| FEIS Home Page |
AUTHORSHIP AND CITATION:
Steinberg, Peter D. 2001. Populus balsamifera subsp. trichocarpa.
In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: https://www.fs.usda.gov
/database/feis/plants/tree/popbalt/all.html
[].
FEIS ABBREVIATION:
POPBALT
POPBAL
SYNONYMS:
Populus trichocarpa Torr. & Gray [70]
Populus balsamifera subsp. trichocarpa (Torr. & Gray) Hult. [74]
NRCS PLANT CODE [153]:
POTR2
COMMON NAMES:
black cottonwood
TAXONOMY:
The scientific name of black cottonwood is Populus balsamifera L. subsp. trichocarpa
(Torr. & Gray) Brayshaw (Salicaceae) [68,78,157]. Balsam poplar
(Populus balsamifera subsp. balsamifera) is covered in a separate FEIS review. Both
subspecies are in the Tacamahaca section of the Populus genus [42].
Black cottonwood commonly hybridizes with other members of the Tacamahaca section, such as narrowleaf cottonwood (P. angustifolia) and balsam poplar [42,84]. Balsam poplar × black cottonwood hybrids have been reported in Alaska, British Columbia, and Alberta [29,50,58,74].
Hybrids with members of the Aigeiros section are also observed. Eastern cottonwood (P. deltoides var. occidentalis) × black cottonwood hybrids have been reported near Flathead Lake, Montana [70], and Fremont cottonwood (P. fremontii) × black cottonwood hybrids (P. × parryi Sarg.) [87] grow in California [36]. Black cottonwood has also hybridized with black poplar (P. nigra), a Eurasian species [42]. Intersectional hybridization does not occur beyond the F1 generation in most cases, but this type of introgression probably occurred in the past where the ranges of eastern and western species overlap [42].
LIFE FORM:In Alaska and the Yukon Territory, black cottonwood is primarily a coastal species, though 2 interior populations have been studied in the Yukon [74]. Black cottonwood is present in British Columbia in a thin strip along the coast in the northern portion of the province and across most of the southern portion [29]. It is present in only in the southwestern part of Alberta, often intergrading with balsam poplar [70]. In Montana, black cottonwood is widespread west of the continental divide, and present in central and eastern Montana in higher elevations [60]. In California black cottonwood is widespread, though absent from the Mojave and Sonoran deserts of the southeastern portion of the state [68]. The species is present throughout Idaho, Washington, and Oregon. Black cottonwood is not widespread in Utah, Wyoming, or North Dakota. It is found only in Utah, Washington, and Wasatch counties in Utah; in Sweetwater, Uinta, and Teton counties of Wyoming; and in Foster, Golden Valley, Grand Forks, Grant, and Griggs counties of North Dakota [38]. Black cottonwood is present only in the northern part of the Baja California in 2 isolated populations [68,99].
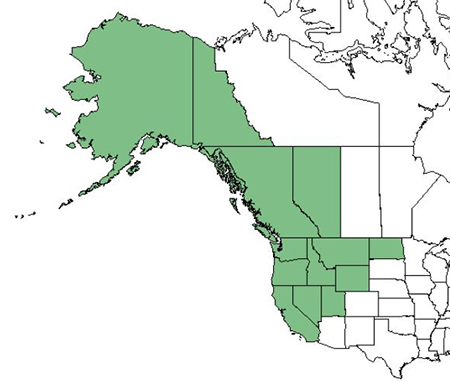 |
| Distribution of black cottonwood. Map courtesy of USDA, NRCS. The PLANTS Database. National Plant Data Team, Greensboro, NC [153]. |
| AK | CA | ID | MT | NV | ND | OR | UT | WA | WY |
| AB | BC | YK |
| MEXICO |
BLM PHYSIOGRAPHIC REGIONS [14]:
2 Cascade Mountains
3 Southern Pacific Border
4 Sierra Mountains
5 Columbia Plateau
6 Upper Basin and Range
8 Northern Rocky Mountains
16 Upper Missouri Basin and Broken Lands
KUCHLER [85] PLANT ASSOCIATIONS:
K001 Spruce-cedar-hemlock forest
K002 Cedar-hemlock-Douglas-fir forest
K003 Silver fir-Douglas-fir forest
K004 Fir- hemlock forest
K005 Mixed conifer forest
K008 Lodgepole pine- subalpine forest
K011 Western ponderosa forest
K012 Douglas-fir forest
K013 Cedar-hemlock-pine forest
K014 Grand fir-Douglas-fir forest
K015 Western spruce- fir forest
K016 Eastern ponderosa forest
K017 Black Hills pine forest
K025 Alder-ash forest
K026 Oregon oakwoods
K028 Mosaic of K002 and K026
K029 California mixed evergreen forest
K030 California oakwoods
K055 Sagebrush steppe
SAF COVER TYPES [43]:
16 Aspen
18 Paper birch
63 Cottonwood
201 White spruce
202 White spruce-paper birch
203 Balsam poplar
205 Mountain hemlock
206 Engelmann spruce-subalpine fir
210 Interior Douglas-fir
211 White fir
212 Western larch
213 Grand fir
215 Western white pine
216 Blue spruce
217 Aspen
218 Lodgepole pine
220 Rocky Mountain juniper
221 Red alder
222 Black cottonwood-willow
223 Sitka spruce
224 Western hemlock
225 Western hemlock-Sitka spruce
226 Coastal true fir-hemlock
227 Western redcedar-western hemlock
228 Western redcedar
229 Pacific Douglas-fir
230 Douglas-fir-western hemlock
233 Oregon white oak
234 Douglas-fir-tanoak-Pacific madrone
235 Cottonwood-willow
237 Interior ponderosa pine
243 Sierra Nevada mixed conifer
244 Pacific ponderosa pine-Douglas-fir
246 California black oak
247 Jeffrey pine
249 Canyon live oak
250 Blue oak-foothills pine
251 White spruce-aspen
252 Paper birch
SRM (RANGELAND) COVER TYPES [130]:
107 Western juniper/big sagebrush/bluebunch wheatgrass
109 Ponderosa pine shrubland
110 Ponderosa pine-grassland
201 Blue oak woodland
202 Coast live oak woodland
203 Riparian woodland
205 Coastal sage scrub
314 Big sagebrush-bluebunch wheatgrass
315 Big sagebrush-Idaho fescue
316 Big sagebrush-rough fescue
324 Threetip sagebrush-Idaho fescue
401 Basin big sagebrush
402 Mountain big sagebrush
403 Wyoming big sagebrush
404 Threetip sagebrush
405 Black sagebrush
406 Low sagebrush
407 Stiff sagebrush
408 Other sagebrush types
409 Tall forb
422 Riparian
901 Alder
917 Tall shrub swamp
921 Willow
HABITAT TYPES AND PLANT COMMUNITIES:
Black cottonwood grows in conifer, hardwood, and mixed conifer-hardwood communities. It is prevalent in riparian communities. Regional occurrences are discussed below.
Alaska: In the Tanana, Yukon, and Susitna valleys, black cottonwood and balsam poplar stands are some of the most productive in Alaska. American green alder (Alnus viridus var. crispa) and thinleaf alder (A. incana subsp. tenuifolia) are usually present throughout stand development; willows (Salix spp.) are less common when cottonwood and balsam poplar are mature. Common associates include prickly rose (Rosa acicularis), viburnum (Viburnum spp.), red-osier dogwood (Cornus sericea), devil's club (Oplopanax horridus), horsetails (Equisetum spp.), and bluejoint (Calamagrostis canadensis) [102]. On Sheep Creek, near Juneau, Alaska, black cottonwood grows with salmonberry (Rubus spectabilis), elderberry (Sambucus spp.), cranberry, and Sitka alder (Alnus viridis subsp. sinuata) [13]. On the Kenai Peninsula black cottonwood is common on wet sites; co-dominants include balsam poplar, black spruce (Picea mariana), Scouler's willow (Salix scouleriana), Barclay's willow (S. barclayi), and American green alder [136,156].
Yukon: Stanek and others [138] describes "Populus balsamifera" (likely including black cottonwood, balsam poplar, and their hybrids) in the mountain alder (Alnus incana)-balsam poplar-field horsetail (Equisetum arvense) vegetation type as well as the balsam poplar-arctic lupine (Lupinus arcticus)-bog birch (Betula nana) vegetation type. The former is confined to permafrost-free terraces and floodplains, and the latter is present on southwest aspects on lower slopes on alluvium.
British Columbia: On floodplains, black cottonwood is dominant, commonly with red-osier dogwood. Western redcedar (Thuja plicata) and hybrid white spruce (Picea glauca) × Engelmann spruce (P. engelmannii), though present, typically do not become dominant because of frequent flooding and sediment deposition [58]. Other codominants in these black cottonwood communities include Pacific willow (Salix lucida subsp. lasiandra), red alder (Alnus rubra), Sitka alder, Himalayan blackberry (Rubus discolor), devil's club, stink currant (Ribes bracteosum), and occasionally Sitka spruce (Picea sitchensis) [82]. Forbs in black cottonwood dominated floodplains include fragrant bedstraw (Galium triflorum), horsetails, liverleaf wintergreen (Pyrola asarifolia), small enchanter's nightshade (Circaea alpina), common lady fern (Athyrium filix-femina), starry false Solomon's-seal (Maianthemum stellatum), sweetcicely (Osmorhiza berteroi), dwarf red blackberry (Rubus pubescens), bride's feathers (Aruncus dioicus), common cowparsnip (Heracleum maximum), stinging nettle (Urtica dioica), slough sedge (Carex obnupta), and arctic sweet coltsfoot (Petasites frigidus var. palmatus) [58]. Along the Blaeberry River near Golden, British Columbia, black cottonwood and white spruce are codominant on "stable" islands; dwarf fireweed (Epilobium latifolium) is dominant in the understory and Hervey's aster (Aster hesperius), Lindley's aster (A. ciliolatus), scarlet Indian paintbrush (Castilleja miniata), northern green orchid (Platanthera hyperborea), and Aleutian selfheal (Prunella vulgaris) are also present [47]. Other woody species in these floodplain communities include quaking aspen (Populus tremuloides), Farr's willow (Salix farriae), and Wolf's willow (S. wolfii).
Western Washington: Black cottonwood is an early seral species in western hemlock (Tsuga heterophylla) communities including western hemlock/skunk cabbage (Lysichiton americanus), western hemlock/common lady fern, and western hemlock/devil's club habitat types. It is also present in wet and/or cool grand fir (Abies grandis), Douglas-fir, and western redcedar habitat types [148]. On the Hoh River on the Olympic Peninsula, a Sitka spruce-bigleaf maple-black cottonwood association occurs on first terraces. Subdominant trees include red alder and Douglas-fir and western hemlock if the site is not disturbed. The understory of these communities includes the grasses redtop (Agrostis gigantea) and fowl bluegrass (Poa palustris) and the forbs Oregon oxalis (Oxalis oregana), western sword fern (Polystichum munitum), and small enchanter's nightshade [45].
Eastern Washington and northern Idaho: Black cottonwood frequently occurs (though never in great abundance) in the seral stages of the development of western hemlock and western redcedar habitat types, and is sometimes a minor component of old-growth stands of these types [100]. Black cottonwood is prevalent at lower elevations on floodplains and terraces where frequently it is mixed with ponderosa pine and Douglas-fir [34,89]. In drier areas, like the Palouse River, black cottonwood is dominant with western water hemlock (Cicuta douglasii) dominant in the understory. Many of these communities have been disturbed by human activities [33]. In mountainous areas of eastern Washington, Idaho, and northwestern Montana, black cottonwood sometimes grows in avalanche chutes with mountain alder, Saskatoon serviceberry, Rocky Mountain maple (Acer glabrum), and quaking aspen [57].
