| FEIS Home Page |
 |
|
| Photo by Miguel Vieira, available on Flickr. |
Chrysolepis chrysophylla var. chrysophylla, typical variety of giant chinquapin
Chrysolepis chrysophylla var. minor (Benth.) Munz, scrub golden chinquapin
Most information on giant chinquapin does not distinguish between varieties. In this review, "giant chinquapin" refers to the species as a whole, and the varieties are referred as "the typical variety" or "scrub golden chinquapin". Shrub forms of giant chinquapin are not necessarily scrub golden chinquapin [54,95]. Although shrub and tree forms may be genetically distinct [54,124], differences in growth form of the typical variety [54] are likely due to site conditions [138,139]. When distinctions were made between shrub and tree growth forms, this will be noted.
Shrub forms of giant chinquapin probably hybridize with bush chinquapin (C. sempervirens) where their distributions overlap [62].
SYNONYMS:for Chrysolepis chrysophylla var. minor:
Castanopsis chrysophylla (Douglas ex Hook.) A. DC. var. minor (Benth.) A. DC. [81,92,122]
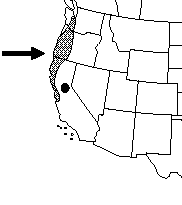 |
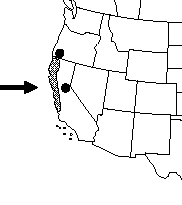 |
| Distribution of the typical variety | Distribution of scrub golden chinquapin |
| Maps courtesy of the Flora of North America Association. 2012, March 20 | |
States (as of 2012) [175]:
United States: CA, OR, WA
Giant chinquapin is native to the Pacific Northwest and California. It is most abundant in the mountain ranges of northwestern California [21,98] and southwestern Oregon [7], including the Coast Ranges [32,40,116], Klamath Mountains [21,85], and western slopes of the Oregon Cascade Range [98]. It is common as far north as the Columbia River [7,82]. Giant chinquapin's distribution extends into the Sierra Nevada [169] including Plumas [112], El Dorado [62,116] and Tulare counties [201]. In Washington, disjunct populations occur in Mason and Skamania counties [27,82,97,98]. Rare dispersal events and occurrence of relict populations from a historically more widespread distribution have been suggested as possible reasons for giant chinquapin's presence in Washington [97].
Scrub golden chinquapin occurs farther south than the typical variety [7,122]. The typical variety is rare south of Marin County [138,139] and is comparatively common from Mendocino and Humboldt counties north to Oregon [3,124]. Scrub golden chinquapin occurs from the Santa Lucia Mountains [122] north along the coast [81] through northern California [81,122,197] to southern Oregon [175,197]. It also occurs in the Sierra Nevada including El Dorado [58,122] and Plumas counties [112].
SITE CHARACTERISTICS AND PLANT COMMUNITIES:
Site characteristics: Giant chinquapin occurs in a wide range of sites from warm, dry, exposed locations [7,47,78,79,82,116] to low-elevation mesic forests [95,116,139]. Shrubby forms are more common in relatively harsh environments [124,138,139], including exposed [82], dry, and high-elevation sites ([7,95,116,138], McMinn 1951 cited in [58]). Giant chinquapin trees are typically associated with low-elevation [116] mesic [95,116,139] forests, often on north-facing slopes [95,139], but they also occur on dry sites [116] and may thrive on moderately dry sites (See Moisture availability).
Although many reports associate giant chinquapin with “xeric” sites within its distribution, this term and others referring to moisture regime are often used in a relative sense, making it difficult to compare reports. For instance, 2 sources [51,73] described the western hemlock/giant chinquapin community as a comparatively xeric old-growth community of the western slope of the Oregon Cascade Range [51,73], while Means [117] described this same community as mesic when compared to dry Douglas-fir- and tanoak-dominated communities of the same region. Giant chinquapin was associated with comparatively moist parts of the ponderosa pine zone in central Oregon [42], and it was an indicator species for "moist" vegetation in the South Umpqua Basin of southwestern Oregon, an area that receives comparatively little rainfall (see Precipitation). Species that indicated dry sites in this study area included hollyleaved barberry (Mahonia aquifolium), California fescue (Festuca californica), and ponderosa pine [119].
The distribution of giant chinquapin occurs within an area of generally mild climate [20,113,116,124]. McKee [116] suggests that some shrubby individuals are an ecotype adapted to heavy snowpacks, cool temperatures, and short growing seasons common in the Oregon Cascade Ranges and eastern Oregon.
Elevation
Topography
Soil
Moisture availability
Precipitation
Temperature
Elevation: Giant chinquapin grows from near sea level [82] in the Coast Ranges of Oregon and California [116] to over 5,000 feet (1,500 m) in the Siskiyou Mountains [193]. It occurred on a site ranging from 660 to 1,150 feet (200-350 m) on the eastern slope of the Coast Ranges in Oregon [132]. In the Siskiyou Mountains of California and Oregon, giant chinquapin was most abundant from 2,500 to 3,500 feet (670-1,070 m) and was common from 1,500 to 4,500 feet (460-1,370 m). It was rare from 4,500 to 5,500 feet (1,370-1,680) and was not observed at higher elevations [193]. In central Oregon, giant chinquapin has been noted up to 5,000 feet (1,520 m) in mixed-conifer/snowbrush ceanothus/long-stolon sedge community (Ceanothus velutinus-Carex inops subsp. inops) [108] and ponderosa pine (Pinus ponderosa) communities [125,183]. Giant chinquapin occurred on a site in the Sierra Nevada at 4,300 feet (1,310 m) [93].
Shrubby giant chinquapins occur at higher elevations than giant chinquapin trees ([95,137,138], McMinn 1951 cited in [58]). In the Willamette, Mt Hood, and Siuslaw National Forests of Oregon, giant chinquapin occurs as a tree at low to midelevations and only occurs as a shrub at high elevations [173]. Giant chinquapin is commonly reported below about 4,500 feet in the Cascade Range ([6,164], Weisberg 1995 cited in [19]), while shrubby giant chinquapin occurs along the crest of the Cascade Range in Oregon up to 6,000 feet (1,830 m) [116]. The importance of giant chinquapin trees in 3 Douglas-fir-hardwood forests of the Klamath Mountains of California declined substantially above 3,900 feet (1,200 m), although tree forms occurred up to 5,250 feet (1,600 m) [95]. McKee [116] suggests that there are 3 giant chinquapin ecotypes, 1 of which is a shrubby form occurring at high elevations in the Oregon Cascade Range and eastern Oregon. Scrub golden chinquapin occurs at high elevations in the southern portion of giant chinquapin's range [7].
Topography: Giant chinquapin may be more common on ridges [47,95,151] and slopes [9,96,145,174,180], including those that are steep [47,84,174], than near streams [145] or in valley bottoms [151]. On 2 west-central Oregon watersheds, giant chinquapin was positively (P=0.008) associated with hillside topography [145], and it was inversely associated with percent slope on the Willamette National Forest [174]. In the western Cascade Range in Oregon, giant chinquapin was the dominant species on a site with a 40° slope [96]. Basal area of giant chinquapin in 63 zero-order stream basins averaged 0.2 m²/ha in valleys, 0.7 m²/ha on slopes, and 0.9 m²/ha on ridges [151]. Giant chinquapin occurrence in valley bottoms may be restricted to the interior and southern boundaries of its distribution [84]. For example, giant chinquapin trees had "significant cover" in ravines and valley bottoms in Douglas-fir-hardwood forests in the Klamath Mountains [95].Although giant chinquapin occurs on all aspects, its growth form [95,139] and occurrence [95,192] suggest that north slopes may be more favorable, at least in some areas. In the Siskiyou Mountains, 47% of giant chinquapin stems were on north- and northeast-facing aspects, while only 5% occurred on south- to west-facing aspects [192]. In Douglas-fir-hardwood communities in the Klamath Mountains, the greatest cover of giant chinquapin trees consistently occurred on north- and northeast-facing aspects, and no substantial giant chinquapin stands occurred on slopes with aspects south of due east or due west [95]. According to Holland [84], giant chinquapins near the southern and interior margins of their distribution are restricted to north slopes. In contrast, on south-facing and southwest-facing slopes in the western Cascade Range in Oregon, giant chinquapin occurred as an overstory dominant [96] and as a midstory dominant under western hemlock (Tsuga heterophylla) [47,73]. In southwestern Oregon, a giant chinquapin-tanoak-sugar pine (Lithocarpus densiflorus-Pinus lambertiana) community usually occurred on south or southwestern aspects [9].
Soil: Giant chinquapin occurs in variable soil types [116] but is commonly associated with rocky [9,10,47,51,76,76,180] and/or unproductive soils [27,116,124,152]. In forests of southwestern Oregon, it often indicates rocky sites [9]. The shrub form commonly occurs on rocky sites [84,86,116]. Within Douglas-fir communities in southwestern Oregon, giant chinquapin was associated with the least productive sites [8].
Soil pH on sites with giant chinquapin is often acidic, with values ranging from around 4.1 [91,190] to 6.2 [5].
Giant chinquapin occurs on sites with various parent materials. Giant chinquapin shrubs occurred in 2 of 3 forest communities occurring on lava flows in Oregon [51]. Giant chinquapin, including scrub golden chinquapin [35,193], is associated with ultramafic [9,134] and serpentine [193] substrates.
It has been suggested that giant chinquapin may be more likely to develop into large trees on sites with deep soils, since these sites generally experience more moderate moisture regimes [116,124].
Moisture availability: Giant chinquapin occurs on sites that experience a wide range of soil moisture regimes, from xeric chaparral communities [116] to mesic redwood (Sequoia sempervirens) forests of the California coast [147]. On the most xeric sites within its range, giant chinquapin occurs mainly as a shrub ([7,95,116,138], McMinn 1951 cited in [58]). Scrub golden chinquapin occurred on dry exposures in central California [7], northwestern California, and southwestern Oregon [116]. The Douglas-fir-giant chinquapin/dwarf Oregon-grape (Pseudotsuga menziesii-Chrysolepis chrysophylla/Mahonia nervosa) association occurs in cool, moist areas of southwestern Oregon [9].
