| FEIS Home Page |
 |
|
Photo © Tom Brock, University of Wisconsin-Madison |
AUTHORSHIP AND CITATION:
Gucker, Corey L. 2011. Quercus macrocarpa.
In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: https://www.fs.usda.gov/database/feis/plants/tree/quemac/all.html
[].
FEIS ABBREVIATION:
QUEMAC
QUEMACD
QUEMACM
NRCS PLANT CODE [251]:
QUMA2
QUMAD
QUMAM
COMMON NAMES:
bur oak
mossy-cup oak
prairie oak
TAXONOMY:
The scientific name of bur oak is Quercus macrocarpa Michx. (Fagaceae) [72,133].
Bur oak belongs to the Quercus subgenus and section. The Quercus section has
also been called the white oak, Leucobalanus, or Lepidiobalanus section
[10,28,72].
Two bur oak varieties are recognized by Kartesz [133] but are considered "clinal
variants" by the Flora of North America [72]:
When the ranges of the parent species ranges overlap, bur oak may hybridize with other white oaks (Quercus section):
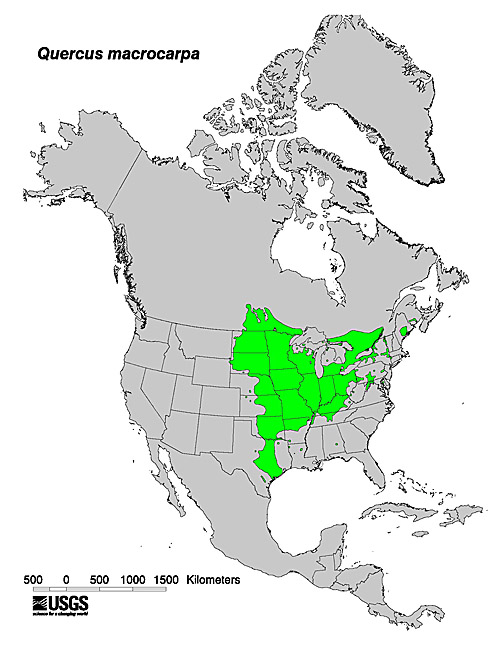 |
| © Elbert Little, United States Forest Service |
The map above illustrates bur oak's distribution in North America as of 1971. Of the North American oaks, bur oak is the most widely distributed [118] and ranges farthest north [168]. General information about the potential distribution of bur oak hybrids is presented in the Introductory section.
Local distribution changes: In many parts of its North American range, the abundance of and area occupied by bur oak has decreased dramatically since European settlement. Conservation and restoration of bur oak has become a management priority for disjunct populations as well as populations within the continuous bur oak range. Agency experts in Canada consider bur oak a conservation priority because it has decreased in abundance, and its habitats are in high demand for development [20]. In New Brunswick, bur oak populations occur about 470 miles (750 km) beyond the continuous North American distribution of the species and about 150 miles (250 km) from the nearest conspecific population in Maine. As of 2009 populations and scattered individuals occupied a combined area of less than 1.9 mi² (5 km²) in this province. All bur oak populations in New Brunswick occurred in narrow areas along floodplains and riverbanks, and many occurred on privately owned lands threatened by waterfront developments [167].
Bur oak savannas in the Midwest and Great Plains were ideal sites for European settlers because they provided wood for homes and fuel, and forage for livestock. Agricultural and urban development together with fire exclusion led to widespread loss of the bur oak savanna ecosystem [34,183,236]. In the early 1900s, oak savannas occupied up to 32 million acres (13 million ha) in the Midwest, and in 1985, only about 6,400 acres (2,600 ha) of "high-quality" oak savanna remained [183]. In Wisconsin, researchers estimated that 5.5 million acres (2.2 million ha) of oak savanna existed before European settlement, but as of the late 1990s, just 500 acres (200 ha) existed (review by [267]). When describing the historical extent of bur oak savannas and a single remaining remnant savanna in southern Wisconsin, Stout [236] called his report "an obituary" for bur oak. In Texas, bur oak occurs on the drier sites within bottomland hardwood forests. Prior to European settlement, these forests likely occurred over 16 million acres (6.5 million ha), but as of early 2000, less than 40% of the forests remain [18]. For more about how fire exclusion and woodland succession contribute to the loss of bur oak trees and savanna ecosystems, see Succession in the absence of fire.
SITE CHARACTERISTICS AND PLANT COMMUNITIES:
In North America, bur oak is most common at elevations of less than 3,300 feet (1,000 m) [72] but tolerates a range of climatic and soil conditions. Bur oak occupies habitats ranging from moist
woodlands and bottomland forests to prairies and sandhills [211]. In the western and northern parts
of its range, bur oak size and growth form may vary with site conditions. In moist woodlands and on alluvial floodplains, bur oak often grows as a tree up to 160 feet (50 m) tall but on dry uplands and bluffs, it may grow as a shrub less than 16 feet (5 m) tall [72,87]. In north-central Nebraska's
Niobrara Valley Preserve, bur oak reaches only 20 feet (6 m) tall on dry sites but may reach 50 feet
(15 m) tall on moist floodplains [99]. Additional bur oak characteristics that can vary by site and distribution are discussed in the Botanical description.
Climate: Bur oak is one of the most cold tolerant of the North American oak species [72]. In one of its northernmost habitats, south-central New Brunswick, bur oak abundance is greatest where the annual growing-day temperature exceeds 40 °F (5 °C) and there are about 150 frost-free days [167]. In bur oak's northwestern range, the average growing season may be only 100 days, but in southern Illinois and Indiana, where bur oak development is considered best, the growing season is 190 days (review by [125]).
Bur oak tolerates a range of moisture regimes. During a severe 7-year drought in the Great Plains, researchers monitoring injury and mortality in the area reported that bur oak "endured drought especially well" [9]. Annual precipitation can be as low as 15 inches (380 mm) in bur oak's northwestern range, while in its southern range annual precipitation can exceed 50 inches (1,270 mm) (review by [125]).
Soils: While bur oak tolerates harsh soil conditions, including poor, dry soils and wet, poorly drained or inundated soils [72,132], bur oak distribution is not necessarily dictated by soil characteristics. Soils in western bur oak habitats are generally Mollisols, in northern habitats are Spodosols, and in central and southern habitats are Alfisols [125]. Although studies have related bur oak's presence and abundance to soil moisture conditions [6,155], comparing soil characteristics without information on past disturbances, land use, and successional change on all but the harshest sites may erroneously indicate bur oak-soil relationships. In a study of structure, composition, and environmental relationships in an oak savanna remnant in northwestern Ohio, researchers predicted bur oak occurrence as a function of disturbance and not of moisture or drainage regimes [27]. Survey records, other historical records, and soil and topographic factors in the Big Woods region of south-central Minnesota indicated that firebreaks (bodies of water and rough topography) were the primary reason for observed vegetation patterns. Bur oak dominated woodlands that experienced frequent fire regardless of soil characteristics [91]. For more on the importance of disturbances and succession on bur oak, see Succession in the absence of fire and Disturbance-related succession.
Although tolerant of some inundation, bur oak does not tolerate prolonged flooding. At Lake Oahe, South Dakota, a field trial showed that bur oak survived at least 2 weeks of growing-season inundation [116]. However, in the northern part of the Mississippi Delta, bur oak is often killed by high water during the growing season. Details about duration of flooding and saturation were not provided [194]. On permanently flooded sites, bur oak trees died within 3 years (review by [125]).
Plant communities and related site characteristics: Bur oak is often a dominant savanna or woodland species in the Great Plains and Great Lakes regions. The Society of American Foresters recognizes western [64] and eastern bur oak forest cover types [65], where bur oak occurs in pure or nearly pure stands. Bur oak savannas are reported nearly throughout the Great Plains and Great Lakes regions [16,142,201,209]. Other wide-ranging forest types in which bur oak is a dominant or codominant include northern oak-hickory (Carya spp.) forests [33,261] and bur oak-chinkapin oak communities [170].
In many cases, time, disturbances, and successional change are more important than climate or site characteristics in determining which type of bur oak community occurs. The exception may be in extremely harsh sites, where shade-tolerant tree species fail to establish and replace bur oak. In the absence of fire or other disturbances, an oak savanna typically transitions into an oak woodland and then to a mixed-deciduous woodland. This transition is described in more detail in the section on Succession in the absence of fire.
Some of the communities discussed below are considered "imperiled" because of their rarity or other factors making them vulnerable to extinction [209] (see Other Management Considerations). Other communities are rare because of land conversions, successional changes, and fire exclusion associated with European settlement [34,183,236]. In some communities, plant associates of bur oak may now be more rare than at the time of the vegetation survey and subsequent publication. For example, Dutch elm disease and phloem necrosis have resulted in high morality levels for American elm (Ulmus americana) [17,162], and shagbark hickory (C. ovata) has been extensively harvested for fuel wood [89].
Northern Great Plains: In the Northern Great Plains, bur oak is common in mixed-conifer forests, deciduous forests, oak and oak-hickory woodlands, oak savannas, and oak shrubland associations. Bur oak is common in bottomlands with rich soils but also occurs on open rocky hillsides with poor soils [234]. In mixed-conifer and mixed-deciduous forest types in the Black Hills of Wyoming and South Dakota, soils are typically sandy loams to clay loams, of calcareous or igneous origin, with pH levels of 5.3 to 7.4 and organic matter contents of 3.6% to 9.5% [216]. Bur oak stands and habitat types at the extreme western part of its range are commonly found at elevations of about 2,300 to 5,300 feet (700-1,600 m) [216,259].
Southern Great Plains: In the Southern Great Plains region, bur oak is common in mixed-deciduous woodlands, oak and oak-hickory woodlands, and oak savannas. These communities are common in floodplain areas.
Oak and oak-hickory savannas and woodlands: Bur oak, mixed-oak, and oak-hickory savannas and woodlands are described in Nebraska, Missouri, Kansas, and Oklahoma. In the savannas, bur oak may dominate the canopy alone or with chinkapin oak. Big bluestem (Andropogon gerardii) is the typical grass associate [148,209]. In oak woodlands, bur oak often shares the canopy with other oaks such as pin oak (Q. palustris), swamp white oak [209], or chinkapin oak. In the Flint Hills of northeastern Kansas, bur oak is most common on the most mesic lowland sites [4]. The growth rate of bur oak was correlated with low topographic slope (r = 0.5) and low soil nitrogen (r = 0.7) (P<0.05) [2]. Oak-hickory woodlands dominated by bur oak are most commonly found on floodplains or other mesic sites [114,148,209]; however, the bur oak-shagbark hickory type occurs on xeric slopes and hilltops and represents the most xerophytic forest association in eastern Nebraska [8].
Great Lakes: In the Great Lakes region, the abundance and area of bur oak habitats declined dramatically with European settlement (see Local distribution changes above). Bur oak was common in frequently burned prairies and savannas. Based on Land Office Survey records from McLean County, Illinois, bur oak was most important in prairies, next most important in savannas, then in open forests, and was least important in closed forests (Rogers and Anderson 1979 as cited in [50]). Persistence of bur oak-dominated habitats depends on fire or other disturbances that limit the establishment of less fire-tolerant and more shade-tolerant woodland species.
Northeastern United States: Bur oak is much less common in the northeastern United States and southeastern Canada than in the Great Plains and Great Lakes regions.
Southern Appalachians: The information available on bur oak habitats in the southern Appalachians is very limited. In Virginia, bur oak is not frequent but is described on calcareous soils [270].
 |
|
| Photo © Paul Wray, Iowa State University, Bugwood.org |
Aboveground description: Bur oak typically grows as a large, spreading tree up to 130 feet (40 m) tall; however, growth form and size can vary by site. Branches in the upper portion of the crown are ascending; in the lower crown, branches are larger and horizontal [68,234,237]. The trunks of mature trees have thick, deeply grooved bark [121] and may measure 8.5 feet (2.6 m) in diameter [63]. In the western part of its range on exposed, harsh sites, bur oak grows as a small tree or shrub [72,90,118] and may produce crooked, gnarled branches [68]. Bur oak growth forms may relate to moisture availability. In the Niobrara Valley Preserve, bur oak may only reach 15 feet (4.6 m) tall on moisture-limited sites but may reach 50 feet (15 m) tall on floodplains [99]. Growth may also be affected by browsing pressure. Bur oak was dwarfed in heavily browsed areas of Manitoba and Saskatchewan [23].
Bur oak is a long-lived tree. It is common to find remnant trees that are 300 to 400 years old [55,93], and in a savanna in Kentucky, a bur oak tree was an estimated 440 years old [38].
The sizes and shapes of bur oak leaves are variable, but generally leaves are deeply lobed and large, up to 12 inches (30 cm) long and about half as wide [90,121,211]. Shallowly lobed leaves may occur on bur oak sprouts or deeply shaded branches [90], and small leaves are common in the Northern Great Plains [234]. Leaves are deep green shiny above and coated with white hairs below [118]. Bur oak produces male flowers in 3- to 4-inch (7-10 cm) long catkins, and female flowers are solitary or in clusters of up to 4 [90,234].
Bur oak acorns are generally 1-seeded with a cup that covers at least 33% of the nut and may, though rarely, cover the entire nut [63,87,90,211,237]. Acorn size and cup coverage can vary by site. In the Northern Great Plains, bur oak produces small fruits and cups with low coverage, which may be the result of past hybridization with Gambel oak [234]. In general, acorn size decreases with increasing latitude [72]; bur oak acorns from a site in Texas averaged 7.5 g, while in Minnesota they averaged 0.9 g [140]. Diameter of acorns in Texas can be 2 inches (5 cm) [60,222]. Bur oak acorn size differences can even occur over small changes in latitude. Acorns produced in Wisconsin were much smaller than those produced in southern Illinois and Missouri [55]. Acorn size can also vary with shadiness of habitat. In east-central Nebraska, bur oak acorns from shady habitats weighed more than those from open habitats. Acorns collected from a closed-canopy floodplain forest weighed 1.3 to 6 g and those from open savannas weighed 0.5 to 2.5 g [144].
Belowground description: Typically bur oak produces extensive root systems with wide-spreading laterals and a deep taproot [8,68,118]. Several studies describe the root system of bur oak from seedling stage to maturity, although mature trees in these studies were not very old (43-80 years old).
Seedlings and saplings: Bur oak rapidly develops deep and wide-spreading roots. At the end of the 1st growing season, bur oak roots may reach 4.6 feet (1.4 m) deep and spread 2.54 feet (0.76 m) (review by [125]). Root systems of bur oak saplings are described in the table below.
| Characteristics of root systems of bur oak saplings at different ages and locations | ||||
| Tree age (yrs) | Tree height | Taproots | Lateral roots | Site |
| 3 | 3.5 ft | 5 ft deep | 2.5-ft spread | silt loam soil in Lancaster County, Nebraska [225] |
| 8 | ----* | 14.6 ft deep; 1.3-in. diameter at 4 ft deep |
11-ft spread; 18-24 laterals from top 14 in. of taproot |
upland clay soil near Fayette, Missouri [24] |
| 12 | 14 ft | 13 ft deep | 11.5-ft spread | silt loam soil in Richardson County, Nebraska [225] |
| *No information. | ||||
Mature trees: In the few excavation studies involving mature bur oak trees, root spread and penetration increased with tree age in clay, loam, and loess soils. A 43-year-old, 20-foot (6 m) tall bur oak tree growing in clay soil in North Dakota produced a taproot that was a little over 8 feet (2.4 m) long. The longest lateral root was 41 feet (12.5 m) [274]. A review reports that a 43-year-old bur oak growing in a prairie had nearly equal weights of above- and belowground biomass [125]. In eastern Nebraska, researchers excavated and described the entire root system of a 65-year-old bur oak tree growing in a deep, fertile, fine-textured loam with a high water-holding capacity. The tree was 37.5 feet (11.4 m) tall with a basal diameter of 14 inches (36 cm). There were 64 main roots with diameters greater than 2 inches (5 cm) that were 3.5 to 15 feet (1.1-4.6 m) long. The taproot was 14 feet (4.3 m) deep. Within a 12-foot (3.6 m) radius of the tree trunk, there were 64 main taproot branches, 82 secondary roots, and an abundance of rootlets. Bur oak aboveground biomass was 1,285 lbs, and the root system weight was nearly the same [262]. In Nance County, Nebraska, an 80-year-old, 20-foot (6.1 m) tall bur oak growing in loess soil produced a root system that reached 16 feet (4.9 m) deep and 72 feet (22 m) wide [225].
Raunkiaer [195]
life form:
Phanerophyte
SEASONAL DEVELOPMENT:
Throughout its range, bur oak flowers sometime between April and early June [7,55,63,90,237,270].
Acorns are produced in the same year as the flowers [55]. Acorns fall as early as August and as
late as November ([7], review by [125]).
REGENERATION PROCESSES:
Bur oak reproduces by seed and is capable of vegetative regeneration from sprouts following top-kill. These topics are discussed in detail below in Seedling establishment and Vegetative regeneration.
Pollination and breeding system: Bur oak is monoecious [90] and dichogamous. Pollen is released before female flowers are receptive, and self pollination is rare, if it occurs at all [62]. Weather conditions may affect bur oak pollination and flowering success. In central Pennsylvania, researchers studied flowering and fruiting in species within the white oak group, including bur oak. Prolonged wet and rainy weather delayed pollen shed, and dry winds and killing freezes reduced or eliminated male flowers and pollen dispersal [215].
Genetic variation: Most studies show high levels of diversity in bur oak populations. In northern Illinois, researchers characterized pollen dispersal using microsatellite analysis. A little more than half of all acorns were pollinated by trees from outside of the study stand. Researchers suggested that bur oak was highly efficient in producing highly outbred individuals [62]. An analysis of 21 bur oak populations from the Great Lakes, Midwest, and Great Plains showed high levels of genetic variation. Genetic similarity of populations tended to decrease with increasing geographic distances between populations, however. Researchers suggested high levels of variation were likely a result of bur oak's wide geographic range, wind-facilitated outcrossed pollination, and long life span, most of which characterize species with high genetic variability [208]. Researchers also found high genetic variation in 14 bur oak stands in central Illinois that were 0.8 to 157 miles (1.3-252.8 km) apart. Researchers predicted that long-distance pollen dispersal would protect bur oak from reproductive isolation in fragmented habitats [49].
Another study suggests that gene flow may have decreased in the last 100 years for bur oak stands in south-central Minnesota. For stands that were 3.1 to 18.6 miles (5-30 km) apart, researchers found that younger populations were more genetically differentiated than older cohorts. Because the younger cohort differs genetically from the older cohort, researchers suspected that the younger cohorts have accumulated different allelic frequencies through reduced gene flow between populations. Fragmentation of stands and an increased density of associated tree species may have restricted gene flow [138].
Seed production: Detailed research specific to bur oak acorn production over time and space is generally lacking. Bur oak is a masting species, producing large acorn crops in most but not all years. Age at first reproduction for bur oak is reported as 35 years in a review [93]. Bur oak may still produce seeds at 400 years old, but the optimum seed bearing years are reported as 75 to 150 (review by [125]). Observations in eastern Nebraska showed that bur oak produced an "abundance" of large acorns [8]. In 3 years of observations near Harvard, Illinois, bur oak trees produced "large" acorn crops in 2 years and almost no acorns in the other year [62]. Weather is one factor that may affect bur oak acorn production. After an early April frost that followed very warm March temperatures in the Trelease Woods of Illinois, bur oak trees with flower buds at the time of the frost failed to produce seed [15].
Bur oak trees sometimes produced multiple-seeded acorns. In east-central Illinois, acorns were collected from 17 bur oak trees. Only 8 trees produced exclusively single-seeded acorns; the frequency of double-seeded acorns was 20% [78]. At the Iowa State University Horticulture Research Station, 7 of 18 bur oak trees produced acorns containing more than 1 seed. A few acorns contained as many as 5 seeds [21].