Oregon: In bottomland forests of the Willamette Valley of western Oregon, black cottonwood, Douglas-fir (Pseudotsuga menziesii), big-leaf maple (Acer macrophyllum), and white ash (Fraxinus americana) are common, all occurring with about the same density. Subdominant trees in these communities include Oregon white oak (Quercus garryana), California laurel (Umbellularia californica), alder (Alnus spp.), bitter cherry (Prunus emarginata), and willows, and the understory usually includes Oregon- grape (Mahonia repens), salmonberry, rose (Rosa spp.), Douglas' spiraea (Spiraea douglasii), Pacific ninebark (Physocarpus capitatus), and Pursh's buckthorn (Frangula purshiana). Many of these community types have declined due to cultivation and hydrologic alterations [55,137]. In the Catherine Creek watershed of eastern Oregon, black cottonwood is dominant with ponderosa pine (Pinus ponderosa) in structurally diverse riparian communities. Subdominants include mountain alder, black hawthorn (Crataegeus douglasii), Woods' rose, Kentucky bluegrass (Poa pratensis), and common snowberry (Symphoricarpos albus). Black cottonwood is commonly the only tree present on recently formed gravel bars [80].
California: In the North Coast Ranges and Klamath Mountains, black cottonwood grows with red alder; older stands with less disturbance also support grand fir, Sitka spruce, Douglas-fir, western redcedar, or western hemlock. Black cottonwood is dominant in montane riparian forests of the Sierra Nevada where Jeffrey pine (Pinus jeffreyi) is a common associate; shrub cover (red-osier dogwood, western juniper (Juniperus occidentalis), willows, and common chokecherry) is commonly about 25% [71]. In southern California, Fremont cottonwood is dominant along washes and streams in lower elevations and black cottonwood is dominant in similar habitats at higher elevations [109,147]. Common associates in the black cottonwood communities include bigleaf maple, white alder (Alnus rhombifolia), willows, and California laurel [147]. In the South Coast Ranges, Fremont cottonwood and California sycamore (Platanus racemosa) are dominant, but black cottonwood and coast live oak (Quercus agrifolia) are also present [71]. Off the coast near Santa Barbara, on Santa Rosa, Santa Cruz, and Santa Catalina islands, riparian woodlands are dominated by black cottonwood with Fremont cottonwood; Tracy willow (Salix lasiolepis var. lasiolepis) and blue elder (Sambucus mexicana) are also present. Herbaceous vegetation consists of saltgrass (Distichlis spicata), annual rabbitsfoot grass (Polypogon monspeliensis), redtop, and tropical medicineplant (Adenostoma verbesina) [28,111]. Near drainageways in the San Dimas Forest near Los Angeles, black cottonwood is a dominant with coast live oak, California sycamore, bigleaf maple, white alder, and willows in open stands [41].
Alberta: In southern Alberta, most riparian communities in the semi-arid portion of the province are dominated by cottonwoods, including black cottonwood, eastern cottonwood (Populus deltoides), narrowleaf cottonwood (P. angustifolia), and balsam poplar. Shrubs include common chokecherry (Prunus virginiana), Saskatoon serviceberry (Amelanchier alnifolia), Wood's rose, and western snowberry (Symphoricarpos occidentalis) [50].
North Dakota: Black cottonwood occurs with green ash (Fraxinus pennsylvanica) and American elm (Ulmus americana) in riparian areas in western North Dakota [72].
Montana: In western Montana, the most common codominant tree with black cottonwood is ponderosa pine. Others include Douglas-fir, Engelmann spruce, western redcedar, Rocky Mountain juniper (Juniperus scopulorum), and quaking aspen. The shrub component includes Wood's rose, mountain alder, Bebb willow (Salix bebbiana), common chokecherry, red-osier dogwood, western snowberry, and common snowberry [61]. Herbaceous species commonly dominant include quackgrass (Elytrigia repens), Kentucky bluegrass, creeping bentgrass (Agrostis stolonifera), starry false Solomon's seal, Virginia strawberry (Fragaria virginiana), and fragrant bedstraw [61,63]. In central and eastern Montana black cottonwood grows with narrowleaf and eastern cottonwoods in transitional zones (narrowleaf and eastern cottonwoods are dominant at lower elevations) [61]. Along the North Fork of the Flathead River, near Glacier National Park, 3 black cottonwood community types were studied by Jenkins and Wright [76]. In early successional communities the black cottonwood overstory is approximately 50%. Coniferous canopy cover is low (<5%), and shrubs are not prominent. Other species present on these sites include common yarrow (Achillea millefolium), mountain alder, asters (Aster spp.), fireweed (Epilobium spp.), prairie Junegrass (Koeleria macrantha), timothy (Phleum pratense), bluegrass (Poa spp.), and Canada goldenrod (Solidago canadensis). In later-successional community types the canopy cover of hybrid spruce (white spruce × Engelmann spruce) increases to approximately 50% and gradually replaces black cottonwood. Shrubs include red-osier dogwood, roses (Rosa spp.), and common snowberry; these species become less prominent with increasing hybrid spruce canopy cover.
Eastern and southern Idaho: Black cottonwood/red-osier dogwood is a common community type; it develops best along large rivers but is also present in narrow bands along small streams in the subalpine zone [59]. Subdominant members of the overstory include narrowleaf cottonwood, lanceleaf cottonwood (P. acuminata), and peachleaf willow (Salix amygdaloides var. wrightii). Common shrub associates include sandbar willow (Salix exigua), water birch (Betula occidentalis), yellow willow (Salix lutea), and Wood's rose [59,75].
Nevada: Confined to riparian areas, black cottonwood commonly grows with lanceleaf and narrowleaf cottonwoods, with these 3 species having approximately 85% canopy cover. Wood's rose is prominent in these habitats (25-90% canopy cover); currants (Ribes spp.) and red-osier dogwood are also common [91]. In central Nevada, black cottonwood is sometimes associated with big sagebrush (Artemisia tridentata) [16].
Classifications describing plant communities in which black cottonwood is a dominant species are as follows:
Alaska [18,154]Flowers grow in catkins. Staminate catkins are usually about 1 inch (2-3 cm) long, though occasionally up to 2 inches (5 cm) long. Pistillate catkins are 3 to 8 inches (8-20 cm) long [69]. Capsules are subsessile and 0.2 to 0.32 inch (5-8 mm) long; each capsule contains 3 (occasionally 2 or 4) carpels [157]. Seeds bear long, white, "cotton" fibers that aid in dispersal via wind and/or water [58]. Each cottonwood seed weighs approximately 0.3 to 0.6 mg [20].
Much genetic variation occurs in black cottonwood with respect to flooding tolerance [131] and cold tolerance [93]; responses to these extremes are influenced by the site from which experimental cuttings are gathered [93,131].
Roots: Black cottonwood develops a "shallow, spreading root system" [11]. In the Tacamahaca section rooting depth is commonly 10 to 16 feet (3-5 m), occasionally deeper [20]. Roots of the Populus genus have well-developed laterals. Eighty percent of the roots of a black cottonwood × plains cottonwood hybrid were horizontal roots (oriented between 0° and 30°). Horizontal roots grow between approximately 2 and 8 inches (5 to 20 cm) below the soil surface. Some roots observed in this study, termed "sinker" roots, originated as horizontal roots, extended laterally a short distance, and then grew downward much like a taproot [114]. Fine root production in black cottonwood is episodic and much more variable than the production and survival of leaves [114].
Mycorrhizal colonization of cottonwoods may be influenced by stand development stage and site characteristics. Members of the Populus genus are typically ectomycorrhizal [39,114,149], but may be colonized by arbuscular mycorrhizal fungi early in stand development or when the site is flooded [39].
RAUNKIAER [115] LIFE FORM:Cottonwood establishment by seed is episodic, depending on seed viability at time of deposition (which depends on the magnitude of flows) and on moisture conditions in the first month of growth [90]. Suitable conditions for seedling establishment sometimes occur (erratically) in approximately 5 to 10 year intervals [20]. Scott and others [129] studied patterns of cottonwood recruitment with respect to different fluvial processes. They found that studying recruitment is often biased by dating trees from growth rings at ground surface; this method was found to underestimate by an average of 5.1 years (range was 0 to 34 years) because of sediment deposition after establishment. Because of light requirements seedling establishment seldom occurs on sites where cottonwood is already dominant and other vegetation is established [20]. Most regeneration in established forests is by "root suckers" or coppice sprouts. On "older islands" studied in southern British Columbia, root suckers were the most common means of regeneration. On more recently exposed sites seedlings were 95% of dense stands; the other 5% were derived from twigs [48].
Scott and others [129] summarized how different geomorphic processes influence the type of cottonwood stand that will be established:
| Fluvial process | Flow | Landform | Community patterns |
| Narrowing | one to several years of flow less than that necessary to mobilize channel bed | channel bed | variable spatial patterns; usually not even-aged stands |
| Meandering | frequent moderate flows | point bars | moderate number of even-aged stands, arranged in narrow, arcuate bands; strong left-bank, right-bank asymmetry in ages based on meander pattern; flood training or stems common |
| Flood deposition | infrequent high flows | flood deposits | small number of linear, even-aged stands; flood training of stems rare |
Asexual regeneration: Cottonwood recruitment is commonly asexual, via root suckering [2,20,42,58], coppice sprouting [58,60], or cladoptosis (the physiological abscission of twigs with leaves still attached) [20,48]. The means of regeneration depends site characteristics and whether or not black cottonwood is already present.
Cladoptosis is an important means of regeneration of black cottonwood on gravel bars [37]. This means of asexual reproduction is most viable in moist climates; because of hot, dry summers it apparently does not occur in Alberta [51]. In laboratory conditions up to 95% of freshly collected, abscised twigs produced roots (the greatest rooting was in twigs shed in October) [48]. Branches of black cottonwood that are broken off often grow when deposited in fresh alluvium; this was observed following the Mt. St. Helens mudflows [1].
Rood and others [121] studied origins of cottonwood saplings along the Oldman River in Alberta. Where sprouting occurred it was most often the result of flood damage. When floods removed stems, root suckering was induced, and when floods buried stems, shoot sprouting was induced [121]. Hansen and others [60] stated that the sprouting ability of black cottonwood is less than that of narrowleaf cottonwood but greater than that of eastern cottonwood [60]; the findings of Rood and others [121], given below, at least partially refute that.
| Species | Seedlings (%) | Root suckers (%) | Shoot suckers (%) | n |
| Narrowleaf cottonwood | 23.7 | 55.9 | 20.3 | 59 |
| Balsam poplar (includes some black cottonwood and hybrids) | 25.6 | 41.9 | 32.6 | 43 |
| Eastern cottonwood | 27.6 | 0 | 72.4 | 76 |
Root suckering in narrowleaf, plains and balsam poplar (subspecies not specified, but likely P. b. subsp. balsamifera) in pots was studied by Schier and Campbell [125]. Results for balsam poplar are presented below (n= 30 to 90):
| Clone 1 | Clone 2 | |
| Root collection date | 9/4 | 10/7 |
| Mean age (years) | 8.5 | - |
| # root segments | 30 | 60 |
| Sucker emergence (days) | 32 | 30 |
| Segments suckering (%) | 60 | 90 |
| Suckers/segment | 16.9 | 13.2 |
| Segments with surface suckers (%) | 100 | 93 |
| Surface suckers/segment | 4.9 | 6.0 |
| Height of tallest sucker (mm) | - | 66 |
Climate: Black cottonwood grows in a wide variety of climates, including coastal areas receiving 140 inches (3,500 mm) of annual precipitation and arid areas receiving 6 to 8 inches (150 to 200 mm), though in the latter climate the species is more restricted to wet habitats [11]. Black cottonwood is not present in the most humid areas of British Columbia (immediately along the coast); it is more common in "marginally subalpine climates" [58].
