| FEIS Home Page |
 |
|
| A black spruce bog in Lake County, MN. © 2005 Jason J. Husveth. |
| This review summarizes information on the general ecology and fire ecology of black spruce that was available in the scientific literature as of
2014. Details and documentation of source materials follow this summary.
Introductory: The Introductory section discusses the taxonomy of black spruce, including hybrids and synonyms. Distribution and Occurrence: Black spruce occurs on a wide range of sites. It grows on wet lowlands and drier uplands, in a variety of soils. It is most common on poorly drained sites underlain with permafrost. Black spruce dominates most spruce-fir ecosystems of boreal North America. Black spruce communities are generally classified into forest and woodland types. Ericaceous shrubs often grow in black spruce understories, with mosses and lichens in ground layers. Additionally, black spruce is important in other boreal mixed-conifer and conifer-hardwood communities, including white spruce-lodgepole pine, jack pine-balsam fir, and quaking aspen-paper birch. Botanical and Ecological Characteristics: The arrangement of black spruce's branches and cones promotes easy ignition and torching while protecting seeds from consumption by fire. The branches are distributed from ground to crown, and the cones grow in tight clusters at the tips of crown branches. Stand structure ranges from very open to closed. Most black spruce regeneration occurs after fire. Black spruce's semiserotinous cones release seeds soon after fire, and dispersal continues through the first few postfire years. Favorable seedbeds include mineral soil and organic soil where most of the moss and/or lichen layers have burned off. Growth is most rapid on open sites such as recent burns or clearcuts. Most succession in black spruce communities occurs after fire. Black spruce generally occurs in all stages of boreal forest succession, although its abundance tends to decrease with time since fire. Fire Effects and Management: Fire of any severity generally kills black spruce. The species regenerates from cone-stored seed after fire; most seeds disperse from the cones of on-site, fire-killed trees. Most establishment occurs within 5 postfire years. Black spruce communities are highly flammable due to the chemical contents of black spruce plants and the multilayered structure of forest stands. Black spruce needles and branches are highly resinous, and fire carries easily from low branches to crowns. Where ericaceous shrubs are present, they provide ladder fuels; however, fire generally spreads rapidly—both vertically and horizontally—in black spruce communities even when shrub layers are sparse or lacking. Groundlayer mosses and lichens provide surface fuels, and low black spruce branches carry fire to the canopy. Fire regimes in black spruce communities are similar across black spruce's distribution. Nearly all fires are stand-replacement. Stands experience mostly mixed crown and surface fires, with some lethal surface fires. Ground fires typically linger after flame fronts have passed. Fire-return intervals in black spruce communities generally range from about 50 to 150 years, averaging about 75 years across black spruce's distribution. Upland communities burn more often than lowland communities; wet, lowland black spruce communities usually burn only during severe fire weather in late summer. Across a landscape, black spruce communities often display a mosaic of unburned to severely burned patches. Most fires in black spruce stands are small, but large wildfires occurring in extreme fire years account for most acreage burned. In western North America, increased fire frequency and/or severity resulting from climate change may cause shifts from black spruce to hardwood types in southern portions of black spruce's range and from black spruce woodland to open tundra in northern portions. Prescribed fire has been used successfully to promote black spruce regeneration and control pests. Eastern dwarf mistletoe can be controlled with prescribed fire. Several Fire Studies on black spruce communities are available in FEIS. They provide fire prescriptions and management recommendations for using prescribed fire in black spruce communities, as well as site-specific information on postfire establishment and growth rates of black spruce and associated species. Management Considerations: A wide variety of wildlife species use black spruce communities as habitat. Some guilds use postfire successional stages preferentially. Among bird guilds, for example, cavity nesters prefer early-seral black spruce stands, while foliage gleaners generally prefer mature stands. While most wildlife species avoid black spruce browse, black spruce provides important winter forage for some species. Many wildlife species consume the seeds. Logging may be used as a fire surrogate. To promote biodiversity, retaining the full spectrum of successional stages is recommended on landscapes where fire is excluded. |
Natural black spruce × red spruce hybrids occur "to a limited extent" [121] in eastern Canada [121,287]. Putative black spruce × white spruce hybrids, sometimes called Rosendahl spruce, have been reported in Minnesota [121,254,395].
See Appendix B for scientific names of plant taxa named in this review and for links to available FEIS reviews.
SYNONYMS: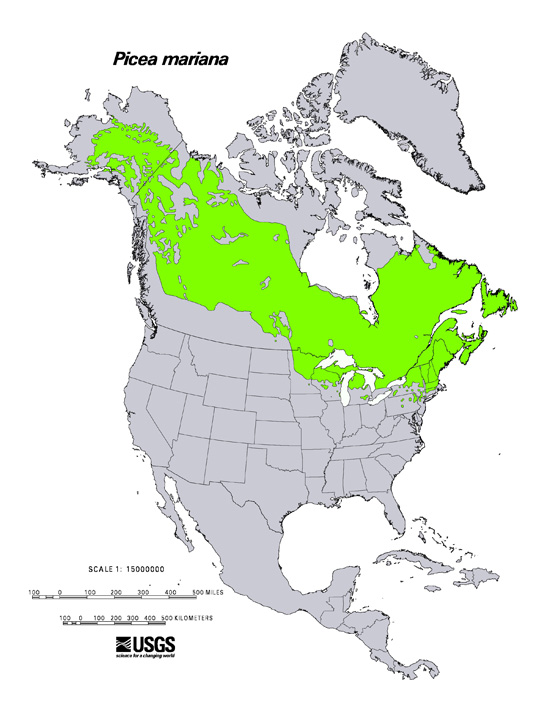 |
| Black spruce distribution. 1971 USDA, Forest Service map provided by [367]. |
Black spruce is native to the United States and Canada [210,253,367]. It is primarily a boreal species, although its distribution extends south into the Great Lakes and Northeast regions of the United States. Black spruce's expansion north is hindered by permanently frozen soils. In interior Alaska, young glacial deposits halt its distribution in the southern Brooks Range [255]. In southern Wisconsin, southern Michigan, Pennsylvania, and New Jersey, black spruce is confined to isolated, cold peatlands [172,395]. Climate warming is apparently favoring expansion of black spruce's distribution in the north [35,75] and shrinkage in the south [172].
Countries, states, and provinces [376]:Topography and elevation: Black spruce most commonly grows on lowlands but also on uplands [129]. Lowland black spruce communities occupy river terraces and gently rolling valleys, while upland communities generally occupy low-gradient and north-facing slopes [162,225,379]. In interior Alaska, black spruce occupies poorly drained lowlands such as cold, wet flats and muskegs. It is also common on north-facing slopes within 5 miles (8 km) of major rivers and—where white spruce is absent—on upland slopes of all exposures that are more than 5 miles from major rivers [124,396]. At the southern end of its range, black spruce is mostly restricted to wet lowlands [142,375,400]. In the Great Lakes states and New England, it is most abundant on low, acidic peatlands, but it is also common on transitional sites between low peatlands and uplands [375].
Except in isolated areas of northern Minnesota and the Upper Peninsula of Michigan [375], black spruce is rare on uplands in the Great Lakes region [198,375]. In the 1830s, township surveyors on the Lower Peninsula of Michigan documented characteristics of sites with black spruce. Analyses of those data in the 1980s showed black spruce was positively associated with depressions, acidic to neutral peats, and areas with very poor drainage (P=0.1) [405].
Blacks spruce occurs from sea level to 5,000 feet (1,500 m) elevation across its range [121] but is generally found from 500 to 2,500 feet (150-760 m) [129]. In Alaska, it ranges up to 2,300 feet (700 m) elevation in Denali National Park and 3,800 feet (1,150 m) in mountainous regions of eastern Alaska [396]. Black spruce-lichen communities tend to occupy relatively high elevations [379] or latitudes. Black spruce is confined to elevations above 2,000 feet (650 m) in the northeastern United States [90].
Soils and soil moisture regimes: Poor drainage and shallow permafrost typify soils supporting black spruce [162,182,225,263,332]; black spruce is one of few conifers that tolerate such conditions [242,243,362,381,387]. However, black spruce tolerates a wide range of soil temperature and moisture regimes: It grows in in relatively warm, dry soils as well as nearly frozen, wet soils that exclude hardwoods and most other conifers [65,242,375]. Substrate moisture varies from saturated in bogs and swamps to wet on bottomlands and flats, wet to moist on lake margins, mesic on north-facing slopes, well-drained on most other slopes, and dry on drained peatlands [121,162,396].
Both lowland and upland black spruce types are most abundant on nutrient-poor soils that are poorly drained and cold [85]. Productivity in black spruce communities is lowest among boreal forest types (review by [85]). The most productive black spruce stands generally occupy south-facing slopes [379].
Although black spruce favors acidic soils, high soil or water pH may be tolerated. In Alberta, black spruce grows in bogs with extremely acidic to neutral soils (pH 4.0-7.0), but it sometimes grows in calcareous bogs with soil pH as high as 8.0 [174].
Black spruce is not well adapted to coastal fog or salt spray, so it is rare in maritime settings (review by [108]).
Permafrost tends to be continuous in black spruce's northernmost distributions, becoming discontinuous to the south [383]. The permafrost layer beneath black spruce is often shallow in boreal regions [85]. In the growing season, soils thaw to a depth of 8 to 35 inches (20-90 cm) [388]. In interior Alaska, black spruce dominates bogs with shallow permafrost [124,182,225]; permafrost tables are often as shallow as 12 inches (30 cm) [104]. Permafrost is often absent on newly deposited alluvium and south slopes and becomes discontinuous south of the Alaska Range, so black spruce is less dominant on such sites [388].
Black spruce stands typically have a thick organic mat overlying the mineral soil layer. Consequently, forest floor temperatures are lower and soil moisture content higher in black spruce muskegs than in white spruce, balsam fir, paper birch, or quaking aspen stands [380]. In Alaska, the live moss and organic soil layers may be up to 20 inches (50 cm) thick [389]. On the Laurentian Shield in Minnesota, the peat layer on black spruce sites is often 10 feet (3 m) deep, and sometimes up to 60 feet (18 m) deep [129]. In the northeastern United States, black spruce grows on peats >12 inches (30 cm) thick [40].
Black spruce grows in all soil textures [129,395] but is mostly restricted to acidic soils [91,108]. It is often reported on loams (for example, [129,205]). In the Great Lakes region, black spruce grows in coarse soils and mucks. It is common in sands, gravels, and coarse to bouldery loams [199] on lakeshores and dune ridges [400]. In northern Minnesota and southern Ontario, black spruce occurs on gravelly and bouldery loams and shallow soils over bedrock [129]. Throughout much of Canada, upland black spruce stands occur on wet to moist clays and clay loams on lowlands and long, gentle slopes. In New Brunswick, Nova Scotia, and parts of Québec, black spruce grows in sands and gravels on outwash plains, river terraces, and eskers [129]. Along a 0.6 × 9-mile (1 × 140-km) transect in southern Québec, all black spruce-lichen woodlands occurred over well-drained podzols formed in fluvial-glacial or glacial deposits [141]. White spruce is more likely than black spruce to occupy alluvial soils [332].
Plant communities: Black spruce communities are nearly ubiquitous in boreal regions of North America but have minor coverage in the Great Lakes states and the Northeast. Many factors determine plant species composition in black spruce communities, including altitude [225,388], slope, drainage, presence and thickness of permafrost, fire history, stage of succession, and canopy openness [388]. Black spruce stands establish after a stand-replacement event; usually fire, although clearcutting, disease, or insect outbreaks may also facilitate such succession.
See Appendix B for scientific names of plant taxa named in this review and for links to available FEIS reviews.
Black spruce grows in pure and mixed stands. Structure of black spruce forests often includes tall shrub and low shrub layers. Mosses and/or lichens usually dominate the ground layer, although graminoids may dominate on some sites. Trees associated with black spruce across its range include tamarack, white spruce, quaking aspen, and paper birch [129,162,395]. Bigtooth aspen is frequently associated in the southeastern portion of black spruce's range [72] and balsam poplar in the southwestern portion [162]. Quaking aspen and birches are more common in southern boreal than in northern boreal black spruce communities [383].
Tall shrubs associated with black spruce across all or most of its range include gray alder, red-osier dogwood, and various willow and birch species. Low shrubs associated with black spruce across its range include prickly rose and the ericaceous shrubs bog blueberry, black crowberry, and bog Labrador tea [383,385,395]. These tall and low shrubs can be ladder fuels during a fire. A review noted that understory diversity usually increases with time since fire and is greatest in old-growth black spruce forest undergoing gap succession [47].
The ground layer is often nearly continuous feather and/or sphagnum mosses. Splendid feather moss, Schreber's moss, juniper haircap moss, and knight's-plume moss are dominant feather mosses [395]. A review noted that in low black spruce peatlands, dominant sphagnum mosses dominant in peatlands include Magellan's sphagnum, deceptive sphagnum, and red sphagnum. Sphagnums dominant on hummocks include hairy-leaved sphagnum, Girgensohn's sphagnum, and fine sphagnum [47]. Lichen layers are sparse to nearly continuous [391] in black spruce-lichen woodlands. Reindeer lichens or green dog lichen usually dominate the ground layer; other lichen species may codominate. Feather mosses may replace lichens as postfire succession proceeds, although lichens may continue to dominate woodlands [383].
Black spruce/feather moss-lichen and black spruce-white spruce forest types occur across boreal Alaska and Canada [108]. "Primarily a Canadian forest cover type of vast extent" [111], the distribution of the Society of American Forester's (SAF) black spruce type stretches across most of Canada [111]. The SAF black spruce type is divided into 6 subtypes [90]:
1) The black spruce/feather moss forest type is most extensive; it is found in the central and southern portions of black spruce's range. Stands are well-stocked to dense spruce stands with a well-developed carpet of Schreber's moss, splendid feather moss, and/or knight's-plume moss.
2) Black spruce-dwarf shrub/Schreber's moss forests occur in the same regions, including Maine, on poor loamy sands and sandy loams. Stands are closed, with a well-developed ericaceous dwarf shrub layer. Mosses and reindeer lichens dominate the forest floor.
3) Black spruce/sphagnum forests occur throughout North America's boreal and Great Lakes regions, on very wet organic or wet mineral soils. Forests are open to closed pure black spruce stands, with a well-developed ericaceous dwarf shrub layer.
4) Black spruce-sedge forests occur across North America's boreal forest on peatlands with moving water; they are infrequent farther south. Stands are very open stands with stunted trees. Sedges, grasses, and a well-developed feather moss layer dominate the ground layer.
5) Black spruce-gray alder forests occur in the same regions, but are confined to mucky depressions where the water table is at or above the surface
during the growing season. Stands are pure or mixed, occurring in areas where the water table is near the surface during the growing season. They have well-developed tall shrub and herbaceous layers.
6) Black spruce-lichen woodlands are frequent throughout black spruce's range at the forest-tundra transition zone. They have a well-developed carpet of reindeer lichens. These woodlands remain open due to cold climate.
Additionally, black spruce is codominant in SAF's balsam fir [130], black spruce-tamarack [111,145], black spruce-white-spruce [391], and black spruce-paper birch [102] forest cover types. The balsam fir type occurs in the Maritime Provinces, eastern Canada, and the northeastern and Great Lakes states on a wide variety of sites [130]. The black spruce-tamarack forest type has a similar distribution. It occupies lowland, minerotrophic streamsides and peat bogs [145]. Tamarack becomes dominant on very wet, nutrient-poor sites [206]. Black spruce-white spruce woodland or forest types occur from Alaska and across Canada to the Hudson Bay. Black spruce-tamarack sites are typically near latitudinal or elevational treelines. Sometimes, these sites have characteristics intermediate to those favored by tamarack (low and acidic) and those favored by spruces (upper floodplain terraces and bases of south slopes) [391]. The black spruce-paper birch forest type occurs in intermittent, small patches in Alaska and Yukon. It is a seral type that occurs after severe ground fire exposes mineral soil. It occupies loess or glacial till slopes with shallow permafrost [102].
See the Fire Regime Table for a list of plant communities in which black spruce may occur and information on the fire regimes associated with those communities. More detailed descriptions of black spruce communities follow by region.
Alaska: Black spruce dominates or codominates most Alaskan taiga landscapes [206]; black spruce is the most common forest type in the state [85]. It has dominated Alaska's boreal region for the past 5,500 years [265]. Black spruce communities presently occupy 39% to 44% of interior Alaska [69]. At the landscape level, Alaskan black spruce communities form mosaics with quaking aspen-birch, white spruce, and mixedwood (spruce-hardwood) stands. Black spruce generally occupies the most poorly drained, coldest portions of these mosaics (see Site characteristics). White spruce and hardwoods generally occupy relatively warm sites, and balsam poplar and black cottonwood dominate floodplains of major rivers [388].Lowland black spruce communities are mostly wetlands, while upland black spruce communities are drier [386]. Lowland black spruce forests are abundant in interior Alaska [122,124] but sporadic on the southern coast (Copper River Basin and Cook Inlet lowlands). Coastal black spruce forests are open and interspersed with shrub tundra [279].
Several species or genera are common in many Alaskan black spruce types including quaking aspen, paper birch, white spruce, and tamarack in the overstory; willows and ericaceous shrubs in the shrub layer; and cottongrasses, feather mosses, sphagnum mosses, and reindeer lichens in the ground layer [124,162,386]. Black spruce branches are often draped with arboreal lichens including Fremont's horsehair lichen and old man's beard [263].
 |
| An upland black spruce forest. Photo by Steve Hillebrand, U.S. Fish and Wildlife Service. |
Foote [122,124] identified several black spruce forest types of interior Alaska. She noted these types might be difficult to distinguish in early postfire succession, but could usually be distinguished from one another by postfire year 50 [124].
Black spruce dominates some riparian areas of interior Alaska. In Kobuk Valley National Park, it dominates poorly drained lowland and some slope forests of the Salmon River valley. Black spruce stands generally dominate river bends where fine-textured sediments are exposed, while balsam poplar or white spruce dominate coarse-textured soils along the riverbank. Black spruce-reindeer lichen woodlands occur on sandy ridges, knolls, and bluffs. Dwarf black spruces have scattered occurrence in heathlands [282]. Along the Kuskokwim River, open black spruce-quaking aspen forests occupy upper terraces, while black spruce/sphagnum muskegs occupy low terraces [99]. On the Tanana River floodplain, open white spruce-black spruce stands were transitional between the active floodplain and upper, older terraces. Canopy cover ranged from 25% to 50%. These stands were 150 to 250 years old and uneven-aged. Open (40%-60% canopy cover) black spruce/bog Labrador tea stands were found on upper terraces of the active floodplain. Both mixed spruce and black spruce bog types were always underlain with permafrost, frequently with a shallow active layer. Black spruce bogs occupied the uppermost, oldest terraces. These poorly drained areas originated on sites where the upper permafrost layer was melting [392].
Black spruce does not always codominate mixed-conifer forests. It is a minor species in many tamarack bogs [101]. In interior Alaska, it is usually a minor component of white spruce forests. In white spruce communities, it is most common on well-drained uplands during early postfire succession [162].
Black spruce is generally a minor type in southern Alaska. It occurs over scattered pockets of permafrost, mainly on muskegs of the Kenai Lowlands. An open to closed black spruce/bunchberry-mountain cranberry/lichen community type has been identified there [329]. Black spruce dominates some hills and ridges of the Ahklun Mountains, and it is the dominant forest type in the Koskokwin Mountains [279]. Open black spruce forests are interspersed with shrub tundra in the Copper River Basin and on Cook Inlet lowlands [279].
Great Lakes: Black spruce dominates some cold forests and wooded bogs of the Great Lakes region. It is common in mixed-conifer and occurs in some maple-beech-birch communities. It often codominates with jack pine in mixed-conifer communities [7]. In the Boundary Waters Canoe Area of Michigan, a black spruce-paper birch-jack pine community was noted on Fishhook Island, and black spruce was a component of a red-maple-balsam fir-eastern white pine community near Hegman Lake [133]. Jack pine-black spruce and black spruce-jack pine communities of Boundary Waters Canoe Area generally differ in understory structure and composition: The jack pine-black spruce community typically has well-developed shrub and herb layers. The black spruce-jack pine community generally lacks those layers but has a feather moss layer that covers nearly 100% of the ground. Both communities occur on southerly, low and midslopes with poorly drained soils, with jack pine becoming less important with time since fire. Black spruce is occasional to important in red maple-quaking aspen-paper birch, eastern white pine, and northern whitecedar communities of the Boundary Waters Canoe Area. It is a minor species in red spruce communities [303].
Four black spruce forest associations are described for Isle Royale National Park, Michigan. They include black spruce/Schreber's moss, jack pine-black spruce/low sweet blueberry/Schreber's moss, black spruce/speckled alder/peatmoss (sphagnum and calliergon mosses) rich swamp, and black spruce/bog Labrador tea/sphagnum poor swamp. These forests are uncommon in the Park but also occur in northern Michigan and northeastern Minnesota. The black spruce/bog Labrador tea forest also occurs in Wisconsin. Canopies of the black spruce/Schreber's moss forest are typically closed (60%-90% canopy cover), while canopies of the jack pine-black spruce forest are open (~60%), with black spruce in the subcanopy. The black spruce/speckled alder forest occurs in wet depressions. It has an open canopy (30%-40%) and a tall shrub layer (20%-40%). Short and dwarf shrubs are sparse. The black spruce/bog Labrador tea swamp forest also occurs in wet depressions, with an open canopy (20%-50%) and a subcanopy of tall shrubs (5%-30%) and black spruce (1%-25%). Black spruce is sometimes codominant in northern whitecedar/speckled alder, jack pine-eastern white pine, and tamarack rich swamp forests of Isle Royale National Park [365].
Black spruce/sphagnum bogs occur on lowlands of northern Minnesota [279] and Michigan. Shrubby black spruce peatlands or black spruce-tamarack swamps dominate sandy glacial lake plains [7]. In Voyageurs National Park, Michigan, black spruce/speckled alder and black spruce/laurel communities occurred on wet, nutrient-poor lowlands. The overstory was nearly pure in black spruce/speckled alder, but the herb layer was often diverse. Black spruce/laurel ecotypes were more open and less diverse, possibly because nutrient levels were very low. Black spruce was an associated to dominant species of red pine-eastern white pine/twinflower and balsam fir-spruce-paper birch/clubmoss ecotypes on drier, richer soils [226].
Mixed forests of this region may have an important black spruce component. Kuchler [224] described a tamarack-black spruce-northern whitecedar conifer bog type that had scattered occurrences in the northern Great Lakes region, New York, and New England. In the mid-1850s in northwestern Wisconsin, surveyors recorded a mosaic of wet sedge meadows interspersed with small patches of lowland tamarack-black spruce woodlands [399]. In northeastern Wisconsin and Michigan's Upper Peninsula, black spruce is successionally dominant in logged or logged-and-burned eastern hemlock-red maple-sugar maple and eastern hemlock-northern whitecedar habitat types [83].
Conifer swamps with black spruce, northern whitecedar, tamarack, and/or eastern hemlock are scattered throughout Wisconsin [86,223]. Tamarack-black spruce communities occur as open bogs, swamps, or closed wet forests in northern Wisconsin. Overstories vary from nearly pure tamarack to nearly pure black spruce. Black spruce usually dominates paludified lake basins completely filled with peat, while tamarack usually dominates where bog mats are still advancing into open water [86].
Northeast: Black spruce dominates some conifer bogs of the Northeast. Dammam and French [91] describe 2 black spruce forest bog community types within this region: black spruce/Magellan's sphagnum of New England and northern Maine and black spruce/threeseeded sedge of New England. Stand structure in Magellan's sphagnum types range from open to closed and dense, with a well-developed sphagnum carpet. The shrub component decreases with increasing overstory density. Ericaceous thickets dominated by highbush blueberry, swamp azalea, bog Labrador tea, and/or catberry may succeed to this forest type. The black spruce/threeseeded sedge type borders northern hardwood or spruce forests; balsam fir may codominate in the overstory [91]. Black spruce also occurs in mixed-conifer, mixedwood, and heathland communities. Its importance decreases in the southern limits of its distribution in this region.
In Maine, black spruce dominates some late-successional conifer communities. Red spruce and balsam fir often cooccur, especially in canopy gaps and on forest edges [228]. In Acadia National Park, black spruce dominates some conifer/heathland communities and occurs in subboreal mixed-conifer forests that are transitional to the boreal spruce-fir forests of Québec. A black spruce/sheep-laurel woodland occurs on rocky sites, and a black spruce/highbush blueberry-black huckleberry/sphagnum woodland occurs in bogs. Black spruce is an important component of red maple woodland swamps and mixed-conifer forests with red spruce, eastern white pine, tamarack, and/or northern whitecedar [259].
In the Adirondack Mountains of New York, black spruce dominates some conifer and mixedwood forests. It replaces red spruce as the dominant tree at high elevations [163,328] (≥1,200 feet (360 m)) [163]. In midelevations of the Adirondack Mountains, black spruce dominates poor fens, while red spruce dominates rich fens by streams [330]. A balsam fir-black spruce/bunchberry/splendid feather moss forest association occurs in the Adirondack and White mountains on very strongly acid soils. Eastern white pine often dominates after fire until black spruce replaces it successionally [163]. On soils that are only seasonally moist, black spruce sometimes dominates mixedwoods with yellow birch, black cherry, and eastern hemlock. These forests occur on flat lowlands or border swamps, lakes, or streams [328]. Above 3,500 feet (1,000 m) in the Adirondack Mountains, black spruce grows as krummholz in balsam fir alpine dwarf woodlands. A black spruce-eastern hemlock talus community occurs in the ice caves (icy rock crevices) of Shawangunk Ridge [328].
Black spruce bogs and swamps are scattered throughout New York. Black spruce-tamarack/sphagnum bogs occur on acidic peatlands in cool, poorly drained depressions. The canopy is open to closed (50%-90%), and the shrub layer is composed of ericaceous species. Stringers of black spruce, often with tamarack, are interspersed with low sphagnum peatlands. The trees are usually dwarfed. In northern and central New York, black spruce is a component of northern whitecedar swamps [328].
In the White Mountains of New Hampshire, black spruce is an associated species of high-elevation balsam fir-quaking aspen forests. Red spruce dominates at low elevations [327]. On the White Mountain National Forest, dwarf black spruce and tamarack cooccur on shrub islands within acidic fens and on small (<2.0 acre (0.8 ha)) forest seeps [336].
Black spruce is occasional in Mud Pond Bog, New Hampshire. Red maple dominates the swampland area of Mud Pond Bog, but black spruce is a component of the swamp. Scattered black spruce trees grow in leatherleaf/sphagnum and black huckleberry-highbush blueberry heathlands, and black spruce grows in dwarf form in sheep-laurel-threeseeded sedge heathlands [100].
In the Allegheny Mountains of northeastern Pennsylvania, black spruce occurs in the transitional zone between oak-hickory and northern mixedwood ecosystems. An eastern hemlock-black spruce bog community was noted at 2,100 feet (640 m) in a flat, poorly drained area. The canopy was so closed that most shrubs were excluded, and the ground layer was thick sphagnum. Woody debris was substantial and the water table was near ground level; often, standing pools of water formed in tip-up pits. A black spruce/tamarack/black highbush blueberry-highbush blueberry/sphagnum bog community was noted at 1,900 feet (580 m) [98].
In New Jersey, where black spruce reaches its lower latitudinal limit, black spruce-tamarack swamps are occasional in lowlands. Black spruce is a component of highbush blueberry bogs [338]. A red maple-black spruce-highbush blueberry palustrine woodland is described in the New Jersey portion of Delaware Water Gap National Recreation Area. The woodland occurs in isolated pockets of upland depressions, within a larger wetland matrix. The woodland soil is very poorly drained and sometimes overlain with muck or peat [322].
Western Canada: In British Columbia and Alberta, black spruce associates with white spruce, Rocky Mountain lodgepole pine (hereafter, lodgepole pine), subalpine fir, and balsam poplar on upland sites. It occurs mostly in pure stands in bogs, although tamarack may be a minor tree. The ground layer is composed of feather and/or sphagnum mosses [174]. In interior portions of the Prince Rupert Forest Region, British Columbia, black spruce/peatmoss communities typically occur in frost-pocket depressions where the peat mat has grown over a pond or lake. Sites range from very acidic bogs to neutral fens, with canopies ranging from very open to closed [153].
In Yukon, black spruce is an indicator of permafrost sites. Black spruce/bog Labrador tea/splendid feather moss forests are multilayered, with grayleaf willow in the tall shrub and ericaceous species in the low shrub layer [305]. Open black spruce-tamarack/bog blueberry/lichen taiga occurs in the foothills of the Richardson Mountains. The shrub canopy is closed. Slopes are gentle and have poor drainage, with permafrost at depths of 13 to 26 inches (33-65 cm) [356].
In the Northwest Territories east of Great Slave Lake, black spruce codominates open parklands with jack pine, white spruce, and paper birch. Ericaceous species (crowberry, Labrador tea, and blueberry spp.) dominate the shrub layer, and lichens (snow, cetraria, and reindeer lichens) dominate the ground layer. Black spruce dominates open, forested peatlands; tamarack is infrequently associated. Plateaus are generally treeless lichen tundra [188].
Central and eastern Canada: In the central portion of black spruce's boreal distribution, jack pine displaces lodgepole pine as the codominant [106]. Black spruce tends to grow on wet lowlands, while jack pine grows on dry uplands. With accumulation of soil organic material due to fire exclusion or long fire-return intervals, black spruce may eventually replace jack pine successionally on uplands [404].
In eastern Canada, black spruce tends to dominate mixed-conifer and mixedwood forests on poorly drained sites. In Ontario, it dominates poorly drained lowlands and codominates with white spruce, quaking aspen, and/or paper birch on uplands. It codominates with jack pine on some sand plains and well-drained, shallow soils [92]. In Quetico Provincial Park, Ontario, jack pine, black spruce, and balsam fir cooccurred in a nearly even-aged stand. Trees ranged from 40 to 69 years old. Jack pine dominated the overstory; black spruce the midstory; and balsam fir the understory. The dense balsam fir understory prevented further conifer establishment. In a 160-year-old stand, black spruce dominated the canopy, with white spruce and jack pine codominating. Paper birch and sugar maple were establishing in the canopy, with the conifers "in the process of breaking up" successionally. Black spruce regeneration was growing slowly, likely due to shade cast by the hardwoods [93]. A mosaic of black spruce-red spruce-balsam spruce and yellow birch-paper birch-red maple occurs on lowland forests in New Brunswick. Black spruce forest occupies poorly drained areas, and the hardwood forest occupies well-drained sites at the periphery of poorly drained sites [402]. In Newfoundland, the black spruce/sheep-laurel forest type is very open (<20% canopy cover), with a ground layer of feather mosses and reindeer lichens. Speckled alder-black spruce swamps occur in mucks with an organic layer >6 inches (15 cm) thick [89].
In Nova Scotia, black spruce bogs occur in a matrix of shrublands and open water. Stunted black spruce are scattered within rocky, black huckleberry-broom crowberry heathlands in southern Nova Scotia [361].
Black spruce dominance increases with latitude in eastern boreal forests. In eastern Québec, black spruce increasingly replaces balsam fir as permafrost thickens [94]. Krummholz black spruce-reindeer lichen woodlands and forests grow at the boreal-arctic treeline interface in Québec. In one study, black spruce occurred as scattered stands on sites that last burned <600 years prior, while reindeer lichens were the primary cover on soils that had not burned for >1,000 years [313].The arrangement of branches and cones promotes easy ignition and torching while protecting seeds. Black spruce trees have a straight bole with little taper and short, compact, drooping branches with upturned ends [113,175,396]. They are short and slender compared to other spruces [113,396]; their form has been described as cigar-shaped [162]. The bole is thin-barked [264,311] and "poorly pruned" [346]: Branches are usually retained after they die [122,383]. Needles are short. Near arctic treeline, needles and cones may be only half the size of those on trees farther south [175]. The cones of black spruce are semiserotinous: they open gradually over time in the absence of fire but open and disperse seeds rapidly when heated by fire [129,152,412]. Cones form dense clusters in the upper part of the tree [2,113]. Generally, they are massed in the upper center of the crown, where they are least likely to sustain fire damage [2]. They are the smallest of the spruce cones [311,346]: only 0.6 to 1.2 inches (1.5-3.2 cm) long [396]. Cones remain on the tree for several years [396]. Seeds support a long, thin wing [396] that is relatively large compared to seed size [395]. Like cones, the seeds are small compared seeds of other spruces [147,242,340], averaging 404,000 seeds/pound [340].
Black spruce has vertical, lateral, and small adventitious roots [65]. The root system tends to be shallow [263], especially in organic soils with a high water table [113] or over permafrost [65]. Most roots grow in the upper 8 inches (20 cm) of organic soil horizons [395], and they are mostly lateral [245]. A review noted that black spruce growing over permafrost may be rooted only in the moss layer [263], but vertical roots sometimes extend into upper permafrost. When this occurs, adventitious roots growing in the organic soil layer help nourish the tree [65].
Stand structure: Structure varies tremendously in black spruce stands, from stringers in open bogs to very open black spruce-lichen woodlands and closed black spruce/feather moss forests. Generally, black spruce density increases with decreasing latitude and is higher on permafrost than nonpermafrost sites. On a site in interior Alaska, black spruce density averaged 2,000 stems/ha on uplands with intermittent permafrost and 11,067 stems/acre on lowlands with permafrost [33]. On the Caribou-Poker Creeks Research Watershed, interior Alaska, black spruce stems are often only 7 feet (2 m) apart. Black spruces rarely exceed 50 (15 m) tall or 10 inches (25 cm) in diameter, and about half are <2 inches (5 cm) in diameter [82].
Most black spruce stands are even-aged and date back to the last fire; however, old stands or stands on poor sites may become uneven-aged [174,197] due to die-off of individual trees [38,174] and subsequent gap succession (review by [47]). In northeastern Ontario, black spruce mortality was not detected in black spruce/feather moss stands <30 years old. Based on a chronosequence of 10 stands ranging from 44 to 82 years old, a self-thinning stage began around postfire year 30 to 40 and lasted about 20 years. Mortality occurred regardless of stand density and did not increase with stand density. It was greatest in drought years [71].
Black spruce generally has a moderate lifespan, although individuals at arctic treeline may be long-lived. Black spruces may live 250 [11,243] to 300 years on favorable lowland sites [11]. In Alberta, maximum recorded ages of black spruce were 200 to 250 years on upper foothills and 270 years in a low foothill bog (review by [245]). In Québec at arctic treeline, where most regeneration is from cloning, researchers dated the oldest stems within a clone at >2,000 years old. The oldest stems within a clone were usually dead, but surrounding live stems were "centuries, if not millennia" old [298].
Raunkiaer [324] life form:| Regional phenology of black spruce | ||
| State or province | Event | Period |
| Alaska, interior | pollination | early to mid-June (review by [412]) |
| Wisconsin | pollination | April |
| female cones ripe & seeds disperse | Sept.-Oct. [86] | |
| Virginia | female cones ripe | late summer [113,346,415] |
| seeds disperse | late summer [383]-early fall [2,346] | |
| Newfoundland | needle drop | mostly in fall; continues through winter [291] |
Seeds disperse either soon after a fire or when ambient air temperatures are warm enough to partially open cones [174]. Cones usually remain attached to and disseminate seeds from burned trees, but sometimes break off after burning. In northern Québec, black spruces burned by a June wildfire had dropped many of their cones by August [37].
Seed rain may occur at any time of year [174,383], but it is greatest in winter and spring and lowest in fall [395]. In northeastern Ontario, 58% of annual seed rain occurred March, April, and May [152]. In Minnesota, proportions of annual seed rain were: 9% in August; 19% in September; 38% from October through April; 13% in May; 14% in June; and 7% in July [129].
Limited data show a spring and summer germination period for black spruce seeds. In northern Québec, seeds hand-sown in late July germinated either just before frost in early summer or after snowmelt in spring. For seeds that germinated, most had done so by May [351]. After winter tree harvest and a follow-up spring prescribed fire in Alberta, most (70%) black spruce germinants emerged in June. About 23% emerged in July and 7% in August [42].