Seed predation: Bur oak acorns are a food source for a variety of birds and mammals [35,125,202], and high levels of predation are common in bur oak habitats. In a floodplain forest in the Flint Hills of Kansas, squirrels removed 72% of bur oak acorns within 24 hours of burial beneath 1 to 2 cm of soil. After 4 days, 97.4% of bur oak acorns were removed. Researchers supposed fox squirrels were the most common seed predator [171]. When 400 bur oak acorns were artificially cached in 3 sites southwest of Chicago, 279 to 397 were removed within a week [74]. When artificial bur oak acorn caches were revisited a year after burial in a south-central Iowa woodland-prairie, none of the cache sites had bur oak seedlings. Tests conducted prior to the caching experiment indicated that 65% of seeds were germinable. Researchers suggested high detection and seed predation rates caused emergence failure [127]. In a tallgrass prairie field study, only 11% of planted acorns escaped predation although protective screens were in place [57].
Insects are also a source of seed predation or loss of seed viability. The frequency of insect infestations was 43% to 100% for acorns collected from bur oak trees in east-central Illinois. The researchers indicated that insect infestations reduced bur oak seed germination but did not report the amount of this reduction [78].
Seed dispersal: Bur oak acorns are animal dispersed. Small mammals are the most likely dispersers. Birds are less likely dispersers, given the large size of bur oak acorns. Likelihood of bird dispersal may increase if only bur oak acorns are available or if small acorns are produced in a given season or on a given site. One study reported that bur oak acorns were dispersed by blue jays in Iowa (Johnson unpublished data [124]), but in a later Iowa study, blue jays avoided bur oak acorns [127].
Animal-mediated dispersal distances as great as 490 feet (150 m) were reported in forest fragments in southern Ontario. Rodents were the suspected dispersal agent. In this study, researchers searched a maximum distance of 490 feet (150 m) between seedlings and the nearest fruiting tree, suggesting that acorn dispersal distances may have been even greater than reported [110]. Near Manhattan, Kansas, fox squirrels cached bur oak acorns an average of 59 feet (18 m) from the source pile [228], but when researchers evaluated the number of seedlings occurring beyond the woodland edge in the same area, bur oak seedlings were found a maximum of 169 feet (51.5 m) from the forest edge [229].
Seed banking: Acorns produced by the white oak group have little to no dormancy and typically germinate soon after falling. White oak acorns do not tolerate dessication below 25% to 30% moisture (review by [28]). A Forest Service nursery handbook reports that bur oak acorns do not store well and may survive only 1 winter in storage [268].
Germination: Conditions considered best for bur oak germination were not well documented in the available literature. In general, germination appears to decrease with desiccation and likely acorn age, but in its northern range, bur oak acorn germination requires 60 days or more of cold stratification ([248], review by [28]).
In controlled conditions, germination of bur oak acorns can be as high as 80%. After 25 to 45 days of alternating temperatures of 86 °F and 68 °F (30/20 °C) in the laboratory, bur oak germination averaged 45% (review [28]). Studies at a greenhouse container nursery showed that bur oak acorns germinated slowly and incompletely. Germination decreased as acorns dried. When acorns were 100%, 80%, and 65% of their fresh weight, germination was about 80%, 35%, and 0%, respectively. Bur oak acorns collected from North Dakota required 90 to 120 days of stratification before germinating. High temperatures were required for continued shoot growth [248].
A Forest Service nursery handbook reports that bur oak acorns have no dormancy and recommends planting acorns immediately follow harvest [268]. In Nebraska, 30% of bur oak seeds germinated within 1 month of falling (review by [125]).
Seedling establishment and plant growth: Bur oak seedlings establish on a variety of sites. Burial in mineral soil improves establishment, but the importance of litter, moisture, and shading in bur oak seedling establishment is less clear. There is no clear pattern to establishment by region or site conditions. It could be that bur oak seedlings exploit harsh sites, where the successful recruitment of other species is limited.
Bur oak seedlings rapidly develop taproots. Researchers monitored the development of shoots and taproots from bur oak acorns collected from northeastern Kansas in the greenhouse. Germinated acorns were planted in soil collected from an annually burned prairie site. The soil was 20% sand, 47% silt, and 33% clay and had a pH of 7.4. About 20 days after germination, bur oak taproots were 5.6 to 11.4 inches (14.4-29.0 cm) long, but shoots had yet to emerge. After 104 days, taproot growth averaged 13 mm/day, and shoot growth averaged 3 mm/day. Researchers thought that rapid taproot growth allowed bur oak to tolerate the temperature and moisture fluctuations characteristic of prairie habitats [56].
Site conditions and inheritance may affect bur oak seedling establishment and plant growth. Bur oak acorns from Menard County, Texas, are said to produce the most drought-tolerant bur oaks in Texas [222].
Acorn burial: Burial of acorns in mineral soil was most important to successful oak seedling establishment in a field experiment in a moderately dense, even-aged oak stand on the Amana Experimental Forest in Iowa. Removal of litter was the second most important factor in successful oak establishment. Researchers planted bur oak, black oak, white oak, and northern red oak in protected and unprotected plots with and without litter. Oak establishment was greatest in plots where litter was removed, acorns were protected from rodents, and acorns were buried beneath 1 inch (2.5 cm) of soil. Protected and unprotected plots without litter had 85% more seedlings than plots with litter and rodent protection [141]. However, in a review of fire and oak relationships, Lorimer [153] suggests that litter can benefit oak seedling establishment.
Caching by small mammals may facilitate seedling establishment through acorn burial. In forest preserves near Chicago, Illinois, researchers observed gray squirrels handling white oak and bur oak acorns. Of 152 acorns handled, 138 were buried. In some cases, however, the growing points of the seed were removed prior to caching [74].
Moisture conditions: Some studies suggest that bur oak establishment is best during drought conditions or on dry, open sites, while other studies indicate that establishment is best on mesic sites. In old fields in Quebec, bur oak seedling growth was evaluated along a soil moisture gradient. Growth was better at wet and dry extremes than at intermediate soil moisture levels [46].
Bur oak establishment coincided with drought conditions in Minnesota, and bur oak recruitment was best in open, xeric habitats in Manitoba. Bur oak recruitment into the Big Woods of central Minnesota peaked during drought conditions prevailing in the 1930s [218]. Analysis of bur oak tree cores from the Helen Allison Savanna, east-central Minnesota, showed that bur oak establishment aligned closely with periods of extended drought. Dry conditions may have limited herbaceous productivity and created openings for establishment. Fire history of the area was not determined, and the researchers acknowledge that fire likely also affected population dynamics [275]. Bur oak recruitment decreased with increasing moisture and shading in Riding Mountain National Park in Manitoba. The driest and most open bur oak-low shrub community type supported the greatest density of bur oak seedlings and saplings. Seedling and sapling densities were least in the most mesic, closed-canopy oak-aspen-ash community [272].
Studies and observations in Illinois suggest that bur oak establishment and survival were best on mesic sites. In the Trelease Woods in Champaign County, bur oak seedling and sapling densities were greatest on wet soils. Only a small number seedlings and saplings occurred on drier soils [81]. In central Illinois, bur oak seedlings were absent from dry and dry-mesic sites but on mesic, wet-mesic, and wet sites there were 56.3, 33.3, and 8.3 bur oak seedlings/ha, respectively [6].
While bur oak seedlings establish well on mesic sites, saturated or flooded conditions are less suitable for establishment. In field and greenhouse studies, bur oak seedlings were taller and had greater biomass in well-drained than saturated soils; however, differences by soil types were much more pronounced in the greenhouse than in the field [59]. In a greenhouse study where 3-month-old bur oak seedlings were flooded for 30 days, root growth was reduced. Flooded seedlings were less drought tolerant when flooding receded [242].
Shade conditions: Bur oak seedlings establish beneath woodland canopies, but studies suggest that bur oak seedling growth may be best in less dense shade.
Recruitment was reported in several shaded habitats in the western part of bur oak's range. Along the Missouri River in central North Dakota, bur oak reproduced beneath an eastern cottonwood-peachleaf willow (Populus deltoides-Salix amygdaloides) canopy. As succession proceeded in the absence of scouring and flooding, bur oak replaced the eastern cottonwood-peachleaf willow community [126]. In another study along the Missouri River, bur oak seedlings and saplings were sparse in floodplain forests, but seedlings and saplings were abundant on lower terraces near the floodplain edge where soils were mesic and fertile [128]. Along the gallery forest-tallgrass prairie ecotone at the Konza Prairie Research Natural Area in northeastern Kansas, bur oak seedlings were much more restricted to shaded microsites than chinkapin oak seedlings were [32].
Bur oak seedling growth was much greater in full sun than deep shade in a common garden experiment in southern Illinois. Researchers reported the height increase between the 1st and 2nd years of growth. In full sun, bur oak seedlings grew 39 inches (100 cm). In 95% shade, bur oak seedlings grew 5 inches (13 cm). One-year-old seedlings cut to ground level were 15.8 inches (129 cm) tall at the end of the 2nd year in full sun. Cut seedlings failed to sprout in 95% shade [13].
Bur oak seedlings established in prairie and oak habitats after acorns were planted in a cleared area of prairie, in an intact oak stand, and in an intact basswood stand near the Missouri River in southeastern Nebraska. After 2 years, the diameters of bur oak seedlings in cleared prairie were 10 times those of seedlings in the oak stand. Seedling heights in the prairie were 7 times those of seedlings in the oak stand. Bur oak seedlings in the basswood stand died by the end of the 2nd growing season. All sites had fine silt loam soils. Soil temperatures were greatest in the prairie and least in the basswood stand. During the growing season, the prairie site experienced full sun, the oak stand averaged 10.4% full sun, and the basswood stand averaged 3.4% full sun. The growth and fate of bur oak seedlings in the 3 sites are summarized in the table below [117]:
| Root growth of 1-, 2-, and 3-year-old bur oak seedlings in 3 different sites in southeastern Nebraska [117] | |||
| Site | Prairie | Oak woodland | Basswood woodland |
| 1-year-old seedlings | |||
| Root length (inches) | 60 | 20 | 11 |
| Root spread (inches) | 30 | 11 | 3 |
| 2-year-old seedlings | |||
| Root length (feet) | 8.5 | 2.3 | * |
| Root spread (feet) | 5.2 | 0.8 | * |
| 3-year-old seedlings | |||
| Root length (feet) | 10 | ~2.3 | * |
| Root spread (feet) | 7.2 | ~1.0 | * |
| * Seedlings died. | |||
Shading and established vegetation did not substantially affect bur oak seedling establishment or first-year growth in the Konza Prairie in northeastern Kansas. Total aboveground biomass/seedling was not significantly different among 3 experimental treatments: 1) undisturbed plant community (control), 2) removal of all aboveground biomass, and 3) removal of all aboveground biomass plus shading. Bur oak seedling survival was high (about 89%) in shaded and control plots and just a little lower (81%) in biomass removal plots. During this study, growing-season precipitation was 35% of the long-term average [57].
Browsing: Bur oak seedling survival is improved when seedlings are protected from browsing. In the Little Missouri National Grasslands, bur oak seedling survival was 90% on sites protected from browsing for 3 years. On unprotected sites, survival was 69% [25]. Browsing by livestock and deer was reported as one reason for a lack of bur oak recruitment in south-central Minnesota. In 4 studied stands, bur oak was the most important of all trees in the large size class (≥9.8 inch (25 cm) DBH) but was never most important in the smaller size class (<9.8 inch (25 cm) DBH). Recruitment between 1910 and 1970 was low to non-existent. Past land-use histories suggested that browsing, fire exclusion, and increased abundance of nonnative and mesic species had reduced recruitment. In stands where fire was reintroduced and livestock were excluded, abundance of bur oak trees less than 40 years old increased [138]. For more on this topic as it relates to more long-term vegetation changes, see Browsing as it relates to succession.
Mature bur oak tree growth: Once bur oaks are established, their growth rate typically increases [108]. Site conditions can dramatically affect bur oak growth. On a "poor" site, bur oak may only have a 4-inch (10 cm) diameter at 100 years old, but on deep rich soils, the diameter of the same-aged tree may be 30 inches (76 cm) [55]. In the Niobrara Valley in Nebraska, similar-aged bur oak trees are 15 to 20 feet (1.5-6 m) tall with a DBH of 2 to 7 inches (5-18 cm) on moisture-limited sites, and are 40 to 50 feet (12-15 m) tall with a DBH of 21 to 34 inches (53-86 cm) on sites with abundant ground water [249]. On a moderately moist site in Kansas, bur oak trees grew 0.7 inch (1.8 cm)/year during a wet period and 0.4 inch (1.1 cm)/year during a dry period [9].
Vegetative regeneration: Bur oak's ability to sprout following aboveground damage is well established, but factors that influence the frequency and abundance of sprouts are not well described. Bur oak sprouting potential appears to decrease with increasing tree age [219,221]. Sprouting of pole-size or smaller bur oak stems is considered "vigorous" after cutting or burning according to Johnson [125]. After an ice storm in a mixed forest near Ottawa, Ontario, just 12% of damaged bur oak trees produced any sprouts, but 1 damaged stem produced 39 sprouts [36]. Vegetative regeneration is also discussed in the Fire Effects and Management section related to Postfire sprouting.
Shading affected sprouting of bur oak seedlings in a common garden in southern Illinois. One-year-old seedlings cut to ground level sprouted and grew more than uncut seedlings after a year in the common garden. In 95% shade, cut seedlings failed to sprout [13].
SUCCESSIONAL STATUS:
In most areas, bur oak is a shade-intolerant, early-seral species that is replaced by shade-tolerant deciduous species in the absence of large-scale disturbances. Individual bur oak trees typically survive disturbance and repeated top-kill. Large canopy gaps are likely necessary for establishment from seed following disturbance, but colonization by seedlings will likely be slow (>30 years).
Shade tolerance: Although bur oak is typically shade intolerant, it can tolerate some shade, in some habitats, at least in the short-term. Patterns of establishment, suppression, and release in mixed-deciduous, old-growth forests in northern Ohio were evaluated from tree core data. Researchers concluded that bur oak was intolerant of shade and became a part of the canopy only where it had established following a large-scale, canopy-removing disturbance [45]. However, other studies report bur oak on shaded sites. Although not abundant in ground layer vegetation in red pine (Pinus resinosa) stands in Minnesota's Chippewa National Forest, bur oak was most frequent in plots receiving a little less than 20% of full sun [217]. Surveys of the Ozark Plateau in eastern Missouri and Arkansas conducted in 1815 showed that bur oak did not occur in open woodlands, savannas, or scrubby oak vegetation, but did occur in dense, closed-canopy forests at low frequency [177]. In North Dakota, bur oak seedlings and saplings were reported in floodplain forests along the Missouri River. Bur oak importance generally increased as scouring and flooding ceased and floodplain forest stand age increased [126,128].
Seral stage: Bur oak stands have been described as early-, mid-, and late-seral as well as subclimax and postclimax, but categorizing bur oak stands into climatic seral stage communities may only be appropriate for areas with harsh site conditions. In the Great Lakes region, bur oak communities are described as peristent vegetation maintained by frequent fire [91].
In the Dakotas, bur oak communities have been classified as early-, mid-, and late-seral and also as subclimax and postclimax. Along the Missouri River in South Dakota, late-seral bur oak woodlands were rare because excessive livestock grazing and/or plant disease made most woodlands early- or mid-seral communities. Cover of grasses decreased from early- to late-seral stages, and bur oak canopy, forb, and shrub cover increased from early- to late-seral communities [207]. In the Black Hills of South Dakota, researchers considered bur oak-sumac communities to be subclimax. A bur oak-deciduous forest, which appeared to be returning to a shrubby subclimax stage was described as "postclimax" [101]. Judd [131] also described a bur oak community in the badlands of western North Dakota as "postclimax".
A review of survey records, other historical records, and edaphic and topographic features of the Big Woods of south-central Minnesota indicated that firebreaks were the primary factor in controlling vegetation patterns. Bur oak represented a persistent vegetation type maintained by fire [91].
Succession in the absence of fire:
Prairies: It is common for bur oak to establish throughout a
prairie if the time between fires extends to 10 years or more. Bur oak may also establish as scattered individuals in safe sites during shorter fire-free periods. Bur oak functions
as a "pioneer along the prairie border" [65]. In the prairie-deciduous forest ecotone that occurs from Minnesota to Texas, bur oak and other woody species invade the prairie at an average rate
of 1 foot (0.3 m)/year without frequent fire [16]. In Kansas, bur oak increased its range during a time
of decreased fire frequency in prairie habitats [80]. In the Wolf Road Prairie in Cook County, Illinois, researchers compared the composition and structure of vegetation over time. Before 1955, the area supported a bur oak savanna. In the next 10 years, a period without fire, the density of bur oak stems increased dramatically. Bur oak grubs, which are burl-like woody structures that develop on the soil surface as young bur oak stems or sprouts are repeatedly top-killed by fire, were released during the fire-free period and produced an abundance of stems. By 1995, the area was dominated by a dense 30-year-old subcanopy of bur oak and northern pin oak. Gaps in the subcanopy were rare [31].
Oak savannas and woodlands: Once bur oak reaches the stage at which it can tolerate repeated fire (12 years or older), it persists indefinitely in savannas or open woodlands with frequent fire [53]. Without fire, bur oak savannas and woodlands are replaced by other deciduous species that are intolerant of fire but tolerant of shade. Changes in bur oak savannas in Wisconsin in the absence of fire were well described by Curtis [53]. After about 10 years without fire in prairies and bur oak savannas, woody saplings and other shrubs become established. After 25 to 30 years without fire, dense oak forests develop. Large, mature bur oaks in the savannas can survive overtopping by other species for about 80 years, at which point they become weakened by wood-rot fungi in the shade-killed lower branches. Most bur oak trees in dense woodlands are snapped by wind storms after 100 to 110 years. Survey records from 1837 to 1840 in Lake County, Illinois, indicated that bur oak was the most common tree species, and bur oak savannas were the most common vegetation type. Surveys in the late 1940s and 1950s showed that, with the exclusion of prairie fires, bur oak savannas were heavily invaded by other woody vegetation. As of 1978, the bur oak savannas of presettlement time in this area were extinct [172]. A comparison of survey records for Stewart's Woods in Wisconsin showed that the area changed from a bur oak-dominated savanna in 1834 to a dense woodland where bur oak was only a minor species in 1946. After evaluating the histories of land use, climate, and diseases, researchers concluded that European settlement and the end of frequent burning by American Indians facilitated the successional change [48]. For a summary of studies documenting changes from open oak savannas and oak-pine woodlands in early land surveys to dense, closed-canopy, mesophytic forest types in more contemporary surveys, see Nowacki and Abrams [182].
The succession from bur oak savanna or woodland to dense, mesic stands has been described in many areas. In his study of vegetation and successional change in Wisconsin, Curtis [55] reported that bur oak fails to reproduce successfully once canopy cover reaches 75%. Climax species that often replace bur oak include sugar maple (Acer saccharum), basswood, and hackberry. The bur oak-chinkapin oak community type that occurs along the Mississippi River drainage system from Kansas and Nebraska to Wisconsin is replaced by sugar maple and basswood in absence of fire or other major disturbances [170]. In southern and western Wisconsin, researchers described a vegetational continuum in upland forest stands. Drought-tolerant, shade-intolerant species such as bur oak, bigtooth aspen (Populus grandidentata), and black oak were first to invade prairie vegetation. Climax species included eastern hophornbeam and sugar maple [52]. In the absence of major disturbances over a 50-year period in the David-Purdue Research Forest in east-central Indiana, bur oak importance decreased and density of American elm and sugar maple increased [187]. After European settlement in about 1840 around the Konza Prairie in northeastern Kansas, the extent, frequency, and/or severity of fires in the area decreased. In the gallery forests, there are old, large bur oaks and chinkapin oaks, but there has been very little oak recruitment for over 50 years. Hackberry dominates the young size classes on moist sites and eastern redbud (Cercis canadensis) on dry sites [4].