Giant chinquapin is often associated with moderately xeric sites. At lower elevations (2,000-3,000 feet (610-915 m)) on diorite in the Siskiyou Mountains of Oregon and California, it had a bimodal distribution across a moisture gradient based on topographic characteristics, with a peak on submesic sites and a larger peak on xeric sites [193]. Giant chinquapin has been considered an indicator of dry sites in Douglas-fir-western hemlock forests of the Pacific Northwest [50], on the Siuslaw [78] and Willamette [79] National Forests, and for the tanoak series of the Siskiyou Region [11]. Based on plant moisture stress in 2 western hemlock/giant chinquapin communities in the western Cascade Range of Oregon, giant chinquapin had high importance values on dry sites. It was not limited by moisture stress on these sites [202]. Giant chinquapin comprised a substantial component of the biomass of old-growth Douglas-fir stands in the H. J. Andrews Experimental Forest in relatively xeric locations [61]. This experimental forest is located on the west slope of the Cascade Range in central Oregon. A Douglas-fir-giant chinquapin community experienced greater water stress in the summer than 3 other major plant communities on a watershed in H. J. Andrews Experimental Forest [61]. In northwestern California, Douglas-fir-giant chinquapin forests occur on drier slopes than Douglas-fir forests [146]. In comparatively moist environments, giant chinquapin may be limited to openings, while it is increasingly competitive on drier sites [116].
Giant chinquapin may grow larger on comparatively xeric sites [30,61,95,137]. It occurred in the overstory of a dry mixed-conifer site with Douglas-fir and Pacific madrone (Arbutus menziesii), while it occurred in the midstory of a more mesic transition site and a wet site in the western hemlock zone [30]. Giant chinquapin reached its greatest development in the lower, drier portions of the Douglas-fir-grand fir association of the Nash Crater Lava Flows in Linn County, Oregon [137], and it occurred as trees on the driest sites of a west-central Oregon Cascade Range study area [187]. “Driest” must be considered in a relative sense, however, considering Roof’s [139] suggestion that development of large giant chinquapin trees requires "ample" winter rainfall and summer fog, and Keeler-Wolf’s [95] statement that giant chinquapin obtains subdominance in Douglas-fir-hardwood forests that receive ≥60 inches (1,520 mm) of precipitation annually.
In Douglas-fir-hardwood forests of the Klamath Region, giant chinquapin sexual reproduction (see Seedling establishment and plant growth) and density were generally greater on mesic than dry sites. The oldest giant chinquapin trees, estimated as 400 to 500 years old, occurred on the driest site, with annual precipitation from 60 to 70 (1,780 mm) inches. Since heart rot was more prevalent on somewhat moister sites, it was suggested that increased heart rot may have shortened giant chinquapin life spans on mesic sites [95].
Precipitation: Average annual precipitation on sites with giant chinquapin ranges from less than 20 inches (510 mm) in southern California [116] and portions of southern Oregon [74] to more than 160 inches (4,000 mm) in coastal Douglas-fir-western hemlock forests of California [84]. Much winter precipitation in the higher elevations of the Cascade Range [116,124] and central Oregon [44,108] falls as snow, while snow is uncommon along the California coast [84,147]. Giant chinquapin's conical shape and deflexed branches led Keeler-Wolf [95] to conclude that it is more tolerant of snow than tanoak or Pacific madrone and is adapted for mesic, upper-elevations of the Douglas-fir-hardwood zone.
| Table 1. Average annual precipitation in regions of the western United States with giant chinquapin | |
| Region | Average annual precipitation |
| Central Oregon | 25-30 inches (635-890 mm) [108,140] |
| Umpqua Valley & Rogue River Basin | 20-30 inches (510-760 mm) [74] |
| Western Cascade Range | foothills: 44 inches (1,120 mm) [19] |
| Southern Cascade Range | 30-68 inches (760-1,730 mm) |
| California and Oregon Coast Ranges | 34-83 inches (865-2,110 mm) [113] 70-100 inches (1,780-2,540 mm) western slope in southern Oregon [74] 48 inches (1,222 mm) east slope in Oregon [132] |
| Klamath Mountains | 30-68 inches (760-1,730 mm) [113] 22.3-64.4 inches (576-1,636 mm) [152] |
| Northern California coast | 20-118 inches (500-3,000 mm) (Miles and Goudey 1997 cited in [162]) ≥98 inches (2,500 mm) east of Cape Mendocino [147] |
| Sierra Nevada | 30-68 inches (760-1,730 mm) [113]
67 inches (1,700 mm) [93] |
| Central California coast | western slopes: ≥39 inches (1,000 mm) [147] |
Throughout most of its range, giant chinquapin experiences moist winters and dry summers [5,44,67,116,124,152,162,164]. This seasonal pattern occurs along the central California coast [147], and in northwestern California [20], southwestern Oregon [74], and central Oregon [44,67,164]. The summers are typically drier in the southern portions of the giant chinquapin's range [116]. In coastal areas, fog may provide additional moisture in the summer months [113,162].
Temperature: Temperatures experienced by giant chinquapin are generally mild [20,74,113,116]. Reported temperatures on sites with or near giant chinquapin range from winter lows of 19 °F (-7 °C) in northwestern California [20] and central Oregon [44] to summer highs of 98 °F (37 °C) on west slopes of the central California coast. On a burned site in central California, July temperatures may have exceeded 110 °F (46 °C) [5]. Number of frost-free days in the interior mountains where giant chinquapin occurs range from 116 to 240, while the number of frost-free days in the Coast Ranges of California and southwestern Oregon ranges from 204 to 338 (McDonald 1981 unpublished report and Stein 1981 unpublished report cited in [113]).
| Table 2. Average temperatures (°F) within the giant chinquapin's distribution in winter and summer. When provided, average minima and maxima for each season are displayed, separated by a slash (/). | ||
| Location | Winter | Summer |
| Central Oregon | 19/42 | 43/84 [44,67] |
| Klamath Mountains (daily max/mins) | 23-36/ 44-54 | 48-63/89-96 [152] |
Klamath Mountains |
33-41 | 62-75 [113] |
| Coast Ranges of California and Oregon | 41-50 | 57-71 [113,132]. |
| Central California | not reported/52 | not reported/87 [162] |
Along the northern distribution of giant chinquapin, there is an east-west gradient with eastern locations getting more snow [8] and having more variable temperatures [8,162] than western locations. This is the case in the Cascade Range [164], the Siskiyou Mountains [8], the Klamath Mountains [152], and the north coastal bioregion of California. In this region, a coastal site had only a 11.5 °F (6.4 °C) difference between winter and summer average maximum temperatures, while a more inland location near Napa Valley had a 34.7 °F (19.3 °C) difference between winter and summer average maximum temperatures [162].
Giant chinquapin is often associated with warm sites [9,77,202], although it can occur on cool sites, where the tree form is more common than shrub form [9,95,139]. It is an indicator of warm sites on the Mt Hood, Willamette [77], and Siuslaw National Forests [78]. The western hemlock-giant chinquapin/salal-Pacific rhododendron (Gaultheria shallon-Rhododendron macrophyllum) community has a warmer climate than western hemlock associations in the Cascade Mountains with less giant chinquapin [9]. Based on soil and air temperatures in western hemlock/giant chinquapin communities in the western Cascade Range of Oregon, giant chinquapin was categorized has having a center of importance on "medium hot" sites [202]. Scrub golden chinquapin occurs on warm exposures in the southern portion of the species' range [7].
Plant communities: Giant chinquapin occurs primarily in the understory of conifer and mixed-conifer-hardwood communities [9,51,73,75,80,86,146,181,194]. Shrub forms, including scrub golden chinquapin, occur in brushfields [74]. See the Fire Regime Table for a list of plant communities in which giant chinquapin may occur and information on the fire regimes associated with those communities.
Forests and woodlands: Giant chinquapin is most common in the midstory under conifers, although it occasionally occurs in the overstory [9,30]. It occurs in the midstory of Douglas-fir-hardwood [146], mixed-conifer [75,181], mixed-evergreen [80,194], western hemlock [9,51,73], white fir (Abies concolor), and Shasta red fir (Abies magnifica var. shastensis) communities [51,86]. A description of giant chinquapin occurrence in forest and woodland communities is provided in Table 3. Overstory cover in these communities ranges from open [98] to fairly dense [82,84]. In western hemlock/giant chinquapin communities, overstory cover was commonly <50% in the western Cascade Range in Oregon [51,73].
Giant chinquapin rarely occurs in pure stands [97,116]. It was the dominant species on 1 of 102 Forest Inventory and Analysis plots in dry hardwood communities of western Oregon [115]. In a survey of California hardwoods, only 1,000 acres (405 ha) of giant chinquapin-dominated stands were reported inside National Forests, and none were reported outside National Forests [21]. Giant chinquapin stands are isolated [7] and rarely exceed 25 acres (10 ha) [116]. In southwestern Oregon, giant chinquapin occurred in the overstory in stands of tanoak, tanoak and Douglas-fir, tanoak and white fir [9], and Douglas-fir and Pacific madrone [30].
In some forest and woodland communities, giant chinquapin has only been reported as a shrub. These include Jeffrey pine (Pinus jeffreyi) [90,184], mountain hemlock (Tsuga mertensiana) [77], and lodgepole pine (P. contorta)-Douglas-fir [99]. Scrub golden chinquapin occurs in knobcone pine (P. attenuata) communities in California [35] and Jeffrey pine communities in the Klamath Mountains [90].
| Table 3. Plant communities in which giant chinquapin is common, including citations distinguishing growth form and/or variety | |
| Communities | Growth form |
Conifer-dominated communities |
|
| Redwood | general [2,7,21,84,122,147] tree [40,62,141,179,190] scrub golden chinquapin [190] |
| Mixed-conifer | general [21,93,97,106,131,164] tree [49,50,51,62] shrub [41,49,50,51,184] scrub golden chinquapin [112] |
| Ponderosa pine | general [125,140,142] shrub [200] scrub golden chinquapin [122] |
| Douglas-fir | general [7,9,13,21,29,50,51,97,122,172,186,196] tree [13,50,117] shrub (large) [143] |
| Western hemlock | general [9,78,117] tree [13,50,51,59,79,117] shrub [47,79,143] |
| White fir | general [8] tree [50] shrub [184] |
| Shasta red fir | general [8] tree [10] shrub [50,51,184] |
| Pacific silver fir (Abies amabilis) | shrub [79] |
| Bishop pine (P. muricata) | general [91,130,190,191] scrub golden chinquapin [122] |
| Port-Orford-cedar (Chamaecyparis lawsoniana) | general [8,157,193] |
Mixed-conifer-hardwood communities |
|
| Mixed-evergreen | general [32,49,51,64,84,100,172,193] tree [9,40,50,62] scrub golden chinquapin [190] |
| Douglas-fir-tanoak | general [50,162] |
Hardwood communities |
|
| Tanoak | general [11,21,84,152] tree [9,50] |
| Mixed-sclerophyll hardwood | general [76] |
GENERAL BOTANICAL CHARACTERISTICS:
 |
|
| Photo by Pete Veilleux, East Bay Wilds |
Botanical description: This description covers characteristics that may be relevant to fire ecology and is not meant for identification. Keys for identification are available (e.g., [7,37,81,82]).