REGENERATION PROCESSES:Cone and seed production: Trees begin producing seed as young as 10 years of age [383] but generally do not produce much seed until they are ≥30 years old [395]. Black spruce usually produces cones at a younger age than white spruce [263,415], which gives it a successional advantage on sites with relatively short fire-return intervals. Maximum seed production occurs in trees around 50 to 150 years old [383]. In Alberta, black spruce first produced cones around 15 years of age, bearing "heavily and regularly" from 50 to past 150 years of age [174]. In open black spruce woodlands in the Northwest Territories, black spruce seed production peaked when stands were 100 to 200 years old [54,55]. Seed production may lessen after that [113].
 |
| Previous-year and current-year (red) female black spruce cones. Photo by Rob Routledge, Sault College, Bugwood.org. |
Black spruce trees produce some seed every year [113]; total seed crop failures are apparently rare [243,263]. Young, healthy trees produce bumper crops about every 2 to 4 years [113,395]. Since seed crops seldom fail and the semiserotinous cones release seeds over several years, stands that are ≥40 years old nearly always have a continuous supply of seeds. In interior Alaska and Yukon, production for stands aged 20 to 150+ years ranged from 0 to 1,138 cones/tree. Sixty-five percent of trees were producing cones. Cone production increased with tree age and basal diameter (P≤0.001). Modeling indicated that 90% to 95% of trees in stands >100 years old were producing cones [398]. A review reports maximum production of 78 seeds/cone and 730 cones/tree for black spruce in Alaska [415].
Annual seed rain in mature black spruce stands has been reported at:
200,000 seeds/acre in Minnesota (review by [395])
344,000 seeds/acre in central Alaska
240,000 to 528,000 seeds/acre near Inuvik, Northwest Territories
990,000 to 1.7 million seeds/acre in Ontario
404,000 to 1.9 million seeds/acre in northeastern Ontario [152]
688,339 seeds/acre, averaged over 5 years in northern Québec [352]
In northern Québec, dispersal from fire-killed black spruces dropped markedly in postfire year 5, indicating that the aerial seed bank was nearly exhausted. Viability of cone-stored seed was low by then [352].
Seed predation, insect attacks, short-interval reburns, and cold climates lower cone and seed production. Red squirrels may devour large quantities of black spruce seed [412]. Small black spruce cones crops were positively correlated with eastern spruce budworm defoliation in stands near Québec City, Québec (R²=0.89). Defoliated stands may take up to 20 years to regain preoutbreak cone production [348].
Black spruce's aerial seed bank is reduced if fire is either severe enough to kill the cone-protected seeds or occurs before the last black spruce cohort is producing many cones [203]. A model based on field data collected in northern Québec predicted that fire-return intervals of <60 years lead to local extinction of black spruce, while fire-return intervals of >220 years lead to local extinction of jack pine [240]. See Fire Management Considerations for further details on the effects of very short fire-return intervals on black spruce.
Cold temperatures during the growing season restrict cone production. Krummholz or prostrate black spruces growing at high elevations or latitudes may not produce cones [25,26,27,55].
Seed banking: Black spruce has a crown-stored seed bank, with seeds stored in semiserotinous cones [138,263,383].
Soil seed banking does not appear important to black spruce. Once dispersed, black spruce seeds remain viable for only a short time. In a clearcut, upland black spruce site in Ontario, portions of a seed lot that were either hand-buried or sown on the soil surface retained viability for up to 10 months, but no seeds were viable 16 months after sowing. Buried seed retained viability longer than seed sown on the soil surface (91% vs. 30% germination, respectively, 10 months after sowing) [131]. On 4 black spruce-lichen woodlands in the Northwest Territories, black spruce seed was collected from the soil, but it did not germinate in the greenhouse [186]. In New Brunswick, however, hand-sown seeds showed delayed emergence. Only 19% of black spruce seed sown on burned areas emerged in the 1st postfire year, while 25% emerged in the 2nd postfire year [366].
Seed dispersal: Wind disperses black spruce's winged seeds [4,263,396]. Since the seeds are small and lightweight [147], they may disperse 70 feet (20 m) or more from the stand edge, although most fall within the stand [197]. Most seeds disperse within about 260 feet (80 m) of their parent [375]. In logged areas, seed sources can include freshly cut slash as well as standing live or dead [5] and nearby trees. Near Ely, Minnesota, black spruce's annual seed rain averaged 3000,000 seeds/ha within a black spruce stand; 18,000 seeds/ha at 100 feet (30 m) away from the stand; and 8,000 seeds/ha at 200 feet (60 m) away. The author suggested that beyond that, seed rain would be too sparse to result in much regeneration [242]. Due to their lighter weight, black spruce seeds can disperse farther than white spruce seeds [263].
The semiserotinous cones release seeds over time [2,113,263,383,388,406,415]. Resin seals mature, second-year cones. If fire melts the resin, cones may open "in a matter of seconds" [195], although postfire dispersal is usually spread over a few years [383,388,415]. Without fire, the cones open slowly as the resin degrades. Warm air temperatures hasten this process [195]. In northeastern Ontario, unburned black spruce cones retained about 50% of their seeds after 5 years. Unburned cones tend to remain closed in cold, wet weather and open in warm, dry weather [152].
Viereck [388] reported that tremendous quantities of black spruce seed disperse during postfire years 1 and 2. In interior Alaska, the quantity of black spruce seed dispersed over 70 postfire days in a burned-over stand was 1.5 times greater than that dispersed in an adjacent unburned stand [394]. How quickly cones open and release seed is apparently related to fire temperatures: High temperatures melt cone resin and open cones quickly [395]. An initial postfire "burst" of seed rain may gradually slow to prefire levels over 2 postfire years [415]. A Saskatchewan study found that 35% of seeds from standing dead black spruces were released by postfire year 2. Only 6% of seed remained by postfire year 6 (review by [151]). Over 2 postfire years in Newfoundland, about half of black spruce's total seed rain occurred in the first 60 postfire days. Seed viability dropped quickly, from an average of 60% immediately after the fire to 20% the next spring [406]. In Minnesota, burned black spruce cones had released about 50% of their seeds within postfire year 1 and about 85% by postfire year 5 [406]. In Québec, black spruce seeds dispersed through postfire year 5. About half had dispersed by postfire year 4 [148].
Because black spruce retains its cones and the cones release seed over several years, the current year's crop is not critically important to regeneration [410]. Some report that fire will only "modestly accelerate" seed dispersal [148].
Germination: Viability of fresh black spruce seed is high, but viability of cone-stored seed drops with age. A variety of substrates may support germination, although rates are generally best on mineral or deeply charred organic soil.
Viability: Coned-stored seeds remain viable for several years [2]. Viability of fresh seed averages about 88% [340]. Some seeds may remain in cones for up to 20 years, but viability drops to nearly zero after about 15 years [174]. A Newfoundland study found viability of crown-stored black spruce seed remained above 90% for 12 years but dropped rapidly after that (review by [395]). In northeastern Ontario, viability of filled seed collected from unburned cones averaged 53% for 1- to 5-year-old seed; 20% for 6- to 10-year-old seed; and 5% for 11- to 15-year-old seed [152].
Seed viability apparently drops quickly after fire opens cones [406]. After the 1971 Wickersham Dome Wildfire in Alaska, germination dropped from 65% for seeds collected in postfire year 1 to 32% for seeds collected in postfire year 2 [411]. After a wildfire at the boreal-arctic interface, Northwest Territories, germination of black spruce seed ranged from 0% to 19% from postfire years 0 to 6 but was ≤1% in postfire year 20 [403]. On burned sites near James Bay, western Québec, black spruce establishment averaged about 4,000 seedlings/ha in postfire year 4, but cone-stored seed was no longer viable and establishment was poor by postfire year 10 [235].
Severe fire kills some of coned-stored seed and apparently reduces overall germination rates of dispersed seed. After the Wickersham Dome Wildfire, germination rates of black spruce seed were significantly higher (P=0.5) on unburned (77%) and low-severity plots (87%, not all needles consumed and some remained green) compared to severely burned plots (54%, needles and fine branches consumed). Researchers collected seeds from postfire months 2 through 12 [411].
Recruitment failure can be due to a lack of seed sources [8] resulting from very short fire-return intervals or due to thick organic soil layers. Natural regeneration of black spruce was poor on a reburned Yukon site that had burned in 1990 or 1991 and again in 2005. The site was artificially seeded to black spruce 3 or 4 years after the last fire. Artificial regeneration resulting from the seeding was similar to or greater than natural establishment on a black spruce site that had burned 94 years prior and therefore had a crown-stored seed bank. Recruitment was poor after artificial seeding of an untreated, 76-year-old stand with a thick moss and lichen layers on the organic horizon. The authors attributed the poor establishment to thick organic horizon layers [64].
Black spruce establishment is generally poor after clearcutting that occurs before seeds disperse (see Seasonal Development) [66,260] or when cone-bearing branches are burned in piles rather than broadcast burned [263]. See the Very frequent fires section of Fire Management Considerations for more information on this topic.
Seed production and viability may be low in the northernmost limits of black spruce's distribution [55,113]. Few trees produced cones near the arctic treeline on the southern Brooks Range, Alaska. Of seeds collected, only 2% germinated in the greenhouse [256].
Seedbeds: Best establishment generally occurs on mineral or thin organic soil. When the seedbed is continually moist, black spruce may successfully germinate on either mineral soil, organic soil, or sphagnum [86,174,255,395]. Germination proceeds "rather quickly" under continually moist conditions. The germination period may be longer if the seedbed substrate dries and re-wets during germination [243].
Fire prepares favorable seedbeds by removing soil organic horizons [54,55], lichen and moss mats [28], other competing vegetation, and by blackening and warming the soil [54,55]. Several authors reported that mineral soil or a thin humus layer provides an ideal seedbed for black spruce [2,22,332]. Generally, severe fires result in good black spruce establishment. On upland burned sites in interior Alaska, good germination occurred on moderately burned (various levels of organic matter burned off but mineral soil not exposed) to heavily burned (mineral soil exposed) sites, while lightly burned (organic layer only scorched) and unburned sites had no black spruce germinants [414]. On 4 sites in interior Alaska and Yukon, plots burned at different severities were then artificially seeded to determine how fire severity affects black spruce's postfire establishment. Although some establishment occurred on unburned plots or plots burned at low severity, black spruce required about 6 times as much seed by weight to produce a viable seedling on unburned organic soil compared to mineral soil. Seedling establishment was negatively associated with depth of the organic soil layer (P<0.025). The authors attributed this to the unstable moisture supply and frequent drought stress of organic soils. However, establishment was lower on the most severely burned site (ash layer with no organic material remaining) than on sites where severe fires left some residual organic material and no ash layer. Establishment was also greater on manually cleared mineral soil than on the most severely burned site. The authors suggested that on the severely burned site, a water-repellant ash layer reduced moisture availability at the soil surface [202]. On a burn in northwestern Québec, black spruce recruitment was highest on skid trails, followed by mineral soil, then very thin charred organic soil. Charred moss, charred sphagnum, and leaf litter had the fewest black spruce germinants [149].
On a 30-year-old burn in Alberta, most black spruces established in rotten wood (40%). About 25% established in mineral soil [245].
A review noted that sphagnums often provide good seedbeds for black spruce. Fire is not always needed for good black spruce establishment on sphagnum seedbeds, although it may help clear a seedbed covered with downed woody debris or slash. In contrast, feather moss is often an unfavorable seedbed, and moderate to severe fire that reduces the feather moss layer is usually required for good black spruce establishment [22]. Sphagnums likely provide a favorable seedbed due to their high capacity to hold moisture: they can absorb about 20 times their dry mass in water [325].
Hydrophobic soil or a heavy ash layer inhibits black spruce germination. After severe fire creates those conditions, the soil must regain permeability before black spruce can successfully germinate and establish. In southeastern Manitoba, black spruce seed hand-sown on wildfire-burned plots showed better establishment in postfire year 3, after mosses and lichens had colonized the mineral soil, than in postfire year 1, when mineral soil was covered by an ash layer [217]. In central Alberta, highest black spruce establishment occurred in postfire year 7, with few trees establishing in postfire year 1. The author attributed this to presence of an inhibitory ash layer in the first few postfire years [252]. Rowe [332] reported that in Riding Mountain National Park, Manitoba, a wildfire in the 1940s—fueled by live vegetation and heavy downed woody debris from a blowdown—left a thick ash layer. By the late 1960s, black spruce had established in "patches of vigorous saplings...conforming to the fired areas". The relatively heavier seeds of black spruce withstood desiccation better than the lightweight seeds of quaking aspen and birch, so even on organic soils, black spruce had higher rates of establishment than the hardwoods [110].
Seedling establishment: Most black spruce establishment occurs after fire, with peak occurrence during postfire years 3 to 5 [186,383]. Paired studies in Alaska and Yukon showed most black spruce establishment occurred within 10 postfire years. There was little difference in the time black spruce took to establish on thick (8-12 inches (20-30 cm)) organic soils in Alaska and thin (0-4 inches (0-10 cm)) organic soils in Yukon [206]. However, seedbed composition and organic horizon depth can influence black spruce rates of establishment and growth.
Most seeds fail to establish. On a site in Alaska, <2% of black spruce seeds that fell in postfire year 3 had produced seedlings (Zasada and others 1979 in [384]).
Rates of postfire establishment are largely determined by seed input and seedbed quality in the first few postfire years [353]. Generally, black spruce establishes best on moist lowlands where most of the moss and/or lichen layer has burned off from organic soils. The summer after winter tree harvest and spring prescribed fire in a white spruce-black spruce forest in Alberta, most spruce germinants (62%) occurred on concave microsites, with 23% on flat and 15% on elevated microsites (data for the 2 spruces were pooled). Severe fire favored spruce establishment: 43% of germinants were on plots that burned at high severity, 30% on medium-severity plots, and 27% on low-severity plots. Spruce density averaged 176,000 germinants/ha. Sixty percent of seedlings survived through the summer, but only 37% of this cohort survived through the next winter. Winter survival was greater for June germinants than for seedlings that germinated later in summer [42].
On 6 burned jack pine-black spruce sites on the Superior National Forest, Minnesota, highest black spruce establishment occurred in postfire years 2 and 3. This establishment peak was 1 or 2 years behind that of white spruce. In postfire year 1, many black spruce germinants died in a thick ash layer. Black spruce seedling survival averaged 54%, with most mortality occurring in the 1st or 2nd growing season after germination. Fires on the study sites varied in severity, with 1 to 6 inches (2.5-15 cm) of the soil organic layer burned off. Some study sites were burned under prescription and some were burned by wildfire; generally, wildfires removed more of the organic soil layer. In all cases, black spruce seedling establishment was higher on burned than on unburned soils [3,4].
In a southern boreal mixedwood in Saskatchewan, black spruce establishment was higher in mineral soil, humus (0.4-2 inches (1-5 cm) thick), or a thin layer of haircap mosses (0.4-2 inches)) than in thick haircap moss (6-8 inches (15-20 cm)), scorched haircap moss, or quaking aspen litter (2-3 inches (5-8 cm)) layers. Through the first growing season, there were no significant differences in black spruce survivorship among mineral soil, humus, and thin haircap moss seedbeds. Black spruce seedling mortality was 100% in quaking aspen litter [78].
A review suggested that on slopes, interactions of litter buildup, geomorphology, and fire may determine conifer seedling establishment and vegetation patterns. For example, black spruce generally establishes on wet slope bottoms of glaciofluvial hillslopes, while jack pine tends to occupy drier middle and top positions. Duff and organic soil layers are thicker in the black spruce stands, so when fire occurs, more ground fuels are available beneath black spruce than beneath jack pine stands. The authors concluded that duff consumption patterns were driving postfire seedling recruitment patterns on the slope bottoms and upper slopes [151].
Requirements for germination may differ from those for black spruce establishment. Although mineral seedbeds are good for germination, black spruce seedlings may grow faster on unburned organic horizons than on mineral soils ([117], review by [115]), presumably because organic horizons hold more moisture. Since black spruce seeds are small, the germinants are also small and can desiccate quickly on porous substrates [147]. However, black spruce germinants grow on a variety of substrates if the seedbed remains moist but not saturated and the site is relatively free of other vegetation [199]. Feather mosses can dry out quickly, but black spruce establishes in feather moss during wet years [129]. In a study comparing different substrates, planted black spruce seedlings grew taller on Schreber's moss or Schreber's moss-humic seedbeds than on sphagnum, decaying wood, or mineral soil seedbeds (P=0.001). The study was conducted in northeastern Ontario and northwestern Québec. Three years prior, study sites had either burned in a wildfire or been logged. Sites were classified as black spruce/bog Labrador tea/feather moss-sphagnum peatlands. Prior to disturbance, time since fire was >120 years for all sites [236].
Lowlands may support higher rates of establishment than uplands. In Alberta, harvesting and prescribed burning resulted in good black spruce seedling establishment on wet to moist low peatlands. Regeneration was "unacceptable" on fresh to moist upland sites. Before tree harvest, the community was a 100-year-old black spruce forest with a continuous feather moss-sphagnum ground layer. Burning was conducted on 29 May 1967, 2 to 3 years after selective logging. In postfire year 22, black spruce density averaged 4,162 stems/ha on unburned sites, 6,203 stems/ha on low-severity plots, and 31,563 stems/ha on moderate-severity plots. Fire severity was determined by level of peat consumption [81].
Drought was the primary cause of black spruce seedling mortality in a burned black spruce-alpine reindeer lichen woodland in Terra Nova National Park, Newfoundland. By postfire year 2, mortality of planted black spruce seedlings averaged 79%. Seedling mortality was similar on substrates that burned at low (73%) and high (76%) severities. Some plots were caged to exclude seedling browsers (voles and snowshoe hares), but there was no significant difference in mortality between caged and uncaged plots in either postfire year 1 or 2. Although mortality was initially high and the burn occurred on the southern edge of the black spruce-lichen woodland latitudinal limit, the authors predicted that black spruce's survivorship would be sufficient to replace the burned stand [293].
Interference by other species can greatly reduce black spruce's rate of establishment. In a fire chronosequence study in Terra Nova National Park, reindeer lichen substrates favored black spruce germination, but black spruce seedling establishment was negligible on that substrate. Mineral soil provided highest rates of black spruce germination (8%), seedling establishment (5%), and growth (0.4-0.8 inch (1-2 cm) at 14 months of age) [272]. Although black spruce seeds readily germinate on sphagnum, black spruce seedlings are often overtopped and engulfed by the faster-growing sphagnums [129]. Conversely, sphagnums may facilitate black spruce establishment on some sites. In the southern Brooks Range of interior Alaska, black spruce establishment was positively correlated with sphagnum (r=0.66, P=0.003). Although sphagnum covered only 15% of the soil surface, 60% of black spruce seedlings were growing in sphagnum [255].
At arctic treeline, black spruce generally establishes at higher rates than other conifers [15]. At an arctic treeline site on the southern Brooks Range, however, white spruce showed better seedling establishment than black spruce [256].
Plant growth: Black spruce seedlings require open conditions for optimal growth [86]. Although the seedlings are shade tolerant, growth is fastest in full sun [199]. Black spruce is often slow-growing [2,243,396]. Seedlings rarely grow more than 1 inch (2.5 cm) in their first growing season. Three-year-old seedlings are commonly 3 to 5 inches (8-13 cm) tall [129]. Roots of 1st-year seedlings may penetrate to 2 inches (5 cm) in upland soils, but roots growing in mosses rarely reach 1.5 inches (3.8 cm) deep in their second year [129].
In a 35-year-old black spruce peatland in north-central Alberta, black spruces that established in the first postfire decade grew faster than those that established later. Most established in postfire years 4 to 7, with little recruitment after postfire year 25. Height growth was "nearly constant" over tree life, although large black spruces tended to attain height growth faster than small black spruces. Most black spruces were <7 feet (2.0 m) tall and 3 inches (8 cm) in diameter. Trees ranged from 3 to 37 years old, with most 31 to 33 years old. Mortality was not evident in this young black spruce stand [252].
Black spruce seedlings do not grow as quickly as hardwood sprouts [373]. On black spruce-alder sites in Ontario, for example, postfire establishment of black spruce is usually poor due to interference from faster-growing alder sprouts [22]. Field manipulations on a 1-year-old burn near Delta Junction, interior Alaska, suggested quaking aspen can outcompete black spruce for light in early postfire succession, reducing black spruce's growth rates [206]. Relative to other conifers, black spruce's slow growth is at least partially due to its relatively small seeds, which have few food reserves to support germinant growth. However, black spruce on upland sites may grow about as quickly as white spruce [113]. On 8 different-aged burns in northeastern Ontario, black spruce took longer to reach breast height (18 years) than jack pine (8 years), quaking aspen, and paper birch (7 years for both hardwoods). Time to reach breast height did not vary with either soil texture or moisture [382]. Black spruce seedlings grow poorly beneath a canopy. Zasada and others [415] report that understory black spruces take 15 to 20 years to attain 5 feet (1.5 m) in height, and it is "not uncommon" for 40-year-old black spruces under a mixedwood canopy to be <4.9 feet tall.
Poor site conditions slow growth. Black spruces on well-drained, upland sites grow tallest [113], while poor drainage [113,175] and cold climates reduce growth. Although nutrient-poor swamps and bogs are tolerated, they are not ideal habitats for black spruce growth. In northeastern Minnesota, black spruce showed progressively slower growth from the border toward the center of a swamp. Eighty-year-old trees at a swamp border averaged 60 feet (18 m) tall, but 120 feet (37 m) away—at the center of the swamp—80-year-old trees were only 20 feet (6 m) tall [243]. In Victory Basin Bog, Vermont, black spruce stringers extended from the raised bog to a nearby stream. Height and density of black spruce decreased towards the bog's center (P<00.1), although there was no corresponding difference in tree ages. Black spruces at the stream edge averaged 39 feet (12 m) tall, while those in the bog center averaged 10 feet (3 m). Age of black spruces ranged from 45 to 77 years; height was not significantly correlated with age. There was a gradient of decreased mineral content and water pH to the bog center. Dominance shifted abruptly from black spruce to speckled alder about 30 feet (10 m) from the stream edge [67]. On the Great Northern Peninsula on the northern tip of Newfoundland, black spruces took an average of 40 years to reach breast height. Tree ages ranged from 25 to 269 years; tree height and age were poorly correlated [278]. Viereck and Little [396] described growth rings of trees in northern boreal Alaska as "very narrow to almost microscopic".
Browsing can slow black spruce growth substantially, and when cone buds are eaten, it can have a "significant impact" on reproduction. Moose, hares, red squirrels, ptarmigan, and grouse are among the animals favoring black spruce twigs and buds [415]. See Importance to Wildlife and Livestock for further details.
Comparing postlogging growth rates of layer-origin vs. seed-origin stems, layered stems were taller because of their prelogging growth. However, postlogging incremental growth was similar among the 2 groups [288].
Vegetative regeneration: Layering occurs after mosses or litter cover black spruce's lower branches [174,396]. It is prevalent on organic soils [113] and is particularly common in swamps, bogs, and at arctic and elevational treelines. Boggy conditions in lowland black spruce/sphagnum sites promote more layering than drier conditions in upland black spruce/feather moss sites [255]. Layering may be particularly important in taiga-tundra transition zones (review by [255,256,415]). Across North America's arctic treeline, black spruce reproduces almost entirely through layering [107,113,383].
Layering may greatly increase black spruce's density in mid- to late succession, although it is not important in early postfire years [383]. In the Brooks Range of Alaska, layering was the primary method of black spruce regeneration for undisturbed stands ([256], review by [415]). Black spruce regenerated from seed in early postfire years, but later-successional regeneration was from layering [255].
Most advanced regeneration present after clearcutting is of layer origin [197,337]. Postclearcut layering is common as long as slash is not broadcast burned [314,337].
Layered branches are ladder fuels [383]. See Fuels for further information.
Root sprouting has been reported in Québec [245] and Alberta. Root sprouting may be more common than is usually realized because it is confused with layering. Excavations in Alberta found more black spruce stems originated from root sprouting (31% of total reproduction) than from branch layering (7%) [174]. Ground fires usually kill black spruce roots [264] (see Immediate Fire Effects on Plant), so root sprouting is likely only incidental in early postfire succession.
SUCCESSIONAL STATUS: Overview and trends: Most succession in black spruce communities is set back by fire. Generally, black spruce communities succeed to black spruce after fire [15,124], and although plant species' frequencies may shift, the same species that were present before fire are still there after fire [383]. A study of 4 burned jack pine-black spruce communities in west-central Québec found prefire basal area of black spruce was positively associated with postfire seedling establishment of black spruce (P<0.0001) [185]. Although black spruce is considered a fire type, fire is not needed to perpetuate black spruce on some cold, poorly drained boreal sites [263].Black spruce is found in all stages of boreal forest succession [87]. Its shade tolerance is intermediate [87]: It grows in open bogs and woodlands [87] as well as in closed-canopy forests [390]. It typically seeds in promptly after fire (see Seed dispersal and Plant response to fire). Although quaking aspen, paper birch, and shrubs frequently sprout or colonize after fire in black spruce types, black spruce usually regains dominance [390] in 90 postfire years or less [383]. However, if fire returns before the black spruce stand has produced many cones (around 30 years old [395]), the site may convert to a hardwood or shrubfield type [162]. The presence of white spruce in nearby, unburned stands increases the change of white spruce becoming codominant with black spruce after fire [203,204,207,383]. Horton and Lees [174] speculated that in the long-term absence of fire in Alberta, black spruce/feather moss bogs would succeed to white spruce-black spruce or subalpine fir types. However, they point out that "fire generally does occur, preventing this succession" [174].
Many factors affect postfire trajectories in black spruce ecosystems, including prefire plant community composition, fire severity, presence of permafrost, and postfire weather [53,203,204,383]. The patchy burn patterns typical of black spruce communities perpetuate numerous seral communities. A review of postfire succession in boreal black spruce/feather moss forest types found this general pattern [383]:
1) Conifer seedling-herb stage, postfire years 1 to 4: black spruce seedlings and common liverwort-fire moss mats often dominate. Ericaceous shrubs and willows sprout in areas that burned at low to moderate severity. Plant cover may increase from 0% to 40% or 50% during this stage.
2) Shrub stage, postfire years 6 to 25: Shrubs establish and/or continue to grow, gaining dominance as cover of mat-forming and herbaceous species decreases.
3) Young tree stage, postfire years 25 to 30: Black spruce gains dominance and may be very dense. Tree density (all species) may exceed 4,000 saplings/ha, with up to 12,000 seedlings/ha. Willows and alders begin to thin, but ericaceous shrubs and paper birch continue to gain cover. Cover of feather mosses and other mosses also increases. Thawed permafrost layers may refreeze as the soil organic layer increases.
4) Mature tree stage, postfire years 60+: Around postfire year 60 to 90, black spruce tends to form closed stands. It self-thins to about 1,700 trees/ha around postfire year 100. Stands have layered branches and are often clumped; shrubs occupy spaces between black spruce clumps. Lichen cover continues to decline.
The degree to which fire burns off organic soil layers greatly influences postfire succession and plant community composition in black spruce communities [207]. In general, intact soil organic layers tend to prolong a graminoid- or shrub-dominated successional phase after fire, while exposed mineral soil promotes postfire establishment of hardwood trees [383]. Hollingsworth (2008 cited in [65]) remarked that in black spruce ecosystems, moisture content of the soil organic layer during fire is an important driver of succession afterwards: "Unless conditions are dry enough to allow the fire to burn down to the organic layer, chances are the system is going to regenerate exactly how it was before the fire". Black spruce is most likely to replace itself successionally after ground fires that leave some organic layers unburned, whereas hardwood trees are most likely to establish with black spruce if they were present before fire and/or if soils are burned down to either shallow organic or mineral layers [203,204,207]. Viereck [383] reported that in Alaska and northern Canada, shrub cover is usually sparse after fires that burn down to mineral soil, but such fires prepare good seed- and sporebeds for black spruce, mosses, and lichens. Postfire sprouting of shrubs is most likely when organic soil layers are not entirely consumed [383].
After severe fire, hardwood trees may dominate for 50 to 80 postfire years before black spruce regains dominance. If hardwoods were on site before low- to moderate-severity fire, postfire sprouting of hardwood trees may prolong the hardwood stage for 60 to 90 postfire years [383]. In the Great Lakes states, hardwoods may replace black spruce on uplands [243]. Some conjecture that black spruce types may succeed to hardwood types following either a fire severe enough to kill seed stored in black spruce crowns or a recurrence of fire before black spruce has matured enough to produce cones [263,388]. See Fire Management Considerations for further discussion on the effects of very short fire-return intervals on black spruce.
Viereck [383] suggested that a quaking aspen community with a black spruce understory develops after a severe fire burns organic alluvium down to mineral soil and quaking aspen and black spruce seedlings establish around the same time. Black spruce likely replaces quaking aspen successionally on these sites unless fire returns within the lifespan of quaking aspen [383], approximately 100 years [196].
Frequent fires (<100-year intervals) often convert fir or spruce-fir forests to spruce forests. In black spruce-balsam fir forests, balsam fir recovers more slowly after fire than black spruce. Like black spruce, it has thin bark and is easily killed, but unlike black spruce, it has nonserotinous cones that experience high seed mortality during fire (review by [137]). After fire, a balsam fir forest with a black spruce seed source either on site or nearby may convert to black spruce [89]. Without fire, balsam fir may replace black spruce on some sites [383]. In subboreal and boreal regions, it is likely that fir-dominated communities will succeed to black spruce or other later-seral conifers, such as tamarack, balsam fir, and northern whitecedar, if late-seral conifers were present before fire (review by [137]).
In southeastern Canada and the northeastern United States, balsam fir and northern whitecedar are more shade tolerant than black spruce and tend to replace it successionally under long fire-return intervals [40,87]. However, black spruce may remain codominant with balsam fir or maintain a "strong presence" [87].
Where jack pine is present before fire, it may establish in larger numbers than black spruce after fire because its cones are totally serotinous (review by [383]). In jack pine communities on the Superior National Forest, Michigan, black spruce advanced regeneration usually gained dominance over jack pine after blowdowns, while jack pine regained dominance after fires [88]. In the Great Lakes states, black spruce often establishes beneath the canopies of pine and mixedwood communities [88]. In northeastern Minnesota, where black spruce often codominates with jack pine, black spruce may replace jack pine successionally on sites that experience long periods between fires or other stand-replacing disturbances [243].
Generally, a site must remain cold for black spruce to maintain dominance (see Site characteristics). Black spruce usually replaces white spruce successionally on permafrost sites [381,387], while white spruce usually replaces black spruce on relatively warm, dry sites. On alluvial sites, periodic fires help prevent development of a permafrost layer, which may prevent succession of white spruce to black spruce [15]. Near rivers, black spruce may replace white spruce on old terraces as the soil organic layer builds up and the permafrost table rises; this may occur on upland sites as well (review by [383]). By the Tanana River, Alaska, black spruce presence was positively correlated with backswamp and other poorly drained permafrost areas and with areas that burned <200 years prior. White spruce was positively correlated with meander belts (P=0.05 for all variables) [274].
Fires that reduce permafrost layers may result in lowered black spruce density or a type shift to hardwoods. Permafrost renders the site wetter and therefore, less likely to experience severe ground fire in most years [207]. However, severe ground fires occur in extreme fire years. When the insulating organic layer is reduced or removed by such fire, the active layer above permafrost often increases [171,212,408]. On some such sites, soils no longer freeze completely in winter, so they drain intermittently and dry out. Plant community composition shifts in response to these drier conditions. While wet soils over shallow permafrost favor black spruce, soils with deeper active layers favor quaking aspen, birches, and alders [171]. This hydrologic-thermal regime is a primary factor controlling plant succession in taiga underlain with permafrost [378].
Tree species composition and abundance may show only short-term change after fires in boreal mixedwoods. On 3 recently burned sites in central Saskatchewan and 1 in central Québec, density of quaking aspen sprouts was greater than densities of black spruce and jack pine seedlings, but relative abundance of the 3 species became similar as quaking aspen sprouts thinned over time. For all 3 species, there was a significant, positive relationship between prefire basal area of mature trees and basal area of postfire regeneration (P=0.05). The authors concluded that for these species, there was little change in pre- and postfire species composition. In Saskatchewan, the prefire forests ranged from 50 to 90 years old and burned in May or June. In Québec, the prefire forest was about 40 years old and burned in early summer [146].
Black spruce may invade the borders of open shrub or herbaceous bogs. In Matanuska Valley near Anchorage, for example, black spruce invaded a bog rosemary-sweetgale-arctic dwarf birch-sphagnum bog [158]. Where permafrost is near the soil surface, an open sphagnum bog may develop into a mosaic of sphagnum bog and black spruce woodland [15]. Pioneering black spruce and tamarack frequently invade sedge mats of filled-lake bogs. In time, they may form stable swamps [129]. In the Great Lakes states, black spruce is usually a late-seral species in bogs and swamps [243]. However, as the peat soil is gradually elevated by the accumulation of organic matter and the fertility of the site improves, balsam fir and northern whitecedar may eventually replace black spruce and tamarack [129,243]. At broad scales, black spruce bogs tend to become more homogenized, less productive, and have smaller trees as paludification progresses [160].
Insects: Insect outbreaks—especially eastern spruce budworm outbreaks—affect successional pathways throughout black spruce's distribution. Since the settlement period, eastern spruce budworm outbreaks have occurred in 40- to 60-year cycles. Prior to the 1800s, they were apparently less frequent and affected less of the landscape [228]. Unlike fire, which kills all or most overstory conifers, eastern spruce budworm outbreaks tend to reduce balsam fir cover over that of black spruce or white spruce [120,216,228]. Among eastern spruces and firs, black spruce is considered the least susceptible to eastern spruce budworm attacks, followed by red spruce, white spruce, and balsam fir, which is most susceptible (review by [270]). Since hardwood species are not attacked, they are favored over all susceptible conifers. However, not all susceptible conifers die, so recovering stands become uneven-aged as black spruce and other conifers establish near recovering conifers [47,94]. In south-central Québec, most black spruce mortality during eastern spruce budworm outbreaks occurred in small, suppressed trees, with mortality increasing with stand density (P<0.001) [261].
A review noted that annually, eastern spruce budworm outbreaks affect succession over a larger area than fire. After eastern spruce budworm attacks, tree mortality and consequent gap succession increased with time since fire. In eastern Canada, increasing postattack abundance of balsam fir was strongly correlated with time since fire [47].
Multiple disturbances over a short time may result in a type shift from black spruce forest to shrubland or woodland. In eastern Québec, black spruce/feather moss stands defoliated by eastern spruce budworm may take 20 years to recover. If fire occurs before then, the site may convert to open black spruce-lichen woodlands [348]. In southeastern Québec, a black spruce/feather moss forest was subject to staggered logging from 1940 to 1980; eastern spruce budworm outbreaks in the late 1970s and mid-1980s; and a wildfire in 1991. Although eastern spruce budworms preferred balsam firs and white spruces, black spruces in the region also showed heavy defoliation or mortality during the outbreaks, and postlogging black spruce regeneration was only beginning to bear cones when the outbreaks occurred. The combined result of these disturbances was a loss of seed-bearing black spruce and consequently, poor postfire regeneration. In postfire year 4, the area was an ericaceous shrub-lichen parkland with few black spruce seedlings [314].
Gap succession: Insect outbreaks, asynchronous mortality of individual trees, windthrow, fungi, eastern dwarf mistletoe infection, and paludification can create gaps in black spruce communities. On a fine scale (≤7 feet (2 m)), this can lead to open patches [160]. In gap succession, black spruce generally remains dominant if it was dominant before the gap-creating disturbance [47]. In in 1930s in northern Minnesota, LeBarron [242] observed uneven-aged black spruce stands in open peatlands and old forests. He attributed this to black spruce seedlings "filling in" canopy gaps where mature black spruces had died [242]. Gaps resulting from windthrow and root-rot fungi are often smaller than those resulting from insect outbreaks [47]. In the Abitibi region of east-central Ontario and southwestern Québec, gaps in boreal black spruce forests increased with time since fire (P=0.05). Eastern dwarf mistletoe might have accelerated the transformation of even-aged to uneven-aged stands. Infection rates usually increased with time since fire [243]. See Fire Management Considerations for information on controlling eastern dwarf mistletoe in black spruce stands.