Other factors affecting succession: While the forest succession described above may be most common, different successional patterns and drivers are also possible. On calcareous soils at Lake Itasca in Minnesota, bur oak is a mid-seral species. Early-seral forests are dominated by quaking aspen, birch (Betula spp.), and jack pine (Pinus banksiana). In the mid-seral, hardwood-eastern white pine (P. strobus) forest, bur oak is common before sugar maple becomes dominant. Climax forest species include white spruce (Picea glauca) and balsam fir (Abies balsamea) [150]. In central Kentucky, dendrochronological analyses indicated that an oak savanna, where bur oak was common, developed from a closed-canopy forest. Historical growth rates and growing conditions estimated from dendrochronologies suggested that savanna trees exhibited suppressed growth rates early in life and were part of a closed-canopy forest. Closed-canopy forests may have developed because American Indian populations in the area suffered extensive losses from pandemics, particularly small pox. Rapid tree growth coincided with Euro-American settlement, which involved extensive land clearing to create pastures [165]. In xeric savannas invaded by nonnative common buckthorn (Rhamnus cathartica) in southeastern Wisconsin, bur oak reproduction is generally lacking. Invasion by common buckthorn coincided with European settlement and fire exclusion. Conditions from 6.6 feet (2 m) above ground to ground level were shadier in invaded than in uninvaded areas [151], which likely limited bur oak recruitment.
On some harsh sites, bur oak may be a late-seral species or may persist for longer periods in the absence of disturbance because successional change occurs slowly on these sites. In Riding Mountain National Park, Manitoba, bur oak forest stands are replacing themselves on excessively drained, gravelly, sandy soils. Researchers doubted that many other tree species could tolerate the dry site conditions [42]. In the Upper Midwest, bur oak often dominates dry calcareous savanna, where soils are shallow or excessively drained. A lack of herbaceous fuel build up on the harsh sites limits the chance of intense fires. Although the density and cover of woody vegetation have increased without fire, harsh soil conditions allow for the persistence of remnant savannas [266]. In the absence of large disturbances in Wisconsin, bur oak typically dominates for just a single generation before being replaced by more shade-tolerant species, but bur oak dominates longer without disturbances on hot, dry sites, where soil organic matter and water retention increase slowly [55]. In south-central Wisconsin, bur oak persisted in the absence of disturbance only in open stands on the most xeric sites [188]. Bur oak may respond to stress from abundant moisture in central Illinois. On mesic sites, bur oak is a pioneer species and is replaced by sugar maple as shade levels increase, but in wet-mesic and floodplain forests, bur oak generally replaces itself and persists through succession [6].
In some areas, researchers think that climate has more influence than fire on succession in bur oak communities. Researchers suggested that climate rather than American Indian fires were responsible for development of oak savannas in southern Ontario, where bur oak occurred but was not dominant [240]. After reviewing current site conditions, historical climate evidence, time since last fires, and European settlement records, researchers suggested that the prevailing climate in Minnesota from 1812 to 1825 was conducive to forest invasion of the prairies and savannas. Although lack of fire was considered important to the succession from prairie or pine-oak savanna to sugar maple-basswood forests, researchers concluded that climate was the most influential factor [39].
Old field succession: Bur oak establishment is slow in old fields, even if an adjacent seed source exists. The following studies suggest that bur oak is unlikely in old fields abandoned less than 30 years. Bur oak did not occur in fields abandoned for 19 to 24 years in southeastern Ontario, even though bur oak occurred in forests adjacent to the fields [51]. In southwestern Ohio, bur oak was uncommon in a 90-year-old field but was not reported in 2-, 10-, 50-year-old fields [255]. On the Anoka Sand Plain in east-central Minnesota, bur oak did not occur in a hayfield abandoned for about 20 years. The area was dominated by bur oak savannas before conversion to agriculture, but the abundance of bur oak in the woodlands surrounding the field was not reported [61]. In the Cedar Creek Natural History Area on the Minnesota sandplain, bur oak was generally absent from fields less than 15 years old [246]; bur oak seedlings and saplings were scattered near the woodland margin of a 48-year-old field; and bur oak seedlings were common but saplings were rare in 60-year-old fields [247]. In east-central Minnesota, researchers surveyed the forest-field margins of 18 fields abandoned less than 65 years. Bur oak was extremely rare in fields less than 31 years old. Abundance in fields increased with increasing abundance of bur oak trees in adjacent forests [149]. In an old field adjacent to mixed-hardwood-oak forests in Ottertail County, Minnesota, bur oak established within 30 years of abandonment [102].
Disturbance-related succession: Large canopy gaps appear necessary for bur oak colonization. Single-tree canopy gaps did not encourage bur oak recruitment in the Brownfield Woods in Champaign County, Illinois. Between 1925 and 1975, the open oak woodland dominated by bur oak and chinkapin oak was being replaced by a closed-canopy woodland dominated by sugar maple. In areas where slippery elm (Ulmus rubra) was killed by disease, sugar maple colonized [169]. During a study of the structure, composition, and environmental relationships of an old-growth remnant in northwestern Ohio, researchers found bur oak trees with DBH greater than 3 feet (1 m) but none with DBH less than 15.8 inches (40 cm). Historical disturbance patterns suggested that bur oak established after 1 or more large disturbances that were more extensive than single- or multiple-tree falls. Occurrence of bur oak was a function of disturbance and not simple edaphic relationships [27]. Bur oak recruitment occurred in canopy gaps created by a "catastrophic windthrow" event in northern pin oak but not in eastern white pine forests in Minnesota. Density of bur oak (>1 inch (2.5 cm) DBH) was 64 stems/ha before and 92 stems/ha 14 years after the storm [12].
Browsing: Livestock and native ungulates can limit bur oak survival and recruitment. Several studies suggest that browsing can maintain open conditions in oak savannas and woodlands. Bur oak seedlings and saplings are commonly browsed by livestock and deer. On a coal mine restoration site in Kansas, bur oak stems within the reach of cattle were nearly browsed to the ground each year [213]. In the Little Missouri National Grasslands, bur oak survival 5 years after planting was 44% in areas grazed by cattle and 82% in protected areas [253]. In ponderosa pine-bur oak forests in the Black Hills of Wyoming and South Dakota, livestock exclusion is suggested to encourage bur oak regeneration [216]. See Importance to Wildlife and Livestock for more on the utilization and palatability of bur oak.
Researchers suggest that loss of large carnivores, introduction of livestock, and browsing by native ungulates limited recruitment of bur oak in Wind Cave National Park, South Dakota. Tree core analyses showed that bur oak recruitment peaked in the 1870s but was nearly nonexistent after the 1890s. Loss of recruitment coincided with large carnivore removal and rapid increases in livestock abundance. When the Park Service removed livestock, however, bur oak recruitment did not improve, likely because of continued heavy browsing by unchecked native ungulate populations. Bur oak trees with a DBH of less than 20 inches (51 cm) were restricted to areas with physical barriers restricting large mammal access [200]. In an upland 230-year-old red pine forest in Itasca State Park, Minnesota, protection from deer browsing allowed for some recruitment of bur oak into the larger size classes. Researchers reported that moderate to high deer browsing levels slowed woody encroachment and succession [204,233].
| Density (stems/ha) of bur oak stems by size class inside and outside exclosures in a red pine forest in Minnesota [204,233] | ||||||||
| Size class (height, unless otherwise reported) | 0.15-2.1 m | 2.1-4.3 m | >4.3 m | overstory (≥20 cm DBH) |
||||
| in* | out | in | out | in | out | in | out | |
| 1969 | 17 | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| 1984 | 15 | 20 | 15 | 0 | 5 | 0 | 0 | 0 |
| *Exclosures constructed in 1937. | ||||||||
Many studies suggest that grazing can maintain open conditions in bur oak habitats in the absence of fire. In the Sheguiandah Township on Manitoulin Island in Ontario, bur oak savannas have remained open because of almost continuous livestock grazing. According to early surveys, these savannas resulted from a "catastrophic fire" in 1865. None of the savannas had burned since 1865, and in areas protected from grazing, a closed woodland has developed [130]. A similar situation was reported in another study in Ontario [199] and the Barton Woods of north-central Illinois. An open-canopy bur oak woodland changed to a closed-canopy forest with an abundance of other deciduous species after 50 to 60 years without grazing. On continually grazed sites, canopy trees, primarily bur oak, were often 39 to 79 feet (12-24 m) apart and sometimes 164 feet (50 m) apart [163]. In the TL Davis Preserve in southwestern Douglas County, Nebraska, just 2 bur oak trees established before 1895 and nearly all other bur oak and other woody species established after 1968. Reasons for the 70- to 80-year gap in tree establishment were not known, but grazing was suspected because of the fencing remnants observed. In 1850, the bur oak-dominated cover was estimated at 23%, and by 2003, it was 99% [92].
Although many suggest that grazing may inhibit bur oak establishment, others suggest the opposite may be true. In western Iowa, periodic overgrazing of prairies during settlement of the area was suggested as the main reason for "spectacular forest advances" [164].
 |
|
| Photo © Tom Brock, Universtiy of Wisconsin-Madison |
FIRE EFFECTS:
Immediate fire effect on plant: Mature bur oak trees are not typically damaged by fire, and bur oak trees only 3 feet (1 m) tall may survive fire [53,55,120]. Bur oak seedling establishment varies on burned sites
and is limited on repeatedly burned sites [3,5,30,139]. Survival of bur oak acorns on burned sites and heat tolerance of acorns were not reported in the literature. Generally, acorns produced by the white
oak group have little to no dormancy and typically germinate or are removed by predators soon after falling, so establishment from soil-stored seeds on burned sites is unlikely (see Seed banking, Germination, and Seed predation).
Postfire regeneration strategy [235]:
Tree with adventitious buds, a sprouting
root crown,
sobols, and/or
root sprouts
Tall shrub, adventitious buds and/or a sprouting
root crown
Initial off-site colonizer (off site, initial community)
Fire adaptations and plant response to fire: Based on prescribed fire studies in the Cedar Creek Natural Area and a review of other fire studies, Peterson and Reich [190] reported that bur oak is a fire "resister". Bur oak typically survives low-severity fire. It is long-lived and persistent at maturity. Bur oak maintains the potential for population growth when spatial or temporal variability in fire allows for seedling establishment or release of grubs [190].
Fire adaptations: Bur oak is well adapted to survive fire, and frequent fires are necessary for bur oak persistence in many habitats. Because the thick bark of mature bur oak trees insulates their cambium from high temperatures [8,239], mature trees rarely suffer any fire damage [5,30]. Young bur oak trees are typically only top-killed by fire [66,219,221]. Once bur oak trees reach 12 to 15 years old, they can survive repeated burning [53].
| Thick bark: Many sources indicate that bur oak trees produce very thick, fire-resistant bark [55,76,121,222]. Large bur oak trees in eastern Nebraska produced bark about 1.5 inches (5 cm) thick [8]. In Funk's Grove in McLean County, Illinois, open-grown bur oak trees 111 to 140 years old, with DBH measurements of 37 to 68 inches (93-172 cm), had bark thicknesses of 1.6 to 2.4 inches (4-6 cm) [239]. |
 |
| Photo © Paul Wray, Iowa State University, Bugwood.org |
In plantations and natural areas in Illinois, researchers evaluated the physical and protective characteristics of bur oak bark. Bark thickness increased with increasing DBH (r² = 0.93), and relatively high rates of bark thickening occurred with radial growth. Maximum bark thickness was 2.9 inches (7.4 cm) for a bur oak with a DBH of 52.9 inches (134.3 cm). Bark moisture was greatest in the summer and lowest in the fall, but differences were not statistically significant. Using a technique designed to mimic conditions produced by low-severity surface fire, researchers found that the average cambial temperature of bur oak during the fire was 134.8 °F (57.1 °C). Cambial temperature exceeded 140 °F (60 °C) for an average of 3.1 minutes in just one bur oak tree [106,107]. Exposure to temperatures of 140 °F (60 °C) for at least 60 s is typically required to kill vascular plant tissue, but tissue can survive 140 °F (60 °C) temperatures for a longer time when moisture content of the tissue is high [273].
| Bark properties of 40-year-old bur oak in a plantation in midsummer and bur oak rank in relation to 10 other plantation species [106] | ||
| Property | Average measurements for bur oak | Ranking among other species* |
| Bark thickness | 1.36 cm | 2 |
| Tree DBH | 33.57 cm | 4 |
| Moisture content | 84.96% | 5 |
| Specific gravity | 0.47 g/cm³ | 8 |
| Thermal conductivity | 2.30 | 7 |
| Volatile matter | 55.43% | 11 |
| Time to 300 °C ignition | 27 s | 5 |
| Time to 600 °C ignition | 8 s | 6 |
| *Other species: black cherry (Prunus serotina), black walnut (Juglas nigra), eastern cottonwood, shingle oak (Quercus imbricaria), silver maple, sugar maple, sweetgum (Liquidambar styraciflua), sycamore (Platanus occidentalis), white ash, white oak (Q. alba), and yellow-poplar (Liriodendron tulipifera). | ||
Additional evidence of the protective power of bur oak bark came during the reconstruction of fire history in an oak savanna remnant in Kenosha County, Wisconsin. Researchers found scars on white oak recording fires that bur oak of similar ages did not record [271].
Sprouting potential: Young and small bur oak trees are often only top-killed by fire. Additional studies and details are needed, however, to determine what factors or combination of factors most influence bur oak sprouting potential and postfire sprout abundance: tree age, tree size, fire conditions, and/or site conditions. Young bur oak shoots or sprouts that are repeatedly top-killed by fire may develop large burl-like woody growth at the soil surface, which are commonly referred to as "grubs". During brief fire-free periods, grubs are released, and bur oak stem density can increase dramatically [53].
In brush-prairie vegetation in northwestern Minnesota, a 9-year-old bur oak was killed by fire, but a 13-year-old bur oak survived. At another site, the researcher found "severe" fire scars on bur oak trees that were 20 years old or slightly younger [66]. According to Curtis [53], bur oak shoots or sprouts that are protected from fire for 12 to 15 years typically survive subsequent fires. In Forest Glen County Preserve, Illinois, 27 bur oak trees that were 9.8 feet (3 m) tall or taller survived 2 sequential prescribed fires without top-kill. Five bur oak trees that were less than 3.3 feet (1 m) tall were top-killed and sprouted. Trees that were 6.6 to 9.8 feet (2-3 m) tall averaged 13 years old. Prescribed fires burned on 3 March 1992 and 30 March 1993. The 1st fire was "hot" and "intense" and burned moderate fuel loads in dry weather; the 2nd fire was less intense because of heavy spring rains and smaller fuel loads [120]. After a spring prescribed fire in Meade County, South Dakota, researchers found that the abundance of bur oak sprouts/tree increased with increasing scorch heights but decreased with tree age [219,221].
Bur oak stems may not sprout immediately after top-kill and may not sprout at all. After a mid-May prescribed fire in the Chippewa National Forest in Minnesota, researchers monitored sprouting for 5 years. Prior to burning the study site, there were 20 bur oak trees with DBH ranging from 3.9 to 16 inches (10-41 cm). Fuels were primarily quaking aspen slash, and the fire energy output rate was 5,800 cal/s-cm. Trees not killed by the fire were cut down. Sixty percent of the bur oak trees that were top-killed or cut down sprouted after the fire. Sprouts were most abundant in the 3rd postfire year. By the 5th postfire year, there was an average of 21 sprouts/clump, and sprouts averaged 8.2 feet (2.5 m) tall [189].
Plant response to fire: Mature bur oak trees are rarely killed or even top-killed by one or more fires [5,30]. Mortality and top-kill by fire typically decrease with increasing tree age or size [122,190,192]. However, postfire sprouting typically decreases with tree age [219,221]. Bur oak seedling establishment on burned sites is variable and limited on repeatedly burned sites [3,5,30,139].
Fire case studies: Various aspects of bur oak survival and recruitment have been studied and reported in areas managed with prescribed fire. These studies provide additional, site-specific details on the effects of fire on bur oak trees, saplings, and seedlings.
In Meade County, South Dakota, fire effects were evaluated on 24 prairie and woodland plots burned by prescribed fires in April. Bur oak was the dominant tree in the plots; there were 1,097 bur oak trees/ha, and the basal area of bur oak was 39 m²/ha. In the understory, bur oak was rare. Fine fuel loads averaged 590 kg/ha; fine fuel moisture averaged 14.6%; woody fuel loads averaged 11 mt/ha; woody fuel moisture averaged 11%; and soil moisture averaged 38%. Fires spread at an average rate of 0.13 foot (0.04 m)/s. Fire spread was "poor" and several ignitions were often necessary [221]. Mortality of bur oak was rare in burned plots, but 2 fire-scarred bur oak trees with heart rot burned for up to 2 weeks. Just 1 large diameter bur oak tree was consumed by fire, and it produced 1 sprout. Bur oak sprouts were more abundant on burned than unburned plots. The number of bur oak sprouts/tree increased with increasing scorch heights but decreased with tree age. Sprouts were more abundant on bottomland sites than on floodplain or slope sites. Bur oak germination was not increased on burned sites, and seedling survival was similar on burned and unburned plots. Seedling survival between the 1st and 2nd postfire growing seasons was 58.5% on burned and 71.8% on unburned plots. The abundance and survival of bur oak sprouts and seedlings on burned and unburned sites are summarized in the table below [219,221].
| Bur oak sprouts and seedlings on burned and unburned plots 1 and 2 growing seasons after a spring prescribed fire in Meade County, South Dakota [219,221] | ||||||
| Time since fire | 1st postfire growing season | 2nd postfire growing season | ||||
| Burned | 2.2 sprouts/tree | 0.4 shrub-sized stems/m² | 706 seedlings/ha | 2.1 sprouts/tree | 0.5 shrub-sized stems/m² | 429 seedlings/ha |
| Unburned | 0.5 sprouts/tree | 0.4 shrub-sized stems/m² | 1,071 seedlings/ha | 0.5 sprouts/tree | 0.4 shrub-sized stems/m² | 692 seedlings/ha |
Effects of spring prescribed fires were studied in oak savannas in east-central Minnesota's Cedar Creek Natural History Area [190,192]. Prescribed fires occurred in April or May, typically under the following weather conditions: air temperatures between 59 °F (15 °C) and 77 °F (25 °C), relative humidity from 25% to 45%, and wind speeds less than 12 miles (20 km)/hour. Prescribed fire frequencies for individual plots ranged from 0 to 26 fires in 32 years.
A study of 1st postfire growing season effects after a single, mid-May prescribed fire revealed that in general, the greater the sapling height at the time of the fire, the greater the number and height of postfire sprouts produced. This "low intensity" prescribed fire burned when the air temperature was 59 °F (15° C) and winds were less than 6.2 miles (10 km)/hour. Dry northern pin oak leaves were the primary surface fuel; flame lengths were 4 to 12 inches (10-30 cm). Before the fire, the site supported 800 bur oak saplings 10 to 26 feet (3-8 m) tall, with basal diameters of 1 to 3.5 inches (3-9 cm). The fire top-killed all but 2 saplings. For saplings greater than 3 feet (1 m) tall, the postfire sprouting frequency was 95% [192].