Form and architecture: Giant chinquapin occurs as a tree or a shrub. In general, it is described as ranging from 16 to 100 feet tall (5-30 m) [2,7,21,27,50,82,96,124,173]. The largest trees are up to 150 feet (45 m) tall [122,124] and 5 feet (1.5 m) in diameter [7,27]. Smaller sizes are much more common [61,73,104]. In several studies of giant chinquapin in western Oregon, average DBH ranged from 3.1 to 8.3 inches (7.9-21 cm), and average heights ranged from 19.2 to 43.4 feet (5.9-13.2 m) [70,104,168]. Over 98% of giant chinquapins in an old-growth Douglas-fir community in a western Cascade Range watershed had a DBH in the 6- to 12-inch (15-30 cm) category [61]. Of 101 giant chinquapins in southwestern Oregon, only 1 had a DBH in the 20- to 25-inch (51-64 cm) class, and most were less than 5 inches (13 cm) in DBH [104].
The extent to which genetics and site characteristics influence the growth form of giant chinquapin is unclear. According to a worldwide flora of the order Fagales, the typical variety can be a tree, large shrub, or shrub (<10 feet (3 m) tall), while scrub golden chinquapin only occurs as a shrub [54]. Two California floras suggest trees greater than 50 feet (15 m) tall are the typical variety, while shrubs and small trees up to about 33 feet (10 m) are scrub golden chinquapin [81,122]. However, giant chinquapin is commonly shrubby in Washington [27,197], where only the typical variety occurs [92]. Good sites or periods of favorable conditions may promote tree growth forms [50,98,139] (see Site characteristics). Roof [138,139] states that individual giant chinquapins may develop from shrubs into trees during favorable periods [138]. He noted a pattern of giant chinquapin shrubs on ridges, trees on sheltered north slopes, and intermediate forms in between. He suggests in these situations, it is impossible to determine where the typical variety ends and scrub golden chinquapin begins [139].
Mature trees typically have a straight trunk [7,21,109,124] with a cone-shaped crown [7,81,124] comprised of stout branches [122] that form right angles from the bole [21]. Trees in open areas may have highly tapered boles and more spreading crowns [124]. The bark is thick and furrowed [7,81,82,173]. Giant chinquapin shrubs have a spreading form [7].
Leaves: Giant chinquapins have sclerophyllous evergreen leaves that are lanceolate to oblong and have entire margins [7,27,82,122]. The undersides are yellow-green to golden and fuzzy [3,7,27,82,122]. Leaves range from 2 to 6 inches (5-15 cm) long [3,7,122]. Leaf area of giant chinquapin saplings at H. J. Andrews Experimental Forest averaged 16 cm² per leaf [96]. Leaves remain on trees for about 3 years [27]. Leaves of scrub golden chinquapin commonly have upturned margins [81,122].
Reproductive structures: Male flowers are 1- to 3-inch (2.5-7.6 cm) long [7,109,116,124] catkins [82,116]. Up to 3 female flowers occur in an involucre at the base of the male catkin or in short separate catkins along the stem [81,82,109,116,124]. The flowers emit a strong musty odor [2,7].
Giant chinquapin fruits are nuts. There are 1 to 3 nuts enclosed [109,116,124,199] in a distinctive 4-valved spiny bur [82,98,122]. The bur typically ranges from 0.6 to 1.0 inch (1.5-2.5 cm) across [82,116,124,173], but may be as large as 2 inches (5 cm) [81]. The nuts range from 6 to 15 mm long [3,7,81,82,122] and from 700 [121] to 1,100 per pound [124].
Roots: Giant chinquapin seedlings may have deep-growing roots [98] and often develop a taproot. As they mature, the lateral root system becomes well developed [124].
Longevity: Giant chinquapin live up to 500 years old [2,7,95,116]. Maximum ages of giant chinquapin in the northern California Coast Ranges [116] and in Douglas-fir-hardwood forest in the Klamath Mountains of northern California [95] were from 400 to 500 years. Giant chinquapin may have longer lifespans on xeric than moist sites because heart rot is less common in these areas [95] (see Site characteristics). Maximum ages of about 150 years have been noted on "better sites" in the Pacific Northwest in general [50] and on the H. J. Andrews Experimental Forest in particular [116]. Because of giant chinquapin's sprouting ability, individual genets may be several centuries old [116].
Raunkiaer [133] life form:Seed production: McKee [116] observed giant chinquapin 40 to 50 years old producing fertile seed, although the age of reproductive maturity is likely earlier. Sprouts that were 6 years old produced some fertile seeds [116]. Fruit production is generally low, with mast seeding occurring cyclically [95,116], at 2-to 5-year intervals [2,95,116]. It takes 2 growing seasons for the fruits to mature [27,81,98,116,122,197,199]. Fertile seeds may be uncommon [7].
Seed production may be locally reduced by insects. For instance, on one site in the H. J. Andrews Experimental Forest nearly 100% of seeds were attacked by insects, while on 2 other sites in the area only 15% of seeds were infested [116].
Seed dispersal: Giant chinquapin seeds are dispersed by gravity [113,116,124] and animals [113,124,182], including birds [113,116] and squirrels [116].
Seed banking: Data on longevity of giant chinquapin seeds in the field were not available as of early 2012. In general, species with large, burred seeds tend to have transient seed banks [15]. Other genera in Fagales, including oaks [23] and chestnuts [22], have seeds that last through only one growing season. Giant chinquapin likely follows this pattern. Giant chinquapin seeds stored at low temperatures remained viable for at least 3 to 5 years [97,109,121].
Occurrence of clumps of seedlings implies that squirrels may cache giant chinquapin nuts [116].
Germination: Giant chinquapin's germination rate of 14% to 53% ([121], USDA Forest Service 1948 cited in [87]) is low compared to other hardwoods of southwestern Oregon and northwestern California [113]. Germination occurs in 16 to 24 days ([121], USDA Forest Service 1948 cited in [87]).
Giant chinquapin germination does not require stratification [121]. The seed, like that of other Fagales [23], may be immediately germinable. Cold stratification did not increase giant chinquapin germination (USDA Forest Service 1948 cited in [87]). This suggests germination could occur in fall, although this was not observed in 3 years of observation on the H. J. Andrews Experimental Forest [116].
On the H. J. Andrews Experimental Forest, giant chinquapin seedlings occurred in comparatively open areas. Based on site characteristics, germination likely occurred in partial shade under a light leaf mulch [116].
Seedling establishment and plant growth: Seedling establishment may be most common on mesic, partially shaded sites with open understories and ground layers. On 3 Douglas-fir-hardwood sites in the Klamath Mountains in California, giant chinquapin seedlings were most common on mesic sites in relatively cool, shady environments, such as valley bottoms, and least common on xeric upper slopes. Increased understory density was negatively associated (P<0.05) with sexual reproduction of giant chinquapin [95]. According to a hardwoods silvics manual, giant chinquapin seedlings from 6 to 18 inches (15-45 cm) tall only occurred in relatively open stands on the H. J. Andrews Experimental Forest [116].
According to McDonald [109], giant chinquapin regeneration is often sparse or absent. Giant chinquapin seedling and sapling occurrence on sites where large individuals occur ranges from 0 [109,117] to 160/ha [95]. Giant chinquapin seedling densities were consistently low in the Klamath Mountains of northern California, typically 0 to 3 seedlings/0.1 ha, although on one site they were as high as 16/0.1 ha [95]. In an inventory of California hardwoods, 70% of hardwood forest types had giant chinquapin seedlings and 35% had giant chinquapin saplings [21]. On sites in the western Oregon Cascade Range, giant chinquapin saplings less than 54 inches (137 cm) tall occurred in 3 of 6 community types, with densities ranging from 6.9 to 124.4/ha. Mature giant chinquapin was present in 5 of these 6 communities [117].
Giant chinquapins, including seedlings [124,165], grow slowly [2,98]. By the July following germination, seedlings are typically 1.6 to 3.9 inches (4-10 cm) tall and have a root system that is ≥5.9 inches (15 cm) deep [165]. In the Pacific Northwest, seedlings may be only 6 to 18 inches (15-46 cm) tall after 4 to 12 years [124]. Giant chinquapin and other hardwoods in the understory of conifer forests often produce 2 or more stems within 10 years. The authors suggest that the growth rate of giant chinquapin in the understory is similar to the growth rate of tanoak in the understory [165]. In 2 stands in the Cascade Range that were about 100 years old, giant chinquapin diameter growth over 10 years averaged 1.8 to 2.0 mm/year [116]. In giant chinquapin-dominated or -codominated stands in northern California, trees may take 15 years to grow 1 inch (2.5 cm) in diameter [113]. On 3 Douglas-fir-hardwood sites in the Klamath Mountains of California, giant chinquapin obtained diameters of 24 inches (60 cm) in about 210 to 260 years and diameters of 48 inches (122 cm) in 400 to 500 years [95]. Sprouts grow comparatively quickly (see Vegetative regeneration).
Vegetative regeneration: Giant chinquapin exhibits "vigorous" [109,116,124,182] sprouting following damage from fire [5,7,55,67,116,124,152], cutting [7,67,116,124], and/or herbicide application [42,55,57,149]. Sprouts may originate from root crowns [109,111], burls [95,139], or roots [94]. In the Siskiyou Mountains of southwestern Oregon, fire increased the rate of sprouting in scrub golden chinquapins that had previously sprouted after herbicide treatment. This was attributed to sprouting from different portions of the same root system, resulting in one individual counting as 2 or more individuals after burning [55].
Sprouts typically grow much faster than seed-originated individuals. In Douglas-fir-hardwood communities in the Klamath Mountains of northern California, it took sprouts about 50 years to reach 18.1 inches (46 cm) DBH, while seed-originated stems this size averaged about 140 years old [95]. In clearcuts in the Siskiyou Mountains near Cave Junction, Oregon, 32 sprout clumps of giant chinquapin averaging 5.6 years old had an average diameter of 11.8 feet (3.6 m), with the tallest sprout reaching 4.9 feet (1.5 m) [120]. Giant chinquapin sprouts commonly grow faster than conifers for several years [109,116]. Sprout stands tend to self-thin rapidly [124].
Based on data from 3 Douglas-fir-hardwood sites in the Klamath Mountains, vegetative regeneration was the primary means of reproduction on relatively xeric upper slopes, where fires occur more frequently and moisture regimes are less conducive to seedling establishment than on more mesic sites [95].