Effects of climate change on succession: Advancement or retreat of black spruce at arctic treeline is controlled primarily by climate. Black spruce woodlands shift northward in warm periods, while tundra shifts southward during cold periods. The last cold shift spanned approximately 500 to 100 years BP. Upheaval of permafrost aggregates in open peatlands can favor black spruce establishment and formation of black spruce krummholz or forest stands as the aggregates dry [313]. See Fire and climate change for further information on this topic.
Regional studies: Examples of succession in black spruce communities follow by region. Alaska: Black spruce is present in all stages of postfire succession in Alaska, although it does not regain dominance for several postfire decades. In interior Alaska, the most common pattern of postfire succession in black spruce forests is rapid replacement of a mature black spruce stand by a dense, young black spruce stand. Stands thin with time since fire, eventually forming open woodlands or scattered muskegs [388]. Throughout much of Alaska, quaking aspen and paper birch dominate seral stands on relatively warm sites. Hardwood stands begin to break up around postfire year 90, when black spruce becomes dominant. Since fires usually occur every 100 years or less, midseral communities codominated by hardwoods and black spruce are widespread [124].Foote [122] noted that in burned black spruce forest communities on the Tanana highlands of interior Alaska, black spruce seedlings were apparent by postfire years 1 to 5, but willow or ericaceous shrubs dominated until about postfire year 25. Hardwoods and small black spruces dominated the canopy from postfire years 26 to 50, after which a dense black spruce/Schreber's moss stage developed. The canopy thinned around postfire year 100. She noted that black spruce-quaking aspen-paper birch/bluejoint reedgrass and quaking aspen-black spruce/red-osier dogwood community types required frequent fire (≤100 years) to maintain the hardwood component. Of 90 black spruce stands surveyed, most were in early postfire succession (<26 years old) [122].
| Table 1. Ages and number of black spruce stands found on the Tanana highlands, interior Alaska [122] | |
| Age (years) | Number of stands represented |
| 0 | 3 |
| 1-5 | 31 |
| 6-25 | 18 |
| 26-50 | 14 |
| 51-100 | 13 |
| >100 | 11 |
In a follow-up study, Foote [124] surveyed 130 black spruce or white spruce stands that were 1 month to 200 years old. Results of that study are discussed in Plant response to fire. See her Research Paper for general information on postfire succession in black spruce communities of interior Alaska and details about her studies on the Tanana highlands [124].
In interior Alaska, patterns of early seedling establishment strongly dictate community composition of black spruce types for at least 2 or 3 postfire decades [206]. Fruticose lichens often colonize dry soils, developing a nearly continuous cover that can delay black spruce establishment. Dry sites eventually succeed to black spruce-lichen woodlands. Feather mosses are most likely to colonize moist sites, which generally succeed to closed-canopy black spruce/feather moss forests [383]. In wildfire-burned black spruce stands in interior Alaska, severe fires favored forbs with wind-dispersed seeds, such as fireweed. Mosses, liverworts, and lichens were most common on moist sites; those groundlayer components were little impacted by moderate-severity fires but declined after severe fires. Deciduous and evergreen shrub cover declined after fire, with evergreen, ericaceous shrubs such as black crowberry decreasing with increasing fire severity (P≤0.03 for all variables) [53]. Postfire species composition in black spruce types appeared highly correlated with fire severity and site moisture 1 year after wildfires in summer 2004. Wet to moist sites were significantly more likely to support residual sprouting and nonvascular plants than dry sites (n=90 sites total). On the 14 sites for which there were prefire data, dry sites and those that had high-severity fire (burned to mineral soil) showed the greatest changes from prefire species composition [173].
Fungal species in black spruce communities also undergo postfire succession. A chronosequence study near Delta Junction found fires did not appreciably reduce abundance of vesicular-arbuscular mycorrhizal fungi. However, ecotmychorrizal fungi required up to 15 years to return to prefire levels. As postfire fungal succession progressed, ecotmychorrizal fungi replaced vesicular-arbuscular mycorrhizal fungi [371].
Several papers are available to help predict successional patterns in black spruce ecosystems of interior Alaska:Northeast: Fire histories and consequent patterns of succession were not well documented for black spruce ecosystems of Maine. Experts suggest that fires occurring early in the growing season or during drought years apparently favor quaking aspen and paper birch, which sprout, over black spruce. After early-season fire, succession to a black spruce-balsam fir overstory may be delayed for as long as 120 years. It is likely that successional patterns in black spruce forests of Maine are similar to those in black spruce forests of southwestern Québec [228], which are discussed in the Eastern Canada section below.
Little information was available on successional patterns in black spruce forests and mires of the Northeast (as of 2014). In a 94-year-old catberry-highbush blueberry mire in central New York, black spruce had the greatest density and basal area of canopy trees; black spruce in the canopy averaged 24 years old. The mire developed after a stand-replacement wildfire [244].
Western Canada: Black spruce tends to replace jack pine and lodgepole pine successionally on relatively dry sites in western Canada [129]. In central-interior British Columbia, black spruce dominated some mature (41-140 years) and old-growth (>140 years) stands. An even-aged lodgepole pine cohort that established after fire dominated most stands <41 years old [96].
A fire chronosequence study on drumlins (compact glacial till forming mounds or low hills) near Great Slave Lake, Northwest Territories, illustrates successional phases of black spruce communities in western boreal Canada. A globose haircap moss phase occurred from postfire years 1 to 20; a reindeer lichen phase from postfire years 21 to about 60; an open-canopy black spruce/Easter snow lichen phase from about postfire years 61 to 130; and a closed-canopy black spruce/splendid feather moss-Schreber's moss phase after about postfire year 130. Succession to the feather moss stage was rare, because fire usually set succession back before then (n=19 sites and 70 plots) [271].
Near Watson Lake, Yukon, succession was studied after stand-replacing wildfires in black spruce-lodgepole pine stands (n=39 stands). In stands that were <30 years old before they burned, black spruce and lodgepole pine had significantly lower rates of early postfire establishment than in stands that were older before they burned (P<0.009). On sites where stands were >80 years old before they burned, black spruce and lodgepole pine densities were significantly related with their prefire basal areas (P≤0.03). Quaking aspen densities did not differ with stand age prior to fire (P<0.77) [206].
Open boreal bogs and marshes may eventually succeed to black spruce bogs. However, because paludification takes centuries to millennia, direct evidence of this is lacking. Moss [292] suggested that in Alberta, "circumstantial evidence...indicates a natural succession from (sedge) marsh, through tamarack swamp, to Sphagnum bog and bog forest dominated by black spruce".
On upland sites across boreal Canada, white spruce may replace black spruce with long fire-return intervals; however, long fire-free periods rarely occur. Typically, upland sites have fire-return intervals short enough to favor black spruce, jack pine, and/or lodgepole pine. Rowe [332] stated that in black spruce's southern distribution, several fires per century may be needed to prevent succession to white spruce, while at the boreal forest-arctic treeline, a fire every few centuries may prevent such succession.
Eastern Canada: The general pattern of succession in conifer forests of this region is early dominance by black spruce, with successional replacement by balsam fir when fire-return intervals are long. If jack pine cooccurs in the overstory, it may dominate or codominate with black spruce in early postfire succession, particularly after severe fire. Sprouting ericaceous shrubs may also dominate early postfire succession in black spruce communities. A chronosequence study in Terra Nova National Park found sheep-laurel dominated burned black spruce sites until about postfire year 60, with 50% to 90% cover from postfire years 1 to 60. Black spruce cover was negligible until postfire year 20, followed by a slow increase, with black spruce cover surpassing that of sheep-laurel after about postfire year 60. Lichen cover increased at a rate similar to that of black spruce [272]. A study in south-central Québec found that in 2,000 miles² (5,000 km²) of black spruce-jack pine-paper birch/feather moss forest, roughly 10% of the landscape was in early postfire succession (0-30 years). Young forests (31-80 years) comprised <1% of the landscape, while mature forests (81-150 years) accounted for 38%. Most (>51%) was old growth (>150 years) [38]. To date (2014), most postfire successional studies in this region were conducted in Québec.
 |
| Sheep-laurel heath, 27 years after a fire in a black spruce forest. Photo taken near Terra Nova National Park, Newfoundland, by A. U. Mallik. |
Relatively long fire-return intervals favor black spruce over jack pine. A model developed for poorly drained black spruce-jack pine boreal forests east of James Bay predicted that a 47-year fire-return interval favored successional replacement of black spruce by jack pine. However, continued dominance of black spruce was predicted with 211- to 270-year fire-return intervals [241]. On 2 sites in Québec that burned 38 and 43 years previously, jack pine had replaced black spruce successionally as the dominant tree species. The authors observed that while black spruce did not "substantially regenerate" in these early postfire decades, jack pine density "noticeably increased in most cases" [235].
In a fire history study in northeastern Québec, black spruce tended to dominate either early postfire communities where it dominated prior to fire or late-seral communities that had not burned for >175 years. Balsam fir often codominated the late-seral stands [94]. Time-series aerial photos of burned sites, taken near the Gulf of St Lawrence in 1930, 1965, and 1987, show 3 patterns of postfire succession. In 1 pattern, black spruce was dominant in early postfire succession, and remained so through late postfire succession. In the other 2 patterns, hardwoods dominated early seres, but either black spruce or balsam fir successionally replaced the hardwoods. Sites where black spruce was dominant throughout successional seres were positively associated with well-drained, thin tills or imperfectly drained tills, while sites that succeeded from hardwoods to black spruce or balsam fir were positively associated with moderate to deep, well-drained tills. However, all 3 patterns of succession were observed on all substrates represented [139].
By analyzing archived and new data, several patterns of succession were apparent in the black spruce/feather moss and balsam fir-paper birch bioclimatic zones of central Québec. Seral sites were compared with sites that had not had fire or insect outbreaks for >100 years. Across sites, time since fire ranged from 10 to 103 years. On glaciofluvial deposits, jack pine and black spruce tended to dominate early-seral stages, with black spruce becoming increasingly dominant with time since fire (P>0.01). Successional patterns were more varied on glacial deposits. More than 30% of glacial-deposit sites in early succession were codominated or dominated by balsam fir, and balsam fir remained dominant throughout succession. About 30% were codominated or dominated by black spruce in early succession, and black spruce retained dominance throughout succession. Hardwood trees dominated early succession on about 25% of the sites; balsam fir and, to a lesser extent, black spruce, replaced the hardwoods. Jack pine dominated or codominated with black spruce on <10% of early-seral sites; black spruce eventually dominated those sites [246].
After >100 postfire years, black spruce-lichen woodlands often succeed to black spruce/moss forests on moist sites [218,219]. Such succession is unlikely on dry sites. In northern Québec, dry black spruce-lichen woodlands remained stable for 125 to 250 years. The canopy remained open, and neither feather mosses nor sphagnum mosses were replacing the lichens successionally [290].
Severe fires can slow or stop paludification. Paludification has been attributed to buildup of the moss layer, resulting in increasingly cold, humid soils and permafrost buildup [362]. Black spruce is favored successionally on cold, wet muskegs, which burn poorly in most years. A study near James Bay, Québec, found that rates of paludification in black spruce/sphagnum peatlands were faster after low-severity fires than after high-severity fires [347]. In the Clay Belt of northern Québec, conifer stands initiated after low-severity fires had low tree recruitment and slow growth relative to stands initiated after severe fires, and stands initiated after low-severity fires tended to remain open throughout succession. Jack pine tended to dominate sites that burned at high severity, while black spruce tended to dominate sites that burned at low severity (P=0.05) [247]. Low-severity fire accelerated paludification; sphagnum usually dominated the ground layer of such sites in <200 postfire years [248]. Postfire successional patterns varied with soil texture, but black spruce dominated all sites that had not burned for ≥100 years. Four burn age classes were investigated: 50 to 100 years; 100 to 150 years; 150 to 200 years; and >200 years, and 953 plots in 781 stands were measured. For sites that burned <100 years prior, black spruce dominated all sites with organic soils, while the shade-intolerant species quaking aspen and jack pine dominated 30% of sites with fine-textured soils and 60% with coarse-textured soils. Tamarack was important on late-successional sites undergoing paludification, while balsam fir was negatively associated with paludification. Overall tree species diversity was highest on burns <100 years old. Mean stand age was 154 years. Stand age was least (108 years) on coarse soils and greatest (188 years) on organics (P=0.5 for all variables) [246]. |
| Black spruce roots killed by ground fire. Mounds in the background are live, scorched clumps of sphagnum. U.S. Forest Service photo by Jill Johnston and Teresa Nettleton Hollingsworth. |
In the foothills of Alberta, "practically all stands containing black spruce over the whole range of sites can be traced back to fire origin. As a result, the stand structure is generally even-aged" [174]. After fires in the summer of 2004 in interior Alaska, surveys conducted in 2005 on 90 burn sites found nearly 100% mortality of black spruce. Based on percent consumption of the organic soil layer, most of these sites burned at high severity [173]. Refer to Fire regimes for detailed discussions of fire severity and patterns of burning in black spruce communities.
Although fires are usually fatal, some black spruce individuals may not torch. In north-central Alberta, Kiil [220] reported that although 100% of black spruces died as a result of an 18 July prescribed fire, only about 54% of black spruce crowns caught fire. Torching of individual black spruces became "increasingly frequent" 10 to 15 minutes after ignition. For trees that were scorched but did not torch, mean scorch height was 9.5 feet (2.9 m). Trees usually torched if scorch height reached more than 16 feet (5 m). Depth of burn into the organic soil layer averaged 3.2 inches (8.1 cm); this completely removed the ground cover of reindeer lichens [220].
On wet to mesic sites, black spruces in clumps or in the interior of stands may escape fire [37,383], leaving a patchwork of stringers or smaller clumps. Foote [124] noted live black spruce stringers on newly burned, mesic black spruce sites in interior Alaska [124]. Lutz [262] reported that in interior Alaska, extensive "upland areas of black spruce are occasionally seen where a fire has killed literally every tree. On relatively wet lowland areas the likelihood of complete destruction of stands is much less. There are individual trees, and trees in irregular groups and stringers, (that) are likely to survive" [262].
Some individual black spruces may survive fire [169] with scarring. Foote [124] noted live, fire-scarred black spruces in black spruce/bog blueberry-bog Labrador tea/splendid feather moss forests of interior Alaska. Scarred trees were either standing alone or in stringers [124]. Some scarred spruces were also found by the Tanana River, Alaska, although the spruces were not identified to species [274]. Rowe and others [335] found a few fire-scarred black spruces in jack pine-black spruce forests in the Northwest Territories. They suggested that scarring was from lingering ground fires that "creep through the peat plateaus in the years after some major conflagration" [335]. In southern Québec, live, fire-scarred black spruces were found in black spruce-lichen woodlands that had stand-replacement fires >100 years prior. The fire scars were thought to have been caused by surface fires that followed stand-replacement fires [141]. Some fire-scarred black spruces were found near arctic treeline in Québec, but the authors noted that fire scars "were rare because subarctic (black) spruces usually display dense foliage at the stem base that is susceptible to burning completely during fire" [298].
Black spruce is most likely to survive low-severity surface fire that leaves unburned patches [205,383]. On 10 sites across interior Alaska that burned in the severe fire years of 2004 or 2005, fires crowned in all stands but effects to the organic soil layer varied from low to high severity. Vegetation and soil data were collected in 2005. Some black spruce foliage remained unconsumed on burn edges or within small islands, and some trees on low-severity plots were alive in either postfire year 1 (for 2004 fires) or postfire week 6 (for 2005 fires). All black spruces on moderately-burned plots were dead. On moderate-severity plots, boles showed "significant charring" and on average, foliage on >50% of stems in tree canopies was consumed. Moderate-severity sites had 30% to 50% mean reduction in total depth of the organic soil layer, and >90% of the layer was charred. High-severity sites had an 80% to 100% average reduction in total depth of the organic soil layer [213].
Despite these exceptions, it is important to note that even low-severity fires typically kill most—or all—black spruces in a stand. Hanson [159] found that all black spruce trees were killed following a low-severity surface fire in an open black spruce-tamarack community in interior Alaska. The site contained 81 to 162 trees/acre; trees ranged from 40 to 178 years old and 1.5 to 3 inches (4-7 cm) in diameter. The fire consumed the top 2 to 4 inches (5-10 cm) of the 6- to 14-inch (15-35 cm) soil organic layer [159].
Effect on seed: Most cone-stored black spruce seeds survive fire. Even though fires usually crown in black spruce stands, they are rarely so hot that all seeds are killed ([263,388], review by [2]). Crown fires kill some seeds [203,208,410,411], but most survive because of the compact nature of individual cones [410] and the tightly packed arrangement of cones on the branch-dense crown [37,243,410]. A study conducted in northern Québec suggests that black spruce cones are more fire-resistant than the fully serotinous cones of jack pine [24]. Mortality of black spruce seeds increases with fire temperature; however, high temperatures also increase the rate of cone opening, ensuring that surviving seeds disperse soon after fire [208,410].
Black spruce seeds subjected to high, prolonged temperatures or those in short-statured individuals are most vulnerable to fire mortality. LeBarron [243] observed that black spruce did not regenerate after slash fires in clearcuts. He concluded that the "intense and prolonged heat" of slash fires kills black spruce seeds [243]. Fire often kills the seed crop of short trees. Natural selection for fast-growing, tall black spruces may occur in areas with fire-return intervals of 100 years [2] or less.
Seed rain of black spruce is usually greatest immediately or soon after fire, with lower levels over ~5 postfire years [410]. Snags, surviving black spruces, and adjacent stands provide seed sources [262]. See Seed dispersal for further information.
Postfire regeneration strategy [358]:
Tree without a sprouting root crown
Prostrate woody plant, stem growing in organic soil
Crown residual colonizer (on site, initial community)
Initial off-site colonizer (off site, initial community)
Secondary colonizer (on- or off-site seed sources)
Fire adaptations and plant response to fire:
Sustained seedfall over several postfire years helps ensure black spruce's establishment during favorable years [366]. Although large amounts of seed disperse during postfire year 1, small amounts of seed rain continue for several more years. For example, seedfall continued for 8 years following fire in a 70-year-old black spruce stand in interior Alaska [390]. See Regeneration Processes for detailed discussions of black spruce's postfire seed dispersal, germination, and establishment.
Plant response to fire: After fire, black spruce establishes from crown-stored seed that disperses from its semiserotinous cones [2,263,383,388,415]. Large quantities of seeds are released soon after fire [2,113,124,263,319,385,406]. In fact, striking recently fire-killed black spruce trees with an axe causes seed rain [243]. Sixty days after a fire in Newfoundland, black spruce seed rain averaged 1,500,000 seeds/acre. Before the upland site burned, the black spruce stand averaged 40 feet (12 m) tall and 188 feet² (17 m²) in basal area [406]. After severe fire in central Alaska, a stand that averaged 909 black spruce snags/ha had an estimated seed rain of 8,200,000 black spruce seeds/ha. Based on the 41% germination rate of a seed lot collected from the burn (n=6,400 seeds), there were approximately 3,400,000 viable seeds/ha [389].
Seed sources are usually on-site trees killed by a recent fire [262,375], supplemented by unburned islands of trees [263]. Seed may also blow in from off-site parents [262,263,290]. In wet lowlands, unburned stringers of black spruce provide off-site seed that disperses onto adjacent burned areas [263]. Some black spruce established from off-site, wind-blown seed after the 1950 Porcupine Wildfire in interior Alaska, which burned 1.7 million acres (0.7 million ha). By postfire year 30, saplings were 7 to 13 feet (2-4 m) tall, and a few trees were producing cones [123,125]. DBH averaged 1.7 inches (4.4 cm) [125]. In Alberta, Eberhart and Woodard [105] found moderate-sized fires left more residual unburned areas than small or large fires.
Black spruce seed production is poor at the taiga-tundra ecotone so generally, little or no seed is available for postfire regeneration. Where seed production is sparse, short-term climatic changes over 1 to 10 years can exhaust the seed population before a fire or prevent seed germination after a fire. Black spruce stands did not regenerate after a fire near arctic treeline in the Northwest Territories [54,55]. At arctic treeline in northern Québec, fires can eliminate or severely reduce black spruce and cause a shift toward arctic tundra. Here, black spruce seedlings are only occasionally found after fire: They are usually in depressions at the edge of burned areas, where cones of nearby living trees contain viable seeds [319].
A type shift from black spruce-lichen woodland to treeless tundra occurred after wildfires at arctic treeline in the Northwest Territories [54] and northern Québec [25,26,27]. A growth-chamber experiment using seed collected in the Northwest Territories found black spruce germination stopped at temperatures below 49 °F (15 °C) [55], suggesting that germination of black spruce is unlikely after fire near arctic treelines.
Following fire, black spruce establishes best on lowland sites with partially burned moist peat or on upland sites where severe fire exposes mineral soil [243,414]. Most fires do not consume the entire forest floor; mosaic fires result in small patches of exposed mineral soil intermixed within larger areas of partially consumed organic material [124], ensuring a variety of potential seedbeds. However, late summer fires sometimes consume the entire organic soil layer and expose extensive areas of mineral soil [390]. Unburned or partially burned sphagnum mosses also provide good seedbeds, but unburned or partially burned feather mosses are generally less favorable [6]. A fire in southern Ontario did not wholly consume fire mosses and hence, did not foster black spruce establishment. However, rotting logs under the feather mosses were often exposed and provided excellent seedbeds [276]. See Seedbeds for further information.
Most black spruce seedling establishment occurs within 10 postfire years [206]. In Québec, most new seedlings established within 3 postfire years [138]. In interior Alaska, Foote [124] found an average of 17,954 black spruce seedlings/acre on black spruce sites that had burned 1 to 5 years prior. In 50-year-old stands, black spruce had self-thinned to 2,595 stems/acre; trees in these stands averaged 2.1 inches (5.4 cm) D.B.H. and were 16.4 to 23 feet (5-7 m) tall [124]. After Alaska's Wickersham Dome Wildfire, most seedling establishment occurred within 3 to 5 postfire years. Even on sites where fire severity was low and some surviving adults produced new cones after fire, most establishment apparently came from fire-opened cones [384]. Johnstone [206] suggested that declines in seedbed quality, such as litter accumulation or sphagnum development, may limit black spruce establishment as postfire succession proceeds. Permanent plots in boreal Alaska and Yukon showed black spruce seedling establishment occurred within 10 postfire years, with most seedlings establishing in postfire years 3 to 7 (review by [151]).
Most studies found postfire density of black spruce seedlings was proportional to prefire basal density of mature stands (for example, [146,147,201]). Dense, pure black spruce stands commonly establish in the first few decades after fire in interior Alaska [396]. On 30-year-old burns, Lutz [263] reported mean black spruce density at 5,000 one-inch- (2.5 cm) diameter stems/acre. In 100-year-old burns, density of stems ≥5-inch (13 cm) diameter ranged from 2,000 to 3,000 stems/acre.
Black spruce density generally drops after the first few postfire decades due to self-thinning. In the Northwest Territories, Rowe and others [335] found mortality of black spruce increased 4-fold from postfire year 80 to postfire year 100.
Fire-return intervals and depth of burn into the organic soil layer (see Seedbeds) affect postfire recruitment of black spruce. Postfire seedling establishment of black spruce is reduced or curtailed when fire-return intervals are so short that few or no trees reach cone-bearing age. Removal of all or most of the soil organic layer reduces black spruce recruitment when hardwood regeneration interferes with black spruce establishment or when the permafrost layer thins after losing the insulating organic soil layers. Sites may experience permafrost thinning where the organic soil layer is <4 inches (10 cm) thick (review by [265]).
A deep permafrost layer helps protect a site from severe ground fire. After fire, there may be fewer changes in species composition on sites with a deep permafrost layer. Studies of burns near the headwaters of the Kobuk River found permafrost on the coldest, wettest sites usually did not thaw deeply after fire. Cold sites included concave positions, low slopes, and north-facing midslopes. Permafrost on warmer or dry sites thawed deeply in some cases, but not in others. Warmer sites with permafrost included convex positions, crests, shoulders, and east-, west-, and south-facing midslopes. Dry sites were on convex positions and upper slopes, usually with sand and gravel at shallow depths. These lacked permafrost regardless of time since fire. Sites where permafrost failed to thaw after fire showed only weak changes in postfire vegetation, while sites where permafrost thawed deeply showed greater changes in postfire plant community composition. Paper birch and quaking aspen were most abundant on dry sites, while black spruce was present on all sites. Feather mosses and sphagnum were most abundant on sites where permafrost remained after fire, while lichens and haircap mosses were most abundant on thawed, dry sites. The burns resulted from fires dating from 1959 to 1991 (≤37 years prior) [363].
See Regeneration Processes for further information, including information on prefire seed production and postfire seed dispersal, germination, establishment, and growth. Successional Status discusses general patterns of postfire succession in black spruce communities. Examples of black spruce response to fire by region follow.
Alaska: In Alaskan studies, black spruce generally showed best establishment on mineral soils or soil where most of the organic layer was burned off. If dominant before fire, it usually regained dominance within 10 postfire years. On 4 wildfire-burned sites in interior Alaska and central Yukon, germination of hand-sown black spruce seed was significantly greater on high-severity plots with exposed mineral soil than on low-severity plots with organic soil (P<0.01). Growth of transplanted seedlings was faster on high-severity than on low-severity plots (P<0.01). Fire severity had less effect on seedling survivorship, although survivorship was about 15% greater on high-severity than on low-severity plots (P=0.07). The prefire communities were mixes of black spruce, white spruce, and lodgepole pine; the study sites were in early postfire succession (postfire year 1 or 2). In general, high-severity plots had greater total tree density than low-severity plots [206].
Across burned black spruce-reindeer lichen woodlands of the central Brooks Range, most postfire seedling establishment of black spruce occurred in postfire years 10 to 35 (n=346 plots). Black spruce height growth averaged 1.3 inches (3.3 cm)/year, which was slightly more than that of white spruce (1.0 inches (2.6 cm)/year) [79].
Foote [124] found that on mesic black spruce sites in the Tanana River valley, black spruce seedlings were about twice as numerous as quaking aspen seedlings and sprouts and about 50 times as numerous as paper birch seedlings and sprouts in postfire years 1 to 5. Quaking aspen and paper birch seedlings and sprouts grew faster than black spruce seedlings until after about postfire year 30, but black spruce was more abundant in all successional stages. Black spruce's density peaked in the moss-herb stage [124].
| Table 2. Mean black spruce density and frequency in the Tanana River valley, Alaska (SD) [124] | ||||
| Postfire years | Successional stage | Age class | Density (stems/ha) | Frequency (%) |
| <1 | newly burned | seedlings | 0 | 0 |
| saplings | 0 | 0 | ||
| trees | 0 | 0 | ||
| 1-5 | moss-herb | seedlings | 17,954 (14,972) | 58 (35) |
| saplings | 13 (23) | 23 (40) | ||
| trees | 48 (154) | 23 (39) | ||
| 6-30 | tall shrub-sapling | seedlings | 12,881 (29,251) | 40 (40) |
| saplings | 4 (7) | 8 (8) | ||
| trees | 2 (5) | 5 (22) | ||
| 31-55 | dense tree | seedlings | 240 (534) | 77 (33) |
| saplings | 1 (1) | 83 (32) | ||
| trees | 358 (736) | 17 (33) | ||
| 56-90 | mixed hardwood-spruce | seedlings | 10 (19) | 750 (1,548) |
| saplings | 417 (718) | 92 (9) | ||
| trees | 1,550 (1,183) | 85 (20) | ||
| 91-200+ | spruce | seedlings | 4,688 (4,942) | 40 (37) |
| saplings | 225 (300) | 52 (55) | ||
| trees | 1,680 (473) | 99 (2) | ||
Three years after an experimental prescribed fire on black spruce/feather moss sites on the Washington Creek Fire Ecology Experimental Area, naturally dispersed and artificially sown black spruce seeds established only where fire had removed part or all of the organic matter. No seedlings were found on unburned, scorched, or charred feather moss substrates. In general, exposed mineral soils provided the best seedbeds. None of the black spruce seedlings that established in areas with some organic soil layer remaining survived past postfire year 3. However, on sites where mineral soil was exposed, seedling frequency of black spruce was 35% in postfire year 1 and 81% in postfire year 3; this was a result of continuing, natural seedfall [414]. For details of this study, see this Research Project Summary: Forest floor and plant responses to experimental fires in an Alaskan black spruce/feather moss community.
Black spruce formed a near-monoculture in the first 2 decades after the Wickersham Dome Wildfire near Fairbanks. The fire burned 15,600 acres (6,300 ha), mostly black spruce stands ranging from 50 to 125 years old. Through postfire year 3, tree seedling establishment was variable regardless of fire severity. Low-severity areas had <50% of the ground surface blackened, litter depth reduced an average of 2.25 inches (5.7 cm), and 40% of ground vegetation alive 1 year after fire. Black spruce seedling establishment was sparse in one high-severity area, with only 20% of plots supporting black spruce seedlings 3 years after the fire. Conversely, another high-severity area contained 21,000 black spruce seedlings/ha 3 years after the fire. In low-severity areas, unburned and partially burned sphagnum mosses provided good seedbeds for black spruce. By postfire year 3, low-severity plots averaged 40,000 black spruce seedlings/ha. Black spruce seedling density peaked at postfire year 10, but 50% of black spruce establishment occurred within the first 3 postfire years. Black spruce comprised 98% of postfire tree establishment, and its density remained "nearly constant" through postfire year 20 [384].
Although black spruce generally regains prefire dominance (for example, [146,147]), competition for substrates—especially from sprouting woody species—may tip postfire succession towards dominance by other tree species. A study in central interior Alaska found no correlation between prefire black spruce basal density and postfire recruitment (r = 0.34, P>0.1), but black spruce recruitment was negatively correlated with quaking aspen regeneration (r = -0.61, P=0.01). Density of quaking aspen regeneration was positively correlated with fire severity (r = 0.49, P=0.02). The study was conducted 7 and 8 years after a summer wildfire in the Alaska Range, near Hajdukovich Creek. The prefire forest was open to closed black spruce, with occasional quaking aspen stands. The fire burned from 14 June to early September 1994. Initially, fire weather was moderate and fire severity was low. In the first 3 weeks in August, however, fire weather escalated to extreme and fire severity was high. Fire severity was assessed by satellite and confirmed on ground plots by postfire depths of organic soil layers. Of 22 stands sampled, 20 were black spruce before the fire; the other 2 were quaking aspen. The quaking aspen stands were on coarse soils; the thick organic soil layer of most sites had apparently deterred quaking aspen growth. Prefire tree age ranged from 60 to 280 years. Two fires had occurred within the previous 200 years, 1 around 1875 and 1 around 1910. Mean prefire organic depth was estimated at >10 inches (25 cm) on severely burned plots, based on depths in an adjacent, unburned forests. Postfire organic depths ranged from 0 to >8 inches (20 cm). The authors attributed differences in postfire organic depths to increasingly dry weather conditions and drying fuels—and therefore, increased consumption of the organic soil layer—as fire severity increased through the summer [211].
Great Lakes: Black spruce seeds in quickly after fire on mesic to dry pine-spruce uplands. However, jack pine and/or red pine also seeds in aggressively and generally overtops black spruce in early postfire years. Thirty-five years after a fire in a mixed-confer forest in northern Minnesota, the overstory was mostly jack pine and black spruce. Most jack pines were 33 to 34 years old and 4 to 6 inches (10-15 cm) in diameter, while most black spruces were 28 to 32 years old and only 1 to 3 inches (2.5-3 cm) in diameter [166]. Black spruce is shade tolerant and can survive suppression for more than 100 years [166], so black spruce may replace the pines in the absence of fire for centuries [46,395].
After the May 1971 Little Sioux Wildfire in northeastern Minnesota, black spruce seedlings gained biomass quickly. In postfire year 1, mean biomass of seedlings was 0.1 g. By postfire year 4, it was 10.2 g [302]. Prior to the wildfire, the area had been recently logged and was a mosaic of stands including lowland and upland black spruce, quaking aspen, jack pine, and eastern spruce budworm-infested balsam fir [342].
Western Canada: A 20-year study after a wildfire in southeastern Yukon found spruce (black spruce and white spruce) seedling density averaged ≥8 stems/m² in postfire year 10. Spruce seedling density remained constant or increased slightly from postfire years 10 to 20 (P=0.08); in contrast, quaking aspen and lodgepole pine densities declined after postfire year 10 (P<0.001). In postfire year 19, mean spruce heights ranged from 0.6 to 4.1 feet (0.2-1.3 m). Tree heights were not correlated with either total tree or individual tree species densities [206].
Although black spruce often regenerates poorly after logging (see Fire Management Considerations), it regenerated successfully in southeastern Manitoba after selective logging was followed by prescribed fire. Two black spruce/feather moss forest sites were thinned from 2,180 to 800 trees/acre in winter, then broadcast burned 2 years later, on 17 May (a low-severity fire) and 29 May (a moderate-severity fire). Black spruce seedling establishment and survival were better on the moderate-severity site than on the low-severity site. Depth of burning into the moss layer averaged 5.5 inches (14.0 cm) on the moderate-severity and 2.5 inches (6.4 cm) on the low-severity site. Five years after burning, black spruce stocking was 94% (16,129 seedlings/acre) in areas where burning depth ranged from 4 to 7 inches (10-18 cm); 70% (3,075 seedlings/acre) in areas where burning depth ranged from 2 to 3 inches (5-8 cm); and 35% (1,898 seedlings/acre) in an unburned control [80]. For details of this study, see this Research Project Summary: Black spruce experimental fires on lowland sites in Manitoba.
 |
|
| A prescribed surface and crown fire in a black spruce-lichen stand on the Caribou Range, Northwest Territories [9]. Photo courtesy of the Northern Forestry Centre. |
Eastern Canada: Black spruce dominates most boreal conifer ecosystems of this region (see Plant communities), but balsam fir and/or northern whitecedar may eventually replace black spruce if fire does not return for centuries. In open woodlands in northern Québec, black spruce tends to regenerate quickly after fire, regaining or exceeding prefire density within 30 years [354]. In the balsam fir-black spruce ecosystem of northwestern Québec, a chronosequence study found that black spruce and northern whitecedar dominated all xeric, exposed-bedrock and some morainal sites from early through late postfire succession. Black spruce frequency declined around postfire year 300; it persisted longer on xeric than on mesic sites. Across sites, balsam fir persisted longer than black spruce, and northern whitecedar persisted longer than balsam fir [46].
A study in northwestern Québec found survivorship of trees was higher after low-severity fire than after moderate-severity fire. Angers and others [19] studied tree recovery after mixed-severity fires in boreal mixedwoods. In stands codominated by black spruce and jack pine, about 60% of those conifers were still alive in postfire year 1 on plots that burned at low severity (data were pooled for the 2 conifers). Conifer survival dropped to about 45% by postfire year 9. On moderate-severity plots, conifer survival was about 20% in postfire year 1 and 8% in postfire year 9. Where conifers and hardwood species codominated, overall tree survival on low-severity plots averaged about 42% in postfire year 1 and 20% in postfire year 9. On moderate-severity plots, overall tree survival averaged about 5% in postfire year 9 [19].
In southern Labrador, black spruce's postfire seedling establishment progresses slowly for 70 to 100 years, resulting in uneven-aged stands. Within spruce stands in this coastal climate, fires generally consume very little organic matter and leave only charred humus. For the first 20 years after fire, black spruce seedling establishment is sporadic and largely restricted to depressions, the edges of water courses, and exposed mineral soils [128].