Bur oak seedlings and sprout densities were similar among plots with varied fire frequency in the Cedar Creek Natural History Area, but seedlings and sprouts were "suppressed" in frequently burned plots (11-26 fires in 32 years). Most sprouts grew from grubs. Generally, bur oak stems reached sapling height (5 feet (1.5 m)) in 3 years in the absence of fire. Dense thickets of bur oak saplings occurred in plots burned at low frequency (4 fires in 32 years). Mature bur oaks, even those in smaller size classes, were rarely killed by fire. When mortality occurred on burned sites it was typically from damage caused by the fall of another tree of a different species that was killed by fire. Mortality of bur oak trees on unburned sites was often the result of shading [190]. In the most frequently burned plots (26 fires in 32 years), the only tree species present were bur oak and northern pin oak [191]. The density and fate of bur oak saplings and trees on burned and unburned plots are summarized below.
| Abundance of bur oak saplings and abundance and fate of bur oak trees on unburned and repeatedly burned plots in the Cedar Creek Natural History Area in east-central Minnesota [190] | |||
| Unburned | Low-frequency fire (4 fires in 32 yrs) |
High-frequency fire (11 fires in 32 yrs) |
|
| Sapling* density (stems/ha) | 89 | 215 | 2 |
| Tree** basal area (m²/ha) | 0.91 | 0.07 | 0.11 |
| Tree density (stems/ha) | 36 | 7 | 3 |
| Tree mortality | 17.5% | 8.3% (all burned plots) | |
| *Saplings: ≥1.5 m tall and <5 cm DBH. **Trees: ≥5 cm DBH. |
|||
Earlier studies of the unburned and burned plots in the Cedar Creek Natural History Area described effects of prescribed fires that ranged from 11 to 17 fires in 17 years. Density of bur oak stems increased on unburned sites. Bur oak mortality averaged 29% across all plots, but recruitment exceeded mortality. Mortality of the oaks (bur oak and northern pin oak) averaged 75% for trees with diameters less than 4 inches (10 cm) and 30% for trees with diameters between 4 and 6.7 inches (10-17 cm). Oak mortality was least on plots burned 2 times in 17 years. Oak mortality was higher on plots burned 6 to 9 times in 17 years than on plots burned 11 times in 17 years. Researchers suggested that fires may have been more severe on the less frequently burned plots due to increases in woody stem densities with longer fire-free periods [122].
Fires every 3 to 5 years limited bur oak recruitment at Allison Savanna in east-central Minnesota, where prescribed fire is used to manage bur oak-northern pin oak barrens. The density of bur oak was 107 stems/ha on unburned plots and 53 stems/ha on high-frequency burned plots (25-year fire-return interval of 1.6-1.9 years). In unburned plots and low-frequency burned plots (25-year fire-return interval of 3.1-5 years), bur oak was abundant in the 4- to 9.8-inch (10-25 cm) DBH size classes. In unburned plots, bur oak trees ranged from 20 to more than 200 years old. In high-frequency burned plots, all bur oak stems were over 70 years old [67].
In the Namekagon River Barrens in northwestern Wisconsin, frequency of bur oak averaged 28% on burned and 16% on unburned sites. Burned sites experienced 1 or 2 spring fires, and postfire sampling occurred in the 1st or 2nd postfire growing season [258]. Bur oak density and basal area were greater on burned than unburned woodland plots in the Marengo Ridge Conservation Area of Illinois. Burned plots experienced 2 fall prescribed fires that were 4 years apart. Fires were low to moderate severity and burned when air temperatures were 60.1 °F (15.6 °C) and 47 °F (8.3 °C), relative humidities averaged 80% and 63%, and winds were 5 miles (8 km)/hour and 15 miles (24 km)/hour, respectively. Two years after the last fire, the density of bur oak (≥2-inch (5 cm) DBH) was 11 stems/ha on burned plots and 3 stems/ha on unburned plots. Basal area was 7.1 ft² (0.66 m²)/ha on burned and 2.9 ft² (0.27 m²)/ha on unburned plots [227].
Postfire seedling establishment: Fires may affect bur oak seedling establishment directly by removing litter and exposing mineral soil and indirectly by influencing the behavior of seed predators. However, the importance or inhibitory effects of litter and moisture on bur oak seedling establishment are unclear (see Seedling establishment). One researcher suggests that litter benefits establishment and survival of oak seedlings [153], while another researcher found that removal of litter improved oak seedling establishment in the field [141]. Because seed predators reduce the number of acorns available for establishment (see Seed predation), Lorimer [153] suggests that acorns on burned, open sites, which are not attractive feeding sites for many small mammals, may avoid predation better than those in unburned areas. However, such avoidance of predation could be counterproductive: The field study conducted by Krajicek [141] found that burial in mineral soil was most important to successful oak seedling establishment, and small mammal caches may be important for burial [74].
Because the combination of factors most conducive to bur oak seedling establishment is unclear, it is not surprising that fire studies fail to report clear patterns of postfire seedling establishment. It does appear however, that annual fires limit bur oak seedling establishment. In Madison County, large bur oak trees were frequent but there were no bur oak seedlings in a prairie remnant burned annually for at least the last 8 years [139]. In the Morton Arboretum in DuPage, Illinois, bur oak trees survived 17 years of annual, dormant-season, low-severity fires, but there was "little evidence for regeneration of oak species" [30]. See the Research Paper by Bowles and others 2007 for further information on prescribed fire and postfire responses of several plant species, including bur oak. On the Konza Prairie, bur oak seedlings were not present before burning in oak gallery forests, but in the 1st growing season after a late-April prescribed fire, the density of bur oak seedlings was 50/ha. The same bur oak seedling density was reported after another prescribed fire in early April of the following year. Prescribed fires moved slowly, 3 to 6.6 feet (1-2 m)/min, produced low flame heights (<1.6 feet (0.5 m)), and did not burn into tree crowns [3]. However, in a later study on the Konza Prairie, bur oak seedlings present before fires were absent 2 years after fire [5].
| Fuels: Several characteristics of oak litter and woody debris make them flammable and important to fire spread. In a controlled experiment, dried bur oak leaves produced temperatures of up to 700 °F (371 °C) [231]. Oak leaves are thick, rigid, and irregularly shaped, which allows for efficient drying and persistence in the litter layer [182]. Leaves curl as they dry and produce a "loose, porous" fuel bed, which can easily carry fire (review by [153]). Oak leaves typically remain curled after snow melt, which allows for drying early in the spring. The high phenolic content of oak leaves means slow decomposition rates, ensuring fuel longevity [182]. Burning oak leaves can also be blown ahead of a fire, potentially igniting spot fires and increasing fire size (review by [153]). When compared, the oak fuel bed is much more "conducive to burning" than that of other hardwoods, which produce thin leaves that stick to the forest floor, trap moisture, provide few air-drying pockets, and decompose rapidly. Woody debris produced by oaks resists decay and provides a long-lasting fuel. Woody debris from other hardwoods decays much more rapidly than that of oak [182]. |
 |
Prescribed fire in bur oak savanna near Vermont,
Wisconsin; primary fuel for the surface fire was bur oak leaves. |
Fuel characteristics in bur oak habitats may be affected by associated vegetation. In riparian areas in central Texas, eastern Oklahoma, southeastern Kansas, southern Missouri, and western Arkansas, bur oak occurred with riverbank grape (Vitis riparia). Often riverbank grape covered the lower branches of bur oak [173]. It is possible that the presence of riverbank grape as a ladder fuel could influence fire behavior and fire effects.
Fire regimes: Bur oak is highly fire adapted, and frequent fires are necessary for bur oak persistence in most habitats. Although very frequent fires (<5-year intervals) typically eliminate bur oak recruitment into large size classes, patchy burn patterns or safe sites in burned areas could allow for limited recruitment and persistence even in very frequently burned sites. Loss of bur oak is much more likely through succession in unburned areas. Throughout bur oak's range, studies indicate that reduced fire frequencies often associated with European settlement have been detrimental to bur oak [104,137,182,183,236].
Fire characteristics in bur oak habitats: Fire regimes in bur oak savannas are characterized by frequent, low-severity surface fires at intervals of less than 25 years. Crown fires and severe surface fires are extremely rare, occurring at intervals of over 1,000 years [75]. In a ponderosa pine forest with bur oak in South Dakota, fires were primarily surface fires. Stand-replacing fires burned only 3.3% of the total study area between 1529 and 1893 [37]. At the Konza Prairie, temperatures were monitored during a mid-April prescribed fire. In the tallgrass prairie, ground-level temperatures during the fire ranged from 66 to 750 °F (19-399 °C). In gallery forests where bur oak was most common, fire temperatures at ground level were lower, ranging from 66 to 365 °F (19-185 °C). Fire temperatures in the prairie were generally highest in areas that were unburned the longest [83].
At the Cedar Creek Natural History Area in Minnesota, researchers found that frequently burned plots had higher litter temperatures, lower litter moisture, and lower soil nitrogen and phosphorus availability than unburned plots. There were 0.3 to 0.8 spring prescribed fires/year in frequently burned plots. Fires were low severity and rarely resulted in complete consumption of the litter layer. Burned plots were dominated by grass, with scattered bur oak and northern pin oak. Unburned plots were closed-canopy oak forests dominated by northern pin oak [109].
Presettlement and contemporary fire regimes: Often in bur oak habitats, fire frequency decreased dramatically with settlement by European Americans. Cessation of burning by American Indians, conversion of land to agricultural use, livestock introductions, and active fire suppression are cited as the primary reasons for reduced fire frequencies and the subsequent loss of bur oak habitats [34,182,183,236,271].
Throughout the Midwest, many fire history studies document reduced fire frequency and severity in bur oak habitats. Fire frequency and fire severity reductions associated with European settlement have resulted in an extensive loss of area occupied by oak savannas, oak woodlands, and oak-pine woodlands. In the early 1900s, prairies and savannas occupied 27 to 32 million acres (11-13 million ha) of the Midwest, but in early 2000, just 0.02% of this area supported prairies and oak savannas. Presettlement and current fire regimes in the Midwest have changed dramatically from grasslands, savannas, and woodlands that experienced frequent and "intense" fires to agricultural lands or closed-canopy forests. As the "pyrogenic" communities remained unburned, the density of woody vegetation and shading increased and areas became increasingly mesic. This "mesophication" process produced communities that do not readily burn and makes restoration through the use of fire unlikely [182].
Burning by American Indians: Several studies suggest that burning by American Indians was important to the maintenance of prairies and oak savannas in the Midwest. American Indians were thought to have burned the prairie region nearly every year. In central North America, all but 1 of 247 prairie fires with known ignition sources was the result of human activity (Moore 1972 cited in [91]).
Studies in the Black Hills suggest that the Oglala Sioux used fire more frequently than the Cheyenne, Kiowa, and Crow tribes. Before 1770, the time between large fires in the Devil's Tower National Monument Area averaged 27 years. From 1770 to 1900, the average time between large fires was 14 years. Fire frequency increased near the time when the Oglala Sioux took over the area, which had been controlled by the Cheyenne, Kiowa, and Crow. The Oglala Sioux had come from prairie-forest border areas where they utilized fire to drive and kill animals. After 1900, the average time between large fires increased dramatically to 42 years. The last area-wide fire occurred in 1937, when the area was already settled by Europeans [69,70].
In an oak savanna remnant in Kenosha County, Wisconsin, 53% of fire scars represented dormant-season fires, which suggested a human ignition source because late summer is the season for lightning-caused fires in the area. Fire history was reconstructed from the cross sections of bur oak and white oak trees cut down to make way for an industrial park. Trees were 153 to 196 years old. During the presettlement period of 1829 to 1839, the mean fire-return interval was 3.7 years. During the peak of European American settlement, from 1840 to 1871, the mean fire-return interval increased to 19.5 years [271].
European settlement: The loss of bur oak habitats with European settlement, which typically coincided with reductions in fire frequency and/or fire severity, is well documented [34,182,183,236,271]. In some cases, activities associated with settlement, such as land clearing and railroad sparks, produced a short-lived increase in fire frequency.
In northwestern Minnesota and west-central Canada, bur oak is common in quaking aspen savannas, where the presettlement fire cycle was estimated at 2 to 15 years. Large, surface fires were most common. After settlement of the area, the fire cycle was estimated at 1,000 years. Fire suppression, logging, land clearing, roads, railroads, utility corridors, and urban areas all factored into the loss of fire in aspen savannas [104]. In the Konza Prairie, increases in forest-dominated areas and decreases in oak recruitment likely resulted from decreased fire frequency and/or severity. In 1858, only about 12 acres (5 ha) of the prairie was forest dominated; in 1939, forest-dominated area had increased to about 274 acres (111 ha); and in 1978, forest-dominated area had increased to 509 acres (206 ha) [3]. Reduced influence of fire was likely associated with European settlement of the area, which occurred in about 1840. Settlement activities that likely contributed to limiting fire occurrence and/or fire effects included construction, expansion of towns, farming, grazing by livestock, and fire suppression (review by [4]).
In a small 15.6-acre (6.3 ha) ponderosa pine forest, where bur oak occurred in the Black Hills of Lawrence County, South Dakota, the last fire occurred in 1879. Fire-scarred cross sections and increment cores indicated that fires were frequent between the mid-1600s and 1879. Mean fire-return intervals were estimated at 11 to 15 years but ranged from 1 to 43 years [265]. At Mount Rushmore, South Dakota, the fire history in a ponderosa pine forest revealed that the last fire occurred in 1893. From 1529 to 1893, the mean fire-return interval for the area was 16 years. The estimated low-severity, surface-fire rotation was 30 years and crown-fire rotation was 846 years [37].
In some cases, fire frequency increases in bur oak habitats were associated with European settlement. Although the overall fire frequency decreased with settlement of an oak savanna remnant in Kenosha County, Wisconsin, fire frequency increased in the mid-1920s with railroad development [271]. In the Brickyard Hill Conservation Area of northwestern Missouri, tree-ring records from bur oak, chinkapin oak, and black oak indicated that fire was rare after the mid-1950s but that a period of very frequent fire coincided with European settlement in the area. Tree cores established a record from 1671 to 2004. From 1672 to 1820, the time before European settlement of the area, the average fire-return interval was 6.6 years. Fires were very frequent from 1825 to 1850, which coincided with European settlement. Researchers found no relationships between fire and drought for any portion of the record. Most fire scars were made in the dormant season, September to March. Fire scars were much less frequent after 1900. Researchers suggested the reduced fire frequency may have been caused by domestic grazing, which would have decreased fuels and thus fire frequency [226].
See the Fire Regime Table for more information on fire regimes of vegetation communities in which bur oak may occur. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
FIRE MANAGEMENT CONSIDERATIONS:
Oak savannas are among the most threatened habitats in the Great Lakes region, and their restoration
will require fire (review by [136]). Cost and effort of restoration with fire will likely be less if implemented before sites are fully converted to dense, mesic, closed-canopy forests. Several prescribed fire programs and fire studies are available [67,190,192,221] and can be used as guides, although adaptations may be necessary to meet local objectives. In some cases, nonnative species and threatened
and endangered species need special consideration in the development of a fire management program.
In the Midwest, millions of acres of what were oak savannas are now mesic, closed-canopy forests. The potential for restoring these forests with fire is limited by their reduced flammability. Seed sources of the once dominant, fire-adapted species may be lacking, and abundance of nonnative invasive species can be high. Opportunities for fire restoration are best in oak- or oak and pine-dominated woodlands. Large contiguous areas of ownership should be restoration priorities because the ability to burn larger areas maximizes cost benefit and allows for variable fire behavior [182].
Observations during and after spring prescribed fires in oak savannas on the Sherburne National Wildlife Refuge in Minnesota revealed that bur oak trees encroaching into grasslands were easily top-killed by fire occurring when air temperature was 80 °F (27 °C) or more and relative humidity was less than 25% [186]. Based on findings from prescribed fire studies in the Cedar Creek Natural History Area, researchers suggest that restoration of "degraded" oak savannas begin with annual burning, which may need to be coupled with mechanical stand thinning, to reduce overstory density. Fire intervals may be lengthened to 2 or more years after the overstory and understory are opened [190]. Prescribed fire results in Meade County, South Dakota, caused researchers to think that increased fire severity and larger burned areas may be necessary to encourage bur oak reproduction. Because their spring prescribed fire did not increase bur oak seedling establishment, researchers suggested several prescription changes to increase fire severity and fire size: 1) burn in the fall instead of the spring, so that grasses are not matted from melted snow, 2) burn after a killing frost to reduce green grass cover to less than 25%, 3) remove grazing to increase fine fuel loads to over 1,500 pounds (700 kg)/ha, and 4) wait for wind speeds of 8 miles (13 km)/hour or more [221].
Prescribed fire considerations: Sensitive and nonnative species are important to consider in the fire management of oak habitats. Insects may also require consideration. In the Upper Midwest, bur oak dominates dry calcareous savannas, where the density and cover of woody vegetation have increased with decreased fire frequency. These habitats are important to many endangered, threatened, or species of concern in Wisconsin [266], which need to be considered before reintroducing fire. Although threatened savanna species are likely adapted to fire, increases in woody fuels may produce fire behaviors and severities different from what occurred historically.
In Wisconsin, reed canarygrass (Phalaris arundinacea), nonnative honeysuckles (Lonicera spp.), and common buckthorn add to the complexity of managing bur oak habitats with fire. In south-central Wisconsin, reed canarygrass forms dense monotypic stands that "appear very shade tolerant and highly competitive" [105]. In an attempt to restore oak svannas and reduce nonnative shrub dominance at the University of Wisconsin Arboretum, thinning, burning, herbicide treatments, and native species plantings were utilized. The understory in the Arboretum was dominated by dense honeysuckle and common buckthorn. None of the treatments, alone or in combination, eliminated common buckthorn. Common buckthorn increased in thinned plots. Although common buckthorn cover was reduced on plots burned twice in 2 years, it was not reduced on plots burned only once in 2 years (P<0.05), and common buckthorn stem densities were not altered significantly [212].
At the Cedar Creek Natural History Area, lace bugs (Corythuca arcuata), which are bur oak specialists, were significantly more abundant in frequently burned than unburned areas (P<0.01). Light available to bur oak branches was significantly greater in burned than unburned areas (P=0.07), and lace bug abundance increased with increasing light availability [134]. Arthropod communities on bur oak bark also differed with fire frequency at the Cedar Creek Natural History Area. Some arthropod taxa were sensitive to frequent fire, and some were not. The number of arthropod species living on bur oak bark was greatest in unburned areas and least on areas burned frequently (20 fires in 25 years) [180].
FEDERAL LEGAL STATUS:
None
OTHER STATUS:
Information on state- and province-level protection status of plants in the United States and
Canada is available at
NatureServe. At the eastern and southern
fringes of its range, bur oak has been described as endangered [156], of special concern [160],
and critically imperiled [154].
IMPORTANCE TO WILDLIFE AND LIVESTOCK:
Bur oak acorns and stems are consumed by a variety of wildlife and livestock species. Bur oak acorns are eaten by black bears, deer, cattle, goats, squirrels, cottontails, mice, and other rodents [26,121,125,157,202,252].
Several bird species utilize bur oak trees or communities for nesting and for foraging (reviews by [88,230]). In Manitoba and Saskatchewan, bur oak is browsed by deer, moose, and rabbits.
When browsing pressure is heavy, bur oak may be dwarfed and/or less abundant [23].
Black bears: Acorns are an important fall food for black bears (review by [203]). In northeastern Minnesota, mature bur oak and northern red oak (Quercus rubra) stands make up only 0.5% of the Superior National Forest, but based on radio-tracked movements, they are very important to black bears. In mast years, acorns are an important food source [202].
Native ungulates: Many studies indicate that bur oak is browsed by deer and elk. Of 8 forest stand types in Riding Mountain National Park, Manitoba, ungulates browsed the bur oak type most heavily [42]. In South Dakota's Wind Cave National Park, bur oak seedlings are browsed by deer and elk, and bison trails occur in bur oak stands [157]. Bur oak is considered moderately important browse and cover for white-tailed deer and mule deer in the Black Hills of South Dakota and Wyoming [79,185]. Browse availability and herbivore stomach content analyses suggest that bur oak is more palatable to white-tailed deer in the fall or winter than in the spring or summer in the Black Hills [111]. Stomach content analyses of white-tailed deer and mule deer from the Black Hills revealed that the frequency and volume of bur oak in diets from January to April was 53% and 2.5%, respectively [112].
