ecology. 39(4): 660-671. [8879]
207. Neiland, Bonita J. 1971. The forest-bog complex of southeast Alaska. Vegetatio. 22(1-3): 1-64. [8383]
208. Nelson, Cara R.; Halpern, Charles B. 2005. Edge-related responses of understory plants to aggregated retention harvest in the Pacific Northwest. Ecological Applications. 15(1): 196-209. [61474]
209. Nguyen-Xuan, Thuy; Bergeron, Yves; Simard, Dan; Fyles, Jim W.; Pare, David. 2000. The importance of forest floor disturbance in the early regeneration patterns of the boreal forest of western and central Quebec: a wildfire versus logging comparison. Canadian Journal of Forest Research. 30(9): 1353-1364. [38061]
210. Nichols, G. E. 1934. The influence of exposure to winter temperatures upon seed germination in various native American plants. Ecology. 15(4): 364-373. [55167]
211. Noble, Mark G.; DeBoer, Linda K.; Johnson, Kenneth L.; Coffin, Barbara A.; Fellows, Lucia G.; Christensen, Neil A. 1977. Quantitative relationships among some Pinus banksiana - Picea mariana forests subjected to wildfire and postlogging treatments. Canadian Journal of Forest Research. 7(2): 368-377. [16532]
212. Nyberg, J. Brian; McNay R, Scott; Kirchoff, Matthew D.; Forbes, Robert D.; Bunnell, Fred L.; Richardson, Edward L. 1989. Integrated management of timber and deer: coastal forests of British Columbia and Alaska. Gen. Tech. Rep. PNW-GTR-226. Ogden, UT: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 65 p. [7468]
213. Ohmann, Lewis F.; Grigal, David F. 1981. Contrasting vegetation responses following two forest fires in northeastern Minnesota. The American Midland Naturalist. 106(1): 54-64. [8285]
214. Orloci, Laszlo; Stanek, Walter. 1979. Vegetation survey of the Alaska Highway, Yukon Territory: types and gradients. Vegetatio. 41(1): 1-56. [12748]
215. Oswald, E. T.; Brown, B. N. 1990. Vegetation establishment during 5 years following wildfire in northern British Columbia and southern Yukon Territory. Information Report BC-X-320. Victoria, BC: Forestry Canada, Pacific and Yukon Region, Pacific Forestry Centre. 46 p. [16934]
216. Outcalt, Kenneth Wayne; White, Edwin H. 1981. Phytosociological changes in understory vegetation following timber harvest in northern Minnesota. Canadian Journal of Forest Research. 11(1): 175-183. [16301]
217. Peel, M. C.; Finlayson, B. L.; McMahon, T. A. 2007. Updated world map of the Koppen-Geiger climate classification. Hydrology and Earth System Sciences. 11(5): 1633-1644. [84176]
218. Peinado, M.; Aguirre, J. L.; Delgadillo, J. 1997. Phytosociological, bioclimatic and biogeographical classification of woody climax communities of western North America. Journal of Vegetation Science. 8(4): 505-528. [28564]
219. Peltzer, Duane A.; Bast, Marcy L.; Wilson, Scott D.; Gerry, Ann K. 2000. Plant diversity and tree responses following contrasting disturbances in boreal forest. Forest Ecology and Management. 127(1-3): 191-203. [48541]
220. Peters, Stuart S. 1958. The ecological effects of fire and its possible application to game management. In: Proceedings, symposium on prescribed burning in forestry, agriculture, and wildlife management. Newfoundland Research Committee Publication No. 1. St. John's, Newfoundland: Memorial University of Newfoundland, Department of Mines and Resources: 41-52. [36589]
221. Pojar, J.; Trowbridge, R.; Coates, D. 1984. Ecosystem classification and interpretation of the sub-boreal spruce zone, Prince Rupert Forest Region, British Columbia. Land Management Report No. 17. Victoria, BC: Province of British Columbia, Ministry of Forests. 319 p. [6929]
222. Pojar, Jim; MacKinnon, Andy, eds. 1994. Plants of the Pacific Northwest coast: Washington, Oregon, British Columbia and Alaska. Redmond, WA: Lone Pine Publishing. 526 p. [25159]