Elevation ranges of black cottonwood are summarized below:
| State | Elevation range | Reference |
| AK | sea level-2,000 feet (0-600 m) | [36] |
| CA | 0-10,000 feet in the southern Sierra Nevada, to 9,200 feet (2,800 m) in the northern part of the state | [53] |
| ID | 4,600-7,400 feet (1,390-2,100 m) | [59] |
| MT (western) | 4,600-7,400 feet (1,390-2,100 m), < 4,600 feet (1,390 m) east of the Continental Divide | [59,60] |
| UT | 4,500-7,700 feet (1,370-2,350 m) | [160] |
| WA (Cascade Range) | 0-5,000 feet (1,500 m) | [36] |
| WY (Wind River Range) | dominant between 4,500 and 5,000 feet (1,370-1,520 m) | [106] |
| BC | 0-6,900 feet (2,100 m) | [58] |
| Baja California | 4,600 feet (1,400 m) and 4,340 feet (1,325 m) | [99] |
Water table: Harrington [64] states that black cottonwood is very tolerant of short-duration flooding, and Hansen and others [61] have found it to be "very tolerant of frequent and prolonged flooding." In any case, the water level is close to the surface in nearly all areas where black cottonwood is dominant [46], though sometimes black cottonwood colonizes avalanche chutes on steep slopes [57]. Because black cottonwood requires water with dissolved oxygen content, it grows better along moving water than near stagnant water [58].
SUCCESSIONAL STATUS:In wet climates in forest clearings on non-alluvial sites black cottonwood is a common pioneer, but these stands are generally small and soon overtaken by secondary succession conifers such as Douglas-fir, western hemlock, western redcedar, Sitka spruce, Engelmann spruce, white spruce, or grand fir. Similarly, black cottonwood, in moist climates like that of the Pacific Northwest, colonizes agricultural clearings where mineral soil is exposed and light penetration is high [20].
In southeastern Alaska, stages of community development include pioneer, mid-successional willow-alder, and climax white spruce-western hemlock communities. The pioneer community includes mosses and lichens (Rhacomitrium and Sterocaulon) with mountainavens (Dryas spp.). The mid-successional willow-alder stage includes Sitka alder, black cottonwood, Sitka willow (Salix sitchensis), and Alaska bog willow (S. fuscescens). Following the willow-alder stage, in the absence of disturbance, white spruce and western hemlock eventually become dominant [6]. In a generalized model of forest succession following fire in Alaska, Viereck [154] stated that balsam poplar and black cottonwood were not "climax" species but were invaders, along with quaking aspen, after fires on relatively warm sites. Stands 70 to 80 years after fire consist "of white spruce and hardwood trees without a closed overstory" [156].
On the Hoh River of the Olympic Mountains in Washington, 5 successional stages are defined with respect to site age. Red alder and Scouler's willow are dominant on the newest gravel bars, red alder alone dominates 80- to 100-year-old floodplains, an Engelmann spruce-bigleaf maple-black cottonwood association is present on "1st terraces" that are approximately 400 years old, Sitka spruce and western hemlock dominate on "2nd terraces" approximately 750 years old, and western hemlock is the dominant on "3rd terraces" [45].
On the McKenzie River of Oregon, 3 early distinct types of early successional vegetation were identified: black cottonwood communities, red alder communities, and willow communities. In the absence of fire or flooding the black cottonwood stage is succeeded by a western hemlock/western sword fern/Oregon oxalis climax; in the presence of fire, the black cottonwood stage is followed by a Douglas-fir/beaked hazelnut (Corylus cornuta)/western sword fern community [65].
In black cottonwood habitat types of southern and eastern Idaho, black cottonwood establishes on recent gravel bars and remains dominant if there is continual flooding and sediment deposition. Without this disturbance the community is gradually replaced by Douglas-fir, Engelmann spruce, subalpine fir, or Rocky Mountain juniper, but occasionally yellow willow or Geyer's willow (Salix geyeriana) will become dominant [59]. Similar succession has been described in California, where it was found that flood control leads to conifer types in California [71].
In the Swan Valley of western Montana, on wet portions of grand fir habitat types, paper birch (Betula papyrifera) and black cottonwood pioneer clearcuts or seed-tree cuts where mineral soil has been exposed; after these partially develop, Douglas-fir, grand fir, western white pine (Pinus monticola), and spruce establish [7]. In Glacier National Park, as well as Oregon, Washington, and British Columbia, black cottonwood is a common, though often short-lived component of early to mid-seral successional stages in western redcedar-western hemlock forests [56]
SEASONAL DEVELOPMENT:Seasonal development of black cottonwood has been studied in detail in British Columbia, Yellowstone National Park, and northern Idaho. Black cottonwood flowers in late March or early April until late May in coastal British Columbia, though in the interior flowering may not occur until the middle of June. Leaves appear after flowers. Fruit ripens about a month after flowers appear; seed dispersal occurs between late April and July [58].
Phenology of black cottonwood was studied in Yellowstone National Park (east of continental divide) [126]:
| Bud burst | Leaves fully grown | Flowers start | Flowers end | Fruit ripe | Leaves start to color or wither | Leaves begin to fall | Leaves fallen and withered | |
| Average date | May 14 | June 20 | May 11 | June 5 | July 2 | September 10 | September 23 | October 14 |
| Earliest date | April 28 | June 10 | April 25 | April 10 | May 17 | July 23 | August 10 | October 5 |
| Latest date | May 29 | July 12 | June 4 | July 12 | July 19 | September 19 | October 3 | October 22 |
| Standard error | 3 | 2 | 3 | 10 | 7 | 4 | 4 | 2 |
| Sample size | 14 | 14 | 10 | 10 | 10 | 14 | 14 | 14 |
Northern Idaho phenology is described below (west of continental divide) [126]:
| Bud burst | Leaves fully grown | Flowers start | Flowers end | Fruit ripe | Seed fall begins | Leaves start to color or wither | Leaves begin to fall | Leaves fallen and withered | |
| Average date | April 30 | May 30 | May 19 | June 11 | August 14 | September 19 | September 15 | September 28 | October 14 |
| Earliest date | April 20 | May 1 | April 1 | May 15 | June 25 | August 18 | August 20 | September 15 | October 2 |
| Latest date | May 14 | July 13 | June 16 | July 2 | September 5 | October 21 | October 25 | October 10 | October 26 |
| Standard error (days) | 2 | 5 | 4 | 3 | 5 | 13 | 5 | 2 | 2 |
| Sample size | 13 | 13 | 15 | 15 | 14 | 5 | 15 | 16 | 14 |
Black cottonwood sprouts from stumps, charred boles, root crowns, or lateral roots following fire [25,29,59,98,110] and because of this has been referred to as a fire "endurer" rather than "resister" [3]. Fire-induced sprouting is more common in the Tacamahaca section than in the Aigeiros section. Rates of sprouting are highest if fire occurs when cottonwoods are dormant [50]; this time also has the highest probability of fire, with late summer and fall, or late winter (in low snow years) most common [59]. In general older trees sprout less than young trees [50], and sprout survival is highest when the water table is close to the surface [59]. In 1986, Coates and Haeussler [29] stated that there was little information regarding black cottonwood sprout vigor after fire, or impact of fire severity on sprouting potential. Though this is still at least partially true, a study of clonal reproduction after fire in Alberta by Gom and Rood [50] provides much useful data that is summarized in the "Fire Effects" section of this species summary.
Black cottonwood is not only a fire "endurer" but also a fire "invader." Fire can improve seedling establishment by increasing light penetration and exposing mineral soil to allow seedling establishment if moisture is available [3,25]. Increases in light penetration following fire also aid establishment [25]. Black cottonwood seedlings have been observed 1 year after stand-replacing fire in upland ponderosa pine/Rocky Mountain Douglas-fir habitat on the Bitterroot National Forest, Montana. The seedlings grew in cavities in mineral soil after the root systems of large trees had burned [132]. A study of seedling establishment (artificially seeded) of balsam poplar after experimental fires in a black spruce habitat demonstrated the dependency of the species on mineral soil exposure for germination and survival. Fire was prescribed on 1 m2 plots. On moderately burned plots 17 black cottonwood germinated, 1 survived 1 year, and 0 survived 3 years. On heavily burned plots 71 germinated, 39 survived 1 year, and 39 survived 3 years [159].
Fire is infrequent on recently formed gravel bars, but when it does occur, damage to cottonwoods is greatest because their root systems have not developed [59].
Fire Regimes: Because of high fuel moisture content and rapid decomposition of litter in riparian forests, historical fire frequency in black cottonwood communities was probably less than in adjacent upland areas. However, wind-driven fires beginning in upland communities may spread to adjacent riparian forests, particularly when fuel accumulation on upland sites has increased as a result of fire exclusion [133]. Arno [10] states that black cottonwood forests along major rivers of the Pacific Northwest likely burned frequently, as they were historically surrounded by communities characterized by high fire frequency, such as ponderosa pine savannas or sagebrush steppes. Fires could have easily spread from adjacent communities, particularly prior to widespread livestock grazing and irrigation, when dry grassy fuels were more continuous than they are currently [10]. When fires do occur in black cottonwood communities, they are most severe in old stands with heavy fuel accumulations [50,133].
Fire return intervals for plant communities and ecosystems in which black cottonwood is dominant are summarized below. Find further fire regime information for the plant communities in which this taxon may occur by entering the plant name in the FEIS home page under "Find Fire Regimes".
| Community or Ecosystem | Dominant Species | Fire Return Interval Range (years) |
| grand fir | Abies grandis | 35-200 [9] |
| sagebrush steppe | Artemisia tridentata/Pseudoroegneria spicata | 20-70 |
| Rocky Mountain juniper | Juniperus scopulorum | < 35 [9] |
| western larch | Larix occidentalis | 25-100 |
| Engelmann spruce-subalpine fir | Picea engelmannii-Abies lasiocarpa | 35 to > 200 |
| blue spruce* | P. pungens | 35-200 [15] |
| Rocky Mountain lodgepole pine* | Pinus contorta var. latifolia | 25-300+ [8,9,119] |
| western white pine* | P. monticola | 50-200 |
| interior ponderosa pine* | P. ponderosa var. scopulorum | 2-10 [9] |
| eastern cottonwood | Populus deltoides | < 35 to 200 [105] |
| quaking aspen (west of the Great Plains) | P. tremuloides | 7-120 [15,54,97] |
| Rocky Mountain Douglas-fir* | Pseudotsuga menziesii var. glauca | 25-100 [9] |
| coastal Douglas-fir* | P. menziesii var. menziesii | 40-240 [9,101,117] |
| western redcedar-western hemlock | Thuja plicata-Tsuga heterophylla | > 200 [9] |
| western hemlock-Sitka spruce | Tsuga heterophylla-Picea sitchensis | > 200 [9,108] |
| elm-ash-cottonwood | Ulmus-Fraxinus-Populus spp. | < 35 to 200 [40,155] |
Hardwood saplings (cottonwood, alder, willow) less than 2 inches (5 cm) in diameter experienced 100% mortality in 168 and 42 BTU/second/foot intensity fires in a white spruce, quaking aspen, and balsam poplar (likely including some black cottonwood and hybrids) stand northeast of Edmonton, Alberta [81].
Hall and Hansen [59] stated that in low and moderate severity fires older black
cottonwood trees
with thick bark may not be top-killed, but little quantitative data examining
the relations between fire severity, tree age, and top-kill or mortality is
available.