Small mammals: Field observations and captive feeding trials indicate that bur oak is important to many small mammals. Scatter-hoarding of bur oak acorns was observed near Manhattan, Kansas [228]. Deer mice and white-footed mice were common in bur oak woodlands along the Missouri River in South Dakota. White-footed mice were most abundant in mid-seral bur oak communities, and deer mice were most abundant in early-seral bur oak communities. Cover of bur oak, forbs, and shrubs increased from early- to late-seral communities [207]. In lab feeding trials, live-trapped eastern gray squirrels and eastern fox squirrels preferred bur oak acorns over many other oak acorns, black walnuts, and shagbark hickory nuts [223]. However, in another feeding trial, bur oak acorns were not preferred by eastern fox squirrels. Bur oak ranked 9th out of the 12 acorn species fed. In the habitats where eastern fox squirrels were trapped, bur oak was scarce to nonexistent, suggesting that squirrels without prior exposure to bur oak might avoid it [184]. In feeding experiments using white-footed mice trapped from eastern Kansas, researchers found that prior experience may affect acorn selection. White-footed mice trapped from a white oak forest in eastern Kansas ate more bur oak acorns than mice from red oak or oak-free habitats [35].
Game birds: Bur oak provides cover and forage for sharp-tailed grouse and wild turkeys ([71,113,178], review by [230]). In Oklahoma, wild turkeys use bur oak for roosting (review by [230]). Grassland-bur oak woodland edges were important habitat for wild turkey broods in Gregory County, South Dakota [58]. Wild turkey hens selected woodlands over grasslands for nesting (P<0.05). Of 8 woodland nests, 4 were next to bur oak and 1 was under a currant (Ribes spp.) next to a bur oak [263]. Frequency of bur oak in crops from wild turkeys killed in the fall was 80% in 1985. Bur oak was lacking from crops in 1984, which likely reflected a failure in bur oak acorn production and not avoidance by wild turkeys. Bur oak woodlands made up 26% of the study area [147].
Other birds: Various other bird species use bur oak for forage and habitat. In east-central Minnesota, bird communities in prairie, oak savanna, and oak woodlands were compared. Bird species presence and abundance were greatest in oak savannas dominated by bur oak, northern pin oak, or northern red oak [14]. In the Black Hills of South Dakota, bur oak was used by several cavity-nesting birds including mountain bluebirds, white-breasted nuthatches, and northern flickers. White-breasted nuthatches used 50 to 100% of bur oak cavities available [82]. Oaks (bur oak, northern red oak, and chinkapin oak) were preferred by winter-foraging birds in the Brownfield Woods of Illinois. White-breasted nuthatches, Eurasian treecreepers, red-bellied woodpeckers, and downy woodpeckers utilized oaks most [269].
Several studies indicate that bur oak is important for birds belonging to the woodpecker (Picidae) family. The winter diet of red-headed woodpeckers is primarily hard mast. Red-headed woodpeckers will migrate out of Kansas when bur oak acorn crops fail (review by [224]). In the Brownfield Woods of Illinois, bur oak was utilized by 17% of red-bellied woodpeckers and 8% of red-headed woodpeckers. Woodlands in the study area were dominated by sugar maple, hackberry, and northern red oak [197]. A review of Illinois birds reports that bur oak provides food and habitat for woodpeckers. Several woodpecker species in Illinois feed on bur oak acorns, although they rarely comprise a large proportion of diets. About 25% to 30% of bur oak were drilled for sap by yellow-bellied sapsuckers. Northern flickers used bur oak for nest sites [88].
Livestock: Both cattle and goats are reported to browse bur oak. On prairie remnants in northwestern Illinois, bur oak was "preferred" by dairy goats [26].
Although cattle may be poisoned by a diet of more than 50% oak (Quercus spp.) [264], studies suggest that cattle do not avoid bur oak. On a coal mine reclamation site in Kansas, researchers reported that bur oak seedlings planted within reach of cattle were browsed almost to ground level each year [213]. Seasonal preferences may occur in some years. In the Black Hills of South Dakota, bur oak made up less than 4% of June and July, 12% of August and October, and 25.6% of September diets [252]. In another year in the Black Hills, bur oak made up 13.2% of June, 8.7% of July, 12.2% of August, and 12.4% of September diets [254].
Palatability and/or nutritional value: Bur oak acorns and browse are considered palatable and nutritious. Bur oak protein was highest in the summer, and fiber was highest in the spring in the Black Hills of South Dakota [79]. Bur oak acorns from Missouri had 4,340 calories/g [223], and bur oak acorns collected in Louisiana had 4,266 calories/g [41]. Nutritional value of bur oak buds, twigs, and acorns collected in the winter from South Dakota and Wyoming is presented by Severson and Kranz [214].
VALUE FOR REHABILITATION OF DISTURBED SITES:
Survival and persistence of bur oak have been reported on revegetated mine sites. Bur oak can be established from seed or seedlings and grows well with herbaceous species but is not recommended for spoils with a pH less than 4. Guidelines for use and planting of bur oak are available in Vogel's guide [257]. Survival of bur oak was as high as 75% on surface coal mine reclamation sites in Wyoming and Colorado; bur oak did not survive on the uranium surface mine site [119]. A review of Forest Service records showed that bur oak survival averaged 39% on coal mine spoils in Missouri, Kansas, and/or Oklahoma. Age of spoils ranged from 1 to 16 years old, and pH ranged from 2.4 to 8.1 (review by [256]).
On coal mine spoils in Ohio, 1st-year bur oak survival was among the best of the tree species seeded [152]. Bur oak survival averaged 28% at the end of the 4th growing season on surface mine sites
in eastern Kentucky, where the pH was 4.5 and available phosphorus was low to very low [193].
On another surface mine site in eastern Kentucky, bur oak survival was reported as 68% in the 5th
year [241]. In Laurel County, Kentucky, bur oak was still present 18 years after planting on a coal surface mine site [245].
OTHER USES:
Acorns produced by species within the white oak group are considered palatable and were preferred by American Indians and early European settlers [159]. The Cheyenne of Montana ate bur oak acorns in a mush mixed with buffalo fat [100].
OTHER MANAGEMENT CONSIDERATIONS:
At the edges of its range, bur oak may be a conservation concern [20,167,209].
Bur oak is also a food source of lepidopterans, of which one species is threatened [179,210].
Given bur oak's fragile existence in some areas as well as its importance to the persistence of other threatened species, its response to predicted climate change is considered important.
Conservation concerns: Bur oak is a conservation concern in Canada [20,167], and several bur oak communities in the Plains region are considered "imperiled" [209]. This topic has also been discussed in Local distribution changes.
The following Great Plains communities are rare and/or vulnerable to extinction [209]:
Bur oak savannas are important for lepidopteran communities. On Iowa's Neal Smith National Wildlife Refuge, smaller forests lacking a prominent bur oak component supported 65 fewer species of moths than larger bur oak-dominated savanna remnants [238]. The barrens dagger moth (Acronicta albarufa) is declining in the northeastern United States. At the caterpillar stage it feeds on bur oak. Land development and fire exclusion threaten the barrens dagger moth [179]. Within the moth's range in Manitoba, bur oak is the only caterpillar food available [210].
Climate change responses: Bur oak range expansions are predicted in many but not all climate change models. A northern expansion of bur oak's range was predicted from climate change models, assuming that bur oak successfully colonized all habitats made suitable by climate change [166]. Based on model simulations, bur oak was expected to increase in abundance with a 9 °F (5 °C) increase in the annual temperature in northwestern Wisconsin [103]. Conversions from boreal forests to grassland savannas or temperate forests are expected with warmer climates in Minnesota's Boundary Waters Canoe Area. Bur oak is expected to expand its range with increasing temperatures in the area. When warm, dry climates prevailed 8,000 to 5,000 years before present, oak species increased their range to the northeast. Oak species ranges decreased in the last 3,000 years with cool climates [77]. In the eastern United States, bur oak's importance is predicted to decrease with climate change and a doubling of current carbon dioxide levels [123].
Although rarely addressed in climate change analyses, the effects of bur oak pests will likely affect distribution changes associated with climate change. For a discussion of current, common bur oak pests and diseases, see the following reviews [125,201].
The following table provides fire regime information that may be relevant to bur oak habitats. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
| Fire regime information on vegetation communities in which bur oak may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [146], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||||||||
|
|||||||||||||
| Northern and Central Rockies | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northern and Central Rockies Grassland | |||||||||||||
| Northern prairie grassland | Replacement | 55% | 22 | 2 | 40 | ||||||||
| Mixed | 45% | 27 | 10 | 50 | |||||||||
| Mountain grassland | Replacement | 60% | 20 | 10 | |||||||||
| Mixed | 40% | 30 | |||||||||||
| Northern and Central Rockies Shrubland | |||||||||||||
| Riparian (Wyoming) | Mixed | 100% | 100 | 25 | 500 | ||||||||
| Northern and Central Rockies Forested | |||||||||||||
| Ponderosa pine (Northern Great Plains) | Replacement | 5% | 300 | ||||||||||
| Mixed | 20% | 75 | |||||||||||
| Surface or low | 75% | 20 | 10 | 40 | |||||||||
| Ponderosa pine (Black Hills, low elevation) | Replacement | 7% | 300 | 200 | 400 | ||||||||
| Mixed | 21% | 100 | 50 | 400 | |||||||||
| Surface or low | 71% | 30 | 5 | 50 | |||||||||
| Ponderosa pine (Black Hills, high elevation) | Replacement | 12% | 300 | ||||||||||
| Mixed | 18% | 200 | |||||||||||
| Surface or low | 71% | 50 | |||||||||||
| Northern Great Plains | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northern Plains Grassland | |||||||||||||
| Nebraska Sandhills prairie | Replacement | 58% | 11 | 2 | 20 | ||||||||
| Mixed | 32% | 20 | |||||||||||
| Surface or low | 10% | 67 | |||||||||||
| Southern mixed-grass prairie | Replacement | 100% | 9 | 1 | 10 | ||||||||
| Central tallgrass prairie | Replacement | 75% | 5 | 3 | 5 | ||||||||
| Mixed | 11% | 34 | 1 | 100 | |||||||||
| Surface or low | 13% | 28 | 1 | 50 | |||||||||
| Northern tallgrass prairie | Replacement | 90% | 6.5 | 1 | 25 | ||||||||
| Mixed | 9% | 63 | |||||||||||
| Surface or low | 2% | 303 | |||||||||||
| Southern tallgrass prairie (East) | Replacement | 96% | 4 | 1 | 10 | ||||||||
| Mixed | 1% | 277 | |||||||||||
| Surface or low | 3% | 135 | |||||||||||
| Oak savanna | Replacement | 7% | 44 | ||||||||||
| Mixed | 17% | 18 | |||||||||||
| Surface or low | 76% | 4 | |||||||||||
| Northern Plains Woodland | |||||||||||||
| Oak woodland | Replacement | 2% | 450 | ||||||||||
| Surface or low | 98% | 7.5 | |||||||||||
| Northern Great Plains wooded draws and ravines | Replacement | 38% | 45 | 30 | 100 | ||||||||
| Mixed | 18% | 94 | |||||||||||
| Surface or low | 43% | 40 | 10 | ||||||||||
| Great Plains floodplain | Replacement | 100% | 500 | ||||||||||
| Great Lakes | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Great Lakes Grassland | |||||||||||||
| Mosaic of bluestem prairie and oak-hickory | Replacement | 79% | 5 | 1 | 8 | ||||||||
| Mixed | 2% | 260 | |||||||||||
| Surface or low | 20% | 2 | 33 | ||||||||||
| Great Lakes Woodland | |||||||||||||
| Great Lakes pine barrens | Replacement | 8% | 41 | 10 | 80 | ||||||||
| Mixed | 9% | 36 | 10 | 80 | |||||||||
| Surface or low | 83% | 4 | 1 | 20 | |||||||||
| Jack pine-open lands (frequent fire-return interval) | Replacement | 83% | 26 | 10 | 100 | ||||||||
| Mixed | 17% | 125 | 10 | ||||||||||
| Northern oak savanna | Replacement | 4% | 110 | 50 | 500 | ||||||||
| Mixed | 9% | 50 | 15 | 150 | |||||||||
| Surface or low | 87% | 5 | 1 | 20 | |||||||||
| Great Lakes Forested | |||||||||||||
| Great Lakes floodplain forest | Mixed | 7% | 833 | ||||||||||
| Surface or low | 93% | 61 | |||||||||||
| Great Lakes pine forest, jack pine | Replacement | 67% | 50 | ||||||||||
| Mixed | 23% | 143 | |||||||||||
| Surface or low | 10% | 333 | |||||||||||
| Maple-basswood | Replacement | 33% | >1,000 | ||||||||||
| Surface or low | 67% | 500 | |||||||||||
| Maple-basswood-oak-aspen | Replacement | 4% | 769 | ||||||||||
| Mixed | 7% | 476 | |||||||||||
| Surface or low | 89% | 35 | |||||||||||
| Oak-hickory | Replacement | 13% | 66 | 1 | |||||||||
| Mixed | 11% | 77 | 5 | ||||||||||
| Surface or low | 76% | 11 | 2 | 25 | |||||||||
| Pine-oak | Replacement | 19% | 357 | ||||||||||
| Surface or low | 81% | 85 | |||||||||||
| Northeast | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Northeast Woodland | |||||||||||||
| Eastern woodland mosaic | Replacement | 2% | 200 | 100 | 300 | ||||||||
| Mixed | 9% | 40 | 20 | 60 | |||||||||
| Surface or low | 89% | 4 | 1 | 7 | |||||||||
| Oak-pine (eastern dry-xeric) | Replacement | 4% | 185 | ||||||||||
| Mixed | 7% | 110 | |||||||||||
| Surface or low | 90% | 8 | |||||||||||
| Northeast Forested | |||||||||||||
| Northern hardwoods (Northeast) | Replacement | 39% | >1,000 | ||||||||||
| Mixed | 61% | 650 | |||||||||||
| Appalachian oak forest (dry-mesic) | Replacement | 2% | 625 | 500 | >1,000 | ||||||||
| Mixed | 6% | 250 | 200 | 500 | |||||||||
| Surface or low | 92% | 15 | 7 | 26 | |||||||||
| Beech-maple | Replacement | 100% | >1,000 | ||||||||||
| South-central US | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| South-central US Grassland | |||||||||||||
| Bluestem-sacahuista | Replacement | 70% | 3.6 | 1 | |||||||||
| Mixed | 30% | 7.7 | 2 | ||||||||||
| Blackland prairie | Replacement | 96% | 4 | ||||||||||
| Surface or low | 4% | 100 | |||||||||||
| Southern tallgrass prairie | Replacement | 91% | 5 | ||||||||||
| Mixed | 9% | 50 | |||||||||||
| Oak savanna | Replacement | 3% | 100 | 5 | 110 | ||||||||
| Mixed | 5% | 60 | 5 | 250 | |||||||||
| Surface or low | 93% | 3 | 1 | 4 | |||||||||
| South-central US Woodland | |||||||||||||
| Oak-hickory savanna (East Texas) | Replacement | 1% | 227 | ||||||||||
| Surface or low | 99% | 3.2 | |||||||||||
| Interior Highlands dry oak/bluestem woodland and glade | Replacement | 16% | 25 | 10 | 100 | ||||||||
| Mixed | 4% | 100 | 10 | ||||||||||
| Surface or low | 80% | 5 | 2 | 7 | |||||||||
| Oak woodland-shrubland-grassland mosaic | Replacement | 11% | 50 | ||||||||||
| Mixed | 56% | 10 | |||||||||||
| Surface or low | 33% | 17 | |||||||||||
| Interior Highlands oak-hickory-pine | Replacement | 3% | 150 | 100 | 300 | ||||||||
| Surface or low | 97% | 4 | 2 | 10 | |||||||||
| South-central US Forested | |||||||||||||
| Interior Highlands dry-mesic forest and woodland | Replacement | 7% | 250 | 50 | 300 | ||||||||
| Mixed | 18% | 90 | 20 | 150 | |||||||||
| Surface or low | 75% | 22 | 5 | 35 | |||||||||
| Southern floodplain | Replacement | 42% | 140 | ||||||||||
| Surface or low | 58% | 100 | |||||||||||
| Cross Timbers | Replacement | 3% | 170 | ||||||||||
| Mixed | 2% | 250 | |||||||||||
| Surface or low | 94% | 6 | |||||||||||
| Southern Appalachians | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Southern Appalachians Grassland | |||||||||||||
| Bluestem-oak barrens | Replacement | 46% | 15 | ||||||||||
| Mixed | 10% | 69 | |||||||||||
| Surface or low | 44% | 16 | |||||||||||
| Eastern prairie-woodland mosaic | Replacement | 50% | 10 | ||||||||||
| Mixed | 1% | 900 | |||||||||||
| Surface or low | 50% | 10 | |||||||||||
| Southern Appalachians Woodland | |||||||||||||
| Oak-ash woodland | Replacement | 23% | 119 | ||||||||||
| Mixed | 28% | 95 | |||||||||||
| Surface or low | 49% | 55 | |||||||||||
| Southern Appalachians Forested | |||||||||||||
| Bottomland hardwood forest | Replacement | 25% | 435 | 200 | >1,000 | ||||||||
| Mixed | 24% | 455 | 150 | 500 | |||||||||
| Surface or low | 51% | 210 | 50 | 250 | |||||||||
| Mixed mesophytic hardwood | Replacement | 11% | 665 | ||||||||||
| Mixed | 10% | 715 | |||||||||||
| Surface or low | 79% | 90 | |||||||||||
| Appalachian oak-hickory-pine | Replacement | 3% | 180 | 30 | 500 | ||||||||
| Mixed | 8% | 65 | 15 | 150 | |||||||||
| Surface or low | 89% | 6 | 3 | 10 | |||||||||
| Oak (eastern dry-xeric) | Replacement | 6% | 128 | 50 | 100 | ||||||||
| Mixed | 16% | 50 | 20 | 30 | |||||||||
| Surface or low | 78% | 10 | 1 | 10 | |||||||||
| Appalachian oak forest (dry-mesic) | Replacement | 6% | 220 | ||||||||||
| Mixed | 15% | 90 | |||||||||||
| Surface or low | 79% | 17 | |||||||||||
| Southeast | |||||||||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||||||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||||||||
| Southeast Forested | |||||||||||||
| Coastal Plain pine-oak-hickory | Replacement | 4% | 200 | ||||||||||
| Mixed | 7% | 100 | |||||||||||
| Surface or low | 89% | 8 | |||||||||||
| Southern floodplain | Replacement | 7% | 900 | ||||||||||
| Surface or low | 93% | 63 | |||||||||||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [94,145]. |
|||||||||||||
1. Abrams, M. D. 1996. Distribution, historical development and ecophysiological attributes of oak species in the eastern United States. Annales des Sciences Forestieres. 53(2-3): 487-512. [27235]
2. Abrams, Marc D. 1985. Age-diameter relationships of Quercus species in relation to edaphic factors in gallery forests of northeast Kansas. Forest Ecology and Management. 13: 181-193. [10377]
3. Abrams, Marc D. 1986. Ecological role of fire in gallery forests in eastern Kansas. In: Koonce, Andrea L., ed. Prescribed burning in the Midwest: state-of-the-art: Proceedings of a symposium; 1986 March 3-6; Stevens Point, WI. Stevens Point, WI: University of Wisconsin, College of Natural Resources, Fire Science Center: 73-80. [16271]
4. Abrams, Marc D. 1986. Historical development of gallery forests in northeast Kansas. Vegetatio. 65: 29-37. [3255]