FUELS AND FIRE REGIMES: Fuels: Black spruce forests are highly flammable: They are the most flammable vegetation types in boreal Alaska [85] and Canada [15]. Jack pine-black spruce forests of the Great Lakes region and Maine are considered the second most flammable ecosystem in the United States, next to chaparral (Frelich personal communication cited in [228,229]). Among black spruce types, upland black spruce stands are generally most flammable and black spruce bogs the least [122]. Fuel loads are usually lower in black spruce-lichen woodlands than in black spruce forests because woodland stands are more park-like and open, often with a component of less flammable white spruce [385]. However, black spruce woodlands can have large accumulations of fine fuels, sometimes >50 tons/acre. During warm summers, these fine fuels dry and become flammable very rapidly [52].Because they are typically wet, lowland black spruce bogs cannot usually carry fire [122] and serve as firebreaks [233] in most years. However, lowland bogs burn in extreme fire years [122]. In Quetico Provincial Park, Ontario, an August 1995 wildfire burned through old-growth red spruce-eastern white pine stands (200-300 years old) into black spruce lowlands that "would normally be considered fire breaks". The crown fire burned 62,000 acres (25,000 ha) and was largest and "most significant" fire in the Park since 1936. Intensity was estimated to have reached 40,000 kW/m in some red pine-eastern white pine stands [267].
Chemical content and arrangement [56] of fuels make black spruce forests highly susceptible to crown fires. Black spruce trees are highly flammable [85,266]. Todd and Jewkes [368] report that black spruces "are an ideal fuel for spreading fire". Black spruces have resinous needles and cones [85,396] and considerable pitch in their wood [368]. Their twigs and needles are "tougher and gummier"—with more resins—than those of white spruce [396]. Branches are usually retained after they die [122,383], encouraging fire spread into crowns [383]. Because black spruce needles are fine and highly flammable fuels, they combust and are consumed in 1 or 2 minutes [37]. Eastern dwarf mistletoe infections increase flammability of black spruce (review by [13]) (see Fire Management Considerations).
Typically, stand structure of black spruce forests provides vertical continuity of surface and ladder fuels [56,85]. Cronan and others [85] report that in black spruce communities, the "combination of continuous surface fuels, frequent ladder fuels, and flammable canopy fuels creates optimal conditions for high-intensity crown fires that can initiate across a wide gradient of weather". The forest floor under most black spruce stands is made up of a thick mat of decomposed organic material [104] and live mosses and lichens. In summer, the mosses and lichens "become dry and tinderlike" [263]. Feather mosses [85] and ground lichens [263] provide surface fuels that can dry out rapidly, becoming highly flammable [85,263]. Because feather mosses retain moisture better than lichens, flammability of surface fuels may decrease with succession as feather mosses replace lichens. Permafrost slows drying of feather mosses [127]. In mature (>50 years) black spruce-lichen tundra communities, lichens often form a continuous mat that ensures surface fuel continuity. These communities are highly flammable, with low rates of decomposition on the forest floor [28]. Typically, most of the litter and much of the soil organic matter are consumed during fire, exposing 30% to 40% of the mineral soil (review by [28]). On the Caribou-Poker Creek Experimental Watershed, Hylocomium feather mosses dried out faster than Schreber's moss, possibly because Hylocomiums have more vertical structure and better drainage. Moisture levels at the surface of the forest floor were more spatially variable in May than in June and July. After the moss layer had dried, it required a "significant rainfall" to rehydrate: Intermittent light rains had little effect on moss moisture levels [116].
Branches of individual black spruces are dense, often extending to the ground [104,368,383]. These branches are ladder fuels, and once fire crowns, it "intensifies and spreads rapidly" [368]. Low, ericaceous shrubs may also carry flames to tree crowns [56,85,383]. Ericaceous shrubs such as black huckleberry, sheep-laurel, and bog blueberry have a high percentage of ether extracts and lipids [28] and are highly flammable [28,383]. They may carry flames 2 to 3 feet (0.5- 1 m) above the soil surface. From that point, black spruce's dead or live lower branches usually carry fire to tree crowns [383]. The branches are often draped with arboreal lichens, which also carry flames to the crown [263,264]. Dyrness and others [103,393] reported that in open-canopy black spruce communities with feather mosses and lichens, a shrub layer is not critical for continuity of ladder fuels; fire easily moves from the moss-lichen ground layer to black spruce canopies.
Johnstone (2008 in [65]) noted that because plant community flammability and fire severity are less in hardwood than in black spruce types, "increasing the proportion of deciduous dominated stands across the landscape reduces the probability of severe fire, not only for deciduous trees but ultimately for black spruce too". Deciduous patches in a black spruce matrix can serve as fire breaks [65].
Woody debris loads are high after crown fires in black spruce stands, and live stands usually accumulate deep organic soil layers [404]. Mutch [295] suggested that accumulation of windfall and other fuels over long time periods renders black spruce communities highly flammable and predisposed to large fires during drought years. In the Northwest Territories, structure of a ~300-year-old black spruce forest was open, with stunted trees and a "high amount of standing dead matter" [301]. In interior Alaska, the litter layer of a black spruce/feather moss-lichen forest had greater mass, was more acidic, and contained fewer total nutrients than the litter layer beneath a quaking aspen-paper birch forest [372].
Moss biomass may be high in all stages of postfire succession in black spruce communities, while snag density tends to decrease and downed woody debris tends to increase over time. In a chronosequence of fuel accumulation in black spruce stands of north-central Manitoba, 8% to 20% of downed woody fuels [273] were completely covered by sphagnum [59] and/or feather mosses [57] in stands of all ages. For stands >70 years old, up to 26% of downed woody fuels were partially to fully buried by mosses. Loads of downed woody debris were greatest from postfire years 20 to 40. Standing dead woody fuels were prevalent before then [273]. Coarse woody debris loads ranged from 9.7 Mg/ha in 43- and 77-year-old stands to 80.4 Mg/ha in an 18-year-old stand. Snag fall was 3 times more frequent in a 12-year-old stand (9.8%/year) than in a 9-year-old stand (2.9%/year) [58]. Net primary production peaked in 12- to 20-year-old stands, and primary production of trees peaked in 37-year-old stands, with both net and tree primary production greater on well-drained than poorly drained burns. In 37-year-old stands, most tree biomass was from sprouting hardwood species such as quaking aspen [59].
Several studies sequenced decay and falling rates of black spruce and other snags. In Labrador, biomass of woody debris in burned black spruce forests peaked around postfire year 20, mostly due to snag fall. The authors provide a model for predicting merchantable wood volume for young (<10-year) to old-growth (>181-year) black spruce stands [154]. In northwestern Québec, most wildfire-burned black spruce snags fell within 19 years of tree death, a rate similar to that of balsam fir. Quaking aspen snags fell quickest (x̄ =15 years), while jack pine snags stood the longest (x̄ =26 years). Nearly all black spruce snags had fallen within 40 years of tree death [20]. For black spruce, snags fell sooner on plots that burned at low severity than on plots that burned at high severity (P<0.0001) [19]. A study near James Bay, Québec, had similar findings. Decomposition rates were slowest for severely burned black spruce snags and highest for lightly burned black spruce logs. The authors found that black spruce snags that burned at high severity shed their bark fastest and had lowest moisture contents, which slowed decay [62]. Rapid decay rates of snags that burned at low severity might be due to wood tunneling by wood-feeding insects and subsequent infections of wood-colonizing fungi [62,341].
In mixedwood stands, boreal hardwood trees are less flammable than associated conifers, including black spruce [170]. A fire history study found that fire was a relatively unimportant disturbance in interior Alaska until about 5,500 years BP, when black spruce replaced birch-alder as the dominant community type in the region [266]. In mixedwood forests, flammability tends to increase as the conifer component of the forest increases [183]. In early postfire stages, however, fuel loads may be greater in hardwood than in black spruce forests. In interior Alaska, total woody fuel loads from postfire years 1 to 5 averaged more in paper birch than in black spruce communities. From postfire years 6 to 25, large woody debris loads were greater in paper birch than black spruce stands, but there were more small-diameter woody fuels in black spruce. After that, black spruce stands had more total downed woody fuels than paper birch stands. Fuel loads in black spruce-hardwood stands were intermediate except from postfire years 51 to 100, when large woody debris loads were greater in mixed stands than in either paper birch or black spruce stands [122].
| Woody fuels loads (T/ha) of black spruce and paper birch communities in interior Alaska. Cells are empty where data were not reported [122]. | |||||
| Postfire years | 1-5 | 6-25 | 26-50 | 51-100 | >100 |
| Small-diameter |
|
7.4 | |||
| Large-diameter | 10.2 | ||||
| Total | 4.9 | 8.1 | 5.7 | 7.3 | |
| Small-diameter | 5.3 | ||||
| Large-diameter | 28.8 | ||||
| Total | 16.3 | 5.4 | 5.2 | 5.0 | |
Distribution and loading of fuel biomass differ with successional stage and substrate moisture. Early-seral herb and shrub stages have less continuous fuels than later-successional, even-aged black spruce stands [368]. In southeastern Labrador, open black spruce-balsam fir forests from 40 to 90 years old were the most flammable vegetation types. At that seral stage, stands were composed of scattered, mature trees; a "fairly luxuriant" shrub layer of bog Labrador tea and sheep-laurel; and a nearly continuous ground layer of reindeer lichens [127]. Surface fuels may increase with soil moisture due to increasing cover of lichens and ericaceous shrubs [385]. Chronosequence surveys near Delta Junction in interior Alaska found biomass production of black spruce communities—including biomass of lichens, feather mosses, and vascular plants—differed with substrate moisture. On dry sites, mean maximum production peaked in a young stand (15 years old) at 410 g/m²/year. On mesic sites, it peaked in a mature stand (>100 years old) at 335 g/m²/year. Production in the young stand was mostly sprouting shrubs, while it was mostly black spruce in the mature stand [269].
Blowdowns resulting from extreme nor'easters can greatly increase loads of downed woody debris in coniferous forests with black spruce. In a review, Flannigan and others [118] noted that an extreme windstorm is "not a rare event" in the Great Lakes region. Windspeeds of ≥60 miles (90 km)/hour occur every 5 to 10 years in Minnesota [118]. In northwestern Ontario, a 1973 tornado in jack pine-black spruce was followed by a 1974 wildfire that consumed blowdown fuels on 79,000 acres (32,000 ha) [118]. A blowdown on 4 July 1999 affected nearly 500,000 acres (200,000 ha) on the Superior National Forest, Michigan. Fuel loads in the Boundary Waters Canoe Area increased from 5-20 tons/acre to 50-100 tons/acre as a result. A blowdown of this size, and the consequent 5- to 10-fold increase in fuel loads, was historically unprecedented. A "very severe prescribed fire" was conducted in September 2002 to reduce fuel loads in the area. The prescribed fire was credited for helping contain the 2006 Cavity Lake Wildfire within the wilderness area. It was a "very high intensity fire" that "burned the soil down to bedrock in some areas", exposing pink granite. In contrast, the 2007, human-ignited Ham Lake Wildfire occurred in an untreated part of the blowdown outside the wilderness and burned 160 structures. The author suggested that climate change may increase the likelihood of severe weather events such as the 1999 blowdown [250].
Several studies across black spruce's distribution measured fuel loads in various strata of black spruce stands. Barney and others [33] provide forest floor fuel loads, depths, and bulk densities for upland and lowland black spruce types of interior Alaska.
In black spruce/Easter snow lichen woodlands of the Northwest Territories, active crown fire developed on 2 plots with crown bulk densities of 0.12 and 0.17 kg/m³ and rates of fire spread of 26.3 and 33.3 m/minute. Active crown fires did not develop on 6 other plots even though crown bulk densities were higher (0.18-0.32 kg/m³); on these plots, rates of fire spread were lower (1.2-6.1 m/minute) (review by [151]).
In Alberta, annual peat accumulation on 2 black spruce/sphagnum bogs ranged from 156 g/m² to 257 g/m². Accumulation was similar on hummocks and in hollows on the Sinkhole Lake Bog, but peat accumulated more rapidly on hummocks than in hollows on the Athabasca Bog. Dark sphagnum dominated the ground layer of hummocks, while lichens, true mosses (Bryopsida), and wet-loving sphagnum species such as narrow-leaved sphagnum dominated the ground layer of hollows [39].
In Newfoundland, litterfall in a mature, 70-year-old black spruce/feather moss forest ranged from 2.4 to 3.5 Mg/ha/year before clearcutting and from 0.2 to 0.3 Mg/ha/year after clearcutting. Rates of litter decay did not differ significantly between uncut and cut plots for 2 to 4.5 years after clearcutting. Splendid feather moss, Schreber's moss, and knight's-plume moss dominated the groundlayer, and there was a balsam fir-hardwood component in the overstory [291].
Zasada and others [103,413,414] demonstrated that feather moss and lichen fuels on the forest floor carry the surface portion of mixed-severity fires in black spruce/feather moss types. Downed woody fuels were too sparse to have much impact on fire spread on the forest floor. The "upper portion of the forest floor readily carried fire across most of every unit" [103]. From there, low branches on black spruce easily carried fire into tree crowns. These experimental fires created a mosaic similar to that created by the mosaic fires typical of black spruce/feather moss forests of interior Alaska. The authors concluded that "wildfires burning on sites similar to the experimental area and within the ranges of variables documented" in their study should produce similar fire behavior and effects on vegetation and soils [103]. For details of this study, see this Research Project Summary: Forest floor and plant responses to experimental fires in an Alaskan black spruce/feather moss community.
Models: Researchers have developed many models to help quantify fuels, decay rates, mortality, and fire behavior in black spruce stands. Models are listed below by region and purpose.
General for the United States and Canada:Fire regimes: Lutz [264] wrote of wildfire that "this powerful ecological factor has operated as long as the boreal forest has existed". Black spruce ecosystems have mostly lightning-ignited, stand-replacing fires. Most wildfires occur in summer. Most or all vegetation is usually killed or top-killed when black spruce communities burn [264,383]. Black spruce woodlands and forests typically burn with mixed, surface-and-crown fires (for example, [37,103,384,394]). Fires spread easily and uniformly through crowns in black spruce forests [28,264,266,383]. Intermittent or passive crown fires occur in more open communities; these occur in combination [9,124] with lethal surface fires [127] on some sites. Consumption of the organic soil layer (ground fire) typically accompanies both crown and lethal surface fires [206,264,383,404]. Fires in late summer—especially those occurring in dry years or with hot winds—often burn down to mineral soil [383]. In black spruce bogs, which generally have deep layers of soil organic material, ground fires may smolder for weeks, months, or even years [404]. Fire frequencies [383] and sizes vary across black spruce's range. Landscapes may burn in a mosaic pattern [124] or more uniformly [221].
In addition to the discussions that follow, see the Fire Regime Table for information on fire regimes of vegetation communities in which black spruce may occur. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
Season: Most fires in black spruce ecosystems occur in late spring and summer, with the most severe fires occurring late in the fire season. In boreal ecosystems of interior Alaska and western Canada, the fire season begins in April and extends until September. May and June fires account for about 90% of the acreage burned (~6.9 million acres (2.8 million ha)/year) [190,266]. May, June, and July are typically the most active fire months in Alaska because they have the highest average temperatures and lowest average humidity and precipitation [388]. Severe fire years are correlated with warm, dry air flows [188]. During the Alaskan wildfires in the summer of 2004—an extreme fire year—sites that burned in late July and August had a larger proportion of high-severity fires than sites that burned earlier in the summer (n=90 sites). That summer, interior Alaska had the largest area burned (6.7 million acres (2.7 million ha)) since fire records were started in the 1940s [173].
Type and severity: Black spruce communities experience nearly 100% stand-replacement fires [29,124,262,263,264,368]. Most are rapidly moving crown fires accompanied by surface and ground fires [37,103,185,285,384,394]. Active crown fires usually occur in closed black spruce forests [193,299], although Norum [299] noted that running crown fires are rare in black spruce forests; surface fuels typically carry the fire, with crowning occurring behind the fire front. Fires that occur early in the fire season, while the ground is still frozen, may burn only in the crown. Late-season fires are most likely to burn deeply into organic soil layers [397].
Lethal surface-ground fires may occur in woodlands [141], but such fires are poorly documented [298]. Active crown fires are rare in black spruce woodlands due to the wide spacing of trees, although individual trees and stringers torch [9,220]. Intermittent or passive crown fires occur in lichen woodlands and other open black spruce communities [193,299]; these may occur in combination [9,124] with lethal surface fires [127] on some sites.
Fire moves quickly between strata, traveling up tree boles and transitioning from surface to crown, or vice versa [124]. In forests, crown fires may move within the canopy and never touch the surface [124]. Hollingsworth (2008 cited in [65]) observed wildfires in interior Alaska that burned in crown, surface, and ground layers. She remarked that "after the fire has burned an individual tree it typically drops down and creeps slowly along the ground, depending on how wet the site is" (Hollingsworth 2008 cited in [65]). Low-severity surface fires sometimes occur in black spruce communities [9], but even low-severity fires are usually lethal to black spruce (see Fire Effects).
As applied to fire in black spruce communities, the term "severe" can be misleading [65]. Patterns of postfire succession in black spruce communities are strongly driven by how deeply fires burn into organic soil layers. Therefore, fire severity in black spruce ecosystems is best measured by how much of the organic soil layer was removed (Hollingsworth 2008 cited in [65]). Because the black spruce overstory is usually killed (for example, [298,317]) even when fire severity is low at ground and surface levels [298], fire severity is not a reliable indicator of black spruce mortality [401]. Ryan and Noste [339] stated that in black spruce communities, "the crown fire phase of a wildfire involves primarily the combustion of fine fuels. It devastates the overstory", but may have only slight effects on understory vegetation and organic soil layers [339]. If fire severity was low to moderate on the forest floor, the landscape may show no postfire changes in species composition [65].
Within 4 burned jack pine-black spruce communities of west-central Québec, Jayen and others [185] found fires on most quadrats were stand-replacement, mixed surface and crown fires, although fires apparently did not crown on some quadrats. They assigned fire-impact classes based on aerial surveys in postfire year 6 or 7. Mortality was not differentiated between jack pine and black spruce [185].
| Variation in forest fire-impact classes in postfire year 6 or 7 in Québec. Data for high-impact classes were lumped for statistical analyses [185]. | ||
| Tree condition | Fire-impact class (fire type)* |
Number of quadrats (n=47) |
| mix of green trees & trees with red crowns; more green than red | light (surface) | 1 |
| mix of green trees & trees with red crowns; more red than green | moderate (surface) | 8 |
| red crowns; generally <25% blowdown | high (surface) | 65 |
| crowns burnt, trees black & bark often detached; generally <40% blowdown | high (crown) | |
| crowns burnt, trees black & bark often detached; generally >40% blowdown | high (crown) | |
| *Fire type (in parentheses) assigned by Fryer based on descriptions in the paper. |
||
Duff, lichen, and/or moss layers in black spruce communities usually support smoldering combustion except when saturated. Video of an experimental fire in a 69-year-old jack pine/black spruce forest in the Northwest Territories showed the continuous flame front was a mix of surface and active crown fire. Rates of spread ranged from 70 to 100 feet (20-30 m)/minute. Tree boles and patches on the forest floor ignited in advance of the flame front, with spot fires from ember rain about 30 feet (10 m) ahead of the flame front. After the flame front passed, residual flaming continued on the forest floor, in downed woody debris, and in tree boles [364].
Ground fires in laurel or other shrublands may result in a type conversion to black spruce. Fires may burn down to mineral soil in extreme fire years, providing a seedbed for black spruce establishment. Once black spruce establishes, the site may become more susceptible to crown fires that perpetuate black spruce. Wein [404] noted areas in Newfoundland that apparently underwent such type conversions. Studies near and within Terra Nova National Park showed that the amount of residual organic matter in the soil was a major driver of postfire succession in this region. Where the residual organic soil layer was >0.8 inch (2 cm) thick in 40- to 100-year-old burns, the site had succeeded to sheep-laurel heathland. Postfire succession was more variable where the residual organic was thinner; such sites succeeded to either sheep-laurel savanna or black spruce forest. The authors suggested that on sites with shallow residual organic matter, an abundance of sprouting shrub species before fire may lead to dominance of sprouting shrubs after fire, while black spruce may dominate sites that had few shrubs before fire [347].
Because of irregular terrain and differences in substrate moisture, fire severity may vary greatly within black spruce communities. A study in central Saskatchewan found fire severity differed at microsite but not landscape levels. The landscape was comprised of closed-canopy black spruce/Schreber's moss-dark sphagnum stands on permafrost bogs and open black spruce/bog Labrador tea/dark sphagnum stands on nonpermafrost bogs. It burned in a March 1999 wildfire. In June 1999, 4 peat cores—2 from each stand type—were collected to determine fire severities. Based on ash concentrations, the researchers found high within-site variations in the amount of burned peat across the landscape (P=0.01). Areas of highly combusted peat often lay within 20 inches (50 cm) of lightly scorched or unburned peat. The authors were unable to account for these differences in fire severity. There were no significant differences in fire severity either across sites or between permafrost and nonpermafrost sites [374].
A study in black spruce and jack pine-black spruce stands of central Saskatchewan showed fire severity was greatest on sites with relatively low duff moisture. Comparing smoldering duff consumption on 2 sites with similar duff characteristics, duff consumption was greater during the Waskesiu Lake Wildfire of July 1998 than during the Bittern Creek Wildfire of June 1996. Both fires crowned, but the ground fire component was more severe on the Waskesiu Lake site. Based on measurements from adjacent unburned sites, this was likely because percent duff moisture was less on the Waskesiu Lake site than on the Bittern Creek site (5%-8% vs. 12%-16%, respectively). Both fires burned down to mineral soil in patches, but the Waskesiu Lake Burn had a wider range of sizes for mineral-soil patches, and average mineral-soil patch size was larger (P<0.001 for all variables). The study provided some additional information on ground fuels that pertains to fire severity: For unburned sites near both burns, low-slope black spruce stands on glaciofluvial deposits had significantly deeper duff layers (4.06 inches (10.3 cm)) compared to upper-slope unburned jack pine-black spruce stands (2.72 inches (6.9 cm), P<0.05). Bulk density of the duff was not significantly different between black spruce and jack pine-black spruce stands. Duff was drier beneath than between trees, probably due to interception of precipitation by tree crowns [285].
Frequency: Black spruce types in general have stand-replacement fires about every 75 years (review by [85]). Fire-return intervals range from 30 to 100 years in black spruce ecosystems [165,233]. Heinselman [167] calculated mean fire-rotation intervals of 100 years for closed black spruce forests and 130 years for open black spruce-lichen woodlands across Alaska. Fire-return intervals tend to increase from west to east [168]. Fire-return intervals increase eastward across boreal Canada, from the relatively dry continental boreal forests toward the Atlantic seaboard. The longest fire-free intervals for spruce stands probably occur in southern Labrador, where the fire-rotation interval for black spruce forests is estimated at 500 years [128]. For a summary of fire-return intervals and rotations across black spruce's distribution, see Table 2 in FEIS's Fire regimes of Alaskan black spruce communities synthesis.
Fire-return intervals lengthen toward arctic treeline. Open black spruce-lichen woodlands in the Northwest Territories and northern Québec have fire-return intervals of ≥100 years [54,271,319]. Some black spruce stands in northern Québec have been aged at >2,000 years old [298]. On a site near Inukjuak, fire-return intervals ranged from 100 to 1,800 years [227].
Upland taiga burns more frequently than lowland taiga. Black spruce stands on muskegs generally experience longer fire-free intervals than nearby upland stands, and they often become uneven-aged with succession [395]. East of Great Slave Lake, black spruce/dark sphagnum peatlands burn about half as often as drier, upland black spruce communities. In observations of >30 burns in the low peatlands, burn patterns were patchy, with peat burned away only around some trees [184]. Johnson [189] reported MRFIs (mean fire-return intervals) of 70 to 100 years for black spruce communities in this region of the Northwest Territories. In the Boundary Waters Canoe Area, black spruce tends to occupy large, upland ridges distant from lakes; these areas burn more frequently than valleys, lower slopes, and lacustrine areas, which are typically occupied by pines and white spruce. Fire-rotation intervals range from 50 to 100 years for black spruce forests in the area, with fires sometimes skirting black spruce peatlands [166]. In southeastern Labrador, fire frequency was less on an interior plateau that was broken up by "extensive peatlands and numerous lakes" than on watersheds with more variable topography and few peatlands [127].
Black spruce peatlands may burn only in dry years. Once ignited, ground fires in peatlands may smolder for weeks, months, or even years. Fire frequencies are highly variable in peatlands and bogs. Small peatlands may burn at the same frequencies as the surrounding plant communities, but generally at lower severity and in a patchy pattern [404].
Based on reviews, Frost [136] mapped black spruce ecosystems of the Great Lakes states and Maine with 26- to 100-year fire-return intervals.
In west-central Alberta, a history of stand-level, crowning wildfire followed by several successive mixed-severity wildfires was documented for 6 mixed-conifer forests dominated by lodgepole pine, white spruce, and black spruce. Crown fires had occurred 120 to 300 years prior. The stand-replacement wildfires were followed by low- to moderate-severity wildfires that left live, fire-scarred lodgepole pine in each of the 6 forests. Return intervals of the low- and moderate-severity wildfires averaged <80 years and ranged from 29 to 167 years [16].
Pattern and size: Patchy fires are common in black spruce communities, with patches burned down to mineral soil intermixed with unburned patches and patches where the soil organic layer was partially burned [124,184]. Upland black spruce communities tend to burn more uniformly than lowland black spruce peatlands. Sphagnum mosses can absorb and sequester water up to about 20 times their own dry mass [325]. This likely explains why black spruce peatlands with >7 feet (2 m) of accumulated sphagnum dry slowly and rarely burn [221].
 |
| A burned black spruce/sphagnum stand in interior Alaska. The brown patches on the forest floor are live, scorched sphagnum. The brown sphagnum patches held so much water that they did not ignite. U.S. Forest Service photo by Jill Johnston and Teresa Nettleton Hollingsworth. |
In burned jack pine/black spruce stands of southeastern Manitoba, most unburned areas were large bogs or fens. Time since fire averaged 70 years on densely treed wetlands—some of which were skirted by previous fires—and >120 years on wetlands with shallow water tables. At the landscape level, this resulted in an uneven-aged forest. Large wildfires accounted for the "vast majority" of area burned from 1955 to 1983. Large fires ranged from 500 to 200,000 acres (200-90,000 ha), with a median of 25,390 acres (10,155 ha). On 6 large burns, residual unburned islands were rare, occurring mostly near burn perimeters and in wetlands. [106].
Fire size varies greatly in black spruce communities (review by [85]). Most fires are small [127]. "Ecologically significant" fires are infrequent, but they can be large (>400 mile² (1,000 km²)) [127]. A few large fires (≥123,500 acres (50,000 ha)) [304,383] occurring in extreme fire years account for most acreage burned (for example, [106,127,383,395]). Burns of 500,000 acres (200, 000 ha) are common [304,333]; in some years, millions of acres may burn [304]. On sites across Alaska, 6 years accounted for 63% of total area burned from 1940 to 1977 [383]. Over 81 years (1863-1944) in the Mackenzie Valley, Northwest Territories, 42% of all fires occurred in 5 dry years, and in the northern portion of the valley, 60% of all fires occurred in 4 dry years. The fire-rotation interval was 80 to 90 years in black spruce communities [333].
Regional studies: Regional trends in fire ignition, season, type and severity, pattern, and size are discussed below. Alaska: Most wildfires in Alaskan black spruce communities are ignited by summer lightning. Documented mean fire-return intervals since the 1700s range from about 40 to 200 years [95,213]. Wet and stringer black spruce communities generally have longer fire-return intervals than mesic communities [85,395]. Fires typically crown, with accompanying surface and ground fire [82,206,264,383,404]. Most fires are small, but a few large fires account for most acreage burned [32,323]. Except near settlements, few Alaskan black spruce communities have been subject to fire exclusion [69]. See the Fire regimes of Alaskan black spruce communities synthesis for detailed information and documentation of fire regimes in this area.Great Lakes: Fires are more frequent in pine-black spruce forests than in black spruce bogs. In Michigan, fire-return intervals of jack pine-black spruce forests are estimated at 50 to 100 years, with a mean of 80 years [132]. Fire-return intervals for jack pine-black spruce forests in the Boundary Waters Canoe Area range from about 50 to 70 years [251]. In pine-spruce forests of the Boundary Waters Canoe Area, Heinselman [164] documented "major fires" from at least 1600 to 1920 . Some areas reburned in 10 years, while others had not burned for 200 to 300 years [164,166]. The longest fire-rotation interval was around 360 years. From the 1920s to the 1970s, only "limited areas of virgin forest ... burned, due to effective fire control" [164]. Fire exclusion in this region may lead to very long fire-return intervals: In the 1990s, the fire-rotation interval for Boundary Waters Canoe Area was estimated at >1,000 years [134].
Because they are so wet, black spruce peatlands do not burn except in extreme fire years. For black spruce swamps in the Great Lakes states, LeBarron [243] noted that "during drought periods when water levels are low they can burn very fiercely, and when they do spruce timber is killed almost entirely". In Itasca State Park, Minnesota, Heinselman [168] found that fire sometimes skirted black spruce and black spruce-tamarack bogs on Laurentian uplands, glacial moraines, and outwashes. Fire-return intervals of upland areas were 20 to 40 years; he was unable to determine how often the bogs burned [168].
Fires typically crown in pine-black spruce and black spruce muskegs of the Great Lakes region [3,229]. In northeastern Minnesota, a mid-July wildfire burned a jack pine-black spruce site with a mixed crown, surface, and ground fire. The ground fire was described as "very hot", burning all litter, much of the duff, and exposing mineral soil on some microsites [3].
On the Pictured Rocks National Lakeshore, Michigan, black spruce is a minor species in mixedwood forests. The historical MFRI for the area is 21.8 years [257], which is too frequent to support black spruce stands (see Cone and seed production).
Northeast: Little information was available on fire regimes of black spruce communities in this region as of 2014, but since black spruce communities are wet, it is likely that fires are infrequent. In northwestern Maine, black spruce forests have an approximate 145-year fire-return interval. Between 20 to 200 acres (10-100 ha) usually burn [228]. Fires can be large in extreme fire years. In southwestern Maine, a 1947 wildfire burned 130,000 acres (53,000 ha) of forestland, about 580 acres (230 ha) of which was balsam fir with black spruce and/or red spruce [31]. Fires on deep, coarse glacial outwash can be intense, and black spruce communities on glacial outwash tend to remain in the heath-lichen stage of postfire succession longer than is typical for black spruce types of Maine [228].
Fire regimes of black spruce communities in Maine are likely similar to those of southern Québec [43,74]. See the Eastern Canada section for information on fire regimes of black spruce communities in Québec.
The red maple-black spruce-highbush cranberry vegetation association of New Jersey rarely burns. It is classified in Fuel Model 0: the ground is saturated and the probability of the vegetation carrying fire is very low [321].
Canada: Throughout much of boreal Canada, spruce stands burn at 50- to 150-year intervals [167]. Across boreal Canada, large fires were least common in the Hudson Plains (0.24 fire/year/4,000 mile² (10,000 km²)) and most common in the Boreal Shield West (Central Plains) region (1.12 fires/year/4,000 mile²). Mean size of fires in the Taiga Plains (west-central) region was significantly larger (31,501 acres (12,748 ha)) than in the Boreal Plains (western interior) (15,458 acres 6,183 ha)) and Boreal Shield East (eastern interior) (12,955 acres (5,182 ha)) regions (P<0.001). Generally, large fires in communities with highly variable substrate moistures, such as black spruce, had proportionately more unburned islands within fire perimeters. Fire seasons arrived later in northern than southern regions. There was a shift toward more spring fires near human settlements [68].
Western Canada: This region has highly variable fire frequencies, types, and patterns. Fire-return intervals range from <20 [73] to >300 [54,356] years. Along the Dempster Highway in central Yukon, fire-return intervals in black spruce woodlands or adjacent shrub tundra communities ranged from 33 to over 300 years. The longest interval detected was 343 years, for a black spruce/carex sedge/fireweed stand [356]. Modeling indicates that black spruce-lichen woodlands of western Canada have a regime of ≥18% crown fire, 8% to 17% intermittent crown fire, and ≤7% surface fire. Intermittent crown fires are defined as those with discontinuous torching. They represent the transition zone between severe surface and active crowning fires, with flames extending from the forest floor to above tree crowns [9].
Studies in the Northwest Territories illustrate variations in fire regimes in black spruce ecosystems of western Canada. The fire season runs from about mid-June to mid-August. In the upper Mackenzie Valley and adjacent uplands, fires were considerably more frequent in jack pine stands (FRI range: 40-60 years) than in black spruce stands (100-200 years) [335]. Time since fire for 19 black spruce/bog blueberry woodlands near Inuvik ranged from 3 to 300 years; most stands were <100 years old [54].
Fire history studies of Nahanni National Park and the Mackenzie Bison Sanctuary, Northwest Territories, found the 2 areas had similar fire-return intervals and fuel loads despite important differences in weather, topography, and fire sizes. Both sites have open black spruce-white spruce boreal forests containing quaking aspen and paper birch. Climate at the Mackenzie Bison Sanctuary is strongly influenced by nearby Great Slave Lake and is harsher than that of Nahanni National Park. Mean fire-return interval of Nahanni National Park was 21.7 years from 1813 to 1974; for the Mackenzie Bison Sanctuary, it was 23.3 years from 1771 to 1977. Nahanni National Park averaged 0.084 large fire/year/200,000 acres (100,000 ha) from 1959 to 1999, while the Mackenzie Bison Sanctuary averaged 0.056 large fire/year/200,000 acres. Large fires were >500 acres (200 ha)). Nahannia National Park had more small fires, while the Mackenzie Bison Sanctuary had fewer but larger fires; this tended to even out the area burned. The authors speculated that steeper topography in Nahanni National Park may have increased fire frequency by increasing fuel continuity. They cautioned that these are conservative estimates, and fires may be more frequent than their results indicate [60].
The Caribou Range area of the Northwest Territories experiences short to moderate fire-return intervals, with discontinuous, infrequent fires in wet areas. From the mid-1970s to the early 1990s, this 1.38 million-acre (5.60 million ha) area averaged 34 fires/year; most were stand-replacement fires. Mean burn size was 226,200 acres (91,533 ha) [126]. From 1966 to 1975, Johnson and Rowe [191] documented 398 fires in the Caribou Range area, 89% of which were lightning-ignited. A total of 1.7 million acres (0.7 million ha) burned within those 10 years, with "large variation" in fire size across years. Mean fire-return intervals ranged from 55.5 to 101.1 years for 4 lacustrine sites. Some sites had not burned for about 150 years [192]. In black spruce-lichen communities of the area, fires did not always carry from open lichen patches into black spruce stands; these discontinuous fires remained on the surface and their severity was low [10].
On sandy soils in northeastern Alberta and northern Saskatchewan, fire-return intervals averaged 38 years (range: 28-54 years) in both upland jack pine-black spruce/bog Labrador tea/green reindeer lichen forests and more open, lowland jack pine-black spruce/bog Labrador tea/green reindeer lichen forests. Lowlands with no fire for ≥90 years showed a shift in groundlayer vegetation from green reindeer lichen to globose haircap moss, with an attendant increase in black spruce [73]. In Wood Buffalo National Park, northern Alberta, the fire-rotation interval for black spruce forests was 78 years: significantly longer than for jack pine or quaking aspen forests. Fire-rotation intervals increased significantly as distance from waterbreaks increased (P=0.05 for all variables) [233].
Eastern Canada: Fires are common and ecologically important in both spruce and mixedwood ecosystems of eastern Canada. Alexander and Euler [8] stated that the eastern boreal mixedwood forest "is a fire-dependent ecosystem that would lose its character, vigor, and faunal and floral diversity in the absence of fire". In boreal western Québec, 71% of the area burned from 1945 to 1998 was due to lightning, but most fires were small and started by humans (62% human ignition vs. 38% lightning) [249]. Generally, wildfires occur only during intervals of low precipitation or low relative humidity (<60%), which allows the litter layer to dry. Ignition and fire spread are most likely when high-atmospheric-pressure systems dominate for at least 3 days, during which rainfall is <1.5 mm. These conditions usually occur from late spring through early summer, after snowmelt but before deciduous leaves have fully flushed out (review by [70]. Because there are fewer flammable conifers in mixedwood stands, ignition is more difficult than in conifer stands [92].