223. Pollett, Frederick C. 1972. Classification of peatlands in Newfoundland. In: Proceedings, 4th International Peat Congress; 1972 June 25-30; Otaniemi, Finland. [Helsinki, Finland]:[International Peat Society]: 101-110. [15403]
224. Powell, Roger A.; Brooks, William S. 1981. Small mammal changes in populations following tornado blowdown in northern mixed forest. Journal of Mammalogy. 62(2): 397-400. [61244]
225. Purdon, Mark; Brais, Suzanne; Bergeron, Yves. 2004. Initial response of understory vegetation to fire severity and salvage-logging in the southern boreal forest of Quebec. Applied Vegetation Science. 7(1): 49-60. [3412]
226. Qian, Hong; Klinka, Karel; Okland, Rune H.; Krestov, Pavel; Kayahara, Gordon J. 2003. Understorey vegetation in boreal Picea mariana and Populus tremuloides stands in British Columbia. Journal of Vegetation Science. 14(2): 173-184. [48547]
227. Quintilio, D.; Alexander, M. E.; Ponto, R. L. 1991. Spring fires in a semimature trembling aspen stand in central Alberta. Information Report NOR-X-323. Edmonton, AB: Forestry Canada, Northwest Region, Northern Forestry Centre. 30 p. [19243]
228. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
229. Rayfield, Bronwyn; Anand, Madhur; Laurence, Sophie. 2005. Assessing simple versus complex restoration strategies for industrially disturbed forests. Restoration Ecology. 13(4): 639-650. [60346]
230. Reagan, Albert B. 1934. Plants used by the Hoh and Quileute Indians. Transactions of the Kansas Academy of Science. 37: 55-70. [66487]
231. Rees, Daniel C.; Juday, Glenn Patrick. 2002. Plant species diversity on logged versus burned sites in central Alaska. Forest Ecology and Management. 155(1-3): 291-302. [40745]
232. Renecker, Lyle A.; Schwartz, Charles C. 2007. Food habits and feeding behavior. In: Franzmann, Albert W.; Schwartz, Charles C.; McCabe, Richard E., eds. Ecology and management of the North American moose. 2nd ed. Boulder, CO: University Press of Colorado: 403-440. [79106]
233. Reynolds, Keith M. 1990. Preliminary classification of forest vegetation of the Kenai Peninsula, Alaska. Res. Pap. PNW-RP-424. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 67 p. [14581]
234. Robbins, C. T.; Hanley, T. A.; Hagerman, A. E.; Hjeljord, O.; Baker, D. L.; Schwartz, C. C.; Mautz, W. W. 1987. Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology. 68(1): 98-107. [5974]
235. Roberts, B. A.; van Nostrand, R. S. 1995. Distribution and site ecology of eastern larch in Newfoundland, Canada. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Ecology and management of Larix forests: a look ahead: Proceedings of an international symposium; 1992 October 5-9; Whitefish, MT. Gen. Tech. Rep. GTR-INT-319. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 349-359. [25331]
236. Roberts, David W. 1980. Forest habitat types of the Bear's Paw Mountains and Little Rocky Mountains, Montana. Missoula, MT: University of Montana. 116 p. Thesis. [29896]
237. Roberts, Mark R.; Zhu, Lixiang. 2002. Early response of the herbaceous layer to harvesting in a mixed coniferous-deciduous forest in New Brunswick, Canada. Forest Ecology and Management. 155(1-3): 17-31. [40747]
238. Robuck, O. Wayne. 1985. The common plants of the muskegs of southeast Alaska. Miscellaneous Publication/July 1985. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 131 p. [11556]
239. Roe, Nicholas A.; Kennedy, Alan J. 1989. Moose and deer habitat use and diet on a reclaimed mine in west-central Alberta. In: Walker, D. G.; Powter, C. B.; Pole, M. W., compilers. Proceedings of the conference: Reclamation, a global perspective; 1989 August 27-31; Calgary, AB. Rep. No. RRTAC 89-2. Vol. 1. Edmonton, AB: Alberta Land Conservation and Reclamation Council: 127-135. [14360]
240. Rogers, Robert S. 1980. Hemlock stands from Wisconsin to Nova Scotia: transitions in understory composition along a floristic gradient. Ecology. 61(1): 178-193. [62813]