PLANT RESPONSE TO FIRE:
One year after the fires along the Oldman River in Alberta (described above),
approximately 75% of the top-killed cottonwoods (including all narrowleaf and
black cottonwoods, and balsam poplar)
produced shoots from remnants of trunks. Sprouting was not observed on
control transects (on adjacent unburned areas). Of the trunks producing sprouts, 90% were from either
narrowleaf or black cottonwood (the 2 species' sprouts were not easily
differentiated). The average number of coppice sprouts was 17 per trunk with a
range of 1 to 60. Height of the tallest sprout on each tree averaged
16 inches (41
cm) and ranged from 3.5 to 30 inches (9-77 cm). The authors speculated that the high
rate of sprouting was in part due to the occurrence of the fires while the
cottonwoods were dormant. The degree of canopy or trunk damage did not have much impact on coppice
sprouting frequency or vigor. Sprouts were even observed from trunks that had burned
to 10 inches (25 cm) below ground. Of trees that experienced "heavy" canopy damage,
60% produced shoots from stumps; 80% of those with "light" damage produced coppice sprouts. The average height of coppice sprouts was not
significantly (p>0.05) affected by the degree of trunk damage, and trunk diameter had no significant (p>0.05)
effect on either the number of coppice
sprouts or the height of sprouts. Over 1,000 root suckers were observed on the study transects, 80% of which were
either narrowleaf or black cottonwood. The density of root suckers (including
all species) was about 1/3 m2). Suckers were
more common near trees that had many coppice sprouts. After 1 growing season the average height of
root suckers was approximately 3 feet (1 m) [50].
Transects on the Oldman River were surveyed again 5 years after fire. At this time there was an average of 4 coppice sprouts per trunk on the 30% of the cottonwoods that still had living sprouts. Average sprout height was approximately 10 feet (3 m) with a range of 5 to 16 feet (1.5 to 5 m). Of the 34 trunks with surviving coppice sprouts 33 were either narrowleaf or black cottonwood and 1 was plains cottonwood. Survival of root suckers over the 5 years was about 50%. A density of 1 sprout per 7 m2 was observed as 550 sprouts still survived on the transects. The average height of root suckers increased from about 3 feet (1 m) to 8 feet (2.4 m). Seven root suckers were present on unburned control sites; the authors stated that these might have been induced by flooding damage 2 years prior to the survey [50].
Gom and Rood [50] summarized the response of several cottonwood species to fire, showing the high sprouting/suckering ability of balsam poplar and black and narrowleaf cottonwoods relative to Fremont and eastern cottonwoods:
| Year of fire | Location | Age when studied (years) | Cottonwoods affected by fire | Success of asexual regeneration |
| about 1988 | Peace River near Fort St. John, BC | ~10 | Balsam poplar | Very good (vigorous sucker sprouts) |
| about 1992 | Red Deer River at Dinosaur Provincial Park, Brooks, AB | ~5 | Eastern cottonwood | Very poor |
| 1990 | Belly River at Lavern, AB | 7 | Narrowleaf cottonwood, black cottonwood | Very good |
| spring 1992 | Oldman River at Lethbridge, AB | 5 | Narrowleaf cottonwood, balsam poplar, black cottonwood, eastern cottonwood | Good (sucker and coppice sprouts) |
| about 1986 | South Saskatchewan River, Police Point Park, Medicine hat, AB | ~10 | Eastern cottonwood | Very poor |
| summer 1973 | Milk River near Manyberries, AB | 24 | Eastern cottonwood | Very poor |
| about 1995 | Lower Truckee River, near Nixon, NV | ~2 | Fremont cottonwood | Poor |
| about 1994 | Kane Springs Creek, south of Moab, UT | ~3 | Fremont cottonwood | Poor (sparse coppice sprouts) |
If the goal of prescribed fire is to maintain a cottonwood stand, 5 years of grazing exclusion after fire are recommended [59]. Herbivory and fire interactions have been well documented for quaking aspen stands. In the Rocky Mountains (study sites ranged from Jasper National Park, Canada to Rocky Mountain National Park, Colorado), White and others [158] found that prolific quaking aspen sprouting after fire could not overcome the effects of elk grazing, and, with a short fire return interval, quaking aspen stands would decline.
Fire can be used to maintain black cottonwood stands, but it may cause negative impacts such as erosion and sedimentation. These impacts both on the site of fire and on sites downstream can be increased by heavy postfire rainfall [133].Uses of black cottonwood include high-grade book and magazine paper [11,36], biomass production for alternative energy [66,67], pallets, boxes, and crates [36], veneer and panelling [107,152], and fiberboard and flakeboard for concealed parts of furniture [36]. Because of their rapid growth rates and propensity for sprouting from stumps after harvest, black cottonwood and its hybrids have been studied for use in intensive culture [66,67,116]. Dickmann and Stuart [37] provide much information about hybridization and genetics, planting considerations, and plantation management of cottonwoods.
IMPORTANCE TO LIVESTOCK AND WILDLIFE:
Streamside black cottonwoods contribute to favorable fish habitat by providing
streambank stability and reduced siltation, maintaining low water temperatures through
shading, increasing debris recruitment for variable stream habitats, and providing nutrient-rich litter for aquatic food webs
[58,62,63]. Black cottonwood is an important source of cover and forage (to a
lesser extent) for wildlife and livestock. Use of black cottonwood by
bird species for nesting and perching is summarized below:
| Bird species/genus | Use of black cottonwood | Other comments | Reference |
| Bald eagle, osprey, great blue heron, Canada geese | common nesting and/or perching site | Bald eagles have been observed spending 44% of perching time in black cottonwood | [58,59] |
| Woodpeckers, great-horned owls, wood ducks | cavity nesting | [11,59] | |
| Owls, hummingbirds, starlings, sapsuckers, flickers, veeries, orioles, grosbeaks, and vireos | nesting | [58] | |
| Common merganser, red-naped sapsucker, hairy woodpecker, three-toed woodpecker, northern flicker, tree swallow, red-breasted nuthatch, and mountain bluebird | cavity nesting | These species were observed using black cottonwood in Glacier National Park; black cottonwood had the 2nd highest selectivity index (% used/% available), behind quaking aspen | [27] |
| Pileated woodpecker | roosting, cavity nesting | All nests were in snags with broken tops | [94,96] |
| Red-tailed hawk | nesting | Black cottonwood and red alder (to a lesser extent) were preferred over western hemlock and Douglas-fir | [135] |
| Holarctic harlequin ducks | cavity nesting | This was observed on the Elwha River, Idaho; it is probably rare | [26] |
| Marbled murrelet | nesting | Black cottonwood is not widely used; Douglas-firs or coastal redwoods are usually selected | [12] |
| Ruffed grouse | foraging | Ruffed grouse use the buds as winter forage | [58] |
Black cottonwood is most important as a source of cover rather than forage for big game species. Elk and deer use is high in black cottonwood stands in Montana, particularly when a shrub canopy layer is well developed [59]. White-tailed deer in the Umatilla River drainage of northeastern Oregon used mature forest structural types during winter. These sites provide hiding cover which is needed in areas that are otherwise rather open. The deer used ponderosa pine, Douglas-fir, Englemann spruce, or grand fir stands during the summer [15]. A study of habitat partitioning among elk, white-tailed deer, and moose near Glacier National Park, Montana found a significant (p<0.1) preference of white-tailed deer for later-successional spruce-black cottonwood communities over early or mid- successional communities. Elk and moose had significantly preferred mid-successional black-cottonwood communities. None of the 3 ungulates was observed to prefer early successional communities [76]. Black cottonwood is used by beavers for forage and dam building [61].
In the Kenai Peninsula of Alaska, many plants are summer moose forage; winter forage consisted of willows (38% of diet in winter), Kenai birch (45%), alder (9%), black cottonwood and quaking aspen (5%) and huckleberries (Vaccinium) (2%) [156]. In British Columbia, Roosevelt elk and white-tailed deer forage on buds during winter [58]. A study of black cottonwood use by Roosevelt elk in Olympic National Park found it to be 9% of diet volume in both winter and autumn [128]. Rocky Mountain mule deer generally do not consume black cottonwood (<1% of diet) [86].
Rabbits and hares also consume black cottonwood inner bark and sometimes girdle stem bases. Black cottonwood is the most palatable species for beavers and is also a frequent source of building materials [58]. Flying squirrels nest in trunk cavities [11,59].
Livestock: Because of proximity to water, livestock use of black cottonwood sites is often heavy, even though the species is of poor to fair palatability. Grazing of black cottonwood community types in Idaho and Montana can lead to the decline of shrubs and maintenance of an open stand with Kentucky bluegrass, timothy, smooth brome (Bromus inermis), and "weedy" forbs in the understory. Ranchers sometimes use these sites as winter ranges for livestock (early season rest is recommended for continued grass productivity) [59].
PALATABILITY:| Montana | North Dakota | Wyoming | |
| Cattle | fair | poor | ---- |
| Domestic sheep | fair | fair | ---- |
| Horse | fair | poor | ---- |
| Elk | good | ---- | fair |
| Mule deer | poor | fair | fair |
| White-tailed deer | poor | fair | fair |
| Pronghorn | poor | poor | poor |
| Upland game birds | poor | poor | poor |
| Waterfowl | poor | poor | poor |
| Nongame birds | ---- | ---- | fair |
| Small mammals | ---- | ---- | good |
| Age | ADF | NDF | Cellulose | Hemicellulose |
| 7-10 years | 38.6 (2.3) | 43.9 (2.3) | 16.6 (2.8) | 24.2 (2.0) |
| 20-30 years | 34.9 (1.9) | 43.3 (3.1) | 14.2 (0.7) | 18.8 (0.9) |
| 70-80 years | 32.9 (2.7) | 39.9 (2.8) | 15.0 (1.0) | 21.5 (0.5) |
McCracken and others [88] observed the nutritional composition of moose diets on the Copper River Delta, Alaska. Here, black cottonwood leaves and twigs were found to be 6.3 % (Standard error= 1.7%) digestible protein, 54.1% (S.E.= 0.5%) digestible dry matter, and 5.1% (S.E.= 1.5%) ash. Cowan and others [30] analyzed the nutritional content of black cottonwood collected in winter in British Columbia: protein was 12.7% dry weight, ether extract was 13.2%, crude fiber was 24.2%, and nitrogen free extract was 43.1%. The energy and protein value of black cottonwood have been rated as "fair" [38].
COVER VALUE:| Montana | North Dakota | Wyoming | |
| Elk | fair | ---- | good |
| Mule deer | ---- | fair | fair |
| White-tailed deer | good | fair | good |
| Pronghorn | poor | poor | poor |
| Upland game birds | ---- | ---- | good |
| Waterfowl | ---- | ---- | poor |
| Small mammals | ---- | ---- | good |
| Nongame birds | ---- | ---- | good |
Plummer [113] evaluated the potential of intermountain plant species for rehabilitation efforts. Black cottonwood was classified as "good" for establishment via transplanting, growth rate, soil stabilization, and adaptation to disturbance, "fair" for natural spread by seed or vegetative reproduction, and "poor" for seed handling and artificial seedling establishment.
Ground scarification is critical for planting to establish a microsite favorable to light requirements. Where a living black cottonwood root system is already present, scarification may also be used to induce sprouting from roots [20].
The following guidelines have been provided for collecting and planting black cottonwood cuttings [59,60]:
McCamant and Black [93] tested black cottonwood clones from different regions for differential cold hardiness. They found considerable variation between sites, and suggested that source of material for hybrid programs or rehabilitation is an important consideration [93]. Variation between populations and even within populations has been observed with respect to flooding tolerance as well. Plants may be monitored for flooding tolerance to select appropriate cutting sources [131]. Similarly, race differentiation in photoperiodicity has also been observed [118]. Regardless of how the selection is chosen, a variety of genotypes is recommended to increase likelihood of population survival [20].
The University of Washington continues experimentation with genetic engineering of members of the Populus genus to produce hybrids that can hyperaccumulate or detoxify industrial pollutants. These species are well suited to environmental rehabilitation of this sort because they have a high transpiration rate and large, spreading root systems. A black cottonwood × plains cottonwood hybrid has been shown to absorb and metabolize trichloroethylene from soil. More trials are needed to test the effectiveness and environmental impacts of such hybrids [39].
