| FEIS Home Page |
 |
| Figure 1—Flowering yellow starthistle in San Jose, California. Photo by Eugene Zelenko and courtesy of Wikimedia Commons. |
| This review summarizes information that was available in the scientific literature as of 2020 on the biology, ecology, and effects of fire and control methods on yellow starthistle in North America.
Yellow starthistle is a nonnative, invasive forb in parts of the western United States. It can occur in dense monocultures that displace native plants; decrease native plant and animal diversity; reduce native wildlife habitat and forage; and alter water cycles, soil microbial community composition, and soil nutrient availability. It is most invasive in annual and perennial grasslands, shrub steppes, oak savannas, open woodlands, and openings in forests. It is especially invasive after disturbance, so limiting disturbance may help prevent yellow starthistle invasion. Yellow starthistle reproduces primarily by seed and may sprout from the root crown after top-kill. It is typically an annual, but sometimes behaves as a biennial. Germination timing depends primarily on the amount and timing of rain. In California, yellow starthistle seeds germinate from October to June, which corresponds to the typical rainy season, but emergence tends to be highest after rain in fall and early winter. Seedlings develop into rosettes with a taproot. Plants bolt then flower, typically between May and December, depending on location. In California, plants begin to produce buds and flowers at the onset of the summer dry period (typically June), and flowering continues until plants senesce from lack of water (typically by mid- to late summer) or are killed by freezing temperatures. In some areas, plants may flower year-round. Yellow starthistle plants can produce hundreds or thousands of seeds. After seeds mature, the plant dies. Seeds are typically dispersed short distances by gravity and wind. Seeds are also spread by animals, water, and vehicles. Yellow starthistle has a large, transient soil seed bank and a small, short- to long-term persistent soil seed bank. Some seeds may remain viable in the soil for up to 10 years. Yellow starthistle seeds germinate and seedlings establish best on moist, disturbed soils. However, seeds can germinate under a wide range of conditions and over an extended period. Once established, yellow starthistle can form monotypic stands on some sites. Most information about yellow starthistle's response to fire comes from field studies in California annual grasslands that used prescribed fire to control invasive populations. Fire usually consumes or kills yellow starthistle plants, although plants occasionally sprout after low-severity fire. Consumption is not necessary to kill the plants, although sufficient heat is required to scorch the foliage, stem-girdle, and kill them. Fires are usually not severe enough to kill yellow starthistle seeds in the soil seed bank, and yellow starthistle can reestablish from surviving seeds. After fire, yellow starthistle abundance may increase because postfire conditions are favorable for germination, seedling establishment, plant growth, and seed production. However, if burned again before yellow starthistle plants set seed, abundance may decrease. In addition to fire, physical and mechanical control, livestock grazing, biological control, and/or chemical control methods may be used in an integrated management program to control yellow starthistle. No matter what method is used to kill yellow starthistle plants, establishment or maintenance of desirable plants is needed for long-term control. |
FEIS abbreviation:
CENSOL
Common names:
yellow star-thistle
yellow starthistle
yellow star thistle
St. Barnaby's thistle
Barnaby's star-thistle
Common names are used throughout this Species Review. For scientific names of plants mentioned in this review and links to other FEIS Species Reviews, see table A1.
 |
| Figure 2—County-level distribution of yellow starthistle. Map courtesy of EDDMapS (2020, 4 March) [91]. |
Yellow starthistle is native to the Mediterranean Basin in southern Europe and the Middle East [70,74,228], and it has been introduced into all other continents, except Antarctica [74,186]. In the United States, the primary range of yellow starthistle is the western-most states, from Washington and Oregon south throughout California and eastward into Idaho, Nevada, Utah, and Arizona. It has been reported in up to 41 states [91,162,207,325] (fig. 2). According to Pitcairn et al. (2004), the “worst infested states” were Washington, Oregon, California, and Idaho [228]. Populations of yellow starthistle in these states and in Nevada can be locally dense [237], while occurrences in the eastern two-thirds of the United States are sporadic and localized and apparently fail to establish and persist [119,186]. According to the PLANTS Database, yellow starthistle occurs in Alberta, Manitoba, Saskatchewan, and Ontario [325], although a 2017 review stated that while a few yellow starthistle plants have been found in British Columbia, Alberta, Saskatchewan, and Ontario, no populations have persisted in Canada [240]. Impacts of yellow starthistle are greatest where the climate is mediterranean, like that of its native range (see Climate).
Yellow starthistle is thought to have been introduced to North America multiple times [12,77,96,109,303]. Erikson et al. (2014) concluded that the native range of the species is likely centered in the eastern Mediterranean region near Turkey, and that it spread from there to other parts of Europe and Asia. California populations came mostly from South America, and South American populations came mostly from Spain, but some populations in both California and South America came from other areas. Introduction of yellow starthistle from disparate parts of its range may have resulted in novel genotypes via intraspecific hybridization [96].
Contaminated alfalfa seed was likely the primary means of introduction into North America [70,74,186,259,262] in the early 1800s. Yellow starthistle seeds were found in adobe bricks from the period between 1824 and 1848 in California (Hendry 1931 cited in [132]). Introduction of yellow starthistle to other western states began in the late 1800s, and it was first collected outside of California, near Bingen, Washington [62,74,283], in 1898. By the 1920s it was widely distributed in eastern Washington [259]. Based on surveys of land managers, Duncan (2001) reported that yellow starthistle occurred on about 19.8 million acres (8 million ha) across 16 western states and provinces in 2000 [86], and Duncan and Jachetta (2005) reported that it occurred on about 14.8 million acres (6.0 million ha) in 17 western states and was "present" in eastern states in 2003 [84,85]. While these studies cover slightly different areas, they suggest that the acreage occupied by yellow starthistle decreased substantially over those 3 years. The reason for this difference in acreage occupied was not addressed by the authors; however, yellow starthistle patch and population size fluctuate from year to year and with timing and amount of precipitation (see Stand Structure), which may explain differences in estimates of area occupied.
SITE CHARACTERISTICSAmount and timing of precipitation affect yellow starthistle establishment and growth. Mean annual precipitation where yellow starthistle occurs in North America ranges from about 10 to 60 inches (250–1,500 mm) [74,169,184] (table 1). In the Pacific Northwest, the most susceptible rangelands are those with 12 to 25 inches (300–635 mm) mean annual precipitation that peaks in winter or spring [283]. In Idaho, it "thrives" in areas with 10 to 30 inches (250–760 mm) mean annual precipitation [40]. Yellow starthistle is intolerant of flooding [108]. Seedlings can survive extended frost periods, but mature plants are not frost tolerant. Cold tolerance appears to be lost during the transition from vegetative to reproductive phases [62].
| Table 1—Mean annual precipitation in some areas where yellow starthistle occurs. | |
| Location | Mean annual precipitation (mm) |
| California | 275–1,520 [29,49,73,93,110,168,169,184,253,307] |
| Idaho | 254–762 [40,113,168,254] |
| Oregon | 490 [168] |
| Utah | 530 [251] |
| Washington | 500–539 [168,334,335] |
Topography
Yellow starthistle occurs on a variety of sites, from flat to steeply sloping, in valleys and on foothill and mountain slopes [23,192,261,265,276,311]. On rangeland in north-central Idaho, it was most common on moderately sloping sites [276]. In southwestern Oregon, yellow starthistle occurs from valley floors up to the conifer transition zone above oak woodlands [261].
Yellow starthistle is most invasive on south- and southwest-facing slopes [100,138,265,276,311] but may spread from these sites onto other aspects [139,261]. For example, at the Garden Creek Ranch Preserve in Idaho, yellow starthistle populations were densest on south-facing slopes but were establishing and spreading on north-facing slopes [139]. At its northern limit in Washington (48° 45' north latitude), yellow starthistle is restricted to south-facing slopes [258], probably due to prolonged soil moisture and reduced light exposure in winter on north-facing slopes [258,260]. Limited solar radiation in winter appears to limit yellow starthistle establishment and spread at the northern extent of its range [257,258].
Yellow starthistle populations occur from sea level to 8,600 feet (2,620 m) (table 2), but most large populations are found below 5,000 feet (1,500 m) [62,186].
| Table 2—Elevational range of yellow starthistle by location. | |
| Location | Elevation (m) |
| California | ~0–2,621 [7,11,134,187,230,286,307,329] |
| Idaho | 259–1,220 [24,46,53,100,168,254,259,289,329] |
| Oregon | 244–1,225 [191,259] |
| Washington | 245–686 [168,259,329] |
| Utah | 915–1,900 [251,330] |
| Four Corners Region | up to 1,980 [133] |
Soils
Yellow starthistle is most invasive in deep, well-drained silt loam and loam soils with few coarse fragments [40,62,172,186,263,311] and high levels of available magnesium [25], but it can also establish in shallow, rocky, nutrient-poor soils [25,184,186]. Yellow starthistle cover appeared to be related to soil depth or total soil moisture-holding capacity on south-facing aspects in eastern Washington grasslands [258,260,311]. Requirements of soil moisture for reproduction during the summer dry period and canopy openness may be critical limiting factors for yellow starthistle establishment and spread [258]. However, too much water harms yellow starthistle. In a greenhouse study that approximated vernal pool conditions in the Central Valley of California, high mortality of yellow starthistle plants occurred after inundation for 38 days [108].
In deep soil, yellow starthistle can extract moisture from depths greater than 6 feet (2 m), and in shallow soil it can extract moisture from fissures in bedrock [74,92,112]. In Yolo County, California, yellow starthistle plants in low-density plots (0.6 plants/m²) used more soil moisture from deep in the profile (≥24 inches (60 cm)) than from the shallowest soil depth examined (12 inches (30 cm)) early in the growing season. In high-density plots (>90 plants/m²), yellow starthistle plants rapidly depleted soil moisture from all soil depths (12–71 inches (180 cm)) by preflowering growth stages. Soil moisture did not recharge in high-density plots, compared with bare-ground plots, after below-average winter and spring precipitation [73].
Yellow starthistle invades and dominates annual grasslands by using deep soil moisture that remains after shallow-rooted annual grasses die in early summer [74,261]. On annual rangelands where yellow starthistle occurs with cheatgrass, population dominance oscillates between yellow starthistle and cheatgrass, depending on precipitation [172,279]. Yellow starthistle is most competitive with cheatgrass for soil moisture on sites with deep soil and in years with wet springs [172]. In shallow soil and during dry springs, cheatgrass is more competitive [279]. However, yellow starthistle is tolerant of drought [82] and can also survive at lower soil water potential than annual grasses such as medusahead [110].
Yellow starthistle competes with perennial grasses for soil moisture more than with annual grasses because they have similar growing seasons [74]. In the Shasta Valley, California, soil water dynamics differed among plots dominated by yellow starthistle, annual grasses, or perennial grasses during 4 years. Total soil water content (across all sampling dates and soil depths) was similar in yellow starthistle and perennial grass plots, and it was significantly lower in yellow starthistle plots than annual grassland plots. This suggests similar use, and thus greater competition between yellow starthistle and perennial grasses for soil moisture, although yellow starthistle depleted soil moisture later into the season and at greater depths than either pernnial or annual grasses [92].
Water use patterns depend on the amount of soil moisture recharge [345]. In Davis, California, during a year with little to no deep soil moisture recharge, yellow starthistle roots were distributed at shallow depths like annual grasses, while in a wet year, yellow starthistle roots were distributed at greater depths like perennial species. Yellow starthistle depletes soil moisture primarily during the short period of lateral root growth from the late rosette to the spiny stages [345].
In California, yellow starthistle grows in serpentine and nonserpentine soils [45,98,106,310], but establishment and growth is better in nonserpentine soils [98,107]. It is rare in serpentine soils [98] and establishment may be limited to disturbed areas with minimal vegetation, such as near roads [45,107] (see Succession).
PLANT COMMUNITIESIn California, yellow starthistle is widely distributed in the Central Valley and adjacent foothills in annual grasslands [29,142], chaparral [48,175], and oak woodlands [23,252], and it is spreading into grasslands and other open plant communities at middle and high elevations in the Sierra Nevada [230,307]. California annual grasslands are dominated by many nonnative species—both grasses and forbs—more than 70% of which originated in the Mediterranean region, including yellow starthistle [163]. At Pinnacles National Park, the largest population of yellow starthistle occurred in a heavily grazed wet meadow [188].
In the Pacific Northwest, yellow starthistle is invasive in native grasslands and disturbed sites historically dominated by perennial grasses—primarily bluebunch wheatgrass, Idaho fescue, and Sandberg bluegrass—and sagebrush [55,283]. In Washington, yellow starthistle can establish and persist, at least in the short-term, in any of the major plant communities below subalpine. However, it is most common in the southeastern part of state on south-facing grassland slopes [255,263,265,311]. In the Columbia Basin, yellow starthistle is common in Idaho fescue, bluebunch wheatgrass, sand dropseed, and Fendler threeawn grasslands [55]. Yellow starthistle commonly codominates with cheatgrass in grassland steppes [280,282]. In the Blue and Ochoco Mountains, Oregon, bluebunch wheatgrass–Sandburg bluegrass communities heavily grazed by elk and cattle are "prime locations" for yellow starthistle establishment and spread [155].
In the Great Basin, yellow starthistle is invasive in bunchgrass and sagebrush ecosystems [51,52,157,225,272]. In a 2004 publication on the status and trends of sagebrush ecosystems, yellow starthistle was classified as highly invasive in bunchgrass communities dominated by wheatgrasses, Fendler threeawn, basin wildrye, and Sandberg bluegrass and in herbaceous wetlands dominated by Nebraska sedge, beaked sedge, and water sedge. It was classified as moderately invasive in basin big sagebrush, mountain big sagebrush, Wyoming big sagebrush, low sagebrush, black sagebrush, and threetip sagebrush communities; dogwood and willow shrub wetlands; and black cottonwood communities. Invasiveness was low in salt desert shrublands [52]. In Great Basin National Park, Nevada, yellow starthistle occurs in the black greasewood/big sagebrush shrubland association. Cover of native grasses and forbs is generally sparse to absent in this association but stands can have "substantial invasion" by cheatgrass [51]. The Nevada Natural Heritage Program described a yellow starthistle semi-natural herbaceous alliance that occurred with and without cheatgrass. This alliance was expected to increase in extent [225].
In the Interior West, Rice et al. (2008) classified yellow starthistle as a high threat in mountain grasslands, open canopy forests, and riparian communities, a potentially high threat in desert shrublands, and a low threat in desert grasslands, sagebrush shrublands, pinyon-juniper woodlands, and closed canopy forests [249]. In Idaho, yellow starthistle primarily occurs on semiarid rangeland and abandoned croplands [174], but it has also established and is spreading in canyon grasslands with Spalding's silene, a federally Threatened species [139,192,193].
In eastern states, yellow starthistle is uncommon. In New England, it generally occurs along roadsides, in fields, and in "waste places" [126]. In Massachusetts, yellow starthistle was described as a "waif" that occurred once or sporadically and was not established on "waste ground" [292].
Yellow starthistle populations in the United States exhibit variations in morphology (plant size and leaf shape), phenology (bolting and flowering time), seed production, and genetic diversity, [186,255,260,277,302,307]. For example, in a common garden experiment where plants were grown from seeds from 34 yellow starthistle populations in four states, wide biotypic variation occurred in rosette area, decurrent leaf width, plant height, stature, number of branches, shape and form, growth stage on four dates, number of buds produced on two dates, flowering rate, date of first flower and first seed, average number of flowers, and seed production rate. None of the variables correlated with precipitation, elevation, or latitude of the source population [255].
 |
| Figure 3—Yellow starthistle plant in California. Photo by J. Smith courtesy of Wikimedia Commons. |
The following description covers characteristics of yellow starthistle that may be relevant to fire ecology and is not meant for identification. Keys for identification are available (e.g., [11,56,99,119,141,162,177,330]).
Yellow starthistle is a winter annual [11,56,67,99,133,177,186,330] or sometimes biennial [56,119,186,330] forb. Plants begin as rosettes with 6 to 28 leaves that lie close to the ground [283]. When crowded and shaded, rosette leaves grow more upright [240,258]. Yellow starthistle stems are typically 0.3 to 3.3 feet (10 to 100 cm) tall [11,56,99,119,133,177,330], sometimes up to 4.9 or 6.6 feet (150 or 200 cm) tall [67,283], depending on site conditions. For example, plants may grow up to 6 feet (1.8 m) tall in wet locations but only 6 inches (15 cm) tall in hot, dry locations [240]. Stems are simple or branched from the base, often forming rounded, bushy plants [11,99,133] (fig. 3). Leaves become progressively smaller up the stem. Lower leaves are 2 to 6 inches (5–15 cm) long [67,99], and upper leaves are 0.4 to 1.2 inches (1–3 cm) long, narrow, and densely covered with cobwebby hairs later in the season [67].
Yellow starthistle flowers are yellow and borne in solitary flowerheads on stem tips [11,119]. The involucre is about 8 to 17 mm long [11,56,67,99,119,133,177,330]. The middle and outer bracts are spine-tipped, and spines radiate from flowerheads in a star shape [283]. The central spine is larger than the others, 10 to 30 mm long [11,56,99,119,133,141,330] (fig. 1).
Yellow starthistle seeds are achenes [56,67,119] or cypselae [99,133]. They are about 2 to 4 mm long [11,67,99,119,133]. Seeds are dimorphic. Most seeds (from about 55% to over 90%) [20,172,184,260] have a 2- to 5-mm long pappus (plumed). Seeds at the periphery of the flowerhead have no pappus (plumeless) [11,56,62,67,99,119,133,177,258,330]. Plumed seeds are larger than plumeless seeds and produce larger plants [116]. See the following publications for more information on yellow starthistle seed weight: [116,295,332].
Yellow starthistle has a taproot with short secondary branches. The taproot grows 4.0 feet (1.2 m) deep or more [73,178,258,262,284] (see Plant Growth and Mortality), allowing access to deep soil moisture during dry summer and fall months [70]. Vesicular arbuscular mycorrhizal fungi have been observed on yellow starthistle roots [39,127,329].
Stand Structure
Yellow starthistle may occur as scattered plants, small patches, or dense stands [70,138,140,188,251,283] that can become monocultures [131,138] or near monocultures [137,251]. Stands with 2 to 3 million plants/acre (5–7.5 million plants/ha) have been reported [40]. In northwestern Idaho, density of yellow starthistle plants ranged from 0 to 400 plants/m² [138]. In north-central Utah, patches were "virtual monocultures" with 1,300 to 2,700 seedlings/m² in spring [251]. Yellow starthistle stand densities are higher in its nonnative range in California than in its native range in central Turkey [327].
Because yellow starthistle seeds germinate over an extended period beginning with the first fall rains and ending with the last spring rains (see Seasonal Development), a typical stand of yellow starthistle includes plants in several stages of development. Dense stands have both large-canopied plants receiving full sunlight and an understory of smaller, shaded plants [29,74].
Yellow starthistle patches expand and contract over time [229,251]. Small patches may be transient, while large patches are more likely to persist from year to year [229]. Precipitation amount and timing affects yellow starthistle abundance [229,251] (see Seedling Establishment and Mortality). In California, high yellow starthistle density in one year is generally followed by low yellow starthistle density in the next, perhaps because dense stands of yellow starthistle deplete soil moisture and create conditions unfavorable for the next generation's survival [92]
Raunkiaer [242] Life FormIn California, yellow starthistle seeds germinate from October to June, which corresponds to the normal rainy season, but emergence tends to be highest after rain in fall and early winter [184]. In Utah, germination in fall appears to be limited by the frequency and timing of rain. In some years, very little germination occurs in fall and most germination occurs in spring [251]. Yellow starthistle plants are insensitive to photoperiod and lack a vernalization requirement, allowing plants to germinate over an extended period as long as adequate moisture is available [266]. In areas with mild winters, plants can behave as biennials. However, in cold-winter, inland areas mature plants rarely survive the winter [74].
Yellow starthistle allocates resources first to root extension, then to leaf expansion, and finally to stem development and flower production [258,277]. In California, Oregon, Washington, and Idaho, a yellow starthistle seedling that establishes in fall will generally overwinter as a basal rosette, and may have a taproot penetrating to 2 feet (0.6 m) or more by spring [258,260,279,284]. In Utah, mortality of seedlings that establish in fall can be high [251]. Seedlings that survive over winter begin rapid, deep, root growth in spring and slowly develop into rosettes [62].
Bolting typically occurs from late spring to early summer, coinciding with increased light availability due to senescence and desiccation of neighboring annual species [74]. Flowering occurs between May and December (table 3), depending on location [99]. A single plant may produce flowers for up to 5 months [111], and frost-free coastal sites may have flowering plants year-round [99]. In California, plants begin to produce buds and flowers at the onset of the summer dry period (typically June) [307]. Flowering continues until plants senesce from lack of water (typically by mid- to late summer) or are killed by freezing temperatures [34,74,124,229,266,307]. In Davis, California, seedlings emerged in January and February, bolted in May, and >25% of plants flowered by 12 June. Flowering continued throughout the summer and into September [34].
| Table 3—Flowering dates in some areas where yellow starthistle occurs. | ||
| Area | Dates | References |
| California | May–October | [11,67,184,229,303] |
| Idaho | mid-July–early August | [186] |
| Illinois | July–September | [197] |
| Montana | June–August | [88] |
| North and South Carolina | June–August | [239] |
| Washington | June–September | [36,257,334] |
| Four Corners Region | June–October | [133] |
| Great Basin | July–September | [57] |
| Great Plains | July–September | [119] |
| Intermountain Region | July–September | [56] |
| New England | August | [274] |
| Pacific Northwest | June–December | [117] |
Benefield et al. (2001) identified 10 phenologically distinct seedhead developmental stages in yellow starthistle in Yolo County, California, beginning with the late bud (full spiny) stage and ending with seed dispersal. On average, seedheads required 21 days to progress from the prebloom stage (after the late bud stage) to petal abscission (before seed dispersal). Flowers remained in full bloom for 2 days before senescence began. Senescence required an additional 14 days. Some germinable seeds were present during the initial senescence stage, about 5 days after flower initiation, although most germinable seeds developed in the late senescence stage, about 8 days after flower initiation. The proportion of germinable seeds peaked at seed dispersal [20]. Near Pullman, Washington, yellow starthistle seedlings emerged in May, plants flowered in July, and seeds dispersed in August [334] (table 4).
| Table 4—Developmental stages of yellow starthistle plants in the Kramer Prairie Natural Area, Pullman, Washington. Plants were visited weekly or biweekly. Data from Woodley et al. (2017) [334]. | |
| Date | Description |
| May 1–2 | Seedling |
| May 14–21 | Rosette |
| May 28–June 4 | Bolting |
| June 3–10 | First floral stage: small buds with yellow-green spines begin to be visible |
| June 19 | Second floral stage: spines protrude more than half of the bud length |
| June 16–25 | Third floral stage: spines are equal to or greater than 45° angle from stem |
| June 2–24 | Fourth floral stage: spines are straw-colored and equal to or greater than 90° angle from stem |
| July 1–24 | Flowering |
| July 23–August 13 | Mature: leaves dry, flowers fade, and the plant turns a straw-color |
| August 19 | Seed dispersal |
Yellow starthistle seeds disperse during two distinct periods [172]. Plumed seeds usually disperse soon after flowers senesce and drop their petals. Plumeless seeds are usually retained in the seedhead until the spiny bracts fall off (about a month), but can be retained well into winter [41,74]. A plant sheds leaves when it begins to flower [304], after which stems dry to a silvery-gray skeleton with cottony-white terminal seedheads [283]. Senesced stems of yellow starthistle degrade slowly and may remain erect for a year [62]. Seeds on or in the soil begin to germinate after rain in fall, and the cycle is repeated [283].
REGENERATION PROCESSESPollination and Breeding System
Yellow starthistle is monoecious, pollinator-dependent, and facultatively xenogamous [128,185,303]. Most yellow starthistle plants are self-incompatible [15,111,128,185,224,303]. Incompatibility varies among individuals and populations [15,128,185,224,303], and self-compatibility may be higher in invasive or "weedy" populations [224]. Yellow starthistle plants may be pollen limited on some sites, such as north-facing but not south-facing slopes in Mount Diablo State Park, California [304].
Yellow starthistle attracts generalist insect pollinators [262]. Nonnative bees (Megachile apicalis and Apis mellifera) are important pollinators [13,15,16,128,176,185,191,307] and may be responsible for >50% of seed set in some areas [15]. Barthell et al. (2001) suggest that nonnative honeybees and yellow starthistle may act as "invasive mutualists" [15,191]. In six shrub-steppe sites in northeastern Oregon, yellow starthistle insect pollinators included 203 species from 41 families in 4 orders. Ten species were considered "key" pollinators based on abundance and pollen carriage: two leafcutting bees (Megachile apicalis and Megachile perihirta), five apid bees (Apis mellifera, Bombus bifarius, Bombus centralis, Svastra obliqua, and Melissodes lutalenta), two sweat bees (Halictus tripartitus and Halictus ligatus), and a tachinid fly (Peleteria malleola). Pollinators varied from site-to-site and year-to-year. The high diversity of yellow starthistle pollinators was attributed to its simple flowers with short corollas, rich nectar reward, and extended flowering season into summer compared with other available plants [191]. See table 5 for studies that provide information about insects that pollinate yellow starthistle flowers at specific locations in the United States.
| Table 5—Publications with information on insects that pollinate yellow starthistle flowers in the United States. | ||
| Location | Title | Reference |
| CA: Contra Costa County, near Brentwood | Pollinator interactions with yellow starthistle (Centaurea solstitialis) across urban, agricultural, and natural landscapes | [176] |
| CA: Davis, Galt, and Santa Cruz Island | Promotion of seed set in yellow star-thistle by honey bees: Evidence of an invasive mutualism | [15] |
| CA: El Dorado National Forest, Loma Alta Open Space Preserve, and Mount Diablo State Park | Complex interactions among biocontrol agents, pollinators, and an invasive weed: A structural equation modeling approach | [307] |
| CA: Mount Diablo State Park | Trait-mediated interactions and lifetime fitness of the invasive plant Centaurea solstitialis | [305] |
| CA: Placer County, near Loomis | Pollination biology of yellow starthistle (Centaurea solstitialis) in California | [185] |
| CA: Santa Cruz Island | Yellow star-thistle, gumplant, and feral honey bees on Santa Cruz Island: A case of invaders assisting invaders | [16] |
| CA and outside the US: Santa Cruz Island, CA, and Lesvos, Greece | Foraging patterns of bees in response to nectar availability in populations of the invasive thistle species Centaurea solstitialis L. in native (Greece) and non-native (USA) island ecosystems | [13] |
| Differing foraging responses by bees to the invasive thistle species Centaurea solstitialis L. in native (Greece) and non-native (USA) island ecosystems | [14] | |
| OR: near Island City | Reproduction and pollination biology of Centaurea and Acroptilon species, with emphasis on C. diffusa | [128] |
| OR: northeastern | Pollinators of the invasive plant, yellow starthistle (Centaurea solstitialis), in north-eastern Oregon, USA | [191] |
Seed Production and Predation
Yellow starthistle seed production varies with soil moisture availability, shading, cover and density of yellow starthistle and associated vegetation, and availability of pollinators. Plants produce more plumed than plumeless seeds [169,260] (see Botanical Description), although the relative proportion of plumed and plumeless seeds also varies with environmental conditions [184]. Seed production is reduced by fire or other disturbances that kill plants during the early flowering stages [130,131]. Biological control insect larvae that consume immature seeds and seedhead tissues can greatly reduce seed production (e.g., [24,54,74,104,228,229,308,339]). Some seeds may also be lost to bird or insect predation after dispersal [20] (see Seed Banking). However, dense yellow starthistle populations produce far more seeds than are necessary for populations to persist and spread [62], and yellow starthistle plants attacked by biological control insects can still produce enough seeds to maintain populations [24,104,228,339] (see Biological Control).
Under field conditions, yellow starthistle plants may produce one to several hundred flowerheads/plant [172,184,251] and more than 30 to 80 seeds/flowerhead [20,172,184,251]. Estimates of seed production in yellow starthistle plants include means of 120 seeds/plant in a dense population (180 plants/m²) near Walla Walla, Washington [172]; 454 seeds/plant (ranging from 0–3,166 seeds/plant) on a site dominated by smooth brome in north-central Utah [251]; 717, 832, and 10,024 seeds/plant in coastal, intercoastal, and valley sites in California, respectively [184]; and 36, 53, and 150 viable seeds/plant in coastal, interior, and Sierra Nevada foothill sites in California, respectively [307]. Under nursery conditions, large plants might produce more than 100,000 seeds [260]. Estimates of seed production in yellow starthistle populations range from 14 million to 100 million seeds/acre (35–250 million seeds/ha) [41,68,172,280]. Proportion of plumed seeds was 70% in Washington [172] and 80% to 91% in California [20]. Ratio of plumed:plumeless seeds in California was about 9:1, 5:1, and 3:1 at coastal, intercoastal, and valley sites, respectively [184].
Studies in Washington indicated that annual seed production depends on available soil moisture, which varies with amount of spring precipitation [172,280,283]. Yellow starthistle populations on one site in Walla Walla produced about 1,940 and 470 seeds/foot² (21,600 and 5,200 seeds/m²) under moist and dry spring conditions, respectively [280]. Another study in Walla Walla found that a yellow starthistle population with 180 plants/m² produced an average 21,000 seeds/m² during a drought year [172], but comparable data from a non-drought year were not available.
Flower and seed production may be lower on north-facing slopes and with shading. At Mount Diablo State Park, California, yellow starthistle on north-facing slopes produced fewer viable seeds (≈150 seeds/plant) than plants on south-facing slopes (≈380 seeds/plant) [304]. In Columbia County, Washington, yellow starthistle plants grown under 6% full sunlight failed to flower, and the average number of flowers/plant increased with increasing sunlight [258].
Some studies found that flowerhead and seed production are positively associated with canopy openness [111] and negatively associated with cover and density of yellow starthistle [227,306] and associated vegetation [227]. In a nonnative annual grassland in Davis, California, number of flowerheads, flowerhead mass, stem mass, and total mass per yellow starthistle plant increased as canopy-gap size increased [111]. At coastal, interior, and Sierra Nevada sites in California, mean seed production per plant was negatively correlated with density of flowering yellow starthistle plants (r²adj = 0.36–0.73; n = 8–23) [306]. Near Davis, the number of seedheads/yellow starthistle plant decreased exponentially with increasing yellow starthistle density (r² = 0.95; n = 8) and decreased with increasing density of all plants (r² = 0.99; n = 5) [227]. In contrast, some studies found no relationship between density of yellow starthistle plants and seed production. In north-central Utah, estimated total viable seed production per plant was not significantly correlated with yellow starthistle density or grass cover [251]. In Yolo County, California, seed production/plot was unchanged across a large range of yellow starthistle plant densities, indicating that a small number of large plants can produce as many seeds as a large number of smaller plants occupying the same area [104].
Flowerhead production may increase after fire [68,137]. See Postfire Flowerhead Production for more information.
Availability of pollinators can affect seed set [15,185,303]. At three sites in California, the total number of honeybees was positively correlated with the mean number of viable seeds/seedhead (correlation coefficient not provided). Exclusion of pollinators using mesh reduced seed set/seedhead at all three sites [15]. In Contra Costa County, California, natural areas had the highest average rates of yellow starthistle seed set among natural, agricultural, and urban areas, although total bee visitation was lowest in natural areas. The authors suggested that bees were more efficient pollinators of yellow starthistle in natural areas because diversity of flowering plants was low in natural areas at the time of the study in August [176].
Seed Dispersal
Plumed and plumeless seeds disperse similar, short distances but at different times. Plumed seeds usually disperse in summer and early fall, after flowers senesce, and they mostly fall within 2 feet (0.6 m) of parent plants [6,194,256]. Plumeless seeds usually remain in seedheads until late fall and winter, and they fall to the soil just below the parent plants as seedheads deteriorate [40,74,256] (see Seasonal Development). Because the pappus of plumed seeds is small relative to seed size, it does little to aid in wind dispersal [6,194,256]. A combination of gusty wind and dry conditions maximizes dispersal distance [256]. Roche (1992) recorded 92% of seeds falling within 2 feet (0.6 m) of the parent plant, and 48% within 1 foot (0.3 m). Maximum wind dispersal was about 16 feet (5 m) over bare ground with wind gusts of 25 miles/hour (40 km/hour). Thus, wind dispersal can serve to increase the area invaded by persistently advancing the perimeter [256].