241. Roland, A. E.; Smith, E. C. 1969. The flora of Nova Scotia. Halifax, NS: Nova Scotia Museum. 746 p. [13158]
242. Rose, Michael; Hermanutz, Luise. 2004. Are boreal ecosystems susceptible to alien plant invasion? Evidence from protected areas. Oecologia. 139(3): 467-477. [48554]
243. Ross, S. Rachel. 1978. The effects of prescribed burning on ground cover vegetation of white pine and mixed hardwood forests in southeastern New Hampshire. Durham, NH: University of New Hampshire. 151 p. Thesis. [20674]
244. Rowe, J. S. 1983. Concepts of fire effects on plant individuals and species. In: Wein, Ross W.; MacLean, David A., eds. The role of fire in northern circumpolar ecosystems. SCOPE 18. New York: John Wiley & Sons: 135-154. [2038]
245. Rowe, J. S.; Bergsteinsson, J. L.; Padbury, G. A.; Hermesh, R. 1974. Fire studies in the Mackenzie Valley. ALUR 73-74-61. Ottawa: Canadian Department of Indian and Northern Development. 123 p. [50174]
246. Safford, L. O. 1980. Paper birch. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 18. [49860]
247. Schaefer, James A. 1993. Spatial patterns in taiga plant communities following fire. Canadian Journal of Botany. 71(12): 1568-1573. [25527]
248. Schmidt, F. J. W. 1936. Winter food of the sharp-tailed grouse and pinnated grouse in Wisconsin. The Wilson Bulletin. 48(3): 186-203. [16729]
249. Schoen, John W.; Kirchhoff, Matthew D. 1990. Seasonal habitat use by Sitka black-tailed deer on Admiralty Island, Alaska. The Journal of Wildlife Management. 54(3): 371-378. [11940]
250. Schwartz, Charles C.; Franzmann, Albert W. 1991. Interrelationship of black bears to moose and forest succession in the northern coniferous forest. Wildlife Monographs No. 113. Washington, DC: The Wildlife Society. 58 p. [67724]
251. Scoggan, H. J. 1978. The flora of Canada. Part 4: Dicotyledoneae (Dictoyledonceae to Compositae). National Museum of Natural Sciences: Publications in Botany, No. 7(4). Ottawa: National Museums of Canada. 1711 p. [78054]
252. Scotter, George W. 1967. The winter diet of barren-ground caribou in northern Canada. The Canadian Field-Naturalist. 81: 33-39. [16672]
253. Scotter, George Wilby. 1964. Effects of forest fires on the winter range of barren-ground caribou in northern Saskatchewan. Wildlife Management Bulletin. Series 1. No. 18. Ottawa, ON: Canadian Wildlife Service, National Parks Branch, Department of Northern Affairs and National Resources. 111 p. [28989]
254. Shiplett, Brian; Neuenschwander, Leon F. 1994. Fire ecology in the cedar-hemlock zone of North Idaho. In: Baumgartner, David M.; Lotan, James E.; Tonn, Jonalea R., compilers. Interior cedar-hemlock-white pine forests: ecology and management: Symposium proceedings; 1993 March 2-4; Spokane, WA. Pullman, WA: Washington State University, Department of Natural Resources: 41-51. [25789]
255. Shirley, Hardy L. 1932. Light intensity in relation to plant growth in a virgin Norway pine forest. Journal of Agricultural Research. 44: 227-244. [10360]
256. Siccama, T. G.; Bormann, F. H.; Likens, G. E. 1970. The Hubbard Brook ecosystem study: productivity, nutrients and phytosociology of the herbaceous layer. Ecological Monographs. 40(4): 389-402. [8875]
257. Simon, Neal P. P.; Schwab, Francis E. 2005. Plant community structure after wildfire in the subarctic forests of western Labrador. Northern Journal of Applied Forestry. 22(4): 229-235. [61221]
258. Sirois, Luc. 1995. Initial phase of postfire forest regeneration in two lichen woodlands of northern Quebec. Ecoscience. 2(2): 177-183. [27068]
259. Skutch, Alexander F. 1929. Early stages of plant succession following forest fires. Ecology. 10(2): 177-190. [21349]