OTHER MANAGEMENT CONSIDERATIONS:| Factor | Impacts |
| 1. Livestock grazing | consumption and trampling of seedlings - death of older trees causes gradual decline |
| 2. Water diversion | drought stress |
| 3. Development | clearing area for home development |
| 4. Nonnative, invasive plants | saltcedar (Tamarix spp.) and Russian-olive (Elaeagnus angustifolia), among others, can reduce recruitment and cover |
| 5. Stream reservoirs | many stands were lost when dams were built in the West |
| 6. Channelization | this reduces meandering, a process critical to cottonwood establishment |
| 7. Agricultural clearing | this was greatest prior to 1950, there has likely been little net change since then |
| 8. Gravel mining | excavated areas and associated infrastructure require forest clearing, but cottonwoods often regenerate asexually |
| 9. Direct harvesting | during early European-American settlement the wood was used more than it is currently |
| 10. Beavers | populations of American beavers are unnaturally high in some areas due to a lack of predators; in other areas, they have crashed to unprecedented lows |
Rood and Mahoney [123] examined decline of cottonwood forests in southern Alberta. They listed many of the above-mentioned activities. Causes of decline were, from largest to smallest impact, 1) livestock grazing, 2) agricultural clearing, 3) water diversion, 4) domestic settlement, 5) stream reservoirs, 6) increase in beaver population, 7) gravel mining, 8) direct harvesting, 9) channelization, and 10) herbicide spraying.
Major causes of decline of black cottonwood stands in eastern Oregon include: conversion of stands for pasture, farmland, or urbanization, conversion of streams from multiple to single channel systems, restriction of lateral movement of streams across floodplains, and control of flooding with dams. Overbrowsing by livestock, elk, and deer, reduced fire frequency, and logging for firewood, lumber, and pulp have also had impacts [32].
Livestock management: Though they require flooding disturbance for establishment, black cottonwood seedlings are generally not tolerant of much grazing disturbance. In southern Alberta, stress from livestock use, including foraging and trampling, is "the greatest overall impact on riparian cottonwood" stands according to Rood and others [122]. In the Wallowa Mountains of eastern Oregon, an exclosure study showed that livestock use slowed the development of black cottonwood stands and delayed succession from shrub-dominated to black cottonwood dominated communities [79]. Gravel bar vegetation surveys in the Catherine Creek watershed of eastern Oregon also highlighted the sensitivity of black cottonwood to livestock pressure. In exclosure plots mean height increased from 6 to 40 inches (15 to 101 cm) in 10 years; on plots with grazing (1.3- 1.8 ha/AUM), mean height increase was only from 5 to 10 inches (12-26 cm) in the same time period. During this study shrub (mountain alder, Bebb willow, and Missouri River willow (Salix eriocephala)) density increased significantly (p<0.01) on sites excluded from grazing, but the density of black cottonwood was not significantly different between grazed and ungrazed sites [52]. Management has sought to control livestock foraging and trampling effects on black cottonwood via short-rotation grazing and exclosure; this allows young trees approximately 5 years to establish [20].
Prolonged heavy grazing of black cottonwood community types can cause decline in shrub canopy cover and thus big game use of the site. Shrub cover can sometimes be restored with a "dramatic change" in management including elimination of grazing. The success of this depends most heavily on the level of the water table. Whether or not the goal is to restore shrub cover, early season rest from grazing is beneficial for forage production alone [59].
Timber management: Though black cottonwood has beneficial effects on bank stability, black cottonwood can have detrimental competitive effects on more economically valuable trees. This effect is greatest during the first 5 years after clearing; self-thinning at this point reduces black cottonwood abundance but many persist throughout rotation times [58]. Logging mature cottonwood produces abundant rapidly growing stump sprouts. Sprouts grow whenever the tree is cut, but cutting in November through February produces the most. Even repeated cutting (up to 5 times in 10 years) does not harm the ability to sprout from stumps. In addition twigs or roots that are covered with soil during logging often take root. When mature trees are still present, seeding is rapid. Because of these characteristics manual treatment is not effective in reducing density or cover of black cottonwood [29].
To increase habitat for nesting birds, habitat management plans are best developed for each individual species, but some general considerations include: within a 1,000 acre (400 ha) area about 50 to 100 acres (20 to 40 ha) with an old-growth structure of ponderosa pine, western larch, or black cottonwood should meet most nesting needs. This 50- to 100-acre area is most effective if scattered throughout the larger area rather than isolated, and in the remaining 900 acres (360 ha) snags are retained. Also suggested for bird habitat maintenance is that the collection of ponderosa pine, western larch, or black cottonwood for firewood be discouraged, particularly collection of snags greater than 15 inches (38 cm) in diameter [95].
Watercourse damming and water diversion: Often listed as a major detriment to cottonwoods, damming and water diversion have a multi-faceted impact on establishment, growth, and mortality. These factors were summarized by Rood and Mahoney [123] as follows:
| Possible cause of decline | Effects | |
| Hydrologic changes | A. Reduced water availability | Diversion of water offstream or well pumping creates a water deficit, resulting in drought stress, slow growth, and increased mortality |
| B. Reduced flooding | Spring flooding is essential to create moist seedbeds for seedling establishment | |
| C. Stabilized flows | Dynamic flows are essential for seedling establishment | |
| Geomorphic changes | A. Reduced meandering and channelization | With reduced flooding, channel migration is reduced and suitable seedbeds are reduced |
Impacts of watercourse damming on cottonwoods are generally negative, but also dependent on the species present, as reported by Rood and Mahoney [123]:
| River | State/province | Species | Effects | Reference |
| Missouri | ND | plains cottonwood | Reduced tree growth and less seedling establishment | [77] |
| several | AZ | Fremont cottonwood, narrowleaf cottonwood | Reduced forest abundance | [24] |
| Colorado | CA | Fremont cottonwood | Reduced forest abundance, absence of seedlings | [103] |
| South Platte | CO | plains cottonwood | Reduced forest abundance | [31] |
| Missouri | MT | plains cottonwood | Reduced forest abundance, absence of seedlings | [104] |
| Owens | CA | Fremont cottonwood | Reduced forest abundance | [23] |
| Rush Creek | CA | balsam poplar | Reduced tree abundance | [142] |
| Sacramento | CA | Fremont cottonwood | Fewer seedlings | [143] |
| Salt | AZ | Fremont cottonwood | Conditions unsuitable for seedling establishment | [44] |
| Milk | MT, AB | plains cottonwood | Reduced forest abundance, fewer saplings | [21] |
| Bighorn | WY | plains cottonwood | Reduced forest abundance | [5] |
| St. Mary, Waterton, Belly | AB | plains cottonwood, balsam poplar, narrowleaf cottonwood | Reduced forest abundance | [120] |
| Rio Grande | NM | Fremont cottonwood | Absence of seedlings | [73] |
| Bishop Creek | CA | Fremont cottonwood, balsam poplar | Smaller leaves, reduced transpiration and water potential | [151] |
| Arkansas | CO | plains cottonwood | Reduced forest abundance | [134] |
The effects of altered flood magnitude, frequency, and duration on the establishment and success of black cottonwood are thought to be less severe than those effects on other cottonwoods [84]. This is because black cottonwood is adapted to flow regimes of smaller streams with more variable flood magnitude and duration than large rivers. Stromberg and Patten [144] noted "subtle" effects of diversion on black cottonwood in the eastern Sierra Nevada, California. On Bishop Creek, where water diversion is nearly complete in normal and dry winters, black cottonwood had higher mortality (and thus lower age and size), lower density, and lower canopy foliage density than on Pine Creek, a similar size watershed with no diversion. Means (standard errors in parentheses) from the two watersheds are presented below [144]:
| Site | Elevation (m) | Canopy foliage density1 | Tree mortality2 (%) | Tree density (#/0.1 ha) | Juvenile density3 (#/0.1 ha) |
| Bishop 2 | 2,020 | 1.73 (0.16) | 27 | 28 | 98 |
| Bishop 3 | 1,800 | 0.51 (0.12) | 14 | 33 | 93 |
| Bishop 4 | 1,470 | 0.35 (0.12) | 33 | 6 | 42 |
| Pine 2 | 2,100 | 2.52 (0.25) | 9 | 41 | 199 |
| Pine 3 | 1,850 | 2.14 (0.19) | 0 | 45 | 170 |
| Pine 4 | 1,580 | 3.13 (0.37) | 11 | 38 | 125 |
Chemical treatments: Black cottonwood is "extremely sensitive" to glyphosate. In southern Alberta this herbicide and picloram are believed to have caused unintended mortality in even large trees [123]. The 2,4-D ester can damage the canopy but does not cause much mortality [29]. High black cottonwood mortality was observed following summer and fall applications of glyphosate. A mid-May spot treatment with hexazinone produced 80% to 95% defoliation and little sprouting in the Nelson Forest region of British Columbia. Broadcast treatments with 2,4-D amine have been observed to cause moderate or severe damage [58]. Balsam poplar in Alberta were susceptible to metsulfuron and 2,4-D, alone or in combination [19].