SUCCESSIONAL STATUS:Giant chinquapin has been observed in recently disturbed stands—as soon as 2 years following fire [24,94]—and in undisturbed stands over 500 years old [13,68]. It was not significantly associated with young (<80), mature (80-180 years), or old-growth (>130 years) forest in the Oregon Cascade Range [68,155] or the Oregon Coast Ranges [68]. In Douglas-fir-hardwood forests of the Klamath Mountains in California, giant chinquapin cover was not associated with total cover of all species, suggesting that giant chinquapin cover has no relationship with seral stage [95]. In Douglas-fir and/or western hemlock communities with Pacific rhododendron/dwarf Oregon-grape understories in the southern Oregon Coast Ranges, giant chinquapin cover averaged 5% in stands 55 to 70 years old, 4% in 190-year-old stands, and 1% in near-climax or climax forests ranging from 300 to 500 years old [13]. In the Coast Ranges in west-central Oregon, giant chinquapin was one of several hardwoods that were prominent in both 55-year-old and >400-year-old stands [132]. Cover of giant chinquapin on the H. J. Andrews Experimental Forest ranged from 0.05% in Douglas-fir plantations that had been clearcut and broadcast burned 5 years previously to 3.85% in 40-year-old plantations. Giant chinquapin cover in 450-year-old undisturbed old growth in this area was 1.42% [148]. Giant chinquapin is commonly reported in stands in intermediate seral stages from about 55 to 200 years old [13,16,30,93,183].
Giant chinquapin may be an important component of early-successional communities. Density of giant chinquapin more than 9 inches (23 cm) DBH in the Coast Ranges of northwestern California was 2.3 trees/ha to 2.9 trees/ha over 30 years following clearcutting and broadcast burning [161]. During 38 years following clearcutting and broadcast burning in 2 watersheds on the H. J. Andrews Experimental Forest, giant chinquapin density peaked at nearly 400 stems/ha in posttreatment years 22 to 25. Giant chinquapin bole biomass increased slowly over the course of the study [107]. In the first 21 years following clearcutting and broadcast burning, giant chinquapin cover was less than 5% [67]. Giant chinquapin has been described as competitive on xeric, unfertile sites early in succession [116] and is a component of early successional brushfields [49,51]. These are either early-successional communities that may slow the establishment of conifers [49,160,163] or edaphic climax communities [40,49,152,163]
Since giant chinquapin is suppressed when overtopped by conifers in some areas [97,107,113], disturbance may be required for it to remain a substantial component of the vegetation [111,116], especially on "good" sites [111,124]. "Suppression mortality" accounted for about half of giant chinquapin stem and bole mortality in the 38 years following clearcutting and broadcast burning on the H. J. Andrews Experimental Forest. "Suppression mortality" was defined as death of small stems in the subcanopy that had slow growth but no damage [107]. Without disturbance, broad-leaved evergreen communities comprised of giant chinquapin, tanoak, and Pacific madrone in southwestern Oregon may slowly succeed to Douglas-fir-tanoak or Douglas-fir forests [76].
On some sites giant chinquapin may be an important component of late-successional and old-growth stands. Cover of giant chinquapin in old-growth Douglas-fir forests (>195 years) on the Oregon Coast Ranges was twice that in mature forests (80-195 years) and more than 10 times that in young (average 55 years) forests (P≤0.1) [155]. Giant chinquapin is considered persistent [68,111] and reproduces in late-successional stages [89,95] in northern California [11,86,89] and the Coast and Cascade ranges in Oregon [68]. Douglas-fir-hardwood [111] and western hemlock-giant chinquapin [47,73,202] are climax communities that may have open structure [47]. In the Klamath Mountains of California, giant chinquapin has been suggested as the hypothetical climax species in the narrow zone above the tanoak and below the white fir series [95]. In contrast, on Douglas-fir and/or western hemlock/Pacific rhododendron/dwarf Oregon-grape forests in the southern Oregon Coast Ranges, giant chinquapin occurrence declined in old-growth stands. It was uncommon and occurred in the shrub layer in canopy gaps within near-climax or climax forests (300-500 years old) [13]. A manual on hardwood silvics also notes giant chinquapin decline in late-successional stages [116].
Shade tolerance: Giant chinquapin has been categorized as tolerant [7,42,88,161], intermediate in tolerance [50,116,124], and intolerant of shade [97,111]. Less photosynthetic tissue per branch volume and slower turnover of foliage of giant chinquapin may contribute to its greater tolerance of shade compared to Arbutus and Quercus [88]. In 4 watersheds in western Oregon, giant chinquapin was not significantly associated with either gaps or forest interiors [145]. In a survey of experts, 50% responded that giant chinquapin had intermediate shade tolerance, 25% responded that it was shade tolerant, and 25% responded that it was shade intolerant. This led Baker [14] to categorize giant chinquapin as intermediately shade tolerant, but he qualified the classification as having a high degree of uncertainty. Tree forms of giant chinquapin [111] and those growing in Washington [97] have been classified as shade intolerant.
Although giant chinquapin can persist in some shaded environments, on at least some sites it has better growth [97], greater abundance [25], and increased sexual reproduction [95] in open conditions. The shrub form has been characterized as more shade-tolerant than the tree form [116,124], and the tree form may be more common in open than in shaded conditions [25]. On sites on the Willamette National Forest and near the Umpqua National Forest, there were "many more" giant chinquapin trees in open clearcuts than in the understory of Douglas fir-western hemlock old-growth, where light intensities ranged from 2.3% to 47.3% of available light [25]. In Washington, the "best" giant chinquapin trees occurred on open sites. Giant chinquapin on an open promontory had sturdy boles and leafy crowns, while giant chinquapin saplings under a Douglas-fir canopy were described as spindly [97]. According to McDonald [109], scrub golden chinquapin growth is typically stiff and upright on exposed sites and low and spreading in shaded areas. Prolonged competition with conifers, including shading in the subcanopy, may lead to mortality of giant chinquapin [107,124]. Sexual reproduction of giant chinquapin was negatively associated (P=0.05) with understory density above 52% cover. High shrub and herb cover may inhibit germination and/or seedling establishment of giant chinquapin [95]. It may be limited to open areas on moist sites [116].
Immediate fire effect on plant:
Giant chinquapins are often top-killed [94,152,162] or killed [94] by fire. In the Sierra Nevada, survival of giant chinquapin following low- and moderate-severity fires in spring and fall ranged from 27% to 78%
(Table 4)
[94]. In a mixed-conifer/snowbrush ceanothus/long-stolon sedge community in central Oregon, all 4 giant chinquapins present sprouted following a moderate-severity, fall fire. Two of these individuals died after a 2nd fall fire within 3 years [108]. Dale and others [43] grouped giant chinquapin as a species in which some individuals survive fire.
Postfire regeneration strategy [159]:
Tree with adventitious buds,
a sprouting root crown and/or root sprouts
Tall shrub, adventitious buds and/or a sprouting root crown
Secondary colonizer (on- or off-site seed sources)
Fire adaptations and plant response to fire:
 |
|
| Scrub golden chinquapin sprouting about 19 months after a June wildfire in manzanita chaparral transitioning to mixed-evergreen and redwood forest in the Bonny Dune Ecological Reserve. Photo © Neal Kramer. |
Limited evidence suggests that seedling establishment of giant chinquapin is not common in early postfire environments. Giant chinquapin seeds in the Klamath Mountains [152] and northern coastal regions [162] of California are described as having "no response" to fire, and seedling establishment was not observed following a prescribed fire in a mixed-conifer forest in the Sierra Nevada [94].
Plant response to fire: Reported responses of giant chinquapin to fire range from nearly 25% mortality following a low-severity prescribed fire in spring [94] to high abundance following severe fires [45,86,181]. Greater survival and faster recovery of giant chinquapin may be more likely in large individuals [72,94], following early spring fires, low-severity fires [94], and/or fires occurring after a fire-free interval long enough to allow sprouts to grow to a fire-resistant size [45,108]. Other explanations for variation in fire responses include variation in prefire plant community composition, treatment history, surface-level fire severity, time frame of investigation, and different sensitivities of shrub and tree growth forms. Data from prescribed fire research in a mixed-conifer forest in the Sierra Nevada are used repeatedly in this review [93,94]. See the Research Project Summary of Kauffman's [93,94] study for more details.
Two years following prescribed fires in a mixed-conifer forest on the Blodgett Research Station, giant chinquapin survival on burned sites was significantly lower than on unburned sites, except for a low-severity, early spring fire [94]. Reductions in giant chinquapin density following prescribed fires were not significant (Table 5).
| Table 4. Top-kill and survival of giant chinquapin on an unburned site and 2 growing seasons after prescribed fire in different seasons in a mixed-conifer forest in the Sierra Nevada [94] | ||
| Treatment | Percent top-kill | Percent survival (SD) |
| Early fall, moderate severity | 100 | 27 (46)a* |
| Late fall, low severity | 94.4 | 39 (50)a |
| Early spring, low severity | 93 | 78 (42)b |
| Late spring, moderate severity | 96 | 40 (50)a |
| Unburned (control) | 0 | 100 (0)b |
| *Different letters indicate a significant difference in survival among treatments (P<0.10). | ||
| Table 5. Density (#/ha) of giant chinquapin on an unburned control and before and after burning in different seasons in a mixed-conifer forest in the Sierra Nevada (no significant differences; P>0.1) [94] | |||
| Treatment | 1983 (prefire) | 1984 | 1985 |
| Early fall, moderate severity | 33 | no data | 0 |
| Late fall, low severity | 83 | 42 | 42 |
| Early spring, low severity | 400 | 233 | 100 |
| Late spring, moderate severity | 200 | 167 | 134 |
| Unburned (control) | 167 | 134 | 100 |
Some general information suggests that fire has little impact on giant chinquapin occurrence. Frequency of giant chinquapin was "somewhat stable" 2 to 3 years after the Biscuit Wildfire on the Rogue River-Siskiyou National Forest [24]. In a ponderosa pine stand in central Oregon, giant chinquapin was present in the understory of an unburned area and an area burned 6 years previously [142].
In some areas, giant chinquapin may be more common on burned sites than unburned sites. On the Rogue River National Forest in southwestern Oregon, giant chinquapin was not present on a harvested site where slash was not burned, but it was common in portions of the same harvested area where slash was burned. Preharvest differences in the 2 treatments were not addressed [114]. On the H. J. Andrews Experimental Forest, giant chinquapin was listed as an invader of burned areas—that is, a species that occurred in clearcut and burned sites not in adjacent forests [198].
The limited data available as of 2012 suggest that giant chinquapin may recover to prefire abundance in less than 15 years after fire. In western Oregon, giant chinquapin cover reached 6.3% on plots subject to clearcutting and moderate-severity slash fires 11 to 16 years previously. Giant chinquapin cover was 5.4% on unburned, clearcut plots [156]. In central Oregon, giant chinquapin recovered prefire canopy cover and density within 5 years of early spring or fall burning (Volland unpublished data cited in [182]). Following logging and burning on 2 watersheds on the H. J. Andrews Experimental Forest, giant chinquapin cover reached predisturbance levels (≤2%) in about 5 to 8 years [67]. In a Douglas-fir forest in the western Oregon Cascade Range, giant chinquapin frequency and cover increased slowly in the 5 years after timber harvest and burning. Giant chinquapin had 11.5% frequency and 0.6% cover before harvest and was absent after slash burning; 5 years after treatments, it had 4.9% frequency and 0.3% cover [46]. In southwestern Oregon and northwestern California, height growth rate of giant chinquapin following harvest and broadcast burning was less than that of Pacific madrone and tanoak. Based on data collected on these sites, Harrington and others [72] developed models of giant chinquapin, tanoak, and Pacific madrone fire response. The giant chinquapin model predicts that the number of sprouts ranging from 0.8 to 3.9 inches (2-10 cm) DBH will increase over the 16 years following harvest and broadcast burning [72].