260. Smith, Marie-Louise. 1995. Community and edaphic analysis of upland northern hardwood communities, central Vermont, USA. Forest Ecology and Management. 72(2-3): 235-249. [27233]
261. Soper, James H.; Heimburger, Margaret L. 1982. Shrubs of Ontario. Life Sciences Miscellaneous Publications. Toronto, ON: Royal Ontario Museum. 495 p. [12907]
262. Southwick, Alrun K. 1981. Cornus canadensis: A boreal species. Botany 510-Boreal flora. Ann Arbor, MI: University of Michigan. 18 p. [83949]
263. Sperka, Marie. 1973. Growing wildflowers: A gardener's guide. New York: Harper & Row. 277 p. [10578]
264. Spies, Thomas A. 1991. Plant species diversity and occurrence in young, mature, and old-growth Douglas-fir stands in western Oregon and Washington. In: Ruggiero, Leonard F.; Aubry, Keith B.; Carey, Andrew B.; Huff, Mark H., technical coordinators. Wildlife and vegetation of unmanaged Douglas-fir forests. Gen. Tech. Rep. PNW-GTR-285. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 111-121. [17309]
265. St. John, Harold; Warren, Fred A. 1937. The plants of Mount Rainier National Park, Washington. The American Midland Naturalist. 18(6): 952-985. [62707]
266. Stark, Kaeli E.; Arsenault, Andre; Bradfield, Gary E. 2006. Soil seed banks and plant community assembly following disturbance by fire and logging in interior Douglas-fir forests of south-central British Columbia. Canadian Journal of Botany. 84(10): 1548-1560. [65962]
267. Stephenson, David E. 1985. The use of charred black spruce bark by snowshoe hare. The Journal of Wildlife Management. 49(2): 296-300. [8451]
268. Stevens, Charles E. 1968. A remarkable disjunct occurrence of Cornus canadensis in the Virginia Blue Ridge. Castanea. 33(3): 247-248. [83933]
269. Stewart, G. H. 1988. The influence of canopy cover on understory development in forests of the western Cascade Range, Oregon, USA. Vegetatio. 76: 79-88. [6631]
270. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in Northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
271. Stocks, B. J. 1989. Fire behavior in mature jack pine. Canadian Journal of Forest Research. 19(6): 783-790. [8672]
272. Strausbaugh, P. D.; Core, Earl L. 1977. Flora of West Virginia. 2nd ed. Morgantown, WV: Seneca Books. 1079 p. [23213]
273. Strong, W. L. 2002. Lodgepole pine/Labrador tea type communities of western Canada. Canadian Journal of Botany. 80(2): 151-165. [51690]
274. Strong, W. L.; Gates, C. C. 2006. Herbicide-induced changes to ungulate forage habitat in western Alberta, Canada. Forest Ecology and Management. 222(1-3): 469-475. [78645]
275. Strong, W. L.; LaRoi, G. H. 1986. A strategy for concurrently monitoring the plant water potentials of spatially separate forest ecosystems. Canadian Journal of Forest Research. 16(2): 346-351. [10805]
276. Strong, W. L.; Redburn, M. J. 2009. Latitude-related variation in understory vegetation of boreal Populus tremuloides stands in Alberta, Canada. Community Ecology. 10(1): 35-44. [83934]
277. Suring, Lowell H.; Goldstein, Michael I.; Howell, Susan M.; Nations, Christopher S. 2008. Response of the cover of berry-producing species to ecological factors on the Kenai Peninsula, Alaska, USA. Canadian Journal of Forest Research. 38(5): 1244-1259. [83935]
278. Suring, Lowell H.; Goldstein, Michael I.; Howell, Susan; Nations, Christopher S. 2006. Effects of spruce beetle infestations on berry productivity on the Kenai Peninsula, Alaska. Forest Ecology and Management. 227(3): 247-256. [62653]
279. Svoboda, Franklin J.; Gullion, Gordon, W. 1972. Preferential use of aspen by ruffed grouse in northern Minnesota. The Journal of Wildlife Management. 36(4): 1166-1180. [16736]
280. Swain, Albert M. 1973. A history of fire and vegetation in northeastern Minnesota as recorded in lake sediments. Quaternary Research. 3(3): 383-396. [38931]