Black spruce lowlands of eastern Canada may be too wet to burn in most years and therefore, have longer fire-return intervals than upland vegetation. In Quetico Parks, Ontario, Heinselman [168] found that fire sometimes skirted black spruce and black spruce-tamarack bogs on Laurentian uplands, glacial moraines, or outwashes. Fire-return intervals of upland areas were 20 to 40 years; he was unable to determine how often the black spruce bogs burned [168].
In Québec, black spruce tends to dominate in early postfire succession in mixed-conifer communities and in late postfire succession in mixedwood communities. In mixedwoods, short fire-return intervals favor hardwoods over black spruce and other conifers because the hardwood species sprout soon after fire, before conifers are old enough produce seed [8]. In southern and central Québec, where black spruce cooccurs with pines, aspens, and birches, black spruce is most common in areas that have moderate to long fire-return intervals [94], ranging from about 70 to 200 years. Balsam fir dominates areas with very long fire-return intervals. Day and Harvey [92] estimated fire-return intervals of approximately 75 years (SD 50) for boreal mixedwoods of southeastern Canada. Black spruces older than 150 to 200 years are scarce in mixedwoods. Lowland mixedwoods [8]—where black spruce is most prevalent—burn less often than upland mixedwoods [8]. In study in boreal eastern Québec, most of the landscape was occupied by mixedwood stands >150 old. Based on field data and modeling, black spruce was associated with sites that had relatively frequent fire (MFRI=164 years), while balsam fir was associated with sites that had infrequent fire (MFRI=722 years, P<0.0001 for both variables) [87]. For the Waswanipi region of central Québec, the fire-rotation interval between the 1940s to the 2000s was estimated at 132 to 153 years [239]. Near James Bay, fire-rotation intervals were estimated at 495 years for black spruce and 3,142 years for white spruce. Occurrence of black spruce was negatively correlated with time since fire (R = -0.75), while occurrence of white spruce was positively correlated with time since fire (R = 0.89) [312]. In boreal mixedwood lowlands of northeastern Québec, fire-return intervals are long enough to allow paludification to occur (see Successional Status). In the Lake Matagami Lowland region, time since fire for black spruce stands ranged from 160 to 1,585 years [20].
Studies in northern Québec show very long fire-return intervals at the boreal-arctic treeline and mostly vegetative regeneration. Black spruce occurred in scattered stands on sites that burned <600 years prior, while reindeer lichen tundra more was common on sites that had not burned for >1,500 years. This slow rate of succession suggests that black spruce communities rarely burn in this region (review by [313]). Tree ring data showed a black spruce-lichen woodland near arctic tree line had not burned for 850 to 900 years [318]. At the present arctic treeline near Chibougamau, fire frequency is low: estimated at 0.04 fire/year in the 20th century. Black spruce grows mostly as layering krummholz, with some stands originating >2,000 years prior [298]. At the northern treeline-tundra interface near Inukjuak, time since fire ranged from 100 to 1,800 years. Black spruces were regenerating solely by layering [227].
A study in a black spruce-lichen woodland in the Boniface River area of northern Québec found a MFRI of around 1,000 years. Black spruce had apparently cloned for 1,500 to 1,700 years before a wildfire deforested the area [25] in the mid-1500s. Carbon-14 dating showed the area had at least 3 fires in 2,500 years: around 350 BC, 10 BC, and in 1567 AD [27]. Black spruce did not regenerate after the 1567 fire; the prefire black spruce clones were likely unable to produce cones due to cold temperatures (see Cone and seed production). Presently, the postfire community is a treeless tundra community dominated by reindeer and other lichens. The authors attributed the type shift from black spruce woodland to lichen tundra to climate warming in the region [25,26,27].
A jack pine-black spruce community in the North Shore area of Québec had a MFRI of 75 years, averaged over the last 1,000 years. Intervals were frequent enough to maintain jack pine dominance and black spruce codominance; longer fire-return intervals would likely shift dominance to black spruce [316].
Spruce-fir and mixedwood ecosystems of eastern Canada often experience mosaic fires [8]. Boreal mixedwood landscapes are usually comprised of small to large patches of different-aged stands that originated after fire. Although black spruce density generally increases with time since fire in mixedwoods [94], closed-canopy black spruce stands rarely have enough time to develop before the next fire [92].
A study of mixed-conifer forests in south-central Québec found a pattern of large fires with long rotations. Over 2,000 miles² (5,000 km²), most of the landscape (55%) was old-growth black spruce-jack pine-paper birch/feather moss forest. Fire size averaged 25,282 acres (10,113 ha), with most detectable fires burning more than 20,000 acres (10,000 ha) (n=176 burns). The authors thought that more small fires occurred than had been detected. Mean fire-rotation interval averaged 247 years from 1734 to 2009 [38].
In a mixedwood forest in Timiskaming, southwestern Québec, no large fires were noted from 1950 to 2000, and <12,000 acres (5,000 ha) of the 1,000-mile² (2,500 km²) study area burned. Mean time since fire averaged 136 years (SD 60); approximately 52% of stands were older than 100 years. Balsam fir and yellow birch were the most common trees throughout the area, but black spruce was most common on xeric sites [150].
A dendrochronological study suggested that fire size and severity are important factors controlling the transition from boreal spruce-fir forests to southern mixedwood forests. Across 315 sites in northwestern Québec, fire frequency decreased around 1850, at the end of the Little Ice Age, compared to the previous 80 years. Fire frequency increased during the settlement period (1910-1920). From 1920 to 1945, there was a trend of large fires in southern mixedwood ecosystems; however, there were few fires in northern boreal spruce-fir ecosystems. Time since fire was not significantly different between the 2 ecosystems, although stands on xeric and surficial-deposit sites were younger than those on moist sites. After 1945, there were more fires in the south than the north, and fires were smaller in the south. Proportions of unburned land were similar for the ecosystems. Among all stands examined, black spruce dominated 25% of mixedwood and 90% of spruce-fir ecosystems, with 69% and 53% frequency, respectively. From 1953 through 1996, MFRIs were 90 years for the mixedwood and 85 years for the spruce-fir ecosystems, respectively; differences were not significant. There were no significant differences in fire weather parameters (for example, relative humidity) between the 2 ecosystems, although the mixedwoods had more lightning strikes [48].
Climate change effects on fire regimes: Fire-return intervals in boreal black spruce communities have lengthened and shortened with long-term patterns of climate cooling and warming, respectively. Sediment-core studies in southwestern Québec found that charcoal concentrations—a surrogate for fire frequency—increased greatly after the mid-Holocene. Analyses of cores from Lac á la Pessiére found MFRIs averaged 64 years (SE 55) from 3,300 to 1,300 BP and 439 years (SE 100) from 1,300 BP to present. Charcoal concentrations increased about 1,000 years earlier in northern boreal spruce-fir forests than in southern boreal mixedwood forests. The authors suggested climate triggered increases in fire frequencies, with cool, dry Pacific air masses creating drier conditions that were conductive to fire ignition and spread. Present-day old growth in the area is dominated by balsam fir and/or black spruce on mesic sites and black spruce on xeric sites [70].
The current trend of increasing fire frequencies and sizes in black spruce ecosystems is well documented (for example, [171,214,368]). A review reported that in the 2000s, 1.90 million acres (767,000 ha)/year of Alaska's boreal forest burned. This was a 50% increase from the 1940s to the 1990s. The area burned in late-season wildfires increased in the 1990s and 2000s. These late-season fires generally resulted in deeper burning of organic soil layers than had occurred previously. Poorly drained, black spruce-sphagnum sites did not experience the deep-burning ground fires [214]. See Fire regimes of Alaskan black spruce communities for further information on this topic.
On the Kenai Peninsula of Alaska, spruce bark beetles were a greater disturbance in black spruce forests than fire. However, the authors suggested that interactions of spruce bark beetle attacks and climate warming may lead to increased fire frequencies (abstract [41]).
Warming climate may not result in increased fire frequencies in parts of black spruce's range in the eastern United States and Canada. A review reported that fire frequency in boreal forests of western Québec has decreased since the end of Little Ice Age (~1850 AD) despite warmer temperatures. This has been attributed to influences of warm, moist air flows during the fire season. Based on surveys of burned sites across Canada, Flannigan and others [119] asserted that "Increased temperature alone does not necessarily translate into greater fire disturbance".
Studies at the arctic treeline in northern Québec showed that from around 300 to 1500 AD, relatively warm temperatures favored black spruce. Size of the black spruce ecosystem contracted during the Little Ice Age (1600s-1850). With warming temperatures in the 19th and 20th centuries, the extent of black spruce ecosystems has shrunk yet again, while open peatlands have expanded [315].
Treeline black spruce communities may recover very slowly after fire, because most regeneration at arctic treeline is from cloning rather than postfire seedling establishment. Based on current, slow rates of black spruce regeneration and postfire recovery at their study site, Payette and others [298] predicted that despite warming temperatures, eastern black spruce-lichen woodlands will not expand into tundra ecosystems of northeastern Canada.
FIRE MANAGEMENT CONSIDERATIONS:On Washington Creek Fire Study and Training Area near Fairbanks, Alaska, Viereck and others [394] found small prescribed fires created a mosaic pattern of different severities that was similar to that created by wildfires. The fires reduced the forest floor by 24% to 62% [394]. For details of this study, see this Research Project Summary: The effects of experimental fires in an Alaskan black spruce/feather moss community.
Experimental prescribed fires were used in the Caribou Range area of the Northwest Territories to relate head fire rate of spread to the Initial Spread Index component of the Canadian Forest Fire Weather Index System for the black spruce-lichen woodland fuel type. The ~160-year-old study forest was near Porter Lake. The canopy was mostly sparsely stocked, stunted black spruce, with some jack pine and paper birch; Easter snow lichen dominated the ground layer. The study design included point-ignition, line-ignition, and wildfire plots. Fires were ignited on 26 June 1982; ground, surface, and crown components were observed during the fires. Head fire rates of spread ranged from 2.0 to 168.6 feet (0.6-51.4 m)/minute, with frontal fire intensities of nearly 33,000 kW/m. Equations presented in the paper help predict of fire behavior in the black spruce-lichen woodland fuel type [10]. See the Research Paper of this study for full details.
After clearcutting black spruce in the Great Lakes states, slash is often broadcast burned to aid natural black spruce regeneration. Burning is generally recommended if there is heavy slash, a feather moss carpet, or abundant tall shrubs, grasses, or sedges [199]. An adequate seed supply is necessary for natural black spruce regeneration, and fuels—including slash, litter, peat, and mosses—need to be sufficiently dry to allow for severe burns [80]. In Minnesota, clearcutting followed by high-severity prescribed fire in early summer produced good black spruce regeneration [375]. Since heavy postfire seed dispersal occurs in postfire years 1 and 2, delaying logging until the second winter after fire may increase natural regeneration of black spruce [149,411]. Black spruce cannot regenerate if cone-bearing branches are removed off site or concentrated in landings [131].
Black spruce may regenerate poorly on sites that are logged without follow-up broadcast burning. In black spruce ecosystems of northeastern Ontario, a chronosequence study comparing stands on 4- to 90-year-old burns to stands that were logged 2 to 126 years prior (and not burned afterward) found that no logged stands had succeeded to black spruce. Quaking aspen and bigtooth aspen had greatest cover on logged sites. Balsam fir cover was higher on logged than on burned sites, although balsam fir was frequent on both sites. Black spruce cover was greatest on burned sites [72].
Tree harvest may be used as a fire surrogate on black spruce sites where prescribed fire is not possible or desired, although black spruce may decline with long-term fire exclusion [45,51]. See Management Considerations for information on this topic.
The seedbed and prefire understory composition can greatly affect black spruce regeneration after logging and/or prescribed burning. Aksamit and Irving [6] studied black spruce regeneration on 27 broadcast-burned clearcuts in northern Minnesota. They found that where sphagnum mosses dominated the ground layer before cutting, black spruce regenerated well regardless of treatment. In fact, adequate regeneration was obtained even without burning. Where feather mosses dominated the ground layer, prescribed burning was necessary for black spruce regeneration [6]. Johnston [199] suggested that to be effective on feather moss sites, prescribed fires are best conducted when 100- and 1,000-hour fuel moistures are <25%. However, burning under these conditions may lead to fire control and mop-up problems, and higher costs. Where speckled alder dominates the understory before logging, natural regeneration of black spruce after broadcast burning is "quite variable". Low-severity fires tend to favor black spruce regeneration, and more severe fires tend to favor tall shrubs [199].
Postfire splitting or checking reduces the salvage value of burned black spruces. In a boreal mixed-conifer forest of central Alberta, order of frequency of splitting (greatest to least) was: balsam fir, black spruce, white spruce, and lodgepole pine. For black spruce trees that split, most split vertically up the bole (53%) but some split in spirals (45%). Thirty-four percent of black spruces did not split. Trees were sampled in severely burned areas of a 1-year-old burn [296].
Salvage logging in burned black spruce communities alters wildlife habitat. Nappi and others [297] review the effects of salvage logging on wildlife biodiversity and provide guidelines for salvage logging on burned boreal sites.
Prescribed burning guidelines are available for managers in the Great Lakes region. Johnston [199] has outlined broadcast burning techniques for lowland black spruce in the Great Lakes states. Humrickhouse [178] presents a prescription for burning cutover black spruce peatlands of Minnesota. McRae [281] provides guidelines for prescribed burning in mixedwood communities in Ontario's Red Clay Belt, where fire spread can be problematic due to discontinuous fuels and many wet areas [281]. See Models for a list of publications providing equations and other tools helpful for predicting fuel loads and fire behavior in different parts of black spruce's range.
Fire Studies available in FEIS:Eastern dwarf mistletoe: Wildfire is the primary factor limiting spread of eastern dwarf mistletoe in unmanaged black spruce stands (review by [13]). Fires of sufficient intensity to kill eastern dwarf mistletoe do not hinder postfire establishment of black spruce [13]. Eastern dwarf mistletoe density tends to increase with stand age [166]. In eastern Minnesota, eastern dwarf mistletoe was not present in black spruce stands <30 years old (Anderson 1949 in [13]). Heinselman [164] stated that "because of the fire exclusion policy and private protection agencies and organizations, we are seeing a vast expansion of dwarf mistletoe in forest areas, particularly on species like black spruce."
 |
| Eastern dwarf mistletoe infection on black spruce. Photo taken in Minnesota by Steven Katovich, USDA Forest Service, Bugwood.org. |
 |
| Prescribed fire in black spruce slash to control eastern dwarf mistletoe and improve the seedbed for black spruce regeneration. Photo taken in Minnesota by Fred Baker, Utah State University, Bugwood.org. |
Prescribed fire helps control eastern dwarf mistletoe in black spruce stands [2]. For such control to be effective, burning must result in 100% black spruce mortality [2,135]. To ensure complete mortality where understories are sparse, live trees can be cut to increase fuels [181].
Eastern spruce budworm: Heinselman [164] speculated that in the Great Lakes area and eastern Canada, outbreaks of eastern spruce budworm may be more frequent than they were historically due to fire exclusion. Outbreaks are most common where old-growth spruce-fir forests are extensive. He wrote that "fire would have frequently curtailed the expansion of these shade-tolerant climax species" (spruces), favoring pine species. A random, but very real fire 'rotation' insured that few stands reached climax", thereby reducing frequency of eastern spruce budworm outbreaks [164].
Eastern spruce budworm outbreaks may increase fuels in black spruce communities [7]. In southern Québec, 7 black spruce stands originated after wildfire occurred shortly after an eastern spruce budworm outbreak that had left many standing dead conifers [141]. In Ontario, a mixed-conifer forest had a 5-year eastern spruce budworm outbreak (1972-1977). Biomass of woody fuels peaked 5 to 8 years after the outbreak (1982-1985). Stand structure changes and increased fuel loads were due to stand mortality, crown breakage, and windthrow. Fire potential was greatest during that time, with fire hazard gradually decreasing as surface fuels—mostly balsam fir debris—decomposed. The infested forest had a sparse eastern white pine-jack pine-white spruce overstory and an understory of budworm-defoliated, dead balsam fir and mostly defoliated, live black spruce [360].
Wildlife: Wildfires in black spruce communities create a mosaic of different forest ages and types, resulting in a mix of habitat types that support a wide variety of wildlife species and guilds. Among the benefits to wildlife [368]:
See Importance to Wildlife and Livestock for further information on this topic.
Very frequent fires: Black spruce normally seeds in prodigiously following fire, but it may be eliminated from an area if a second fire occurs before black spruce regeneration reaches cone-bearing age [1,263]. Lutz [263] wrote that in Alaska, "repeated fires at intervals shorter than 20 to 30 years (that is, seed-bearing age for black spruce) may result in replacement of the species by treeless communities of sedges, rushes, grasses, and low shrubs". Black spruce regenerated quickly following a 1923 fire in northern Ontario, but no black spruce seedlings were found after a second fire passed through the area in 1929 [283]. In northern British Columbia and southern Yukon, black spruce stands that burned when <25 years old tended to succeed to quaking aspen. For stands that burned when >75 years old, postfire black spruce recruitment tended to be correlated with prefire basal area of black spruce (P=0.02) [201].
Fire occurring within a few decades of an eastern spruce budworm outbreak may also result in poor black spruce recruitment because cone-bearing black spruces do not have sufficient time to recover from defoliation. For example, a 1920 wildfire burned in a black spruce/feather moss habitat type in central Québec. Although the history of eastern spruce budworm outbreaks on the study site was unknown, 3 documented eastern spruce budworm outbreaks occurred nearby between 1930 to 1992. In spring 1995, black spruce cover averaged 14%, but a wildfire that summer killed most black spruces on the study site. In 2011 (postfire year 16), black spruce's cover was only 3.5%. The authors suggested this poor recruitment was due to wildfires in 1920 and 1995, with successive eastern spruce budworm defoliations occurring between the wildfires [84].
Fire and climate change: In western North America, increased fire frequency and/or severity resulting from climate change may cause shifts from black spruce to hardwood types in the southern portion of black spruce's range [75,173,207] and from black spruce woodland to open tundra in the northern portion [30,35,75,315]. Studies in interior Alaska suggest that postfire succession is most likely to shift toward hardwoods when severe fire occurs on moderate- to well-drained sites [204].
Postfire permafrost melting can affect patterns of postfire succession in black spruce communities. In interior Alaska, failure of the permafrost layer to re-form after severe fire may result in type switches from black spruce to hardwood [75,173,207]. See Yoshikawa and others [408] for a discussion and model of fire effects on ground albedo and the active permafrost layer. The model was developed using data from the 1983 Rosie Creek Burn near the Alaska Range [408].
See the Fire regimes of Alaskan black spruce communities synthesis for more information on fire-climate interactions in Alaska.
A model developed for black spruce forests of the Waswanipi region of west-central Québec predicted that compared to wildfire activity from 1975 to 2005, the likelihood of August wildfires would double by 2100. Due to a predicted shift in the fire season, however, the likelihood of May wildfires was predicted to decrease by 20%. The total number of forest fires was predicted to increase slightly in the region [238].
Black spruce may become more abundant in southern boreal regions with climate warming. This trend has been noted in southern Québec. From 1948 to 1995, the total area occupied by black spruce within 16 sphagnum bog-black spruce mosaic bogs in the Bas-St Lawrence region increased from 22.5% to 56.5%. The authors attributed this change to a combination of climate warming, human-engineered drainage affecting portions of all 16 bogs, and wildfires in 11 of the 16 bogs [320].See Appendix C for links to FEIS reviews available for animal species mentioned in this section.
Many wildlife species are adapted to particular successional stages in black spruce communities. For example, moose [164,251,268], snowshoe hares [164,215], black-backed woodpeckers [176,222,294,298], and other woodpeckers [294,298] use early postfire stages. American martens use midseral [234] and late-seral [18,143,144] black spruce communities. Woodland caribou use late-seral black spruce communities as winter habitat and forage on arboreal lichens draping black spruce branches [77].
Some wildlife species move between and use different-aged black spruce stands. For example, woodland caribou use open burns when migrating and as predator escape routes [284]. A study in central-interior Alaska found snowshoe hares and Canada lynx preferred 30-year-old (midseral) black spruce forests and woodlands, but the authors noted that Canada lynx might use late-seral black spruce stands during denning and when snowshoe hare numbers are low [310].
Postfire population trends of mammals, birds, herptiles, fish, and arthropods using black spruce communities follow:
In north-central Ontario, deer mouse populations increased after logging and slash fires in black spruce forests. Southern red-back vole populations declined [277].
Short-term studies in the Great Lakes region showed postfire shifts in bird guilds, a temporary decrease in small mammals, habitat improvements for larger mammals, and no effect on fish populations. Surveys in northeastern Minnesota found foliage gleaners such as Blackburnian warbler were most important in a 70-year-old jack pine-black spruce stand. A wildfire burned the stand in August 1976. In May 1977, ground- and shrub-foragers such as gray-cheeked thrush had become most important [21]. A study conducted 3 years after a 1979 wildfire on the Seney National Wildlife Refuge, Michigan, also found postfire shifts in bird guilds. The fire created a mosaic of open bogs in lowlands interspersed with tamarack-black spruce-red maple peatlands on uplands. In postfire year 3, bird species that forage in early-seral forests, especially brown thrashers and song sparrows, used the upland peatlands. Small mammal numbers dropped the fall after the fire, and no small mammal species used burned areas more than unburned areas. Small mammal numbers increased 17-fold in the second postfire fall. By postfire year 3, American beavers were concentrating in alder thickets, which were dense with new sprouts. Likely due to heavy berry crops, survival of American black bear cubs was good in postfire year 3. An inventory in postfire year 2 showed no significant postfire drop in northern pike, yellow perch, or brown bullhead populations. This was attributed to the ability of remaining peat to hold silt and minerals, leaving the mineral content of the water similar to that of prefire levels [17].
 |
| Great gray owl partially hidden behind a black spruce bole. Photo ©Sparky Stensaas, www.ThePhotonaturalist.com. |
Newly burned black spruces provide foraging opportunities for insectivorous birds, snags for cavity nesters, and open structure for foraging raptors. A study comparing bird use on 1) unburned, 2) burned, and 3) burned and salvage logged sites in mixedwoods of Alberta found that the 3 sites supported distinctly different assemblages. Canopy nesters, cavity nesters, and insectivores (for example, yellow-rumped warbler, boreal chickadee, ovenbird, respectively) were most common in burns and least likely to use burned and salvage logged sites. Ground nesters, shrub nesters, omnivores, and habitat generalists (for example, white-throated sparrow, chipping sparrow, gray jay, and dark-eyed junco, respectively) were most common in burned and salvage logged sites. The mixedwoods were a mosaic of jack pine and quaking aspen on uplands and black spruce and tamarack on lowlands. The wildfire occurred in the summer of 1995, and salvage logging was conducted in postfire year 2. Bird surveys were conducted in the spring and summer of postfire year 3 [289].
Bird counts in black spruce/feather moss forests of southern Québec showed woodpeckers were abundant in early postfire succession. Secondary cavity nesters such as boreal chickadees and brown creepers were most abundant in mid- and late succession [258]. Overall, bird species diversity was 60% higher 30 years after a wildfire than before in a jack pine-black spruce forest in the Boundary Waters Canoe Area. From postfire years 1 through 5, the number of primary and secondary cavity nesters—such as black-backed woodpeckers and tree swallows, respectively—increased. Because shrub cover increased in early postfire years, the importance of shrub-foraging species such as white-throated sparrow and chipping sparrow also increased. The forest was 73 years old prior to the late-August, lightning-ignited fire that burned 554 acres (1,368 ha). Permanent plots had been established the year before the wildfire [156].
In boreal black spruce forests of east-central Alberta, northern hawk owls used burned snags in early postfire seres for perching and nesting. Their abundance was negatively associated with time since fire (P=0.05), and no northern hawk owls were detected in forests >110 years old. Based on surveys and a model, the authors suggested that burned black spruce forests were suitable northern hawk owl habitat only until around postfire year 8 [157].
Few studies were available on herptile use of black spruce communities. In a New York study, the eastern massasauga rattlesnake [194], a Federal Candidate species [377], was found exclusively within open portions of a black spruce-tamarack-heathland that had been logged and pile-burned a year prior [194].
Fire in subarctic black spruce/sphagnum forests apparently had little effect on small-lake fish populations of Alberta. Two years after summer wildfires, <3% of the variation in fish assemblages in 6 small lakes within a burned area was due to disturbance (fire alone or fire and salvage logging). The fish assemblages in the burned area were compared to those within an adjacent unburned area encompassing 9 lakes. The difference in population sizes was not statistically significant (P=0.18). However, white sucker populations were slightly higher [370] and artic grayling slightly lower [369] in lakes within the burned area than in lakes in the adjacent unburned area.
Arthropod guilds use burned and unburned black spruce habitats based, in part, on their foraging or hunting strategies. Several families of beetles (for example, Buprestidae) contain species that are obligate feeders on dead or decaying wood [140,298,341]. These beetles congregate in recent burns, and woodpeckers prey upon them. In black spruce/feather moss stands of northwestern Québec, wood-feeding beetles were found in areas that burned from low to high severity. They were most abundant where fire severity was low [298]. In Grand-Jardins Provincial Park, Québec, stalking spiders were positively associated with burns and clearcuts, while web builders were about equally common on burns, clearcuts, and undisturbed sites. Total spider species diversity was greater on burns and clearcuts than on unburned sites (P≤0.01 for all variables) [232].
Palatability and nutritional value: Wild ungulates and livestock rarely browse black spruce. Moose occasionally browse saplings [129,412], but white-tailed deer browse black spruce only under starvation conditions [129]. Barren grounds caribou use black spruce-lichen woodlands and black spruce/shrub forests as winter rangeland [345].
Black spruce is important browse for some small mammals and bird species. It is a major food of snowshoe hares, especially in winter. Snowshoe hares in interior Alaska consume black spruce's bark, twigs, and needles most heavily in fall and winter [407]. Near Yellowknife, Northwest Territories, they browsed the bark of charred black spruces more than expected based on availability (P>0.05). The study was conducted August, in a black spruce-paper birch-tamarack forest that had burned 1 year prior [357]. Spruce grouse feed entirely on spruce needles during winter. In Alaska, they eat spruce needles from early November through March [109].
See Ellison [109] for information on the nutritional content of black spruce needles.
Numerous mammals and birds feed on black spruce seed. Red squirrels harvest the cones and eat the seeds within [113,243,263,412]. Mice, voles, shrews, and chipmunks consume seeds off the ground. Chickadees, nuthatches, crossbills, grosbeaks, and the pine siskin extract seeds from open spruce cones and eat seeds off the ground [155,200].
Black spruce seeds do not provide as much energy as white spruce seeds. In Alaska, black spruce seeds averaged 6,053 cal/g, about 9% fewer calories than white spruce seeds [63].
Cover value: Black spruce provides good cover for moose. It often grows in dense stands and on moist substrates, conditions that provide cool bedding in summer [14]. Black spruce also provides good cover for spruce grouse [199]. In the Great Lakes states, spruce grouse use black spruce stands regularly [200].
The ruby-crowned kinglet, magnolia warbler, Cape May warbler, and ovenbird commonly nest in black spruces in the Great Lakes region [200].
VALUE FOR REHABILITATION OF DISTURBED SITES:Special procedures have been developed for removing black spruce seed from the semiserotinous cones [340]. Seeds retain their viability for several years when stored in sealed containers in a cool, dry environment [340]. They require no stratification prior to sowing, which is recommended soon after snowmelt [55]. On peatlands, black spruce seedling establishment is best when surface organic layers are exposed by burning or mechanical scarification. On upland sites, it is best to expose mineral soils before sowing [200].
On well-drained soils, 8- to 12-inch-tall (20-43 cm) bareroot transplants showed good growth and survival when planted directly into organic soil layers. Armson [23] recommended against removing soil organic layers when transplanting black spruce on uplands. Transplant survival and growth are generally better following summer than spring outplanting [23]. In northeastern Alberta, overwinter survival of container-grown and transplanted black spruce seedlings was "satisfactory" on amended oil sand tailings [114].
Black spruce can be propagated from root cuttings [23].
OTHER USES:A study across 30,956 miles² (57,332 km²) in eastern Québec found that generally, balsam fir dominated stands originated after clearcutting; quaking aspen and paper birch dominated stands originating after clearcutting followed by wildfire; and black spruce dominated stands originating after fire (prescribed or wild). Sites subjected to clearcutting from 1920 to 2000 or to burning from 1800 to 2000 were selected for study [61].
Not all studies indicate that fire is important for black spruce regeneration. In a mixedwood forest near Thunder Bay, Ontario, black spruce establishment was greater 5 to 15 years after clearcutting (~190 trees/ha) than 5 to 15 years after fire (~60 trees/ha). The researchers concluded that for black spruce, the "natural regeneration process after fire appears to be emulated by clearcutting". Density of black spruce after disturbance was positively related to its density before disturbance (P<0.001) [180].
Eastern dwarf mistletoe: Eastern dwarf mistletoe is the most serious disease of black spruce in the Great Lakes states and eastern Canada [129,395]. A 1986 publication estimated that 10% to 20% of black spruce in the Great Lakes states was infested with eastern dwarf mistletoe [135]. It is less frequent in the West, and it is absent in northwestern Canada and Alaska [129,376,395]. Infection results in reduced vigor, witches' brooms, deformed trees, and death. Control is possible through silvicultural treatments and use of fire [129,395] (see Fire Management Considerations).
Other damaging agents: Black spruce is susceptible to numerous needle rusts, fungi, and insect infestations that result in defoliation, reduced vigor, or death. These diseases usually remain at low levels but may become epidemic [395]. Wind breakage caused by butt and heart rots is common in 70- to 100-year-old stands on uplands and 100- to 130-year-old stands on low peatlands [129,199]. The eastern spruce budworm defoliates black spruce; however, black spruce is less susceptible than balsam fir, white spruce, or red spruce. Black spruce trees most likely to be attacked are those growing with balsam fir and white spruce [129]. Numerous other insects attack black spruce but only occasionally cause serious damage [129,395].
Climate change and distributions of black spruce communities: Black spruce and adjacent boreal ecosystems are expected to be disproportionately affected by climate change (for example, [75,76,85]). With warming temperatures, black spruce is apparently expanding into some tundra communities (e.g., [30,35,75]). In some boreal regions, reduced permafrost layers have favored hardwood species at the expense of black spruce [75,173,207]. Some boreal wetlands are drying out, reducing the area available for black spruce bogs. In lowlands of the Kenai Peninsula, Alaska, cover of black spruce-white spruce-quaking aspen forests increased while cover of open wetlands decreased from 1950 to 1996, and two-thirds of water bodies showed loss of area. The authors stated that "it appears likely that climate change is driving wetland drying and vegetative shifts to predominantly woodland and forest" [221]. See Fire Management Considerations and Fire regimes of Alaskan black spruce communities for further discussions on this topic.