Plant size: Evidence from 2 studies suggests that the prefire size of giant chinquapin is positively associated with postfire response. Large giant chinquapins produced 100 to 300 sprouts 2 years following prescribed fires in a mixed-conifer forest in the Sierra Nevada, and the author noted survival increased with prefire tree size [94]. Models developed from 8 giant chinquapin clumps in southwestern Oregon and northwestern California predict that giant chinquapin sprout production will increase with the size of the parent tree. Sixteen years after harvest and broadcast burning, 6 sprout clumps of giant chinquapin are predicted when parent-tree basal area is 30 cm², and about 10 sprout clumps of giant chinquapin are predicted when parent-tree basal area is 150 cm² [72].
Site influences on plant response: Few data address the impacts of site characteristics on giant chinquapin's response to fire. Within the 660- to 3,900-foot (200-1,200 m) elevation range impacted by the 2002 Biscuit Wildfire in southern Oregon, the number of regenerating giant chinquapin stems generally increased with elevation, suggesting postfire response at higher elevations of this range was somewhat stronger than that at low elevations [66]. Site characteristics such as elevation or topography explained ≤2% of variation in sprout diameter or hardwood crown size in the 16 years following clearcutting and broadcast burning in southwestern Oregon and northwestern California [72].
Season: Short-term negative impacts of fire on giant chinquapin may be minimal following spring burning [94]. Following both early spring and fall fires in central Oregon, giant chinquapin density and cover reached prefire levels within 5 years (Volland unpublished data cited in [182]). In a mixed-conifer forest in the Sierra Nevada, giant chinquapin survival 2 growing seasons after a low-severity prescribed fire in early spring was not significantly different from that on the unburned control and was greater than that on a low-severity, fall fire (P<0.10) (see Table 4).
Severity: There is conflicting information regarding the impact of severe fire on giant chinquapin, perhaps due in part to variation in use of the term “severe”. Giant chinquapin was considered an increaser following severe fires in shrub and mixed-conifer communities in central [28,181] and southern Oregon [45,86] and the Klamath Region of northern California [152]. Two growing seasons following a 2nd severe wildfire within 15 years on a site in the Klamath-Siskiyou Mountains of southern Oregon, giant chinquapin frequency on the reburn was more than twice that in unburned old-growth vegetation. Giant chinquapin cover was slightly greater on the burned sites [45]. In a ponderosa pine stand just east of the Cascade Range crest in central Oregon, giant chinquapin was a primary shrub species before and after a summer wildfire that killed 95% of trees within 75% of the burned area [28]. Based on plots within the area burned in the Biscuit Wildfire in southern Oregon, giant chinquapin was generally associated with exposed mineral soil, a metric that may indicate high fire severity [66]. Giant chinquapin and greenleaf manzanita and/or pinemat manzanita (Arctostaphylos nevadensis) were mentioned as the first species to establish following "severe logging and burning" in mixed-conifer/snowbrush ceanothus-kinnikinnick (Arctostaphylos uva-ursi) communities in southern Oregon. Giant chinquapin may increase to the point of being problematic for forest regeneration following severe disturbance, including burning, in white fir/giant chinquapin-Oregon boxwood-prince's-pine (Paxistima myrsinites-Chimaphila umbellata) and Shasta red fir-white fir/giant chinquapin-prince's-pine/long-stolon sedge communities of southern Oregon. Giant chinquapin also exhibits large increases following "severe" opening of white fir/snowberry/ strawberry (Symphoricarpos spp.-Fragaria spp.) stands in this area [86] and in the mixed-conifer/common snowberry (Symphoricarpos albus)/forb association of central Oregon [181]. It is a component of shrublands that develop following severe fires that remove canopy cover on "poor" sites in the Klamath Mountain Region of northern California [152].
In contrast, there is evidence that increased fire severity may be detrimental to giant chinquapin, at least in the short term. Twenty-eight years after harvesting and broadcast burning on a watershed in the H. J. Andrews Experimental Forest, giant chinquapin had survived or reestablished in locations that were “lightly” burned but not in the “heavily” burned locations [68]. Scrub golden chinquapin burned severely in a chaparral fire in Sonoma County, California, did not sprout [5]. In a mixed-conifer community in the Sierra Nevada, survival of giant chinquapin 2 years following moderate-severity prescribed fire (as measured by consumption of fuels) ranged from 27% to 40% and was significantly less than survival following a low-severity spring prescribed fire (P<0.1). Survival of giant chinquapin after a moderate-severity spring prescribed fire was 40%, significantly less than the 78% survival after a low-severity spring prescribed fire (see Table 4). Low-severity prescribed fires may have reduced giant chinquapin size in the 1st postfire growing season less than moderate-severity fires (Table 6). After 2 years, giant chinquapin in the low-severity fall prescribed fire treatment had the greatest recovery, 67% of prefire height and 54% of prefire basal diameter [94]. For percent of prefire shrub size 1 year following these prescribed fires, see Table 6. Basal diameter was significantly smaller (P<0.10) after the low-severity spring burn (Table 7) [93]. For more information regarding this study, see the Research Project Summary.
| Table 6. Percent of prefire shrub size one growing season after prescribed fire treatments in a mixed-conifer forest in the northern Sierra Nevada [94] | |||||
| Treatment | Crown volume | Crown area | Basal Diameter | Height | Stems |
| Early fall, moderate severity | 0.7 | 3.1 | 11.9 | 22.7 | 206.9 |
| Late fall, low severity | 7.1 | 13.1 | 38.7 | 45.9 | 111.4 |
| Early spring, low severity | 14.2 | 29.9 | 33.7 | 45.0 | 285.6 |
| Late spring, moderate severity | 5.9 | 10.5 | 28.4 | 36.0 | 186.0 |
| Unburned (control) | 127.8a | 109.4a | 102.0a | 114.7a | 178.5 |
| Table 7. Average (SD) of several giant chinquapin characteristics before and 2 growing seasons following late spring prescribed fires [93] | |||
| Characteristic | 1983 (prefire) | 1984 | 1985 |
| Basal diameter | 6.8 (5.1)a* | 2.0 (1.1)b | 3.5 (0.9)c |
| Number of stems | 4.0 (5.0)a | 14.0 (20.0)b | 9.0 (11.0)b |
| Height | 34.7 (34.8) | 19.3 (15.4) | 16.4 (9.1) |
| Crown area | 0.24 (0.42) | 0.04 (0.07) | 0.05 (0.07) |
| *Different letters indicate significant differences between years (P<0.10). | |||
Frequency Giant chinquapin has been described as well adapted to frequent fire [116] and occurs in areas that burn regularly [8] (see Fire regimes), but it may be negatively impacted when consecutive fires occur within a few years. In the Klamath-Siskiyou Mountains of southern Oregon, an area burned in severe wildfires in both 1987 and 2002. Frequency of giant chinquapin on reburned sites was more than twice that on sites that burned in only one of these fires, while cover was similar [45]. In contrast, 2 fall prescribed fires within 3 years in a mixed-conifer/snowbrush ceanothus/long-stolon sedge community in central Oregon resulted in the death of 2 of 4 giant chinquapins. The 2 sprouting giant chinquapins went from dominating a 16- to 26-foot (5-8 m) diameter area to individuals with 2 or 3 sprouts per plant each occupying a 10-inch (25 cm) diameter area [108]. For more information regarding this study including detailed information on fuel characteristics and consumption, see the Research Paper by Martin.
Because giant chinquapin is shade tolerant, fire exclusion may favor it in some plant communities. Greater frequency of giant chinquapin in the younger age classes compared to the oldest age class (established before 1950), implies that it responded positively to cessation of annual burning in coniferous forests in the Willamette Valley. Giant chinquapin was reproducing in this community in the absence of fire [34].
FUELS AND FIRE REGIMES: Fuels: Weatherspoon and Skinner [185] categorized giant chinquapin as having "intermediate flammability" compared to other hardwoods. It was ranked as having lower flammability than California black oak and greater flammability than canyon live oak (Quercus chrysolepis) or interior live oak (Q. wislizeni) [185]. Based on fuel load and fuel drying rating variables, plant communities of southwestern Oregon where giant chinquapin was most common had high estimated likelihood of fire occurrence compared to other communities in the area [8].Giant chinquapin’s contribution to the fuel load varies by site. Out of 17 species in 6 communities, giant chinquapin had the greatest biomass of any large shrub, reaching 10,838 kg/ha in the western hemlock/giant chinquapin community [143]. In old-growth Douglas-fir communities on a watershed on the H. J. Andrews Experimental Forest, giant chinquapin comprised 1.6% of the 1,168,890 kg/ha total tree biomass on a south ridge and 2.5% of the 798,100 kg/ha total tree biomass on a north slope. In 2 other old-growth Douglas-fir communities in this watershed, giant chinquapin comprised 0.3% to 0.4% of the total tree biomass. On an old-growth Douglas-fir site, giant chinquapin had the 2nd highest contribution to annual biomass accumulation (14.6%) and the 3rd highest on a south ridge (9.5%). Table 8 provides information on the organic matter distribution in these communities [61]. Biomass [168] and crown area [96,176] equation parameters for giant chinquapin are available.
| Table 8. Organic matter distribution (kg) in Douglas-fir-giant chinquapin communities of a watershed on the H. J. Andrews Experimental Forest [61] | |||
| Organic matter component | North slope | South ridge | Watershed average |
| Total aboveground | 671,570 | 982,510 | 717,900 |
| Roots, coarse (>5 mm) | 132,900 | 193,040 | 141,270 |
Roots, fine (<5 mm) |
10,900 | 11,000 | 11,270 |
| Forest floor (ash free dry weight) | 57,170 | 49,270 | 51,160 |
| Standing dead | 34,500 | 3,800 | 24,600 |
| Fallen logs | 124,800 | 55,200 | 190,000 |
| Organic matter at 0-100 cm soil depths | 101,000 | 90,000 | 112,960 |
| Ecosystem total | 1,132,840 | 1,384,820 | 1,249,160 |
Evergreen hardwood communities of southwestern Oregon were given a fairly low woody debris rating (2 of 5) [65].
Average annual leaf litter decay rate of giant chinquapin collected from 5 western Oregon sites was 0.54. This was an intermediate rate, faster than that of most species investigated but substantially slower than those of vine maple (Acer circinatum) and Pacific dogwood (Cornus nuttallii), the 2 fastest decaying species [177].