281. Sweetman, Harvey L. 1949. Further studies of the winter feeding habits of cottontail rabbits. Ecology. 30(3): 371-376. [72521]
282. Tappeiner, J. C.; Alaback, P. B. 1989. Early establishment and vegetative growth of understory species in the western hemlock-Sitka spruce forests of southeast Alaska. Canadian Journal of Botany. 67(2): 318-326. [8931]
283. The Nature Conservancy. 1999. Classification of the vegetation of Isle Royale National Park. USGS-NPS Vegetation Mapping Program. Minneapolis, MN: The Nature Conservancy; Arlington, VA: The Nature Conservancy. 140 p. Available online: http://biology.usgs.gov/npsveg/isro/isrorpt.pdf [2007, October 3]. [68269]
284. Todd, John B. 1927. Winter food of cottontail rabbits. Journal of Mammalogy. 8(3): 222-228. [74541]
285. Topik, Christopher; Halverson, Nancy M.; Brockway, Dale G. 1986. Plant association and management guide for the western hemlock zone: Gifford Pinchot National Forest. R6-ECOL-230A. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 132 p. [2351]
286. Topik, Christopher; Hemstrom, Miles A., comps. 1982. Guide to common forest-zone plants: Willamette, Mt. Hood, and Siuslaw National Forests. R6-Ecol 101-1982. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 95 p. [3234]
287. Troth, John L.; Deneke, Frederick J.; Brown, Lloyd M. 1976. Upland aspen/birch and black spruce stands and their litter and soil properties in interior Alaska. Forest Science. 22(1): 33-44. [8161]
288. Turner, Nancy Chapman; Bell, Marcus A. M. 1971. The ethnobotany of the Coast Salish Indians of Vancouver Island. Economic Botany. 25(3): 63-104. [21014]
289. U.S. Department of Agriculture, Forest Service, Alaska Region. [n.d.]. Preliminary forest plant associations of the Stikine Area, Tongass National Forest. R10-TP-72. Portland, OR: U.S. Department of Agriculture, Forest Service, Alaska Region. 126 p. [19016]
290. U.S. Department of Agriculture, Natural Resources Conservation Service. 2012. PLANTS Database, [Online]. Available: https://plants.usda.gov /. [34262]
291. Usui, Masayuki; Kakuda, Yukio; Kevan, Peter G. 1994. Composition and energy values of wild fruits from the boreal forest of northern Ontario. Canadian Journal of Plant Science. 74(3): 581-587. [24583]
292. Van Cleve, Keith; Viereck, Leslie A. 1981. Forest succession in relation to nutrient cycling in the boreal forest of Alaska. In: Fire and succession in conifer forests of North America. New York: Springer-Verlag: 185-211. [50633]
293. Van Horne, B.; Hanley, T. A.; Cates, R. G.; McKencrick, J. D.; Horner, J. D. 1988. Influence of seral stage and season on leaf chemistry of southeastern Alaska deer forage. Canadian Journal of Forest Research. 18(1): 90-99. [8873]
294. Van Nostrand, R. S. 1965. Results of experimental seeding of balsam fir on a recent burn. Department of Forestry Publication No. 1103. Ottawa, ON: Canadian Department of Forestry, Research Branch. 10 p. [41914]
295. Viereck, L. A.; Dyrness, C. T. 1979. Ecological effects of the Wickersham Dome fire near Fairbanks, Alaska. Gen. Tech. Rep. PNW-90. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 71 p. [6392]
296. Viereck, Leslie A. 1970. Forest succession and soil development adjacent to the Chena River in interior Alaska. Arctic and Alpine Research. 2(1): 1-26. [12466]
297. Viereck, Leslie A.; Johnston, William F. 1990. Picea mariana (Mill.) B.S.P. black spruce. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 227-237. [13386]
298. Viereck, Leslie A.; Little, Elbert L., Jr. 1972. Alaska trees and shrubs. Agric. Handb. 410. Washington, DC: U.S. Department of Agriculture, Forest Service. 265 p. [6884]
299. Voss, Edward G. 1985. Michigan flora. Part II. Dicots (Saururaceae--Cornaceae). Bulletin 59. Bloomfield Hills, MI: Cranbrook Institute of Science; Ann Arbor, MI: University of Michigan Herbarium. 724 p. [11472]