Across the western half of the southern boreal forest region, climate change could limit peatland development in black spruce ecosystems. This may limit black spruce recruitment. Because peatlands develop when water levels remain high enough to support anaerobic conditions and a slow rate of peat decay, they cannot develop or subsist under extended drought [172,184]. In southwestern Saskatchewan, moisture deficiencies during the seedling stage may increase rates of black spruce seedling mortality [172].| Fire regime information on vegetation communities in which black spruce may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [231], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||
|
|||||||
| Great Lakes | |||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||
| Great Lakes Forested | |||||||
| Conifer lowland (embedded in fire-prone ecosystem) | Replacement | 45% | 120 | 90 | 220 | ||
| Mixed | 55% | 100 | |||||
| Conifer lowland (embedded in fire-resistant ecosystem) | Replacement | 36% | 540 | 220 | >1,000 | ||
| Mixed | 64% | 300 | |||||
| Eastern white pine-eastern hemlock | Replacement | 54% | 370 | ||||
| Mixed | 12% | >1,000 | |||||
| Surface or low | 34% | 588 | |||||
| Great Lakes floodplain forest | Mixed | 7% | 833 | ||||
| Surface or low | 93% | 61 | |||||
| Great Lakes pine forest, eastern white pine-eastern hemlock (frequent fire) | Replacement | 52% | 260 | ||||
| Mixed | 12% | >1,000 | |||||
| Surface or low | 35% | 385 | |||||
| Great Lakes pine forest, jack pine | Replacement | 67% | 50 | ||||
| Mixed | 23% | 143 | |||||
| Surface or low | 10% | 333 | |||||
| Great Lakes spruce-fir | Replacement | 100% | 85 | 50 | 200 | ||
| Maple-basswood | Replacement | 33% | >1,000 | ||||
| Surface or low | 67% | 500 | |||||
| Maple-basswood mesic hardwood forest (Great Lakes) | Replacement | 100% | >1,000 | >1,000 | >1,000 | ||
| Minnesota spruce-fir (adjacent to Lake Superior and Drift and Lake Plain) | Replacement | 21% | 300 | ||||
| Surface or low | 79% | 80 | |||||
| Northern hardwood-eastern hemlock forest (Great Lakes) | Replacement | 99% | >1,000 | ||||
| Northern hardwood maple-beech-eastern hemlock | Replacement | 60% | >1,000 | ||||
| Mixed | 40% | >1,000 | |||||
| Red pine-eastern white pine (frequent fire) | Replacement | 38% | 56 | ||||
| Mixed | 36% | 60 | |||||
| Surface or low | 26% | 84 | |||||
| Red pine-eastern white pine (less frequent fire) | Replacement | 30% | 166 | ||||
| Mixed | 47% | 105 | |||||
| Surface or low | 23% | 220 | |||||
| Northeast | |||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||
| Northeast Forested | |||||||
| Eastern white pine-northern hardwood | Replacement | 72% | 475 | ||||
| Surface or low | 28% | >1,000 | |||||
| Northern hardwoods (Northeast) | Replacement | 39% | >1,000 | ||||
| Mixed | 61% | 650 | |||||
| Northern hardwoods-eastern hemlock | Replacement | 50% | >1,000 | ||||
| Surface or low | 50% | >1,000 | |||||
| Northern hardwoods-spruce | Replacement | 100% | >1,000 | 400 | >1,000 | ||
| Northeast spruce-fir forest | Replacement | 100% | 265 | 150 | 300 | ||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [36,230]. |
|||||||
| Common name | Scientific name |
| Lichens | |
| alpine reindeer lichen | Cladonia stellaris |
| cetraria lichens | Cetraria spp. |
| Easter snow lichen | Stereocaulon paschale |
| felt lichens | Peltigera spp. |
| Fremont's horsehair lichen | Bryoria fremontii |
| gray reindeer lichen | Cladonia rangiferina |
| green reindeer lichen | Cladonia mitis |
| old man's beard | Usnea subfloridana |
| reindeer lichens | Cladonia spp. |
| snow lichens | Stereocaulon spp. |
| toothed felt lichen | Peltigera canina |
| Bryophytes | |
| calliergon mosses | Calliergon spp. |
| common liverwort | Marchantia polymorpha |
| dark sphagnum | Sphagnum fuscum |
| deceptive sphagnum | Sphagnum fallax |
| feather mosses | Hylocomiaceae |
| fine sphagnum | Sphagnum subtile |
| fire moss | Ceratodon purpureus |
| Girgensohn's sphagnum | Sphagnum girgensohnii |
| globose haircap moss | Polytrichum piliferum |
| haircap mosses | Polytrichum spp. |
| hairy-leaved sphagnum | Sphagnum capillifolium |
| juniper haircap moss | Polytrichum juniperinum |
| knight's-plume moss | Ptilium crista-castrensis |
| Magellan's sphagnum | Sphagnum magellanicum |
| red sphagnum | Sphagnum rubellum |
| Schreber's moss | Pleurozium schreberi |
| sphagnums | Sphagnum spp. |
| splendid feather moss | Hylocomium splendens |
| Fern allies | |
| clubmosses | Lycopodium spp. |
| wood horsetail | Equisetum sylvaticum |
| Forbs | |
| twinflower | Linnaea borealis |
| Graminoids | |
| carex sedges | Carex spp. |
| cottongrasses | Eriophorum spp. |
| grasses | Poaceae |
| sedges | Cyperaceae |
| threeseeded sedge | Carex trisperma |
| Shrubs | |
| alders | Alnus spp. |
| arctic dwarf birch | Betula nana subsp. exilis |
| black crowberry | Empetrum nigrum |
| black highbush blueberry | Vaccinium fuscatum |
| black huckleberry | Gaylussacia baccata |
| blueberries | Vaccinium spp. |
| bog blueberry | Vaccinium uliginosum |
| bog Labrador tea | Ledum groenlandicum |
| bog rosemary | Andromeda polifolia |
| bog-laurel | Kalmia polifolia |
| broom crowberry | Corema conradii |
| bunchberry | Cornus canadensis |
| catberry | Ilex mucronata |
| crowberries | Empetrum spp. |
| eastern dwarf mistletoe | Arceuthobium pusillum |
| ericaceous shrubs | Ericaceae |
| gray alder | Alnus incana |
| grayleaf willow | Salix glauca |
| highbush blueberry | Vaccinium corymbosum |
| Labrador teas | Ledumspp. |
| laurels | Kalmia spp. |
| leatherleaf | Chamaedaphne calyculata |
| low sweet blueberry | Vaccinium angustifolium |
| mountain azalea | Rhododendron canadense |
| mountain cranberry | Vaccinium vitis-idaea |
| mountain-avens | Dryas spp. |
| prickly rose | Rosa acicularis |
| sheep-laurel | Kalmia angustifolia |
| speckled alder | Alnus incana subsp. rugosa |
| swamp azalea | Rhododendron viscosum |
| sweetgale | Myrica gale |
| thinleaf alder | Alnus incana subsp. tenuifolia |
| willows | Salix spp. |
| Trees | |
| balsam fir | Abies balsamea |
| balsam poplar | Populus balsamifera subsp. balsamifera |
| beeches | Fagus spp. |
| bigtooth aspen | Populus grandidentata |
| birches | Betula spp. |
| black cherry | Prunus serotina |
| black cottonwood | Populus balsamifera subsp. trichocarpa |
| black spruce | Picea mariana |
| eastern hemlock | Tsuga canadensis |
| eastern white pine | Pinus strobus |
| hickories | Carya spp. |
| jack pine | Pinus banksiana |
| maples | Acer spp. |
| northern whitecedar | Thuja occidentalis |
| oaks | Quercus spp. |
| paper birch | Betula papyrifera |
| quaking aspen | Populus tremuloides |
| red maple | Acer rubrum |
| red spruce | Picea rubens |
| Rocky Mountain lodgepole pine | Pinus contorta var. latifolia |
| spruces | Picea spp. |
| subalpine fir | Abies lasiocarpa |
| sugar maple | Acer saccharum |
| tamarack | Larix laricina |
| white spruce | Picea glauca |
| yellow birch | Betula alleghaniensis |
| Common name | Scientific name |
| Mammals | |
| American beaver | Castor canadensis |
| American black bear | Ursus americanus |
| American marten | Martes americana |
| Canada lynx | Lynx canadensis |
| caribou | Rangifer tarandus |
| deer mouse | Peromyscus maniculatus |
| moose | Alces americanus |
| red squirrel | Tamiasciurus hudsonicus |
| snowshoe hare | Lepus americanus |
| Birds | |
| black-backed woodpecker | Picoides arcticus |
| great gray owl | Strix nebulosa |
1. A. D. Revill Associates. 1978. Ecological effects of fire and its management in Canada's national parks: a synthesis of the literature. Volume two, annotated bibliography. Ottawa, ON: Parks Canada, National Parks Branch, Natural Resources Division. 345 p. [3416]
2. Ahlgren, C. E. 1974. Effects of fires on temperate forests: north central United States. In: Kozlowski, T. T.; Ahlgren, C. E., eds. Fire and ecosystems. New York: Academic Press: 195-223. [7198]
3. Ahlgren, Clifford E. 1959. Some effects of fire on forest reproduction in northeastern Minnesota. Journal of Forestry. 57(3): 194-200. [208]
4. Ahlgren, Clifford E. 1960. Some effects of fire on reproduction and growth of vegetation in northeastern Minnesota. Ecology. 41(3): 431-445. [207]
5. Ahlgren, Clifford E. 1960. Vegetational development following burning in the northern coniferous forest of Minnesota. In: Proceedings, annual meeting of the Society of American Foresters; 1959 November 15-19; San Francisco, CA. Bethesda, MD: Society of American Foresters: 21-22. [29104]
6. Aksamit, Scott E.; Irving, Frank D. 1984. Prescribed burning for lowland black spruce regeneration in northern Minnesota. Canadian Journal of Forest Research. 14(1): 107-113. [7298]
7. Albert, Dennis A. 1995. Regional landscape ecosystems of Michigan, Minnesota, and Wisconsin: a working map classification--4th revision: July 1994. Gen. Tech. Rep. NC-178. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 250 p. [27980]
8. Alexander, M. E.; Euler, D. L. 1981. Ecological role of fire in the uncut boreal mixedwood forest. In: Whitney, R. D.; McClain, K. M., compilers. Boreal mixedwood symposium. Proceedings of a symposium; 1980 September 16-18; Thunder Bay, ON. COJFRC Symposium Proceedings O-P-9. Sault Ste. Marie, ON: Environment Canada, Canadian Forestry Service, Great Lakes Forestry Research Centre: 42-64. [13597]
9. Alexander, M. E.; Lanoville, R. A. 1989. Predicting fire behavior in the black spruce-lichen woodland fuel type of western and northern Canada. Edmonton, AB: Forestry Canada, Northern Forestry Centre; Fort Smith, NT: Government of the Northwest Territories, Department of Renewable Resources, Territorial Forest Fire Centre. Poster. [21077]
10. Alexander, M. E.; Stocks, B. J.; Lawson, B. D. 1991. Fire behavior in black spruce-lichen woodland: the Porter Lake project. NOR-X-310. Edmonton, AB: Forestry Canada, Northwest Region, Northern Forestry Centre. 44 p. [18823]
11. Alexander, Martin E. 1980. Forest fire history research in Ontario: a problem analysis. In: Stokes, Marvin A.; Dieterich, John H., technical coordinators. Proceedings of the fire history workshop; 1980 October 20-24; Tucson, AZ. Gen. Tech. Rep. RM-81. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 96-109. [16049]
12. Alexander, Martin E.; Cruz, Miguel G. 2006. Evaluating a model for predicting active crown fire rate of spread using wildfire observations. Canadian Journal of Forest Research. 36(11): 3015-3028. [67213]
13. Alexander, Martin E.; Hawksworth, Frank G. 1975. Wildland fires and dwarf mistletoes: a literature review of ecology and prescribed burning. Gen. Tech. Rep. RM-14. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 12 p. [15583]
14. Allen, Arthur W.; Jordan, Peter A.; Terrell, James W. 1987. Habitat suitability index models: moose--Lake Superior region. Biol. Rep. 82 (10.155). Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service. 47 p. [11710]
15. Allison, Lorraine. 1979. The relationship of fire and wildlife to fire management in the Northwest Territories. Fire Management Contract Report No. 1. [Place of publication unknown]: Government of the Northwest Territories, Northwest Territories Wildlife Service. 45 p. [8397]
16. Amoroso, Mariano M.; Daniels, Lori D.; Bataineh, Mohammad; Andison, David W. 2011. Evidence of mixed-severity fires in the foothills of the Rocky Mountains of west-central Alberta, Canada. Forest Ecology and Management. 262(12): 2240-2249. [84144]
17. Anderson, Stanley H. 1978. Wildlife habitat changes following 1976 wildfire on the Seney National Wildlife Refuge. Washington, DC: U.S. Department of the Interior, U.S. Fish and Wildlife Service. 4 p. [12127]
18. Andruskiw, Mark; Fryxell, John M.; Thompson, Ian D.; Baker, James A. 2008. Habitat-mediated variation in predation risk by the American marten. Ecology. 89(8): 2273-2280. [76045]
19. Angers, Virginie A.; Gaulthier, Sylvie; Drapeau, Pierre; Jayen, Karelle; Bergeron, Yves. 2011. Tree mortality and snag dynamics in North American boreal tree species after a wildfire: a long-term study. International Journal of Wildland Fire. 20(6): 751-763. [84626]
20. Angers, Virginie Arielle; Drapeau, Pierre; Bergeron, Yves. 2010. Snag degradation pathways of four North American boreal tree species. Forest Ecology and Management. 259(3): 246-256. [80800]
21. Apfelbaum, Steven; Haney, Alan. 1981. Bird populations before and after wildfire in a Great Lakes pine forest. The Condor. 83(4): 347-354. [8556]
22. Archibald, David J.; Baker, William D. 1989. Prescribed burning for black spruce regeneration in northwestern Ontario. Technical Report #14. Thunderbay, ON: Ontario Ministry of Natural Resources, Northwestern Ontario Forest Technology Development Unit. 21 p. [45593]
23. Armson, K. A. 1975. Establishment and early development of black spruce. In: Fraser, J. W.; Jeglum, J. K.; Ketcheson, D. E.; Robinson, F. C.; Van Bers H. P. G.; McLain, K. M.; Auld, J. M., tech. coords. Black spruce symposium; 1975 September 23-25; Thunder Bay, ON. Symposium Proceedings 0-P-4. Sault Ste. Marie, ON: Department of the Environment, Canadian Forestry Service, Great Lakes Forest Research Centre: 45-60. [8825]
24. Arseneault, Dominique. 2001. Impact of fire behavior on postfire forest development in a homogeneous boreal landscape. Canadian Journal of Forest Research. 31(8): 1367-1374. [66204]
25. Arseneault, Dominique; Payette, Serge. 1992. A postfire shift from lichen-spruce to lichen-tundra vegetation at tree line. Ecology. 73(3): 1067-1081. [18741]
26. Arseneault, Dominique; Payette, Serge. 1997. Landscape change following deforestation at the Arctic tree line in Quebec, Canada. Ecology. 78(3): 693-706. [85731]
27. Arseneault, Dominique; Payette, Serge. 1997. Reconstruction of millennial forest dynamics from tree remains in a subarctic tree line peatland. Ecology. 78(6): 1873-1883. [29229]
28. Auclair, A. N. D. 1983. The role of fire in lichen-dominated tundra and forest-tundra. In: Wein, Ross W.; MacLean, David A., eds. The role of fire in northern circumpolar ecosystems. Scope 18. New York: John Wiley & Sons: 235-256. [18510]
29. Auclair, Allan N. D. 1985. Postfire regeneration of plant and soil organic pools in a Picea mariana-Cladonia stellaris ecosystem. Canadian Journal of Forest Research. 15(1): 279-291. [66004]
30. Bachelet, D.; Lenihan, J.; Neilson, R.; Drapek, R.; Kittel, T. 2005. Simulating the response of natural ecosystems and their fire regimes to climatic variability in Alaska. Canadian Journal of Forest Research. 35(9): 2244-2257. [85380]
31. Banks, Wayne G.; Rettie, James C. 1949. Restocking conditions on the burned-over forest lands of southwestern Maine, June 1949. Station Paper No. 30. Upper Darby, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 9 p. [29096]
32. Barney, R. J.; Stocks, B. J. 1983. Fire frequencies during the suppression period. In: Wein, Ross W.; MacLean, David A., eds. The role of fire in northern circumpolar ecosystems. New York: John Wiley & Sons: 45-61. [12079]
33. Barney, Richard J.; Bevins, Collin D.; Bradshaw, Larry S. 1981. Forest floor fuel loads, depths, and bulk densities in four interior Alaskan cover types. Res. Note INT-304. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 7 p. [8182]
34. Barney, Richard J.; Van Cleve, Keith, Schlentner, Robert. 1978. Biomass distribution and crown characteristics in two Alaskan Picea mariana ecosystems. Canadian Journal of Forest Research. 8(1): 36-41. [8163]
35. Barrett, K.; Kasischke, E. S.; McGuire, A. D.; Turetsky, M. R.; Kane, E. S. 2010. Modeling fire severity in black spruce stands in the Alaskan boreal forest using spectral and non-spectral geospatial data. Remote Sensing of Environment. 114(7): 1494-1503. [85401]
36. Barrett, S.; Havlina, D.; Jones, J.; Hann, W.; Frame, C.; Hamilton, D.; Schon, K.; Demeo, T.; Hutter, L.; Menakis, J. 2010. Interagency Fire Regime Condition Class Guidebook. Version 3.0, [Online]. In: Interagency Fire Regime Condition Class (FRCC). U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior; The Nature Conservancy (Producers). Available: http://www.frcc.gov/ [2013, May 13]. [85876]
37. Begin, Yves; Marguerie, Dominique. 2002. Characterization of tree macroremains production in a recently burned conifer forest in northern Quebec, Canada. Plant Ecology. 159(2): 143-152. [45872]
38. Belisle, Annie Claude; Gauthier, Silvie; Cyr, Dominic; Bergeron, Yves; Morin, Hubert. 2011. Fire regime and old-growth boreal forests in central Quebec, Canada: an ecosystem management perspective. Silva Fennica. 45(5): 889-908. [84908]
39. Benscoter, Brian W.; Vitt, Dale H.; Wieder, R. Kelman. 2005. Association of postfire peat accumulation and microtopography in boreal bogs. Canadian Journal of Forest Research. 35(9): 2188-2193. [61539]
40. Benzie, John W.; Blum, Barton M. 1989. Silviculture of northeastern conifers. In: Burns, Russell M., compiler. The scientific basis for silvicultural and management decisions in the National Forest System. Gen. Tech. Rep. WO-55. Washington, DC: U.S. Department of Agriculture, Forest Service: 18-30. [10243]
41. Berg, Edward E.; Anderson, R. S.; De Volder, A. D. 2003. Fire and spruce bark beetle disturbance regimes on the Kenai Peninsula, Alaska, [Online]. In: Proceedings, 2nd international wildland fire ecology and fire management congress held concurrently with the 5th symposium on fire and forest meteorology; 2003 November 16-20; Orlando, FL. Boston, MA: American Meteorology Society (Producer): Available: http://ams.confex.com/ams/FIRE2003/techprogram/paper_65661.htm [2014, April 17]. [64198]
42. Berger, Carrie A.; Gilmore, Daniel W. 2003. Germination and survival of spruce seedlings following fire in northwestern Alberta. Northern Journal of Applied Forestry. 20(1): 45-47. [44059]
43. Bergeron, Yves. 2000. Species and stand dynamics in the mixed woods of Quebec's southern boreal forest. Ecology. 81(6): 1500-1516. [37017]
44. Bergeron, Yves. 2004. Is regulated even-aged management the right strategy for the Canadian boreal forest? The Forestry Chronicle. 80(4): 458-462. [55789]
45. Bergeron, Yves; Cyr, Dominic; Drever, C. Ronnie; Flannigan, Mike; Gauthier, Sylvie; Kneeshaw, Daniel; Lauzon, Eve; Leduc, Alain; Le Goff, Heloise; Lesieur, Daniel; Logan, Kimberley. 2006. Past, current, and future fire frequencies in Quebec's commercial forests: implications for the cumulative effects of harvesting and fire on age-class structure. Canadian Journal of Forest Research. 36(11): 2737-2744. [67198]
46. Bergeron, Yves; Dubuc, Michelle. 1989. Succession in the southern part of the Canadian boreal forest. Vegetatio. 79(1-2): 51-63. [5042]
47. Bergeron, Yves; Fenton, Nicole J. 2012. Boreal forests of eastern Canada revisited: old growth, nonfire disturbances, forest succession, and biodiversity. Botany. 90(6): 509-523. [87230]
48. Bergeron, Yves; Gauthier, Sylvie; Flannigan, Mike; Kafka, Victor. 2004. Fire regimes at the transition between mixedwood and coniferous boreal forest in northwestern Quebec. Ecology. 85(7): 1916-1932. [50076]
49. Bergeron, Yves; Gauthier, Sylvie; Kafka, Victor; Lefort, Patrick; Lesieur, Daniel. 2001. Natural fire frequency for the eastern Canadian boreal forest: consequences for sustainable forestry. Canadian Journal of Forest Research. 31(3): 384-391. [86886]
50. Bergeron, Yves; Leduc, Alain. 1998. Relationships between change in fire frequency and mortality due to spruce budworm outbreak in the southeastern Canadian boreal forest. Journal of Vegetation Science. 9(4): 492-500. [30345]
51. Bergeron, Yves; Leduc, Alain; Harvey, Brian D.; Gauthier, Sylvie. 2002. Natural fire regime: a guide for sustainable management of the Canadian boreal forest. Silva Fennica. 36(1): 81-95. [45874]
52. Berglund, Erwin R.; Barney, Richard J. 1977. Air temperature and wind profiles in an Alaskan lowland black spruce stand. Research Note PNW-305. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 12 p. [85738]
53. Bernhardt, Emily Louise. 2008. The effects of fire severity and site moisture on species composition and functional properties of black spruce forests in interior Alaska. Fairbanks, AK: University of Alaska Fairbanks. 124 p. Thesis. [85608]
54. Black, R. A.; Bliss, L. C. 1978. Recovery sequence of Picea mariana - Vaccinium uliginosum forests after burning near Inuvik, Northwest Territories, Canada. Canadian Journal of Botany. 56(17): 2020-2030. [7448]
55. Black, R. Alan; Bliss, L. C. 1980. Reproductive ecology of Picea mariana (Mill.) BSP., at tree line near Inuvik, Northwest Territories, Canada. Ecological Monographs. 50(3): 331-354. [8413]
56. Bonan, Gordon B.; Shugart, Herman H. 1989. Environmental factors and ecological processes in boreal forests. Annual Review of Ecology and Systematics. 20: 1-28. [14170]
57. Bond-Lamberty, B.; Wang, C.; Gower, S. T.; Norman, J. 2002. Leaf area dynamics of a boreal black spruce fire chronosequence. Tree Physiology. 22(14): 993-1002. [43580]
58. Bond-Lamberty, Ben; Gower, Stith T. 2008. Decomposition and fragmentation of coarse woody debris: re-visiting a boreal black spruce chronosequence. Ecosystems. 11(6): 831-840. [85742]
59. Bond-Lamberty, Ben; Wang, Chuankuan; Gower, Stith T. 2004. Net primary production and net ecosystem production of a boreal black spruce wildfire chronosequence. Global Change Biology. 10(4): 473-487. [48379]
60. Bothwell, P. M.; de Groot, W. J.; Dube, D. E.; Chowns, T.; Carlsson, D. H.; Stefner, C. N. 2004. Fire regimes in Nahanni National Park and the Mackenzie Bison Sanctuary, Northwest Territories, Canada. In: Engstrom, R. Todd; Galley, Krista E. M.; de Groot, William J., eds. Fire in temperate, boreal, and montane ecosystems: Proceedings of the 22nd Tall Timbers fire ecology conference: an international symposium; 2001 October 15-18; Kananaskis Village, AB. No. 22. Tallahassee, FL: Tall Timbers Research: 43-54. [52302]
61. Bouchard, Mathieu; Pothier, David. 2011. Long-term influence of fire and harvesting on boreal forest age structure and forest composition in eastern Quebec. Forest Ecology and Management. 261(4): 811-820. [85744]
62. Boulanger, Yan; Sirois, Luc. 2006. Postfire dynamics of black spruce coarse woody debris in northern boreal forest of Quebec. Canadian Journal of Forest Research. 36(7): 1770-1780. [67206]
63. Brink, C. Holden; Dean, Frederick C. 1966. Spruce seed as a food of red squirrels and flying squirrels in interior Alaska. The Journal of Wildlife Management. 30(3): 503-512. [13253]
64. Brown, Carissa D.; Johnstone, Jill F. 2012. Once burned, twice shy: repeat fires reduce seed availability and alter substrate constraints on Picea mariana regeneration. Forest Ecology and Management. 266: 34-41. [84139]
65. Brown, Marjie. 2008. Fire and ice: fire severity of future flammability in Alaskan black spruce forests. Joint Fire Science Program Fire Science Brief. In: JFSP Project Details--Project: 05-1-2-06. Boise, ID: Joint Fire Science Program. 6 p. Available online: http://www.firescience.gov/projects/briefs/05-1-2-06_FSBrief10.pdf [2011, January 6]. [81494]
66. Brumelis, G.; Carleton, T. J. 1989. The vegetation of post-logged black spruce lowlands in central Canada. II. Understory vegetation. Journal of Applied Ecology. 26(1): 321-339. [7864]
67. Bubier, Jill L. 1991. Patterns of Picea mariana (black spruce) growth and raised bog development in Victory Basin, Vermont. Bulletin of the Torrey Botanical Club. 118(4): 399-411. [66067]
68. Burton, Philip J.; Parisien, Marc-Andre; Hicke, Jeffrey A.; Hall, Ronald J.; Freeburn, Jason T. 2008. Large fires as agents of ecological diversity in the North American boreal forest. International Journal of Wildland Fire. 17(6): 754-767. [81052]
69. Calef, M. P.; McGuire, A. D.; Chapin, F. S., III. 2008. Human influences on wildfire in Alaska from 1988 through 2005: an analysis of the spatial patterns of human impacts. Earth Interactions. 12(1): 1-17. [77035]
70. Carcaillet, Christopher; Bergeron, Yves; Richard, Pierre J. H.; Frechette, Bianca; Gauthier, Sylvie; Prairie, Yves T. 2001. Change of fire frequency in the eastern Canadian boreal forests during the Holocene: does vegetation composition or climate trigger the fire regime? Journal of Ecology. 89(6): 930-946. [40141]
71. Carleton, T. J.; Wannamaker, Brenda A. 1987. Mortality and self-thinning in postfire black spruce. Annals of Botany. 59(6): 621-628. [7879]
72. Carleton, Terence J.; MacLellan, Patricia. 1994. Woody vegetation responses to fire versus clear-cutting logging: a comparative survey in the central Canadian boreal forest. Ecoscience. 1(2): 141-152. [26054]
73. Carroll, S. B.; Bliss, L. C. 1982. Jack pine - lichen woodland on sandy soils in northern Saskatchewan and northeastern Alberta. Canadian Journal of Botany. 60(11): 2270-2282. [7283]
74. Chandler, Craig; Cheney, Phillip; Thomas, Philip; Trabaud, Louis; Williams, Dave. 1983. Fire in forestry: Vol. I. Forest fire behavior and effects. New York: John Wiley & Sons. 450 p. [12241]
75. Chapin, F. S., III; McGuire, A. D.; Ruess, R. W.; Hollingsworth, T. N.; Mack, M. C.; Johnstone, J. F.; Kasischke, E. S.; Euskirchen, E. S.; Jones, J. B.; Jorgenson, M. T.; Kielland, K.; Kofinas, G. P.; Turetsky, M. R.; Yarie, J.; Lloyd, A. H.; Taylor, D. 2010. Resilience of Alaska's boreal forest to climatic change. Canadian Journal of Forest Research. 40(7): 1360-1370. [82445]
76. Chapin, F. Stuart, III; Shaver, Gaius R.; Giblin, Anne E.; Nadelhoffer, Knute J.; Laundre, James A. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology. 76(3): 694-711. [66118]
77. Chapin, F. Stuart, III; Trainor, Sarah F.; Huntington, Orville; Lovecraft, Amy L.; Zavaleta, Erika; Natcher, David C.; McGuire, A. David; Nelson, Joanna L.; Ray, Lily; Calef, Monika; Fresco, Nancy; Huntington, Henry; Rupp, T. Scott; DeWilde, La'ona; Naylo. 2008. Increasing wildfire in Alaska's boreal forest: pathways to potential solutions of a wicked problem. BioScience. 58(6): 531-540. [71423]
78. Charron, I; Greene, D. F. 2002. Post-wildfire seedbeds and tree establishment in the southern mixedwood boreal forest. Canadian Journal of Forest Research. 32(9): 1607-1615. [43075]
79. Christiansen, Janet S. 1988. A spruce-lichen woodland in northern Alaska: post-fire regeneration and community dynamics. Seattle, WA: University of Washington. 94 p. Thesis. [85885]
80. Chrosciewicz, Z. 1976. Burning for black spruce regeneration on a lowland cutover site in southeastern Manitoba. Canadian Journal of Forest Research. 6(2): 179-186. [7280]
81. Chrosciewicz, Z. 1990. Post-cut burning and black spruce regeneration. In: Titus, B. D.; Lavigne, M. B.; Newton, P. F.; Meades, W. J., eds. The silvics and ecology of boreal spruces: 1989 IUFRO working party S1.05-12 symposium proceedings; 1989 August 12-17; Newfoundland, Canada. Forest Canada Information Report N-X-271. St. John's, NL: Forestry Canada, Newfoundland and Labrador Region: 35-44. [86441]
82. Coen, Janice; Mahalingham, Shankar; Daily, John. 2004. Infrared imagery of crown-fire dynamics during FROSTFIRE. Journal of Applied Meterology. 43(9): 1241-1259. [87188]
83. Coffman, Michael S.; Alyanak, Edward; Resovsky, Richard. 1980. Habitat classification system field guide: Northern Lake States region--Upper Peninsula of Michigan and northeast Wisconsin. Houghton, MI: Michigan Technological University. 112 p. [Developed by: Cooperative research on forest soils.]. [8997]
84. Cote, Damien; Girard, Francois; Hebert, Francois; Bouchard, Sylvie; Gagnon, Rejean; Lord, Daniel. 2013. Is the closed-crown boreal forest resilient after successive stand disturbances? A quantitative demonstration from a case study. Journal of Vegetation Science. 24(4): 664-674. [87168]
85. Cronan, James; McKenzie, Donald; Olson, Diana. [2012]. Fire regimes of the Alaska boreal forest. Draft manuscript. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 124 p. In cooperation with: Seattle, WA: University of Washington, School of Forest Resources; New Haven, CT: Yale School of Forestry and Environmental Studies; Moscow, ID: University of Idaho; Fairbanks, AK: U.S. Department of the Interior, Bureau of Land Management, Alaska Fire Service. Available online: http://www.frames.gov/documents/alaska/fire_history/fire_regimes_alaskan_boreal_forest_draft_gtr.zip [2012, September 4]. [85879]
86. Curtis, John T. 1959. The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press. 657 p. [7116]
87. Cyr, Dominic; Gauthier, Sylvie; Bergeron, Yves. 2012. The influence of landscape-level heterogeneity in fire frequency on canopy composition in the boreal forest of eastern Canada. Journal of Vegetation Science. 23(1): 140-150. [85755]
88. D'Amato, Anthony W.; Fraver, Shawn; Palik, Brian J.; Bradford, John B.; Patty, Laura. 2011. Singular and interactive effects of blowdown, salvage logging, and wildfire in sub-boreal pine systems. Forest Ecology and Management. 262(11): 2070-2078. [84141]
89. Damman, A. W. H. 1964. Some forest types of central Newfoundland and their relation to environmental factors. Forest Science Monograph 8. Washington, DC: Society of American Foresters. 62 p. [14281]
90. Damman, A. W. H.; Johnston, William F. 1980. Black spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 11-14. [49853]
91. Damman, Antoni W. H.; French, Thomas W. 1987. The ecology of peat bogs of the glaciated northeastern United States: a community profile. Biological Report 85(7.16). Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service, Research and Development, National Wetlands Research Center. 100 p. [9238]
92. Day, R. J.; Harvey, E. M. 1981. Forest dynamics in boreal mixedwood. In: Whitney, R. D.; McClain, K. M., compilers. Boreal mixedwood symposium. Proceedings of a symposium; 1980 September 16-18; Thunder Bay, ON. COJFRC Symposium Proceedings O-P-9. Sault Ste. Marie, ON: Environment Canada, Canadian Forestry Service, Great Lakes Forestry Research Centre: 29-41. [14204]
93. Day, R. J.; Woods, G. T. 1977. The role of wildfire in the ecology of jack and red pine forests in Quetico Provincial Park. Quetico Provincial Park fire ecology study report 5. Toronto, ON: Ontario Ministry of Natural Resources, North Central Region, Atikokan District. 79 p. [85878]
94. De Grandpre, Louis; Morissette, Jacques; Gauthier, Sylvie. 2000. Long-term post-fire changes in the northeastern boreal forest of Quebec. Journal of Vegetation Science. 11(6): 791-800. [40148]
95. De Volder, Andrew. 1999. Fire and climate history of lowland black spruce forests, Kenai National Wildlife Refuge, Alaska. Flagstaff, AZ: Northern Arizona University. 128 p. Thesis. [85887]
96. DeLong, S. Craig; Kessler, Winifred B. 2000. Ecological characteristics of mature forest remnants left by wildfire. Forest Ecology and Management. 131(1): 93-106. [35986]
97. DeWilde, La'ona; Chapin, F. Stuart, III. 2006. Human impacts on the fire regime of interior Alaska: interactions among fuels, ignition sources, and fire suppression. Ecosystems. 9(8): 1342-1353. [69884]
98. Donahue, William H. 1954. Some plant communities in the Anthracite Region of northeastern Pennsylvania. The American Midland Naturalist. 51(1): 203-231. [64481]
99. Drury, William H., Jr. 1956. Bog flats and physiographic processes in the upper Kuskokwim River region, Alaska. Contributions from the Gray Herbarium No. 178. Cambridge, MA: Harvard University, The Gray Herbarium. 127 p. [12996]
100. Dunlop, D. A. 1987. Community classification of the vascular vegetation of a New Hampshire peatland. Rhodora. 89(860): 415-440. [20275]
101. Dutro, Ruth; Cohoe, Edith. 1938. An ecological study of Wolf's Bog, Cheboygan County, Michigan. Transactions of the Kansas Academy of Science. 41(4): 87-95. [80285]
102. Dyrness, C. T. 1980. Black spruce-paper birch. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 85. [50021]
103. Dyrness, C. T.; Norum, Rodney A. 1983. The effects of experimental fires on black spruce forest floors in interior Alaska. Canadian Journal of Forest Research. 13(5): 879-893. [7299]
104. Dyrness, C. T.; Viereck, L. A.; Van Cleve, K. 1986. Fire in taiga communities of interior Alaska. In: Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Ecological Studies 57. New York: Springer-Verlag: 74-86. [3881]
105. Eberhart, Kevin E.; Woodard, Paul M. 1987. Distribution of residual vegetation associated with large fires in Alberta. Canadian Journal of Forest Research. 17(10): 1207-1212. [10464]
106. Ehnes, James; Keenan, Vince. 2002. Implementing wildfire-based timber harvest guidelines in southeastern Manitoba. The Forestry Chronicle. 78(5): 680-685. [43633]
107. Elliott, Deborah L. 1979. The current regenerative capacity of the northern Canadian trees, Keewatin, N.W.T., Canada: some preliminary observations. Arctic and Alpine Research. 11(2): 243-251. [8419]
108. Elliott-Fisk, Deborah L. 1988. The boreal forest. In: Barbour, Michael G.; Billings, William Dwight, eds. North American terrestrial vegetation. New York: Cambridge University Press: 33-62. [13878]
109. Ellison, Laurence. 1966. Seasonal foods and chemical analysis of winter diet of Alaskan spruce grouse. The Journal of Wildlife Management. 30(4): 729-735. [9735]
110. Epting, Justin; Verbyla, David. 2005. Landscape-level interactions of prefire vegetation, burn severity, and postfire vegetation over a 16-year period in interior Alaska. Canadian Journal of Forest Research. 35(6): 1367-1377. [55816]
111. Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters. 148 p. [905]
112. Famous, Norman C.; Spencer, M. 1989. Revegetation patterns in mined peatlands in central and eastern North America studies. Restoration and Management Notes. 7(2): 95-96. [10171]
113. Farrar, John Laird. 1995. Trees of the northern United States and Canada. Ames, IA: Blackwell Publishing. 502 p. [60614]
114. Fedkenheuer, A. W.; Heacock, H. M.; Lewis, D. L. 1980. Early performance of native shrubs and trees planted on amended Athabasca oil sand tailings. Reclamation Review. 3(1): 47-55. [12468]
115. Feller, M. C. 1982. The ecological effects of slashburning with particular reference to British Columbia: a literature review. Victoria, BC: Ministry of Forests. 60 p. [10470]
116. Ferguson, Sue A.; Ruthford, Julia; Rorig, Miriam; Sandberg, David V. 2003. Measuring moss moisture dynamics to predict fire severity. In: Galley, Krista E. M.; Klinger, Robert C.; Sugihara, Neil G., eds. Proceedings of fire conference 2000: the 1st national congress on fire ecology, prevention, and management; 2000 November 27-December 1; San Diego, CA. Miscellaneous Publication No. 13. Tallahassee, FL: Tall Timbers Research Station: 211-217. [51398]
117. Filion, Jacques; Morin, Hubert. 1996. The spatial distribution of Picea mariana seedlings 8 years after a fire in boreal forest (Quebec). Canadian Journal of Forest Research. 26(4): 601-610. [29248]