Fire regimes: Fires in communities with giant chinquapin typically occur in summer or early fall [162,167]. Fires are commonly lightning-caused. Typical fire regimes range from frequent low-severity fires to infrequent moderate- or high-severity fires. Fire-return intervals have lengthened in several portions of giant chinquapin's range compared to historical intervals. The impacts of this change on giant chinquapin have not been addressed. See the Fire Regime Table for further information on fire regimes of vegetation communities in which giant chinquapin may occur. For information on fire occurrence and spatial extent in western Oregon from 1200 through 2000, see Berkley [19]. Volland and Dell [182] provide brief general summaries of fire regimes in the Pacific Northwest by region. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
Lightning was and is a common source of ignition in some parts of the giant chinquapin's range. Ignitions by American Indians were common before European settlement [8]. Most of the area burned in the Klamath Mountains in recent decades has been the result of lightning-caused fires (e.g., 1977, 1987, 1999, and 2002) [152]. In contrast, lightning-caused fires are rare in northern coastal California. In a roughly 148,000-acre (60,000 ha) area, only 22 lightning-caused fires were reported from 1960 to 1978 (Sibley unpublished cited in [179]). Lightning is more frequent in southwestern Oregon mixed-conifer-hardwood forests [32] and the Siskiyou Mountains [1] than elsewhere in Oregon or in Washington. There is an average of 12.8 lightning strikes/year/km² in the Klamath Mountains [152] and 1.2 lightning strikes/year/km² in the north coastal bioregion of California [162]. However, the number lightning strikes is not necessarily related to the number of lightning-caused fires, since storms that create the most lightning strikes are typically too wet to start fires [152]. Lightning strikes in Oregon and western Washington are most common in July and August [1].
Fire-return intervals in areas occupied by giant chinquapin vary widely from about 20 years in mixed-evergreen forests [32,167], Douglas-fir-tanoak [162], and Douglas-fir communities [166] of southwestern Oregon to around 150 years in coastal forests of southwestern Oregon [1] and forest dominated or codominated by western hemlock the western Oregon Cascade Range [117]. Giant chinquapin is a component of redwood forests (see Plant communities), where fire-return intervals vary from around 50 years in warm, dry locations up to 500 years in cool, moist, coastal areas [162]. In the west-central Cascade Range in Oregon, mean fire-return interval of the dry, low-elevation sites where giant chinquapin was most likely to occur ranged from 81 to 97 years [186]. Fire frequency ranges from 90 to 150 years in coastal forests of southwestern Oregon and declines to about 50 years along the crest of the Coast Ranges [1]. In the Siskiyou Mountains, fire-return intervals were highly variable, with an average of 20 years [8]. Moisture availability [1,32,41,162] and aspect [166,167,187] probably influence the variability of fire-return intervals.
Giant chinquapin is typically exposed to fires of low to moderate severity. Within the perimeter of the Biscuit Wildfire, most giant chinquapin areas burned at "very low" or "low" severity. Only 4% of individual giant chinquapins and 11% of giant chinquapin community types burned at moderate to high severity [12]. Historically, mixed-conifer-pine (Pinus spp.) communities mostly experienced frequent, low-severity fires, and mixed-conifer-fir (Abies spp.) communities were mostly subject to moderate-severity fire. In both cases, increases in fuels from long periods without fire increase severity. Fire behavior in montane chaparral is influenced by species composition, ratio of dead to live vegetation, and live fuel moistures. When live fuel moistures are high in this community, fire severity is typically low and fires are patchy [163]. In mixed-conifer-hardwood vegetation, fire severity generally ranges from low on dry, hot sites to moderate on cool, moist sites [32]; this trend is presumably due to more frequent fires on dry, hot sites. Fires in the Douglas-fir-tanoak zone are often low- to moderate-severity surface fires, and fires in redwood forests are generally low-severity surface fires, although intensities may be moderate (345-1,730 kW/m) [162]. The Klamath Mountains experience a regime of frequent, low- to moderate-severity fires [152]. Patterns of past fire severity in Douglas-fir forests in the Klamath Mountains in California suggest that upper slopes, ridgetops, and south- and west-facing aspects experienced more severe fires than other topographic positions from 1850 to 1950, a period characterized by infrequent fire and fuel accumulation [166].
In several areas within giant chinquapin's range, including the coastal mountains of northwestern California [161], the western Oregon Cascade Range [187], and the Klamath Mountains [152,166,167], fire-return intervals have increased due to fire exclusion. Possible consequences of reduced fire frequency include altering species composition—for example, Douglas-fir and ponderosa pine stands becoming dominated by grand fir (Abies grandis) [41]—and increased fire severity [8,163]. However, in mixed-evergreen forests of the Klamath-Siskiyou region, severe fire was associated with recently burned areas [126]. Potential explanations for this result include increased presence of shade-intolerant, early-successional species that have greater flammability [126,127] and increased temperatures in open areas [126].
FIRE MANAGEMENT CONSIDERATIONS:Reducing fuel loads may result in lower fire severity in communities where giant chinquapin occurs. In a mixed-evergreen community in the Coast Ranges of southwestern Oregon, total tree mortality was highest (80%-100%) in areas that had been thinned 6 years before the fire, intermediate in unthinned controls (53%-54%), and lowest (5%) in areas that were thinned 6 years before and underburned 1 year before the Biscuit Wildfire. Fine fuels (≤3 inch diameter (7.6 cm)) were more abundant on thinned-only sites (27.6 Mg/ha) than on thinned-underburned (5.1 Mg/ha) sites, suggesting that heavy fine fuels led to higher tree mortality on thinned-only sites [135]. Guidelines that were developed in the mid-1970s for managing fuels in the Pacific Northwest include broadcast burning [131]. Amaranthus and McNabb [4] recommend incorporating duff layer protection into prescriptions to minimize bare soil exposure.
Bray [26] reviewed impacts of forest burning on air quality in Washington and Oregon.Palatability and nutritional value: Birds, small mammals [113,116,124], and insects [113] eat giant chinquapin nuts. Squirrels may cache them [116]. McDonald and others [113] note that giant chinquapin nuts are probably nutritious.
Giant chinquapin is not a palatable browse species for domestic sheep [60,170]. Evergreen hardwood communities of southwestern Oregon, which are frequently occupied by giant chinquapin, were ranked low (2 of 5) in browse production [65].
Cover value: Giant chinquapin provides important cover for birds and small- to medium-sized mammals. Pine siskins, red-breasted nuthatches, and flycatchers (Empidonax spp.) were positively associated with giant chinquapin in the Oregon Cascade Range (P<0.05) [53]. On the Winema National Forest, northern goshawks, pileated woodpeckers, and spotted owls used a Shasta red fir-white fir/giant chinquapin-prince's-pine/yellow sedge community for feeding and nesting [86]. Rarely, spotted owls in California nested in giant chinquapin [101]. In an 8-acre (3 ha) giant chinquapin area in the western Cascade Range of Oregon, nesting birds used 55% of snags. They likely selected snags based on availability [188]. Small mammals, such as red tree voles and Pacific shrews, were associated with giant chinquapin in the Oregon Cascade Range [52]. In coastal northwestern California, a fisher used giant chinquapin for its natal den [171], and 13% of American marten "rest structures" were in giant chinquapin trees or snags, with 3 being used as maternal dens by a single female [154]. Giant chinquapin was typical in [124] and often dominated the dense understories used by American martens [153] and many other animals [124].
VALUE FOR REHABILITATION OF DISTURBED SITES:Giant chinquapin often germinates and grows fairly well in containers, but it rarely survives outplanting. Germination in peat is recommended by Roof [138] and Mirov and Kraebel [121]. In several decades of work, Roof [138] did not observe a single outplanted giant chinquapin that lived more than a few years, and most lived less than a few months. Witt [197] implies transplanting is difficult, but possible, and provides outplanting recommendations [197].
OTHER USES:Nuts: The nuts of giant chinquapin are edible [7,199], tasty [3,138,197], and were eaten by American Indians, most notably in northern portions of California [33].
OTHER MANAGEMENT CONSIDERATIONS:Timber harvesting: Recovery of giant chinquapin following clearcutting or thinning may be fast or rather slow. Giant chinquapin sprouts grow quickly on some clearcuts [110] and may slow the establishment of conifers (see Control). It was 1 of 3 woody perennials that dominated the ground cover 3 to 28 years after clearcuts and partial cuts in the southern Oregon Cascade Range [158]. In Douglas-fir forests of western Oregon, mortality of giant chinquapin with DBH of ≥2 inches (5 cm) was lower 5 years after heavy thinning than 5 years after lightly thinning or no treatment (P<0.1) [17,18]. In contrast, on a site in the western Cascade Range in Oregon, giant chinquapin increased slowly in the 5 years following harvest and broadcast burning, from 0% frequency and cover to 4.9% frequency and 0.3% cover. Before harvesting, frequency of giant chinquapin was 11.5% and cover was 0.6% [46]. In the Douglas-fir region on northwestern California, giant chinquapin occurred at densities of 4 and 15 individuals/acre on 2 sites in undisturbed forest, but it was not present in areas harvested in the previous 5 years [63].
Little information on harvesting of giant chinquapin was available as of 2012. McDonald and others [113] state that it is not known whether clearcutting promotes giant chinquapin seedling establishment, and Hamilton [69] notes that giant chinquapin's rank as intermediate in tolerance to single tree selection was highly uncertain. It has been estimated that there are 151 million board feet of giant chinquapin in west-central and southwestern Oregon [128].
Control: Since hardwoods, including giant chinquapin, may slow conifer regeneration [149,160], giant chinquapin has been the target of control treatments, including bulldozing [149], ground scarification with a tractor [116], and herbicides [36,42,58,118,123]. Early in succession, giant chinquapin sprouts may interfere with conifer growth [109,116], particularly that of Douglas-fir [118,160,165]. The low light intensities under giant chinquapin are likely to reduce conifer seedling growth [120]. Douglas-fir size was negatively associated with hardwood density 10 years following harvesting in plantations in southwestern Oregon (Harrington 1989 cited in [165]). Bulldozing at least 6 inches (15 cm) of soil to remove giant chinquapin's small sprouting roots [149] or scarification by tractor [116] have been suggested as effective control techniques.
Giant chinquapin is resistant to herbicides [76,116,149,160]. Multiple applications may increase effectiveness; 3 treatments in 5 years resulted in 60% mortality in southeastern Oregon [57]. In contrast, spraying followed by burning followed by 2 more applications of herbicide in a 4-year period resulted in increases in scrub golden chinquapin in a brushfield in the Siskiyou Mountains [55]. Several articles address the effectiveness of various herbicides and application methods in controlling giant chinquapin, including Conard and Emmingham [36], Dahms [42], and Newton and Roberts [123].