300. Wali, M. K.; Krajina, V. J. 1973. Vegetation-environment relationships of some sub-boreal spruce zone ecosystems in British Columbia. Vegetatio. 26(4/6): 237-381. [9856]
301. Wang, G. Geoff; Kemball, Kevin J. 2003. The effect of fire severity on early development of understory vegetation following a stand replacing wildfire--3B.2, [Online]. In: Proceedings, 2nd international wildland fire ecology and fire management congress held concurrently with the 5th symposium on fire and forest meteorology; 2003 November 16-20; Orlando, FL. Boston, MA: American Meteorology Society (Producer): Available: http://ams.confex.com/ams/FIRE2003/techprogram/paper_65430.htm [2006, October 27]. [64194]
302. Wang, G. Geoff; Kemball, Kevin J. 2005. Effects of fire severity on early development of understory vegetation. Canadian Journal of Forest Research. 35(2): 254-262. [60329]
303. Waterman, W. G. 1922. Development of plant communities of a sand ridge region in Michigan. Botanical Gazette. 74(1): 1-31. [63565]
304. Weatherbee, Pamela B.; Crow, Garrett E. 1992. Natural plant communities of Berkshire County, Massachusetts. Rhodora. 94(878): 171-209. [19726]
305. Weber, William A.; Wittmann, Ronald C. 1996. Colorado flora: eastern slope. 2nd ed. Niwot, CO: University Press of Colorado. 524 p. [27572]
306. Weckwerth, Richard P.; Hawley, Vernon D. 1962. Marten food habits and population fluctuations in Montana. The Journal of Wildlife Management. 26(1): 55-74. [76088]
307. Welch, Christy A.; Keay, Jeffrey; Kendall, Katherine C.; Robbins, Charles T. 1997. Constraints on frugivory by bears. Ecology. 78(4): 1105-1119. [27896]
308. Wetzel, John F.; Wambaugh, James R.; Peek, James M. 1975. Appraisal of white-tailed deer winter habitats in northeastern Minnesota. The Journal of Wildlife Management. 39(1): 59-66. [64397]
309. Wherry, E. T. 1934. Temperature relations of the bunchberry, Cornus canadensis L. Ecology. 15(4): 440-443. [8929]
310. Whitaker, D. L.; Webster, L. A.; Edwards, J. 2007. The biomechanics of Cornus canadensis stamens are ideal for catapulting pollen vertically. Functional Ecology. 21(2): 219-225. [83939]
311. Wiant, Harry V., Jr. 1980. Eastern hemlock. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 27. [49891]
312. Wilde, S. A. 1933. The relation of soils and forest vegetation of the Lake States region. Ecology. 14(2): 94-105. [66064]
313. Wolff, Jerry O. 1978. Food habits of snowshoe hares in interior Alaska. The Journal of Wildlife Management. 42(1): 148-153. [7443]
314. Yerkes, Vern P. 1960. Occurrence of shrubs and herbaceous vegetation after clear cutting old-growth Douglas-fir. Res. Pap. PNW-34. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 12 p. [8937]
315. Yole, D.; Lewis, T.; Inselberg, A.; Pojar, J.; Holmes, D. 1989. A field guide for identification and interpretation of the Engelmann spruce-subalpine fir zone in the Prince Rupert Forest Region, BC. Victoria, BC: Ministry of Forests, Research Branch. 81 p. [17095]
316. Zasada, John C. 1980. Paper birch. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 83. [50015]
317. Zasada, John C. 1980. White spruce-paper birch. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 82. [50014]
318. Zobel, Donald B.; McKee, Arthur; Hawk, Glenn M.; Dyrness, C. T. 1976. Relationships of environment to composition, structure, and diversity of forest communities of the central western Cascades of Oregon. Ecological Monographs. 46(2): 135-156. [8767]
319. Zogg, Gregory P.; Barnes, Burton, V. 1995. Ecological classification and analysis of wetland ecosystems, northern Lower Michigan, U.S.A. Canadian Journal of Forest Research. 25(11): 1865-1875. [26166]
320. Zoladeski, Christopher A.; Maycock, Paul F. 1990. Dynamics of the boreal forest in northwest Ontario. The American Midland Naturalist. 124(2): 289-300. [13496]