Humans, animals, and water can also transport yellow starthistle seeds. Because pappus bristles are covered with stiff, microscopic barbs that readily adhere to clothing and fur, plumed seeds may be dispersed by humans and animals over potentially long distances [40,74,194] with the movements of wildlife, livestock, vehicles, and equipment [67,74]. Birds such as ring-necked pheasants, California quail, house finches, and American goldfinches feed heavily on yellow starthistle seeds [256,260] (see Importance to Wildlife and Livestock). A small percentage of yellow starthistle seeds remain viable after being digested by animals [114,256]. About 3% of yellow starthistle seeds were viable after digestion by domestic goats [114]. The pappus may aid flotation in moving water [262].
Seed Banking
Yellow starthistle has a large, transient soil seed bank and a small, short- to long-term persistent soil seed bank. Transient seed bank density is directly related to the amount of seed produced in a given year. Its density generally peaks after plumed seed dispersal in late summer to fall, then fluctuates in late fall and winter as seeds are lost through germination and gained when plumeless seeds disperse [41,74] (see Seasonal Development). Most yellow starthistle seeds germinate soon after dispersal if conditions are suitable (see Germination), and seed bank density declines rapidly after fall and winter rains stimulate germination. Remaining viable seeds are either lost to predation, desiccation, or decay [20,62,159,160,256], or they are dormant and form a persistent soil seed bank [74,159]. Estimates of yellow starthistle seed longevity in the persistent seed bank range from about 4 years in California [20,159,160] up to about 10 years in Washington [41].
Yellow starthistle's persistent soil seed bank contributes little to recruitment relative to the current year’s seed rain [104,172,280,283,308]. On a bunchgrass rangeland dominated by cheatgrass and yellow starthistle in Washington, yellow starthistle seed density in the soil seed bank just prior to seed dispersal was approximately 13% of annual seed production and consisted mostly of plumeless seeds. Density of flowering yellow starthistle plants ranged from 16 to 21 plants/foot² (180–236 plants/m²), and yellow starthistle seed bank density was about 252 to 378 seeds/foot² (2,800–4,200 seeds/m²) in the top 3 inches (8 cm) of soil [172,280,283].
Yellow starthistle seed bank density appears to increase with increased precipitation, likely due to increased seed production in relatively wet years [172,280,283] (see Seed Production). During 4 years at Sugarloaf Ridge State Park, density of yellow starthistle seeds in the soil seed bank in late summer was positively correlated with rainfall in the preceding year (r² = 0.61; n = 4) (Kyser, unpublished data cited in [74]). Seed density in the soil under yellow starthistle plants in fall varied from 309 to 911 seeds/foot² (3,438–10,127 seeds/m²) during 3 years, and was lowest during a year when spring moisture was lacking [68].
Sites with a high density of yellow starthistle plants tend to have high density of both plumed and plumeless seeds in the soil seed bank [159,180]. In three grasslands in Yolo County, California, for example, density of seeds in persistent and transient soil seed banks decreased with decreasing density of yellow starthistle plants (r²= 0.57;n= 12) [180]. Near Davis, density of viable plumed seeds under a "moderately dense" yellow starthistle stand peaked at 8,292 seeds/m² in early October before the first fall rain and declined to 849 seeds/m² after the first fall rain in mid-November. Density of viable plumed seeds under a “dense” yellow starthistle stand peaked at 18,882 seeds/m² in late September before the first fall rain and declined to 4,756 seeds/m² by mid-October, after the first fall rain [159].
Viable seed bank density fluctuates in response to weather conditions that stimulate germination. It generally peaks after seed dispersal—from late summer to winter—and decreases throughout the following year [159,280]. For example, in southeastern Washington, about 29,150 seeds/cm² were collected in seed traps in fall, and about 20 seeds/cm² occurred in the top 1 inch (2.5 cm) of soil the following May [256]. Observations near Davis, California, indicated that density of viable plumed seeds in the soil peaked after dispersal in fall, declined rapidly when seeds germinated after the first fall rains, and generally declined thereafter [159] (fig. 4).
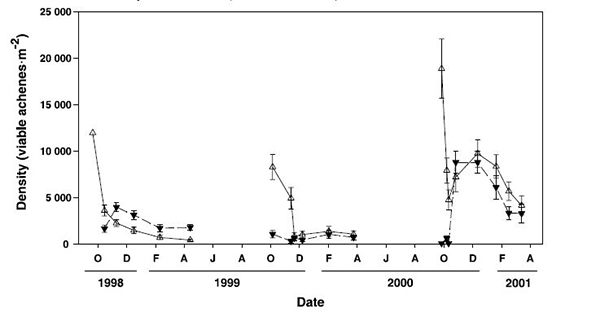 |
| Figure 4—Mean density of viable plumed (open triangles) and plumeless (solid inverted triangles) seeds recovered from soil cores at three sites during 3 years near Davis, California. Data were collected every other month starting in October (O) and ending in April (A) for each stand. Yellow starthistle stand density was "high" in the 1998 stand, "moderate" in the 1999 stand, and "high" in the 2000 stand. Error bars indicate 99% confidence intervals. Image courtesy of Joley et al. (2003) [159]. |
Prescribed fire, livestock grazing, physical and mechanical controls, and biological controls can all reduce seed bank density by reducing current-year seed production (see Postfire Seed Banks and Control). A 4-year study near Davis, California, showed that preventing yellow starthistle seed rain can reduce seed bank density. Seed rain was permitted during the first year of the study and prevented during the subsequent 3 years by removing yellow starthistle plants in and around the plot before they set seed. Density of viable plumed seeds was 3,614 seeds/m² in October of the first year and declined throughout the sampling period to 4 seeds/m² in November of the fourth year. Density of viable plumeless seeds was 1,662 seeds/m² in October of the first year, increased to 3,975 seeds/m² in November of that year, then declined throughout the rest of the sampling period to 11 seeds/m² in November of the fourth year [159]. While 2 to 3 years of preventing seed production can dramatically reduce yellow starthistle populations and presence in the soil seed bank (e.g., [68,159,160,189], survival of even a small number of seeds can lead to reinvasion of a site [41,68,160].
It is unclear how burial affects yellow starthistle seed longevity because few studies reported this. After 13 months of burial at 0.2, 0.4, 1.0, and 2.0 inches (0.5, 1, 2.5, and 5 cm) under a fallow orchard in northeastern California, yellow starthistle seed viability was 0.5%, 4%, 63%, and 88%, respectively. The loss of viable seeds at the two shallowest depths was due primarily to germination. A similar study at the same site found no difference in viability of plumed and plumeless seeds after 6 years of burial at 2 inches (5 cm). Seed viability was highly variable, ranging from 0% to 96%, compared with 99% viability of seeds held in dry storage [160]. Viability of yellow starthistle seeds buried 1, 2, and 6 inches (2.5, 5, and 15 cm) deep in sandy loam soil under an annual grass community in southeastern Washington decreased over time, but burial depth did not affect seed longevity. Estimated longevity for buried seeds (at any depth) averaged 10 years for plumed and 6 years for plumeless seeds [41]. At the botanical garden of Aix-Marseille University, France, viability of yellow starthistle seeds was similar between seeds buried 2.0, 3.9, and 9.8 inches (5, 10, and 25 cm) deep both 85 and 136 days after burial [270].
Germination
Germinable yellow starthistle seeds may be present in flowerheads during the late flowering stage [20,185], and mean number of germinable seeds increases consistently with advancing phenological stage thereafter (see Seasonal Development). Most yellow starthistle seeds (95%) are viable at maturity [220,283] and germinate soon after dispersal with adequate soil moisture. At optimum temperatures, germination can begin in 1 day, and at minimum temperatures for germination, it begins within 10 days [266]. A laboratory study found that under similar conditions of moisture and temperature and no light, 95% of plumeless seeds and 73% of plumed seeds germinated within 84 hours. Plumeless seeds reached maximum germination (100%) at 108 hours and plumed seeds at 132 hours [277,284]. High germination rates of fresh seeds suggest that most seeds have no innate dormancy, while seasonal changes in germinability suggest that some seeds may develop conditional dormancy if they do not germinate soon after dispersal [158,159].
Timing and rates of yellow starthistle germination in the field depend primarily on available moisture, ambient temperature, and exposure to light. Plumed and plumeless seeds have different temperature and moisture optima for germination [158,159,173,260,284,343]. Due to conditional dormancy in some seeds and differences in germination optima between plumed and plumeless seeds, germination occurs under a wide range of conditions and over an extended period of time [173,251]. Heat from fire may kill some yellow starthistle seeds in the soil seed bank [1,247] (see Immediate Fire Effects on Plant), but fire may also stimulate germination of surviving seeds [69,74,75,130] (see Postfire Germination). Postfire germination and extended periods of germination and seedling establishment are important considerations for controlling yellow starthistle populations [74] (see Fire Management Considerations and Control).
Moisture: Plumed seeds generally disperse prior to the first fall rains in California, and germinate in large numbers afterward [20,70,158,184,251,283,306] (see Seasonal Development). Summer precipitation adequate to trigger germination is rare in California, but germination may occur following August thunderstorms in north-central Idaho and eastern Oregon and Washington. Germination may occur along streams or in irrigated areas any time of year [262].
Temperature: Yellow starthistle seeds germinate at both low and high temperatures [158,159,173,260,284,343]. Germination occurs in both fall and spring [40], and in mediterranean climates where temperatures remain above freezing, yellow starthistle seeds can germinate throughout winter [158]. Plumed and plumeless seeds collected from Davis, California, germinated at similar numbers and rates in a common garden [134].
Young et al. (2005) developed 85 germination temperature profiles for yellow starthistle seeds collected from 15 sites in California, Nevada, and Oregon, and tested immediately after harvest. For most profiles, some germination occurred at all temperature regimes except a constant 104 °F (40 °C). No single temperature regime always supported optimum germination when all the profiles were combined. Plumed seeds tended to germinate better at colder temperatures than plumeless seeds [343]. Joley et al. (1997) observed nearly 100% germination at constant temperatures of 50, 59, and 68 °F (10, 15, and 20 °C), and at alternating temperatures of 59/41 and 68/50 °F (15/5 and 20/10 °C) [158].
Total germination is generally reduced at very low and very high temperatures [1,158,247,266,284,343], and temperatures above 239 °F (115 °C) can be lethal to yellow starthistle seeds [247] (see Immediate Fire Effects on Plant).
Light: Effects of light on yellow starthistle seed germination are unclear. Germination rates of seeds collected from California and Nevada and exposed to alternating temperatures reached nearly 100% without light [343], and germination of seeds collected from Spain was higher in shade (45%) than in full sunlight (28%) regardless of seed type [247]. However, germination of yellow starthistle seeds appears to be stimulated by white and red light [158,159,208] and may be reduced in the dark [158]. Germination differences between seed types were more prevalent in the dark than under light for seeds collected from California [158].
Seed Dormancy and Afterripening: Nearly all viable seeds are germinable under a range of conditions at dispersal [343], suggesting that most yellow starthistle seeds have no innate dormancy and do not require a period of afterripening [20,260]. For example, 88% and 95% of seeds collected from two California sites were viable at the seed dispersal stage, and germination rates were 88% and 84% in a laboratory [20]. Another study found that, of seeds collected from six California sites, stored for 100 to 120 days, and then sown in field soils, 100% of plumed seeds germinated (i.e., 0% were dormant), and 85% of plumeless seeds germinated (i.e., 15% were dormant) [111].
A period of afterripening can break dormancy and broaden the range of conditions where germination can occur. Germination rates of yellow starthistle seeds collected from Loomis, California, and stored for 2 months at room temperature were higher than those of freshly ripe seeds when germinated at 68 and 77 °F (20 and 25 °C) [158]. Yellow starthistle seeds collected from 15 sites in California, Nevada, and Oregon, showed no evidence of afterripening requirements, except for plumeless seeds exposed to very cold temperature regimes (constant or alternating temperatures from 32 to 41 °F (0-5 °C)) [343].
If conditions are not favorable for germination, conditional dormancy may be induced, which would render seeds “able to germinate under a narrow range of conditions” [159]. Dormant seeds may then become part of the soil seed bank and, although this represents a small portion of seeds produced annually, it may be sufficient to reinvade a site [41,68,160] (see Seed Banking).
Seedling Establishment and Mortality
High seed production and germination rates can result in extremely dense yellow starthistle seedling populations, especially in disturbed areas [172,311]. However, seedling mortality can be high, and only a small portion of seedlings typically survive to maturity [74,251,256,262,280,283]. In Walla Walla, Washington, 4,080 yellow starthistle seedlings/m² were recorded in mid-November, and about 1,000 seedlings established each week over the next 6 weeks. Seedling density peaked in late January at 7,562 seedlings/m² and declined to 4,740 seedlings/m² by mid-March. The adult population peaked at 940 individuals/m² in mid-June and declined 75% to 236 individuals by mid-July [280] (see Plant Growth and Mortality).
In dense yellow starthistle populations, intraspecific competition for light and soil moisture can result in higher rates of seedling mortality than interspecific competition [62,67,234,279,281,282,344].
Disturbance creates favorable conditions for yellow starthistle seedling establishment by increasing bare ground, reducing vegetation, increasing sunlight to the soil surface, and increasing nutrient availability [74]. Large disturbances may be more favorable to yellow starthistle establishment than small disturbances [251]. Abundant yellow starthistle seedlings often emerge with the first fall rains after fire in California grasslands [167] (see Postfire Seedling Establishment). During 2 years, yellow starthistle seedling establishment across nine sites in Washington tended to be greater in disturbed plots—where either biological soil crusts were removed or soil crusts and plants were removed—than in undisturbed plots [220]. In southeastern Washington, yellow starthistle did not establish in areas with undisturbed perennial grasses, and areas of clipped bunchgrasses were more susceptible to yellow starthistle establishment than were areas with clipped sod-forming grasses. Yellow starthistle seedlings in plots shaded by standing grasses and grass litter appeared "weak and spindly" compared to the "vigorous" rosettes grown in grass-free control plots [258].
Propagule pressure from yellow starthistle (i.e., viable seed input) is an important predictor of yellow starthistle seedling establishment [45]. A 9-year study of planted serpentine grassland communities near Morgan Hill, California, indicated that propagule pressure from yellow starthistle was the most important predictor of yellow starthistle establishment success. In early years of the study, yellow starthistle could only establish in the absence of other plants. In later years, as yellow starthistle abundance increased, increased propagule pressure from established yellow starthistle plants appeared to overcome the initial ecological resistance of the plant community to yellow starthistle establishment [45].
High yellow starthistle cover is positively associated with high yellow starthistle seedling establishment. In north-central Utah, the percentage of yellow starthistle plants that survived to flowering was highest when plants were near patches of flowering yellow starthistle or when individuals were surrounded by high yellow starthistle cover and low grass cover. Elevated levels of soil inorganic nitrogen beneath adult yellow starthistle plants may have created a positive feedback loop, where the presence of adults encouraged the establishment of seedlings [251].
High spring precipitation appears to favor yellow starthistle seedling establishment [220,297], while below-average precipitation in spring and throughout the year may lead to high seedling mortality [69,251] and reduced cover of yellow starthistle plants [93,229,251,344]. Yellow starthistle establishment from seeds sown in undisturbed plots across nine steppe, shrub-steppe, and ponderosa pine sites in Washington was positively correlated with April to June precipitation during 2 years (r² = 0.49 and 0.26; n = 9); and about twice the number of yellow starthistle, diffuse knapweed, and spotted knapweed seedlings established during a wet year than a dry year. Yellow starthistle seedling establishment (3.3% of seeds sown) was nearly twice that of diffuse knapweed and spotted knapweed (1.5% each) at the three driest sites, and it was about half that of spotted knapweed at the two wettest sites (12% each for yellow starthistle and diffuse knapweed and 20% for spotted knapweed) [220]. In north-central Utah, about 13% of yellow starthistle plants survived to flower during a drought year. Cover and extent of yellow starthistle patches varied over 3 years when annual precipitation was below the 100-year average, and was lowest during the year with the fewest summer rainfall events [251].
At three sites in California, yellow starthistle seedling establishment did not appear strongly related to precipitation, except in dry years at two sites (table 6). Proportion of seedlings surviving to maturity ranged from 11% to 58% in years of average or above-average precipitation and from 0% to 43% in years with below-average precipitation [69]. In Sacramento County, yellow starthistle cover was <25% during a year with spring drought and 96% during a year with a wet spring [229]. Severe drought (<46% mean annual precipitation) resulted in mortality of all yellow starthistle plants in a north-central California site by midsummer [93].
| Table 6—Density of yellow starthistle seedlings and mature plants and precipitation patterns during 3 years. Seedlings were counted in late spring and mature plants in midsummer. Years with average or above-average precipitation are highlighted in bold. Table modified from DiTomaso et al. (2006) [69]. | |||||
| Site | Year | Seedling density (SD) (plants/m²) | Mature plant density (SD) (plants/m²) | Survival (%) | % of mean annual precipitation |
| San Benito | 1999 | 857 (195) | 205 (171) | 24 | 75 |
| 2000 | 810 (224) | 345 (150) | 43 | 80 | |
| 2001 | 1192 (357) | 174 (75) | 15 | 91 | |
| Yuba | 1999 | 419 (217) | 55 (29) | 13 | 92 |
| 2000 | 170 (96) | 98 (51) | 58 | 111 | |
| 2001 | 68 (33) | 3 (1) | 4 | 72 | |
| Siskiyou | 1999 | 140 (38) | 28 (17) | 20 | 115 |
| 2000 | 214 (44) | 23 (12) | 11 | 100 | |
| 2001 | 76 (30) | 0 | 0 | 38* | |
| *Precipitation was lowest on record. | |||||
Yellow starthistle plants are not tolerant of flooding. In a greenhouse, yellow starthistle mortality increased and total mass decreased with increased water levels. No yellow starthistle plants survived at the greatest water depth (2.8 inches (7 cm) deep) [108].
Seedlings can survive extended frost periods [74]; however, frost heaving can kill seedlings. Near Walla Walla, Washington, about 2,500 yellow starthistle seedlings/foot² (26,875 seedlings/m²) were recorded in mid-January. Subsequent frost heaving reduced seedling density by about 40% [262,280,283]. Mature yellow starthistle plants are not frost tolerant [74].
Plant Growth and Mortality
Yellow starthistle roots can grow quickly and deeply in full sunlight and at mild temperatures, but growth is slower when plants are shaded or temperatures are cold [34,73,178,247,258,262,284]. In Columbia County, Washington, roots grew at a mean rate of 0.2 inch (0.5 cm)/day and as fast as 0.8 inch (2.1 cm)/day. Roots exceeded 48 inches (120 cm) in length 140 days after germinants were planted [258]. In Yolo County, California, roots grew at a mean rate 0.5 inch (1.3 cm)/day, and root depth increased exponentially with time. Roots reached an average depth of 26 inches (65 cm) 68 days after seeding, and roots in most plots exceeded 39 inches (100 cm) 80 days after seeding. When plants were grown under 80% and 92% shading, roots grew 45% and 64% slower, respectively, compared to unshaded plants [73].
Yellow starthistle roots grow faster and reach greater depths than associated annual grasses [34,279,282,296], and yellow starthistle has a deeper root system than many associated perennial grasses, such as nonnative pubescent wheatgrass [234]. After 46 days in a growth chamber, yellow starthistle and cheatgrass roots averaged 30 inches (77 cm) and 16 inches (40 cm), respectfully [279]. In an agricultural field on 10 May, yellow starthistle and soft brome roots were 41 inches (105 cm) and 22 inches (55 cm) deep, respectively [34]. Rapid, deep root growth in yellow starthistle makes resources available into late summer, long after seasonal rainfall has ended and shallow-rooted annual grasses have senesced, thus reducing competition for resources with associated plants at the reproductive stage [74,104,284]. Because yellow starthistle has a deeper root system, it can access water from greater depths than many associated perennial grasses during dry conditions in late summer [234].
Sunlight affects yellow starthistle morphology and growth [258]. Under experimental conditions in Pullman, Washington, rosettes grown in full sunlight were compact and flattened to the soil surface, while those grown in shade were more erect and had larger leaves. In November, rosette leaf size and rosette height were negatively correlated with light level (r = −0.95 and r = −0.93, respectively), while rosette root length (r = 0.93) and rosette area (correlation coefficient not provided) were positively correlated with light level. The following July, rosette height (r = 0.80), rosette area (r = 0.91), and aboveground biomass (r = 0.93; n = 8 for all correlations) were positively correlated with light level. Aboveground biomass of individual plants in July averaged about 0.35 ounces (10 g) at 53% sunlight, about 0.46 ounces (13 g) at 70% sunlight, and about 1.41 ounces (40 g) at 100% sunlight [258].
Yellow starthistle can grow rapidly on burned sites. At Fort Hunter Liggett, California, yellow starthistle seedlings were 2.0 to 4.7 inches (5-12 cm) tall in March following October prescribed fires at two sites [167]. However, trends in postfire plant growth are inconsistent among studies. Yellow starthistle plants may be larger [137,247], smaller, or similar [68,69,83] in size on burned than unburned sites.
Rosette and adult mortality may be high. According to a review, 60% to 75% of yellow starthistle rosettes die by July in most years [283]. In Walla Walla, Washington, 40% of seedlings present in late January died before reaching the rosette stage in late April, 75% of rosettes present in late April died before reaching the adult stage in mid-June, and 75% of adults present in mid-June died by mid-July [280].
Vegetative Regeneration
Yellow starthistle may sprout from the root crown after top-kill. It can sprout after mowing [19,26] and livestock grazing [150,283,318], but burned plants do not usually sprout [26,188]. After a June prescribed fire that scorched yellow starthistle plants, some plants "were able to resprout from the base" but, overall, "sprouting was minimal" [188]. After mowing to a height of 2 inches (5 cm), yellow starthistle can recover if leaves and buds are still attached [19].
Succession
Yellow starthistle is an early successional species [190] that establishes best on disturbed sites (e.g., [29,38,135,137,220,251]), including burned areas [61,167], newly formed sandbars [161], eroded gullies [131], and animal-created soil mounds [139]. However, disturbance is not always required for successful establishment [251].
Yellow starthistle may establish on undisturbed sites in some areas and may spread onto these sites from nearby disturbed sites. For example, yellow starthistle often establishes along roadsides, where it then spreads into adjacent disturbed and undisturbed plant communities [38,106,107]. In Napa, Lake, and Colusa counties, California, yellow starthistle occurred more frequently in plots close to roads (33 feet (10 m)) than in plots distant from roads (328 and 3,280 feet (100 m and 1000 m)) on both serpentine and nonserpentine soils (P < 0.001) [106].
In the mountainous ecoregions of Washington, Oregon, Idaho, and Montana, yellow starthistle was classified as "invasive with disturbance" on low-elevation to alpine sites, and it was classified as "invasive without disturbance" in riparian areas [222].
 |
| Figure 5—Field in Bitterwater, California, after prescribed fire to control yellow starthistle in early June, when yellow starthistle first began to flower. Most yellow starthistle survived because burning was incomplete due to high soil moisture, high humidity on the day of the fire, and a substantial number of still-green shortpod mustard plants. Photo by Devii Rao, University of California Cooperative Extension, Hollister, California. |
Fire usually consumes or kills yellow starthistle plants [26,68], although plants may sprout after low-severity fire [188] (see Vegetative Regeneration). Surviving yellow starthistle plants may remain green for up to 4 days following burning, which could allow seeds to mature if burning is conducted too late in the flowering stage [130]. Consumption of yellow starthistle plants by fire is not necessary to kill the plants, although sufficient heat is required to scorch the foliage, stem-girdle, and kill them [130,131]. For example, at Sugarloaf Ridge State Park, a prescribed fire was "too cool" (averaging 394–401 °F (201–205 °C) at the soil surface) and did not fully consume all yellow starthistle plants. However, of the yellow starthistle plants not consumed by the fire, nearly 100% had "complete foliar scorch" 2 days after the fire. Although flowers and immature seedheads remained on the plants, seeds did not mature [130,131]. Similar patterns of consumption and scorching were observed after low-severity prescribed fire in Marin County, California [214]. Fuels may be too moist in spring to carry fire such that burning is incomplete and yellow starthistle mortality is low (fig. 5).
Only two published studies examined the effects of temperature or smoke on yellow starthistle seed germination. One found that germination rate of yellow starthistle seeds decreased with increasing temperature when exposed to 5-minute heat treatments of ≈185, 221, 257, and 293 °F (85, 105, 125, and 145 °C) in a drying oven. Smoke treatment had no effect on germination rates, except when combined with a heat treatment of 221 °F, germination of smoke-treated seeds was 10% lower than that of untreated control seeds. Exposure to aqueous charate did not affect germination after heat treatment at any temperature [1]. In contrast, another study found that temperatures higher than about 239 to 248 °F (115–120 °C) were lethal to yellow starthistle seeds, and that germination rates for both plumed and plumeless seeds were similar among lower temperature treatments: ambient (control), 158 °F (70 °C), and 230 °F (110 °C) [247].
Fires are usually not severe enough to kill yellow starthistle seeds in the soil seed bank [69,74,75,130], but postfire germination from the soil seed bank reduces seed bank density [68,69]. See Postfire Seed Banks for more information.
Postfire Regeneration Strategy [301]
Yellow starthistle is a winter annual that produces abundant seeds (see Seed Production). It can establish after fire either from undamaged seeds in the soil seed bank [68], or from seeds dispersed from off-site sources. Fire is likely to create conditions that are favorable for yellow starthistle establishment by increasing bare ground, reducing vegetation, increasing sunlight at the soil surface, and increasing nutrient availability [74].
 |
| Figure 6—Fort Hunter Liggett Fire Department conducts a prescribed burn of yellow starthistle in fall to prepare for herbicide application in spring. Photo courtesy of US Army Garrison Fort Hunter Liggett. |
Most information about yellow starthistle's response to fire comes from field studies using prescribed fire—alone and in combination with other methods—to control invasive populations of yellow starthistle. Information regarding yellow starthistle response to fire alone is summarized below. For a summary of its response to fire in combination with other control methods, see Integrated Management with Prescribed Fire. Most studies about yellow starthistle's response to fire were conducted in California annual grasslands, and only two were conducted in perennial grasslands in Idaho. Additional information comes from greenhouse and laboratory studies comparing germination and growth in soils from burned and unburned sites. Yellow starthistle's response to fire may vary among plant communities, with differences in fire characteristics (timing, frequency, pattern, and severity), and with postfire weather. Table A3 provides a summary of publications with information on yellow starthistle's response to fire.
California Annual Grasslands:
Postfire Seed Banks: Results from three field studies on postfire yellow starthistle seed banks in California annual grasslands are described in five publications [68,69,130,131,166]. These suggest that burning yellow starthistle in summer—during the early flowering stage and prior to seed set—can reduce yellow starthistle seed bank density that fall [68,69]. Consecutive annual burning can further reduce seed bank density [68,131], as long as fuels are sufficient to carry fires that kill all reproductive yellow starthistle plants before seeds mature (e.g., [69]). Without additional burning or follow-up treatment, yellow starthistle can reestablish from any remaining viable seeds in the soil seed bank [166].
On two sites at Sugarloaf Ridge State Park, one prescribed fire at the early flowering stage (late June to early July) reduced the yellow starthistle seed bank by 75% compared to an unburned control, and prescribed fires in 3 consecutive years—with no further seed production—reduced the yellow starthistle seed bank by >99% [68,130,131]. Additional years of postfire data from one of these study sites showed yellow starthistle seed bank density increased 1 year after the last prescribed fire, as new plants established and set seed. Seed bank density continued to increase over the next 3 years, and it was 80% of an unburned control after 4 years [166] (table A3). In San Benito County, summer prescribed fires in 2 consecutive years did not reduce the yellow starthistle soil seed bank the subsequent fall (≈4 months after the second fire) (1,600 seeds/m²) compared with untreated controls (2,200 seeds/m²). The second fire was incomplete due to lack of fine fuels, and surviving plants added seeds to the soil seed bank [69].
Postfire Germination: Heat from fire may damage or kill yellow starthistle seeds [1,247] (see Immediate Fire Effects on Plant) and thus reduce germination rates of seeds in the soil seed bank. However, observations of increased germination and high seedling densities in burned areas suggest that burning may "stimulate germination" of surviving seeds [69,74,75,130], and thus reduce the density of germinable seeds in the soil seed bank [68,69] (see Postfire Seed Banks). For example, DiTomaso (2006) cited unpublished data that showed that yellow starthistle germination "increased dramatically" in fall after prescribed fire [61] and Kyser et al. (2013) observed a "heavy flush" of yellow starthistle seedlings with the first fall rains after October prescribed fire (i.e., after seed dispersal) [167].
One study examined germination rates of yellow starthistle seeds in burned soil. Germination rates of yellow starthistle seeds collected from Spain and sown in pots were similar in soil from burned (38.3%) and unburned (40.8%) sites for plumeless seeds, while germination rates were lower in soil from burned (21.7%) than unburned (44.1%) sites for plumed seeds. Soil organic matter was lower and ammonium, nitrate, and pH were higher in burned than unburned soil [247].
Postfire Seedling Establishment: Fire is likely to create conditions that are favorable for yellow starthistle seedling establishment by increasing bare soil, reducing vegetation, increasing sunlight at the soil surface, and increasing nutrient availability [74] (Seedling Establishment and Mortality and Plant Growth and Mortality). At Jasper Ridge Biological Preserve, mean establishment from yellow starthistle seeds sown in fall was higher in burned (≈15 plants) than unburned (≈11.5 plants) plots in winter and spring probably due to reduced litter and increased sunlight on the soil surface [83]. In northern California, yellow starthistle establishment in summer from seeds sown in fall was greater in burned plots than in control plots [137]. In contrast, mortality of yellow starthistle seedlings grown from seeds collected in Spain was similar in soil from burned and unburned sites [247]
After a single prescribed fire, yellow starthistle seedling establishment and survival may exceed that on unburned sites [69]. However, after two or more fires in consecutive years that kill all reproductive yellow starthistle plants before seeds mature, seedling establishment and survival are likely to be reduced on burned sites [68,69]. After a single summer prescribed fire at Fort Hunter Liggett in Monterey County (fig. 6), mean yellow starthistle seedling density in late winter (≈7 to 8 months after fire) was 2.3 times greater on burned than on unburned plots, but it was similar on burned and unburned plots at a site in San Benito County [69]
After two consecutive summer prescribed fires in Siskiyou County, where annual grass fuel loads and continuity were sufficient to carry fire in the second year (and the burn was complete), mean yellow starthistle seedling densities were lower on burned (27 seedlings/m²) than unburned (76 seedlings/m²) sites. In contrast, in San Benito County, where yellow starthistle was so dense that fine fuels were insufficient to carry fire in the second year (and the burn was incomplete), seedling density was greater on burned (1,792 seedlings/m²) than unburned (1,192 seedlings/m²) sites [69]. Differences in timing and amount of precipitation may have also contributed to differences in seedling establishment between these sites [69] (table 6). After three consecutive annual fires at Sugarloaf Ridge State Park, fewer seedlings occurred on burned than unburned plots in the first year [68,130], and fewer rosettes occurred on burned than unburned plots up to 5 years after the last fire [166].
Postfire Plant Growth: Postfire soil conditions may favor yellow starthistle growth, such that plants grow fast [167] and large [247] on burned sites. However, results are inconsistent (e.g., [68,69,83]). In northern California, yellow starthistle patches were taller in burned (≈28 inches (70 cm)) than unburned (≈16 inches (40 cm)) plots the first summer after burning [137]. The number and size of leaves on yellow starthistle seedlings grown in a greenhouse was greater in soil from burned than unburned sites [247].