Diseases and insect pests: Generally, diseases and insects have little impact on giant chinquapin [7,116,124]. Leaf fungi do little damage and root rots are rare [116]. Giant chinquapin is most susceptible to heart-rotting fungi, such as Phellinus igniarius. The filbertworm (Melissopus latiferreanus) may impact reproduction [116,124] (see Seed production). See these sources for further information: [25,31,38,39,105,116,124,144,150,195].| Fire regime information on vegetation communities in which giant chinquapin may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [103], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||||
|
|||||||
| Pacific Northwest | |||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||
| Pacific Northwest Woodland | |||||||
| Pine savannah (ultramafic) | Replacement | 7% | 200 | 100 | 300 | ||
| Surface or low | 93% | 15 | 10 | 20 | |||
| Ponderosa pine | Replacement | 5% | 200 | ||||
| Mixed | 17% | 60 | |||||
| Surface or low | 78% | 13 | |||||
| Pacific Northwest Forested | |||||||
| Douglas-fir (Willamette Valley foothills) | Replacement | 18% | 150 | 100 | 400 | ||
| Mixed | 29% | 90 | 40 | 150 | |||
| Surface or low | 53% | 50 | 20 | 80 | |||
| Oregon coastal tanoak | Replacement | 10% | 250 | ||||
| Mixed | 90% | 28 | 15 | 40 | |||
| Dry ponderosa pine (mesic) | Replacement | 5% | 125 | ||||
| Mixed | 13% | 50 | |||||
| Surface or low | 82% | 8 | |||||
| Douglas-fir-western hemlock (dry mesic) | Replacement | 25% | 300 | 250 | 500 | ||
| Mixed | 75% | 100 | 50 | 150 | |||
| Douglas-fir-western hemlock (wet mesic) | Replacement | 71% | 400 | ||||
| Mixed | 29% | >1,000 | |||||
| Mixed conifer (southwestern Oregon) | Replacement | 4% | 400 | ||||
| Mixed | 29% | 50 | |||||
| Surface or low | 67% | 22 | |||||
| California mixed evergreen (northern California and southern Oregon) | Replacement | 6% | 150 | 100 | 200 | ||
| Mixed | 29% | 33 | 15 | 50 | |||
| Surface or low | 64% | 15 | 5 | 30 | |||
| Mountain hemlock | Replacement | 93% | 750 | 500 | >1,000 | ||
| Mixed | 7% | >1,000 | |||||
| Mixed conifer (eastside dry) | Replacement | 14% | 115 | 70 | 200 | ||
| Mixed | 21% | 75 | 70 | 175 | |||
| Surface or low | 64% | 25 | 20 | 25 | |||
| Mixed conifer (eastside mesic) | Replacement | 35% | 200 | ||||
| Mixed | 47% | 150 | |||||
| Surface or low | 18% | 400 | |||||
| Red fir | Replacement | 20% | 400 | 150 | 400 | ||
| Mixed | 80% | 100 | 80 | 130 | |||
| California | |||||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||||
| California Shrubland | |||||||
| Chaparral | Replacement | 100% | 50 | 30 | 125 | ||
| Montane chaparral | Replacement | 34% | 95 | ||||
| Mixed | 66% | 50 | |||||
| California Woodland | |||||||
| Ponderosa pine | Replacement | 5% | 200 | ||||
| Mixed | 17% | 60 | |||||
| Surface or low | 78% | 13 | |||||
| California Forested | |||||||
| California mixed evergreen | Replacement | 10% | 140 | 65 | 700 | ||
| Mixed | 58% | 25 | 10 | 33 | |||
| Surface or low | 32% | 45 | 7 | ||||
| Coast redwood | Replacement | 2% | ≥1,000 | ||||
| Surface or low | 98% | 20 | |||||
| Mixed conifer (north slopes) | Replacement | 5% | 250 | ||||
| Mixed | 7% | 200 | |||||
| Surface or low | 88% | 15 | 10 | 40 | |||
| Mixed conifer (south slopes) | Replacement | 4% | 200 | ||||
| Mixed | 16% | 50 | |||||
| Surface or low | 80% | 10 | |||||
| Jeffrey pine | Replacement | 9% | 250 | ||||
| Mixed | 17% | 130 | |||||
| Surface or low | 74% | 30 | |||||
| Interior white fir (northeastern California) | Replacement | 47% | 145 | ||||
| Mixed | 32% | 210 | |||||
| Surface or low | 21% | 325 | |||||
| Red fir-white fir | Replacement | 13% | 200 | 125 | 500 | ||
| Mixed | 36% | 70 | |||||
| Surface or low | 51% | 50 | 15 | 50 | |||
| Red fir-western white pine | Replacement | 16% | 250 | ||||
| Mixed | 65% | 60 | 25 | 80 | |||
| Surface or low | 19% | 200 | |||||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [71,102]. |
|||||||
1. Agee, James K. 1991. Fire history of Douglas-fir forests in the Pacific Northwest. In: Ruggiero, Leonard F.; Aubry, Keith B.; Carey, Andrew B.; Huff, Mark H., tech. coords. Wildlife and vegetation of unmanaged Douglas-fir forests. Gen. Tech. Rep. PNW-GTR-285. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 25-33. [17303]
2. Alden, Harry A. 1995. Hardwoods of North America. Gen. Tech. Rep. FPL-GTR-83. Madison, WI: U.S. Department of Agriculture, Forest Service, Forest Products Laboratory. 136 p. Available online: http://www.fpl.fs.usda.gov/documnts/fplgtr/fplgtr83.pdf [2004, January 6]. [46270]
3. Alderman, DeForest C. 1974. Native edible fruits, nuts, vegetables, herbs, spices, and grasses of California: I. Fruit trees and nuts. Leaflet 75-LE/ 2226. Berkeley, CA: University of California, Division of Agricultural Sciences, Cooperative Extension. 14 p. [67651]
4. Amaranthus, M.; McNabb, D. H. 1983. Bare soil exposure following logging and prescribed burning in southwest Oregon. In: New forests for a changing world: Proceedings of the 1983 convention of the Society of American Foresters; 1983 October 16-20; Portland, OR. Bethedsa, MD: Society of American Foresters: 234-237. [12362]
5. Ammirati, Joseph Frank, Jr. 1967. The occurrence of annual and perennial plants on chaparral burns. San Francisco, CA: San Francisco State College. 140 p. Thesis. [29202]
6. Applegate, Elmer I. 1939. Plants of Crater Lake National Park. The American Midland Naturalist. 22(2): 225-314. [75979]
7. Arno, Stephen F.; Hammerly, Ramona P. 1977. Northwest trees. Seattle, WA: The Mountaineers. 222 p. [4208]
8. Atzet, Thomas. 1979. Description and classification of the forests of the upper Illinois River drainage of southwestern Oregon. Corvallis, OR: Oregon State University. 211 p. Dissertation. [6452]
9. Atzet, Thomas; White, Diane E.; McCrimmon, Lisa A.; Martinez, Patricia A.; Fong, Paula Reid; Randall, Vince D., tech. coords. 1996. Field guide to the forested plant associations of southwestern Oregon. Tech. Pap. R6-NR-ECOL-TP-17-96. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. Available online: http://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5319837.pdf [2012, March 16]]. [49881]
10. Atzet, Tom; Wheeler, David; Riegel, Gregg; Smith, Brad; Franklin, Jerry. 1984. The mountain hemlock and Shasta red fir series of the Siskiyou Region of southwest Oregon. Forestry Intensified Research Report. Corvallis, OR: Oregon State University. 6(1): 4-7. [9486]
11. Atzet, Tom; Wheeler, David; Smith, Brad; Riegel, Gregg; Franklin, Jerry. 1985. The tanoak series of the Siskiyou region of southwest Oregon. Forestry Intensified Research. Corvallis, OR: Oregon State University. 6(4): 6-10. [8594]
12. Azuma, David L.; Donnegan, Joseph; Gedney, Donald. 2004. Southwest Oregon Biscuit Fire: an analysis of forest resources and fire severity. Res. Pap. PNW-RP-560. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 32 p. [50121]
13. Bailey, Arthur Wesley. 1966. Forest associations and secondary succession in the southern Oregon Coast Range. Corvallis, OR: Oregon State University. 166 p. Thesis. [5786]
14. Baker, Frederick S. 1949. A revised tolerance table. Journal of Forestry. 47(3): 179-181. [20405]
15. Baskin, Carol C.; Baskin, Jerry M. 2001. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press. 666 p. [60775]
16. Battles, John J.; Shlisky, Ayn J.; Barrett, Reginald H.; Heald, Robert C.; Allen-Diaz, Barbara H. 2001. The effects of forest management on plant species diversity in a Sierran conifer forest. Forest Ecology and Management. 146(1/3): 211-222. [45769]
17. Beggs, Liane R. 2005. Vegetation response following thinning in young Douglas-fir forests of western Oregon: can thinning accelerate development of late-successional structure and composition? Corvallis, OR: Oregon State University. 95 p. Thesis. [63617]
18. Beggs, Liane R.; Puettmann, Klaus J.; Tucker, Gabriel F. 2005. Vegetation response to alternative thinning treatments in young Douglas-fir stands. In: Peterson, C. E.; Maguire, D. A., eds. Balancing ecosystem values: innovative experiments for sustainable forestry; 2004 August 15-20; Portland, OR. Gen. Tech. Rep. PNW-GTR-635. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 243-248. [54801]
19. Berkley, Evelyn L. 2000. Temporal and spatial variability of fire occurrence in western Oregon, A.D. 1200 to present. Eugene, OR: University of Oregon. 110 p. Thesis. [41941]
20. Bingham, Bruce B.; Sawyer, John O., Jr. 1991. Distinctive features and definitions of young, mature, and old-growth Douglas-fir/hardwood forests. In: Ruggiero, Leonard F.; Aubry, Keith B.; Carey, Andrew B.; Huff, Mark H., technical coordinators. Wildlife and vegetation of unmanaged Douglas-fir forests. Gen. Tech. Rep. PNW-GTR-285. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 363-377. [17328]
21. Bolsinger, Charles L. 1988. The hardwoods of California's timberlands, woodlands, and savannas. Resour. Bull. PNW-RB-148. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 148 p. [5291]
22. Bonner, Franklin T. 2008. Castenea P. Mill.: chestnut. In: Bonner, Franklin T.; Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 338-341. [79075]
23. Bonner, Franklin T. 2008. Quercus L.: oak. In: Bonner, Franklin T.; Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 928-938. [62581]
24. Bormann, Bernard T.; Darbyshire, Robyn. 2005. Ecosystem effects of the Biscuit Fire. In: Taylor, Lagene; Zelnik, Jessica; Cadwallader, Sara; Hughes, Brian, comps. Mixed severity fire regimes: ecology and management: symposium proceedings; 2004 November 17-19; Spokane, WA. Pullman, WA: Washington State University Extension: 79-88. [61399]