Disease and other management considerations: Black cottonwood is susceptible to Cytospora canker, particularly in fire-damaged stands or in young trees or cuttings in nurseries. Wood-decaying fungi also grow on black cottonwood; the most significant parasites of this sort include Polyporus delectans and Philota destruens [118]. Young seedlings are particularly susceptible to pathogenic fungal infection [114]. Trees, particularly young saplings, may be injured or killed by late frost either directly or indirectly via increased susceptibility to fungal decay. High winds sometimes cause damage on sites where adjacent vegetation is much shorter [36], and scouring by ice during spring runoff sometimes kills or induces sprouting from young trees [20].1. Adams, A. B.; Dale, V. H.; Smith, E. P.; Kruckeberg, A. R. 1987. Plant survival, growth form and regeneration following the 18 May 1980 eruption of Mount St. Helens, Washington. Northwest Science. 61(3): 160-170. [6886]
2. Agee, James K. 1988. Successional dynamics in forest riparian zones. In: Raedeke, Kenneth J., ed. Streamside management: riparian wildlife and forestry interactions. Institute of Forest Resources Contribution No. 58. Seattle, WA: University of Washington, College of Forest Resources: 31-43. [7657]
3. Agee, James K. 1991. Fire history of Douglas-fir forests in the Pacific Northwest. In: Ruggiero, Leonard F.; Aubry, Keith B.; Carey, Andrew B.; Huff, Mark H., tech. coords. Wildlife and vegetation of unmanaged Douglas-fir forests. Gen. Tech. Rep. PNW-GTR-285. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 25-33. [17303]
4. Agee, James K. 1993. Fire ecology of Pacific Northwest forests. Washington, DC: Island Press. 493 p. [22247]
5. Akashi, Yoshiko. 1988. Riparian vegetation dynamics along the Bighorn River, Wyoming. Laramie, WY: University of Wyoming. 245 p. Thesis. [39266]
6. Alaback, Paul B. 1984. Plant succession following logging in the Sitka spruce-western hemlock forests of southeast Alaska. Gen. Tech. Rep. PNW-173. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 26 p. [7849]
7. Antos, Joseph A.; Shearer, Raymond C. 1980. Vegetation development on disturbed grand fir sites, Swan Valley, northwestern Montana. Res. Pap. INT-251. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 26 p. [7269]
8. Arno, Stephen F. 1980. Forest fire history in the Northern Rockies. Journal of Forestry. 78(8): 460-465. [11990]
9. Arno, Stephen F. 2000. Fire in western forest ecosystems. In: Brown, James K.; Smith, Jane Kapler, eds. Wildland fire in ecosystems: Effects of fire on flora. Gen. Tech. Rep. RMRS-GTR-42-vol. 2. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 97-120. [36984]
10. Arno, Stephen F. 2001. [Personal communication]. December 12. Regarding fire regime information for cottonwood stands. Missoula, MT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory [Retired]. In: FEIS log book. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [38714]
11. Arno, Stephen F.; Hammerly, Ramona P. 1977. Northwest trees. Seattle, WA: The Mountaineers. 222 p. [4208]
12. Bell, Jack H.; Lauer, Jerry L.; Peek, James M. 1992. Habitat use patterns of white-tailed deer, Umatilla River, Oregon. Northwest Science. 66(3): 160-171. [19276]
13. Bermejo, Teresa; Traveset, Anna; Willson, Mary F. 1998. Post-dispersal seed predation in the temperate rainforest of southeast Alaska. Canadian Field Naturalist. 112(3): 510-512. [30895]
14. Bernard, Stephen R.; Brown, Kenneth F. 1977. Distribution of mammals, reptiles, and amphibians by BLM physiographic regions and A.W. Kuchler's associations for the eleven western states. Tech. Note 301. Denver, CO: U.S. Department of the Interior, Bureau of Land Management. 169 p. [434]
15. Binford, Laurence C.; Elliott, Bruce G.; Singer, Steven W. 1975. Discovery of a nest and the downy young of the marbled murrelet. Wilson Bulletin. 87(3): 303-319. [23735]
16. Blackburn, Wilbert H.; Tueller, Paul T.; Eckert, Richard E., Jr. 1968. Vegetation and soils of the Mill Creek Watershed. Reno, NV: University of Nevada, College of Agriculture. 71 p. [12500]
17. Boes, Teresa K.; Strauss, Steven H. 1994. Floral phenology and morphology of black cottonwood, Populus trichocarpa (Salicaceae). American Journal of Botany. 81(5): 562-567. [24158]
18. Boggs, Keith. 2000. Classification of community types, successional sequences, and landscapes of the Copper River Delta, Alaska. Gen. Tech. Rep. PNW-GTR-469. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 244 p. [38491]
19. Bowes, G. G.; Spurr, D. T. 1996. Control of aspen poplar, balsam poplar, prickly rose and western snowberry with metsulfuron-methyl and 2,4-D. Canadian Journal of Plant Science. 76(4): 885-889. [27519]
20. Braatne, Jeffrey H.; Rood, Stewart B.; Heilman, Paul E. 1996. Life history, ecology, and conservation of riparian cottonwoods in North America. In: Stettler, R. F.; Bradshaw, H. D., Jr.; Heilman, P. E.; Hinckley, T. M., eds. Biology of Populus and its implications for management and conservation: Part 1. Ottawa, ON: National Research Council of Canada, NRC Research Press: 57-85. [29693]
21. Bradley, Cheryl E.; Smith, Derald G. 1986. Plains cottonwood recruitment and survival on a prairie meandering river floodplain, Milk River, southern Alberta and northern Montana. Canadian Journal of Botany. 64: 1433-1442. [8920]
22. Brayshaw, T. C. 1965. The status of the black cottonwood (Populus trichocarpa Torrey and Gray). Canadian Field-Naturalist. 79(2): 91-95. [6285]
23. Brothers, Timothy S. 1984. Historical vegetation change in the Owens River riparian woodland. In: Warner, Richard E.; Hendrix, Kathleen M., eds. California riparian systems: Ecology, conservation, and productive management: Proceedings of the conference; 1981 September 17-19; Davis, CA. Berkeley, CA: University of California Press: 75-84. [5827]
24. Brown, David E.; Lowe, Charles H.; Hausler, Janet F. 1977. Southwestern riparian communities: their biotic importance and management in Arizona. In: Johnson, R. Roy; Jones, Dale A., tech. coords. Importance, preservation and management of riparian habitat: a symposium: Proceedings; 1977 July 9; Tucson, AZ. Gen. Tech. Rep. RM-43. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 201-211. [5348]
25. Brown, James K. 1996. Fire effects on aspen and cottonwood. In: Aspen and cottonwood in the Blue Mountains: Workshop; 1996 April 2-4; La Grande, OR. La Grande, OR: Blue Mountains Natural Resources Institute: 11 p. [35823]
26. Cassirer, E. Francis; Schirato, Greg; Sharpe, Fred; Groves, Craig R.; Anderson, Rusty N. 1993. Cavity nesting by harlequin ducks in the Pacific Northwest. The Wilson Bulletin. 105(4): 691-694. [22778]
27. Caton, Elaine L. 1996. Effects of fire and salvage logging on the cavity-nesting bird community in northwestern Montana. Missoula, MT: The University of Montana. 115 p. Dissertation. [28661]
28. Clark, Ronilee A.; Halvorson, William L.; Sawdo, Andell A.; Danielsen, Karen C. 1990. Plant communities of Santa Rosa Island, Channel Islands National Park. Tech. Rep. No. 42. Davis, CA: University of California, Institute of Ecology, Cooperative National Park Resources Studies Unit. 93 p. [18246]
29. Coates, D.; Haeussler, S. 1986. A preliminary guide to the response of major species of competing vegetation to silvicultural treatments. Land Management Handbook No. 9. Victoria, BC: Ministry of Forests, Information Services Branch. 88 p. [17453]
30. Cowan, I. M.; Hoar, W. S.; Hatter, J. 1950. The effect of forest succession upon the quantity and upon the nutritive values of woody plants used by moose. Canadian Journal of Research. 28(5): 249-271. [12820]
31. Crouch, Glenn L. 1979. Long-term changes in cottonwoods on a grazed and an ungrazed plains bottomland in northeastern Colorado. Res. Note RM-370. Fort Collins, CO: U.S. Department of of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 4 p. [3496]
32. Crowe, Elizabeth. 1996. Historical and present-day distribution of quaking aspen and black cottonwood in the Blue Mountains. In: Aspen and cottonwood in the Blue Mountains: Workshop; 1996 April 2-4; La Grande, OR. La Grande, OR: Blue Mountains Natural Resources Institute: 3 p. [35822]
33. Daubenmire, R. 1970. Steppe vegetation of Washington. Tech. Bull. 62. Pullman, WA: Washington State University, College of Agriculture; Washington Agricultural Experiment Station. 131 p. [733]
34. Daubenmire, Rexford F.; Daubenmire, Jean B. 1968. Forest vegetation of eastern Washington and northern Idaho. Tech. Bull. 60. Pullman, WA: Washington State University, Agricultural Experiment Station. 104 p. [749]
35. Dearness, John; Hansbrough, J. R. 1934. Cytospora infection following fire injury in western British Columbia. Canadian Journal of Research. 10(1): 125-128. [33069]
36. DeBell, Dean S. 1990. Populus trichocarpa Torr. & Gray black cottonwood. In: Burns, Russell M.; Honkala, Barbara H., tech. coords. Silvics of North America: Volume 2, Hardwoods. Agriculture Handbook 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 555-569. [38483]
37. Dickmann, Donald I.; Stuart, Katherine W. 1983. The culture of poplars in eastern North America. East Lansing, MI: Michigan State University, Department of Forestry. 168 p. [6317]
38. Dittberner, Phillip L.; Olson, Michael R. 1983. The Plant Information Network (PIN) data base: Colorado, Montana, North Dakota, Utah, and Wyoming. FWS/OBS-83/86. Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service. 786 p. [806]
39. Dix, Mary Ellen; Klopfenstein, Ned B.; Zhang, Jian-Wei; Workman, Sarah W.; Kim, Mee-Sook. 1997. Potential use of Populus for phytoremediation of environmental pollution in riparian zones. In: Klopfenstein, Ned B.; Chun, Young Woo; Kim, Mee-Sook; Ahuja, M. Raj, eds. Micropropagation, genetic engineering, and molecular biology of Populus. Gen. Tech. Rep. RM-GTR-297. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 206-211. [27768]
40. Duchesne, Luc C.; Hawkes, Brad C. 2000. Fire in northern ecosystems. In: Brown, James K.; Smith, Jane Kapler, eds. Wildland fire in ecosystems: effects of fire on flora. Gen. Tech. Rep. RMRS-GTR-42-vol. 2. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 35-51. [36982]
41. Dunn, Paul H.; Barro, Susan C.; Wells, Wade G., II; Poth, Mark A.; Wohlgemuth, Peter M.; Colver, Charles G. 1988. The San Dimas Experimental Forest: 50 years of research. Gen. Tech. Rep. PSW-104. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 49 p. [8400]
42. Eckenwalder, James E. 1996. Systematics and evolution of Populus. In: Stettler, R. F.; Bradshaw, H. D., Jr.; Heilman, P. E.; Hinckley, T. M., eds. Biology of Populus and its implications for management and conservation. Ottawa, ON: National Research Council of Canada, NRC Research Press: 7-32. [28505]
43. Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters. 148 p. [905]
44. Fenner, Pattie; Brady, Ward W.; Patton, David R. 1985. Effects of regulated water flows on regeneration of Fremont cottonwood. Journal of Range Management. 38(2): 135-138. [5489]
45. Fonda, R. W. 1974. Forest succession in relation to river terrace development in Olympic National Park, Washington. Ecology. 55(5): 927-942. [6746]
46. Foote, Geoffrey G. 1965. Phytosociology of the bottomland hardwood forests in western Montana. Missoula, MT: University of Montana. 140 p. Thesis. [17369]
47. Fyles, J. W.; Bell, M. A. M. 1986. Vegetation colonizing river gravel bars in the Rocky Mountains of southeastern British Columbia. Northwest Science. 60(1): 8-14. [5981]
48. Galloway, Glen; Worrall, J. 1979. Cladoptosis: a reproductive strategy in black cottonwood. Canadian Journal of Forest Research. 9: 122-125. [6311]
49. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; Lewis, Mont E.; Smith, Dixie R. 1977. Vegetation and environmental features of forest and range ecosystems. Agric. Handb. 475. Washington, DC: U.S. Department of Agriculture, Forest Service. 68 p. [998]
50. Gom, Lori A.; Rood, Stewart B. 1999. Fire induces clonal sprouting of riparian cottonwoods. Canadian Journal of Botany. 77(11): 1604-1616. [38169]
51. Gom, Lori A.; Rood, Stewart B. 1999. The discrimination of cottonwood clones in a mature grove along the Oldman River in southern Alberta. Canadian Journal of Botany. 77(8): 1084-1094. [33062]