5. Abrams, Marc D. 1988. Effects of prescribed fire on woody vegetation in a gallery forest understory in northeastern Kansas. Transactions of the Kansas Academy of Science. 91(3-4): 63-70. [10796]
6. Adams, Dwight E.; Anderson, Roger C. 1980. Species response to a moisture gradient in central Illinois forests. American Journal of Botany. 67(3): 381-392. [13295]
7. Ahlgren, C. E. 1957. Phenological observations of nineteen native tree species in northeastern Minnesota. Ecology. 38(4): 622-628. [74]
8. Aikman, John M. 1926. Distribution and structure of the forests of eastern Nebraska. Nebraska University Studies. 26(1-2): 1-75. [6575]
9. Albertson, F. W.; Weaver, J. E. 1945. Injury and death or recovery of trees in prairie climate. Ecological Monographs. 15: 393-433. [4328]
10. Alden, Harry A. 1995. Hardwoods of North America. Gen. Tech. Rep. FPL-GTR-83. Madison, WI: U.S. Department of Agriculture, Forest Service, Forest Products Laboratory. 136 p. Available online: http://www.fpl.fs.usda.gov/documnts/fplgtr/fplgtr83.pdf [2004, January 6]. [46270]
11. Alexander, Robert R. 1986. Classification of the forest vegetation of Wyoming. Res. Note RM-466. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 10 p. [304]
12. Arevalo, Jose Ramon; DeCoster, James K.; McAlister, Suzanne D.; Palmer, Michael W. 2000. Changes in two Minnesota forests during 14 years following catastrophic windthrow. Journal of Vegetation Science. 11(6): 833-840. [49619]
13. Ashby, W. Clark. 1976. Basswood seedlings outgrow red and bur oak in full light or heavy shade. Tree Planters' Notes. 27(4): 24-26. [22183]
14. Au, Leakhena; Andersen, David E.; David, Mark. 2008. Patterns in bird community structure related to restoration of Minnesota dry oak savannas and across a prairie to oak woodland ecological gradient. Natural Areas Journal. 28(4): 330-341. [82454]
15. Augspurger, Carol K. 2009. Spring 2007 warmth and frost: phenology, damage, and refoliation in a temperate deciduous forest. Functional Ecology. 23(6): 1031-1039. [81314]
16. Barbour, Michael G.; Burk, Jack H.; Pitts, Wanna D. 1980. Major vegetation types of North America. In: Terrestrial plant ecology. Menlo Park, CA: The Benjamin/Cummings Publishing Company: 486-583. [45729]
17. Barnes, Burton V. 1976. Succession in deciduous swamp communities of southeastern Michigan formerly dominated by American elm. Canadian Journal of Botany. 54: 19-24. [4914]
18. Barry, Dwight; Kroll, Andrew J. 2003. A phytosociological description of a remnant bottomland hardwood forest in Denton, Texas. LLELA Research Note 5. Lewisville, TX: Lewisville Lake Environmental Learning Area. 9 p. Available online: http://www.ias.unt.edu/llela/main.htm. [49461]
19. Bartlett, H. H. 1951. Regression of X Quercus deamii toward Quercus macrocarpa and Quercus muhlenbergii. Rhodora. 53(635): 249-264. [10664]
20. Beardmore, Tannis; Loo, Judy; McAfee, Brenda; Malouin, Christian; Simpson, Dale. 2006. A survey of tree species of concern in Canada: the role for genetic conservation. The Forestry Chronicle. 82(3): 351-363. [63693]
21. Beck, Allan R.; Weigle, Jack L. 1970. Plural-seeded acorns in bur oak (Quercus macrocarpa Michx.). HortScience. 5(1): 10-11. [3282]
22. Bird, Ralph D. 1927. A preliminary ecological survey of the district surrounding the entomological station at Treesbank, Manitoba. Ecology. 8(2): 207-220. [63548]
23. Bird, Ralph D. 1961. Ecology of the aspen parkland of western Canada in relation to land use. Contribution No. 27. Ottawa: Canada Department of Agriculture, Research Branch. 153 p. [15620]
24. Biswell, Harold H. 1935. Effects of environment upon the root habits of certain deciduous forest trees. Botanical Gazette. 96(4): 676-708. [3076]
25. Bjugstad, Ardell J.; Girard, Michele. 1984. Wooded draws in rangelands of the northern Great Plains. In: Henderson, F. R., ed. Guidelines for increasing wildlife on farms and ranches: With ideas for supplemental income sources for rural families. Manhattan, KS: Kansas State University, Cooperative Extension Service; Great Plains Agricultural Council, Wildlife Resources Committee: 27B-36B. [4239]
26. Blackmore, Mary. 1999. Dairy goats as tools for controlling woody vegetation on prairie remnants. In: Springer, J. T., ed. The central Nebraska loess hills prairie: Proceedings of the 16th North American prairie conference; 1998 July 26-29; Kearney, NE. No. 16. Kearney, NE: University of Nebraska: 243-249. [46837]
27. Boerner, Ralph E. J.; Cho, Do-Soon. 1987. Structure and composition of Goll Woods, an old-growth forest remnant in northwestern Ohio. Bulletin of the Torrey Botanical Club. 114(2): 173-179. [8711]
28. Bonner, Franklin T. 2008. Quercus L.: oak. In: Bonner, Franklin T., Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 928-938. [62581]
29. Booth, W. E.; Wright, J. C. 1962. [Revised]. Flora of Montana: Part II--Dicotyledons. Bozeman, MT: Montana State College, Department of Botany and Bacteriology. 280 p. [47286]
30. Bowles, Marlin L.; Jacobs, Karel A.; Mengler, Jeffrey L. 2007. Long-term changes in an oak forest's woody understory and herb layer with repeated burning. Journal of the Torrey Botanical Society. 134(2): 223-237. [69792]
31. Bowles, Marlin L.; McBride, Jenny L. 1998. Vegetation composition, structure, and chronological change in a decadent midwestern North American savanna remnant. Natural Areas Journal. 18(1): 14-27. [27556]
32. Bragg, Wendy K.; Knapp, Alan K.; Briggs, John M. 1993. Comparative water relations of seedling and adult Quercus species during gallery forest expansion in tallgrass prairie. Forest Ecology and Management. 56: 29-41. [20946]
33. Braun, E. Lucy. 1950. The oak-hickory forest region. In: Deciduous forests of eastern North America. Philadelphia, PA: Blakiston Books: 162-191. [28381]
34. Breining, Greg. 1993. The case of the missing ecosystem. Nature Conservancy. 43(6): 11-15. [22100]
35. Briggs, John M.; Smith, Kimberly G. 1989. Influence of habitat on acorn selection by Peromyscus leucopus. Journal of Mammalogy. 70(1): 35-43. [10387]
36. Brommit, Angela G.; Charbonneau, Neil; Contreras, Thomas A.; Fahrig, Lenore. 2004. Crown loss and subsequent branch sprouting of forest trees in response to a major ice storm. Journal of the Torrey Botanical Society. 131(2): 169-176. [82135]
37. Brown, Peter M.; Wienk, Cody L.; Symstad, Amy J. 2008. Fire and forest history at Mount Rushmore. Ecological Applications. 18(8): 1984-1999. [74211]
38. Bryant, William S.; Wharton, Mary E.; Martin, William H.; Varner, Johnnie B. 1980. The blue ash-oak savanna: Woodland, a remnant of presettlement vegetation in the Inner Bluegrass of Kentucky. Castanea. 45(3): 149-165. [10375]
39. Buell, Murray F.; Cantlon, John E. 1951. A study of two forest stands in Minnesota with an interpretation of the prairie-forest margin. Ecology. 32(2): 294-316. [3251]
40. Buell, Murray F.; Facey, Vera. 1960. Forest-prairie transition west of Itasca Park, Minnesota. Bulletin of the Torrey Botanical Club. 87(1): 46-58. [14171]
41. Burns, Thomas A.; Viers, Charles E., Jr. 1973. Caloric and moisture content values of selected fruits and mast. Journal of Wildlife Management. 37(4): 585-587. [41689]
42. Caners, R. T.; Kenkel, N. C. 2003. Forest stand structure and dynamics at Riding Mountain National Park, Manitoba, Canada. Community Ecology. 4(2): 185-204. [49546]
43. Catling, Paul M.; Brownell, Vivian R. 1995. A review of the alvars of the Great Lakes region: distribution, floristic composition, biogeography, and protection. The Canadian Field-Naturalist. 109(2): 143-171. [80535]
44. Chechowitz, Naomi; Chappell, Dorothy M. 1990. Morphological, electrophoretic, and ecological analysis of Quercus macrocarpa populations in the Black Hills of South Dakota and Wyoming. Canadian Journal of Botany. 68: 2185-2194. [13503]
45. Cho, Do-Soon; Boerner, R. E. J. 1995. Dendrochronological analysis of the canopy history of two Ohio old-growth forests. Vegetatio. 120: 173-183. [82137]
46. Cogiastro, Alain; Gagnon, Daniel; Bouchard, Andre. 1997. Experimental determination of soil characterisitics optimal for the growth of ten hardwoods planted on abandoned farmland. Forest Ecology and Management. 96: 49-63. [27430]
47. Costello, David F. 1931. Comparative study of river bluff succession on the Iowa and Nebraska sides of the Missouri River. Botanical Gazette. 91(3): 295-307. [82138]
48. Cottam, Grant. 1949. The phytosociology of an oak woods in southwestern Wisconsin. Ecology. 30(3): 271-287. [229]
49. Craft, Kathleen J.; Ashley, Mary V. 2007. Landscape genetic structure of bur oak (Quercus macrocarpa) savannas in Illinois. Forest Ecology and Management. 239(1-3): 13-20. [65503]
50. Crow, T. R. 1988. Reproductive mode and mechanisms for self-replacement of northern red oak (Quercus rubra)--a review. Forest Science. 34(1): 19-40. [8730]
51. Crowder, A.; Harmsen, R. 1998. Notes on forest succession in old fields in southeastern Ontario: the woody species. The Canadian Field-Naturalist. 112(3): 410-418. [35971]
52. Curtis, J. T.; McIntosh, R. P. 1951. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology. 32: 476-496. [6927]
53. Curtis, John T. 1959. Savanna. In: The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press: 325-351. [60528]
54. Curtis, John T. 1959. Southern forests--lowland: General distribution. In: The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press: 156-168. [60520]
55. Curtis, John T. 1959. Southern forests--xeric. In: The vegetation of Wisconsin. Madison, WI: The University of Wisconsin Press: 132-155. [60519]
56. Danner, Brett T.; Knapp, Alan K. 2001. Growth dynamics of oak seedlings (Quercus macrocarpa Michx. and Quercus muhlenbergii Engelm.) from gallery forests: implications for forest expansion into grasslands. Trees. 15(5): 271-277. [39418]
57. Danner, Brett T.; Knapp, Alan K. 2003. Abiotic constraints on the establishment of Quercus seedlings in grassland. Global Change Biology. 9: 266-275. [82139]
58. Day, Keith S.; Flake, Lester D.; Tucker, W. Lee. 1991. Movements and habitat use by wild turkey hens with broods in a grassland-woodland mosaic in the northern plains. Prairie Naturalist. 23(2): 73-83. [18061]
59. Deitz, Karen B. 1997. Seed germination and seedling growth of woody plant species under saturated and well-drained conditions in the field and greenhouse. Syracuse, NY: State University of New York, College of Environmental Science and Forestry. 84 p. Thesis. [82455]
60. Diggs, George M., Jr.; Lipscomb, Barney L.; O'Kennon, Robert J. 1999. Illustrated flora of north-central Texas. Sida Botanical Miscellany, No. 16. Fort Worth, TX: Botanical Research Institute of Texas. 1626 p. [35698]
61. Dovciak, Martin; Frelich, Lee E.; Reich, Peter B. 2005. Pathways in old-field succession to white pine: seed rain, shade, and climate effects. Ecological Monographs. 75(3): 363-378. [55525]
62. Dow, B. D.; Ashley, M. V. 1998. High levels of gene flow in bur oak revealed by paternity analysis using microsatellites. The Journal of Heredity. 89(1): 62-70. [82140]
63. Duncan, Wilbur H.; Duncan, Marion B. 1988. Trees of the southeastern United States. Athens, GA: The University of Georgia Press. 322 p. [12764]
64. Dyas, Robert W. 1980. Bur oak--Forest Cover Type 236. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 113. [50050]
65. Dyas, Robert W. 1980. Bur oak--Forest Cover Type 42. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 39-40. [49907]
66. Ewing, J. 1924. Plant successions of the brush-prairie in north-western Minnesota. Journal of Ecology. 12: 238-266. [11122]
67. Faber-Langendoen, Don; Davis, Mark A. 1995. Effects of fire frequency on tree canopy cover at Allison Savanna, eastcentral Minnesota, USA. Natural Areas Journal. 15(4): 319-328. [26527]
68. Farrar, John Laird. 1995. Trees of the northern United States and Canada. Ames, IA: Blackwell Publishing. 502 p. [60614]
69. Fisher, R. F.; Jenkins, M. J.; Fisher, W. F. 1985. Fire and the vegetative mosaic at Devils Tower National Monument. In: Long, James N., ed. Fire management: The challenge of protection and use symposium: Proceedings; 1985 April 17-19; Logan, UT. Logan, UT: Utah State University: 11-24. [6905]
70. Fisher, R. F.; Jenkins, M. J.; Fisher, William F. 1986. Fire and the prairie-forest mosaic of Devils Tower National Monument. The American Midland Naturalist. 117(2): 250-257. [15638]
71. Flake, Lester D.; Lehman, Chad P.; Leif, Anthony P.; Rumble, Mark A.; Thompson, Daniel J. 2006. The wild turkey in South Dakota. B747. Brookings, SD: South Dakota State University, College of Agriculture and Biological Sciences; South Dakota Agricultural Experiment Station. 189 p. [67887]
72. Flora of North America Editorial Committee, eds. 2011. Flora of North America North of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
73. Foster, J. H.; Krausz, H. B.; Leidigh, A. H. 1917. General survey of Texas woodlands including a study of the commercial possibilities of mesquite. Bulletin of the Agricultural and Mechanical College of Texas. Bulletin 3: Department of Forestry. Third Series 3(9): 1-47. [11796]
74. Fox, J. F. 1982. Adaptation of gray squirrel behavior to autumn germination by white oak acorns. Evolution. 36(4): 800-809. [10518]
75. Frelich, Lee E. 2002. The forest setting. In: Forest dynamics and disturbance regimes: Studies from temperate evergreen-deciduous forests. Cambridge: Cambridge University Press: 1-14. [43732]
76. Frelich, Lee E.; Faber-Langendoen, Don; Tester, John; Tilman, David. 1992. Changes in age structure of oak woodlands along a topographic and disturbance gradient. Bulletin of the Ecological Society of America. 73(2)Suppl: 180. Abstract. [82153]
77. Frelich, Lee E.; Reich, Peter B. 2009. Wilderness conservation in an era of global warming and invasive species: a case study from Minnesota's Boundary Waters Canoe Area Wilderness. Natural Areas Journal. 29(4): 385-393. [77775]
78. Garrison, W. J.; Augspurger, C. K. 1983. Double- and single-seeded acorns of bur oak (Quercus macrocarpa): frequency and some ecological consequences. Bulletin of the Torrey Botanical Club. 110(2): 154-160. [82154]
79. Gastler, George F.; Moxon, Alvin L.; McKean, William T. 1951. Composition of some plants eaten by deer in the Black Hills of South Dakota. Journal of Wildlife Management. 15(4): 352-357. [3996]
80. Gates, F. C. 1939. Trends of tree migration in Kansas. Transactions of the Kansas Academy of Science. 42: 127-132. [79795]
81. Geis, James W.; Boggess, William R. 1970. Soil-vegetation relationships in a prairie grove remnant. Bulletin of the Torrey Botanical Club. 97(4): 196-203. [82155]
82. Gentry, Dale J.; Vierling, Kerri T. 2008. Reuse of woodpecker cavities in the breeding and non-breeding seasons in old burn habitats in the Black Hills, South Dakota. The American Midland Naturalist. 160(2): 413-429. [73021]
83. Gibson, David J.; Hartnett, David C.; Merrill, Gary L. S. 1990. Fire temperature heterogeneity in contrasting fire prone habitats: Kansas tallgrass prairie and Florida sandhill. Bulletin of the Torrey Botanical Club. 117(4): 348-356. [14138]
84. Girard, Michele M.; Goetz, Harold; Bjugstad, Ardell J. 1984. Upland hardwood habitat types in southwestern North Dakota. In: Noble, Daniel L; Winokur, Robert P.,eds. Wooded draws: characteristics and values for the Northern Great Plains: Symposium proceedings; 1984 June 12-13; Rapid City, SD. Great Plains Agricultural Council Publication No. 111. Rapid City, SD: South Dakota School of Mines and Technology, Biology Department: 10-14. [1024]
85. Girard, Michele M.; Goetz, Harold; Bjugstad, Ardell J. 1989. Native woodland habitat types of southwestern North Dakota. Res. Pap. RM-281. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 36 p. [6319]
86. Girard, Michele Marie. 1985. Native woodland ecology and habitat classification of southwestern North Dakota. Fargo, ND: North Dakota State University. 314 p. Dissertation. [1025]
87. Gleason, Henry A.; Cronquist, Arthur. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd ed. New York: New York Botanical Garden. 910 p. [20329]
88. Graber, Jean W.; Graber, Richard R.; Kirk, Ethelyn L. 1977. Illinois birds: Picidae. Biological Notes No. 102. Urbana, IL: State of Illinois, Department of Registration and Education; Natural History Survey Division, Natural History Survey. 73 p. [64983]
89. Graney, David L. 1990. Carya ovata (Mill.) K. Koch shagbark hickory. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 219-225. [13976]
90. Great Plains Flora Association. 1986. Flora of the Great Plains. Lawrence, KS: University Press of Kansas. 1392 p. [1603]
91. Grimm, Eric C. 1984. Fire and other factors controlling the Big Woods vegetation of Minnesota in the mid-nineteenth century. Ecological Monographs. 54(3): 291-311. [22170]
92. Guetersloh, Erika Rose. 2008. Historic savanna and extant east-central Nebraska loess bluff bur oak communities. Omaha, NE: University of Nebraska. 41 p. Thesis. [82456]
93. Guyette, Richard P.; Muzika, Rose-Marie; Kabrick, John; Stambaugh, Michael C. 2004. A perspective on Quercus life history characteristics and forest disturbance. In: Spetich, Martin A., ed. Upland oak ecology symposium: history, current conditions, and sustainability: Proceedings; 2002 October 7-10;Fayetteville, AR. Gen. Tech. Rep. SRS-73. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station: 138-142. [82156]
94. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org/niftt/released/FRCC_Guidebook_2010_final.pdf. [81749]
95. Hansen, Henry L.; Kurmis, Vilis. 1972. Natural succession in north-central Minnesota. In: Aspen: Symposium proceedings; [Date of conference unknown]; Duluth, MN. Gen. Tech. Rep. NC-1. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: 59-66. [12040]
96. Hansen, Paul L.; Hoffman, George R.; Steinauer, Gerry A. 1984. Upland forest and woodland habitat types of the Missouri Plateau, Great Plains Province. In: Noble, Daniel L.; Winokur, Robert P., eds. Wooded draws: characteristics and values for the Northern Great Plains: Symposium proceedings; 1984 June 12-13; Rapid City, SD. Great Plains Agricultural Council Publ. No. 111. Rapid City, SD: South Dakota School of Mines and Technology, Biology Department: 15-26. [1078]
97. Hardin, James W. 1975. Hybridization and introgression in Quercus alba. Journal of the Arnold Arboretum. 56: 336-363. [10553]
98. Harms, W. R. 1990. Quercus virginiana Mill. live oak. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Vol. 2: Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 751-754. [18139]