However, plant size and biomass were smaller or similar on burned versus unburned sites in other studies. For example, at Sugarloaf Ridge State Park, yellow starthistle height and biomass were similar on unburned plots and burned plots 1 year after a summer prescribed fire and 1 year after 3 consecutive summer prescribed fires [68]. At Jasper Ridge Biological Preserve, mean yellow starthistle stem diameter was 32% smaller for yellow starthistle plants growing in burned than unburned plots and mean aboveground yellow starthistle biomass and height were similar [83]. At three sites in San Benito, Siskiyou, and Yuba counties, biomass of yellow starthistle in summer was similar on burned and unburned plots 1 year after two consecutive summer prescribed fires [69].
Postfire Flowerhead Production: Two studies in California annual grasslands found increased or similar fecundity of yellow starthistle in burned compared to unburned areas. Yellow starthistle plants in burned plots in northern California had more flowerheads the following summer (58 flowerheads/plant) than plants in unburned plots (16 flowerheads/plant) [137]. One year after a single summer prescribed fire at Sugarloaf Ridge State Park, yellow starthistle plants in burned plots averaged 3.8 seedheads/plant, and 1 year after 3 consecutive summer prescribed fires yellow starthistle plants averaged 5.1 seedheads/plant, compared to an average of 2.2 seedheads/plant in unburned plots. These differences were not statistically significant [68].
Postfire Cover and Density: Fire kills yellow starthistle plants (see Immediate Fire Effects on Plant) and can reduce yellow starthistle seed banks; thus, yellow starthistle cover and density may be reduced soon after a single fire. However, yellow starthistle germination, seedling establishment, plant growth, and seed production may increase after a single fire due to favorable postfire conditions; thus, a single fire may increase yellow starthistle cover and density soon after fire [69,137,214]. In Marin County, yellow starthistle cover was lower on burned plots the spring after a summer prescribed fire (≈40%) than before the fire (≈60%) [214]. At Fort Hunter Liggett, mature plant density in early summer was lower on burned plots (≈0 and 10 plants/m², respectively) 1 year after a summer prescribed fire than on unburned plots (≈45 and 550 plants/m²) [69]. In contrast, in northern California, yellow starthistle cover in summer was greater on 1-m² burned plots where yellow starthistle seeds were sown in fall (80%) than on unburned, seeded plots (8%) [137].
If burned again before plants set seed, yellow starthistle abundance may decrease after two or more consecutive annual fires [68,69,130,146,214,347]. Yellow starthistle abundance on burned plots after two or more consecutive annual fires was similar to or lower than that on unburned control plots in three studies. In Marin County, yellow starthistle cover was lower in spring after two (≈20%) and three (<10%) consecutive summer prescribed fires than before the fires (≈60%) [214]. One year after two consecutive summer prescribed fires, cover of yellow starthistle was lower on burned (9%) than unburned plots (22%) in San Benito County, and cover was similar on burned (0.9%) and unburned (4.7%) plots in Yuba County. Plant density and biomass were similar on burned and unburned plots at both sites. No yellow starthistle plants occurred in either burned or control plots in summer at a site in Siskiyou County, likely due to drought [69]. Ten months after two consecutive summer prescribed fires in Colusa County, yellow starthistle cover was lower on burned (9%) than unburned (30%) plots (table 7). Yellow starthistle plant density was similar on burned and unburned plots ≈4 months after (zero or near zero plants in both) and ≈16 months after (≈2.5 plants/m² in burned and ≈3.0 plants/m² in unburned plots) the second fire [347].
Perennial Grasslands: Two field studies in Idaho found no change in yellow starthistle cover 1 to 3 years after fire [120,121,193]. In a bluebunch wheatgrass-Sandberg bluegrass-arrowleaf balsamroot habitat type at the Craig Mountain Wildlife Area, yellow starthistle cover in late May or early June was similar on burned (6.3%–7.1%) and unburned (4.4%–13.9%) plots 2 years before and 3 years after an August wildfire (P > 0.05). However, yellow starthistle cover generally decreased over time on unburned plots and was relatively unchanged on burned plots. Above-average postfire precipitation likely facilitated postfire recovery of native perennial bunchgrasses [120,121]. For further information on this study, see Gucker and Bunting's Research Project Summary. Hill (2004) cites an unpublished report about a pilot study conducted in an Idaho fescue-bluebunch wheatgrass community that found that cover, density, and frequency of yellow starthistle in burned plots was higher than in unburned plots following an August wildfire [139], but this report and details about the study could not be obtained.
Riparian and Wetland Communities: Two studies in California found reduced yellow starthistle abundance about 1 year after fire in riparian and wet meadow communities. On the Klamath National Forest, yellow starthistle density was lower 1 year after fire than before fire at two sites in riparian narrowleaf willow communities [169]. In a wet meadow dominated by nonnative annual grasses in Pinnacles National Park, yellow starthistle density was reduced by more than 98% following an early June, mixed-severity prescribed fire. Prior to the fire in May, the 10-acre (4-ha) area had an estimated 450,000 yellow starthistle plants [188].
FUELS
Senesced stems of yellow starthistle degrade slowly and may remain erect for a year [40,62]. Dried skeletons of yellow starthistle can provide fuel for late summer wildfires, but green plants are too moist to carry fire, and dense populations may have insufficient grass fuels to carry fire [64,69,131,226]. In an eroded gully at Sugarloaf Ridge State Park, where yellow starthistle cover was 100%, fuels were insufficient to carry a surface fire in July during either of 2 years. Yellow starthistle was described as "nonflammable" and "fleshy" at this time and grass fuels were lacking [131]. In San Benito County, California, a second consecutive year of summer prescribed fire was incomplete because the first-year prescribed fire removed all litter, and little vegetation other than yellow starthistle was available to provide fuel the second year. At a site in Yuba County, California, however, "a good stand of annual grasses" provided enough fuel to carry a second-year summer prescribed fire, and burning was complete [69].
FIRE REGIMES
Yellow starthistle is most invasive in annual and perennial grasslands, shrub steppes, oak savannas, and open woodlands in California, the Pacific Northwest, and the Great Basin (see Plant Communities). Invasion of native perennial grasslands by yellow starthistle at Sugarloaf Ridge State Park, California, has been attributed, in part, to fire exclusion and infrequent fire in these ecosystems [64,130]. DiTomaso et al. (2006) suggested that prescribed fire intervals that mimic historical fire intervals of 2 to 10 years may increase native plant diversity and control yellow starthistle at Sugarloaf Ridge State Park [74]. Following initiation of an annual prescribed fire program in this community, yellow starthistle cover was reduced and native plant diversity increased [68,130].
While it is unclear how fire regimes of invaded plant communities might affect or be affected by yellow starthistle populations, yellow starthistle fuels do not carry fire as well as grass fuels [131] (see Fuels). Therefore, dense yellow starthistle populations alter fuel characteristics on invaded grassland sites, and thus alter fire regime characteristics. FEIS publications with information on fire regimes in Biophysical Settings where yellow starthistle is invasive include the following:
FIRE MANAGEMENT CONSIDERATIONS
Fire as a Control Agent
Most information about controlling yellow starthistle with prescribed fire comes from field studies in California annual grasslands and a few literature reviews. No published information was available regarding use of prescribed fire to control yellow starthistle in other regions or vegetation types.
The effectiveness of fire for killing yellow starthistle and other invasive plants or reducing their population growth depends on fire characteristics (pattern, severity, timing, and frequency) [58,74], as well as the species present in the prefire plant community and soil seed bank. Because postfire conditions stimulate germination, yellow starthistle seed bank density tends to decrease after prescribed fire in the short term. However, consecutive annual prescribed fires (or other follow-up treatments) are needed to prevent subsequent seed production and thus reduce yellow starthistle populations in the long term. Fires are most effective for controlling yellow starthistle populations when they are spatially continuous (i.e., consuming or scorching and stem-girdling all plants) and timed to kill yellow starthistle plants before they produce seeds (see Prescribed Fire Timing). Patchy fires leave surviving plants that can produce seeds and replenish the soil seed bank [69,74]. Follow-up monitoring and treatment of seedlings may be needed for several years to prevent reestablishment [166]. Sufficient fuels may not be available to carry consecutive annual fires (see Prescribed Fire and Fuels), and fires of this frequency may have severe impacts on desired plants [69] (see Prescribed Fire Frequency). Seeding may be necessary in areas where desirable plant populations are depleted [62,240,283] (see Revegetation). Prescribed fire is most effective at reducing yellow starthistle populations when it is used in combination with other control methods [240] (see Integrated Management with Prescribed Fire).
Prescribed Fire Timing: Yellow starthistle flowers and sets seed later than most associated vegetation in California annual grasslands [65,72,74] and in canyons and foothills of the Pacific Northwest in north-central Idaho, northeastern Oregon, and eastern Washington [262]. The optimal time to burn for the most effective control of yellow starthistle in these communities is after annual grasses have senesced and before yellow starthistle produces viable seeds. This generally corresponds with early to mid-summer in California annual grasslands [65,72,74] and mid-summer in the Pacific Northwest [262], and there may be only a narrow window of time when grass fuels can carry fire through still-green yellow starthistle stands (see Fuels). For example, prescribed fires in Marin County, California—with its relatively wet climate and late-curing grasses—were of low severity and difficult to sustain because fuels were too moist to carry fire, resulting in patchy burns. Nonetheless, nearly 100% of the yellow starthistle plants not consumed by the fire were scorched and killed [214] (see Immediate Fire Effects on Plant).
Air quality concerns and a high potential for fire to escape can limit opportunities for prescribed burning when timing is optimal for effective yellow starthistle control [72,188,196]. Burning in fall or spring to reduce the risk of fire escape may not effectively control yellow starthistle [261]. An early June prescribed fire (earlier than typically recommended) in Pinnacles National Park, California, reduced yellow starthistle density by 98%. The fire was conducted when most yellow starthistle plants were in the rosette and seedling stages, a small percentage had bolted, and none were flowering. Nonnative annual grasses provided abundant surface fuels [188]. Yellow starthistle seedlings can also be killed by burning small areas (e.g., 4-m² plots) with a handheld torch in winter or early spring [74,269]. This approach reduces risk of escaped fire and avoids some air quality issues, but it is somewhat nonselective and provides inconsistent control of yellow starthistle. It is most effective when followed by a dry spring, but may be a "complete failure" when followed by a wet spring, especially if desired species are suppressed [74].
Timing of prescribed fire can influence populations of nontarget species. Species that complete their life cycle before burns are conducted will generally be selected for, whereas those that flower and seed later will generally be selected against [65]. A concern with using prescribed fire to reduce yellow starthistle is that nonnative annuals, which complete their life cycle before burns are conducted, are likely to increase. In Marin County, California, yellow starthistle cover decreased after 3 consecutive years of prescribed fires, while cover of nonnative annual grasses increased, and cover of native herbs and grasses was unchanged. The authors suggested that these results were acceptable because, although nonnative annual grasses increased, "these at least provided forage value", unlike yellow starthistle [214]. About 10 months after two consecutive prescribed fires in Colusa County, California, yellow starthistle cover was lower in burned than unburned plots, cover of annuals (nonnative and native combined) was greater in burned than unburned plots, and native perennial grass cover was similar between plots. The authors concluded that fire favored germination and establishment of annuals in spring by reducing cover of yellow starthistle thatch [347] (table 7). In contrast, the first growing season after three consecutive annual prescribed fires at Sugarloaf Ridge State Park, cover and frequency of yellow starthistle and three nonnative annual grasses (purple false brome, ripgut brome, and soft brome) were lower, and that of one native annual grass (wild oat) was higher in burned than unburned sites. Most species (92%) that declined in cover and frequency were nonnative grasses and forbs, while 52% of the species that increased in cover and frequency were California natives [68].
| Table 7—Cover in roadside plant communities in Colusa County, California, in April 2006, 2 years after initial treatments to control yellow starthistle. Plots were treated in spring and summer of 2004 and 2005. Burns occurred August 2004 and June 2005. Within columns, cover values with different letters are significantly different (P < 0.05). Table modified from Young and Claassen (2008) [347]. | ||||
| Treatment | ||||
| Yellow starthistle | Nonnative and native annuals | Native perennial grasses | Thatch | |
| Untreated control | 30.0a | 34.8a | 7.3a | 27.6a |
| Burn | 9.0b | 63.6b | 10.5a | 16.6ab |
| Mow | 13.6b | 65.3b | 14.8a | 6.0b |
| Clopyralid | 0.8b | 73.0b | 7.3a | 19.0ab |
| Mow + fire | 10.5a | 82.8b | 2.1b | 4.6a |
| Clopyralid + fire | 1.1b | 77.8b | 11.0a | 9.8a |
| Clopyralid + mow | 0.0b | 77.8b | 11.5a | 10.6a |
| Clopyralid + mow + fire | 0.3b | 72.8b | 14.1a | 12.6a |
Prescribed Fire Frequency: Yellow starthistle plants emerge from surviving seeds in the soil seed bank the fall or spring after prescribed fires. To reduce yellow starthistle populations, it is critical to kill these emerging plants before they produce seeds, as they are likely to be highly productive due to decreased competition for resources [130]. Three or more consecutive years of prescribed fire can deplete the soil seed bank and reduce yellow starthistle populations [65,70,74,75]. However, after annual burning ceases, yellow starthistle may establish from the few remaining seeds in the soil seed bank or from seeds dispersed from off-site sources [166] (see Plant Response to Fire). Therefore, follow-up monitoring and treatment of seedlings may be needed for several years to prevent reestablishment [65,70,74,75].
Consecutive annual burning may be detrimental to native plant communities [146]. Two consecutive years of burning in grassland sites in the Lassen foothills of northern California was effective in reducing cover of yellow starthistle and medusahead without reducing overall plant diversity. However, plant diversity decreased after the third year of burning, causing concern for several sensitive plant species in the area [146].
Prescribed Fire and Fuels: Sufficient fuels are needed for prescribed burning to be effective at killing yellow starthistle plants, and fuels may be insufficient where dry grasses are lacking, such as in dense yellow starthistle stands or in frequently burned stands [69,72,74,131,188] (see Fuels). Prefire herbicides or mowing can enhance the effectiveness of burning by increasing the amount of dry fuels [61] (see Prescribed Fire and Herbicides). Deferring livestock grazing may allow fuels to build up prior to burning [146]. Sowing grasses in yellow starthistle stands can add fuel for prescribed fires [131,188]. At Pinnacles National Park, a sterile, annual wheat × wheatgrass hybrid was seeded in the fall after an early June prescribed fire to provide fuel for subsequent fires [188]. However, no information was given on the efficacy of this approach.
Integrated Management with Prescribed Fire:| a. | b. | c. |
 |
 |
 |
| Figure 7—Field invaded by yellow starthistle at US Army Garrison Fort Hunter Liggett, Monterey County, California, a) before treatments, b) during prescribed fire, and c) after treatments (6 June 2013). Treatments were prescribed fire (26 November 2012), mowing and aminopyralid application to emerging rosettes (14 March 2013). Yellow starthistle appears as silvery grey skeletons in a and b. Results of these treatments were not available as of this writing. Photos courtesy of the Monterey County Agricultural Commissioner's Office. Details about this project can be found at the County of Monterey website. | ||
In areas where consecutive annual burning may be constrained by inadequate fuels, or in areas where frequent fire is likely to have negative impacts on desirable plants or result in soil erosion, a first-year prescribed fire followed by other control methods may be most effective for long-term management [72,74,130,167,240,250,261]. Information on management of yellow starthistle comes predominantly from California annual grasslands; management information from other plant communities is lacking. Table A3 lists studies that investigated yellow starthistle's response to prescribed fire alone and in combination with other control methods in California annual grasslands.
Prescribed Fire and Herbicides: Literature reviews suggest that herbicides may be used before or after fire to control yellow starthistle. Herbicide applied before fire may increase dead fuel loads to better carry fire [65]. Herbicide applied after fire may be more effective than herbicide without fire because target weeds may be better seen and herbicides may have better contact with them [240,261].
Prescribed fire can be used in the first, second, or third year of a long-term yellow starthistle management strategy. If burning can be used only once, DiTomaso et al. (2006) recommend burning in the first year and applying herbicide the second year. Because yellow starthistle seedling establishment may increase after fire, burned areas must be monitored and establishing plants killed before they produce seeds [69,74]. Prescribed fire can be used to stimulate germination of yellow starthistle seeds, and herbicide can then be used to kill these seedlings (e.g., [167]. This depletes the seed bank more rapidly than 2 consecutive years of herbicide application or a first-year herbicide application followed by a second-year prescribed fire [65,69]. In San Benito County, yellow starthistle seed bank density on untreated control plots (2,200 seeds/m²) was higher than that on treated plots that included two consecutive years of clopyralid application, first-year summer prescribed fire followed by second-year clopyralid application, and first-year clopyralid application followed by second-year summer prescribed fire (all ≤100 seeds/m²). Two consecutive years of summer prescribed fire did not reduce yellow starthistle seed bank density the subsequent fall (≈4 months after fire) (1,600 seeds/m²) relative to the control, because the second-year fire was incomplete due to lack of fine fuels, and surviving plants added seeds to the soil seed bank [69]. At two sites at Fort Hunter Liggett, density of yellow starthistle seeds in the soil seed bank in the fall following a first-year summer prescribed fire and a second-year spring clopyralid application was lower than that in untreated control plots. After the final treatments at both sites, which included either a third-year prescribed fire or a third-year clopyralid application, densities remained lower in treated than untreated plots [69] (table 8).
| Table 8—Mean yellow starthistle seed density in the soil seed bank (seeds/m²) in fall on two annual grassland sites at Fort Hunter Liggett, California, in untreated control plots and in plots treated with prescribed fire and clopyralid applications. Cells within the same rows with different letters are significantly different (P ≤ 0.05). Data estimated from DiTomaso et al. (2006) [69]. | ||||
| Sites | ||||
| Control | Treatment | Control | Treatment | |
| TA15a | 1,900a | 250b | 1,800a | 100b |
| TA27b | 4,300a | 400b | 4,100a | 200b |
| aTreatments were prescribed fire (summer 1999) clopyralid application (spring 2000), and clopyralid application (spring 2001). | ||||
| bTreatments were prescribed fire (summer 1999), clopyralid application (spring 2000), and prescribed fire (summer 2001). | ||||
A combination of herbicide plus prescribed fire may be more effective for reducing yellow starthistle abundance than prescribed fire alone, but results are inconsistent. Effects of treatment combinations vary with location, timing of herbicide application, and posttreatment precipitation [69,167,347]. Yellow starthistle abundance 1 year after prescribed fire and/or herbicide treatments varied among treatments and sites in three California counties (table 9). In San Benito County, yellow starthistle cover, density, and biomass in summer was highest in plots with second-year prescribed fire, a result attributed to higher germination and plant growth relative to plots with second-year herbicide application. In Yuba County, variability was high among plots and differences were not always statistically significant. In Siskiyou County, absence of yellow starthistle from all sites by summer was attributed to drought-induced mortality [69]. Two years after initiation of various combinations of burning, mowing, and herbicide treatments in Colusa County, California, mean yellow starthistle cover averaged ≤1.1% in all treatments that included herbicide application and ≥9.0% in all treatments that included only prescribed fire and/or mowing (table 7). The authors concluded that a combination of herbicide, mowing, and burning appeared to be most effective for reducing yellow starthistle cover while increasing native perennial grass cover, although cover of annuals also increased [347]. On two sites at Fort Hunter Liggett, mean yellow starthistle cover was lower in plots burned under prescription in October and sprayed with herbicide in November, January, or March than in untreated control plots the first July after treatments. By August the following year, cover remained lower in the January- and March-sprayed plots than in the untreated plots at both sites. However, cover in the November-sprayed plots was high at both sites, which was attributed to the "relatively long rainy season" that resulted in low soil residual herbicide activity and failure control late-season germinants [167].
| Table 9—Mean yellow starthistle abundance at three annual grassland sites 1 year after the second (final) year of treatments. Values within a column followed by different letters are significantly different (P ≤ 0.05). Blank cells indicate insufficient data for analysis. Table modified from DiTomaso et al. (2006) [69]. | |||||
| Site and treatment | Early spring | Late spring | |||
| Density (seedlings/m²) | Cover (%) | Cover (%) | Density (plants/m²) | Biomass (g dry wt/m²) |
|
| San Benito County | |||||
| Control | 1,192b | 73.8a | 22.4a | 174.2ab | 38.2b |
| Two consecutive years of summer prescribed firea | 1,792a | 60.7b | 9.3b | 105.8b | 32.0bc |
| First-year summer prescribed fire; second-year clopyralid application | 0d | 0c | 0b | 0b | 0c |
| First-year clopyralid application; second-year summer prescribed fire | 563c | 59.3b | 22.0a | 333.3a | 87.1a |
| Two consecutive years of clopyralid | 55d | 2.4c | 2.0b | 1.8b | 1.3c |
| Yuba Countyb | |||||
| Control | 68 | 11.6ab | 4.7b | 2.7 | 1.8b |
| Two consecutive years of summer prescribed fire | 0 | 0.2b | 0.9b | 1.8 | 2.2b |
| First-year summer prescribed fire; second-year clopyralid application | 0 | 0.2b | 0b | 0 | 0b |
| First-year clopyralid application; second-year summer prescribed fire | 90 | 20a | 25.3a | 106.2 | 77.3a |
| Two consecutive years of clopyralid | 1 | 0.7b | 1.6b | 0.4 | 0.4b |
| Siskiyou Countyc | |||||
| Control | 76a | 2.9 | 0 | 0 | |
| Two consecutive years of summer prescribed fire | 27b | 0 | 0 | 0 | |
| First-year summer prescribed fire; second-year clopyralid application | 5b | 0 | 0 | 0 | |
| First-year clopyralid application; second-year summer prescribed fire | 15b | 0.4 | 0 | 0 | |
| Two consecutive years of clopyralid application | 6b | 0 | 0 | 0 | |
| aThe second-year prescribed fire was incomplete because the first-year prescribed fire removed all litter and there was little vegetation other than yellow starthistle for fuel. | |||||
| bA lack of significance in seedling density among treatments was attributed to highly variable densities in early spring and summer. | |||||
| cSpring drought resulted in high mortality of yellow starthistle plants in all plots. | |||||
Prescribed Fire and Biological Control Agents: Prescribed fire can be harmful to biological control agents in the short term, particularly yellow starthistle rust and insects that feed in seedheads [240]. However, biological control populations may recover quickly after fire [61,69,74]. In San Benito and Siskiyou counties, California, attack rates of two introduced biological control insects, Eustenopus villosus and Chaetorellia succinea, on yellow starthistle seedheads in burned plots were greater than or similar to those in unburned plots 1 year after 2 consecutive years of summer prescribed fire. Thus, despite the likely death of larvae within the seedheads of yellow starthistle on burned sites, recruitment of biological control insects the following year was rapid [69].
Preventing Postfire Establishment and Spread
If yellow starthistle is present on or near a site before fire, there is potential for its establishment and spread after fire (see Plant Response to Fire) unless desirable vegetation is established to replace it [93,240]. For example, after 3 consecutive years of burning an annual grassland in early July at Sugarloaf Ridge State Park, yellow starthistle seeds in the soil seed bank were reduced by 99%, seedling density was reduced by 99%, yellow starthistle cover was reduced by 91%, and total plant diversity and species richness increased, particularly that of native forbs [68,166]. However, following cessation of annual burning, density of yellow starthistle seeds in the soil seed bank, seeding density, and cover on burned plots increased. Four years after the last fire, seed bank density on burned plots was ≈80%, seedling density was ≈10%, and cover was ≈10% of unburned controls. While yellow starthistle abundance was significantly lower on burned than unburned plots in postfire year 4, total forb cover (particularly of native species), total plant cover, and plant diversity were also lower on burned than unburned plots, and grass cover did not show any consistent response. Yellow starthistle established from seeds that were dispersed onto the burn from adjacent areas, and the absence of many original dominant grassland species in the community made the grassland "at a constant risk of invasion" by yellow starthistle [166].
General recommendations for preventing postfire establishment and spread of invasive plants, including yellow starthistle, include the following:
For more detailed information on these topics, see the following publications: [10,31,115,326].
Several authors caution against the use of "certified weed-free" hay because of contamination with nonnative invasive species such as yellow starthistle. Dombeck et al. (2004) remarked that the use of certified weed-free hay for erosion control "may be suspect" when hay is only 95% or even 99% weed-free [78]. The use of weed-free hay on postfire recovery efforts on the Klamath National Forest, California, resulted in the introduction and spread of yellow starthistle on forest lands (J. Perkins, person communication cited in [78]). After the 2001 Darby Fire on the Stanislaus National Forest, California, 62 acres (25 ha) were aerially mulched with weed-free rice straw and 63 acres (25.5 ha) of yellow starthistle and Maltese starthistle were mapped the following year [50]. See Prevention for more information.
Yellow starthistle is toxic to horses, causing a neurological disease called equine nigropallidal encephalomalacia, or "chewing disease", that can be fatal with prolonged yellow starthistle consumption [74,221]. Poisoning is most likely when yellow starthistle is the only forage available (e.g., on poor condition range) or when yellow starthistle is a substantial contaminant of dried hay. In some cases, horses acquire a taste for yellow starthistle and seek it out even when other forage is available [221]. Sesquiterpene lactones are the compounds in yellow starthistle responsible for equine nigropallidal encephalomalacia [5] and also account for its allelopathic potential [151] (see Impacts). Mules and burros are not susceptible to the toxic effects [43,74]. See the review by DiTomaso et al. (2006) for more information about toxicity of yellow starthistle [74].
Yellow starthistle foliage may be eaten by grasshoppers [154], and yellow starthistle seeds are consumed by many species of birds including ring-necked pheasants, California quail, house finches, and American goldfinches [67,260].
Palatability and Nutritional Value
Yellow starthistle leaves are nutritious and highly digestible to domestic ruminants before flowering [4,316], but as plants mature, palatability and nutritional value tend to decline [4,100,186] and sesquiterpene lactone concentration tends to increase [4]. For example, at two locations in the Sacramento Valley, California, mean protein content of yellow starthistle in the rosette stage (17%) was higher than that in the bolting (12%) and flowering (10%) stages [4]. In Nez Perce County, Idaho, crude protein content was higher before (15.7%) than after (5.5%–12.8%) the bolting stage [100]. In Colusa County, California, protein content in the rosette stage (13%) was higher than that in the bolting to early bud stages (11%). In contrast, in Tehama County, mean protein content was lower in the rosette stage (10%) than in the bolting to early bud stages (13%). However, statistical differences were not determined [316]. See the following publications for more information on yellow starthistle nutritional value: [4,74,100,316].
Cover Value
Value of yellow starthistle as cover for birds varies among species. Gray and Greaves [118] and Olson and Gray [215] report some use of yellow starthistle for cover and nesting by least Bell's vireo in California. In the Santa Cruz Mountains in northern California, nonnative wild turkeys used yellow starthistle-dominated habitat more frequently than would be expected based on availability. Yellow starthistle cover was tall enough to conceal wild turkey polts from predators but short enough for adult wild turkeys to see over [216]. In contrast, areas in west-central Idaho with >5% yellow starthistle cover were used 33% less by chukars than expected based upon availability, and areas with ≤5% yellow starthistle cover were used more than expected. Chukars likely avoided dense yellow starthistle patches due to the spines on yellow starthistle flowerheads that "often produce a nearly impassable field", and the near absence of other vegetation under dense yellow starthistle canopies [179].
Yellow starthistle may provide cover for some rodents. In Yuba County, California, five species of small mammals were captured in annual grasslands with varying cover of yellow starthistle. Overall abundance of small mammals differed by season, but it did not differ among low (≤33%), medium (34%–67%), and high (≥68%) categories of yellow starthistle cover or low (≤9.8 inches (25 cm)), medium (10.2–19.3 inches (26–49 cm)), and high (≥19.7 inches (50 cm)) categories of starthistle height. Western harvest mouse and California vole were the only rodent species whose abundance differed with yellow starthistle cover and height, but differences depended on season. Most western harvest mice were captured in winter, and 91% of these were captured in plots with at least 40% yellow starthistle cover. In contrast, most California voles were captured in fall and the highest percentage of these (48%) was caught in plots with low (<33%) yellow starthistle cover [49].
OTHER USES
Yellow starthistle is regarded as an important nectar source for honey production in California and other western states [62].
Yellow starthistle has a variety of constituents that have been investigated for use in medicine. See table A4 for a list of these and other publications with information on uses of yellow starthistle.
Yellow starthistle is used in Turkish folk medicine for the treatment of ulcers and herpes infections around lips, among other ailments [102,123,143,342]. In a laboratory study, aqueous extracts of fresh or dried yellow starthistle flowers given orally showed significant antiulcerogenic activity in rats (P < 0.01) [342]. In addition, yellow starthistle has shown antiviral, antibacterial, and antifungal activities [35,291], and extracts from yellow starthistle plants were shown to have antiproliferative activities on rat brain tumor cells and human carcinoma cells [3,95].
The histopathology of horse brain tissue with equine nigropallidal encephalomalacia is the same as for human Parkinson’s disease, so researchers have attempted to isolate and identify neurotoxic substances from yellow starthistle and Russian knapweed, the only Centaurea species in the world that are toxic to horses, looking for connections with Parkinson’s disease [47,202,271].
Yellow starthistle can be converted into thermoplastics as a sustainable alternative to thermoplastics produced from petrochemicals [323].
IMPACTS |
| Figure 8—A dense yellow starthistle population. Photo by Steve Dewey, Utah State University, Bugwood.org. |
Yellow starthistle can occur in dense monocultures (fig. 8) (see Stand Structure) that displace native plants; decrease native plant and animal diversity; reduce native wildlife habitat and forage; and alter water cycles, soil microbial community composition, and soil nutrient availability [17,25,62,64,139,264]. Dense yellow starthistle stands can fragment sensitive plant and animal habitat [62]. Several rare and sensitive plant species such as agate desertparsley on the Agate Desert Preserve in southwestern Oregon, California bearpoppy on the Ash Meadow Preserve in Nevada, and Spalding's silene at the Garden Creek Ranch Preserve in Idaho, are considered threatened by yellow starthistle establishment and spread [138,140,241,248].
Once spines appear, cattle and domestic sheep avoid grazing in dense yellow starthistle stands [4,70,113,186,283,298,316,317,318] (see Importance to Wildlife and Livestock). Substantial economic losses are attributed to low forage yield and quality on invaded rangelands and associated treatment costs [62,74,89].
Large populations of yellow starthistle can deplete soil moisture and alter water cycles in annual grasslands and foothill woodlands in California [18,62,72,92,94,112] and in foothill perennial grasslands in Oregon [27] because yellow starthistle uses more water to a greater soil depth than native or desirable vegetation (see Soils). Enloe (2002) estimated a potential water loss of more than 65 m³/ha/year in a 3,800-acre (1,538-ha) area with 10% cover of yellow starthistle in Siskiyou County, California [94]. In the Sacramento Valley of California, estimated soil moisture losses in annual grasslands are at least 1,200 m³/ha/year for sites with deep soil and 1,050 m³/ha/year for sites with shallow soil [79,110].
Yellow starthistle can change soil microbial communities and soil nutrient availability in areas they invade [17,25]. These changes have the potential to alter native plant fitness and/or ecosystem function [17].
Invasion Success
Invasion success of yellow starthistle in the western United States has been attributed to allelopathy (e.g., [184,186,293]), life history traits [111,206,262,340], propagule pressure [45,147,204], local morphological and phenological adaptations [97,116,134,181,199,201,332], escape from soil pathogens [7,8,9,44,137,182], escape from the strong competitive effects of grasses in its native range [200,340], and occupation of an unfilled niche created when native perennial grasses began to be replaced by nonnative annual grasses starting in the late 1800s [33,76,144,296]. See table A5 for a list of publications providing information on factors facilitating or inhibiting yellow starthistle invasion in specific locations.