52. Green, Douglas M.; Kauffman, J. Boone. 1995. Succession and livestock grazing in a northeastern Oregon riparian ecosystem. Journal of Range Management. 48(4): 307-313. [25925]
53. Griffin, James R.; Critchfield, William B. 1972. The distribution of forest trees in California. Res. Pap. PSW-82. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 118 p. [1041]
54. Gruell, G. E.; Loope, L. L. 1974. Relationships among aspen, fire, and ungulate browsing in Jackson Hole, Wyoming. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 33 p. In cooperation with: U.S. Department of the Interior, National Park Service, Rocky Mountain Region. [3862]
55. Habeck, James R. 1961. The original vegetation of the mid-Willamette Valley, Oregon. Northwest Science. 35: 65-77. [11419]
56. Habeck, James R. 1968. Forest succession in the Glacier Park cedar-hemlock forests. Ecology. 49(5): 872-880. [6479]
57. Habeck, James R. 1994. Dynamics of forest communities used by great gray owls. In: Hayward, Gregory D.; Verner, Jon, tech. eds. Flammulated, boreal, and great gray owls in the United States: a technical conservation assessment. Gen. Tech. Rep. RM-253. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 176-201. [27356]
58. Haeussler, S.; Coates, D.; Mather, J. 1990. Autecology of common plants in British Columbia: a literature review. FRDA Report 158. Victoria, BC: Forestry Canada, Pacific Forestry Center; British Columbia Ministry of Forests, Research Branch. 272 p. [18033]
59. Hall, James B.; Hansen, Paul L. 1997. A preliminary riparian habitat type classification system for the Bureau of Land Management districts in southern and eastern Idaho. Tech. Bull. No. 97-11. Boise, ID: U.S. Department of the Interior, Bureau of Land Management; Missoula, MT: University of Montana, School of Forestry, Riparian and Wetland Research Program. 381 p. [28173]
60. Hansen, Paul L.; Boggs, Keith; Pfister, Robert D.; Joy, John; Cook, Brad. 1994. Classification and management of riparian and wetland sites in Montana. In: Hamre, R. H., ed. Workshop on western wetlands and riparian areas: public/private efforts in recovery, management, and education: Proceedings; 1993 September 9-11; Snowbird, UT. Boulder, CO: Thorne Ecological Institute: 1-17. [27800]
61. Hansen, Paul L.; Chadde, Steve W.; Pfister, Robert D. 1988. Riparian dominance types of Montana. Misc. Publ. No. 49. Missoula, MT: University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station. 411 p. [5660]
62. Hansen, Paul L.; Pfister, Robert D.; Boggs, Keith; Cook, Bradley J.; Joy, John; Hinckley, Dan K. 1995. Classification and management of Montana's riparian and wetland sites. Misc. Publ. No. 54. Missoula, MT: The University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station. 646 p. [24768]
63. Hansen, Paul; Boggs, Keith; Pfister, Robert; Joy, John. 1990. Classification and management of riparian and wetland sites in central and eastern Montana. Draft Version 2. Missoula, MT: University of Montana, School of Forestry, Montana Forest and Conservation Experiment Station, Montana Riparian Association. 279 p. [12477]
64. Harrington, Constance A. 1987. Responses of red alder and black cottonwood seedlings to flooding. Physiological Plantarum. 69: 35-48. [7654]
65. Hawk, G. M.; Zobel, D. B. 1974. Forest succession on alluvial landforms of the McKenzie River Valley, Oregon. Northwest Science. 48(4): 245-265. [9686]
66. Heilman, Paul E.; Stettler, R. F. 1990. Genetic variation and productivity of Populus trichocarpa and its hybrids. IV. Performance in short-rotation coppice. Canadian Journal of Forest Research. 20: 1257-1264. [13788]
67. Heilman, Paul; Peabody, D. V., Jr. 1981. Effect of harvest cycle and spacing on productivity of black cottonwood in intensive culture. Canadian Journal of Forest Research. 11(1): 118-123. [6288]
68. Hickman, James C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p. [21992]
69. Hitchcock, C. Leo; Cronquist, Arthur. 1964. Vascular plants of the Pacific Northwest. Part 2: Salicaceae to Saxifragaceae. Seattle, WA: University of Washington Press. 597 p. [1166]
70. Hitchcock, C. Leo; Cronquist, Arthur. 1973. Flora of the Pacific Northwest. Seattle, WA: University of Washington Press. 730 p. [1168]
71. Holland, Robert F. 1986. Preliminary descriptions of the terrestrial natural communities of California. Sacramento, CA: California Department of Fish and Game. 156 p. [12756]
72. Hopkins, Rick B.; Cassel, J. Frank; Bjugstad, Ardell J. 1986. Relationships between breeding birds and vegetation in four woodland types of the Little Missouri National Grasslands. Res. Pap. RM-270. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 12 p. [2758]
73. Howe, W. H.; Knopf, F. L. 1991. On the imminent decline of Rio Grande cottonwoods in central New Mexico. The Southwestern Naturalist. 36(2): 218-224. [18278]
74. Hulten, Eric. 1968. Flora of Alaska and neighboring territories. Stanford, CA: Stanford University Press. 1008 p. [13403]
75. Jankovsky-Jones, Mabel; Rust, Steven K.; Moseley, Robert K. 1999. Riparian reference areas in Idaho: A catalog of plant associations and conservation sites. Gen. Tech. Rep. RMRS-GTR-20. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 141 p. [29900]
76. Jenkins, K. J.; Wright, R. G. 1988. Resource partitioning and competition among cervids in the northern Rocky Mountains. Journal of Applied Ecology. 25: 11-24. [16289]
77. Johnson, W. Carter; Burgess, Robert L.; Keammerer, Warren R. 1976. Forest overstory vegetation and environment on the Missouri River floodplain in North Dakota. Ecological Monographs. 46(1): 59-84. [6313]
78. Kartesz, John T. 1999. A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. 1st ed. In: Kartesz, John T.; Meacham, Christopher A. Synthesis of the North American flora (Windows Version 1.0), [CD-ROM]. Chapel Hill, NC: North Carolina Botanical Garden (Producer). In cooperation with: The Nature Conservancy; U.S. Department of Agriculture, Natural Resources Conservation Service; U.S. Department of the Interior, Fish and Wildlife Service. [36715]
79. Kauffman, J. Boone; Krueger, W. C.; Vavra, M. 1983. Effects of late season cattle grazing on riparian plant communities. Journal of Range Management. 36(6): 685-691. [16965]
80. Kauffman, J. Boone; Krueger, W. C.; Vavra, M. 1985. Ecology and plant communities of the riparian areas associated with Catherine Creek in northeastern Oregon. Tech. Bull. 147. Corvallis, OR: Oregon State University, Agricultural Experiment Station. 35 p. [6174]
81. Kiil, A. D. 1970. Effects of spring burning on vegetation in old partially cut spruce-aspen stands in east-central Alberta. Information Report A-X-33. Edmonton, AB: Canadian Forestry Service, Department of Fisheries and Forestry, Forest Research Laboratory. 12 p. [12997]
82. Klinka, Karel; Qian, Hong; Pojar, Jim; Meidinger, Del V. 1996. Classification of natural forest communities of coastal British Columbia, Canada. Vegetatio. 125(2): 149-168. [28530]
83. Kovalchik, Bernard L. 1987. Riparian zone associations: Deschutes, Ochoco, Fremont, and Winema National Forests. R6 ECOL TP-279-87. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 171 p. [9632]
84. Kranjcec, Julie; Mahoney, John M.; Rood, Stewart B. 1998. The responses of three riparian cottonwood species to water table decline. Forest Ecology and Management. 110: 77-87. [35957]
85. Kuchler, A. W. 1964. United States: Map, [Potential natural vegetation of the conterminous United States]. Special Publication No. 36. New York: American Geographical Society. 1:3,168,000; colored. [3455]
86. Kufeld, Roland C.; Wallmo, O. C.; Feddema, Charles. 1973. Foods of the Rocky Mountain mule deer. Res. Pap. RM-111. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 31 p. [1387]
87. Little, Elbert L., Jr. 1979. Checklist of United States trees (native and naturalized). Agric. Handb. 541. Washington, DC: U.S. Department of Agriculture, Forest Service. 375 p. [2952]
88. MacCracken, James G.; Van Ballenberghe, Victor; Peek, James M. 1993. Use of aquatic plants by moose: sodium hunger or foraging efficiency? Canadian Journal of Zoology. 71(12): 2345-2362. [23423]
89. Mack, Richard N. 1988. First comprehensive botanical survey of the Columbia Plateau, Washington: the Sandberg and Leiberg Expedition of 1893. Northwest Science. 62: 118-128. [5171]
90. Malanson, George P.; Butler, David R. 1991. Floristic variation among gravel bars in a subalpine river, Montana, U.S.A. Arctic and Alpine Research. 23(3): 273-278. [16470]
91. Manning, Mary E.; Padgett, Wayne G. 1989. Preliminary riparian community type classification for Nevada. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Region. Preliminary draft. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 135 p. [11531]
92. Marston, Richard A.; Anderson, Jay E. 1991. Watersheds and vegetation of the Greater Yellowstone Ecosystem. Conservation Biology. 5(3): 338-346. [21676]
93. McCamant, Thaddeaus; Black, R. Alan. 2000. Cold hardiness in coastal, montane, and inland populations of Populus trichocarpa. Canadian Journal of Forest Research. 30(1): 91-99. [38196]
94. McClelland, B. Riley. 1980. Influences of harvesting and residue management on cavity-nesting birds. In: Environmental consequences of timber harvesting in Rocky Mountain coniferous forests: Symposium proceedings; 1979 September 11-13; Missoula, MT. Gen. Tech. Rep. INT-90. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 469-514. [10315]
95. McClelland, B. Riley; Frissell, Sidney S.; Fischer, William C.; Halvorson, Curtis H. 1979. Habitat management for hole-nesting birds in forests of western larch and Douglas-fir. Journal of Forestry. August: 480-483. [9491]
96. McClelland, B. Riley; McClelland, Patricia T. 1999. Pileated woodpecker nest and roost trees in Montana: links with old-growth and forest "health". Wildlife Society Bulletin. 27(3): 846-857. [31155]
97. Meinecke, E. P. 1929. Quaking aspen: A study in applied forest pathology. Tech. Bull. No. 155. Washington, DC: U.S. Department of Agriculture. 34 p. [26669]
98. Miller, Melanie. 2000. Fire autecology. In: Brown, James K.; Smith, Jane Kapler, eds. Wildland fire in ecosystems: Effects of fire on flora. Gen. Tech. Rep. RMRS-GTR-42-vol. 2. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 9-34. [36981]
99. Minnich, Richard A. 1987. The distribution of forest trees in northern Baja California, Mexico. Madrono. 34(2): 98-127. [6985]
100. Moeur, Melinda. 1992. Baseline demographics of late successional western hemlock/western redcedar stands in northern Idaho Research Natural Areas. Res. Pap. INT-456. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 16 p. [19177]
101. Morrison, Peter H.; Swanson, Frederick J. 1990. Fire history and pattern in a Cascade Range landscape. Gen. Tech. Rep. PNW-GTR-254. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 77 p. [13074]
102. Neiland, Bonita J.; Viereck, Leslie A. 1977. Forest types and ecosystems. In: North American forest lands at latitudes north of 60 degrees: Proceedings of a symposium; 1977 September 19-22; Fairbanks, AK. Fairbanks, AK: University of Alaska, Fairbanks: 109-136. [19933]
103. Ohmart, Robert D.; Deason, Wayne O.; Burke, Constance. 1977. A riparian case history: the Colorado River. In: Johnson, Roy; Jones, Dale A., technical coordinators. Importance, preservation and management of riparian habitat: A symposium; 1977 July 9; Tucson, AZ. Gen. Tech. Rep. RM-43. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 35-47. Available from: NTIS, Springfield, VA 22151; PB-274 582. [5334]
104. Old, Sylvia M. 1969. Microclimate, fire, and plant production in an Illinois prairie. Ecological Monographs. 39(4): 355-384. [154]
105. Olson, Matthew S.; Platt, William J. 1995. Effects of habitat and growing season fires on resprouting of shrubs in longleaf pine savannas. Vegetatio. 119: 101-118. [26979]
106. Olson, R. A.; Gerhart, W. A. 1982. A physical and biological characterization of riparian habitat and its importance to wildlife in Wyoming. Cheyenne, WY: Wyoming Game and Fish Department. 188 p. [6755]