99. Harrison, A. Tyrone. 1980. The Niobrara Valley Preserve: its biogeographic importance and description of its biotic communities. Unpublished report to the Nature Conservancy. On file at: U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Fire Sciences Laboratory, Missoula, MT. 116 p. [5736]
100. Hart, Jeffrey A. 1981. The ethnobotany of the Northern Cheyenne Indians of Montana. Journal of Ethnopharmacology. 4: 1-55. [35893]
101. Hayward, Herman E. 1928. Studies of plants in the Black Hills of South Dakota. Botanical Gazette. 85(4): 353-412. [1110]
102. Hazelwood, Donna. 2001. Preliminary examination of species of an abandoned farm field in tallgrass mixed hardwood forest in Ottertail County, Minnesota. In: Bernstein, Neil P.; Ostrander, Laura J., eds. Seeds for the future; roots of the past: Proceedings of the 17th North American prairie conference; 2000 July 16-20; Mason City, IA. Mason City, IA: North Iowa Community College: 42-47. [46492]
103. He, Hong S.; Mladenoff, David J.; Gustafson, Eric J. 2002. Study of landscape change under forest harvesting and climate warming-induced fire disturbance. Forest Ecology and Management. 155: 257-270. [40715]
104. Heinselman, Miron L. 1981. Fire intensity and frequency as factors in the distribution and structure of northern ecosystems. In: Mooney, H. A.; Bonnicksen, T. M.; Christensen, N. L.; Lotan, J. E.; Reiners, W. A., technical coordinators. Fire regimes and ecosystem properties: Proceedings of the conference; 1978 December 11-15; Honolulu, HI. Gen. Tech. Rep. WO-26. Washington, DC: U.S. Department of Agriculture, Forest Service: 7-57. [4390]
105. Henderson, Richard A. 1991. Reed canary grass poses threat to oak savanna restoration and maintenance. Restoration & Management Notes. 9(1): 32. [15451]
106. Hengst, Gretel E.; Dawson, Jeffrey G. 1993. Bark thermal properties of selected central hardwood species. In: Gillespie, Andrew R.; Parker, George R.; Pope, Phillip E., eds. Proceedings, 9th central hardwood forest conference; 1993 March 8-10; West Lafayette, IN. Gen. Tech. Rep. NC-161. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: 55-75. [27002]
107. Hengst, Gretel E.; Dawson, Jeffrey O. 1994. Bark properties and fire resistance of selected tree species from the central hardwood region of North America. Canadian Journal of Forest Research. 24: 688-696. [23452]
108. Herman, Dale E. 1993. Quercus macrocarpa, bur oak. Arbor Age. 13(7): 48-49. [21657]
109. Hernandez, Daniel L.; Hobbie, Sarah E. 2008. Effects of fire frequency on oak litter decomposition and nitrogen dynamics. Oecologia. 158(3): 535-543. [72978]
110. Hewitt, Nina; Kellman, Martin. 2002. Tree seed dispersal among forest fragments: II. Dispersal abilities and biogeographical controls. Journal of Biogeography. 29(3): 351-363. [82159]
111. Hill, Ralph R. 1946. Palatability ratings of Black Hills plants for white-tailed deer. Journal of Wildlife Management. 10(1): 47-54. [3270]
112. Hill, Ralph R.; Harris, Dave. 1943. Food preferences of Black Hills deer. Journal of Wildlife Management. 7(2): 233-235. [67155]
113. Hillman, Conrad N.; Jackson, Warren W. 1973. The sharp-tailed and prairie grouse in South Dakota. Technical Bulletin Number 3. Pierre, SD: South Dakota Department of Game, Fish, and Parks. 61 p. [76559]
114. Hoagland, Bruce. 2000. The vegetation of Oklahoma: a classification for landscape mapping and conservation planning. The Southwestern Naturalist. 45(4): 385-420. [41226]
115. Hoffman, George R.; Alexander, Robert R. 1987. Forest vegetation of the Black Hills National Forest of South Dakota and Wyoming: a habitat type classification. Res. Pap. RM-276. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 48 p. [1181]
116. Hoffman, George R.; Shetron, Stephen G.; Klimas, Charles V.; Allen, Hollis H. 1986. Lakeshore vegetation, studies at Lake Oahe, South Dakota--Final report. Technical Report E-86-3. Vicksburg, MS: U.S. Army Engineer Waterways Experiment Station, Environmental Laboratory. 16 p. [+ appendices]. [78218]
117. Holch, A. E. 1931. Development of roots and shoots of certain deciduous tree seedlings in different sites. Ecology. 12(2): 259-298. [3734]
118. Hosie, R. C. 1969. Native trees of Canada. 7th ed. Ottawa, ON: Canadian Forestry Service, Department of Fisheries and Forestry. 380 p. [3375]
119. Howard, Gene S.; Rauzi, Frank; Schuman, Gerald E. 1979. Woody plant trials at six mine reclamation sites in Wyoming and Colorado. Production Res. Rep. PRR 177/1/79. Washington, DC: U.S. Department of Agriculture. 14 p. [42428]
120. Hruska, Mary C.; Ebinger, John E. 1995. Monitoring a savanna restoration in east-central Illinois. Transactions of the Illinois State Academy of Science. 88(3&4): 109-117. [41436]
121. Hunter, Carl G. 1989. Trees, shrubs, and vines of Arkansas. Little Rock, AR: The Ozark Society Foundation. 207 p. [21266]
122. Irving, F. D.; Aksamit, S. E. 1983. Tree mortality by fire in oak savanna restoration. Restoration & Management Notes. 1(4): 18-19. Abstract. [50360]
123. Iverson, Louis R.; Prasad, Anantha M. 1998. Predicting abundance of 80 tree species following climate change in the eastern United States. Ecological Monographs. 68(4): 465-485. [29327]
124. Johnson, Carter W.; Webb, Thompson, III. 1989. The role of blue jays (Cyanocitta cristata L.) in the postglacial dispersal of fagaceous trees in eastern North America. Journal of Biogeography. 16: 561-571. [27818]
125. Johnson, Paul S. 1990. Quercus macrocarpa Michx. bur oak. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 686-692. [82254]
126. Johnson, W. Carter. 1992. Dams and riparian forests: case study from the upper Missouri River. Rivers. 3(4): 229-242. [49559]
127. Johnson, W. Carter. 1997. Nut caching by blue jays (Cyanocitta cristata L.): implications for tree demography. The American Midland Naturalist. 138(2): 357-370. [27816]
128. Johnson, W. Carter; Burgess, Robert L.; Keammerer, Warren R. 1976. Forest overstory vegetation and environment on the Missouri River floodplain in North Dakota. Ecological Monographs. 46(1): 59-84. [6313]
129. Johnson, Warren Carter. 1971. The forest overstory vegetation on the Missouri River floodplain in North Dakota. Fargo, ND: North Dakota State University. 185 p. Dissertation. [49512]
130. Jones, Judith. 2000. Fire history of the bur oak savannas of Sheguiandah Township, Manitoulin Island, Ontario. The Michigan Botanist. 39: 3-15. [82451]
131. Judd, B. Ira. 1939. Plant succession on scoria buttes of western North Dakota. Ecology. 20(2): 335-336. [55047]
132. Kaminski, D. A.; Jackson, M. T. 1978. A light and moisture continuum analysis of the presettlement prairie-forest border region of eastern Illinois. The American Midland Naturalist. 99(2): 280-289. [49635]
133. Kartesz, John T. 1999. A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. 1st ed. In: Kartesz, John T.; Meacham, Christopher A. Synthesis of the North American flora (Windows Version 1.0), [CD-ROM]. Chapel Hill, NC: North Carolina Botanical Garden (Producer). In cooperation with: The Nature Conservancy; U.S. Department of Agriculture, Natural Resources Conservation Service; U.S. Department of the Interior, Fish and Wildlife Service. [36715]
134. Kay, Adam D.; Schade, John D.; Ogdahl, Megan; Wesserle, Eleonore O.; Hobbie, Sarah E. 2007. Fire effects on insect herbivores in an oak savanna: the role of light and nutrients. Ecological Entomology. 32(6): 754-761. [70287]
135. Keammerer, Warren R.; Johnson, W. Carter; Burgess, Robert L. 1975. Floristic analysis of the Missouri River bottomland forest in North Dakota. The Canadian Field-Naturalist. 89: 5-19. [7447]
136. Keane, Robert E.; Agee, James K.; Fule, Peter; Keeley, Jon E.; Key, Carl; Kitchen, Stanley G.; Miller, Richard; Schulte, Lisa A. 2008. Ecological effects of large fires on US landscapes: benefit or catastrophe? International Journal of Wildland Fire. 17: 696-712. [73387]
137. Kipfmueller, Kurt F.; Hepola, Tim. 2007. Fire history and age structure analysis in the Sherburne National Wildlife Refuge: establishing reference conditions in a remnant oak savanna woodland. In: Butler, Bret W.; Cook, Wayne, comps. The fire environment--innovations, management, and policy; conference proceedings; 2007 March 26-30; Destin, FL. Proceedings RMRS-P-46CD. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 507-514. [71114]
138. Kittelson, Pamela M.; Pinahs, Christopher; Dwyer, Joshua; Ingersoll, Angela; Mans, Elaine; Rieke, Jennifer; Rutman, Brady; Volenec, Matthew. 2009. Age structure and genetic diversity of four Quercus macrocarpa (Michx.) populations in fragmented oak savanna along the central Minnesota River Valley. The American Midland Naturalist. 161(2): 301-312. [74295]
139. Knoop, Jeffrey D. 1986. Floristic and vegetational survey of the W. Pearl King Prairie Grove, a prairie remnant in Madison County, Ohio. The prairie: past, present and future: Proceedings of the 9th North American prairie conference; 1984 July 29 - August 1; Moorhead, MN. Fargo, ND: Tri-College University Center for Environmental Studies : 44-49. [3513]
140. Koenig, Walter D.; Knops, Johannes M. H.; Dickinson, Janis L.; Zuckerberg, Benjamin. 2009. Latitudinal decrease in acorn size in bur oak (Quercus macrocarpa) is due to environmental constraints, not avian dispersal. Botany. 87(4): 349-356. [75465]
141. Krajicek, John E. 1960. Some factors affecting oak and black walnut reproduction. Iowa State Journal of Science. 34(4): 631-634. [82449]
142. Kuchler, A. W. 1964. Oak savanna (Quercus-Andropogon). In: Manual to accompany the map of potential vegetation of the conterminous United States. Special Publication No. 36. New York: American Geographical Society: 81. [67800]
143. Kurmis, Vilis; Webb, Sara L.; Merriam, Lawrence C., Jr. 1986. Plant communities of Voyageurs National Park, Minnesota, U.S.A. Canadian Journal of Botany. 64: 531-540. [16088]
144. Laing, Charles L. 1966. Bur oak seed size and shadiness of habitat in southeastern Nebraska. The American Midland Naturalist. 76(2): 534-536. [82160]
145. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: http://www.landfire.gov/downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
146. LANDFIRE Rapid Assessment. 2007. Rapid assessment reference condition models, [Online]. In: LANDFIRE. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; The Nature Conservancy (Producers). Available: http://www.landfire.gov/models_EW.php [2008, April 18] [66533]
147. Laudenslager, Scott L.; Flake, Lester D. 1987. Fall food habits of wild turkeys in south central South Dakota. Prairie Naturalist. 19(1): 37-40. [251]
148. Lauver, Chris L.; Kindscher, Kelly; Faber-Langendoen, Don; Schneider, Rick. 1999. A classification of the natural vegetation of Kansas. The Southwestern Naturalist. 44(4): 421-443. [38847]
149. Lawson, Dan; Inouye, Richard S.; Huntly, Nancy; Carson, Walter P. 1999. Patterns of woody plant abundance, recruitment, mortality, and growth in a 65 year chronosequence of old-fields. Plant Ecology. 145(2): 267-279. [36110]
150. Lee, Shun Ching. 1924. Factors controlling forest succession at Lake Itasca, Minnesota. Botanical Gazette. 78(2): 129-174. [41396]
151. Leitner, Lawrence Arthur. 1985. An alien shrub in a changing landscape: the European buckthorn (Rhamnus cathartica L.) in southeastern Wisconsin. Milwaukee, WI: University of Wisconsin, Milwaukee. 387 p. Dissertation. [72588]
152. Limstrom, G. A.; Merz, R. W. 1949. Rehabilitation of lands stripped for coal in Ohio. Tech. Pap. No. 113. Columbus, OH: The Ohio Reclamation Association. 41 p. In cooperation with: U.S. Department of Agriculture, Forest Service, Central States Forest Experiment Station. [4427]
153. Lorimer, Craig G. 1985. The role of fire in the perpetuation of oak forests. In:, Johnson, J. E., ed. Challenges in oak management and utilization. Madison, WI: University of Wisconsin, Cooperative Extension Service: 8-25. [19543]
154. Louisiana Department of Wildlife and Fisheries, Natural Heritage Program. 1999. Rare plant species of Louisiana--December 1999. Baton Rouge, LA: Louisiana Department of Wildlife and Fisheries. 20 p. [35364]
155. Mack, John J.; Boerner, R. E. J. 2004. At the tip of the Prairie Peninsula: vegetation of Daughmer Savannah, Crawford County, Ohio. Castanea. 69(4): 309-323. [82162]
156. Magee, Dennis W.; Ahles, Harry E. 2007. Flora of the Northeast: A manual of the vascular flora of New England and adjacent New York. 2nd ed. Amherst, MA: University of Massachusetts Press. 1214 p. [74293]
157. Magruder, T. L. 1985. Wind Cave's riparian habitats. South Dakota Conservation Digest. 52(3): 20-23. [13752]
158. Manske, Llewellyn Leo. 1980. Habitat, phenology and growth of selected sandhills range plants. Fargo, ND: North Dakota State University. 154 p. Dissertation. [4549]
159. Martin, Alexander C.; Zim, Herbert S.; Nelson, Arnold L. 1951. American wildlife and plants. New York: McGraw-Hill Book Company. 500 p. [4021]
160. Massachusetts Division of Fisheries and Wildlife, Department of Fish and Game. 2008. Fact sheet: Bur oak--Quercus macrocarpa A. Michaus, [Online]. In: Massachusetts list of endangered, threatened and special concern species. Boston, MA: Natural Heritage and Endangered Species Program (Producer). Available: http://www.mass.gov/dfwele/dfw/nhesp/species_info/nhfacts/quercus_macrocarpa.pdf [2011, May 27]. [82727]
161. Maze, Jack. 1968. Past hybridization between Quercus macrocarpa and Quercus gambelii. Brittonia. 20: 321-333. [1559]
162. McBride, Joe. 1973. Natural replacement of disease-killed elms. The American Midland Naturalist. 90(2): 300-306. [8868]
163. McClain, William E.; Jenkins, Michael A.; Jenkins, Sean E.; Ebinger, John E. 1993. Changes in the woody vegetation of a bur oak savanna remnant in central Illinois. Natural Areas Journal. 13(2): 108-114. [21443]
164. McComb, A. L.; Loomis, W. E. 1944. Subclimax prairie. Bulletin of the Torrey Botanical Club. 71(1): 46-76. [4341]
165. McEwan, Ryan W.; McCarthy, Brian C. 2008. Anthropogenic disturbance and the formation of oak savanna in central Kentucky, USA. Journal of Biogeography. 35: 965-975. [82458]
166. McKenney, Daniel W.; Pedlar, John H.; Lawrence, Kevin; Campbell, Kathy; Hutchinson, Michael F. 2007. Potential impacts of climate change on the distribution of North American trees. BioScience. 57(11): 939-948. [70374]
167. McPhee, Donnie A.; Loo, Jude A. 2009. Past and present distribution of New Brunswick bur oak populations: a case for conservation. Northeastern Naturalist. 16(1): 85-100. [82165]
168. McWilliams, William H.; O'Brien, Renee A.; Reese, Gordon C.; Waddell, Karen L. 2002. Distribution and abundance of oaks in North America. In: McShea, William J.; Healy, William M., eds. Oak forest ecosystems: Ecology and management for wildlife. Baltimore, MD: The Johns Hopkins University Press: 13-33. [43465]
169. Miceli, J. C.; Rolfe, G. L.; Pelz, D. R.; Edgington, J. M. 1977. Brownfield Woods, Illinois: woody vegetation and changes since 1960. The American Midland Naturalist. 98(2): 469-176. [10371]
170. Monk, Carl D.; Imm, Donald W.; Potter, Robert L. 1990. Oak forests of eastern North America. Castanea. 55(2): 77-96. [82166]
171. Monzyk, Frederick R.; Smith, Christopher C. 1991. Fox squirrel rate of seed removal in comparison to that of nocturnal animals. Transactions of the Kansas Academy of Science. 94(1-2): 30-32. [82167]
172. Moran, Robbin C. 1978. Presettlement vegetation of Lake County, Illinois. In: Glenn-Lewin, David C.; Landers, Roger Q., Jr., eds. Proceedings, 5th Midwest prairie conference; 1976 August 22-24; Ames, IA. Ames, IA: Iowa State University: 12-18. [3290]
173. Morano, L. D.; Walker, M. A. 1995. Soils and plant communities associated with three Vitis species. The American Midland Naturalist. 134(2): 254-263. [75534]
174. NatureServe. 2002. International classification of ecological communities: terrestrial vegetation of the United States--National forests in Texas final report, [Online]. [Extracted from Natural Heritage Central Databases]. In: NatureServe--Publications. 285 p. Arlington, VA: NatureServe; Durham, NC: NatureServe-South Community Ecology Group (Producers). Available: http://www.natureserve.org/library/TexasNF.doc [2010, March 30]. [78740]
175. NatureServe. 2004. International ecological classification standard: Terrestrial ecological classifications--Land Between the Lakes National Recreation Area (Kentucky, Tennessee). Interim report. [Extracted from NatureServe Central Databases]. Arlington, VA: NatureServe; Durham, NC: NatureServe Ecology South. 39 p. [79666]
176. NatureServe. 2004. International ecological classification standard: Terrestrial ecological classifications--Sumter National Forest final report. [Extracted from NatureServe Central Databases]. Arlington, VA: NatureServe. 114 p. [79642]
177. Nelson, John C. 1997. Presettlement vegetation patterns along the 5th Principal Meridian, Missouri Territory, 1815. The American Midland Naturalist. 137(1): 79-94. [63029]
178. Nemick, Joseph J. 1987. Sharp-tailed grouse management and ecology in Wyoming. In: Fisser, Herbert G., ed. Wyoming shrublands: Proceedings, 16th Wyoming shrub ecology workshop; 1987 May 26-27; Sundance, WY. Laramie, WY: University of Wyoming, Department of Range Management, Wyoming Shrub Ecology Workshop: 45-47. [13920]
179. New York Natural Heritage Program. 2008. New York Natural Heritage Program Conservation Guide: Barrens dagger moth (Acronicta albarufa), [Online]. In: Animal guides. New York Natural Heritage Program (Producer). Available: http://acris.nynhp.org/guide.php?id=8081 [2008, September 8]. [71014]
180. Nicolai, Volker. 1991. Reactions of the fauna on the bark of trees to the frequency of fires in a North American savanna. Oecologia. 88(1): 132-137. [16715]
181. Nixon, Elray S.; Willett, R. Larry. 1974. Vegetative analysis of the floodplain of the Trinity River, Texas. Contract No. DACW6-74-C-0030. [Preliminary report prepared for U.S. Army Corps of Engineers, Fort Worth District, Fort Worth, Texas]. Nacogdoches, TX: Stephen F. Austin State University. 267 p. On file at: U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [20420]
182. Nowacki, Gregory J.; Abrams, Marc D. 2008. The demise of fire and "mesophication" of forests in the eastern United States. BioScience. 58(2): 123-138. [70112]
183. Nuzzo, Victoria A. 1986. Extent and status of Midwest oak savanna: presettlement and 1985. Natural Areas Journal. 6(2): 6-36. [50415]
184. Ofcarcik, R. P.; Burns, E. E.; Teer, J. G. 1973. Acceptance of selected acorns by captive fox squirrels. The Southwestern Naturalist. 17(4): 349-355. [11365]