118. Flannigan, M. D.; Lynham, T. J.; Ward, P. C. 1989. An extensive blowdown occurrence in northwestern Ontario. In: MacIver, D. C.; Auld, H.; Whitewood, R., eds. Proceedings of the 10th conference on fire and forest meteorology; 1989 April 17-21; Ottawa, ON. Boston: American Meteorology Society: 60-71. [13517]
119. Flannigan, Mike; Stocks, Brian; Weber, Mike. 2003. Fire regimes and climate change in Canadian forests. In: Veblen, Thomas T.; Baker, William L.; Montenegro, Gloria; Swetnam, Thomas W., eds. Fire and climatic change in temperate ecosystems of the western Americas. Ecological Studies, Vol. 160. New York: Springer: 97-119. [45407]
120. Flexner, J. Lindsey; Bassett, John R.; Montgomery, Bruce A.; Simmons, Gary A.; Witter, John A. 1983. Spruce-fir silviculture and the spruce budworm in the Lake States. Handbook 83-2. [Ann Arbor, MI]: Michigan Cooperative Forest Pest Management Program. 30 p. [8664]
121. Flora of North America Editorial Committee, eds. 2014. Flora of North America north of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
122. Foote, Joan. 1976. Classification, description, and dynamics of plant communities following fire in the taiga of interior Alaska. Final report: "Fire effects study"; BLM Contract No. 53500-CT2-244. Juneau, AK: U.S. Department of the Interior, Bureau of Land Management. 211 p. [36000]
123. Foote, Joan. 1985. Natural revegetation following the 1950 Porcupine River fire in northeast Alaska: 1951-81. In: Lotan, James E.; Kilgore, Bruce M.; Fischer, William C.; Mutch, Robert W., tech. coords. Proceedings--symposium and workshop on wilderness fire; 1983 November 15-18; Missoula, MT. Gen. Tech. Rep. INT-182. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 332. [45359]
124. Foote, M. Joan. 1983. Classification, description, and dynamics of plant communities after fire in the taiga of interior Alaska. Res. Pap. PNW-307. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 108 p. [7080]
125. Foote, M. Joan. 1993. Revegetation following the 1950 Porcupine River Fire: 1950-1981. Fairbanks, AK: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Institute of Northern Forestry. 71 p. Review draft. [19874]
126. Forster, W.; Epp, H.; Lanoville, R. A. 1994. Fire ecology of the caribou range of northwest Canada. In: Proceedings, 12th conference on fire and forest meteorology; 1993 October 26-28; Jekyll Island, GA. Bethesda, MD: Society of American Foresters: 620-627. [26333]
127. Foster, David R. 1983. The history and pattern of fire in the boreal forest of southeastern Labrador. Canadian Journal of Botany. 61(9): 2459-2471. [9683]
128. Foster, David R. 1985. Vegetation development following fire in Picea mariana (black spruce)-Pleurozium forests of south-eastern Labrador, Canada. Journal of Ecology. 73(2): 517-534. [7222]
129. Fowells, H. A., compiler. 1965. Silvics of forest trees of the United States. Agric. Handb. 271. Washington, DC: U.S. Department of Agriculture, Forest Service. 762 p. [12442]
130. Frank, Robert M.; Majcen, Zoran; Gagnon, Gilles. 1980. Balsam fir--Forest Cover Type 5. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 10-11. [49851]
131. Fraser, J. W. 1976. Viability of black spruce seed in or on a boreal forest seedbed. The Forestry Chronicle. 52(5): 229-231. [86641]
132. Frelich, Lee E. 2002. Forest dynamics and disturbance regimes: Studies from temperate evergreen-deciduous forests. Cambridge: Cambridge University Press. 266 p. [43731]
133. Frelich, Lee E.; Reich, Peter B. 1995. Neighborhood effects, disturbance, and succession in forests of the western Great Lakes Region. Ecoscience. 2(2): 148-158. [27778]
134. Frelich, Lee E.; Reich, Peter B. 1995. Spatial patterns and succession in a Minnesota southern-boreal forest. Ecological Monographs. 63(3): 325-346. [26533]
135. French, David W.; Irving, Frank. 1986. Dwarf mistletoe eradication by burning. In: Koonce, Andrea L., ed. Prescribed burning in the Midwest: state-of-the-art: Proceedings of a symposium; 1986 March 3-6; Stevens Point, WI. Stevens Point, WI: University of Wisconsin, College of Natural Resources, Fire Science Center: 116-118. [16279]
136. Frost, Cecil C. 1998. Presettlement fire frequency regimes of the United States: a first approximation. In: Pruden, Teresa L.; Brennan, Leonard A., eds. Fire in ecosystem management: shifting the paradigm from suppression to prescription: Proceedings, Tall Timbers fire ecology conference; 1996 May 7-10; Boise, ID. No. 20. Tallahassee, FL: Tall Timbers Research Station: 70-81. [35605]
137. Furyaev, V. V.; Wein, Ross W.; MacLean, David A. 1983. Fire influences in Abies-dominated forests. In: Wein, Ross W.; MacLean, David A., eds. The role of fire in northern circumpolar ecosystems. Scope 18. New York: John Wiley & Sons: 221-234. [14610]
138. Gagnon, Rejean; Morin, Jubert; St-Pierre, Helene. 1991. Natural seed regeneration of black spruce (Picea mariana) stands in the Quebec boreal forest. In: Simpson, C. M, ed. Proceedings of the conference on natural regeneration management; 1990 March 27-28; Fredericton, NB. Fredericton, NB: Forestry Canada, Maritimes Region: 103-113. [17193]
139. Gauthier, Sylvie; Boucher, Dominique; Morissette, Jacques; De Grandpre, Louis. 2010. Fifty-seven years of composition change in the eastern boreal forest of Canada. Journal of Vegetation Science. 21(4): 772-785. [82490]
140. Gervais, David J.; Greene, David F.; Work, Timothy T. 2012. Causes of variation in wood-boring beetle damage in fire-killed black spruce (Picea mariana) forests in the central boreal forest of Quebec. Ecoscience. 19(4): 398-403. [87731]
141. Girard, Francois; Payette, Serge; Gagnon, Rejean. 2009. Origin of the lichen-spruce woodland in the closed-crown forest zone of eastern Canada. Global Ecology and Biogeography. 18(3): 291-303. [85773]
142. Gleason, Henry A.; Cronquist, Arthur. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd ed. New York: New York Botanical Garden. 910 p. [20329]
143. Golden, Howard N. 1987. Survey of furbearer populations on the Yukon Flats National Wildlife Refuge. Final report: Cooperative Agreement Project 14-16-007-84-7416. Fairbanks, AK: Alaska Department of Fish and Game; U.S. Fish and Wildlife Service. 86 p. [77607]
144. Gosse, John W.; Cox, Rodney; Avery, Shawn W. 2005. Home-range characteristics and habitat use by American martens in eastern Newfoundland. Journal of Mammalogy. 86(6): 1156-1163. [76061]
145. Grandtner, M. M.; Ducruc, Jean-Pierre. 1980. Black spruce-tamarack. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 14. [49855]
146. Greene, D. F.; Johnson, E. A. 1999. Modelling recruitment of Populus tremuloides, Pinus banksiana, and Picea mariana following fire in the mixedwood boreal forest. Canadian Journal of Forest Research. 29(4): 462-473. [30617]
147. Greene, D. F.; Noel, J.; Bergeron, Y.; Rousseau, M.; Gauthier, S. 2004. Recruitment of Picea mariana, Pinus banksiana, and Populus tremuloides across a burn severity gradient following wildfire in the southern boreal forest of Quebec. Canadian Journal of Forest Research. 34(9): 1845-1857. [55392]
148. Greene, D. F.; Splawinski, T. B.; Gauthier, S.; Bergeron, Y. 2013. Seed abscission schedules and the timing of post-fire salvage of Picea mariana and Pinus banksiana. Forest Ecology and Management. 303: 20-24. [86742]
149. Greene, David F.; Gauthier, Sylvie; Noel, Josee; Rousseau; Bergeron, Yves. 2006. A field experiment to determine the effect of post-fire salvage on seedbeds and tree regeneration. Frontiers in Ecology and the Environment. 4(2): 69-74. [65003]
150. Grenier, Daniel J.; Bergeron, Yves; Kneeshaw, Daniel; Gauthier, Sylvie. 2005. Fire frequency for the transitional mixedwood forest of Timiskaming, Quebec, Canada. Canadian Journal of Forest Research. 35(3): 656-666. [61484]
151. Gutsell, Sheri L.; Johnson, Edward A. 2007. Wildfire and tree population processes. In: Johnson, Edward A.; Miyanishi, Kiyoko, eds. Plant disturbance ecology: The process and the response. Amsterdam; Boston, MA: Academic Press: 441-485. [69470]
152. Haavisto, V. F. 1975. Peatland black spruce seed production and dispersal in northeastern Ontario. In: Fraser, J. W.; Jeglum, J. K.; Ketcheson, D. E.; Robinson, F. C.; Van Bers H. P. G.; McLain, K. M.; Auld, J. M., tech. coords. Black spruce symposium; 1975 September 23-25; Thunder Bay, ON. Symposium Proceedings 0-P-4. Sault Ste. Marie, ON: Department of the Environment, Canadian Forestry Service, Great Lakes Forest Research Centre: 250-264. [8839]
153. Haeussler, S.; Pojar, J.; Geisler, B. M.; Yole, D.; Annas, R. M. 1985. A guide to the interior cedar-hemlock zone, northwestern transitional subzone (ICHg), in the Prince Rupert forest region, British Columbia. Land Management Report Number 26. Victoria, BC: British Columbia, Ministry of Forests. 263 p. [6930]
154. Hagemann, Ulrike; Moroni, Martin T.; Makeschin, Franz. 2009. Deadwood abundance in Labrador high-boreal black spruce forests. Canadian Journal of Forest Research. 39(1): 131-142. [83739]
155. Halvorson, Curtis H. 1986. Influence of vertebrates on conifer seed production. In: Shearer, Raymond C., compiler. Proceedings--conifer tree seed in the Inland Mountain West symposium; 1985 August 5-6; Missoula, MT. Gen. Tech. Rep. INT-203. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 201-222. [13115]
156. Haney, Alan; Apfelbaum, Steven; Burris, John M. 2008. Thirty years of post-fire succession in a southern boreal forest bird community. The American Midland Naturalist. 159(2): 421-433. [74331]
157. Hannah, Kevin C.; Hoyt, Jeff S. 2004. Northern hawk owls and recent burns: does burn age matter? The Condor. 106(2): 420-423. [50287]
158. Hanson, Herbert C. 1951. Characteristics of some grassland, marsh, and other plant communities in western Alaska. Ecological Monographs. 21(4): 317-378. [62710]
159. Hanson, William A. 1979. Preliminary results of the Bear Creek fire effects studies. Proposed open file report. Anchorage, AK: U.S. Department of the Interior, Bureau of Land Management, Anchorage District Office. 83 p. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [6400]
160. Harper, Karen A.; Bergeron, Yves; Drapeau, Pierre; Gauthier, Sylvie; De Grandpre, Louis. 2006. Changes in spatial pattern of trees and snags during structural development in Picea mariana boreal forests. Journal of Vegetation Science. 17(5): 625-636. [64976]
161. Hayasaka, Hiroshi; Lynch, Mary. 2003. Probability of lightning-ignited forest fires in Alaska. In: Galley, Krista E. M.; Klinger, Robert C.; Sugihara, Neil G., eds. Proceedings of fire conference 2000: the 1st national congress on fire ecology, prevention, and management; 2000 November 27-December 1; San Diego, CA. Miscellaneous Publication No. 13. Tallahassee, FL: Tall Timbers Research Station: 225-226. Abstract. [51400]
162. Hegg, Karl M. 1967. A photo identification guide for the land and forest types of interior Alaska. RP-NOR-3. Juneau, AK: U.S. Department of Agriculture, Forest Service, Northern Forest Experiment Station. 55 p. [86769]
163. Heimburger, Carl C. 1934. Forest-type studies in the Adirondack Region. Memoir 165. Ithaca, NY: Cornell University, Agricultural Experiment Station. 122 p. [21495]
164. Heinselman, Miron L. 1970. The natural role of fire in northern coniferous forests. The Naturalist. 21(4): 15-23. [34705]
165. Heinselman, Miron L. 1973. Fire in the virgin forests of the Boundary Waters Canoe Area, Minnesota. Quaternary Research. 3(3): 329-382. [282]
166. Heinselman, Miron L. 1973. Restoring fire to the canoe country. Naturalist. 24: 21-31. [15810]
167. Heinselman, Miron L. 1981. Fire intensity and frequency as factors in the distribution and structure of northern ecosystems. In: Mooney, H. A.; Bonnicksen, T. M.; Christensen, N. L.; Lotan, J. E.; Reiners, W. A., technical coordinators. Fire regimes and ecosystem properties: Proceedings of the conference; 1978 December 11-15; Honolulu, HI. Gen. Tech. Rep. WO-26. Washington, DC: U.S. Department of Agriculture, Forest Service: 7-57. [4390]
168. Heinselman, Miron L. 1985. Fire regimes and management options in ecosystems with large high-intensity fires. In: Lotan, James E.; Kilgore, Bruce M.; Fischer, William C.; Mutch, Robert W., tech. coords. Proceedings--symposium and workshop on wilderness fire; 1983 November 15-18; Missoula, MT. Gen. Tech. Rep. INT-182. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 101-109. [45352]
169. Hely, Christelle; Flannigan, Mike; Bergeron, Yves. 2003. Modeling tree mortality following wildfire in the southeastern Canadian mixed-wood boreal forest. Forest Science. 49(4): 566-576. [45263]
170. Hely, Christelle; Flannigan, Mike; Bergeron, Yves; McRae, Douglas. 2001. Role of vegetation and weather on fire behavior in the Canadian mixedwood boreal forest using two fire behavior prediction systems. Canadian Journal of Forest Research. 31(3): 430-441. [40168]
171. Hinzman, Larry D.; Fukuda, Masami; Sandberg, David V.; Chapin, F. Stuart, III; Dash, David. 2003. FROSTFIRE: an experimental approach to predicting the climate feedbacks from the changing boreal forest fire regime. Journal of Geophysical Research. 108(D1): 8153. doi:10.1029/2001JD000415. [50283]
172. Hogg, Edward H. 1994. Climate and the southern limit of the western Canadian boreal forest. Canadian Journal of Forest Research. 24(9): 1835-1845. [24509]
173. Hollingsworth, Teresa; Johnstone, Jill; Chapin, F. S., III; Mack, Michelle; Schuur, Edward; Verbyla, David. 2007. Managing fire with fire in Alaskan black spruce forests: impacts of fire severity on successional trajectory and future forest flammability. Final Report to Joint Fire Science Program, Project #05-1-2-06. Boise, ID: Joint Fire Science Program. 16 p. [87173]
174. Horton, K. W.; Lees, J. C. 1961. Black spruce in the foothills of Alberta. Tech. Note 110. Ottawa, ON: Canadian Department of Forestry, Forest Research Branch, the Queen's Printer and Controller of Stationery. 17 p. [87105]
175. Hosie, R. C. 1969. Native trees of Canada. 7th ed. Ottawa, ON: Canadian Forestry Service, Department of Fisheries and Forestry. 380 p. [3375]
176. Hoyt, Jeff S.; Hannon, Susan J. 2002. Habitat associations of black-backed and three-toed woodpeckers in the boreal forest of Alberta. Canadian Journal of Forest Research. 32(10): 1881-1888. [43215]
177. Hulten, Eric. 1968. Flora of Alaska and neighboring territories. Stanford, CA: Stanford University Press. 1008 p. [13403]
178. Humrickhouse, A. Bruce. 1986. Aerial ignition for prescribed burning in Minnesota. In: Koonce, Andrea L., ed. Prescribed burning in the Midwest: state-of-the-art: Proceedings of a symposium; 1986 March 3-6; Stevens Point, WI. Stevens Point, WI: University of Wisconsin, College of Natural Resources, Fire Science Center: 138-145. [16281]
179. Hustich, Ilmari. 1953. The boreal limits of conifers. Arctic. 6(2): 149-162. [8257]
180. Ilisson, Triin; Chen, Han Y. H. 2009. Responses of six boreal tree species to stand replacing fire and clearcutting. Ecosystems. 12(5): 820-829. [81498]
181. Irving, F. D.; French, D. W. 1971. Control by fire of dwarf mistletoe in black spruce. Journal of Forestry. 69: 28-30. [14006]
182. Islam, M. Anisul; McDonald, S. Ellen; Zwiazek, Janusz J. 2003. Responses of black spruce (Picea mariana) and tamarack (Larix laricina) to flooding and ethylene. Tree Physiology. 23(8): 545-552. [86736]
183. Janke, Robert A.; Lowther, John L. 1980. Post-fire succession in the boreal forest type of Isle Royale National Park. In: Proceedings, 2nd conference on scientific research in the National Parks; 1979 November 26-30; San Francisco, CA. Volume 7: Ecosystem Studies/Interdisciplinary Studies. Washington, DC: U.S. Department of the Interior, National Park Service; The American Institute of Biological Sciences: 99-135. [19929]
184. Jasieniuk, M. A.; Johnson, E. A. 1982. Peatland vegetation organization and dynamics in the western subarctic, Northwest Territories, Canada. Canadian Journal of Botany. 60(12): 2581-2593. [70178]
185. Jayen, Karelle; Leduc, Alain; Bergeron, Yves. 2006. Effect of fire severity on regeneration success in the boreal forest of northwest Quebec, Canada. Ecoscience. 13(2): 143-151. [66967]
186. Johnson, E. A. 1975. Buried seed populations in the subarctic forest east of Great Slave Lake, Northwest Territories. Canadian Journal of Botany. 53(24): 2933-2941. [6466]
187. Johnson, E. A. 1979. Fire recurrence in the subarctic and its implications for vegetation composition. Canadian Journal of Botany. 57(12): 1374-1379. [18744]
188. Johnson, E. A. 1980. Fire recurrence and vegetation in the lichen woodlands of the Northwest Territories, Canada. In: Stokes, Marvin A.; Dieterich, John H., technical coordinators. Proceedings of the fire history workshop; 1980 October 20-24; Tucson, AZ. Gen. Tech. Rep. RM-81. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 110-114. [16050]
189. Johnson, E. A. 1981. Vegetation organization and dynamics of lichen woodland communities in the Northwest Territories, Canada. Ecology. 62(1): 200-215. [19244]
190. Johnson, E. A.; Miyanishi, K.; O'Brien, N. 1999. Long-term reconstruction of the fire season in the mixedwood boreal forest of western Canada. Canadian Journal of Botany. 77(8): 1185-1188. [33061]
191. Johnson, E. A.; Rowe, J. S. 1975. Fire in the subarctic wintering ground of the Beverly caribou herd. The American Midland Naturalist. 94(1): 1-14. [8384]
192. Johnson, E. A.; Rowe, J. S. 1977. Fire and vegetation change in the western subarctic. ALUR 75-76-61. Ottawa, ON: Arctic Land Use Research Program, Northern Natural Resources and Environment Branch, Department of Indian Affairs and Northern Development. 58 p. [69456]
193. Johnson, Edward A. 1992. Fire and vegetation dynamics: Studies from the North American boreal forest. Cambridge Studies in Ecology. Cambridge, UK: Cambridge University Press. 129 p. [19950]
194. Johnson, Glenn; Breisch, Alvin R. 1993. The eastern massasauga rattlesnake in New York: occurrence and habitat management. In: Johnson, Bob; Menzies, Vi, eds. International symposium and workshop on the conservation of the eastern massasauga rattlesnake, Proceedings; 1992 May 8-9; Toronto, ON. West Hill, ON: Metropolitan Toronto Zoo: 48-54. [26682]
195. Johnson, J. S.; Johnson, E. A. 1994. Opening of semi-serotinous cones of Picea mariana by fire and ambient heating. In: Science and public policy: Proceedings, 79th annual meeting of the Ecological Society of America; 1994 August 7-11; Knoxville, TN. In: Bulletin of the Ecological Society of America. 75(2): 123. (Supplement). [Abstract]. [23737]
196. Johnston, B. C.; Hendzel, L. 1985. Examples of aspen treatment, succession, and management in western Colorado. Lakewood, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Region. 164 p. [18670]
197. Johnston, William F. 1971. Management guide for the black spruce type in the Lake States. NC-64. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 12 p. [8687]
198. Johnston, William F. 1975. Reproducing lowland conifer forests. Journal of Forestry. 73(1): 17-20. [14277]
199. Johnston, William F. 1977. Manager's handbook for black spruce in the North Central States. Gen. Tech. Rep. NC-34. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 18 p. [8684]
200. Johnston, William F.; Smith, Thomas M. 1983. Black spruce. In: Burns, Russell M., tech. comp. Silvicultural systems for the major forest types of the United States. Agric. Handb. No. 445. Washington, DC: U.S. Department of Agriculture, Forest Service: 96-98. [13821]
201. Johnstone, J. F.; Chapin, F. S., III. 2006. Fire interval effects on successional trajectory in boreal forests of northwest Canada. Ecosystems. 9(2): 268-277. [63209]
202. Johnstone, Jill F.; Chapin, F. Stuart, III. 2006. Effects of soil burn severity on post-fire tree recruitment in boreal forest. Ecosystems. 9(1): 14-31. [62101]
203. Johnstone, Jill F.; Hollingsworth, Teresa N.; Chapin, F. Stuart, III. 2008. A key for predicting postfire successional trajectories in black spruce stands of Interior Alaska. Gen. Tech. Rep. PNW-GTR-767. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 37 p. [77041]
204. Johnstone, Jill F.; Hollingsworth, Teresa N.; Chapin, Stuart F., III; Mack, Michelle C. 2009. Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Global Change Biology. 16(4): 1281-1295. [81455]
205. Johnstone, Jill F.; Kasischke, Eric S. 2005. Stand-level effects of soil burn severity on postfire regeneration in a recently burned black spruce forest. Canadian Journal of Forest Research. 35(5): 2151-2163. [61462]
206. Johnstone, Jill Frances. 2003. Fire and successional trajectories in boreal forest: implications for response to a changing climate. Fairbanks, AK: University of Alaska Fairbanks. 201 p. Dissertation. [85591]
207. Johnstone, Jill; Hollingsworth,Teresa; Chapin, Terry. 2007. Workbook of potential successional trajectories in burned stands of black spruce in interior Alaska [Draft]. Joint Fire Science Project No. 05-1-2-06. Fairbanks, AK: University of Alaska Fairbanks; Portland. OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Boreal Ecology Cooperative Research Unit. 16 p. [87182]
208. Johnstone, Jill; Boby, Leslie; Tissier, Emily; Mack, Michelle; Verbyla, Dave; Walker, Xanthe. 2009. Postfire seed rain of black spruce, a semiserotinous conifer, in forests of interior Alaska. Canadian Journal of Forest Research. 39(8): 1575-1588. [83030]
209. Joly, Kyle; Dale, Bruce W.; Collins, William B.; Adams, Layne G. 2003. Winter habitat use by female caribou in relation to wildland fires in interior Alaska. Canadian Journal of Zoology. 81(7): 1192-1201. [65772]
210. Kartesz, J. T.; The Biota of North America Program (BONAP). 2014. North American plant atlas, [Online]. Chapel Hill, NC: The Biota of North America Program (Producer). Available: http://bonap.org/. [Maps generated from Kartesz, J. T. 2010. Floristic synthesis of North America, Version 1.0. Biota of North America Program (BONAP). [In press]. [84789]
211. Kasischke, Eric S.; Johnstone, Jill F. 2005. Variation in postfire organic layer thickness in a black spruce forest complex in interior Alaska and its effects on soil temperature and moisture. Canadian Journal of Forest Research. 35(9): 2164-2177. [61464]
212. Kasischke, Eric S.; Turetsky, Merritt R.; Kane, Evan S. 2012. Effects of trees on the burning of organic layers on permafrost terrain. Forest Ecology and Management. 267: 127-133. [85448]
213. Kasischke, Eric S.; Turetsky, Merritt R.; Ottmar, Roger D.; French, Nancy H. F.; Hoy, Elizabeth E.; Kane, Evan S. 2008. Evaluation of the composite burn index for assessing fire severity in Alaskan black spruce forests. International Journal of Wildland Fire. 17(4): 515-526. [75579]
214. Kasischke, Eric S.; Verbyla, David L.; Rupp, T. Scott; McGuire, A. David; Murphy, Karen A.; Jandt, Randi; Barnes, Jennifer L.; Hoy, Elizabeth E.; Duffy, Paul A.; Calef, Monida; Turetsky, Merritt R. 2010. Alaska's changing fire regime--implications for the vulnerability of its boreal forests. Canadian Journal of Forest Research. 40(7): 1313-1324. [82442]
215. Keith, Lloyd B.; Surrendi, Dennis C. 1971. Effects of fire on a snowshoe hare population. The Journal of Wildlife Management. 35(1): 16-26. [124]
216. Kemball, Kevin J.; Wang, G. Geoff; Dang, Qing-Lai. 2005. Response of understory plant community of boreal mixedwood stands to fire, logging, and spruce budworm outbreak. Canadian Journal of Botany. 83(12): 1550-1560. [63193]
217. Kemball, Kevin J.; Wang, G. Geoff; Westwood, A. Richard. 2006. Are mineral soils exposed by severe wildfire better seedbeds for conifer regeneration? Canadian Journal of Forest Research. 36(8): 1943-1950. [66364]
218. Kershaw, K. A. 1977. Studies on lichen-dominated systems. XX. An examination of the northern boreal lichen woodlands in Canada. Canadian Journal of Botany. 55(4): 393-410. [69098]
219. Kershaw, K. A. 1978. The role of lichens in the boreal tundra transition areas. The Bryologist. 81(2): 294-306. [87111]
220. Kiil, A. D. 1975. Fire spread in a black spruce stand. Bi-Monthly Research Notes. Ottawa: Environment Canada, Forestry Service. 31(1): 2-3. [50643]
221. Klein, Eric; Berg, Edward E.; Dial, Roman. 2005. Wetland drying and succession across the Kenai Peninsula Lowlands, south-central Alaska. Canadian Journal of Forest Research. 35(8): 1931-1941. [61037]
222. Koivula, Matti J.; Schmiegelow, Fiona K. A. 2007. Boreal woodpecker assemblages in recently burned forested landscapes in Alberta, Canada: effects of post-fire harvesting and burn severity. Forest Ecology and Management. 242(2-3): 606-618. [66563]
223. Kotar, John; Kovach, Joseph A.; Locey, Craig T. 1988. Field guide to forest habitat types of northern Wisconsin. Madison, WI: University of Wisconsin, Department of Forestry; Wisconsin Department of Natural Resources. 217 p. [11510]
224. Kuchler, A. W. 1964. Conifer bog (Larix-Picea-Thuja). In: Kuchler, A. W. Manual to accompany the map of potential vegetation of the conterminous United States. Special Publication No. 36. New York: American Geographical Society: 94. [67842]
225. Kurkowski, Thomas A.; Mann, Daniel H.; Rupp, T. Scott; Verbyla, David L. 2008. Relative importance of different secondary successional pathways in an Alaskan boreal forest. Canadian Journal of Forest Research. 38(7): 1911-1923. [77039]
226. Kurmis, Vilis; Webb, Sara L.; Merriam, Lawrence C., Jr. 1986. Plant communities of Voyageurs National Park, Minnesota, U.S.A. Canadian Journal of Botany. 64(3): 531-540. [16088]
227. Laberge, Marie-Josee; Payette, Serge; Bousquet, Jean. 2000. Life span and biomass allocation of stunted black spruce clones in the subarctic environment. Journal of Ecology. 88(4): 584-593. [35778]
228. LANDFIRE Biophysical Settings. 2009. Biophysical setting 661340: Boreal jack pine-black spruce forest. In: LANDFIRE Biophysical Setting Model: Map zone 66, [Online]. In: Vegetation Dynamics Models. In: LANDFIRE. Washington, DC: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory; U.S. Geological Survey; Arlington, VA: The Nature Conservancy (Producers). Available: http://www.landfire.gov/national_veg_models_op2.php [2013, July 10]. [87062]
229. LANDFIRE Biophysical Settings. 2009. Biophysical setting 6613440: Boreal jack pine-black spruce forest. In: LANDFIRE Biophysical Setting Model: Map zone 41, [Online]. In: Vegetation Dynamics Models. In: LANDFIRE. Washington, DC: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory; U.S. Geological Survey; Arlington, VA: The Nature Conservancy (Producers). Available: http://www.landfire.gov/national_veg_models_op2.php [2013, July 10]. [87061]
230. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: http://www.landfire.gov/downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
231. LANDFIRE Rapid Assessment. 2007. Rapid assessment potential natural vegetation groups (PNVGs): associated vegetation descriptions and geographic distributions. Washington, DC: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; Arlington, VA: The Nature Conservancy. 84 p. [66533]
232. Larrivee, Maxim; Fahrig, Lenore; Drapeau, Pierre. 2005. Effects of a recent wildfire and clearcuts on ground-dwelling boreal forest spider assemblages. Canadian Journal of Forest Research. 35(11): 2575-2588. [61439]
233. Larsen, C. P. S. 1997. Spatial and temporal variations in boreal forest fire frequency in northern Alberta. Journal of Biogeography. 24(5): 663-673. [29316]
234. Latour, Paul B.; Maclean, Norm; Poole, Kim G. 1994. Movements of martens, Martes americana, in burned and unburned taiga in the Mackenzie Valley, Northwest Territories. The Canadian Field-Naturalist. 108(3): 351-354. [25918]
235. Lavoie, Luc; Sirois, Luc. 1998. Vegetation changes caused by recent fires in the northern boreal forest of eastern Canada. Journal of Vegetation Science. 9(4): 483-492. [30104]
236. Lavoie, Martin; Pare, David; Bergeron, Yves. 2007. Relationships between microsite type and the growth and nutrition of young black spruce on post-disturbed lowland black spruce sites in eastern Canada. Canadian Journal of Forest Research. 37(1): 62-73. [67202]
237. Lawson, B. D.; Frandsen, W. H.; Hawkes, B. C.; Dalrymple, G. N. 1997. Probability of sustained smoldering ignition for some boreal forest duff types. Forest Management Note 63: Edmonton, AB: Natural Resources Canada, Canadian Forest Service, Northern Forestry Centre. 11 p. [66240]
238. Le Goff, Heloise; Flannigan, Mike D.; Bergeron, Yves. 2009. Potential changes in monthly fire risk in the eastern Canadian boreal forest under future climate change. Canadian Journal of Forest Research. 39(12): 2369-2380. [82193]
239. Le Goff, Heloise; Flannigan, Mike D.; Bergeron, Yves; Giradin, Martin P. 2007. Historical fire regime shifts related to climate teleconnections in the Waswanipi area, central Quebec, Canada. International Journal of Wildland Fire. 16(5): 607-618. [70362]
240. Le Goff, Heloise; Sirois, Luc. 2004. Black spruce and jack pine dynamics simulated under varying fire cycles in the northern boreal forest of Quebec, Canada. Canadian Journal of Forest Research. 34(12): 2399-2409. [55447]
241. Le Goff, Heloise; Sirois, Luc. 2004. The influence of fire interval on the regeneration of black spruce and jack pine in the northern boreal forest of Quebec. In: Engstrom, R. Todd; Galley, Krista E. M.; de Groot, William J., eds. Fire in temperate, boreal, and montane ecosystems: Proceedings of the 22nd Tall Timbers fire ecology conference: an international symposium; 2001 October 15-18; Kananaskis Village, AB. No. 22. Tallahassee, FL: Tall Timbers Research: 127. Abstract. [52308]
242. LeBarron, R. K. 1939. The role of forest fires in the reproduction of black spruce. Proceedings of the Minnesota Academy of Science. 7: 10-14. [14454]
243. LeBarron, Russell K. 1948. Silvicultural management of black spruce in Minnesota. Circular No. 791. Washington, DC: U.S. Department of Agriculture. 60 p. [13554]
244. LeBlanc, Cheryl M.; Leopold, Donald J. 1992. Demography and age structure of a central New York shrub-carr 94 years after fire. Bulletin of the Torrey Botanical Club. 119(1): 50-64. [18208]
245. LeBlanc, Joseph-Henri. 1955. A mode of vegetative reproduction in black spruce. Pulp and Paper Magazine of Canada. 56: 146-153. [87109]
246. Lecomte, N.; Bergeron, Y. 2005. Successional pathways on different surficial deposits in the coniferous boreal forest of the Quebec Clay Belt. Canadian Journal of Forest Research. 35(8): 1984-1995. [61039]
247. Lecomte, Nicolas; Simard, Martin; Bergeron, Yves. 2006. Effects of fire severity and initial tree composition on stand structural development in the coniferous boreal forest of northwestern Quebec, Canada. In: Bergeron, Y.; Macdonald, E.; Engelmark, O.; Kuuluvainen, T.; Shorohova, E. J., eds. 5th international workshop on disturbance dynamics in boreal forests; 2004 August 1-5; Dubna, Russia. In: Ecoscience. 13(2): 152-163. [66346]
248. Lecomte, Nicolas; Simard, Martin; Bergeron, Yves; Larouche, Alayn; Asnong, Hans; Richard, Pierre J. H. 2005. Effects of fire severity and initial tree composition on understorey vegetation dynamics in a boreal landscape inferred from chronosequence and paleoecological data. Journal of Vegetation Science. 16(6): 665-674. [61833]
249. Lefort, Patrick; Leduc, Alain; Gauthier, Sylvie; Bergeron, Yves. 2004. Recent fire regime (1945-1998) in the boreal forest of western Quebec. Ecoscience. 11(4): 433-445. [55646]
250. LeQuire, Elise. 2009. Forecast for southern boreal forest: an increasing incidence of severe disturbance. Joint Fire Science Program Fire Science Brief. In: JFSP Project Details--Project: 00-2-23. Boise, ID: Joint Fire Science Program. 6 p. Available online: http://www.firescience.gov/projects/briefs/00-2-23_FSBrief23.pdf [2011, January 6]. [81524]
251. LeResche, Robert E.; Davis, James L. 1973. Importance of nonbrowse foods to moose on the Kenai Peninsula, Alaska. The Journal of Wildlife Management. 37(3): 279-287. [13123]
252. Lieffers, V. J. 1986. Stand structure, variability in growth and intraspecific competition in a peatland stand of black spruce Picea mariana. Holarctic Ecology. 9(1): 58-64. [85805]
253. Little, Elbert L., Jr. 1979. Checklist of United States trees (native and naturalized). Agric. Handb. 541. Washington, DC: U.S. Department of Agriculture, Forest Service. 375 p. [2952]
254. Little, Elbert L., Jr.; Pauley, Scott S. 1958. A natural hybrid between black and white spruce in Minnesota. The American Midland Naturalist. 60(1): 202-211. [85786]
255. Lloyd, Andrea H.; Fastie, Christopher L.; Eisen, Hilary. 2007. Fire and substrate interact to control the northern range limit of black spruce (Picea mariana) in Alaska. Canadian Journal of Forest Research. 37(12): 2480-2493. [70541]
256. Lloyd, Andrea H.; Wilson, Alexis E.; Fastie, Christopher L.; Landis, R. Matthew. 2005. Population dynamics of black spruce and white spruce near the arctic tree line in the southern Brooks Range, Alaska. Canadian Journal of Forest Research. 35(9): 2073-2081. [62408]
257. Loope, Walter L. 1991. Interrelationships of fire history, land use history, and landscape pattern within Pictured Rocks National Seashore, Michigan. The Canadian Field-Naturalist. 105(1): 18-28. [5950]
258. Lowe, Jeovanna; Pothier, David; Savard, Jean-Pierre L.; Rompre, Ghislain; Bouchard, Mathieu. 2011. Snag characteristics and cavity-nesting birds in the unmanaged post-fire northeastern Canadian boreal forest. Silva Fennica. 45(1): 55-67. [85693]
259. Lubinski, Sara; Hop, Kevin; Gawler, Susan. 2003. U.S. Geological Survey-National Park Service Vegetation Mapping Program: Acadia National Park, Maine. Final report. [Revised edition]. La Crosse, WI: U.S. Department of the Interior, U.S. Geological Survey, Upper Midwest Environmental Studies Center. 50 p. [+ appendices]. Available online: http://biology.usgs.gov/npsveg/acad/acadrpt.pdf [2012, January 12]. [79619]
260. Lussier, Jean-Martin; Morin, Hubert; Gagnon, Rejean. 1991. Growth performance of mature second-growth black spruce in the Saguenay-Lac St-Jean region, Quebec: preliminary results. In: Simpson, C. M, ed. Proceedings of the conference on natural regeneration management; 1990 March 27-28; Fredericton, NB. Fredericton, NB: Forestry Canada, Maritimes Region: 133-146. [17195]
261. Lussier, Jean-Martin; Morin, Hubert; Gagnon, Rejean. 2002. Mortality in black spruce stands of fire or clear-cut origin. Canadian Journal of Research. 32(3): 539-547. [41798]
262. Lutz, H. J. 1953. The effects of forest fires on the vegetation of interior Alaska. Station Paper No. 1. Juneau, AK: U.S. Department of Agriculture, Forest Service, Alaska Forest Research Center. 36 p. [7076]