Centaurea spp. are commonly allelopathic [238]. Researchers have proposed that allelopathy could be a contributing factor to yellow starthistle's invasiveness [184,186,293], and some studies indicate allelopathic effects of yellow starthistle (e.g., [125,151,300]); however, others suggest that yellow starthistle is not allelopathic (e.g., [137,238]). Differences among studies may be due to differences in methods (e.g., timing, sesquiterpene lactone concentrations used, soil characteristics, plant parts tested, and species included in tests) [293,300,349] and differences in allelopathic expression [151] among plants from different locations.
Although Muth and Pigliucci (2006) found few phenotypic traits that distinguished invasive and noninvasive Centaurea spp. [205], life history traits that could contribute to yellow starthistle's invasiveness in the western United States include high seed production, dimorphic achenes, minimal seed dormancy, the ability of seeds to germinate over a wide temperature range, long-distance dispersal potential, rapid germination, an extended period of seedling establishment, and ability to compensate for severe defoliation caused by disturbances such as clipping [39,45,111,262] (see Regeneration Processes). Rapid growth of a deep taproot allows yellow starthistle to access deeper soil moisture than associated shallow-rooted species when moisture availability is limited near the soil surface [82,92,111,144,262,296,345] (see Seasonal Development and Plant Growth and Mortality).
Yellow starthistle is most competitive in high-light environments, such as disturbed areas where vegetation and litter have been removed [38,74,136,262,340] (see Successional Status). Establishment, growth, and reproduction are higher on disturbed than undisturbed soils [111]. The size and intensity of the disturbance [137,147,251] (see Regeneration Processes) as well as site characteristics [107] influence yellow starthistle's response to disturbance.
Site invasibility or resistance to yellow starthistle invasion is influenced by plant community composition [39,200,340], history of disturbance and management [39,107,220,251], seed input and germination of yellow starthistle and associated species [45,147,148,297], and soil characteristics such as depth, moisture and nutrient availability, and microbial community composition [7,8,9,39,44,137,182,223,246,329]. For more information on site invasibility, see Maintaining Desirable Vegetation.
PREVENTION
Preventing yellow starthistle invasion is the most economically and ecologically effective management strategy [74,183,285]. Minimizing soil disturbance and maintaining desirable vegetation, limiting yellow starthistle seed dispersal, and establishing a program for monitoring and early detection can help prevent its establishment, persistence, and spread. If disturbance cannot be avoided, establishing desirable species on disturbed areas as soon as possible may reduce yellow starthistle establishment and spread [74,240,283,322] (see Revegetation). Although still vulnerable to yellow starthistle establishment and spread, undisturbed sites are more resistant to invasion than those where soil, vegetation, or both are disturbed (e.g., [39,107,220,240,251]) (see Succession).
Maintaining Desirable Vegetation
Plant communities that include species that are functionally similar to yellow starthistle, such as those that share late-season phenology and similar root morphologies, are more resistant to its establishment and spread [33,82,147,148,149,344,350]. Annual plant communities (either grasses or forbs) comprised of shallow-rooted, early-season plants that senesce before yellow starthistle are generally less resistant to invasion by yellow starthistle than deep-rooted, perennial plant communities that senesce later and share similar water use patterns (e.g., [246,275,344,345,346]). In invaded annual grasslands in Santa Clara County, California, litter removal coupled with high seeding rates of native species with phenological and morphological traits similar to yellow starthistle was necessary to reduce yellow starthistle abundance and increase native plant species abundance [147].
Plant communities dominated by shallow-rooted, early-season annuals are generally less resistant to yellow starthistle invasion than those dominated by deep-rooted perennials [90,246,275,344,345,346]. Among perennial grass communities in Idaho dominated by Idaho fescue, sheep fescue, bluebunch wheatgrass, or intermediate wheatgrass, those dominated by bluebunch wheatgrass were most susceptible and those dominated by fescues were least susceptible to yellow starthistle invasion. Annual grass communities were the most susceptible to yellow starthistle invasion, overall [236]. In Davis, California, annual plant communities were susceptible to yellow starthistle invasion, while communities with perennial blue wildrye resisted yellow starthistle by a combination of shading the soil surface and competition for soil water [346].
According to a review, cover of yellow starthistle and perennial grasses are negatively correlated, and maintaining perennial grass cover is important for preventing yellow starthistle invasion [74]. At the Garden Creek Preserve in Idaho, bluebunch wheatgrass-Sandberg bluegrass communites were more susceptible to yellow starthistle invasion in early-seral, annual-dominated stages than in late-seral perennial-dominated stages. When perennial plant and cryptogam cover was above 10% to 15% (i.e., in mid- to late-seral stages), yellow starthistle cover was low (mean cover 2.19% in mid-seral and 0.57% in late-seral communities) [254]. Grazing management to maintain cover of perennial grasses and other desirable plants can help limit yellow starthistle establishment and spread (see Livestock Grazing).
Species richness and diversity of the plant community may (e.g., [33,80,82]) or may not (e.g., [45,204]) affect resistance of a site to yellow starthistle invasion. Site characteristics (e.g., soil type and fertility) and the particular species or combinations of species may affect resistance [33,82].
Reducing yellow starthistle propagule pressure and increasing native species propagule pressure are important to reducing site invasibility and maintaining desirable vegetation [45,147]. Invasibility of serpentine grasslands by yellow starthistle was not significantly impacted by species diversity, level of disturbance, or functional similarity between yellow starthistle and planted species; rather, long-term propagule pressure from yellow starthistle was the single most important predictor of yellow starthistle abundance and seemed to "overwhelm" these other factors [45].
Limiting Spread
Yellow starthistle spread can be reduced by limiting seed dispersal, controlling established plants in transportation corridors, and by detecting and eradicating new populations when they are small. Seed dispersal can be limited by restricting vehicle, human, and livestock travel from yellow starthistle populations to areas without yellow starthistle, especially after seeds have matured and plants have died. Washing the undercarriage of vehicles leaving areas with weeds is recommended [74,283]. Livestock can move yellow starthistle seeds from one area to another in their feces or by transporting seeds attached to fur or mud. Seed dispersal by animals can be minimized by preventing livestock grazing during flowering and seeding stages or by holding animals for 7 days before moving them from invaded areas [74,240]. Public awareness of the identity and characteristics of yellow starthistle, support of local weed management programs, and restrictions for using only certified weed-free hay for livestock entering the backcountry can also help prevent seed dispersal [74,240,283]. Although, some researchers have noted spread of yellow starthistle following use of contaminated "weed-free" hay [50,78] (see Preventing Postfire Establishment and Spread). Because yellow starthistle often spreads along transportation corridors (highways, roads, and trails) (e.g., [106]) (see Succession), controlling established plants in transportation corridors can help limit yellow starthistle spread [74,283]. Detecting new populations when they are small improves chances for eradication and preventing persistence and spread on new sites. This may be achieved with regular monitoring of susceptible areas, such as areas near established populations and along roads [74,156,283].
Control of yellow starthistle requires preventing seed production, depleting the yellow starthistle soil seed bank, and establishing desired vegetation. The choice of control techniques depends on management objectives, site conditions, and available resources. Timing treatments to prevent seed production is critical. Defoliation treatments (e.g., prescribed burning and mowing) timed around yellow starthistle flower initiation—before germinable seeds are present (see Seasonal Development)—are most practical and effective for preventing viable seed production [20,74].
A brief discussion of each control method used for yellow starthistle follows. Detailed discussions and tables displaying management options and their relative advantages, risks, timing, and fit into a strategic management plan for yellow starthistle can be seen online at the University of California Weed Research and Information Center (WRIC), the book titled "Weed Control in Natural Areas in the Western United States" by DiTomaso et al. (2013) [70], and the "Yellow starthistle Management Guide" by DiTomaso et al. (2006) [74]. Table A6 provides a list of publications from 1999 to 2020 that provide information on yellow starthistle's response to nonfire control treatments.
Fire
For information on use of prescribed fire to control yellow starthistle, see Fire Management Considerations.
Physical or Mechanical Control
Removal of, or damage to, yellow starthistle plants by physical or mechanical methods may offer some degree of control depending on the timing and frequency of treatment, the condition of desired vegetation, and the degree of soil disturbance imposed by the treatment itself.
Pulling: Pulling (e.g., hand pulling and digging) are practical methods for removing yellow starthistle when plants are scattered, patches are small. or where other control methods are not feasible [70,74,240,333]. Pulling by volunteers reduced populations of yellow starthistle in the Agate Desert Preserve in southwestern Oregon [241]. To ensure that plants do not recover, it is important to remove all aboveground stem material. A stem cut to 2 inches (5 cm) tall can recover if leaves and buds are still attached [19,70]. Pulling in dense yellow starthistle populations can cause considerable soil disturbance [138]. It is important to minimize soil disturbance because it can create sites for reestablishment of yellow starthistle or other undesirable species [74]. For example, at Garden Creek Ranch, Idaho, Japanese brome established in disturbed areas soon after yellow starthistle was pulled [139].
Pulling yellow starthistle after plants have bolted but before they produce viable seeds (early flowering) is recommended [20,70]. Pulling can also be effective on young plants before root systems are well developed [240]. In areas where yellow starthistle is growing with established vegetation, it tends to develop a more erect and slender stem and usually lacks basal leaves [70,258]. Under these conditions, yellow starthistle can be relatively brittle and easy to remove and will rarely recover, even when a portion of the stem is left intact [19,70]. The Bradley method [103] can be used to control yellow starthistle populations up to about 40 acres (16 ha) in size by removing plants from the outer edge of the population and moving in. Multiple visits are required throughout the growing season to ensure that no new seeds are produced [70,74,283].
Small, scattered yellow starthistle populations are unlikely to have a large soil seed bank, making them relatively easy to reduce by pulling. Large, dense yellow starthistle populations are likely to have a large soil seed bank and require many years of pulling before populations are reduced [140]. In dense yellow starthistle populations in northwestern Idaho, three to four pulling events during the growing season reduced yellow starthistle density the following year. Repeated pulling was necessary to remove seedlings emerging from the soil seed bank and small plants that were easy to miss initially. Annual follow-up treatments are needed until the soil seed bank is depleted, which could take 4 years or more [140] (see Seed Banking).
Mowing or Cutting: Mowing or cutting (e.g., weed whipping) can reduce yellow starthistle growth (biomass), seed production, and seed bank size but typically does not kill plants [189,294]. Mowing is most effective for reducing large yellow starthistle populations in relatively flat, accessible areas under intensive management, such as along roadsides [74,240,261]. Cutting plants below the height of the lowest branches can be effective for reducing small yellow starthistle populations [70,140]. Anecdotal information suggests that mowing standing skeletons in fall, before the first fall rains, can form a mulch that blocks light and suppresses subsequent yellow starthistle germination [70].
Successful control with mowing or cutting depends on timing, the size and growth form of yellow starthistle plants [19,94], availability of desirable plants to fill the emptied niche [315,317], posttreatment precipitation, and site conditions that affect moisture availability [315]. Mowing too early or too late usually increases yellow starthistle abundance [19,74,261,315,317]. Mowing when plants are bolting can result in "vigorous" regrowth (and subsequent seed production) and may suppress desired vegetation, while mowing after seed set can disperse seeds [70,240]. Repeated mowing may be most effective [240]. For example, at two sites in northern California, mowing once at the early flowering stage and again at the floral bud stage (4 to 6 weeks later) reduced yellow starthistle canopy size, flowerhead production, seed production, plant density, and seedling density. Results were improved when mowing was combined with planting subterranean clover [315].
Mowing may be more likely to reduce yellow starthistle on sites where plants have an erect, high-branching growth form than on sites where plants have a low-branching growth form [19,70]. In contrast, weed whipping may be more likely to reduce yellow starthistle on sites where yellow starthistle plants have a low-branching growth form. For example, during a drought year at the Garden Creek Ranch, Idaho, weed whipping was more effective for controlling short (2–6 inches (5–15 cm)), small-stemmed yellow starthistle plants than tall (10–15 inches (25–38 cm)), large-stemmed yellow starthistle plants. Small plants did not sprout after weed whipping, while large plants sprouted from remaining leaf axils and grew side-branches that often produced seeds [140].
Mowing may have adverse effects on nontarget species. For example, it may decrease reproduction of biological control insects [70,74,240], injure late-season native forb species [70,269], and/or reduce fall and winter forage for wildlife and livestock [70,74,75].
Tillage: Any tillage operation that severs the roots below the soil surface can effectively control yellow starthistle, which is probably why it is not a common cropland weed [70,74,283]. Tillage is sometimes used for yellow starthistle control on roadsides, but it is not appropriate in wildlands or rangelands because it damages desirable plant species, alters soil structure, and increases erosion. Yellow starthistle seedlings can rapidly establish on exposed mineral soil if rainfall occurs after tillage [70,74]. Tillage in early summer, before viable seeds are set, and repeated tillage after rainfall and germination [283] can deplete the yellow starthistle seed bank, but may also deplete the seed bank of desirable species [70,74]. Tillage is not compatible with biological control agents [240].
Livestock Grazing
Yellow starthistle can provide nutritious forage for most livestock (cattle, domestic sheep, and domestic goats, but not horses) when grazed at the proper time (see Importance to Livestock and Wildlife), and properly timed grazing can reduce yellow starthistle growth, survival, and seed production [113,240,316,328]. Excessive or improperly timed grazing may reduce desirable grasses, slow grass recovery rates [74], and facilitate yellow starthistle establishment and spread by increasing bare soil and reducing shade [75,150,261,298,314]. Allowing desirable rangeland plants to recover fully before regrazing an area [283] helps to shade yellow starthistle rosettes during the critical root development phase [258,266,316,318]. Avoiding grazing after seeds are mature helps to limit seed dispersal [240] (see Seed Dispersal), although grazing by domestic goats when yellow starthistle was in full spine and soil moisture was inadequate to support regrowth reduced yellow starthistle density and seed production in Idaho [113]. Livestock grazing has the potential to be a valuable tool in an integrated management program although this has been little studied [70,74,316].
Timing of grazing is critical for yellow starthistle control [74,150,253,316,318,328], because if plants are allowed to produce and disperse seeds, yellow starthistle density may increase on grazed sites relative to ungrazed controls [94,145,328]. Livestock grazing is most effective for reducing yellow starthistle abundance when yellow starthistle plants are palatable and nutritious and other annual species have senesced. This occurs in late May and June in California, when yellow starthistle plants have bolted but before they produce spiny heads [70,94,150] (see Palatability and Nutritional Value). However, most yellow starthistle plants regrow when grazed at the bolting and early flowering stages, and repeated grazing (up to four times at about 2-week intervals under rotational grazing) is often needed to reduce subsequent growth and seed production. Areas receiving moisture after grazing are likely to require repeated grazing [150,283,318]. Livestock grazing in late winter or early spring is not effective for yellow starthistle control, because livestock select young grasses with erect growth forms rather than yellow starthistle seedlings and rosettes. This reduces shading of yellow starthistle juveniles and results in increased growth [150]. In Colusa County, California, for example, a moderately stocked rotational cattle grazing system implemented from January through May for 6 years did not affect yellow starthistle cover [253].
Targeted grazing may need to be repeated annually for several years to control yellow starthistle plants that establish from on- and off-site sources. High-intensity, short-duration grazing by cattle, domestic sheep, and domestic goats in dense yellow starthistle populations on California rangeland between mid-May and early July reduced yellow starthistle biomass, canopy size, and seed production. However, sufficient seeds were produced or already present in the soil seed bank for new plants to establish in subsequent years, suggesting that this prescription would need to be continued for at least 3 years to reduce the yellow starthistle seed bank. Grazing at this time also allowed annual grasses, legumes, and most other resident annuals to complete their life cycle and set seed, thus allowing for seed bank replenishment of other associated species [316,318].
Although few studies have examined the impact of livestock grazing on yellow starthistle biological control agents, it appears to be compatible with biological control insects [240]. However, timing of grazing may impact biocontrol activity and efficacy [328].
Biological Control
Six insects and a pathogen that attack yellow starthistle are established in the United States (table 10). The insects include three species of weevils and three species of flies [70,240], all of which attack the flowerheads of yellow starthistle and produce larvae that develop and feed within the seedhead [70]. The pathogen, Puccinia jaceae var. solstitialis, which attacks leaves and young stems is established in California and Oregon [240]. A root-boring weevil, Ceratapion basicorne, whose larvae feed on the midrib, root crown, and stem tissues at the rosette stage has not yet been federally approved for release as of 2020 [70,124]. Researchers continue to look for new yellow starthistle biological control agents [22,74,122,124,228,331] (table A6). See Randall et al. (2017) [240] and Pitcairn et al. (2004) [228] for information on the biology of federally approved yellow starthistle biological control agents and developing, implementing, and managing biological control programs for yellow starthistle.
| Table 10—Yellow starthistle biological control agents [70,86,231,240,283,336] and their distribution and impact on yellow starthistle reproduction [70,74,240]. Those with the greatest impact are in bold. | ||
| Scientific name | Common name | Distribution and impact |
| Insects | ||
| Bangasternus orientalis | yellow starthistle bud weevil | wide distribution, low impact |
| Chaetorellia australis | yellow starthistle peacock fly | limited distribution, low impact |
| Chaetorellia succinea* | false yellow starthistle peacock fly | wide distribution, moderate impact |
| Eustenopus villosus | yellow starthistle hairy weevil | wide distribution, moderate impact |
| Larinus curtus | yellow starthistle flower weevil | limited distribution, low impact |
| Urophora jaculata** | yellow starthistle gall fly | failed to establish |
| Urophora sirunaseva | banded yellow starthistle gall fly | wide distribution, low impact |
| Pathogens | ||
| Puccinia jaceae var. solstitialis | yellow starthistle rust fungus | limited distribution, low impact |
*Chaetorellia succinea was released accidentally and is not a federally approved biological control insect [74]. |
||
Biological control agents are unlikely to eliminate yellow starthistle populations [74,240,305,321], but they can suppress yellow starthistle along with other control methods over time by reducing yellow starthistle reproduction and growth [232,240]. For this reason, Randall et al. (2017) recommended an integrated weed management program that includes biological control agents, particularly for large (>1 acre (0.4 ha)), widespread, and long-established yellow starthistle populations [240].
Biological control agents with the greatest impact on yellow starthistle in the western United States are Eustenopus villosus and Chaetorellia succinea [70,74,231,240]. Both larvae and adult Eustenopus villosus damage plants. Larvae can kill up to 100% of seeds within attacked seedheads. Adults feed on yellow starthistle buds, which delays flower production by 2 to 3 weeks and often kills young flower buds. Chaetorellia succinea was not federally approved for release, but it occurs almost everywhere that yellow starthistle occurs. It reduces seed production and the rate of spread of yellow starthistle populations, and is most effective in areas where garden coneflower, another nonnative invasive Centaurea spp., also occurs. Larval feeding on yellow starthistle kills up to 80% of seeds within attacked seedheads and decreases pollinator visitation [240]. In contrast, Bangasternus orientalis larvae typically kill only 60% of seeds within attacked seedheads, the attack rate is only 1% of available capitula, and adult feeding has a negligible effect on plants [240].
Yellow starthistle biological control insects may not reduce yellow starthistle populations because plants can still produce enough seeds to replace the population [24,104,228,339]. For example, in the Salmon River Canyon of Idaho, six biological control insects were introduced over 3 years. A "rapid build-up" of insect populations followed, and 6 years after the last year of release, yellow starthistle seed mortality fluctuated around 90%. Vegetation monitoring plots, however, showed no consistent decline in the yellow starthistle population, indicating that seed production was still sufficient to allow full replacement. However, the rate of spread and number of new, isolated pockets of yellow starthistle were reduced [24].
The effects of biological control agents on yellow starthistle abundance depend on many factors, including the amount of intraspecific and interspecific competition for resources. At many locations, yellow starthistle plants compensate for any decreases in density by growing larger and producing more seedheads [104,309]. Models indicate that the impact of a biological control agent on yellow starthistle populations is likely to be lower in dense stands because unattacked plants (experiencing less competition for resources) are more likely to survive to flowering and produce seeds [309]. In Yolo County, California, the combined effects of three biological control insects reduced yellow starthistle seed production by 70%. However, self-thinning reduced seedling numbers such that densities of yellow starthistle plants in plots with biological control insects present and absent were similar. In addition, seed production/plot was unchanged across a large range of yellow starthistle densities [104]. In the California Central Valley, Puccinia jaceae var. solstitialis reduced yellow starthistle growth when grown with wild oat but not when grown in monocultures [212].
A combination of biological control agents may result in greater declines in yellow starthistle abundance than a single agent, but results depend on many factors, such as the type of biological control agents and their life stages, presence of biological control parasitoids, climate and weather, and site characteristics (e.g., soil temperature and moisture regimes and soil type). See the following publications for information on the effects of multiple biological control agents: [213,240,305,307,308,310,335].
The use of biological control agents can be an important component of an integrated management program [70] and may be used in combination with prescribed fire, livestock grazing, and herbicides.
Chemical Control
Herbicides may be effective in controlling yellow starthistle populations, but they are rarely a complete or long-term solution to weed management [37]. Control with herbicides is temporary, as it does not change conditions that allowed invasions to occur in the first place [261,348]. According to Randall et al. (2017), herbicides are most effective when applied to small populations, including newly established populations and recently established satellite patches arising from nearby older, larger yellow starthistle populations [240]. Other reviews recommended use of herbicides on large populations [63,283], but Randall et al. (2017) considered this prohibitively expensive and damaging to desired vegetation over large areas [240]. Herbicides are more effective when incorporated into long-term management plans that include replacement of weeds with desired species, careful land use management, and prevention of new invasions [37,66,72,75,250]. See the Weed Control Methods Handbook [320] for considerations on the use of herbicides in natural areas and detailed information on specific chemicals.
Many herbicides have been tested for controlling yellow starthistle, and their application, efficacy, and length of control depend on a number of factors including the soil residual activity of the herbicide, site characteristics, weather, and the present and desired plant community [63,74,240]. Abundance of nonnative invasive annual grasses, such as medusahead, cheatgrass, and barbed goatgrass, often increases after herbicide treatment of yellow starthistle [69,75]. Continuous use of herbicides can select for tolerance or resistance in yellow starthistle (e.g., [43,101,195,299]). Integrating herbicide use with other control methods, such as prescribed fire (see Prescribed Fire and Herbicides), can decrease herbicide use and the incidence of herbicide resistant biotypes [65,75]. Improper use of herbicides can lead to other potential problems, including off-site chemical movement, injury to desirable plants, reduction in plant diversity, and changes in nutrient balance. See DiTomaso et al. (2006) [74], Randall et al. (2017) [240], and the publications in table A6 for information on the use and efficacy of specific chemicals on yellow starthistle.
Biological control insects may be used in combination with herbicides. In Sacramento County, California, a single application of clopyralid in March resulted in a 97% reduction in yellow starthistle plant density; however, individual surviving plants were larger and produced more seedheads than plants in untreated control plots. One year after treatment, the attack rate by biological control insects (Bangasternus orientalis, Chaetorellia succinea, Eustenopus villosus, and Urophora sirunaseva) was similar between treated and untreated plots [229]. Populations of yellow starthistle near Dayton, Washington, developed herbicide resistance in response to repeated applications of picloram and other auxin-type herbicides. Herbicide resistance did not create host incompatibility or reduce the effectiveness of Eustenopus villosus [268].
REVEGETATION
Reducing yellow starthistle abundance is important to grassland restoration efforts [73]. No matter what method is used to reduce yellow starthistle abundance (see Control), establishment or maintenance of desirable plants is needed for long-term control [62,240,283]. When desired plants emerge before yellow starthistle, their shade restricts the growth of yellow starthistle seedlings and rosettes [240]. No single species can suppress yellow starthistle on all sites at all times [171]. Species that are functionally similar to yellow starthistle, such as those that share late-season phenology and similar root morphologies are most competitive for resources with yellow starthistle [33,82,147,149,153,344,350]. Subterranean clover [284,315,317], tarweeds [82,149,153], purple needlegrass [33,245], blue wildrye [344], Idaho fescue [28], orchardgrass [28,59,170,171], tall oatgrass [171], tall fescue [59], thickspike wheatgrass [170], and intermediate wheatgrass [93,234,258] are examples of species competitive for resources with yellow starthistle in California, Oregon, and Washington. However, the most suitable plant species to use for revegetation efforts depends on habitat type, site conditions, climate, management treatments and goals, and future land uses [74,240].
Establishing perennial grasses may help prevent reinvasion by yellow starthistle. In northern California annual rangeland, seeded perennial grasses were more likely than seeded annual grasses and forbs to establish long-term (>3 years) dominance in medusahead-yellow starthistle communities. Yellow starthistle cover was nearly zero in established perennial grass plots, where dry matter production was more than three times that in unseeded control plots [59]. In southeastern Washington, perennial grasses can limit yellow starthistle establishment and growth by shading yellow starthistle seedlings and depleting soil moisture before yellow starthistle matures. Shallow soils may not support enough vegetation to interfere with yellow starthistle establishment and growth [258].
Seeding a combination of grasses and forbs with various growth forms and ecological traits may be more effective for long-term yellow starthistle control than seeding only perennial grasses because plant communities comprised of diverse species that occupy most of the above- and below-ground niches are more weed resistant [74,75,203,240]. Late-season, deep-rooting forbs may effectively compete with yellow starthistle for water [82,149]. However, herbicides that kill yellow starthistle likely also kill desirable forbs [74,75]. For this reason, DiTomaso et al. (2006) recommended a two-phased revegetation management strategy that involves 1) seeding with perennial grasses soon after control treatments to reduce the time the site is suitable for yellow starthistle establishment, and 2) subsequent seeding with forbs [74].
Plant communities with shallow-rooted, early-season annuals are generally less resistant to invasion by yellow starthistle than plant communities with deep-rooted, perennials [90,246,275,344,345,346] (see Maintaining Desirable Vegetation). In oak woodland-annual grasslands in Napa County, California, perennial and mixed perennial seeding mixes, and, to a lesser extent, a native perennial seeding mix, provided greater barriers to yellow starthistle invasion than an annual seeding mix and a unseeded control [90]. In Davis, California, during 5 years, plots seeded with early-season native annual forbs and plots seeded with early-season nonnative annual grasses were susceptible to yellow starthistle invasion, while plots seeded with late-season native perennials in combinations with annual forbs maintained low cover (<10%) of yellow starthistle, except in posttreatment year 1. Communities with blue wildrye, a species functionally similar to yellow starthistle, better resisted invasion than communities lacking a functional analog. In contrast, Great Valley gumweed, which is also functionally similar to yellow starthistle, failed to survive. The authors recommended selecting species for restoration that are functionally similar to the invader but also demonstrate successful establishment and survival [344]. Seeded species must be adapted to local site conditions, including soils, climate, and topography [59,74].
Many nonnative species used for revegetation are nonnative because they tend to establish better than native species; however, seeding nonnative species may reduce plant community diversity [74]. At two study sites in valley bottom grassland within mixed oak-foothill pine woodland at Fort Hunter Liggett, native plants failed to establish from seeds broadcast after prescribed fire (October 2009) and broadleaf herbicide (aminopyralid) application (November 2009, January 2010, or March 2010), regardless of seeding date (December 2009, January 2010, or March 2010). Only one native perennial grass species, nodding needlegrass, established from drill seeding on the same dates after the same treatments, despite above-average rainfall during the previous 2 years [167].
Mature perennial bunchgrass communities with large plants are more resistant to invasion by yellow starthistle than young perennial bunchgrass communities with small plants, but resistance depends on soil depth and water availability [245,246]. In both years of a 2-year study in Yolo County, California, yellow starthistle number, biomass, and seedhead production declined as purple needlegrass size increased. However, even yellow starthistle plants grown with the largest purple needlegrass plants produced some seeds, especially during the year with the highest rainfall [245]. In another study in Yolo County, soil depth, water availability, and purple needlegrass size influenced the impact of yellow starthistle on purple needlegrass performance (i.e., survival, growth, "reproduction", and predawn water potential). High water availability increased yellow starthistle performance and decreased purple needlegrass performance in both shallow and deep soil, although the effects were stronger in shallow soil. Purple needlegrass performance was better in deep soil, and plants were able to suppress yellow starthistle growth more strongly by shading as they grew older and larger. Shallow soil combined with low water availability reduced yellow starthistle performance and impacts [246].
Revegetation with desired native or nonnative perennial grasses can not only inhibit reinvasion by yellow starthistle but also provide forage for livestock (e.g., [90,170,171,198,209,210,211,233,234,235]). However, full recovery of desirable rangeland plants should be allowed before the area is grazed to help minimize reinvasion by yellow starthistle [75] (see Livestock Grazing).
INTEGRATED MANAGEMENT
The use of multiple control methods is important when implementing any weed management program [63], because multiple approaches can create a cumulative stress on target plants, and reduce their reproduction and spread. Many control methods cannot (such as prescribed fire because of sparse fuels) or should not (such as herbicides because of the potential to develop resistance) be used continuously, and integrated methods can provide needed diversity in management methods [74]. With combinations of treatments, timing is critical and must be customized to the plant community, present and desired, and to site conditions. Integrated management includes a long-term commitment to replacing weed-dominated plant communities with more desirable plant communities. Methods selected for control of yellow starthistle on a specific site are determined by land use objectives, environmental factors, economics, extent, and effectiveness of the control techniques on yellow starthistle [74,243,283,317]. DiTomaso et al. (2000, 2001) stated that the goal of any management plan should be not only controlling the invasive plants, but also developing an ecologically healthy plant community that is weed resistant and meets other land-use objectives such as livestock forage, wildlife habitat, or recreation [62,72]. Once the desired plant community has been determined, an integrated weed management strategy can be developed to direct succession toward that plant community by identifying key mechanisms and processes directing plant community dynamics (site availability, species availability, and species performance) and predicting plant community response to control measures [278]. Some examples of combined approaches are presented within the preceding sections, in Integrated Management with Prescribed Fire, and in table A6.