185. Olson, Rich. 1992. White-tailed deer habitat requirements and management in Wyoming. B-964. Laramie, WY: University of Wyoming, Cooperative Extension Service. 17 p. [20678]
186. Papike, R. V. 1984. Experimental burns, reintroductions in savanna restoration project (Minnesota). Restoration & Management Notes. 2(2): 73. [50403]
187. Parker, G. R.; Leopold, D. J.; Eichenberger, J. K. 1985. Tree dynamics in an old-growth, deciduous forest. Forest Ecology and Management. 11(1&2): 31-57. [13314]
188. Peet, Robert K.; Loucks, Orie L. 1977. A gradient analysis of southern Wisconsin forests. Ecology. 58(3): 485-499. [82168]
189. Perala, Donald A. 1974. Growth and survival of northern hardwood sprouts after burning. Res. Note NC-176. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 4 p. [7349]
190. Peterson, David W.; Reich, Peter B. 2001. Prescribed fire in oak savanna: fire frequency effects on stand structure and dynamics. Ecological Applications. 11(3): 914-927. [39627]
191. Peterson, David W.; Reich, Peter B. 2008. Fire frequency and tree canopy structure influence plant species diversity in a forest-grassland ecotone. Plant Ecology. 194: 5-16. [70391]
192. Peterson, David Wassell. 1998. Fire effects on oak savanna and woodland vegetation in Minnesota. Minneapolis, MN: University of Minnesota. 130 p. Dissertation. [82453]
193. Plass, William T. 1975. An evaluation of trees and shrubs for planting surface-mine spoils. Res. Pap. NE-317. Upper Darby, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 8 p. [46129]
194. Putnam, John A. 1951. Management of bottomland hardwoods. Occasional Paper 116. New Orleans, LA: U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station. 60 p. [6748]
195. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
196. Reid, M.; Schulz, K.; Schindel, M.; Comer, P.; Kittel, G.; [and others], compilers. 2000. International classification of ecological communities: Terrestrial vegetation of the western United States--Chihuahuan Desert subset. Report from Biological Conservation Datasystem and working draft of April 23, 2000. Boulder, CO: Association for Biodiversity Information/The Nature Conservancy, Community Ecology Group. 154 p. In: Southwestern Regional Gap Analysis Project. Reston, VA: U.S. Geological Survey, Gap Analysis Program (Producer). Available online: http://fws-nmcfwru.nmsu.edu/swregap/nm/Chihuahua.pdf [2005, May 6]. [52906]
197. Reller, Ann Willbern. 1972. Aspects of behavioral ecology of red-headed and red-bellied woodpeckers. The American Midland Naturalist. 88(2): 270-290. [61970]
198. Reschke, Carol. 1990. Ecological communities of New York State. Latham, NY: New York State Department of Environmental Conservation, Natural Heritage Program. 96 p. [21441]
199. Reschke, Carol; Reid, Ron; Jones, Judith; Feeney, Tom; Potter, Heather, comps. 1999. Conserving Great Lakes alvars. Final technical report of the International Alvar Conservation Initiative. Chicago, IL: The Nature Conservancy. 230 p. Available online: http://www.epa.gov/ecopage/shore/alvars/alvar.pdf [2011, January 19]. [80830]
200. Ripple, William J.; Beschta, Robert L. 2007. Hardwood tree decline following large carnivore loss on the Great Plains, USA. Frontiers in Ecology and the Environment. 5(5): 241-246. [82169]
201. Risser, P. G.; Birney, E. C.; Blocker, H. D.; May, S. W.; Parton, W. J.; Wiens, J. A. 1981. The true prairie ecosystem. US/IBP Synthesis Series 16. Stroudsburg, PA: Hutchinson Ross Publishing. 557 p. [16874]
202. Rogers, Lynn L. 1987. Effects of food supply and kinship on social behavior, movements, and population growth of black bears in northeastern Minnesota. Wildlife Monographs No. 97. Washington, DC: The Wildlife Society. 72 p. [68405]
203. Rogers, Lynn L.; Allen, Arthur W. 1987. Habitat suitability index models: black bear, upper Great Lakes region. Biological Report 82(10.144). Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service. 54 p. [11711]
204. Ross, Bruce A.; Bray, J. Roger; Marshall, William H. 1970. Effects of long-term deer exclusion on a Pinus resinosa forest in north-central Minnesota. Ecology. 51(6): 1088-1093. [41652]
205. Rothenberger, Steven J. 1985. Community analysis of the forest vegetation in the Lower Platte River Valley, eastern Nebraska. Prairie Naturalist. 17(1): 1-14. [2031]
206. Rothenberger, Steven J. 1995. Plant community analysis of Schultz Prairie, Webster County, Nebraska. In: Hartnett, David C., ed. Prairie biodiversity: Proceedings, 14th North American prairie conference; 1994 July 12-16; Manhattan, KS. Manhattan, KS: Kansas State University: 35-41. [28225]
207. Rumble, Mark A.; Gobeille, John E. 2001. Small mammals in successional prairie woodlands of the northern Great Plains. Res. Pap. RMRS-RP-28. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 9 p. [38088]
208. Schnabel, Andrew; Hamrick, J. L. 1990. Comparative analysis of population genetic structure in Quercus macrocarpa and Q. gambelii (Fagaceae). Systematic Botany. 15(2): 240-251. [11727]
209. Schneider, Rick E.; Faber-Langendoen, Don; Crawford, Rex C.; Weakley, Alan S. 1997. The status of biodiversity in the Great Plains: Great Plains vegetation classification--Supplemental document 1, [Online]. [Cooperative Agreement # X 007803-01-3]. In: Ostlie, Wayne R.; Schneider, Rick E.; Aldrich, Janette Marie; Faust, Thomas M.; McKim, Robert L. B.; Chaplin, Stephen J., comps. The status of biodiversity in the Great Plains. Arlington, VA: The Nature Conservancy, Great Plains Program (Producer). 75 p. Available: http://conserveonline.org/docs/2005/02/greatplains_vegclass_97.pdf [2006, May 16]. [62020]
210. Schweitzer, Dale F. 2007. Comprehensive species report - Acronicta albarufa, Barrens dagger moth, [Online]. In: NatureServe Explorer: an online encyclopedia of life. Version 7.0. Arlington, VA: NatureServe (Producer). Available: http://www.natureserve.org/explorer/servlet/NatureServe?loadTemplate= tabular_report.wmt&paging=home&save=all&sourceTemplate=reviewMiddle.wmt [2008, September 17]. [71015]
211. Scoggan, H. J. 1978. The flora of Canada. Part 3: Dicotyledoneae (Saururaceae to Violaceae). National Museum of Natural Sciences: Publications in Botany, No. 7(3). Ottawa: National Museums of Canada. 1115 p. [75493]
212. Scriver, Bryn Muree. 2005. Consequences of oak savanna restoration techniques on the re-invasion of non-native invasive shrubs, particularly Rhamnus cathartica L. (common buckthorn). Madison, WI: University of Wisconsin-Madison. 153 p. Thesis. [80743]
213. Seidel, Kenneth W.; Brinkman, Kenneth A. 1962. Mixed or pure walnut plantings on strip-mined land in Kansas? Tech. Pap. 187. Columbus, OH: U.S. Department of Agriculture, Forest Service, Central Forest Experiment Station. 10 p. [22161]
214. Severson, Kieth E.; Kranz, Jeremiah J. 1978. Management of bur oak on deer winter range. Wildlife Society Bulletin. 6(4): 212-216. [82170]
215. Sharp, Ward M.; Chisman, Henry H. 1961. Flowering and fruiting in the white oaks. I. Staminate flowering through pollen dispersal. Ecology. 42: 365-372. [3910]
216. Shepperd, Wayne D.; Battaglia, Michael A. 2002. Ecology, silviculture, and management of Black Hills ponderosa pine. Gen. Tech. Rep. RMRS-GTR-97. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 112 p. [44794]
217. Shirley, Hardy L. 1932. Light intensity in relation to plant growth in a virgin Norway pine forest. Journal of Agricultural Research. 44: 227-244. [10360]
218. Shuman, Bryan; Henderson, Anna K.; Plank, Colin; Stefanova, Ivanka; Ziegler, Susy S. 2009. Woodland-to-forest transition during prolonged drought in Minnesota after ca. AD 1300. Ecology. 90(10): 2792-2807. [81308]
219. Sieg, Carolyn Hull. 1991. Ecology of bur oak woodlands in the foothills of the Black Hills, South Dakota. Lubbock, TX: Texas Tech University. 198 p. Dissertation. [82461]
220. Sieg, Carolyn Hull. 1993. A classification of soils in bur oak woodlands in the foothills of the Black Hills, South Dakota. Soil Science. 155(2): 131-147. [82171]
221. Sieg, Carolyn Hull; Wright, Henry A. 1996. The role of prescribed burning in regenerating Quercus macrocarpa and associated woody plants in stringer woodlands in the Black Hills, South Dakota. International Journal of Wildland Fire. 6(1): 21-29. [26769]
222. Simpson, Benny J. 1988. A field guide to Texas trees. Austin, TX: Texas Monthly Press. 372 p. [11708]
223. Smith, Christopher C.; Follmer, David. 1972. Food preferences of squirrels. Ecology. 53: 82-91. [2942]
224. Smith, Kimberly G.; Withgott, James H.; Rodewald, Paul G. 2000. Red-headed woodpecker--Melanerpes erythrocephalus. In: Poole, A.; Gill, F., eds. The birds of North America. No. 518. Philadelphia, PA: The Academy of Natural Sciences; Washington, DC: The American Ornithologists' Union: 1-27. [61928]
225. Sprackling, John A.; Read, Ralph A. 1979. Tree root systems in eastern Nebraska. Nebraska Conservation Bulletin Number 37. Lincoln, NE: The University of Nebraska, Institute of Agriculture and Natural Resources, Conservation and Survey Division. 71 p. [50196]
226. Stambaugh, Michael C.; Guyette, Richard P.; McMurry, Erin R.; Dey, Daniel C. 2006. Fire history at the eastern Great Plains margin, Missouri River Loess Hills. Great Plains Research. 16: 149-159. [82172]
227. Stan, Amanda B.; Rigg, Lesley S.; Jones, Linda S. 2006. Dynamics of a managed oak woodland in northeastern Illinois. Natural Areas Journal. 26(2): 187-197. [63290]
228. Stapanian, Martin A.; Smith, Christopher C. 1984. Density-dependent survival of scatterhoarded nuts: an experimental approach. Ecology. 65(5): 1387-1396. [10380]
229. Stapanian, Martin A.; Smith, Christopher C. 1986. How fox squirrels influence the invasion of prairies by nut-bearing trees. Journal of Mammalogy. 67(2): 326-332. [11978]
230. Steffen, David E.; Lafon, Nelson W.; Norman, Gary W. 2002. Turkeys, acorns, and oaks. In: McShea, William J.; Healy, William M., eds. Oak forest ecosystems: Ecology and management for wildlife. Baltimore, MD: The Johns Hopkins University Press: 241-255. [43534]
231. Steffen, Jim. 1993. Study examines the heat of combustion of deciduous tree leaf litter. Restoration & Management Notes. 11(2): 152. [22788]
232. Steinauer, Gerald A. 1981. A classification of the Cercocarpus montanus, Quercus macrocarpa, Populus deltoides, and Picea glauca habitat types of the Black Hills National Forest. Vermillion, SD: University of South Dakota. 95 p. Thesis. [86]
233. Steingraber, Sandra Kathyrn. 1989. Deer browsing, plant competition and succession in a red pine forest, Itasca State Park, Minnesota. Ann Arbor, MI: University of Michigan. 204 p. Dissertation. [64360]
234. Stephens, H. A. 1973. Woody plants of the north Central Plains. Lawrence, KS: The University Press of Kansas. 530 p. [3804]
235. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in Northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
236. Stout, A. B. 1944. The bur oak openings in southern Wisconsin. Transactions of the Wisconsin Academy of Science. 36: 141-161. [264]
237. Strausbaugh, P. D.; Core, Earl L. 1977. Flora of West Virginia. 2nd ed. Morgantown, WV: Seneca Books. 1079 p. [23213]
238. Summerville, Keith S.; Steichen, Renae M.; Lewis, Michelle N. 2005. Restoring lepidopteran communities to oak savannas: contrasting influences of habitat quantity and quality. Restoration Ecology. 13(1): 120-128. [60397]
239. Szafoni, Robert E.; Phipps, Richard L.; Harty, Francis M. 1994. Large, open-grown trees as indicators of presettlement savanna. Natural Areas Journal. 14(2): 107-112. [23925]
240. Szeicz, J. M.; MacDonald, G. M. 1991. Postglacial vegetation history of oak savanna in southern Ontario. Canadian Journal of Botany. 69: 1507-1519. [16607]
241. Tackett, Edward M.; Graves, Donald H. 1983. Evaluation of direct-seeding of tree species on surface mine spoils after five years. In: Symposium on surface mining, hydrology, sedimentology and reclamation: Proceedings; 1983 November 27 - December 2; Lexington, KY. [Lexington, KY]: [University of Kentucky, College of Engineering]: 437-441. [72089]
242. Tang, Z. C.; Kozlowski, T. T. 1982. Some physiological and morphological responses of Quercus macrocarpa seedlings to flooding. Canadian Journal of Forest Research. 12: 196-202. [42024]
243. Texas Natural Heritage Program. 1993. Plant communities of Texas (Series level). Austin, TX: Texas Parks and Wildlife Department. Unpublished report on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 26 p. [23810]
244. Thilenius, John F. 1972. Classification of deer habitat in the ponderosa pine forest of the Black Hills, South Dakota. Res. Pap. RM-91. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 28 p. [2317]
245. Thompson, Ralph L.; Vogel, Willis G.; Taylor, David D. 1984. Vegetation and flora of a coal surface-mined area in Laurel County, Kentucky. Castanea. 49(3): 111-126. [75263]
246. Tilman, David. 1987. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecological Monographs. 57(3): 189-214. [27331]
247. Tilman, David. 1988. Plant strategies and the dynamics and structure of plant communities. Monographs in Population Biology 26. Princeton, NJ: Princeton University Press. 360 p. [16944]
248. Tinus, Richard W. 1978. Production of container-grown hardwoods. Tree Planters' Notes. 29(4): 3-9. [63157]
249. Tolstead, W. L. 1942. Vegetation of the northern part of Cherry County, Nebraska. Ecological Monographs. 12: 255-292. [4470]
250. Tucker, J. M.; Maze, J. R. 1966. Bur oak (Quercus macrocarpa) in New Mexico? The Southwestern Naturalist. 11(3): 402-405. [82173]
251. U.S. Department of Agriculture, Natural Resources Conservation Service. 2011. PLANTS Database, [Online]. Available: https://plants.usda.gov /. [34262]
252. Uresk, Daniel W. 1987. Diets of cattle in the Black Hills of South Dakota. In: Fisser, Herbert G., ed. Wyoming shrublands: Proceedings, 16th Wyoming shrub ecology workshop; 1987 May 26-27; Sundance, WY. Laramie, WY: University of Wyoming, Department of Range Management, Wyoming Shrub Ecology Workshop: 33-35. [13916]
253. Uresk, Daniel W.; Boldt, Charles E. 1986. Effect of cultural treatments on regeneration of native woodlands on the Northern Great Plains. Prairie Naturalist. 18(4): 193-201. [3836]
254. Uresk, Daniel W.; Paintner, Wayne W. 1985. Cattle diets in a ponderosa pine forest in the northern Black Hills. Journal of Range Management. 38(5): 440-442. [2401]
255. Vankat, John L.; Snyder, Gary W. 1991. Floristics of a chronosequence corresponding to old field--deciduous forest succession in southwestern Ohio. I. Undisturbed vegetation. Bulletin of the Torrey Botanical Club. 118(4): 365-376. [18758]
256. Vogel, Willis G. 1977. Revegetation of surface-mined lands in the East. In: Forests for people: A challenge in world affairs: Proceedings of the Society of American Foresters 1977 national convention; 1977 October 2-6; Albuquerque, NM. Washington, DC: Society of American Foresters: 167-172. [9949]
257. Vogel, Willis G. 1981. A guide for revegetating coal minespoils in the eastern United States. Gen. Tech. Rep. NE-68. Broomall, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 190 p. [15577]
258. Vogl, Richard J. 1971. Fire and the northern Wisconsin pine barrens. In: Proceedings, annual Tall Timbers fire ecology conference; 1970 August 20-21; Fredericton, NB. No. 10. Tallahassee, FL: Tall Timbers Research Station: 175-209. [2432]
259. Wali, M. K.; Killingbeck, K. T.; Bares, R. H.; Shubert, L. E. 1980. Vegetation-environment relationships of woodland and shrub communities, and soil algae in western North Dakota. North Dakota Regional Environmental Assessment Program (REAP): ND REAP Project No. 7-01-1. Grand Forks, ND: University of North Dakota, Department of Biology. 159 p. [7433]
260. Wasser, C. H.; Hess, Karl. 1982. The habitat types of Region 2--U.S. Forest Service: a synthesis. Final report: Cooperative Agreement No. 16-845-CA. Lakewood, CO: U.S. Department of Agriculture, Forest Service, Region 2. 140 p. [5594]
261. Weaver, J. E. 1968. Studies in woodlands. In: Prairie plants and their environment: A fifty-year study in the Midwest. Lincoln, NE: University of Nebraska Press. 121-145. [55097]
262. Weaver, J. E.; Kramer, Joseph. 1932. Root system of Quercus macrocarpa in relation to the invasion of prairie. Botanical Gazette. 94: 51-85. [274]
263. Wertz, Tara L.; Flake, Lester D. 1988. Wild turkey nesting ecology in south central South Dakota. Prairie Naturalist. 20(1): 29-37. [9335]
264. Whitson, Thomas D. 1987. Weeds in Wyoming causing livestock poisoning. In: Fisser, Herbert G., ed. Wyoming shrublands: Proceedings, 16th Wyoming shrub ecology workshop; 1987 May 26-27; Sundance, WY. Laramie, WY: University of Wyoming, Department of Range Management: 55-57. [13922]
265. Wienk, Cody L.; Sieg, Carolyn Hull; McPherson, Guy R. 2004. Evaluating the role of cutting treatments, fire and soil seed banks in an experimental framework in ponderosa pine forests of the Black Hills, South Dakota. Forest Ecology and Management. 192(2-3): 375-393. [48628]
266. Will-Wolfe, Susan; Stearns, Forest. 1998. Characterization of dry site oak savanna in the Upper Midwest. Transactions of the Wisconsin Academy of Sciences, Arts and Letters. 86: 223-234. [39626]
267. Willert, Jolene M. 2000. Oak savanna restoration: management techniques to inhibit exotic shrub reinvasion. Madison, WI: University of Wisconsin-Madison. 142 p. Thesis. [80742]
268. Williams, Robert D.; Hanks, Sidney H. 1976. Hardwood nurseryman's guide. Agric. Handb. 473. Washington, DC: U.S. Department of Agriculture, Forest Service. 78 p. [4182]
269. Willson, Mary F. 1970. Foraging behavior of some winter birds of deciduous woods. The Condor. 72(2): 169-174. [61918]
270. Wofford, B. Eugene. 1989. Guide to the vascular plants of the Blue Ridge. Athens, GA: The University of Georgia Press. 384 p. [12908]
271. Wolf, Joy. 2004. A 200-year fire history in a remnant oak savanna in southeastern Wisconsin. The American Midland Naturalist. 152(2): 201-213. [55434]
272. Wolfe, Kim. 2001. Bur oak (Quercus macrocarp Michx.) in Riding Mountain National Park. Winnipeg, MB: University of Manitoba. 162 p. Thesis. [82463]
273. Wright, Henry A.; Bailey, Arthur W. 1982. Temperature and heat effects. In: Fire ecology: United States and southern Canada. New York: John Wiley & Sons: 8-23. [18417]
274. Yeager, A. F. 1935. Root systems of certain trees and shrubs grown on prairie soils. Journal of Agricultural Research. 51(12): 1085-1092. [3748]
275. Ziegler, Susy Svatek; Larson, Evan R.; Rauchfuss, Julia; Elliott, Grant P. 2008. Tree establishment during dry spells at an oak savanna in Minnesota. Tree-Ring Research. 64(1): 47-54. [82174]