107. Overholser, J. L. 1977. Oregon hardwood timber. Corvallis, OR: Oregon State University, Forest Research Laboratory. 43 p. [16165]
108. Parminter, John. 1991. Fire history and effects on vegetation in three biogeoclimatic zones of British Columbia. In: Nodvin, Stephen C.; Waldrop, Thomas A., eds. Fire and the environment: ecological and cultural perspectives: Proceedings of an international symposium; 1990 March 20-24; Knoxville, TN. Gen. Tech. Rep. SE-69. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southeastern Forest Experiment Station: 263-272. [16651]
109. Paysen, Timothy E.; Derby, Jeanine A.; Black, Hugh, Jr.; Bleich, Vernon C.; Mincks, John W. 1980. A vegetation classification system applied to southern California. Gen. Tech. Rep. PSW-45. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 33 p. [1849]
110. Peterson, E. B.; Peterson, N. M. 1992. Ecology, management, and use of aspen and balsam poplar in the prairie provinces, Canada. Special Report 1. Edmonton, AB: Forestry Canada, Northwest Region, Northern Forestry Centre. 252 p. [22689]
111. Philbrick, Ralph N., Haller, J. R. 1977. The southern California islands. In: Barbour, Michael G.; Malor, Jack, eds. Terrestrial vegetation of California. New York: John Wiley and Sons: 893-906. [7210]
112. Pierce, John; Johnson, Janet. 1986. Wetland community type classification for west-central Montana. [Review draft]. Missoula, MT: U.S. Department of Agriculture, Forest Service, Northern Region, Ecosystem Management Program. 158 p. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [7436]
113. Plummer, A. Perry. 1977. Revegetation of disturbed Intermountain area sites. In: Thames, J. C., ed. Reclamation and use of disturbed lands of the Southwest. Tucson, AZ: University of Arizona Press: 302-337. [27411]
114. Pregitzer, Kurt S.; Friend Alexander L. 1996. The structure and function of Populus root systems. In: Steller, R. F., ed. Biology of Populus and its implications for management and conservation. Ottawa, ON: NRC Research Press: 331-354. [28723]
115. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford, England: Clarendon Press. 632 p. [2843]
116. Rhodenbaugh, E. J.; Pallardy, S. G. 1993. Water stress, photosynthesis and early growth patterns of cuttings of three Populus clones. Tree Physiology. 13(3): 213-226. [22505]
117. Ripple, William J. 1994. Historic spatial patterns of old forests in western Oregon. Journal of Forestry. 92(11): 45-49. [33881]
118. Roe, Arthur L. 1958. Silvics of black cottonwood. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 18 p. [7179]
119. Romme, William H. 1982. Fire and landscape diversity in subalpine forests of Yellowstone National Park. Ecological Monographs. 52(2): 199-221. [9696]
120. Rood, Stewart B.; Heinze-Milne, Sig. 1989. Abrupt downstream forest decline following river damming in southern Alberta. Canadian Journal of Botany. 67: 1744-1749. [39202]
121. Rood, Stewart B.; Hillman, Craig; Sanche, Trevor; Mahoney, John M. 1994. Clonal reproduction of riparian cottonwoods in southern Alberta. Canadian Journal of Botany. 72: 1766-1774. [26548]
122. Rood, Stewart B.; Kalischuk, Andrea R.; Mahoney, John M. 1998. Initial cottonwood seedling recruitment following the flood of the century of the Oldman River, Alberta, Canada. Wetlands. 18(4): 557-570. [38485]
123. Rood, Stewart B.; Mahoney, John M. 1993. River damming and riparian cottonwoods: management opportunities and problems. In: Tellman, Barbara; Cortner, Hanna J.; Wallace, Mary G.; DeBano, Leonard F.; Hamre, R. H., tech. coords. Riparian management: common threads and shared interests: A western regional conference on river management strategies: Proceedings; 1993 February 4-6; Albuquerque, NM. Gen. Tech. Rep. RM-226. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 134-143. [22186]
124. Rood, Stewart B.; Patino, Sandra; Coombs, Krista; Tyree, Melvin T. 2000. Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees. 14: 248-257. [35961]
125. Schier, George A.; Campbell, Robert B. 1976. Differences among Populus species in ability to form adventitious shoots and roots. Canadian Journal of Forest Research. 6: 253-261. [3919]
126. Schmidt, Wyman C.; Lotan, James E. 1980. Phenology of common forest flora of the Northern Rockies--1928 to 1937. Res. Pap. INT-259. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 20 p. [2082]
127. Schreiner, Ernst J. 1974. Populus L. Poplar. In: Schopmeyer, C. S., ed. Seeds of woody plants in the United States. Agriculture Handbook No. 450. Washington, D.C.: U. S. Department of Agriculture, Forest Service: 645-655. [7731]
128. Schwartz, John E., II; Mitchell, Glen E. 1945. The Roosevelt elk on the Olympic Peninsula, Washington. The Journal of Wildlife Management. 9(4): 295-319. [8878]
129. Scott, Michael L.; Auble, Gregor T.; Friedman, Jonathan M. 1997. Flood dependency of cottonwood establishment along the Missouri River, Montana, USA. Ecological Applications. 7(2): 677-690. [28708]
130. Shiflet, Thomas N., ed. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p. [23362]
131. Smit, Barbara A. 1988. Selection of flood-resistant and susceptible seedlings of Populus trichocarpa. Canadian Journal of Forest Research. 18: 271-275. [2943]
132. Smith, Jane Kapler. 2001. [Personal communication]. October 31. Regarding black cottonwood postfire seedling establishment. Missoula, MT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Fire Modeling Institute. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [38481]
133. Smith, Jane Kapler; Fischer, William C. 1997. Fire ecology of the forest habitat types of northern Idaho. Gen. Tech. Rep. INT-GTR-363. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 142 p. [27992]
134. Snyder, Warren D.; Miller, Gary C. 1991. Changes in plains cottonwoods along the Arkansas and South Platte Rivers--eastern Colorado. Prairie Naturalist. 23(3): 165-176. [39203]
135. Speiser, Robert. 1990. Nest site characteristics of red-tailed hawks in western Washington. Northwestern Naturalist. 71(3): 95-97. [22702]
136. Spencer, David L.; Hakala, John B. 1964. Moose and fire on the Kenai. In: Proceedings, 3rd annual Tall Timbers fire ecology conference; 1964 April 9-10; Tallahassee, FL. Tallahassee, FL: Tall Timbers Research Station: 10-33. [5970]
137. Sprague, F. LeRoy; Hansen, Henry P. 1946. Forest succession in the McDonald Forest, Willamette Valley, Oregon. Northwest Science. 20(4): 89-98. [35027]
138. Stanek, W.; Alexander, K.; Simmons, C. S. 1981. Reconnaissance of vegetation and soils along the Dempster Highway, Yukon Territory: I. Vegetation types. BC-X-217. Victoria, BC: Environment Canada, Canadian Forestry Service, Pacific Forest Research Centre. 32 p. [16526]
139. Stettler, R. F.; Fenn, R. C.; Heilman. P. E.; Stanton, B. J. 1988. Populus trichocarpa X Populus deltoides hybrids for short rotation culture: variation patterns and 4-year field performance. Canadian Journal of Forest Research. 18: 745-753. [6308]
140. Stettler, Reinhard F. 1971. Variations in sex expression of black cottonwood and related hybrids. Silvae Genetica. 20(1-2): 42-46. [38204]
141. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
142. Stine, Scott; Gaines, David; Vorster, Peter. 1984. Destruction of riparian systems due to water development in the Mono Lake watershed. In: Warner, Richard E.; Hendrix, Kathleen M., eds. California riparian systems: Ecology, conservation, and productive management: Proceedings of the conference; 1981 September 17-19; Davis, CA. Berkeley, CA: University of California Press: 528-533. [5857]
143. Strahan Jan. 1984. Regeneration of riparian forests of the Central Valley. In: Warner, Richard E.; Hendrix, Kathleen M., eds. California riparian systems: Ecology, conservation, and productive management: Proceedings of the conference; 1981 September 17-19; Davis, CA. Berkeley, CA: University of California Press: 58-67. [5825]
144. Stromberg, Juliet C.; Patten, Duncan T. 1992. Mortality and age of black cottonwood stands along diverted and undiverted streams in the eastern Sierra Nevada, California. Madrono. 39(3): 205-223. [19148]
145. Strong, Terry. 1989. Rotation length and repeated harvesting influence Populus coppice production. Res. Note NC-350. Rhinelander, WI: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 4 p. [10907]
146. Szaro, Robert C.; Pase, Charles P. 1983. Short-term changes in a cottonwood-ash-willow association on a grazed and an ungrazed portion of Little Ash Creek in central Arizona. Journal of Range Management. 36(3): 382-384. [6520]
147. Thorne, Robert F. 1976. The vascular plant communities of California. In: Latting, June, ed. Symposium proceedings: Plant communities of southern California; 1974 May 4; Fullerton, CA. Special Publication No. 2. Berkeley, CA: California Native Plant Society: 1-31. [3289]
148. Topik, Christopher; Halverson, Nancy M.; Brockway, Dale G. 1986. Plant association and management guide for the western hemlock zone: Gifford Pinchot National Forest. R6-ECOL-230A. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 132 p. [2351]
149. Trappe, James M. 1962. Fungus associates of ectotrophic mycorrhizae. Botanical Review. 28: 538-606. [20401]
150. Tuhy, Joel S.; Jensen, Sherman. 1982. Riparian classification for the Upper Salmon/Middle Fork Salmon River drainages, Idaho. Final report. Smithfield, UT: White Horse Associates. Unpublished report on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station Fire Sciences Laboratory, Missoula, MT. 153 p. [8380]
151. U.S. Department of Agriculture, Forest Service, Intermountain Region. 1972. Range analysis handbook. Forest Service Handbook 2209.21. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Region. 234 p. [2892]
152. U.S. Department of Agriculture, Forest Service. 1964. Veneer cutting and drying properties of cottonwood. Research Note FPL-044. Madison, WI: U.S. Department of Agriculture, Forest Service, Forest Products Laboratory. 4 p. In cooperation with: University of Wisconsin, Madison. [38209]
153. USDA, NRCS. 2019. The PLANTS Database, [Online]. U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team, Greensboro, NC (Producer). Available: https://plants.usda.gov/. [34262]
154. Viereck, L. A.; Dyrness, C. T.; Batten, A. R.; Wenzlick, K. J. 1992. The Alaska vegetation classification. Gen. Tech. Rep. PNW-GTR-286. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 278 p. [2431]
155. Wade, Dale D.; Brock, Brent L.; Brose, Patrick H.; Grace, James B.; Hoch, Greg A.; Patterson, William A., III. 2000. Fire in eastern ecosystems. In: Brown, James K.; Smith, Jane Kapler, eds. Wildland fire in ecosystems: Effects of fire on flora. Gen. Tech. Rep. RMRS-GTR-42-vol. 2. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 53-96. [36983]
156. Weixelman, David A.; Bowyer, R. Terry; Van Ballenberghe, Victor. 1998. Diet selection by Alaskan moose during winter: effects of fire and forest succession. In: Ballard, W. B.; Rodgers, A. R. J., eds. Proceedings, 33rd North American moose conference and workshop/4th international moose symposium; 1997 May 17-23; Fairbanks, AK. In: Alces. 34(1): 213-238. [30325]
157. Welsh, Stanley L.; Atwood, N. Duane; Goodrich, Sherel; Higgins, Larry C., eds. 1987. A Utah flora. The Great Basin Naturalist Memoir No. 9. Provo, UT: Brigham Young University. 894 p. [2944]
158. White, Clifford A.; Olmsted, Charles E.; Kay, Charles E. 1998. Aspen, elk, and fire in the Rocky Mountain national parks of North America. Wildlife Society Bulletin. 26(3): 449-462. [30387]
159. Zasada, John C.; Norum, Rodney A.; Van Veldhuizen, Robert M.; Teutsch, Christian E. 1983. Artificial regeneration of trees and tall shrubs in experimentally burned upland black spruce/feather moss stands in Alaska. Canadian Journal of Forest Research. 13(5): 903-913. [6991]
160. Zasada, John C.; Van Cleve, Keith; Werner, Richard A.; McQueen, John A.; Nyland, Edo. 1978. Forest biology and management in high-latitude North American forests. In: North American forest lands at latitudes north of 60 degrees: Proceedings of a symposium; 1977 September 19-22; Fairbanks, AK. Fairbanks, AK: U.S. Department of Agriculture, Alaska Region: 137-195. [13613]