MANAGEMENT UNDER A CHANGING CLIMATE
Several publications are available that provide information on how climate change affects yellow starthistle (table 11). Ongoing and predicted increases in disturbance and elevated atmospheric carbon dioxide and temperature are likely to facilitate yellow starthistle invasion throughout the western United States by increasing the amount of climatically suitable habitat [30]. Yellow starthistle growth responds positively to increased carbon dioxide and nitrogen deposition, suggesting that it is likely to become more competitive for resources with native species [81,83,351], perhaps especially under resource-rich conditions and with increased precipitation at the end of the rainy season in spring [98]. At Jasper Ridge Biological Preserve, aboveground biomass of yellow starthistle was more than six times higher in response to elevated carbon dioxide and more than three times higher in response to nitrate deposition, which allowed it to become more competitive for resources with native species that responded less strongly or did not respond to these treatments [83].
| Table 11—Publications from 1999 to 2020 that provide information on climate change effects on yellow starthistle. | ||
| Study location | Title | Reference |
| CA: Napa and Lake counties, Donald and Sylvia McLaughlin Natural Reserve | Exotic plant invasions under enhanced rainfall are constrained by soil nutrients and competition | [98] |
| CA: San Mateo County, Jasper Ridge Biological Preserve | In-field yellow starthistle (Centaurea solstitialis) volatile composition under elevated temperature and CO2 and implications for future control | [217] |
| Strong response of an invasive plant species (Centaurea solstitialis L.) to global environmental changes | [83] | |
| Comparison of the effect of elevated CO2 on an invasive species (Centaurea solstitialis) in monoculture and community settings | [81] | |
| US: western | Climate change and plant invasions: Restoration opportunities ahead? | [30] |
| Laboratory: seeds obtained from unnamed commercial seed source | Evaluation of the growth response of six invasive species to past, present and future atmospheric carbon dioxide | [351] |
| Table A1—Common and scientific names of plant species mentioned in this review. For further information on fire ecology of these taxa, follow the highlighted links to FEIS Species Reviews. Nonnative species are indicated with an asterisk (*). | |
| Common name | Scientific name |
| Forbs | |
| agate desertparsley | Lomatium cookii |
| alfalfa* | Medicago sativa |
| arrowleaf balsamroot | Balsamorhiza sagittata |
| California bearpoppy | Arctomecon californica |
| diffuse knapweed* | Centaurea diffusa |
| garden coneflower* | Centaurea cyanus |
| Great Valley gumweed | Grindelia camporum |
| gumplant | Grindelia spp. |
| Maltese starthistle* | Centaurea melitensis |
| red starthistle | Centaurea calcitrapa |
| Russian knapweed* | Acroptilon repens |
| shortpod mustard* | Hirschfeldia incana |
Spalding's catchfly |
Silene spaldingii |
| spotted knapweed* | Centaurea stoebe subsp. micranthos |
| subterranean clover* | Trifolium subterraneum |
| sulfur knapweed* | Centaurea sulphurea |
| tarweed | Centromadia spp., Hemizonia spp., Holocarpha spp. |
| Graminoids | |
| annual wheat* | Eremopyrum triticeum |
| barbed goatgrass* | Aegilops triuncialis |
| basin wildrye | Leymus cinereus |
| beaked sedge | Carex rostrata |
| bluebunch wheatgrass | Pseudoroegneria spicata |
| blue wildrye | Elymus glaucus |
| cheatgrass* | Bromus tectorum |
| Fendler threeawn | Aristida purpurea var. longiseta |
| Idaho fescue | Festuca idahoensis |
| intermediate wheatgrass* | Thinopyrum intermedium |
| Japanese brome* | Bromus japonicus |
| medusahead* | Taeniatherum caput-medusae |
| Nebraska sedge | Carex nebraskensis |
| nodding needlegrass | Nassella cernua |
| orchardgrass | Dactylis glomerata |
| purple false brome* | Brachypodium distachyon |
| purple needlegrass | Nassella pulchra |
| ripgut brome* | Bromus diandrus |
| Sandberg bluegrass | Poa secunda |
| sand dropseed | Sporobolus cryptandrus |
| sheep fescue* | Festuca ovina |
| smooth brome* | Bromus inermis |
| soft brome* | Bromus hordeaceus |
| tall oatgrass | Arrhenatherum elatius |
| water sedge | Carex aquatilis |
| wheatgrass | Agropyron spp. |
| wild oat* | Avena fatua |
| Shrubs | |
| big sagebrush basin big sagebrush mountain big sagebrush Wyoming big sagebrush |
Artemisia tridentata Artemisia tridentata subsp. tridentata Artemisia tridentata subsp. vaseyana Artemisia tridentata subsp. wyomingensis |
| black greasewood | Sarcobatus vermiculatus |
| black sagebrush | Artemisia nova |
| dogwood | Cornus spp. |
| low sagebrush | Artemisia arbuscula |
| narrowleaf willow | Salix exigua |
| threetip sagebrush | Artemisia tripartita |
| willow | Salix spp. |
| Trees | |
| black cottonwood | Populus balsamifera subsp. trichocarpa |
| blue oak | Quercus douglasii |
| juniper | Juniperus spp. |
| oak | Quercus spp. |
| pine | Pinus spp. |
| pinyon | Pinus spp. |
ponderosa pine |
Pinus ponderosa |
| Table A2—Ecosystems, Associations, Cover Types, and BLM Regions where yellow starthistle likely occurs. The following biogeographic classification systems are presented as a guide to demonstrate where yellow starthistle might be found or is likely to be invasive, based on reported occurrence and biological tolerance to factors that are likely to limit its distribution. Precise distribution information is limited, especially in the southwestern, southern, central, midwestern, and eastern states. Therefore, these lists are somewhat speculative and probably not exhaustive. | |
| Ecosystems [105] | |
| FRES21 | Ponderosa pine |
| FRES28 | Western hardwoods |
| FRES29 | Sagebrush |
| FRES33 | Southwestern shrubsteppe |
| FRES34 | Chaparral-mountain shrub |
| FRES35 | Pinyon-juniper |
| FRES36 | Mountain grasslands |
| FRES38 | Plains grasslands |
| FRES39 | Prairie |
| FRES40 | Desert grasslands |
| FRES41 | Wet grasslands |
| FRES42 | Annual grasslands |
| BLM Physiographic Regions [21] | |
| 1 | Northern Pacific Border |
| 2 | Cascade Mountains |
| 3 | Southern Pacific Border |
| 4 | Sierra Mountains |
| 5 | Columbia Plateau |
| 6 | Upper Basin and Range |
| 7 | Lower Basin and Range |
| 8 | Northern Rocky Mountains |
| 9 | Middle Rocky Mountains |
| 10 | Wyoming Basin |
| 11 | Southern Rocky Mountains |
| 12 | Colorado Plateau |
| 13 | Rocky Mountain Piedmont |
| 14 | Great Plains |
| 15 | Black Hills Uplift |
| 16 | Upper Missouri Basin and Broken Lands |
| Kuchler [165] | |
| K010 | Ponderosa shrub forest |
| K022 | Great Basin pine forest |
| K023 | Juniper-pinyon woodland |
| K024 | Juniper steppe woodland |
| K025 | Alder-ash forest |
| K026 | Oregon oakwoods |
| K030 | California oakwoods |
| K033 | Chaparral |
| K035 | Montane chaparral |
| K036 | Coastal sagebrush |
| K038 | Mosaic of K030 and K035 |
| K047 | Great Basin sagebrush |
| K048 | Fescue-oatgrass |
| K050 | California steppe |
| K051 | Wheatgrass-bluegrass |
| K055 | Sagebrush steppe |
| SAF Forest Cover Types [290] | |
| 220 | Rocky Mountain juniper |
| 221 | Red alder |
| 222 | Black cottonwood-willow |
| 233 | Oregon white oak |
| 235 | Cottonwood-willow |
| 236 | Bur oak |
| 237 | Interior ponderosa pine |
| 238 | Western juniper |
| 239 | Pinyon-juniper |
| 245 | Pacific ponderosa pine |
| 246 | California black oak |
| 247 | Jeffrey pine |
| 249 | Canyon live oak |
| 250 | Blue oak-foothills pine |
| 255 | California coast live oak |
| SRM (Rangeland) Cover Types [287] | |
| 101 | Bluebunch wheatgrass |
| 102 | Idaho fescue |
| 103 | Green fescue |
| 104 | Antelope bitterbrush-bluebunch wheatgrass |
| 105 | Antelope bitterbrush-Idaho fescue |
| 107 | Western juniper/big sagebrush/bluebunch wheatgrass |
| 109 | Ponderosa pine shrubland |
| 110 | Ponderosa pine-grassland |
| 201 | Blue oak woodland |
| 202 | Coast live oak woodland |
| 203 | Riparian woodland |
| 204 | North coastal shrub |
| 205 | Coastal sage shrub |
| 206 | Chamise chaparral |
| 207 | Scrub oak mixed chaparral |
| 208 | Ceanothus mixed chaparral |
| 209 | Montane shrubland |
| 210 | Bitterbrush |
| 214 | Coastal prairie |
| 215 | Valley grassland |
| 235 | Cottonwood-willow |
| 311 | Rough fescue-bluebunch wheatgrass |
| 312 | Rough fescue-Idaho fescue |
| 314 | Big sagebrush-bluebunch wheatgrass |
| 315 | Big sagebrush-Idaho fescue |
| 316 | Big sagebrush-rough fescue |
| 317 | Bitterbrush-bluebunch wheatgrass |
| 318 | Bitterbrush-Idaho fescue |
| 319 | Bitterbrush-rough fescue |
| 322 | Curlleaf mountain-mahogany-bluebunch wheatgrass |
| 401 | Basin big sagebrush |
| 402 | Mountain big sagebrush |
| 403 | Wyoming big sagebrush |
| 404 | Threetip sagebrush |
| 405 | Black sagebrush |
| 406 | Low sagebrush |
| 407 | Stiff sagebrush |
| 408 | Other sagebrush types |
| 409 | Tall forb |
| 412 | Juniper-pinyon woodland |
| Table A3—Publications from 1999 to 2020 providing information on yellow starthistle's response to fire, with and without other treatments. | |||
| Study or collection location; plant community | Title | Treatments investigated | Reference |
| Annual grasslands and oak woodlands | |||
| CA: Colusa County; former purple needlegrass and blue wildrye stand along a road currently dominated by yellow starthistle | Release of roadside native perennial grasses following removal of yellow starthistle | Single June or late August prescribed fire, mowing, and herbicide (clopyralid) application | [347] |
| CA: Marin County (Rock Springs Meadow, Mount Tamalpais); North Coastal prairie grasslands with patches of yellow starthistle | Use of short rotation burning to combat non-natives and their seed banks in California north coastal prairie | Consecutive annual July prescribed fires | [214] |
| CA: Monterey County (Fort Hunter Liggett); 2 study sites (Mission and Back) in valley bottom grasslands within mixed oak-foothill pine woodland with "high yellow starthistle infestations" | Integration of prescribed burning, aminopyralid, and reseeding for restoration of yellow starthistle (Centaurea solstitialis)-infested rangeland | Single late October prescribed fire, herbicide (aminopyralid) application, and seeding native species | [167] |
| CA and outside the US: nonnative annual grasslands in northern California and perennial grasslands in Argentina and Turkey | Disturbance facilitates invasion: The effects are stronger abroad than at home | Burning of 1-m² plots and seeding yellow starthistle | [137] |
CA: San Benito, Siskiyou, and Yuba counties in annual grasslands "heavily infested with yellow starthistle"; and Monterey County (Fort Hunter Liggett) in annual grasslands with high yellow starthistle cover (20%-70%) |
Integrating prescribed burning and clopyralid for the management of yellow starthistle (Centaurea solstitialis) | Single and consecutive annual summer prescribed fires and herbicide (clopyralid) application | [69] |
| CA: Sonoma County (Sugarloaf Ridge State Park); annual grasslands (predominantly yellow starthistle and annual grasses) with a "substantial component of native perennial herbs and grasses" | Fire controls yellow starthistle in California grasslands: Test plots at Sugarloaf Ridge State Park | Single and consecutive annual July prescribed fires | [130] |
| The use of fire for yellow starthistle (Centaurea solstitialis) management and the restoration of native grasslands at Sugarloaf Ridge State Park | [131] | ||
| Increasing biodiversity through the restoration of a natural process by prescribed burning in California state parks: "A Sonoma County experience" | [129] | ||
| CA: Sonoma County (Sugarloaf Ridge State Park); annual grasslands | Prescribed burning for control of yellow starthistle (Centaurea solstitialis) and enhanced native plant diversity | Consecutive annual July prescribed fires | [68] |
| Instability in a grassland community after the control of yellow starthistle (Centaurea solstitialis) with prescribed burning | Consecutive annual July prescribed fires | [166] | |
| CA: Woodside (Jasper Ridge Biological Preserve); annual grasslands | Strong response of an invasive plant species (Centaurea solstitialis L.) to global environmental changes | July fire | [83] |
| Perennial grasslands | |||
| ID: Craig Mountain Wildlife Area; bluebunch wheatgrass-Sandberg bluegrass-arrowleaf balsamroot habitat type | Canyon grassland vegetation changes following the Maloney Creek wildfire | August wildfire | [121] |
| Canyon grassland vegetation changes following fire in northern Idaho | [120] | ||
| ID: Garden Creek Ranch Preserve; canyon grassland communities dominated by Idaho fescue and bluebunch wheatgrass | Short-term influence of wildfire on canyon grassland plant communities and Spalding's catchfly, a threatened plant | October wildfire | [193] |
| Riparian and wetland communities | |||
| CA: Klamath National Forest, along the lower to mid-Klamath River; narrowleaf willow communities with grass and yellow starthistle in the understory | Traditional ecological knowledge to develop and maintain fire regimes in northwestern California, Klamath-Siskiyou bioregion: Management and restoration of culturally significant habitats | Single or two consecutive October prescribed fires | [169] |
| CA: Pinnacles National Park; wet meadow dominated by yellow starthistle, ripgut brome, and soft brome | Prescribed burning and competitive reseeding as tools for reducing yellow starthistle (Centaurea solstitialis) at Pinnacles National Monument | Single June prescribed fire and hand pulling | [188] |
| Greenhouse and laboratory | |||
| Greenhouse: seeds collected from Montsec, Spain, and soils collected from Barcelona | Fire and species range in Mediterranean landscapes: An experimental comparison of seed and seedling performance among Centaurea taxa | Heating, shading, and burned soils |
[247] |
| Laboratory: seeds collected from San Luis National Wildlife Refuge, California | Heat and smoke effects on the seeds of common San Joaquin Valley grassland plants | Heating and smoke | [1] |
| Table A4—Publications with information on other uses of yellow starthistle, including the biological activities of sesquiterpene lactones and other volatile constituents extracted from yellow starthistle. | ||
| Collection location of plants | Title | Citation |
| Croatia: Split | Phytochemical and cytogenetic characterization of Centaurea solstitialis L. (Asteraceae) from Croatia | [42] |
| Italy: Apulia | Qualitative characterisation of cultivated and wild edible plants: Mineral elements, phenols content and antioxidant capacity | [60] |
| Italy: southern | Volatile components from flower-heads of Centaurea nicaeensis All., C. parlatoris Helder and C. solstitialis L. ssp. schouwii (DC.) Dostal growing wild in southern Italy and their biological activity | [273] |
| Turkey: Afyon, Kütahya, Denizli, Muğla, Aydın provinces, Turkey | Traditional medicine in Turkey VI. Folk medicine in West Anatolia: Afyon, Kütahya, Denizli, Muğla, Aydın provinces | [143] |
| Turkey: Ankara | Evaluation of the antiulcerogenic effect of the sesquiterpene lactones from Centaurea solstitialis ssp. solstitialis by using various in vivo and biochemical techniques | [123] |
| Turkey: Ankara | Screening of Turkish antiulcerogenic folk remedies for anti-Helicobacter pylori activity | [342] |
| Turkey: Ankara | Sesquiterpene lactones with antinociceptive and antipyretic activity from two Centaurea species | [2] |
| Turkey: Golbasi | Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis | [219] |
| Turkey: Golbasi | Isolation of antiulcerogenic sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis through bioassay-guided fractionation procedures in rats | [341] |
| Turkey: Isparta region | Centaurea solstitialis and Silybum marianum weeds conversion into value‑added thermoplastic materials by benzylation process | [323] |
| Turkey: Kastamonu | The potential medicinal value of plants from Asteraceae family with antioxidant defense enzymes as biological targets | [164] |
| Turkey: Konya | Antibacterial activities of extracts from twelve Centaurea species from Turkey | [313] |
| Turkey: Konya | Antioxidant property of Centaurea solstitialis L. from Konya, Turkey | [312] |
| Turkey: the middle and west Black Sea | Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions | [102] |
| Turkey: Mugla | The anticancer and anti-inflammatory effects of Centaurea solstitialis extract on human cancer cell lines | [3] |
| Turkey: Tasliciflik | Bioactivity-guided isolation of antiproliferative sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis | [95] |
| Table A5—Publications from 1999 to 2020 with information on factors facilitating or inhibiting yellow starthistle invasion success in specific locations. | ||
| Study or collection location | Title | Citation |
Field studies |
||
| CA: Colusa, Lake, and Napa counties | Invasibility of roadless grasslands: an experimental study of yellow starthistle | [107] |
| CA: Lake and Napa counties, Donald and Sylvia McLaughlin Natural Reserve | Site conditions determine a key native plant’s contribution to invasion resistance in grasslands | [148] |
| CA: San Mateo County, Jasper Ridge Biological Preserve (mesocosms) | Abundance declines of a native forb have nonlinear impacts on grassland invasion resistance | [149] |
| CA: San Mateo County, Jasper Ridge Biological Preserve (microcosms) | Biodiversity and invasibility in grassland microcosms | [80] |
| CA: San Mateo County, Jasper Ridge Biological Preserve (microcosms) | Realistic variation in species composition affects grassland production, resource use and invasion resistance | [350] |
| CA: San Mateo County, Jasper Ridge Biological Preserve (microcosms) | Species composition and diversity affect grassland susceptibility and response to invasion | [82] |
| CA: Santa Clara County | Functional composition controls invasion success in a California serpentine grassland | [144] |
| CA: Santa Clara County | Long-term propagule pressure overwhelms initial community determination of invader success | [45] |
| CA: Santa Clara County | Using filter-based community assembly models to improve restoration outcomes | [147] |
CA: Shasta Valley |
Soil water dynamics differ among rangeland plant communities dominated by yellow starthistle (Centaurea solstitialis), annual grasses, or perennial grasses | [92] |
| CA: Tehama County | Forage seeding in rangelands increases production and prevents weed invasion | [59] |
| CA: Yolo County | Back to the basics: Using density series to test regulation versus limitation for invasive plants | [204] |
| CA: Yolo County | Centaurea solstitialis invasion success is influenced by Nassella pulchra size | [245] |
| CA: Yolo County | Effects of belowground resource use complementarity on invasion of constructed grassland plant communities | [33] |
| CA: Yolo County | Functionally similar species confer greater resistance to invasion: Implications for grassland restoration | [344] |
| CA: Yuba County | Separating sources of density-dependent and density-independent establishment limitation in invading species | [297] |
| CA: Yolo County | Spatio-temporal relationship between water depletion and root distribution patterns of Centaurea solstitialis and two native perennials | [345] |
CA: Yolo County |
Testing life history correlates of invasiveness using congeneric plant species | [111] |
| CA: Yolo County | The role of light and soil moisture in plant community resistance to invasion by yellow starthistle (Centaurea solstitialis) | [346] |
| CA: Yolo County | Variation in resource availability changes the impact of invasive thistles on native bunchgrasses | [246] |
| CA and outside the US: Davis, CA and Santa Rosa, Argentina | Resistance to Centaurea solstitialis invasion from annual and perennial grasses in California and Argentina | [136] |
ID: Nez Perce and Lewis counties, Garden Creek Preserve |
Effects of perennial plant competition on the invasibility of canyon grassland communities by Centaurea solstitialis | [254] |
| UT: South Ogden | Yellow starthistle in Utah: An investigation of Centaurea solstitialis invasion patterns, processes, and population dynamics | [251] |
Common garden and greenhouse |
||
| CA: plants from near Gilroy, Triangle, Lebec, Red Bluff, and Clovis | Evidence for a growth-defense trade-off in Centaurea solstitialis | [44] |
| CA: seeds from Solono County | Abiotic constraints eclipse biotic resistance in determining invasibility along experimental vernal pool gradients | [108] |
| CA: seeds from Solono County and an unknown location | Implementation of a novel framework for assessing species plasticity in biological invasions: Responses of Centaurea and Crepis to phosphorus and water availability | [206] |
| CA: seeds from Winters | Interactive effects of plant-plant competition and an introduced biological control agent on yellow starthistle (Centaurea solstitialis) seed production | [153] |
| CA: seeds from Yolo County | Does superior competitive ability explain yellow starthistle's (Centaurea solstitialis) successful invasion of annual grasslands in California? | [296] |
| CA: seeds from unknown location, soils from Santa Barbara County | Defoliation of Centaurea solstitialis stimulates compensatory growth and intensifies negative effects on neighbors |
[39] |
| CA, ID and outside the US: seeds from near Davis, Putah Creek, and Alameda, CA, Hell's Canyon, ID, and France, Greece, Italy, Russia, Sardinia, Sicily, and Turkey | Enhanced growth and seed properties in introduced vs. native populations of yellow starthistle (Centaurea solstitialis) | [332] |
| CA, ID, and WA and outside the US: seeds from 12 sites in US and in 12 sites in Georgia, Hungary, Romania, and Turkey | Reduced mycorrhizal responsiveness leads to increased competitive tolerance in an invasive exotic plant | [329] |
| CA and outside the US: plants from 7 sites in CA and 8 sites in Spain, France, and Hungary | Native and invading yellow starthistle (Centaurea solstitialis) microbiomes differ in composition and diversity of bacteria | [182] |
| CA and outside the US: seeds from 6 sites in CA and 16 sites in Argentina, Georgia, and Turkey | Disturbance facilitates invasion: The effects are stronger abroad than at home | [137] |
| CA and outside the US: seeds from 7 sites near San Francisco, CA, and 7 sites in Spain | Invasive species grows faster, competes better, and shows greater evolution toward increased seed size and growth than exotic non-invasive congeners | [116] |
| CA and outside the US: seeds from 7 sites in CA and 7 sites in Armenia and Georgia | Species interactions contribute to the success of a global plant invader | [7] | xc
| CA and outside the US: seeds from 8 sites in CA and 8 sites in Spain | Evidence for evolution of increased competitive ability for invasive Centaurea solstitialis, but not for naturalized C. calcitrapa | [200] |
| CA and outside the US: seeds from 8 sites CA and 8 sites in Spain | Modeling the relative importance of ecological factors in exotic invasion: The origin of competitors matters, but disturbance in the non-native range tips the balance | [340] |
| CA and outside the US: seeds collected from 10 sites in CA and 10 sites in Argentina | Cage matching: Head to head competition experiments of an invasive plant species from different regions as a means to test for differentiation | [181] |
| CA and outside the US: seeds from 11 sites in CA and 8 sites in Spain | Neo-allopatry and rapid reproductive isolation | [201] |
| CA and outside the US: seeds from 11 sites in CA and in 8 sites in Spain | Traits correlate with invasive success more than plasticity: A comparison of three Centaurea congeners | [199] |
| CA and outside US: seeds from 15 sites in CA and 58 sites in Argentina, Spain, France, Crete, Turkey, Armenia, Hungary, Romania, and Georgia. | Germination responses of an invasive species in native and non-native ranges | [134] |
| CA and outside the US: seeds from 16 sites CA, Argentina, Georgia, and Turkey | Morphological differentiation in a common garden experiment among native and non-native specimens of the invasive weed yellow starthistle (Centaurea solstitialis) | [97] |
| CA and outside the US: seeds from 19 sites in CA, 1 site in OR, and in Armenia, Bulgaria, France, Greece, Hungary, Romania, Spain, and Turkey | Evolution of invasiveness through increased resource use in a vacant niche | [76] |
CA and outside the US: seeds from Yuba County, CA, Argentina, Armenia, Chili, and Georgia |
Geographic mosaics of plant-soil microbe interactions in a global plant invasion | [8] |
| CA and outside the US: seeds from Yuba County, CA, Argentina, Armenia, Chili, and Georgia | Range-expanding populations of a globally introduced weed experience negative plant-soil feedbacks | [9] |
| MT and NV: seeds from an unknown location, soils from northern NV western MT, and eastern MT | Invasive grasses consistently create similar plant-soil feedback types in soils collected from geographically distant locations | [223] |
| Seeds from an unknown location | Traits of invasives reconsidered: Phenotypic comparisons of introduced invasive and introduced noninvasive plant species within two closely related clades | [205] |
| Table A6—Publications from 1999 to 2020 with information on nonfire control treatment effects on yellow starthistle. | |||
| Study or collection location; plant community | Title | Treatments investigated | Reference |
| Annual grasslands | |||
| CA: Brooks and Davis; a fallow agricultural field and an undescribed site in a riparian reserve | Population-level compensation by an invasive thistle thwarts biological control from seed predators | Biological control (Chaetorellia succinea and Eustenopus villosus) | [104] |
| CA: Butte, Calaveras, Shasta, Siskiyou, and Yolo counties; annual grasslands | Success of mowing to control yellow starthistle depends on timing and plant's branching form | Mowing | [19] |
| CA: Colusa County; annual rangelands and blue oak woodlands | Plant community responses to cattle grazing in a noxious weed-dominated rangeland | Grazing with cattle | [253] |
| CA: Lake and Napa counties, Donald and Sylvia McLaughlin Natural Reserve; nonnative annual grasslands with yellow starthistle on serpentine and nonserpentine soils | Soil type mediates indirect interactions between Centaurea solstitialis and its biological control agents | Biological control (Chaetorellia australis, Chaetorellia succinea, Eustenopus villosus, Puccinia jaceae var. solstitialis, and Urophora sirunaseva) | [310] |
| CA: Loma Alta Open Space Preserve, Mount Diablo State Park, and El Dorado National Forest; modeled yellow starthistle populations | Spatiotemporal variation in the strength of density dependence: Implications for biocontrol of Centaurea solstitialis | Biological control (hypothetical) | [309] |
| CA: Loma Alta Open Space Preserve, Mount Diablo State Park, and El Dorado National Forest; undescribed yellow starthistle stands | Complex interactions among biocontrol agents, pollinators, and an invasive weed: A structural equation modeling approach | Biological control (Chaetorellia australis and Eustenopus villosus) | [307] |
| CA: Marin and Contra Costa counties; undescribed yellow starthistle stands | Variable effects of a generalist parasitoid on a biocontrol seed predator and its target weed | Biological control (Eustenopus villosus) | [308] |
| CA: Mendocino County; yellow starthistle stands with 100% starthistle cover along seasonal creek beds | Response of biomass and seedbanks of rangeland functional groups to mechanical control of yellow starthistle | Mowing with and without removal of mowed biomass and shading with black plastic | [189] |
| CA: Mount Diablo State Park; undescribed yellow starthistle stand | Trait-mediated interactions and lifetime fitness of the invasive plant Centaurea solstitialis | Biological control (Eustenopus villosus and Puccinia jaceae var. solstitialis) | [305] |
| CA: Mount Diablo State Park; undescribed yellow starthistle stand | Biocontrol attack increases pollen limitation under some circumstances in the invasive plant Centaurea solstitialis | Biological control (Puccinia jaceae var. solstitialis) | [304] |
| CA: Place, Sonoma, and Yolo counties; undescribed grasslands with yellow starthistle | Population buildup and combined impact of introduced insects on yellow starthistle (Centaurea solstitialis L.) in California | Biological control (Bangasternus orientalis, Chaetorellia succinea, Chaetorellia succinea, Eustenopus villosus, Larinus curtus and Urophora sirunaseva) | [232] |
| CA: Sacramento County; undescribed yellow starthistle stand | Rangeland and uncultivated areas: Integrating biological control agents and herbicides for starthistle control | Herbicide (clopyralid) and biological control (Bangasternus orientalis, Chaetorellia succinea, Eustenopus villosus, and Urophora sirunaseva) | [229] |
| CA: Ukiah and Winters; undescribed "grassy hillsides" with yellow starthistle | Seasonal phenology and impact of Urophora sirunaseva on yellow starthistle seed production in California | Biological control (Urophora sirunaseva) | [339] |
| CA: Yolo County; nonnative annual grasslands | New growth regulator herbicide provides excellent control of yellow starthistle | Herbicide (clopyralid) | [71] |
| CA: Yreka; nonnative annual grasslands | Perennial grass establishment integrated with clopyralid treatment for yellow starthistle management on annual range | Herbicide (clopyralid) and seeding perennial grasses | [93] |
| CA: Yreka; nonnative annual grasslands and intermediate wheatgrass communities | Chapter 3: Yellow starthistle response to clipping in annual and improved perennial grassland communities in California | Clipping | [94] |
| Perennial grasslands | |||
| CA: Solano County, Jepson Prairie Reserve; bunchgrass prairie with "moderate to dense" yellow starthistle populations | Clopyralid effects on yellow starthistle (Centaurea solstitialis) and nontarget species | Herbicide (clopyralid) | [244] |
| ID: Bentz Ridge; former bluebunch wheatgrass/Sandberg bluegrass plant association with 37% yellow starthistle cover | Late-season targeted grazing of yellow starthistle (Centaurea solstitialis) with goats in Idaho | Grazing with domestic goats | [113] |
| ID: Genesee; former perennial grassland dominated by annual grasses and yellow starthistle | The effect of targeted grazing and biological control on yellow starthistle (Centaurea solstitialis) in canyon grasslands of Idaho | Biological control (Bangasternus orientalis, Chaetorellia australis, Eustenopus villosus, Larinus curtus, and Urophora sirunaseva) and grazing with domestic sheep and cattle | [328] |