263. Lutz, H. J. 1956. Ecological effects of forest fires in the interior of Alaska. Tech. Bull. No. 1133. Washington, DC: U.S. Department of Agriculture, Forest Service. 121 p. [7653]
264. Lutz, H. J. 1960. Fire as an ecological factor in the boreal forest of Alaska. Journal of Forestry. 58(6): 454-460. [16603]
265. Lynch, Jason A.; Clark, James S.; Bigelow, Nancy H.; Edwards, Mary E.; Finney, Bruce P. 2003. Geographic and temporal variations in fire history in boreal ecosystems in Alaska. Journal of Geophysical Research. 107: 8152. doi:10.1029/2001JD000332. [85464]
266. Lynch, Jason Anthony. 2001. Fire history of boreal forests: implications for past climate change. Durham, NC: Duke University. 175 p. Dissertation. [85587]
267. Lynham, T. J.; Curran, T. R. 1998. Vegetation recovery after wildfire in old-growth red and white pine. Frontline: Forestry Research Applications/Technical Note No. 100. Sault Ste. Marie, ON: Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre. 4 p. [30685]
268. MacCracken, James G.; Viereck, Leslie A. 1990. Browse regrowth and use by moose after fire in interior Alaska. Northwest Science. 64(1): 11-18. [10803]
269. Mack, Michelle C.; Treseder, Kathleen K.; Manies, Kristen L.; Harden, Jennifer W.; Schuur, Edward A. G.; Vogel, Jason G.; Randerson, James T.; Chapin, F. Stuart, III. 2008. Recovery of aboveground plant biomass and productivity after fire in mesic and dry black spruce forest of interior Alaska. Ecosystems. 11(2): 209-225. [69973]
270. MacLean, David A. 1980. Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: a review and discussion. The Forestry Chronicle. 56(5): 213-221. [14609]
271. Maikawa, E.; Kershaw, K. A. 1976. Studies on lichen-dominated systems. XIX. The postfire recovery sequence of black spruce-lichen woodland in the Abitau Lake region, N.W.T. Canadian Journal of Botany. 54(23): 2679-2687. [7225]
272. Mallik, Azim U.; Bloom, Robin G.; Whisenant, Steve G. 2010. Seedbed filter controls post-fire succession. Basic and Applied Ecology. 11(2): 170-181. [85808]
273. Manies, K. L.; Harden, J. W.; Bond-Lamberty, B. P.; O'Neill, K. P. 2005. Woody debris along an upland chronosequence in boreal Manitoba and its impact on long-term carbon storage. Canadian Journal of Forest Research. 35(2): 472-482. [60393]
274. Mann, Daniel H.; Fastie, Christopher L.; Rowland, Erika L.; Bigelow, Nancy H. 1995. Spruce succession, disturbance, and geomorphology on the Tanana River floodplain, Alaska. Ecoscience. 2(2): 184-199. [26577]
275. Manning, G. H.; Massie, M. R. C.; Rudd, F. 1984. Metric single-tree weight tables for the Yukon Territory. Information Report BC-X-250. Victoria, BC: Environment Canada, Canadian Forestry Service, Pacific Forest Research Centre. 60 p. [20766]
276. Marek, G. T. 1975. Ecosystem management of black spruce on shallow sites in the Lake Nipigon-Beardmore area. In: Fraser, J. W.; Jeglum, J. K.; Ketcheson, D. E.; Robinson, F. C.; Van Bers H. P. G.; McLain, K. M.; Auld, J. M., tech. coords. Black spruce symposium; 1975 September 23-25; Thunder Bay, ON. Symposium Proceedings 0-P-4. Sault Ste. Marie, ON: Department of the Environment, Canadian Forestry Service, Great Lakes Forest Research Centre: 195-200. [8834]
277. Martell, Arthur M. 1984. Changes in small mammal communities after fire in northcentral Ontario. The Canadian Field-Naturalist. 98(2): 223-226. [10507]
278. McCarthy, John W.; Weetman, Gordon. 2006. Age and size structure of gap-dynamic, old-growth boreal forest stands in Newfoundland. Silva Fennica. 40(2): 209-230. [64046]
279. McNab, W. Henry; Avers, Peter E., comps. 1994. Ecological subregions of the United States: section descriptions. Administrative Publication WO-WSA-5. Washington, DC: U.S. Department of Agriculture, Forest Service, Ecosystem Management. 267 p. [64271]
280. McRae, D. J. 1980. Preliminary fuel consumption guidelines for prescribed burning in Ontario slash fuel complexes. Rep. O-X-316. Sault Ste. Marie, ON: Department of the Environment, Canadian Forestry Service, Great Lakes Forest Research Centre. 25 p. [12356]
281. McRae, Douglas J. 1986. Guidelines for prescribed burning in Ontario's Clay Belt boreal mixedwood slash. In: Koonce, Andrea L., ed. Prescribed burning in the Midwest: state-of-the-art: Proceedings of a symposium; 1986 March 3-6; Stevens Point, WI. Stevens Point, WI: University of Wisconsin, College of Natural Resources, Fire Science Center: 28-33. [16265]
282. Melchior, Herbert R. 1976. Biological survey of the proposed Kobuk Valley National Monument. Final Report CX-9000-3-0136. Change Order No. 3. Fairbanks, AK: U.S. Department of the Interior, National Park Service; University of Alaska, Alaska Cooperative Park Studies Unit, Biological and Resource Management Program. 215 p. [70200]
283. Millar, J. B. 1939. Spruce regeneration in northern Ontario. The Forestry Chronicle. 15(11): 93-96. [13255]
284. Miller, D. R. 1980. Wildfire effects on barren-ground caribou wintering on the taiga of northcentral Canada: a reassessment. In: Reimers, Eigil; Gaare, Eldar; Skjenneberg, Sven, eds. Proceedings of the 2nd international reindeer/caribou symposium; 1979 September 17-21; Roros, Norway. Trondheim, Norway: Direktoratet for vilt og ferskvannsfisk: 84-98. [65849]
285. Miyanishi, K.; Johnson, E. A. 2002. Process and patterns of duff consumption in the mixedwood boreal forest. Canadian Journal of Forest Research. 32(7): 1285-1295. [42238]
286. Moerman, Dan. 2003. Native American ethnobotany: A database of foods, drugs, dyes, and fibers of Native American peoples, derived from plants, [Online]. Dearborn, MI: University of Michigan (Producer). Available: http://herb.umd.umich.edu/ [2013, October 28]. [37492]
287. Morgenstern, E. Kristian; Farrar, J. L. 1964. Introgressive hybridization in red spruce and black spruce. Technical Report No. 4. Toronto, ON: University of Toronto, Faculty of Forestry. 46 p. [85783]
288. Morin, Hubert; Gagnon, Rejean. 1992. Comparative growth and yield of layer- and seed-origin black spruce (Picea mariana) stands in Quebec. Canadian Journal of Forest Research. 22(4): 465-473. [18681]
289. Morissette, J. L.; Cobb, T. P.; Brigham, R. M.; James, P. C. 2002. The response of boreal forest songbird communities to fire and post-fire harvesting. Canadian Journal of Forest Research. 32(12): 2169-2183. [43889]
290. Morneau, Claude; Payette, Serge. 1989. Postfire lichen-spruce woodland recovery at the limit of the boreal forest in northern Quebec. Canadian Journal of Botany. 67(9): 2770-2782. [9270]
291. Moroni, M. T.; Zhu, X. 2012. Litter-fall and decomposition in harvested and un-harvested boreal forests. The Forestry Chronicle. 88(5): 613-621. [86440]
292. Moss, E. H. 1953. Marsh and bog vegetation in northwestern Alberta. Canadian Journal of Botany. 31(4): 448-470. [5117]
293. Moss, Melissa; Hermanutz, Luise. 2009. Postfire seedling recruitment at the southern limit of the lichen woodland. Canadian Journal of Forest Research. 39(12): 2299-2306. [82197]
294. Murphy, Edward C.; Lehnhausen, William A. 1998. Density and foraging ecology of woodpeckers following a stand replacement fire. The Journal of Wildlife Management. 62(4): 1359-1372. [30131]
295. Mutch, Robert W. 1970. Wildland fires and ecosystems--a hypothesis. Ecology. 51(6): 1046-1051. [5631]
296. Nakamura, Nobutaka; Woodard, Paul M.; Bach, Lars. 2003. Splitting in fire-killed trees in the boreal forest of Alberta. Northern Journal of Applied Forestry. 20(4): 167-174. [48534]
297. Nappi, A.; Drapeau, P.; Savard, J.-P. L. 2004. Salvage logging after wildfire in the boreal forest: is it becoming a hot issue for wildlife? The Forestry Chronicle. 80(1): 67-74. [48414]
298. Nappi, Antoine; Drapeau, Pierre; Saint-Germain, Michel; Angers, Virginie A. 2010. Effect of fire severity on long-term occupancy of burned boreal conifer forests by saproxylic insects and wood-foraging birds. International Journal of Wildland Fire. 19(4): 500-511. [81779]
299. Norum, Rodney A. 1982. Predicting wildfire behavior in black spruce forests in Alaska. Res. Note PNW-401. Portland, OR: U.S. Department of Agriculture, Forest Fire, Pacific Northwest Forest and Range Experiment Station. 10 p. [10463]
300. Norum, Rodney A. 1983. Wind adjustment factors for predicting fire behavior in three fuel types in Alaska. Res. Pap. PNW-309. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 5 p. [14618]
301. Nowak, Stephanie; Kershaw, G. Peter; Kershaw, Linda J. 2002. Plant diversity and cover after wildfire on anthropogenically disturbed and undisturbed sites in subarctic upland Picea mariana forest. Arctic. 55(3): 269-280. [46067]
302. Ohmann, Lewis F.; Grigal, David F. 1977. Some individual plant biomass values from northeastern Minnesota. Res. Note NC-227. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 2 p. [8151]
303. Ohmann, Lewis F.; Ream, Robert R. 1971. Wilderness ecology: virgin plant communities of the Boundary Waters Canoe Area. Res. Pap. NC-63. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 55 p. [9271]
304. Olson, Diana L.; Cronan, James B.; McKenzie, Donald; Barnes, Jennifer L.; Camp, Ann E. 2011. Compiling, synthesizing and analyzing existing boreal forest fire history data in Alaska, [Online]. Final report to the Joint Fire Science Program: Project 06-3-1-26. Moscow, ID: Forest Research and Management Exchange System. 23 p. (+ appendices). In: Alaska Boreal Forest Fire History Project. In: FRAMES. Alaska Fire Science Consortium (Producer). Available: http://www.firescience.gov/projects/06-3-1-26/project/06-3-1-26_final_report.pdf [2013, October 28]. [85880]
305. Orloci, Laszlo; Stanek, Walter. 1979. Vegetation survey of the Alaska Highway, Yukon Territory: types and gradients. Vegetatio. 41(1): 1-56. [12748]
306. Osborne, Timothy O. 1987. Biology of the great gray owl in interior Alaska. In: Nero, Robert W.; Clark, Richard J.; Knapton, Richard J.; Hamre, R. H., eds. Biology and conservation of northern forest owls: Symposium proceedings; 1987 February 3-7; Winnipeg, MB. Gen. Tech. Rep. RM-142. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 91-95. [17929]
307. Ostrom, Arnold J. 1983. Tree and shrub biomass estimates for Michigan, 1980. Res. Note NC-302. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 7 p. [8162]
308. Ottmar, Roger D.; Sandberg, David V. 2003. Predicting forest floor consumption from wildland fire in boreal forests of Alaska--preliminary results. In: Galley, Krista E. M.; Klinger, Robert C.; Sugihara, Neil G., eds. Proceedings of fire conference 2000: the 1st national congress on fire ecology, prevention, and management; 2000 November 27-December 1; San Diego, CA. Miscellaneous Publication No. 13. Tallahassee, FL: Tall Timbers Research Station: 218-224. [51399]
309. Paragi, Thomas F.; Johnson, W. N.; Katnik, Donald D.; Magoun, Audrey J. 1996. Marten selection of postfire seres in the Alaskan taiga. Canadian Journal of Zoology. 74(12): 2226-2237. [28567]
310. Paragi, Thomas F.; Johnson, W. N.; Katnik, Donald D.; Magoun, Audrey J. 1997. Selection of post-fire seres by lynx and snowshoe hares in the Alaskan taiga. Northwestern Naturalist. 78(3): 77-86. [66449]
311. Parish, Roberta; Thompson, Sandra. 1960. Black spruce (Picea mariana). In: Tree book: learning to recognize trees of British Columbia. Victoria, BC: Canadian Forest Service: 73-75. Available online: http://www.for.gov.bc.ca/hfd/library/documents/treebook/treebook.pdf [2013, July 15]. [87097]
312. Parisien, Marc-Andre; Sirois, Luc. 2003. Distribution and dynamics of tree species across a fire frequency gradient in the James Bay region of Quebec. Canadian Journal of Forest Research. 33(2): 243-256. [44221]
313. Payette, Serge. 1980. Fire history at the treeline in northern Quebec: a paleoclimatic tool. In: Stokes, Marvin A.; Dieterich, John H., technical coordinators. Proceedings of the fire history workshop; 1980 October 20-24; Tucson, AZ. Gen. Tech. Rep. RM-81. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 126-131. [16053]
314. Payette, Serge; Delwaide, Ann. 2003. Shift of conifer boreal forest to lichen-heath parkland caused by successive stand disturbances. Ecosystems. 6(6): 540-550. [55710]
315. Payette, Serge; Delwaide, Ann. 2004. Dynamics of subarctic wetland forests over the past 1500 years. Ecological Monographs. 74(3): 373-391. [85812]
316. Payette, Serge; Delwaide, Ann; Schaffhauser, Alice; Magnan, Gabriel. 2012. Calculating long-term fire frequency at the stand scale from charcoal data. Ecosphere. 3(7): doi.org/10.1890/ES12-00026.1. [87466]
317. Payette, Serge; Filion, Louise; Delwaide, Ann. 2008. Spatially explicit fire-climate history of the boreal forest-tundra (eastern Canada) over the last 2000 years. Philosophical Transactions of the Royal Society. 363(1501): 2301-2314. [86057]
318. Payette, Serge; Filion, Louise; Gauthier, Line; Boutin, Yves. 1985. Secular climate change in old-growth tree-line vegetation of northern Quebec. Nature. 315(9): 135-138. [87110]
319. Payette, Serge; Morneau, Claude; Sirois, Luc; Desponts, Mireille. 1989. Recent fire history of the northern Quebec biomes. Ecology. 70(3): 656-673. [9704]
320. Pellerin, Stephanie; Lavoie, Claude. 2003. Reconstructing the recent dynamics of mires using a multitechnique approach. Journal of Ecology. 91(6): 1008-1021. [55712]
321. Perles, Stephanie J.; Podniesinski, Gregory S.; Eastman, E.; Sneddon, Lesley A.; Gawler, Sue C. 2007. Classification and mapping of vegetation and fire fuel models at Delaware Water Gap National Recreation Area: Volume 1 of 2, [Online]. Technical Report NPS/NER/NRTR2007/076. Philadelphia, PA: U.S. Department of the Interior, National Park Service, Northeast Region, Natural Resource Stewardship and Science. (Producer) 187 p. Available: http://www.nps.gov/nero/science/FINAL/DEWA_veg_map/DEWA_veg_map.htm [2010, March 3]. [78999]
322. Perles, Stephanie J.; Podniesinski, Gregory S.; Eastman, E.; Sneddon, Lesley A.; Gawler, Sue C. 2007. Classification and mapping of vegetation and fire fuel models at Delaware Water Gap National Recreation Area: Volume 2 of 2--Appendix G, [Online]. Technical Report NPS/NER/NRTR--2007/076. Philadelphia, PA: U.S. Department of the Interior, National Park Service, Northeast Region, Natural Resource Stewardship and Science (Producer). 400 p. Available: http://www.nps.gov/nero/science/FINAL/DEWA_veg_map/DEWA_veg_map.htm [2010, March 3]. [79090]
323. Racine, Charles H.; Dennis, John G.; Patterson, William A., III. 1985. Tundra fire regimes in the Noatak River watershed, Alaska: 1956-83. Arctic. 38(3): 194-200. [9709]
324. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
325. Raven, Peter H.; Evert, Ray F.; Eichorn, Susan E. 1992. Bryophytes. In: Biology of plants. 5th ed. New York: Worth Publishers: 298-308. [87106]
326. Rehder, Alfred. 1907. Some new or little known forms of New England trees. Rhodora. 9(103): 109-117. [87206]
327. Reiners, William A.; Lang, Gerald E. 1979. Vegetational patterns and processes in the balsam fir zone, White Mountains, New Hampshire. Ecology. 60(2): 403-417. [14869]
328. Reschke, Carol. 1990. Ecological communities of New York State. Latham, NY: New York State Department of Environmental Conservation, Natural Heritage Program. 96 p. [21441]
329. Reynolds, Keith M. 1990. Preliminary classification of forest vegetation of the Kenai Peninsula, Alaska. Res. Pap. PNW-RP-424. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 67 p. [14581]
330. Roman, John Ross. 1980. Vegetation-environment relationships in virgin, middle elevation forests in the Adirondack Mountains, New York. Dissertation Abstracts International. Syracuse, NY: State University of New York. 41(3): 807-B. Dissertation abstract. [21154]
331. Rothermel, Richard C. 1972. A mathematical model for predicting fire spread in wildland fuels. Res. Pap. INT-115. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 40 p. [16845]
332. Rowe, J. S. 1971. Spruce and fire in northwest Canada and Alaska. In: Proceedings, annual Tall Timbers fire ecology conference; 1970 August 20-21; Fredericton, NB, Canada. No. 10. Tallahassee, FL: Tall Timbers Research Station: 245-254. [12895]
333. Rowe, J. S.; Bergsteinsson, J. L.; Padbury, G. A.; Hermesh, R. 1974. Fire studies in the Mackenzie Valley. ALUR 73-74-61. Ottawa, ON: Canadian Department of Indian and Northern Development. 123 p. [50174]
334. Rowe, J. S.; Scotter, G. W. 1973. Fire in the boreal forest. Quaternary Research. 3(3): 444-464. [72]
335. Rowe, J. Stan; Spittlehouse, David; Johnson, Edward; Jasieniuk, Marie. 1975. Fire studies in the upper Mackenzie Valley and adjacent Precambrian uplands. ALUR Rep. 74-75-61. Ottawa, ON: Indian and Northern Affairs. 128 p. [69483]
336. Royte, Joshua L.; Sperduto, Daniel D.; Lortie, John P. 1996. Botanical reconnaissance of Nancy Brook Research Natural Area. Gen. Tech. Rep. NE-216. Radnor, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 23 p. [26999]
337. Ruel, J.-C.; Horvath, R.; Ung, C. H.; Munson, A. 2004. Comparing height growth and biomass production of black spruce trees in logged and burned stands. Forest Ecology and Management. 193(3): 371-384. [48556]
338. Russell, Emily W. B. 1981. Vegetation of northern New Jersey before European settlement. The American Midland Naturalist. 105(1): 1-12. [8737]
339. Ryan, Kevin C.; Noste, Nonan V. 1985. Evaluating prescribed fires. In: Lotan, James E.; Kilgore, Bruce M.; Fischer, William C.; Mutch, Robert W., technical coordinators. Proceedings--symposium and workshop on wilderness fire; 1983 November 15-18; Missoula, MT. Gen. Tech. Rep. INT-182. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 230-238. [12456]
340. Safford, L. O. 1974. Picea A. Dietr. spruce. In: Schopmeyer, C. S., ed. Seeds of woody plants in the United States. Agric. Handb. 450. Washington, DC: U.S. Department of Agriculture, Forest Service: 587-597. [7728]
341. Saint-Germain, Michel; Drapeau, Pierre; Hebert, Christian. 2004. Comparison of Coleoptera assemblages from a recently burned and unburned black spruce forests of northeastern North America. Biological Conservation. 118(5): 583-592. [51234]
342. Sando, Rodney W.; Haines, Donald A. 1972. Fire weather and behavior of the Little Sioux fire. Res. Pap. NC-76. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Experiment Station. 6 p. [68055]
343. Sando, Rodney W.; Wick, Charles H. 1972. A method of evaluating crown fuels in forest stands. Res. Pap. NC-84. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 10 p. [20756]
344. Scoggan, H. J. 1978. The flora of Canada. Part 2: Pteridophyta, Gymnospermae, Monocotyledoneae. National Museum of Natural Sciences: Publications in Botany, No. 7(2). Ottawa, ON: National Museums of Canada. 545 p. [75494]
345. Scotter, George W. 1971. Wildfires in relation to the habitat of barren-ground caribou in the taiga of northern Canada. In: Proceedings, annual Tall Timbers fire ecology conference; 1970 August 20-21; Fredericton, NB. No. 10. Tallahassee, FL: Tall Timbers Research Station: 85-105. [18935]
346. Seiler, John; Jensen, Edward; Niemiera, Alex; Peterson, John. 2011. Fact sheet: Black spruce, [Online]. In: Gymnosperm families. In: Forest biology and dendrology education: Tree identification fact sheets. Blacksburg, VA: Virginia Polytechnic Institute and State University, College of Natural Resources and Environment, Department of Forest Resources and Environmental Conservation (Producer). Available: http://dendro.cnre.vt.edu/dendrology/syllabus/factsheet.cfm?ID=104 [2013, October 28]. [85787]
347. Siegwart Collier, Laura C.; Mallik, Azim U. 2010. Does post-fire abiotic habitat filtering create divergent plant communities on black spruce forests of eastern Canada? Oecologia. 164(2): 465-477. [81167]
348. Simard, Martin; Payette, Serge. 2005. Reduction of black spruce seed bank by spruce budworm infestation compromises postfire stand regeneration. Canadian Journal of Forest Research. 35(7): 1686-1696. [61214]
349. Singh, T. 1983. Weight tables for important tree species in the Northwest Territories. Forest Management Note No. 27. Edmonton, AB: Environment Canada, Canadian Forestry Service, Northern Forest Research Centre. 4 p. [20752]
350. Singh, T. 1984. Biomass equations for six major tree species of the Northwest Territories. Information Report NOR-X-257. Edmonton, AB: Environment Canada, Canadian Forestry Service, Northern Forest Research Centre. 22 p. [20754]
351. Sirois, Luc. 1993. Impact of fire on Picea mariana and Pinus banksiana seedlings in subarctic lichen woodlands. Journal of Vegetation Science. 4(6): 795-802. [23018]
352. Sirois, Luc. 1995. Initial phase of postfire forest regeneration in two lichen woodlands of northern Quebec. Ecoscience. 2(2): 177-183. [27068]
353. Sirois, Luc; Payette, Serge. 1989. Postfire black spruce establishment in subarctic and boreal Quebec. Canadian Journal of Forest Research. 19(12): 1571-1580. [10110]
354. Sirois, Luc; Payette, Serge. 1991. Reduced postfire tree regeneration along a boreal forest--forest-tundra transect in northern Quebec. Ecology. 72(2): 619-627. [13954]
355. Skoog, Ronald Oliver. 1968. Ecology of the caribou (Rangifer tarandus granti) in Alaska. Berkeley, CA: University of California, Berkeley. 699 p. Dissertation. [37914]
356. Stanek, W.; Alexander, K.; Simmons, C. S. 1981. Reconnaissance of vegetation and soils along the Dempster Highway, Yukon Territory: I. Vegetation types. BC-X-217. Victoria, BC: Environment Canada, Canadian Forestry Service, Pacific Forest Research Centre. 32 p. [16526]
357. Stephenson, David E. 1985. The use of charred black spruce bark by snowshoe hare. The Journal of Wildlife Management. 49(2): 296-300. [8451]
358. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
359. Stocks, B. J. 1980. Black spruce crown fuel weights in northern Ontario. Canadian Journal of Forest Research. 10(4): 498-501. [85840]
360. Stocks, B. J. 1987. Fire potential in the spruce budworm-damaged forests of Ontario. The Forestry Chronicle. 63(1): 8-14. [12369]
361. Strang, R. M. 1971. The ecology of the rocky heathlands of western Nova Scotia. In: Proceedings, annual Tall Timbers fire ecology conference; 1970 August 20-21; Fredericton, NB. No. 10. Tallahassee, FL: Tall Timbers Research Station: 287-292. [5466]
362. Strang, R. M.; Johnson, A. H. 1981. Fire and climax spruce forests in central Yukon. Arctic. 34(1): 60-61. [7079]
363. Swanson, David K. 1996. Susceptibility of permafrost soils to deep thaw after forest fires in interior Alaska, U.S.A., and some ecologic implications. Arctic and Alpine Research. 28(2): 217-227. [26903]
364. Taylor, S. W.; Wotton, B. M.; Alexander, M. E.; Dalrymple, G. N. 2004. Variation in wind and crown fire behaviour in a northern jack pine - black spruce forest. Canadian Journal of Forest Research. 34(8): 1561-1576. [55669]
365. The Nature Conservancy. 1999. Classification of the vegetation of Isle Royale National Park. USGS-NPS Vegetation Mapping Program. Minneapolis, MN: The Nature Conservancy, Midwest Regional Office; Arlington, VA: The Nature Conservancy. 140 p. Available online: http://www1.usgs.gov/vip/isro/isrorpt.pdf [2014, April 18]. [68269]
366. Thomas, P. A.; Wein, Ross W. 1985. Delayed emergence of four conifer species on postfire seedbeds in eastern Canada. Canadian Journal of Forest Research. 15(4): 727-729. [7882]
367. Thompson, Robert S.; Anderson, Katherine H.; Bartlein, Patrick J. 1999. Digital representations of tree species range maps from "Atlas of United States trees" by Elbert L. Little, Jr. (and other publications), [Online]. In: Atlas of relations between climatic parameters and distributions of important trees and shrubs in North America--GIS files of tree species range maps. U.S. Geological Survey Professional Paper 1650 A&B. Reston, VA: U.S. Geological Survey, Geology and Environmental Change Science Center, Earth Surface Processes (Producer). Available: http://esp.cr.usgs.gov/data/atlas/little/ [2011, June 8]. [82831]
368. Todd, Susan K.; Jewkes, Holly Ann. 2006. Wildland fire in Alaska: a history of organized fire suppression and management in the last frontier. Bulletin No. 114. Fairbanks, AK: University of Alaska Fairbanks, Agricultural and Forestry Experiment Station. 63 p. [85899]
369. Tonn, William M.; Boss, Shelly M.; Aku, Peter K. M.; Scrimgeour, Garry J.; Paszkowski, Cynthia A. 2004. Fish assemblages in subarctic lakes: does fire affect fish-environment relations in northern Alberta? Transactions, American Fisheries Society. 133(1): 132-143. [48584]
370. Tonn, William M.; Paszkowski, Cynthia A.; Scrimgeour, Garry J.; Aku, Peter K. M.; Lange, Marc; Prepas, Ellie E.; Westcott, Kim. 2003. Effects of forest harvesting and fire on fish assemblages in boreal plains lakes: a reference condition approach. Transactions, American Fisheries Society. 132(3): 514-523. [47331]
371. Treseder, Kathleen K.; Mack, Michelle C.; Cross, Alison. 2004. Relationships among fires, fungi, and soil dynamics in Alaskan boreal forests. Ecological Applications. 14(6): 1826-1838. [55375]
372. Troth, John L.; Deneke, Frederick J.; Brown, Lloyd M. 1976. Upland aspen/birch and black spruce stands and their litter and soil properties in interior Alaska. Forest Science. 22(1): 33-44. [8161]
373. Tsuyuzaki, Shiro; Narita, Kenji; Sawada, Yuki; Kushida, Keiji. 2014. The establishment patterns of tree seedlings are determined immediately after wildfire in a black spruce (Picea mariana) forest. Plant Ecology. 215(3): 327-337. [87729]
374. Turetsky, M. R.; Wieder, R. K. 2001. A direct approach to quantifying organic matter lost as a result of peatland wildfire. Canadian Journal of Forest Research. 31(2): 363-366. [40776]
375. Tuskan, Gerald A.; Laughlin, Kevin. 1991. Windbreak species performance and management practices as reported by Montana and North Dakota landowners. Journal of Soil and Water Conservation. 46(3): 225-228. [15084]
376. U.S. Department of Agriculture, Natural Resources Conservation Service. 2014. PLANTS Database, [Online]. Available: https://plants.usda.gov /. [34262]
377. U.S. Department of the Interior, Fish and Wildlife Service. 2013. Candidate species report. In: Environmental Conservation Online System, [Online]. In: Species reports. [Washington, DC]: U.S. Department of the Interior, Fish and Wildlife Service (Producer). Available: http://ecos.fws.gov/tess_public/pub/candidateSpecies.jsp. [86537]
378. Van Cleve, K.; Chapin, F. S., III; Dyrness, C. T.; Viereck, L. A. 1991. Element cycling in taiga forests: state-factor control. Bioscience. 41(2): 78-88. [13192]
379. Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. 1986. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Vol. 57. New York: Springer-Verlag. 230 p. [86253]
380. Van Cleve, K.; Dyrness, C. T.; Viereck, L. A.; Fox, J.; Chapin, F. S., III; Oechel, W. 1983. Taiga ecosystems in interior Alaska. BioScience. 33(1): 39-44. [7884]
381. Van Cleve, Keith; Viereck, Leslie A. 1981. Forest succession in relation to nutrient cycling in the boreal forest of Alaska. In: Fire and succession in conifer forests of North America. New York: Springer-Verlag: 185-211. [50633]
382. Vasiliauskas, S.; Chen, Han Y. H. 2002. How long do trees take to reach breast height after fire in northeastern Ontario? Canadian Journal of Forest Research. 32(10): 1889-1892. [43122]
383. Viereck, L. A. 1983. The effects of fire in black spruce ecosystems of Alaska and northern Canada. In: Wein, Ross W.; MacLean, David A., eds. The role of fire in northern circumpolar ecosystems. New York: John Wiley and Sons: 201-220. [7078]
384. Viereck, L. A.; Dyrness, C. T., tech. eds. 1979. Ecological effects of the Wickersham Dome fire near Fairbanks, Alaska. Gen. Tech. Rep. PNW-90. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 71 p. [6392]
385. Viereck, L. A.; Dyrness, C. T.; Batten, A. R.; Wenzlick, K. J. 1992. The Alaska vegetation classification. Gen. Tech. Rep. PNW-GTR-286. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 278 p. [2431]
386. Viereck, L. A.; Van Cleve, K.; Dyrness, C. T. 1986. Forest ecosystem distribution in the taiga environment. In: Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Vol. 57. New York: Springer-Verlag: 22-43. [85917]
387. Viereck, Leslie A. 1970. Forest succession and soil development adjacent to the Chena River in interior Alaska. Arctic and Alpine Research. 2(1): 1-26. [12466]
388. Viereck, Leslie A. 1973. Ecological effects of river flooding and forest fires on permafrost in the taiga of Alaska. In: Pewe, Troy L.; Mackay, J. Ross, chairs. Permafrost: second international conference, North American contribution; 1973 July 13-28; Yakutsk, U.S.S.R. Washington, DC: National Academy of Sciences: 60-67. [86121]
389. Viereck, Leslie A. 1973. Wildfire in the taiga of Alaska. Quaternary Research. 3(3): 465-495. [7247]
390. Viereck, Leslie A. 1975. Forest ecology of the Alaska taiga. In: Proceedings of the circumpolar conference on northern ecology; 1975 September 15-18; Ottawa, ON. Washington, DC: U.S. Department of Agriculture, Forest Service: 1-22. [7315]
391. Viereck, Leslie A. 1980. Black spruce-white spruce. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 84-85. [50019]
392. Viereck, Leslie A. 1989. Flood-plain succession and vegetation classification in interior Alaska. In: Ferguson, Dennis E.; Morgan, Penelope; Johnson, Frederic D., comps. Proceedings, land classifications based on vegetation: applications for resource management; 1987 November 17-19; Moscow, ID. Gen. Tech. Rep. INT-257. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 197-203. [6959]
393. Viereck, Leslie A.; Dyrness, C. T. 1980. A preliminary classification system for vegetation of Alaska. Gen. Tech. Rep. PNW-106. U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 38 p. [79782]
394. Viereck, Leslie A.; Foote, Joan; Dyrness, C. T.; Van Cleve, Keith; Kane, Douglas; Seifert, Richard. 1979. Preliminary results of experimental fires in the black spruce type of interior Alaska. Res. Note PNW-332. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 27 p. [7077]
395. Viereck, Leslie A.; Johnston, William F. 1990. Picea mariana (Mill.) B.S.P. black spruce. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 227-237. [13386]
396. Viereck, Leslie A.; Little, Elbert L., Jr. 1972. Alaska trees and shrubs. Agric. Handb. 410. Washington, DC: U.S. Department of Agriculture, Forest Service. 265 p. [6884]
397. Viereck, Leslie A.; Schandelmeier, Linda A. 1980. Effects of fire in Alaska and adjacent Canada--a literature review. BLM-Alaska Tech. Rep. 6; BLM/AK/TR-80/06. Anchorage, AK: U.S. Department of the Interior, Bureau of Land Management, Alaska State Office. 124 p. [28862]
398. Viglas, Jayme N.; Brown, Clarissa D.; Johnstone, Jill F. 2013. Age and size effects on seed productivity of northern black spruce. Canadian Journal of Forest Research. 43(6): 534-543. [87023]
399. Vogl, Richard J. 1964. Vegetational history of Crex Meadows, a prairie savanna in northwestern Wisconsin. The American Midland Naturalist. 72(1): 157-175. [61264]
400. Voss, Edward G. 1972. Michigan flora. Part I: Gymnosperms and monocots. Bulletin 55. Bloomfield Hills, MI: Cranbrook Institute of Science; Ann Arbor, MI: University of Michigan Herbarium. 488 p. [11471]
401. Wang, G. Geoff; Kemball, Kevin J. 2010. Effects of fire severity on early survival and growth of planted jack pine, black spruce and white spruce. The Forestry Chronicle. 86(2): 193-199. [81219]
402. Warner, Barry G.; Tolonen, Kimmo; Tolonen, Mirjami. 1991. A postglacial history of vegetation and bog formation at Point Escuminac, New Brunswick. Canadian Journal of Earth Science. 28(10): 1572-1582. [70203]
403. Wein, R. W. 1975. Vegetation recovery in Arctic tundra and forest-tundra after fire. In: ALUR Rep. 74-75-62. Ottawa, ON: Department of Indian Affairs and Northern Development, Arctic Land Use Research Program: 1-61. [12990]
404. Wein, Ross W. 1983. Fire behaviour and ecological effects in organic terrain. In: Wein, Ross W.; MacLean, David A., eds. The role of fire in northern circumpolar ecosystems. Scope 18. New York: John Wiley & Sons: 81-95. [18505]
405. Whitney, Gordon G. 1986. Relation of Michigan's presettlement pine forests to substrate and disturbance history. Ecology. 67(6): 1548-1559. [8713]
406. Wilton, W. C. 1963. Black spruce seedfall immediately following fire. The Forestry Chronicle. 39(4): 477-478. [12801]
407. Wolff, Jerry O. 1978. Food habits of snowshoe hares in interior Alaska. The Journal of Wildlife Management. 42(1): 148-153. [7443]
408. Yoshikawa, Kenji; Bolton, William R.; Romanovsky, Vladimir E.; Fukuda, Masami; Hinzman, Larry D. 2003. Impacts of wildfire on the permafrost in the boreal forests of Interior Alaska. Journal of Geophysical Research. 108: 8148. doi:10.1029/2001JD000438. [85496]
409. Youngblood, Andrew; Safford, Lawrence O. 2008. Picea A. Dietr.: spruce. In: Bonner, Franklin T.; Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 793-806. [79477]
410. Zasada, J. C.; Viereck, L. A.; Foote, M. J. 1979. Biotic factors: Annual pattern of dispersal. In: Viereck, L. A.; Dyrness, C. T., tech. eds. Ecological effects of the Wickersham Dome fire near Fairbanks, Alaska. Gen. Tech. Rep. PNW-90. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: 46-50. [85967]
411. Zasada, J. 1986. Natural regeneration of trees and tall shrubs on forest sites in interior ALaska. In: Van Cleve, K.; Chapin, F. S., III; Flanagan, P. W.; Viereck, L. A.; Dyrness, C. T., eds. Forest ecosystems in the Alaskan taiga. A synthesis of structure and function. Vol. 57. New York: Springer-Verlag: 44-73. [86265]
412. Zasada, John C.; Norum, Rodney A.; Teutsch, Christian E.; Densmore, Roseann. 1987. Survival and growth of planted black spruce, alder, aspen and willow after fire on black spruce/feather moss sites in interior Alaska. The Forestry Chronicle. 63(2): 84-88. [85354]
413. Zasada, John C.; Norum, Rodney A.; Van Veldhuizen, Robert M.; Teutsch, Christian E. 1983. Artificial regeneration of trees and tall shrubs in experimentally burned upland black spruce/feather moss stands in Alaska. Canadian Journal of Forest Research. 13(5): 903-913. [6991]
414. Zasada, John C.; Sharik, Terry L.; Nygren, Markku. 1992. The reproductive process in boreal forest trees. In: Shugart, H. H.; Leemans, R.; Bonan, G., eds. A systems analysis of the global boreal. A systems analysis of the global boreal forest. Cambridge, New York: Cambridge University Press: 85-125. [86611]
415. Zasada, John C. [n.d.]. Taiga community structure as influenced by patterns of succession, [Draft]. Fairbanks, AK: U.S. Department of Agriculture, Forest Service, Institute of Northern Forestry. 119 p. [87170]