| ID: Genesee; former perennial grassland dominated by annual grasses and yellow starthistle | The response of yellow starthistle (Centaurea solstitialis), annual grasses, and smooth brome (Bromus inermis) to imazapic and picloram | Herbicide (imazapic and picloram) | [288] |
| ID: Lenore; "dense" stand of yellow starthistle | Phenological synchrony of Eustenopus villosus (Coleoptera: Curculionidae) with Centaurea solstitialis in Idaho | Biological control (Eustenopus villosus) | [53] |
| ID: Lewiston; former perennial grassland dominated by annual grasses and yellow starthistle | Grazing by cattle and sheep affect yellow starthistle | Grazing with domestic sheep and cattle | [145] |
| ID: Nez Perce and Lewis counties, Garden Creek Preserve; yellow starthistle stands with up to 100 plants/0.25m² | Conservation of Spalding's catchfly (Silene spaldingii) in the Lower Corral Creek study area, Garden Creek Ranch, Craig Mountain, Idaho. 2000 field season | Hand pulling, weed clipping/whipping, herbicide (2,4-D), and biological control (Bangasternus orientalis and Eustenopus villosus) | [140] |
| ID: Salmon River Canyon; bluebunch wheatgrass/Sandberg bluegrass/arrowleaf balsamroot community dominated by yellow starthistle and cheatgrass | Herbicides reduce seed production in reproductive-stage yellow starthistle (Centaurea solstitialis) | Herbicide (picloram) | [46] |
| ID: Salmon River Canyon; bunchgrass steppe dominated by nonnative annual grasses and yellow starthistle | Biological control of yellow starthistle (Centaurea solstitialis) in the Salmon River canyon of Idaho | Biological control (Bangasternus orientalis, Chaetorellia australis, Eustenopus villosus, Larinus curtus, and Urophora sirunaseva) | [24] |
| ID: Tammany; "unimproved pastureland" with yellow starthistle | The response of yellow starthistle (Centaurea solstitialis), spotted knapweed (Centaurea maculosa), and meadow hawkweed (Hieracium caespitosum) to imazapic | Herbicide (imazapic and picloram) | [289] |
| WA: Kramer Prairie Natural Area; undescribed steppe | Percent infestation and seed consumption of Centaurea solstitialis L. (Asteraceae: Cardueae) by Eustenopus villosus and Larinus curtus (Coleoptera: Curculionidae) in Washington, USA | Biological control (Eustenopus villosus and Larinus curtus) | [335] |
| Multiple locations | |||
| CA, OR, WA, and ID; various, undescribed plant communities | Control of yellow starthistle (Centaurea solstitialis) and coast fiddleneck (Amsinckia menziesii) with aminopyralid | Herbicide (aminopyralid and clopyralid) | [168] |
| CA, OR, WA, ID, and MT; various, undescribed plant communities | Yellow starthistle management with herbicides | Herbicide (aminopyralid andclopyralid) | [87] |
| US: 11 western states; modeled yellow starthistle populations | Assessing the biological control of yellow starthistle (Centaurea solstitialis L): Prospective analysis of the impact of the rosette weevil (Ceratapion basicorne (Illiger)) | Biological control (Ceratapion basicorne) | [124] |
| Common garden, greenhouse, laboratory, and livestock pen | |||
| Common garden; information on seed source not provided | Yellow starthistle leaf loss due to early season infection by the Puccinia jaceae var. solstitialis | Biological control (Puccinia jaceae var. solstitialis) | [337] |
| Common garden; yellow starthistle seeds collected from Winters, CA | Interactive effects of plant-plant competition and an introduced biocontrol agent on yellow starthistle (Centaurea solstitialis) seed production | Biological control (Eustenopus villosus) | [153] |
| Common garden; yellow starthistle seeds collected from Yolo County, CA | Effects of the rust Puccinia jaceae var. solstitialis on Centaurea solstitialis (yellow starthistle) growth and competition | Biological control (Puccinia jaceae var. solstitialis) | [212] |
| Common garden; yellow starthistle seeds collected from Yolo County, CA | The effect of Puccinia jaceae var. solstitialis on the yellow starthistle biological control insects Eustenopus villosus and Chaetorellia succinea | Biological control (Chaetorellia succinea, Eustenopus villosus, and Puccinia jaceae var. solstitialis) | [213] |
| Common garden; seeds collected from Yolo County, CA | Impacts of mowing and bud destruction on Centaurea solstitialis growth, flowering, root dynamics and soil moisture | Mowing, clipping, and manually destroying flowerheads with pliers | [294] |
| Greenhouse; plants from Dayton, WA | Cross-resistance in and chemical control of auxinic herbicide-resistant yellow starthistle (Centaurea solstitialis) | Herbicide (2,4-D, carfentrazone-ethyl, clopyralid, dicamba, fluroxypyr, metsulfuron methyl, picloram, quinclorac, sodium diflufenzopry, triasulfuron, and triclopyr) | [195] |
| Greenhouse; seeds collected from Central Grade, ID, and Dayton, WA | Picloram-resistant and -susceptible yellow starthistle accessions have similar competitive ability | Herbicide (picloram) | [299] |
| Laboratory; biocontrols collected from Turkey | Natural history studies for the preliminary evaluation of Larinus filiformis (Coleoptera: Curculionidae) as a prospective biological control agent of yellow starthistle | Biological control (Larinus filiformis) | [122] |
| Laboratory; yellow starthistle plants collected near Dayton, WA | Eustenopus villosus (Coleoptera: Curculionidae) feeding of herbicide-resistant yellow starthistle (Centaurea solstitialis L.) | Biological control (Eustenopus villosus) | [268] |
| Laboratory; yellow starthistle plants and biocontrols collected from Greece | First report of leaf spot caused by Cladosporium herbarum on Centaurea solstitialis in Greece | Biological control (Cladosporium herbarum) | [22] |
| Laboratory; yellow starthistle seeds collected from CA and France | Filtrates of rhizosphere bacteria suppressive to Centaurea solstitialis seed germination | Biological control (Streptomyces griseus) | [331] |
| Laboratory and greenhouse; yellow starthistle seeds collected throughout CA | Susceptibility of yellow starthistle to Puccinia jaceae var. solstitialis and greenhouse production of inoculum for classical biological control programs | Biological control (Puccinia jaceae var. solstitialis) | [338] |
| Livestock pen; yellow starthistle plants grown in a common garden and fed to domestic sheep in a pen | Sheep grazing to control yellow starthistle: Role of sesquiterpene lactones | Grazing with domestic sheep | [4] |
1. Akiona, Reyn. 2014. Heat and smoke effects on the seeds of common San Joaquin Valley grassland plants. Turlock, CA: California State University, Stanislaus. 57 p. Thesis. [88564]
2. Akkol, Esra Kupeli; Arif, Reyhan; Ergun, Fatma; Yesilada, Erdem. 2009. Sesquiterpene lactones with antinociceptive and antipyretic activity from two Centaurea species. Journal of Ethnopharmacology. 122(2): 210-215. [94012]
3. Alper, Mehlika; Gunes, Hatice. 2019. The anticancer and anti-inflammatory effects of Centaurea solstitialis extract on human cancer cell lines. Turkish Journal of Pharmaceutical Sciences. 16(3): 273-281. [93992]
4. Alvarez, Pelayo. 2008. Sheep grazing to control yellow starthistle: Role of sesquiterpene lactones. Davis, CA: University of California, Davis. 83 p. Dissertation. [94049]
5. Anand, Sridhar. 2008. Structure-toxicity relationship studies of the sequiterpene lactone repin. Oxford, MI: The University of Mississippi. 173 p. Dissertation. [93985]
6. Andersen, Mark C. 1993. Diaspore morphology and seed dispersal in several wind-dispersed Asteraceae. American Journal of Botany. 80(5): 487-492. [21433]
7. Andonian, Krikor; Hierro, Jose L. 2011. Species interactions contribute to the success of a global plant invader. Biological Invasions. 13(12): 2957-2965. [94087]
8. Andonian, Krikor; Hierro, Jose L.; Khetsuriani, Liana; Becerra, Pablo I.; Janoyan, Grigor; Villareal, Diego; Cavieres, Lohengrin, A.; Fox, Laurel R.; Callaway, Ragan M. 2012. Geographic mosaics of plant–soil microbe interactions in a global plant invasion. Journal of Biogeography. 39(3): 600-608. [94088]
9. Andonian, Krikor; Hierro, Jose L.; Khetsuriani, Liana; Becerra, Pablo; Janoyan, Grigor; Villarreal, Diego; Caviers, Lohengrid; Fox, Laurel R.; Callaway, Ragan M. 2011. Range-expanding populations of a globally introduced weed experience negative plant-soil feedbacks. PLoS ONE. 6(5): e20117. [94086]
10. Asher, Jerry; Dewey, Steven; Olivarez, Jim; Johnson, Curt. 1998. Minimizing weed spread following wildland fires. In: Christianson, Kathy, ed. Proceedings of the Western Society of Weed Science; 1998 March 10-12; Waikoloa, HI. [Journal unknown] 51: 49. Abstract. [40409]
11. Baldwin, Bruce G.; Goldman, Douglas H.; Keil, David J.; Patterson, Robert; Rosatti, Thomas J.; Wilken, Dieter H., eds. 2012. The Jepson manual. Vascular plants of California, second edition. Berkeley, CA: University of California Press. 1568 p. [86254]
12. Barker, Brittany S.; Andonian, Krikor; Swope, Sarah M.; Luster, Douglas G.; Dlugosch, Katrina M. 2017. Population genomic analyses reveal a history of range expansion and trait evolution across the native and invaded range of yellow starthistle (Centaurea solstitialis). Molecular Ecology. 26(4): 1131-1147. [93984]
13. Barthell, J. F.; Clement, M. L. ; Wells H.; Crocker, K. C.; Becker, E. C.; Leavitt, K. D.; McCall, B. T.; Mills-Novoa, M.; Walker, C. M.; Petanidou T. 2009. Foraging patterns of bees in response to nectar availability in populations of the invasive thistle species Centaurea solstitialis L. in native (Greece) and non-native (USA) island ecosystems. Integrative and Comparative Biology 49: E10. [93986]
14. Barthell, John F.; Clement, Meredith L. ; Giannoni, Manual A.; Liu, Lucy; Presky, Miyeon E.; Redd, JeAnna R.; Ricci, Paige R.; Stevison, Blake K.; Freeman, Brett; Petanidou, Theodora; Hranitz, John M.; Wells, Harrington. 2010. Differing foraging responses by bees to the invasive thistle species Centaurea solstitialis L. in native (Greece) and non-native (USA) island ecosystems. Integrative and Comparative Biology 50: E203. [93987]
15. Barthell, John F.; Randall, John M.; Thorp, Robbin W.; Wenner, Adrian M. 2001. Promotion of seed set in yellow star-thistle by honey bees: Evidence of an invasive mutualism. Ecological Applications. 11(6): 1870-1883. [43146]
16. Barthell, John F.; Thorp, Robbin W.; Wenner, Adrian M.; Randall, John M. 2000. Yellow star-thistle, gumplant, and feral honey bees on Santa Cruz Island: A case of invaders assisting invaders. In: Browne, David R.; Mitchell, Kathryn L.; Chaney, Henry W., eds. Proceedings of the 5th California islands symposium; 1999 March 29 - April 1; Camarillo, CA. Santa Barbara, CA: Santa Barbara Museum of Natural History: 269-273. [40412]
17. Batten, Katharine M.; Scow, Kate M.; Davies, Kendi F.; Harrison, Susan P. 2006. Two invasive plants alter soil microbial community composition in serpentine grasslands. Biological Invasions. 8(2): 217-230. [94090]
18. Benefield, Carri B.; DiTomaso, Joseph M.; Kyser, Guy B. 1998. Impacts of yellow starthistle density on the soil moisture profile and rangeland management. Proceedings of the Western Society of Weed Science. 51: 66. [40408]
19. Benefield, Carri B.; DiTomaso, Joseph M.; Kyser, Guy B.; Orloff, Steve; Churches, Kenneth R.; Marcum, Daniel B.; Nader, Glenn A. 1999. Success of mowing to control yellow starthistle depends on timing and plant's branching form. California Agriculture. 53(2): 17-21. [40407]
20. Benefield, Carri B.; DiTomaso, Joseph M.; Kyser, Guy B.; Tschohl, Alison. 2001. Reproductive biology of yellow starthistle: Maximizing late-season control. Weed Science. 49(1): 83-90. [40406]
21. Bernard, Stephen R.; Brown, Kenneth F. 1977. Distribution of mammals, reptiles, and amphibians by BLM physiographic regions and A.W. Kuchler's associations for the eleven western states. Tech. Note 301. Denver, CO: U.S. Department of the Interior, Bureau of Land Management. 169 p. [434]
22. Berner, D. K.; Smallwood, E. L.; McMahon, M. B.; Luster, D. G. 2007. First report of leaf spot caused by Cladosporium herbarum on Centaurea solstitialis in Greece. Plant Disease. 91(4): 463. [93988]
23. Bernhardt, Elizabeth A.; Swiecki, Tedmund J. 1991. Minimum input techniques for valley oak restocking. In: Standiford, Richard B., technical coordinator. Proceedings of the symposium on oak woodlands and hardwood rangeland management; 1990 October 31 - November 2; Davis, CA. Gen. Tech. Rep. PSW-126. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: 2-8. [17379]
24. Birdsall, Jennifer L.; Markin, George P. 2010. Biological control of yellow starthistle (Centaurea solstitialis) in the Salmon River canyon of Idaho. Invasive Plant Science and Management. 3(4): 462-469. [94050]
25. Blank, Robert R.; Young, James A. 2004. Influence of three weed species on soil nutrient dynamics. Soil Science. 169(5): 385-397. [94091]
26. Borgias, Darren. 2002. [Email to Kristin Zouhar]. March 23. Regarding yellow starthistle and fire. Medford, OR: The Nature Conservancy of Oregon. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [40503]
27. Borman, M. M.; Johnson, D. E.; Krueger, W. C. 2002. Soil moisture extraction by vegetation in a Mediterranean/maritime climatic regime. Agronomy Journal. 84(5): 897-904. [94282]
28. Borman, M. M.; Krueger, W. C.; Johnson, D. E. 1991. Effects of established perennial grasses on yields of associated annual weeds. Journal of Range Management. 44(4): 318-322. [16119]
29. Bowcutt, Frederica S. 1999. A floristic study of Sugarloaf Ridge State Park, Sonoma County, California. Aliso. 18(1): 19-34. [40636]
30. Bradley, Bethany A.; Oppenheimer, Michael; Wilcove, David S. 2009. Climate change and plant invasions: Restoration opportunities ahead? Global Change Biology. 15(6): 1511-1521. [73490]
31. Brooks, Matthew L. 2008. Effects of fire suppression and postfire management activities on plant invasions. In: Zouhar, Kristin; Smith, Jane Kapler; Sutherland, Steve; Brooks, Matthew L., eds. Wildland fire in ecosystems: Fire and nonnative invasive plants. Gen. Tech. Rep. RMRS-GTR-42. Vol. 6. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 269-280. [70909]
32. Brooks, Matthew L.; Pyke, David A. 2001. Invasive plants and fire in the deserts of North America. In: Galley, Krista E. M.; Wilson, Tyrone P., eds. Proceedings of the invasive species workshop: The role of fire in the control and spread of invasive species; Fire conference 2000: 1st national congress on fire ecology, prevention, and management; 2000 November 27 - December 1; San Diego, CA. Misc. Publ. No. 11. Tallahassee, FL: Tall Timbers Research Station: 1-14. [40491]
33. Brown, Cynthia S.; Rice, Kevin J. 2010. Effects of belowground resource use complementarity on invasion of constructed grassland plant communities. Biological Invasions. 12(5): 1319-1334. [94093]
34. Brownsey, Rachel N.; Kyser, Guy B.; DiTomaso, Joseph M. 2014. Growth and phenology of Dittrichia graveolens, a rapidly spreading invasive plant in California. Biological Invasions. 16(1): 43-52. [94032]
35. Bruno, Maurizio; Bancheva, Svetlana; Rosselli, Sergio; Maggio, Antonella. 2013. Sesquiterpenoids in subtribe Centaureinae (Cass.) Dumort (tribe Cardueae, Asteraceae): Distribution, 13C NMR spectral data and biological properties. Phytochemistry. 95: 19-93. [94011]
36. Buckley, Katharine Denise. 2019. Native habitat restoration in eastern Washington wine vineyards as a pest management strategy. Pullman, WA: Washington State University. 160 p. Dissertation. [94017]
37. Bussan, Alvin J.; Dyer, William E. 1999. Herbicides and rangeland. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 116-132. [35716]
38. California Invasive Plant Council. 2006. California invasive plant inventory, [Online]. Cal-IPC Publication 2006-02. Berkeley, CA: California Invasive Plant Council (Producer). Available: https://www.cal-ipc.org/plants/inventory/ [2021, February 3]. [74015]
39. Callaway, Ragan M.; Kim, Judy; Mahall, Bruce E. 2006. Defoliation of Centaurea solstitialis stimulates compensatory growth and intensifies negative effects on neighbors. Biological Invasions. 8(6): 1389-1397. [94052]
40. Callihan, R. H.; Prather, T. S.; Northam, F. E. 1989. Invasion by yellow starthistle. In: Fay, Peter K.; Lacey, John R., eds. Proceedings: Knapweed symposium; 1989 April 4-5; Bozeman, MT. Bozeman, MT: Montana State University: 73-76. [37798]
41. Callihan, Robert H.; Prather, Timothy S.; Northam, Francis E. 1993. Longevity of yellow starthistle (Centaurea solstitialis) achenes in soil. Weed Technology. 7(1): 33-35. [24469]
42. Carev, Ivana; Ruscic, Mirko; Skocibusic, Mirjana; Maravic, Ana; Siljak-Yakovlev, Sonja; Politeo, Olivera. 2017. Phytochemical and cytogenetic characterization of Centaurea solstitialis L. (Asteraceae) from Croatia. Chemistry & Biodiversity. 14(2): e1600213. [94010]
43. Carlson, John E.; Willis, David B.; Michalson, Edgar L.; Callihan, Robert H. 1990. Yellow starthistle in North-Central Idaho: A survey of farmers' and ranchers' behavior and attitudes. Bulletin No. 712. Moscow, ID: University of Idaho, College of Agriculture, Agricultural Experiment Station. 11 p. [40405]
44. Carpenter, Elizabeth Marjorie. 2019. Evidence for a growth-defense trade-off in Centaurea solstitialis. Tucson, AZ: University of Arizona. 23 p. Thesis. [94033]
45. Carr, Amanda N.; Hooper, David U.; Dukes, Jeffrey S. 2019. Long-term propagule pressure overwhelms initial community determination of invader success. Ecosphere. 10(8): e02826. [94094]
46. Carrithers, Vanelle F.; Roche, Cindy Talbott; Gaiser, Dean R.; Horton, Denise; Duncan, Celestine L.; Scherer, Peter N. 2004. Herbicides reduce seed production in reproductive-stage yellow starthistle (Centaurea solstitialis). Weed Technology. 18(4): 1065-1071. [94053]
47. Chang, H. T.; Rumbeiha, W. K.; Patterson, J.S.; Puschner, B.; Knight, A. P. 2012. Toxic equine Parkinsonism: An immunohistochemical study of 10 horses with nigropallidal encephalomalacia. Veterinary Pathology. 49(2): 398-402. [93982]
48. Chappell, Christopher B.; Kagan, Jimmy. 2001. 12. Ceanothus-manzanita shrubland. In: Chappell, Christopher B.; Crawford, Rex C.; Barrett, Charley; Kagan, Jimmy; Johnson, David H.; O'Mealy, Mikell; Green, Greg A.; Ferguson, Howard L.; Edge, W. Daniel; Greda, Eva L.; O'Neil, Thomas A. Wildlife habitats: Descriptions, status, trends, and system dynamics. In: Johnson, David H.; O'Neil, Thomas A., managing directors. Wildlife-habitat relationships in Oregon and Washington. Corvallis, OR: Oregon State University Press: 43-44. [68102]
49. Christopherson, Kirsten; Morrison, Michael L. 2004. Influence of yellow starthistle (Centaurea solstitialis) on small mammals in central California. Western North American Naturalist. 64(2): 202-207. [56138]
50. Clines, Joanna. 2005. Preventing weed spread in contaminated hay and straw. In: Cal-IPC Symposium. Prevention reinvention: Protocols, information, and partnerships to stop the spread of invasive plants; Chico, CA; Chico State University. Berkeley, CA: California Invasive Plant Council: Available: https://www.cal-ipc.org/wp-content/uploads/2017/12/Clines-symposium-2005.pdf [2020, January 7]. [93961]
51. Cogan, Dan; Taylor, Jeanne E.; Schulz, Keith. 2012. Vegetation Inventory Project: Great Basin National Park. Natural Resource Rep. NPS/MOJN/NRR-2012/568. Fort Collins, CO: U.S. Department of the Interior, National Park Service. 79 p. [+ appendices]. [89265]
52. Connelly, J. W.; Knick, S. T.; Schroeder, M. A. Stiver, S. J. 2004. Conservation assessment of greater sage-grouse and sagebrush habitats. Cheyenne, WY: Western Association of Fish and Wildlife Agencies. Unpublished report. 535 p. [+ supplementary information]. Available: https://www.usgs.gov/media/files/conservation-assessment-greater-sage-grouse-and-sagebrush-habitats. [91549]
53. Connett, J. F.; Wilson, L. M.; McCaffrey, J. P.; Harmon, B. L. 2001. Phenological synchrony of Eustenopus villosus (Coleoptera: Curculionidae) with Centaurea solstitialis in Idaho. Biological Control. 30(2): 439-442. [94054]
54. Coombs, Eric M.; Clark, Janet K.; Piper, Gary L.; Cofrancesco, Alfred F., Jr., eds. 2004. Biological control of invasive plants in the United States. Corvallis, OR: Oregon State University Press. 467 p. [52973]
55. Crawford, Rex C.; Kagan, Jimmy. 2001. 15. Eastside grasslands. In: Chappell, Christopher B.; Crawford, Rex C.; Barrett, Charley; Kagan, Jimmy; Johnson, David H.; O'Mealy, Mikell; Green, Greg A.; Ferguson, Howard L.; Edge, W. Daniel; Greda, Eva L.; O'Neil, Thomas A. Wildlife habitats: Descriptions, status, trends, and system dynamics. In: Johnson, David H.; O'Neil, Thomas A., managing directors. Wildlife-habitat relationships in Oregon and Washington. Corvallis, OR: Oregon State University Press: 48-49. [68104]
56. Cronquist, Arthur; Holmgren, Arthur H.; Holmgren, Noel H.; Reveal, James L.; Holmgren, Patricia K. 1984. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Vol. 4: Subclass Asteridae, (except Asteraceae). New York: The New York Botanical Garden. 573 p. [718]
57. Cronquist, Arthur; Holmgren, Arthur H.; Holmgren, Noel H.; Reveal, James L.; Holmgren, Patricia K. 1994. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Vol. 5: Asterales. New York: The New York Botanical Garden. 496 p. [28653]
58. D'Antonio, Carla M. 2000. Fire, plant invasions, and global changes. In: Mooney, Harold A.; Hobbs, Richard J., eds. Invasive species in a changing world. Washington, DC: Island Press: 65-93. [37679]
59. Davy, Josh S.; Dykier, Katherine; Turri, Tony; Gornish, Elise. 2017. Forage seeding in rangelands increases production and prevents weed invasion. California Agriculture. 71(4): 239-248. [94055]
60. Disciglio, Grzaia; Tarantino, Annalisa; Frabboni, Laura; Gagliardi, Anna; Giuliani, Marcella Michela; Tarantino, Emanuele; Gatta, Giuseppe. 2017. Qualitative characterisation of cultivated and wild edible plants: Mineral elements, phenols content and antioxidant capacity. Italian Journal of Argonomy. 12(1036): 383-394. [93998]
61. DiTomaso, J. M. 2006. Using prescribed burning in integrated strategies. In: DiTomaso, J. M.; Johnson, D. W., eds. The use of fire as a tool for controlling invasive plants. Cal-IPC Publication 2006-01. Berkeley, CA: California Invasive Plant Council: 19-27. [90715]
62. DiTomaso, Joe. 2001. Element stewardship abstract: Centaurea solstitialis L. In: Weeds on the web: The Nature Conservancy wildland invasive species program, [Online]. https://www.invasive.org/weedcd/pdfs/tncweeds/centsol.pdf [2020, March 4]. [40416]
63. DiTomaso, Joseph M. 2000. Invasive weeds in rangelands: Species, impacts, and management. Weed Science. 48(2): 255-265. [37419]
64. DiTomaso, Joseph M. 2005. Yellow starthistle--Centaurea solstitialis L. In: Duncan, Celestine L.; Clark, Janet K., eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. WSSA Special Publication. Lawrence, KS: Weed Science Society of America: 36-50. [60230]
65. DiTomaso, Joseph M.; Brooks, Matthew L.; Allen, Edith B.; Minnich, Ralph; Rice, Peter M.; Kyser, Guy B. 2006. Control of invasive weeds with prescribed burning. Weed Technology. 20(2): 535-548. [63695]
66. DiTomaso, Joseph M.; Enloe, Steven F. 2001. Integrated approaches for the management of yellow starthistle. In: Smith, Lincoln, ed. Proceedings, 1st international knapweed symposium of the 21st century; 2001 March 15-16; Coeur d'Alene, ID. Albany, CA: U.S. Department of Agriculture, Agricultural Research Service: 63-64. Abstract. [37836]
67. DiTomaso, Joseph M.; Gerlach, John D., Jr. 2000. Centaurea solstitialis. In: Bossard, Carla C.; Randall, John M.; Hoshovsky, Marc C., eds. Invasive plants of California's wildlands. Berkeley, CA: University of California Press: 101-106. [40301]
68. DiTomaso, Joseph M.; Kyser, Guy B.; Hastings, Marla S. 1999. Prescribed burning for control of yellow starthistle (Centaurea solstitialis) and enhanced native plant diversity. Weed Science. 47: 233-242. [40403]
69. DiTomaso, Joseph M.; Kyser, Guy B.; Miller, Jessica R.; Garcia Sergio; Smith, Richard F.; Nader, Glenn; Connor, J. Michael; Orloff, Steve B. 2006. Integrating prescribed burning and clopyralid for the management of yellow starthistle (Centaurea solstitialis). Weed Science. 54: 757-767. [64333]
70. DiTomaso, Joseph M.; Kyser, Guy B.; Oneto, Scott R.; Wilson, Rob G.; Orloff, Steve B.; Anderson, Lars W.; Wright, Steven D.; Roncoroni, John A.; Miller, Timothy L.; Prather, Timothy S.; Ransom, Corey; Beck, K. George; Duncan, Celestine; Wilson, Katherine A.; Mann, J. Jeremiah. 2013. Yellow starthistle. In: Weed control in natural areas in the Western United States. Davis, CA: University of California, Weed Research and Information Center: 100-104. [93959]
71. DiTomaso, Joseph M.; Kyser, Guy B.; Orloff, Steve B.; Enloe, Stephen F.; Nader, Glenn A. 1999. New growth regulator herbicide provides excellent control of yellow starthistle. California Agriculture. 53(2): 12-16. [94275]
72. DiTomaso, Joseph M.; Kyser, Guy B.; Orloff, Steve B.; Enloe, Steven F. 2000. Integrated strategies offer site-specific control of yellow starthistle. California Agriculture. 54(6): 30-36. [40404]
73. DiTomaso, Joseph M.; Kyser, Guy B.; Pirosko, Carri B. 2003. Effect of light and density on yellow starthistle (Centaurea solstitialis) root growth and soil moisture use. Weed Science. 51(3): 334-341. [94034]
74. DiTomaso, Joseph M.; Kyser, Guy B.; Pitcairn, Michael J. 2006. Yellow starthistle management guide. Cal-IPC Publication 2006-03. California Invasive Plant Council: Berkeley, CA. 78 p. Available: https://www.cal-ipc.org/docs/ip/management/pdf/YSTMgmtweb.pdf [2020, February 12]. [94040]
75. DiTomaso, Joseph. 2001. Yellow starthistle information. Davis, CA: University of California, Weed Research and Information Center. Document on file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Missoula Fire Sciences Laboratory, Missoula, MT; FEIS files. [40415]
76. Dlugosch, Katrina M.; Cang, F. Alice; Barker, Brittany S.; Andonian, Krikor; Swope, Sarah M.; Rieseberg, Loren H. 2015. Evolution of invasiveness through increased resource use in a vacant niche. Nature Plants. 1(6): 15066. [94095]
77. Dlugosch, Katrina M.; Lai, Zhao; Bonin, Aurelie; Hierro, Jose; Rieseberg, Loren H. 2013. Allele identification for transcriptome-based population genomics in the invasive plant Centaurea solstitialis. Genes, Genomes, Genetics. 3(2): 359-367. [94228]
78. Dombeck, Michael P.; Williams, Jack E.; Wood, Christopher A. 2004. Wildfire policy and public lands: Integrating scientific understanding with social concerns across landscapes. Conservation Biology. 18(4): 883-889. [50265]
79. Dudley, David R. 2000. Wicked weed of the West. California Wild. 53(4): 32-35. [40401]
80. Dukes, Jeffrey S. 2001. Biodiversity and invasibility in grassland microcosms. Oecologia. 126(3): 563-568. [94096]
81. Dukes, Jeffrey S. 2002. Comparison of the effect of elevated CO2 on an invasive species (Centaurea solstitialis) in monoculture and community settings. Plant Ecology. 160(2): 225-234. [94013]
82. Dukes, Jeffrey S. 2002. Species composition and diversity affect grassland susceptibility and response to invasion. Ecological Applications. 12(2): 602-617. [94097]
83. Dukes, Jeffrey S.; Chiariello, Nona R.; Loarie, Scott R.; Field, Christopher B. 2011. Strong response of an invasive plant species (Centaurea solstitialis L.) to global environmental changes. Ecological Applications. 21(6): 1887-1894. [83506]
84. Duncan, Celestine A. 2005. Diffuse knapweed--Centaurea diffusa Lam. In: Duncan, Celestine L.; Clark, Janet K., eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. WSSA Special Publication. Lawrence, KS: Weed Science Society of America: 26-35. [60229]
85. Duncan, Celestine A.; Jachetta, John J. 2005. Introduction. In: Duncan, Celestine L.; Clark, Janet K., eds. Invasive plants of range and wildlands and their environmental, economic, and societal impacts. WSSA Special Publication. Lawrence, KS: Weed Science Society of America: 1-7. [60226]
86. Duncan, Celestine Lacey. 2001. Knapweed management: Another decade of change. In: Smith, Lincoln, ed. Proceedings, 1st international knapweed symposium of the 21st century; 2001 March 15-16; Coeur d'Alene, ID. Albany, CA: U.S. Department of Agriculture, Agricultural Research Service: 1-7. [37824]
87. Duncan, Celestine. 2011. Yellow starthistle management with herbicides. TechLine News, TechNotes. Article number: 010-50830. Available: https://uploads-ssl.webflow.com/5cdc7da45f4fbe2e6bcda1f2/5e53e85564688e90a06119c0_Yellow%20Starthistle%20Management%20with%20Herbicides.pdf [2021, February 03]. [94057]
88. Duncan, Celestine; Story, Jim; Sheley, Roger. 2001. Montana knapweeds: Identification, biology, and management. Circular 311 [Revised from 1998]. Bozeman, MT: Montana State University, Extension Service. 17 p. [38344]
89. Eagle, Alison J.; Eiswerth, Mark E.; Johnson, Wayne S.; Schoenig, Steve E.; van Kooten, G. Cornelis. 2007. Costs and losses imposed on California ranchers by yellow starthistle. Rangeland Ecology & Management. 60(4): 369-377. [93965]
90. Eastburn, D. J.; Roche, L. M.; Doran, Morgan P.; Blake, Rhilip R.; Bouril, Chip S.; Gamble, George; Gornish, Elise S. 2018. Seeding plants for long-term multiple ecosystem service goals. Journal of Environmental Management. 211: 191-197. [93966]
91. EDDMapS. 2020. Early detection & distribution mapping system. Athens, GA: The University of Georgia, Center for Invasive Species and Ecosystem Health. Available: http://www.eddmaps.org. [93957]
92. Enloe, Stephen F.; DiTomaso, Joseph M.; Orloff, Steve B.; Drake, Daniel J. 2004. Soil water dynamics differ among rangeland plant communities dominated by yellow starthistle (Centaurea solstitialis), annual grasses, or perennial grasses. Weed Science. 52: 929-935. [51119]
93. Enloe, Stephen F.; DiTomaso, Joseph M.; Orloff, Steve B.; Drake, Daniel J. 2005. Perennial grass establishment integrated with clopyralid treatment for yellow starthistel management on annual range. Weed Technology. 19(1): 94-101. [94058]
94. Enloe, Stephen Frederick. 2002. The ecology and management of yellow starthistle in annual and perennial grassland communities. Davis, CA: University of California, Davis. 108 p. Dissertation. [94059]
95. Erenler, Ramazan; Sen, Ozkan; Yaglioglu, Ayse Sahin; Demirtas, Ibrahim. 2015. Bioactivity-guided isolation of antiproliferative sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Combinatorial Chemistry & High Throughput Screening. 19(1): 66-72. [94008]
96. Eriksen, Renee L.; Hierro, Jose L.; Eren, Ozkan; Andonian, Krikor; Torok, Katalin; Becerra, Pablo I.; Montesinos, Daniel; Khetsuriani, Liana; Diaconu, Alecu; Kesseli, Rick. 2014. Dispersal pathways and genetic differentiation among worldwide populations of the invasive weed Centaurea solstitialis L. (Asteraceae). PLoS ONE. 9(12): e114786. [94039]
97. Eriksen, Renne L.; Desronvil, Theodora; Hierro, Jose L.; Kesseli, Rick. 2012. Morphological differentiation in a common garden experiment among native and non-native specimens of the invasive weed yellow starthistle (Centaurea solstitialis). Biological Invasions. 14(7): 1459-1467. [94035]
98. Eskelinen, Anu; Harrison, Susan. 2014. Exotic plant invasions under enhanced rainfall are constrained by soil nutrients and competition. Ecology. 95(3): 682-692. [94098]
99. Flora of North America Editorial Committee, eds. 2021. Flora of North America north of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
100. Frost, Rachel A.; Wilson, Linda M.; Launchbaugh, Karen L.; Hovde, Elayne M. 2008. Seasonal change in forage value of rangeland weeds in northern Idaho. Invasive Plant Science and Management. 1(4): 343-351. [80549]
101. Fuerst, E. P.; Sterling, T. M.; Norman, M. A.; Prather, T. S.; Irzyk, G. P.; Wu, Y.; Lownds, N. K.; Calliham, R. H. 1996. Physiological characterization of Picloram resistance in yellow starthistle. Pesticide Biochemistry and Physiology. 56(2): 149-161. [94070]
102. Fujita, Tetsuro; Sezik, Ekrem; Tabata, Mamoru; Yesilada, Erdem; Honda, Gisho; Takesa, Yoshio; Tanaka, Toshihiro; Takaishi, Toshishisa. 1995. Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Economic Botany. 49(4): 406-422. [94007]
103. Fuller, T. C.; Barbe, G. Douglas. 1985. The Bradley method of eliminating exotic plants from natural reserves. Fremontia. 13(2): 24-25. [40504]
104. Garren, Julie M.; Strauss, Sharon Y. 2009. Population-level compensation by an invasive thistle thwarts biological control from seed predators. Ecological Applications. 19(3): 709-721. [94062]
105. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; Lewis, Mont E.; Smith, Dixie R., comps. 1977. Forest and range ecosystems of the United States. Washington, DC: U.S. Department of Agriculture, Forest Service. 1:7,5000,000; map, colored. [86817]
106. Gelbard, Jonathan L.; Harrison, Susan. 2003. Roadless habitats as refuges for native grasslands: Interactions with soil, aspect, and grazing. Ecological Applications. 13(2): 404-415. [44585]
107. Gelbard, Jonathan L.; Harrison, Susan. 2005. Invasibility of roadless grasslands: An experimental study of yellow starthistle. Ecological Applications. 15(5): 1570-1580. [55829]
108. Gerhardt, Fritz; Collinge, Sharon K. 2007. Abiotic constraints eclipse biotic resistance in determining invasibility along experimental vernal pool gradients. Ecological Applications. 17(3): 922-933. [94099]
109. Gerlach, John D., Jr. 2004. The impacts of serial land-use changes and biological invasions on soil water resources in California, USA. Journal of Arid Environments. 57(3): 365-379. [94100]
110. Gerlach, John D., Jr.; Rice, Kevin J. 2003. Testing life history correlates of invasiveness using congeneric plant species. Ecological Applications. 13(1): 167-179. [94016]
111. Gerlach, John D. 1997. How the West was lost: Reconstructing the invasion dynamics of yellow starthistle and other plant invaders of western rangelands and natural areas. In: Kelly, M.; Wagner, E.; Warner, P., eds. Proceedings, California Exotic Pest Plant Council symposium; 1997 October 2-4; Concord, CA. Volume 3. Berkeley, CA: California Exotic Pest Plant Council: 67-72. [94230]
112. Gerlach, John; Dyer, Andrew; Rice, Kevin. 1998. Grassland and foothill woodland ecosystems of the Central Valley. Fremontia. 26(4): 39-43. [40400]
113. Goehring, Brianna J.; Launchbaugh, Karen L.; Wilson, Linda M. 2010. Late-season targeted grazing of yellow starthistle (Centaurea solstitialis) with goats in Idaho. Invasive Plant Science and Management. 3(2): 148-154. [94063]
114. Goehring, Brianna. 2009. The effects of targeted grazing on yellow starthistle by domestic goats in northern Idaho and an examination of seed survival in the ruminant digestive tract. Moscow, ID: University of Idaho. 65 p. Thesis. [94304]
115. Goodwin, Kim; Sheley, Roger; Clark, Janet. 2002. Integrated noxious weed management after wildfires, [Online]. EB-160. Bozeman, MT: Montana State University, Extension Service (Producer). 46 p. Available: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=1586&context=govdocs [2021, January 28]. [45303]
116. Graebner, Ryan C.; Callaway, Ragan M.; Montesinos, Daniel. 2012. Invasive species grows faster, competes better, and shows greater evolution toward increased seed size and growth than exotic non-invasive congeners. Plant Ecology. 213(4): 545-553. [94101]
117. Gray, Andrew N.; Barndt, Katie; Reichard, Sarah H. 2011. Nonnative invasive plants of Pacific Coast forests. Gen. Tech. Rep. PNW-GTR-817. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 91 p. [84388]
118. Gray, M. Violet; Greaves, James M. 1984. Riparian forest as habitat for the least Bell's vireo. In: Warner, Richard E.; Hendrix, Kathleen M., eds. California riparian systems: Ecology, conservation, and productive management: Proceedings of a conference; 1981 September 17-19; Davis, CA. Berkeley, CA: University of California Press: 605-611. [5862]
119. Great Plains Flora Association. 1986. Flora of the Great Plains. Lawrence, KS: University Press of Kansas. 1392 p. [1603]
120. Gucker, Corey L.; Bunting, Stephen C. 2011. Canyon grassland vegetation changes following fire in Northern Idaho. Western North American Naturalist. 71(1): 97-105. [82531]
121. Gucker, Corey. 2004. Canyon grassland vegetation changes following the Maloney Creek wildfire. Moscow, ID: University of Idaho. 80 p. Thesis. [51512]
122. Gultekin, L.; Cristofaro, M.; Tronci, C.; Smith, L. 2008. Natural history studies for the preliminary evaluation of Larinus filiformis (Coleoptera: Curculionidae) as a prospective biological control agent of yellow starthistle. Population Ecology. 37(5): 1185-1199. [93794]
123. Gurbuz, Ilhan; Yesilada, Erdem. 2007. Evaluation of the anti-ulcerogenic effect of sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis by using various in vivo and biochemical techniques. Journal of Ethnopharmacology. 112(2): 284-291. [93996]
124. Gutierrez, Andrew Paul; Ponti, Luigi; Cristofaro, Massimo; Smith, Lincoln; Pitcairn, Michael J. 2017. Assessing the biological control of yellow starthistle (Centaurea solstitialis L): Prospective analysis of the impact of the rosette weevil (Ceratapion basicorne (Illiger)). Agricultural and Forest Entomology. 19(3): 257-273. [94064]
125. Hachani, Chadlia; Lamhamedi, Mohammed S.; Abassi, Mejda; Bejaoui, Zoubeir. 2020. Inhibitory effect of aqueous extracts of Centaurea solstitialis subsp. schouwii on seed germination and growth of Sulla coronaria. Botany. 98(5): 273-281. [94980]
126. Haines, Arthur. 2011. Flora novae angliae: A manual for the identification of native and naturalized higher vascular plants of New England. New Haven, CT: Yale University Press. 973 p. [94359]
127. Harris, P.; Clapperton, M. J. 1997. An exploratory study on the influence of vesicular-arbuscular mycorrhizal fungi on the success of weed biological control with insects. Biocontrol Science and Technology. 7(2): 193-201. [38345]
128. Harrod, Richy J.; Taylor, Ronald J. 1995. Reproduction and pollination biology of Centaurea and Acroptilon species, with emphasis on C. diffusa. Northwest Science. 69(2): 97-105. [28487]
129. Hastings, Maria; Barry, W. James. 2002. Increasing biodiversity through the restoration of a natural process by prescribed burning in California state parks: "A Sonoma County experience". In: Sugihara, Neil G.; Morales, Maria; Morales, Tony, eds. Proceedings of the symposium: Fire in California ecosystems: Integrating ecology, prevention and management; 1997 November 17-20; San Diego, CA. Misc. Pub. No. 1. [Berkeley, CA]: Association for Fire Ecology: 350-351. [46236]
130. Hastings, Marla S.; DiTomaso, Joseph M. 1996. Fire controls yellow starthistle in California grasslands: Test plots at Sugarloaf Ridge State Park. Restoration & Management Notes. 14(2): 124-128. [40398]
131. Hastings, Marla; DiTomaso, Joseph. 1996. The use of fire for yellow starthistle (Centaurea solstitialis) management and the restoration of native grasslands at Sugarloaf Ridge State Park. Project summary as presented to the California Weed Conference, January 1996. CalEPPC News. Berkeley, CA: California Exotic Plant Pest Council. 4(1): 4-6. [93963]
132. Heady, H. F.; Bartolome, J. W.; Pitt, M. D.; Savelle, G. D.; Stroud, M. C. 1992. California prairie. In: Coupland, R. T., ed. Natural grasslands: Introduction and western hemisphere. Ecosystems of the World 8A. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.: 313-335. [23831]
133. Heil, Kenneth D.; O'Kane, Steve L. Jr.; Reeves, Linda Mary; Clifford, Arnold. 2013. Flora of the Four Corners Region. Vascular plants of the San Juan River Drainage: Arizona, Colorado, New Mexico, and Utah. St. Louis, MO: Missouri Botanical Garden Press. 1098 p. [94189]
134. Hierro, Jose L.; Eren, Ozkan; Khetsuriani, Liana; Diaconu, Alecu; Torok, Katalin; Montesinos, Daniel; Andonian, Krikor; Kikodze, David; Janoian, Levan; Villarreal, Diego; Estanga-Mollica, Maria E.; Callaway, Ragan M. 2009. Germination responses of an invasive species in native and non-native ranges. Oikos. 118(4): 529-538. [74207]
135. Hierro, Jose L.; Knetsuriani, Liana; Andonian, Krikor; Eren, Ozkan; Villarreal, Diego; Janoian, Grigor; Reinhart, Kirt O.; Callaway, Ragan M. 2017. The importance of factors controlling species abundance and distribution varies in native and non-native ranges. Ecography. 40(8): 991-1002. [94065]
136. Hierro, Jose L.; Lortie, Christopher J.; Villarreal, Diego; Estanga-Mollica, Maria E.; Callaway, Ragan M. 2011. Resistance to Centaurea solstitialis invasion from annual and perennial grasses in California and Argentina. Biological Invasions. 13(10): 2249-2259. [94102]
137. Hierro, Jose L.; Villarreal, Diego; Eren, Ozkan; Graham, Jon M.; Callaway, Ragan M. 2006. Disturbance facilitates invasion: The effects are stronger abroad than at home. The American Naturalist. 168(2): 144-156. [94038]
138. Hill, Janice L.; Gray, Karen L. 2000. Conservation of Spalding's catchfly (Silene spaldingii) in the Lower Corral Creek study area, Garden Creek Ranch, Craig Mountain, Idaho. 1999 field season. The Nature Conservancy of Idaho. North Idaho Progarm. 19 p. [+Appendices]. [94215]
139. Hill, Janice L.; Gray, Karen L. 2004. Conservation strategy for Spalding's catchfly (Silene spaldingii Wats.). Boise, ID: Idaho Department of Fish and Game, Conservation Data Center (Producer). 149 p. Available: https://idfg.idaho.gov/ifwis/idnhp/cdc_pdf/u04hil01.pdf [2011, April 12]. [82420]
140. Hill, Janice L.; Gray, Karen L.; Fuchs, Sam J. 2000. Conservation of Spalding's catchfly (Silene spaldingii) in the Lower Corral Creek study area, Garden Creek Ranch, Craig Mountain, Idaho. 2000 field season. The Nature Conservancy of Idaho. North Idaho Progarm. 15 p. [+Appendices]. [94216]
141. Hitchcock, C. Leo; Cronquist, Arthur. 2018. Flora of the Pacific Northwest. 2nd ed. Seattle, WA: University of Washington Press. 882 p. [94186]
142. Holmes, Katherine A.; Veblen, Kari E.; Young, Truman P.; Berry, Alison M. 2008. California oaks and fire: A review and case study. In: Merenlender, Adina; McCreary, Douglas; Purcell, Kathryn L., tech. eds. Proceedings of the 6th symposium on oak woodlands: Today's challenges, tomorrow's opportunities; 2006 October 9-12; Rohnert Park, CA. Gen. Tech. Rep. PSW-GTR-217. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: 551-565. [80982]
143. Honda, Gisho; Yesilada, Erdem; Tabata, Mamoru; Sezik, Ekrem; Fujiat, Tetsuro; Takeda, Yoshio; Takaishi, Yoshihisa; Tanaka, Toshihiro. 1996. Traditional medicine in Turkey VI. Folk medicine in West Anatolia: Afyon, Kütahya, Denizli, Muğla, Aydin provinces. Journal of Ethnopharmacology. 53(2): 75-87. [94000]
144. Hooper, David U.; Dukes, Jeffrey S. 2010. Functional composition controls invasion success in a California serpentine grassland. Journal of Ecology. 98(4): 764-777. [81197]
145. Hovde, Elayne M. 2006. Grazing by cattle and sheep affect yellow starthistle on Idaho rangelands. Moscow, ID: University of Idaho. 69 p. Thesis. [94305]
146. Hujik, Peter. 2002. [Personal communication]. January 25. Regarding yellow starthistle and medusahead burns. Los Molinos, CA: The Nature Conservancy, Dye Creek Preserve. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [40501]
147. Hulvey, Kristin B.; Aigner, Paul A. 2014. Using filter-based community assembly models to improve restoration outcomes. Journal of Applied Ecology. British Ecological Society. 51(4): 997-1005. [94104]
148. Hulvey, Kristin B.; Teller, Brittany J. 2018. Site conditions determine a key native plant's contribution to invasion resistance in grasslands. Ecology. 99(6): 1257-1264. [94105]
149. Hulvey, Kristin B.; Zavaleta, Erika S. 2012. Abundance declines of a native forb have nonlinear impacts on grassland invasion resistance. Ecology. 93(2): 378-388. [94103]
150. Huntsinger, Lynn; Bartolome, James W.; D'Antonio, Carla M. 2007. Grazing management on California's mediterranean grasslands. In: Stromberg, Mark; Corbin, Jeffrey.; D'Antonio, Carla, eds. California Grasslands: Ecology and Management. Berkeley, CA: University of California Press: 233-253. [94311]
151. Irimia, Romona E.; Lopes, Susana M. M.; Sotes, Gaston; Cavieres, Lohengrin A.; Eren, Ozkan; Lortie, Christopher J.; French, Kristine; Hierro, Jose L.; Rosche, Christoph; Callaway, Ragan M.; Pinho e Melo, Teresa M. V. D.; Montesinos, Daniel. 2019. Biogeographic differences in the allelopathy of leaf surface extracts of an invasive weed. Biological Invasions. 21(10): 3151-3168. [94006]
152. Jacobs, J.; Mangold, J.; Parkinson, H.; Graves, M. 2011. Plant Guide: Yellow Star-thistle--Centaurea solstitialis L. [Online]. Bozeman, MT: U.S. Department of Agriculture, Natural Resources Conservation Service, Montana State Office (Producer). Available: http://plants.usda.gov/plantguide/pdf/pg_ceso3.pdf [2015, August 7]. [89177]
153. Janes, Benjamin Robert. 2012. Interactive effects of plant-plant competition and an introduced biocontrol agent on yellow starthistle (Centaurea solstitialis) seed production. Davis, CA: University of California, Davis. 39 p. Thesis. [94066]
154. Jogesh, Tania; Carpenter, David; Cappuccino, Naomi. 2008. Herbivory on invasive exotic plants and their non-invasive relatives. Biological Invasions. 10(6): 797-804. [72436]
155. Johnson, Charles Grier, Jr.; Swanson, David K. 2005. Bunchgrass plant communities of the Blue and Ochoco Mountains: A guide for managers. Gen. Tech. Rep. PNW-GTR-641. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 119 p. [63805]
156. Johnson, Douglas E. 1999. Surveying, mapping, and monitoring noxious weeds on rangelands. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 19-36. [35707]
157. Johnson, Mara; Rew, Lisa J.; Maxwell, Bruce D.; Sutherland, Steve. 2006. The role of wildfire in the establishment and range expansion of nonnative plant species into natural areas: A review of current literature. Bozeman, MT: Montana State University Center for Invasive Plant Management. 80 p. Available: https://www.fs.usda.gov/treesearch/pubs/27808 [2021, February 3]. [68196]
158. Joley, D. B.; Maddox, D. M.; Mackey, B. E.; Schoenig, S. E.; Casanave, K. A. 1997. Effect of light and temperature on germination of dimorphic achenes of Centaurea solstitialis in California. Canadian Journal of Botany. 75(12): 2131-2139. [28551]
159. Joley, D. B.; Maddox, D. M.; Schoenig, S. E.; Mackey, B. E. 2003. Parameters affecting germinability and seed bank dynamics in dimorphic achenes of Centaurea solstitialis in California. Canadian Journal of Botany. 81(10): 993-1007. [47085]
160. Joley, Donald B.; Maddox, Donald M.; Supkoff, David M.; Mayfield, Aubrey. 1992. Dynamics of yellow starthistle (Centaurea solstitialis) achenes in field and laboratory. Weed Science. 40(2): 190-194. [19446]
161. Kan, Tamara. 1998. The Nature Conservancy's approach to weed control. Fremontia. 26(4): 44-48. [47115]
162. Kartesz, J. T. The Biota of North America Program (BONAP). 2015. Taxonomic Data Center, [Online]. Chapel Hill, NC: The Biota of North America Program (Producer). Available: http://bonap.net/tdc [Maps generated from Kartesz, J. T. 2010. Floristic synthesis of North America, Version 1.0. Biota of North America Program (BONAP). [in press]. [84789]
163. Keeley, Jon E. 1990. The California valley grassland. In: Schoenherr, Allan A., ed. Endangered plant communities of southern California: Proceedings of the 15th annual symposium; 1989 October 28; Fullerton, CA. Special Publication No. 3. Claremont, CA: Southern California Botanists: 2-23. [21317]
164. Koc, Suheda; Isgor, Belgin S.; Isgor, Yasemin G.; Moghaddam, Naznoosh Shomali; Yildirim, Ozlem. 2015. The potential medicinal value of plants from Asteraceae family with antioxidant defense enzymes as biological targets. Pharmaceutical Biology. 53(5): 746-751. [93995]
165. Kuchler, A. W. 1964. United States: Map, [Potential natural vegetation of the conterminous United States]. Special Publication No. 36. New York: American Geographical Society. 1:3,168,000; colored. [3455]
166. Kyser, Guy B.; DiTomaso, Joseph M. 2002. Instability in a grassland community after the control of yellow starthistle (Centaurea solstitialis) with prescribed burning. Weed Science. 50(5): 648-657. [43620]
167. Kyser, Guy B.; Hazebrook, Arthur; DiTomaso, Joseph M. 2013. Integration of prescribed burning, aminopyralid, and reseeding for restoration of yellow starthistle (Centaurea solstitalis)-infested rangeland. Invasive Plant Science and Management. 6(4): 480-491. [87805]
168. Kyser, Guy B.; Peterson, Vanelle; Orloff, Steve B.; Wright, Steven D.; DiTomaso, Joseph M. 2011. Control of yellow starthistle (Centaurea solstitialis) and coast fiddleneck (Amsinckia menziesii) with Aminopyralid. Invasive Plant Science and Management. 4(3): 341-348. [94067]
169. Lake, Frank K. 2007. Traditional ecological knowledge to develop and maintain fire regimes in northwestern California, Klamath-Siskiyou bioregion: Management and restoration of culturally significant habitats. Corvallis, OR: Oregon State University. 732 p. Dissertation. [88360]
170. Larson, L. L.; McInnis, M. L. 1989. Impact of grass seedings on establishment and density of diffuse knapweed and yellow starthistle. Northwest Science. 63(4): 162-166. [9278]
171. Larson, Larry L.; McInnis, Michael L. 1989. Response of yellow starthistle (Centaurea solstitialis) and grass biomass to grass, picloram, and fertilizer combinations. Weed Technology. 3(3): 497-500. [9345]
172. Larson, Larry L.; Sheley, Roger L. 1994. Ecological relationships between yellow starthistle and cheatgrass. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 92-94. [24260]
173. Larson, Larry; Kiemnec, Gary. 1997. Differential germination by dimorphic achenes of yellow starthistle (Centaurea solstitialis L.) under water stress. Journal of Arid Environments. 37(1): 107-114. [40397]
174. Lass, Larry; Prather, Tim. 2007. A scientific evaluation for noxious and invasive weeds of the Highway 95 construction project between the Uniontown Cutoff and Moscow. Vegetation technical reports, final Environmental Impact Statement, US-95 Thorncreek Road to Moscow. Project No. DHP-NH-4110(156); Key No 09294. FHWA-ID-EIS-12-01-F. Missoula, MT: AquilaVision Inc. 78 p. [90997]
175. Lentile, Leigh B.; Morgan, Penelope; Hudak, Andrew T.; Bobbitt, Michael J.; Lewis, Sarah A.; Smith, Alistair M. S.; Robichaud, Peter R. 2007. Post-fire burn severity and vegetation response following eight large wildfires across the western United States. Fire Ecology. 3(1): 91-108. [70228]
176. Leong, Misha; Kremen, Claire; Roderick, George K. 2014. Pollinator interactions with yellow starthistle (Centaurea solstitialis) across urban, agricultural, and natural landscapes. PLoS ONE. 9(1): e86357. [93980]
177. Lesica, Peter. 2012. Manual of Montana vascular plants. Fort Worth, TX: Brit Press. 771 p. [92949]
178. Lieffers, V. J.; Landhausser, S. M.; Hogg, E. H. 2001. Is the wide distribution of aspen a result of its stress tolerance? In: Shepperd, Wayne D.; Binkley, Dan; Bartos, Dale L.; Stohlgren, Thomas J.; Eskew, Lane G., comps. Sustaining aspen in western landscapes: Symposium Proceedings; 2000 June 13-15; Grand Junction, CO. Proceedings RMRS-P-18. Fort Collins, CO: U.S. Dept. of Agriculture, Forest Service, Rocky Mountain Research Station: 311-323. [40685]
179. Lindbloom, Andrew J.; Reese, Kerry P.; Zager, Peter. 2003. Nesting and brood-rearing characteristics of chukars in West Central Idaho. Western North American Naturalist. 63(4): 429-439. [56115]
180. Lortie, Christopher J.; Munshaw, Michael; DiTomaso, Joseph; Hierro, Jose L. 2010. The small-scale spatiotemporal pattern of the seedbank and vegetation of a highly invasive weed, Centaurea solstitialis: Strength in numbers. Oikos. 119(3): 428-436. [81081]
181. Lortie, Christopher J.; Munshaw, Michael; Zikovitz, Andrea; Hierro, Jose. 2009. Cage matching: Head to head competition experiments of an invasive plant species from different regions as a means to test for differentiation. PLoS ONE. 4(3): e4823. [94029]
182. Lu-Irving, Patricia; Harencar, Julia G.; Sounart, Hailey; Welles, Shana R.; Swope, Sarah M.; Baltrus, Davis A.; Dlugosch, Katrina M. 2019. Native and invading yellow starthistle (Centaurea solstitialis) microbiomes differ in composition and diversity of bacteria. mSphere. 4(2): e00088-19. [94069]
183. Mack, Richard N.; Simberloff, Daniel; Lonsdale, W. Mark; Evans, Harry; Clout, Michael; Bazzaz, Fakhri A. 2000. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecological Applications. 10(3): 689-710. [48324]
184. Maddox, D. M. 1981. Introduction, phenology, and density of yellow starthistle in coastal, intercoastal, and central valley situations in California. Agricultural Research Results ARR-W-20. Oakland, CA: U.S. Department of Agriculture, Agricultural Research Service, Western Region. 24 p. [40411]
185. Maddox, D. M.; Joley, D. B.; Supkoff, D. M.; Mayfield, A. 1996. Pollination biology of yellow starthistle (Centaurea solstitialis) in California. Canadian Journal of Botany. 74(2): 262-267. [26714]
186. Maddox, Donald M.; Mayfield, Aubrey; Poritz, Noah H. 1985. Distribution of yellow starthistle (Centaurea solstitialis) and Russian knapweed (Centaurea repens). Weed Science. 33(3): 315-327. [23467]
187. Marler, Marilyn. 2000. A survey of exotic plants in federal wilderness areas. In: Cole, David N.; McCool, Stephen F.; Borrie, William T.; O'Loughlin, Jennifer, comps. Wilderness science in a time of change conference--Volume 5: Wilderness ecosystems, threats, and management; 1999 May 23-27; Missoula, MT. Proceedings RMRS-P-15-VOL-5. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 318-327. [40580]
188. Martin, Eveline L.; Martin, Ian D. 1999. Prescribed burning and competitive reseeding as tools for reducing yellow starthistle (Centaurea solstitialis) at Pinnacles National Monument. In: Kelly, Mike; Howe, Melanie; Neill, Bill, eds. Proceedings, California Exotic Pest Plant Council; 1999 October 15-17; Sacramento, CA. Volume 5. [Davis, CA]: California Exotic Pest Plant Council: 51-56. [40413]
189. Matzek, Virginia; Hill, Shannon. 2012. Response of biomass and seedbanks of rangeland functional groups to mechanical control of yellow starthistle. Rangeland Ecology & Management. 65(1): 96-100. [94020]
190. McClain, Charles D.; Holl, Karen D.; Wood, David M. 2011. Successional models as guides for restoration of riparian forest understory. Restoration Ecology. 19(2): 280-289. [85672]
191. McIver, James; Thorp, Robbin; Erickson, Karen. 2009. Pollinators of the invasive plant, yellow starthistle (Centaurea solstitialis), in north-eastern Oregon, USA. Weed Biology and Management. 9(2): 137-145. [93978]
192. Menke, Carolyn A.; Muir, Patricia S. 2004. Patterns and influences of exotic species invasion into the grassland habitat of the threatened plant Silene spaldingii. Natural Areas Journal. 24(2): 119-128. [60026]
193. Menke, Carolyn A.; Muir, Patricia S. 2004. Short-term influence of wildfire on canyon grassland plant communities and Spalding's catchfly, a threatened plant. Northwest Science. 78(3): 192-203. [60415]
194. Miguel, M. Florencia; Lortie, Christopher J.; Callaway, Ragan M.; Hierro, Jose L. 2017. Competition does not come at the expense of colonization in seed morphs with increased size and dispersal. American Journal of Botany. 104(9): 1323-1333. [92319]
195. Miller, Timothy W.; Shinn, Sandra L.; Thill, Donald C. 2001. Cross-resistance in and chemical control of Auxinic herbicide-resistant yellow starthistle (Centaurea solstitialis). Weed Technology. 15(2): 293-299. [94071]
196. Minnich, Ralph. 2006. Planning and implementing prescribed burns. In: Di Tomaso, J. M.; Johnson, D. W., eds. The use of fire as a tool for controlling invasive plants. Cal-IPC Publication 2006-01. Berkeley, CA: California Invasive Plant Council: 3-6. [90713]
197. Mohlenbrock, Robert H. 1986. Guide to the vascular flora of Illinois. [Revised edition]. Carbondale, IL: Southern Illinois University Press. 507 p. [17383]
198. Monsen, Stephen B.; McArthur, E. Durant. 1995. Implications of early Intermountain range and watershed restoration practices. In: Roundy, Bruce A.; McArthur, E. Durant; Haley, Jennifer S.; Mann, David K., compilers. Proceedings: Wildland shrub and arid land restoration symposium; 1993 October 19-21; Las Vegas, NV. Gen. Tech. Rep. INT-GTR-315. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 16-25. [24819]
199. Montesinos, Daniel; Callaway, Ragan M. 2018. Traits correlate with invasive success more than plasticity: A comparison of three Centaurea congeners. Ecology and Evolution. 8(15): 7378-7385. [94031]
200. Montesinos, Daniel; Graebner, Ryan C.; Callaway, Ragan M. 2019. Evidence for evolution of increased competitive ability for invasive Centaurea solstitialis, but not for naturalized C. calcitrapa. Biological Invasions. 21(1): 99-110. [94107]
201. Montesinos, Daniel; Santiago, Gilberto; Callaway, Ragan M. 2012. Neo-allopatry and rapid reproductive isolation. The American Naturalist. 180(4): 529-533. [94022]
202. Moret, Sabrina; Populin, Tizina; Lanfranco, Conte S.; Cosens, Gladys. 2005. HPLC determination of free nitrogenous compounds of Centaurea solstitialis (Asteraceae), the cause of equine nigropallidal encephalomalacia. Toxicon. 46(6): 651-657. [93977]
203. Mullin, Barbara. 1992. Meeting the invasion: Integrated weed management. Western Wildlands. 18(2): 33-38. [19462]
204. Munshaw, Michael G.; Lortie, Christopher J. 2010. Back to the basics: Using density series to test regulation versus limitation for invasive plants. Plant Ecology. 211(1): 1-5. [94154]
205. Muth, Norris Z.; Pigliucci, Massimo. 2006. Traits of invasives reconsidered: Phenotypic comparisons of introduced invasive and introduced noninvasive plant species within two closely related clades. American Journal of Botany. 93(2): 188-196. [61906]
206. Muth, Norris Z.; Pigliucci, Massimo. 2007. Implementation of a novel framework for assessing species plasticity in biological invasions: Responses of Centaurea and Crepis to phosphorus and water availability. Journal of Ecology. 95(5): 1001-1013. [68198]
207. NatureServe. 2019. NatureServe Explorer: An online encyclopedia of life, [Online]. Version 7.1. Arlington, VA: NatureServe (Producer). Available: http://explorer.natureserve.org/. [69873]
208. Nolan, Daryl G.; Upadhyaya, Mahesh K. 1988. Primary seed dormancy in diffuse and spotted knapweed. Canadian Journal of Plant Science. 68(3): 775-783. [5593]
209. Northam, F. E.; Callihan, R. H. 1988. Yellow starthistle presence in 29 month-old stands of eight grasses. Western Society of Weed Science. Research Progress Report: 60-63. [40393]
210. Northam, F. E.; Callihan, R. H. 1990. Evaluation of soil conservation plant materials for herbicide tolerance and revegetating semi-arid land infested with yellow starthistle. Western Society of Weed Science. Research Progress Report: 75-78. [40392]
211. Northam, F. E.; Callihan, R. H. 1990. Grass adaptation to semi-arid, yellow starthistle infested canyonland. Western Society of Weed Science--Research Progress Report: 79-82. [24471]
212. O'Brien, Jon M.; Kyser, Guy B.; Woods, Dale M.; DiTomaso, Joseph M. 2010. Effects of the rust Puccinia jaceae var. solstitialis on Centaurea solstitialis (yellow starthistle) growth and competition. Biological Control. 52(2): 174-181. [94073]
213. O'Brien, Jon M.; Kyser, Guy B.; Woods, Dale M.; DiTomaso, Joseph M. 2010. The effect of Puccinia jaceae var. solstitialis on the yellow starthistle biological control insects Eustenopus villosus and Chaetorellia succinea. Biological Control. 52(2): 182-187. [94072]
214. Odion, Dennis C.; Alexander, Janice; Swezy, Michael. 2004. Use of short rotation burning to combat non-natives and their seed banks in California north coastal prairie. In: Morales, Maria E.; Morales, Tony J. Proceedings of the Symposium: Fire management: Emerging policies and new paradigms. 1999 Nov 16-19; San Diego, CA. Eugene, OR: The Association for Fire Ecology: 46-57. [93967]
215. Olson, Thomas E.; Gray, M. Violet. 1989. Characteristics of least Bell's vireo nest sites along the Santa Ynez River. In: Abell, Dana L., technical coordinator. Proceedings of the California riparian systems conference: Protection, management, and restoration for the 1990's; 1988 September 22-24; Davis, CA. Gen. Tech. Rep. PSW-110. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 278-284. [14447]
216. Ornelas, Sara. 2009. Habitat selection and activities of non-native Rio Grande turkeys in an open space preserve. San Jose, CA: San Jose State University. 57 p. Thesis. [93976]
217. Oster, Marina; Beck, John J.; Furrow, Robert E.; Yeung, Kara; Field, Christopher B. 2015. In-field yellow starthistle (Centaurea solstitialis) volatile composition under elevated temperature and CO2 and implications for future control. Chemoecology. 25(6): 313-323. [94014]
218. Otfinowski, R.; Kenkel, N. C.; Dixon, P.; Wilmshurst, J. F. 2007. Integrating climate and trait models to predict the invasiveness of exotic plants in Canada's Riding Mountain National Park. Canadian Journal of Plant Science. 87(5): 1001-1012. [70500]
219. Ozcelik, Berrin; Gurbuz, Ilhan; Karaoglu, Taner; Yesilada, Erdem. 2009. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiological Research. 164(5): 545-552. [93997]
220. Pankey, Joel Robert. 2009. Centaurea in the Columbia Basin ecoregion: Disturbance, invasion, and competition. Pullman, WA: Washington State University. 148 p. Dissertation. [93939]
221. Panter, Kip E. 1991. Neurotoxicity of the knapweeds (Centaurea spp.) in horses. In: James, Lynn F.; Evans, John O., eds. Noxious range weeds. Westview Special Studies in Agriculture Science and Policy. Boulder, CO: Westview Press: 316-324. [23468]
222. Parks, Catherine G.; Radosevich, Steven R.; Endress, Bryan A.; Naylor, Bridgett J.; Anzinger, Dawn; Rew, Lisa J.; Maxwell, Bruce D.; Dwire, Kathleen A. 2005. Natural and land-use history of the Northwest mountain ecoregions (USA) in relation to patterns of plant invasions. Perspectives in Plant Ecology, Evolution and Systematics. 7(3): 137-158. [70353]
223. Perkins, Lora B.; Hatfield, Gary; Espeland, Erin K. 2016. Invasive grasses consistently create similar plant-soil feedback types in soils collected from geographically distant locations. Journal of Plant Ecology. 9(2): 180-186. [94155]
224. Petanidou, Theodora; Godfree, Robert C.; Song, Daniel S.; Kantsa, Aphrodite; Dupont, Yoko L.; Waser, Nickolas M. 2012. Self-compatibility and plant invasiveness: Comparing species in native and invasive ranges. Perspectives in Plant Ecology, Evolution and Systematics. 14(1): 3-12. [94015]
225. Peterson, Eric B. 2008. International vegetation classification alliances and associations occurring in Nevada with proposed additions. Carson City, NV: Nevada Natural Heritage Program. 347 p. [77864]
226. Piper, Gary L. 2001. The biological control of yellow starthistle in the western U.S.: Four decades of progress. In: Smith, Lincoln, ed. Proceedings, 1st international knapweed symposium of the 21st century; 2001 March 15-16; Coeur d'Alene, ID. Albany, CA: U.S. Department of Agriculture, Agricultural Research Service: 48-55. [37831]
227. Pitcairn, M. J.; Joley, D. B.; Woods, D. M. 1997. Impact of plant density on yellow starthistle seedhead production. In: Woods, Dale M., ed. Biological Control Program Annual Summary, 1996. Sacramento, CA: California Department of Food and Agriculture, Division of Plant Industry: 59-61. [94236]
228. Pitcairn, M. J.; Piper, G. L.; Coombs, E. M. 2004. Yellow starthistle. In: Coombs, Eric M.; Clark, Janet K.; Piper, Gary L.; Cofrancesco, Alfred F., Jr., eds. Biological control of invasive plants in the United States. Corvallis, OR: Oregon State University Press: 421-423. [53090]
229. Pitcairn, Michael J.; DiTomaso, Joseph M. 2000. Rangeland and uncultivated areas: Integrating biological control agents and herbicides for starthistle control. In: California Conference on Biological Control II; 2000 July 11-12; Riverside, CA. [Riverside, CA]: Center for Biological Control, College of Natural Resources, University of California: 65-72. [94077]
230. Pitcairn, Michael J.; Schoenig, Steve; Yacoub, Rosei; Gendron, John. 2006. Yellow starthistle continues its spread in California. California Agriculture. 60(2): 83-90. [94231]
231. Pitcairn, Michael J.; Smith, Lincoln; Moran, Patrick. 2014. Weed biological control agents approved for California. Cal-IPC News. 22(1): 6-7. [94292]
232. Pitcairn, Michael J.; Woods, Dale M.; Joley, Donald B.; Turner, Charles E.; Balciunas, Joseph K. 2000. Population buildup and combined impact of introduced insects on yellow starthistle (Centaurea solstitialis L.) in California. In: Spencer, N. R., ed. Proceedings of the X international symposium on biological control of weeds; 1999 July 4-14; Montana State University, Bozeman, MT. Bozeman, MT: Montana State University: 747-751. [94076]
233. Prather, T. S.; Callihan, R. H. 1989. Yellow starthistle trends in perennial and annual communities. Western Society of Weed Science. Research Progress Report: 130. [40389]
234. Prather, T. S.; Callihan, R. H. 1991. Interference between yellow starthistle and pubescent wheatgrass during grass establishment. Journal of Range Management. 44(5): 443-447. [16285]
235. Prather, T. S.; Callihan, R. H.; Thill, D. C. 1988. Revegetating yellow starthistle infested land with intermediate wheatgrass. Western Society of Weed Science. Research Progress Report: 68-69. [40391]
236. Prather, Timothy S.; Wilson, Linda. 2001. Ability of annual and perennial grass communities to withstand invasion by yellow starthistle. In: Smith, Lincoln, ed. Proceedings, 1st international knapweed symposium of the 21st century; 2001 March 15-16; Coeur d'Alene, ID. Albany, CA: U.S. Department of Agriculture, Agricultural Research Service: 89-90. Abstract. [37865]
237. Pyke, David A. 2000. Invasive exotic plants in sagebrush ecosystems of the Intermountain West. In: Entwistle, P. G.; DeBolt, A. M.; Kaltenecker, J. H.; Steenhof, K., compilers. Sagebrush steppe ecosystems symposium: Proceedings; 1999 June 21-23; Boise, ID. Publ. No. BLM/ID/PT-001001+1150. Boise, ID: U.S. Department of the Interior, Bureau of Land Management, Boise State Office: 43-54. [42719]
238. Qin, Bo; Lau, Jennifer A.; Kopshever, Joseph; Callaway, Ragan M.; McGray, Heather; Perry, Laura G.; Weir, Tiffany L.; Paschke, Mark W.; Hierro, Jose L.; Yoder, John; Vivanco, Jorge M.; Strauss, Sharon. 2007. No evidence for root-mediated allelopathy in Centaurea solstitialis, a species in a commonly allelopathic genus. Biological Invasions. 9(8): 897-907. [71234]
239. Radford, Albert E.; Ahles, Harry E.; Bell, C. Ritchie. 1968. Manual of the vascular flora of the Carolinas. Chapel Hill, NC: The University of North Carolina Press. 1183 p. [7606]
240. Randall, Carol Bell; Winston, Rachel L.; Jette, Cynthia A.; Pitcairn, Michael J.; DiTomaso, Joseph M. 2017. Biology and biological control of yellow starthistle. 4th edition. Morgantown, WV: USDA Forest Service, Forest Health Technology Enterprise Team. FHTET-2016-08. 110. [94294]
241. Randall, John M. 1995. Weeds and natural areas management. In: Brenton, Robert; Sherlock, Joe, tech. coords. Proceedings: 16th annual forest vegetation management conference; 1995 January 10-12; Sacramento, CA. Redding, CA: Forest Vegetation Management Conference: 23-28. [27750]
242. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford, England: Clarendon Press. 632 p. [2843]
243. Rees, N. E.; Quimby, P. C., Jr.; Mullin, B. H. 1996. Section I. Biological control of weeds. In: Rees, Norman E.; Quimby, Paul C., Jr.; Piper, Gary L.; [and others], eds. Biological control of weeds in the West. Bozeman, MT: Western Society of Weed Science. In cooperation with: U.S. Department of Agriculture, Agricultural Research Service; Montana Department of Agriculture; Montana State University: 3-24. [38273]
244. Reever Morghan, Kimberly J.; Leger, Elizabeth A.; Rice, Kevin J. 2003. Clopyralid effects on yellow starthistle (Centaurea solstitialis) and nontarget species. Weed Science. 51(4): 596-600. [94078]
245. Reever Morghan, Kimberly J.; Rice, Kevin J. 2005. Centaurea solstitialis invasion success is influenced by Nassella pulchra size. Restoration Ecology. 13(3): 524-528. [94152]
246. Reever Morghan, Kimberly J.; Rice, Kevin J. 2006. Variation in resource availability changes the impact of invasive thistles on native bunchgrasses. Ecological Applications. 16(2): 528-539. [62278]
247. Riba, Miquel; Rodrigo, Anselm; Colas, Bruno; Retana, Javier. 2002. Fire and species range in Mediterranean landscapes: An experimental comparison of seed and seedling performance among Centaurea taxa. Journal of Biogeography. 29(1): 135-146. [46082]
248. Rice, Barry Meyers; Randall, John, compilers. 2001. Weed report: Centaurea solstitialis: Yellow starthistle. In: Wildland weeds management and research: 1998-99 weed survey. Davis, CA: The Nature Conservancy, Wildland Invasive Species Program. 21 p. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [40829]
249. Rice, Peter M.; McPherson, Guy R.; Rew, Lisa J. 2008. Fire and nonnative invasive plants in the Interior West bioregion. In: Zouhar, Kristin; Smith, Jane Kapler; Sutherland, Steve; Brooks, Matthew L., eds. Wildland fire in ecosystems: Fire and nonnative invasive plants. Gen. Tech. Rep. RMRS-GTR-42-vol. 6. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 141-173. [70332]
250. Rice, Peter; Flynn, Sarah Wilhelm. 2000. Integration of herbicides and prescribed burning for plant community restoration--Pesticide Impact Assessment Program (FS-PIAP). Unpublished study plan on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 11 p. [38261]
251. Rieder, Julie Patrice. 2005. Yellow starthistle in Utah: An investigation of Centaurea solstitialis invasion patterns, processes, and population dynamics. Logan, UT: Utah State University. 207 p. Dissertation. [94151]
252. Rissman, Adena R.; Reed, Sarah, E.; Hughes, Chuck; Reiner, Richard. 2008. Monitoring understory composition of blue oak woodlands on conservation easements. In: Merenlender, Adina; McCreary, Douglas; Purcell, Kathryn L., tech. eds. Proceedings of the 6th symposium on oak woodlands: Today's challenges, tomorrow's opportunities; 2006 October 9-12; Rohnert Park, CA. Gen. Tech. Rep. PSW-GTR-217. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: 589-602. [80984]
253. Robertson, Alexis Vertolli. 2012. Plant community responses to cattle grazing in a noxious weed-dominated rangeland. Davis, CA: University of California Davis. 22 p. Thesis. [93697]
254. Robins, Sandra S. 2001. Effects of perennial plant competition on the invasibility of canyon grassland communities by Centaurea solstitialis. Moscow, ID: University of Idaho. 54 p. Thesis. [66570]
255. Roche, Ben F., Jr. 1989. Ecological notes on yellow starthistle. In: Fay, Peter K.; Lacey, John R., eds. Proceedings: Knapweed symposium; 1989 April 4-5; Bozeman, MT. Bozeman, MT: Montana State University: 77-78. [37799]
256. Roche, Ben F., Jr. 1992. Achene dispersal in yellow starthistle (Centaurea solstitialis L.). Northwest Science. 66(2): 62-65. [18414]
257. Roche, Ben F., Jr.; Piper, Gary L.; Talbott, Cindy Jo. 1986. Knapweeds of Washington. Pullman, WA: Washington State University, Cooperative Extension, College of Agriculture and Home Economics. 41 p. [2015]
258. Roche, Ben F., Jr.; Roche, Cindy T.; Chapman, Roger C. 1994. Impacts of grassland habitat on yellow starthistle (Centaurea solstitialis L.) invasion. Northwest Science. 68(2): 86-96. [23144]
259. Roche, Ben F., Jr.; Talbott, Cindy Jo. 1986. The collection history of Centaureas found in Washington state. Research Bulletin XB 0978. Pullman, WA: Washington State University, College of Agriculture and Home Economics, Agriculture Research Center. 36 p. [2016]
260. Roche, Ben Francis, Jr. 1965. Ecologic studies of yellow star-thistle (Centaurea solstitialis L.). Moscow, ID: University of Idaho. 78 p. Dissertation. [4033]
261. Roche, C.; White, G. R. 2000. Managing yellow starthistle in southwestern Oregon. EM 8750. Corvallis, OR: Oregon State University. 8 p. [40378]
262. Roche, Cindy Talbot; Thill, Donald C. 2001. Biology of common crupina and yellow starthistle, two Mediterranean winter annual invaders in Western North America. Weed Science. 49(4): 439-447. [94019]
263. Roche, Cindy Talbott. 1990. Knapweed: Major populations in Washington. In: Roche, Ben F.; Roche, Cindy Talbott, eds. Range weeds revisited: Proceedings of a symposium: A 1989 Pacific Northwest range management short course; 1989 January 24-26; Spokane, WA. Pullman, WA: Washington State University, Department of Natural Resource Sciences, Cooperative Extension: 23-28. [14829]
264. Roche, Cindy Talbott; Roche, Ben F. Jr. 1988. Distribution and amount of four knapweed (Centaurea L.) species found in Eastern Washington. Northwest Science. 62(5): 242-253. [6439]
265. Roche, Cindy Talbott; Roche, Ben F., Jr. 1991. Meadow knapweed invasion in the Pacific Northwest, U.S.A., and British Columbia, Canada. Northwest Science. 65(1): 53-61. [14144]
266. Roche, Cindy Talbott; Thill, Donald C.; Shafii, Bahman. 1997. Reproductive phenology in yellow starthistle (Centaurea solstitialis). Weed Science. 45(6): 763-770. [40388]
267. Roche, Cindy. 2001. Reproductive phenology in yellow starthistle. In: Smith, Lincoln, ed. Proceedings, 1st international knapweed symposium of the 21st century; 2001 March 15-16; Coeur d'Alene, ID. Albany, CA: U.S. Department of Agriculture, Agricultural Research Service: 93. Abstract. [37870]
268. Roche, Cynthia Talbot; Harmon, Bradley L.; Wilson, Linda M.; McCaffrey, Joseph P. 2001. Eustenopus villosus (Coleoptera: Curculionidae) feeding of herbicide-resistant yellow starthistle (Centaurea solstitialis L.). Biological Control. 20(3): 279-286. [94079]
269. Rusmore, John T. 1995. Use of fire and cutting to control yellow starthistle (Preliminary results of a yellow starthistle control experiment). In: Lovich, Jeff; Randall, John; Kelly, Mike, eds. Proceedings, California Exotic Pest Plant Council: Symposium '95; 1995 October 6-8; Pacific Grove, CA. Berkeley, CA: California Exotic Pest Plant Council: 13-19. [44116]
270. Saatkamp, Arne; Affre, Laurence; Baumberger, Teddy; Dumas, Pierre-Jean; Gasmi, Aida; Gachet, Sophie; Arene, Fabien. 2011. Soil depth detection by seeds and diurnally fluctuating temperatures: Different dynamics in 10 annual plants. Plant Soil. 349(1-2): 331-340. [94028]
271. Sanders, Sean Gary. 2003. The isolation, identification and characterization of a toxin from Centaurea solstitialis that interacts with the dopamine transporter. Pullman, WA: Washington State University. 114 p. Dissertation. [94004]
272. Sawtooth National Forest. 2003. Sawtooth National Forest land and resource management plan, revised. Stanley, ID: U.S. Department of Agriculture, Forest Service, Sawtooth National Forest. 551 p. [89717]
273. Senatore, Felice; Formisano, Carmen; Raio, Aida; Bellone, Gabriella; Bruno, Maurizio. 2008. Volatile components from flower-heads of Centaurea nicaeensis All., C. parlatoris Helder and C. solstitialis L. ssp. schouwii (DC.) Dostál growing wild in southern Italy and their biological activity. Natural Product Research. 22(10): 825-832. [94003]
274. Seymour, Frank Conkling. 1982. The flora of New England. 2nd ed. Phytologia Memoirs 5. Plainfield, NJ: Harold N. Moldenke and Alma L. Moldenke. 611 p. [7604]
275. Shaffer, Kevin M. 2006. Fire and at-risk species. In: Sugihara, Neil G.; van Wagtendonk, Jan W.; Shaffer, Kevin E.; Fites-Kaufman, Joann; Thode, Andrea E., eds. Fire in California's ecosystems. Berkeley, CA: University of California Press: 520-537. [65570]
276. Shafii, Bahman; Price, William J.; Prather, Timothy S.; Lass, Lawrene W.; Thill, Donald C. 2003. Predicting the likelihood of yellow starthistle (Centaurea solstitialis) occurrence using landscape characteristics. Weed Science. 51(5): 748-751. [94025]
277. Sheley, R. L.; Callihan, R. H.; Huston, C. H.; Thill, D. C. 1983. Genotypic variation among yellow starthistle populations. Western Society of Weed Science. Research Progress Report: 53-54. [40387]
278. Sheley, Roger L. 2001. Ecological principles for managing knapweed. In: Smith, Lincoln, ed. Proceedings, 1st international knapweed symposium of the 21st century; 2001 March 15-16; Coeur d'Alene, ID. Albany, CA: U.S. Department of Agriculture, Agricultural Research Service: 62. Abstract. [37834]
279. Sheley, Roger L.; Larson, Larry L. 1994. Comparative growth and interference between cheatgrass and yellow starthistle seedlings. Journal of Range Management. 47(6): 470-474. [40385]
280. Sheley, Roger L.; Larson, Larry L. 1994. Observation: Comparative life-history of cheatgrass and yellow starthistle. Journal of Range Management. 47(6): 450-456. [40386]
281. Sheley, Roger L.; Larson, Larry L. 1995. Interference between cheatgrass and yellow starthistle at 3 soil depths. Journal of Range Management. 48(5): 392-397. [94237]
282. Sheley, Roger L.; Larson, Larry L. 1997. Cheatgrass and yellow starthistle growth at 3 soil depths. Journal of Range Management. 50(2): 146-150. [27429]
283. Sheley, Roger L.; Larson, Larry L.; Jacobs, James S. 1999. Yellow starthistle. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 408-416. [35760]
284. Sheley, Roger L.; Larson, Larry L.; Johnson, Douglas E. 1993. Germination and root dynamics of range weeds and forage species. Weed Technology. 7: 234-237. [24473]
285. Sheley, Roger; Manoukian, Mark; Marks, Gerald. 1999. Preventing noxious weed invasion. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 69-72. [35711]
286. Shepperd, Wayne C.; Rogers, Paul C.; Burton, David; Bartos, Dale L. 2006. Ecology, biodiversity, management, and restoration of aspen in the Sierra Nevada. RMRS-GTR-178. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 122 p. [65072]
287. Shiflet, Thomas N., ed. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p. [23362]
288. Shinn, Sandra L.; Thill, Donald C. 2002. The response of yellow starthistle (Centaurea solstitialis), annual grasses, and smooth brome (Bromus inermis) to imazapic and picloram. Weed Technology. 16(2): 366-370. [93701]
289. Shinn, Sandra L.; Thill, Donald C. 2003. The response of yellow starthistle (Centaurea solstitialis), spotted knapweed (Centaurea maculosa), and meadow hawkweed (Hieracium caespitosum) to imazapic. Weed Technology. 17(1): 94-101. [80616]
290. Society of American Foresters. 1954. Forest cover types of North America (exclusive of Mexico). Washington, D.C.: Society of American Foresters. 67 p. [2196]
291. Sokovic, Marina; Ciric, Ana; Glamoclija, Jasmina; Skaltsa, Helen. 2017. Biological activities of sesquiterpene lactones isolated from the genus Centaurea L. (Asteraceae). Current Pharmaceutical Design. 23(19): 2767-2786. [93990]
292. Sorrie, Bruce A. 2005. Alien vascular plants in Massachusetts. Rhodora. 107(931): 284-329. [76467]
293. Sotes, Gaston J.; Cavieres, Lohengrin A.; Montesinos, Daniel; Coutinho, Antonio Zavier Pereira; Pelaez, Walter Jose; Lopes, Susana M. M.; Pinho e Melo, Teresa M.V.D. 2015. Inter-regional variation on leaf surface defenses in native and non-native Centaurea solstitialis plants. Biochemical Systematics and Ecology. 62: 208-218. [93975]
294. Spencer, D. F.; Enloe, S. F.; Pitcairn, M. J.; DiTomaso, J. M. 2014. Impacts of mowing and bud destruction on Centaurea solstitialis growth, flowering, root dynamics and soil moisture. Weed Research. 54(2): 140-150. [94080]
295. Spencer, D. F.; Liow, P-S.; Pitcairn, M. J.; Villegas, B. 2014. Variation in seed characteristics and growth for thistles (Cardueae: Asteraceae) in California and Oregon. Madrono. 61(4): 339-349. [94024]
296. Spencer, David; Enloe, Stephen; Liow, Pul-Sze; Ksander, Greg; Carruthers, Raymond. 2011. Does superior competitive ability explain yellow starthistle's (Centaurea solstitialis) successful invasion of annual grasslands in California? Invasive Plant Science and Management. 4(3): 284-295. [94150]
297. Spotswood, Erica N.; Mariotte, Pierre; Farrer, Emily C.; Nichols, Liana; Suding, Katharine N. 2017. Separating sources of density-dependent and density-independent establishment limitation in invading species. Journal of Ecology. 105(2): 436-444. [91824]
298. Stannard, Mark. 1993. Overview of the basic biology, distribution and vegetative suppression of four knapweed species in Washington. Plant Materials Center Tech. Notes #25. Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 8 p. [38351]
299. Sterling, Tracy M.; Lownds, Norman K.; Murray, Leigh W. 2001. Picloram-resistant and -susceptible yellow starthistle accessions have similar competitive ability. Weed Science. 49(1): 42-47. [94081]
300. Stevens, K. L.; Merrill, G. B. 1985. Sequiterpene lactones and allelochemicals from Centaurea species. American Chemical Society Symposium Series. 286: 83-98. [39240]
301. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Missoula Fire Sciences Laboratory. 10 p. [20090]
302. Sun, Mei. 1997. Population genetic structure of yellow starthistle (Centaurea solstitialis), a colonizing weed in the Western United States. Canadian Journal of Botany. 75(9): 1470-1478. [28320]
303. Sun, Mei; Ritland, Kermit. 1998. Mating system of yellow starthistle (Centaurea solstitialis), a successful colonizer in North America. Heredity. 80(2): 225-232. [40384]
304. Swope, Sarah M. 2014. Biocontrol attack increases pollen limitation under some circumstances in the invasive plant Centaurea solstitialis. Oecologia. 174(1): 205-215. [94085]
305. Swope, Sarah M.; Parker, Ingrid M. 2010. Trait-mediated interactions and lifetime fitness of the invasive plant Centaurea solstitialis. Ecology. 91(8): 2284-2293. [94082]
306. Swope, Sarah M.; Parker, Ingrid M. 2010. Widespread seed limitation affects plant density but not population trajectory in the invasive plant Centaurea solstitialis. Oecologia. 164(1): 117-128. [94023]
307. Swope, Sarah M.; Parker, Ingrid M. 2012. Complex interactions among biocontrol agents, pollinators, and an invasive weed: A structural equation modeling approach. Ecological Applications. 22(8): 2122-2134. [94084]
308. Swope, Sarah M.; Satterthwaite, William H. 2012. Variable effects of a generalist parasitoid on a biocontrol seed predator and its target weed. Ecological Applications. 22(1): 20-34. [85304]
309. Swope, Sarah M.; Satterthwaite, William H.; Parker, Ingrid M. 2017. Spatiotemporal variation in the strength of density dependence: Implications for biocontrol of Centaurea solstitialis. Biological Invasions. 19(9): 2675-2691. [94048]
310. Swope, Sarah M.; Stein, Ilana R. 2012. Soil type mediates indirect interactions between Centaurea solstitialis and its biocontrol agents. Biological Invasions. 14(8): 1697-1710. [94083]
311. Talbott, C. J. 1987. Distribution and ecologic amplitude of selected Centaurea species in eastern Washington. Pullman, WA: Washington State University. 186 p. Thesis. [38546]
312. Tekeli, Yener; Sezgin, Mehmet; Aktumsek, Abdurrahman. 2008. Antioxidant property of Centaurea solstitialis L. from Konya, Turkey. Asian Journal of Chemistry. 20(6): 4831-4835. [93994]
313. Tekeli, Yener; Zengin, Gokhan; Aktumsek, Abdurrahman; Sezgin, Mehmet; Torlak, Emrah. 2011. Antibacterial activities of extracts from twelve Centaurea species from Turkey. Archives of Biological Sciences. 63(3): 685-690. [93993]
314. Thill, Donald C.; Roche, Cindy T.; Zamora, David L. 1999. Common crupina. In: Sheley, Roger L.; Petroff, Janet K., eds. Biology and management of noxious rangeland weeds. Corvallis, OR: Oregon State University Press: 189-201. [35723]
315. Thomsen, Craig D.; Vayssieres, Marc P.; Williams, William A. 1997. Mowing and subclover plantings suppress yellow starthistle. California Agriculture. 51(6): 15-20. [40381]
316. Thomsen, Craig D.; Williams, William A.; George, Melvin R.; McHenry, W. B.; Bell, Fremont L.; Knight, Ronald S. 1989. Managing yellow starthistle on rangeland. California Agriculture. 43(5): 4-7. [11180]
317. Thomsen, Craig D.; Williams, William A.; Olkowski, William; Pratt, Dave W. 1996. Grazing, mowing and clover plantings control yellow starthistle. The IPM Practitioner: Monitoring the field of pest management. 18(2): 1-4. [40382]
318. Thomsen, Craig D.; Williams, William A.; Vayssieres, Marc; Bell, Fremont L.; George, Melvin R. 1993. Controlled grazing on annual grassland decreases yellow starthistle. California Agriculture. 47(6): 36-40. [40383]
319. Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. 2001. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press. 440 p. [36690]
320. Tu, Mandy; Hurd, Callie; Randall, John M., eds. 2001. Weed control methods handbook: Tools and techniques for use in natural areas. Davis, CA: The Nature Conservancy. 194 p. [37787]
321. Turner, Charles E. 1991. Biological control of yellow starthistle, Centaurea solstitialis L.: A progress report. In: Proceedings, 43rd California weed conference; [Date unknown]; Fremont, CA. [Place of publication unknown]: [Publisher unknown]: 78-82. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [24476]
322. Tyser, Robin W.; Worley, Christopher A. 1992. Alien flora in grasslands adjacent to road and trail corridors in Glacier National Park, Montana (U.S.A.). Conservation Biology. 6(2): 253-262. [19435]
323. Uner, Birol; Dorak, Sinem; Ismailoglu, Yunus; Tufan, M. Zakir; Kurul, Fatih; Yalcin, Omer Umit. 2016. Centaurea solstitialis and Silybum marianum weeds conversion into value ‑ added thermoplastic materials by benzylation process. Iranian Polymer Journal. 25(1): 37-43. [93991]
324. USDA, Animal and Plant Health Inspection Service. 2010. Federal noxious weed list, [Online]. In: Plant health--Noxious weeds program. Washington, DC: U.S. Department of Agriculture, Animal and Plant Health Inspection Service (Producer). Available: https://www.aphis.usda.gov/plant_health/plant_pest_info/weeds/downloads/weedlist.pdf [2018, January 31]. [36689]
325. USDA, NRCS. 2021. The PLANTS Database, [Online]. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team (Producer). Available: https://plants.usda.gov/. [34262]
326. USDA. 2001. Guide to noxious weed prevention practices, [Online]. Washington, DC: U.S. Department of Agriculture, Forest Service (Producer). 25 p. Available: https://www.fs.usda.gov/invasivespecies/documents/FS_WeedBMP_2001.pdf [2021, February 3]. [37889]
327. Uygur, Sibel; Smith, Lincoln; Uygur, F. Nezihi; Cristofaro, Massimo; Balciunas, Joe. 2004. Population densities of yellow starthistle (Centaurea solstitialis) in Turkey. Weed Science. 52(5): 746-753. [94227]
328. Wallace, John M.; Wilson, Linda M.; Launchbaugh, Karen L. 2008. The effect of targeted grazing and biological control on yellow starthistle (Centaurea solstitialis) in canyon grasslands of Idaho. Rangeland Ecology & Management. 61(3): 314-320. [94046]
329. Waller, Lauren P.; Callaway, Ragan M.; Klironomos, John N.; Ortega, Yvette K.; Maron, John L. 2016. Reduced mycorrhizal responsiveness leads to increased competitive tolerance in an invasive exotic plant. Journal of Ecology. 104(6): 1599-1607. [94149]
330. Welsh, Stanley L.; Atwood, N. Duane; Goodrich, Sherel; Higgins, Larry C., eds. 2015. A Utah flora. 5th ed. Provo, UT: Brigham Young University. 987 p. [94185]
331. Widmer, T. L.; Guermache, F. 2004. Filtrates of rhizosphere bacteria suppressive to Centaurea solstitialis seed germination. Journal of Plant Diseases and Protection. Switzerland: Springer Nature. 19 (Special Issue): 497-502. [94001]
332. Widmer, Timothy L.; Guermache, Fatiha; Dolgovskaia, Margarita Yu; Reznik, Sergey Ya. 2007. Enhanced growth and seed properties in introduced vs. native populations of yellow starthistle (Centaurea solstitialis). Weed Science. 55(5): 465-473. [71425]
333. Woo, Isa; Swiadon, Laurie; Drlik, Tanya; Quarles, William. 1999. Integrated management of yellow starthistle. The IPM Practitioner: Monitoring the field of pest management. 21(7): 1-10. [40379]
334. Woodley, S. E.; Zamora, B. A.; Coffey, T. 2017. Environmental variables influence the association of Eustenopus villosus and Larinus curtus (Coleoptera: Curculionidae) adults with different growth stages of the invasive thistle Centaurea solstitialis (Asteraceae: Cardueae) in Washington state. Physiological Ecology. 46(2): 383-392. [94045]
335. Woodley, Steven E.; Zamora, Benjamin A.; Coffey, Todd. 2019. Percent infestation and seed consumption of Centaurea solstitialis L. (Asteracea: Cardueae) by Eustenopus villosus and Larinus curtus (Coleoptera: Curculionidae) in Washington, USA. Biological Control. 134: 38-44. [94044]
336. Woods, D. M.; Bruckart, W. L., III. 2004. Puccinia jaceae var. solstitialis. In: Coombs, Eric M.; Clark, Janet K.; Piper, Gary L.; Cofrancesco, Alfred F., Jr., eds. Biological control of invasive plants in the United States. Corvallis, OR: Oregon State University Press: 431-432. [53095]
337. Woods, D. M.; Popescu, V. 2007. Yellow starthistle leaf loss due to early season infection by the Puccinia jaceae var. solstitialis. Phytopathology. 97(7): S124. [94217]
338. Woods, Dale M.; Bruckart, William L. III; Pitcairn, Michael; Popescu, Viola; O'Brien, Jon. 2009. Susceptibility of yellow starthistle to Puccinia jaceae var. solstitialis and greenhouse production of inoculum for classical biological control programs. Biological Control. 50(3): 275-280. [94043]
339. Woods, Dale M.; Pitcairn, Michael J.; Joley, Donald B.; Turner, Charles E. 2008. Seasonal phenology and impact of Urophora sirunaseva on yellow starthistle seed production in California. Biological Control. 47(2): 172-179. [94074]
340. Xiao, Sa; Callaway, Ragan M.; Graebner, Ryan; Hierro, Jose L.; Montesinos, Daniel. 2016. Modeling the relative importance of ecological factors in exotic invasion: The origin of competitors matters, but disturbance in the non-native range tips the balance. Ecological Modeling. 335: 39-47. [94148]
341. Yesilada, Erdem; Gurbuz, Ilhan; Behir, Erdal; Tatli, Irem; Khan, Ikhlas A. 2004. Isolation of anti-ulcerogenic sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis through bioassay-guided fractionation procedures in rats. Journal of Ethnopharmacology. 95(2-3): 213-219. [93999]
342. Yesilada, Erdem; Sezik, Ekrem; Fujita, Tetsuro; Tanaka, Shigeo; Tabata, Mamoru. 1993. Screening of some Turkish medicinal plants for their antiulcerogenic activities. Phytotherapy Research. 7(3): 263-265. [28718]
343. Young, James A.; Clements, Charlie D.; Pitcairn, Michael J.; Balciunas, Joe; Enloe, Steve; Turner, Charles; Harmon, Daniel. 2005. Germination-temperature profiles for achenes of yellow starthistle (Centaurea solstitialis). Weed Technology. 19(4): 815-823. [94027]
344. Young, Stephen L.; Barney, Jacob N.; Kyser, Guy B.; Jones, Tracy S.; DiTomaso, Joseph M. 2008. Functionally similar species confer greater resistance to invasion: Implications for grassland restoration. Restoration Ecology. 17(6): 884-892. [94218]
345. Young, Stephen L.; Kyser, Guy B.; Barney, Jacob N.; Claassen, Victor P.; DiTomaso, Joseph M. 2010. Spatio-temporal relationship between water depletion and root distribution patterns of Centaurea solstitialis and two native perennials. Restoration Ecology. 18(S2): 323-333. [94146]
346. Young, Stephen L.; Kyser, Guy B.; Barney, Jacob N.; Claassen, Victor P.; DiTomaso, Joseph M. 2011. The role of light and soil moisture in plant community resistance to invasion by yellow starthistle (Centaurea solstitialis). Restoration Ecology. 19(5): 599-606. [83526]
347. Young, Stephen, L.; Claassen, Victor P. 2008. Release of roadside native perennial grasses following removal of yellow starthistle. Ecological Restoration. 26(4): 357-364. [93970]
348. Youtie, Berta; Soll, Jonathan. 1990. Diffuse knapweed control on the Tom McCall Preserve and Mayer State Park. Grant proposal prepared for the Mazama Research Committee, Portland OR. Unpublished paper on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Missoula Fire Sciences Laboratory. 18 p. [38353]
349. Zamora, David L. 1984. The allelopathic potential of yellow starthistle. Moscow, ID: University of Idaho. 32 p. Thesis. [94214]
350. Zavaleta, E. S.; Hulvey, K. B. 2007. Realistic variation in species composition affects grassland production, resource use and invasion resistance. Plant Ecology. 188(1): 39-51. [94143]
351. Ziska, Lewis H. 2003. Evaluation of the growth response of six invasive species to past, present and future atmospheric carbon dioxide. Journal of Experimental Botany. 54(381): 395-404. [74986]