25. Bowden, Richard I., Jr. 2003. The influence of light intensity on the phytochemistry of Chrysolepis chrysophylla (Fagaceae) and the relationship to the herbivory of Habrodais grunus herri (Lycaenidae). Corvallis, OR: Oregon State University. 61 p. Thesis. [83668]
26. Bray, David C. 1978. Impact of forestry burning upon air quality: A state-of-the-knowledge characterization in Washington and Oregon. EPA 910/9-78-052. Seattle, WA: U.S. Environmental Protection Agency, Region 10. 253 p. [17153]
27. Brockman, C. Frank. 1958. Golden chinkapin, (Castanopsis chrysophylla [Hook.] DC.). University of Washington Arboretum Bulletin. 21: 46-47, 70. [12176]
28. Cahall, Rebecca E.; Hayes, John P. 2009. Influences of postfire salvage logging on forest birds in the Eastern Cascades, Oregon, USA. Forest Ecology and Management. 257(3): 1119-1128. [74062]
29. Cairns, Michael A.; Lajtha, Kate; Beedlow, Peter A. 2009. Dissolved carbon and nitrogen losses from forests of the Oregon Cascades over a successional gradient. Plant Soil. 318(1-2): 185-196. [74647]
30. Carey, Andrew B.; Kershner, Janet; Biswell, Brian; Dominguez de Toledo, Laura. 1999. Ecological scale and forest development: squirrels, dietary fungi, and vascular plants in managed and unmanaged forests. Wildlife Monographs No. 142. Washington, DC: The Wildlife Society. 71 p. [30476]
31. Carmean, David; Miller, Jeffrey C. Scaccia, Brian. 1989. Overwintering of Phryganidia californica in the Oregon Cascades and notes on its parasitoids (Lepidoptera: Dioptidae). Pan-Pacific Entomologist. 65(1): 74-76. [83669]
32. Chappell, Christopher B.; Kagan, Jimmy. 2001. 3. Southwest Oregon mixed conifer-hardwood forest. In: Chappell, Christopher B.; Crawford, Rex C.; Barrett, Charley; Kagan, Jimmy; Johnson, David H.; O'Mealy, Mikell; Green, Greg A.; Ferguson, Howard L.; Edge, W. Daniel; Greda, Eva L.; O'Neil, Thomas A. Wildlife habitats: descriptions, status, trends, and system dynamics. In: Johnson, David H.; O'Neil, Thomas A., managing directors. Wildlife-habitat relationships in Oregon and Washington. Corvallis, OR: Oregon State University Press: 28-30. [68066]
33. Chesnut, V. K. 1902. Plants used by the Indians of Mendocino County, California. Contributions from the U.S. National Herbarium. [Washington, DC]: U.S. Department of Agriculture, Division of Botany. 7(3): 295-408. [54917]
34. Cole, David. 1977. Ecosystem dynamics in the coniferous forest of the Willamette Valley, Oregon, U.S.A. Journal of Biogeography. 4(2): 181-192. [10195]
35. Colwell, Wilmer L., Jr. 1980. Knobcone pine. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 124-125. [50059]
36. Conard, Susan G.; Emmingham, W. H. 1984. Herbicides for forest brush control in southwestern Oregon. Corvallis, OR: Oregon State University, College of Forestry. 7 p. [10817]
37. Cooke, Wm. Bridge. 1940. Flora of Mount Shasta. The American Midland Naturalist. 23(3): 497-572. [64906]
38. Cooke, Wm. Bridge. 1944. Notes on the ecology of the fungi of Mount Shasta. The American Midland Naturalist. 31(1): 237-249. [84126]
39. Cooke, Wm. Bridge. 1979. The 1975 Oregon foray and workshop. Mycologia. 71(5): 1078-1081. [84124]
40. Cooper, William Skinner. 1922. The broad-sclerophyll vegetation of California: An ecological study of the chaparral and its related communities. Publ. No. 319. Washington, DC: The Carnegie Institution of Washington. 145 p. [6716]
41. Crawford, Rex C. 2001. 5. Eastside mixed conifer forest. In: Chappell, Christopher B.; Crawford, Rex C.; Barrett, Charley; Kagan, Jimmy; Johnson, David H.; O'Mealy, Mikell; Green, Greg A.; Ferguson, Howard L.; Edge, W. Daniel; Greda, Eva L.; O'Neil, Thomas A. Wildlife habitats: descriptions, status, trends, and system dynamics. In: Johnson, David H.; O'Neil, Thomas A., managing directors. Wildlife-habitat relationships in Oregon and Washington. Corvallis, OR: Oregon State University Press: 32-33. [68068]
42. Dahms, Walter B. 1961. Chemical control of brush in ponderosa pine forests of central Oregon. Res. Pap. 39. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 17 p. [66103]
43. Dale, Virginia H.; Hemstrom, Miles A.; Franklin, Jerry F. 1984. The effect of disturbance frequency on forest succession in the Pacific Northwest. In: New forests for a changing world: Proceedings of the 1983 convention of the Society of American Foresters; 1983 October 16-20; Portland, OR. Bethesda, MD: Society of American Foresters: 300-304. [4781]
44. Dixon, Rita Dianne. 1995. Ecology of white-headed woodpeckers in the central Oregon Cascades. Moscow, ID: University of Idaho. 148 p. Thesis. [61425]
45. Donato, Daniel C.; Fontaine, Joseph B.; Robinson, W. Douglas; Kauffman, J. Boone; Law, Beverly E. 2009. Vegetation response to a short interval between high-severity wildfires in a mixed-evergreen forest. Journal of Ecology. 97(1): 142-154. [73207]
46. Dyrness, C. T. 1973. Early stages of plant succession following logging and burning in the western Cascades of Oregon. Ecology. 54(1): 57-69. [7345]
47. Dyrness, C. T.; Franklin, J. F.; Moir, W. H. 1974. A preliminary classification of forest communities in the central portion of the western Cascades in Oregon. Bulletin No. 4. Seattle, WA: University of Washington, Ecosystem Analysis Studies, Coniferous Forest Biome. 123 p. [8480]
48. Flora of North America Editorial Committee, eds. 2012. Flora of North America north of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
49. Franklin, Jerry F. 1979. Vegetation of the Douglas-fir region. In: Heilman, Paul E.; Anderson, Harry W.; Baumgartner, David M., eds. Forest soils of the Douglas-fir region. Pullman, WA: Washington State University, Cooperative Extension Service: 93-112. [8207]
50. Franklin, Jerry F. 1988. Pacific Northwest forests. In: Barbour, Michael G.; Billings, William Dwight, eds. North American terrestrial vegetation. New York: Cambridge University Press: 103-130. [13879]
51. Franklin, Jerry F.; Dyrness, C. T. 1973. Natural vegetation of Oregon and Washington. Gen. Tech. Rep. PNW-8. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 417 p. [961]
52. Gilbert, Frederick F.; Allwine, Rochelle. 1991. Small mammal communities in the Oregon Cascade Range. In: Ruggiero, Leonard F.; Aubry, Keith B.; Carey, Andrew B.; Huff, Mark H., technical coordinators. Wildlife and vegetation of unmanaged Douglas-fir forests. Gen. Tech. Rep. PNW-GTR-285. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 257-267. [17317]
53. Gilbert, Frederick F.; Allwine, Rochelle. 1991. Spring bird communities in the Oregon Cascade Range. In: Ruggiero, Leonard F.; Aubry, Keith B.; Carey, Andrew B.; Huff, Mark H., technical coordinators. Wildlife and vegetation of unmanaged Douglas-fir forests. Gen. Tech. Rep. PNW-GTR-285. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 145-158. [17311]
54. Govaerts, Rafael; Frodin, David G. 1998. World checklist and bibliography of Fagales (Betulaceae, Corylaceae, Fagaceae and Ticodendraceae). Kew, UK: The Royal Botanic Gardens. 497 p. [60947]
55. Gratkowski, H. J.; Philbrick, J. R. 1965. Repeated aerial spraying and burning to control sclerophyllous brush. Journal of Forestry. 63(12): 919-923. [8797]
56. Gratkowski, H. 1974. Brushfield reclamation and type conversion. In: Cramer, Owen P., ed. Environmental effects of forest residues management in the Pacific Northwest: A state-of-knowledge compendium. Gen. Tech. Rep. PNW-24. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: I-1 to I-31. [6418]
57. Gratkowski, H. 1975. Silvicultural use of herbicides in Pacific Northwest forests. Gen. Tech. Rep. PNW-37. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 44 p. [10998]
58. Gratkowski, H. 1978. Herbicides for shrub and weed control in western Oregon. Gen. Tech. Rep. PNW-77. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 48 p. [6539]
59. Gratkowski, Henry John. 1962. Heat as a factor in germination of seeds of Ceanothus velutinus var. laevigatus T. & G. Corvallis, OR: Oregon State University. 122 p. Dissertation. [34941]
60. Greiman, Harley L. 1988. Sheep grazing in conifer plantations. Rangelands. 10(3): 99-101. [5411]
61. Grier, Charles C.; Logan, Robert S. 1977. Old-growth Pseudotsuga menziesii communities of a western Oregon watershed: biomass distribution and production budgets. Ecological Monographs. 47(4): 373-400. [8762]
62. Griffin, James R.; Critchfield, William B. 1972. The distribution of forest trees in California. Res. Pap. PSW-82. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 118 p. [1041]
63. Hagar, Donald C. 1960. The interrelationships of logging, birds, and timber regeneration in the Douglas-fir region of northwestern California. Ecology. 41(1): 116-125. [34500]
64. Hall, Frederick C. 1984. Ecoclass coding system for Pacific Northwest plant associations. R6-Ecol-173. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 83 p. [80572]
65. Hall, Frederick C.; Brewer, Larry W.; Franklin, Jerry F.; Werner, Richard L. 1985. Plant communities and stand conditions. In: Brown, E. Reade, tech. ed. Management of wildlife and fish habitats in forests of western Oregon and Washington: Part 1--Chapter narratives. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region: 17-31. In cooperation with: U. S. Department of the Interior, Bureau of Land Management. [74822]
66. Halofsky, Jessica E.; Hibbs, David E. 2009. Controls on early post-fire woody plant colonization in riparian areas. Forest Ecology and Management. 258(7): 1350-1358. [77249]
67. Halpern, C. B. 1989. Early successional patterns of forest species: interactions of life history traits and disturbance. Ecology. 70(3): 704-720. [6829]
68. Halpern, Charles B.; Spies, Thomas A. 1995. Plant species diversity in natural and managed forests of the Pacific Northwest. Ecological Applications. 5(4): 913-934. [62677]
69. Hamilton, Ronald C. 1991. Single-tree selection method: An uneven-aged silviculture system. In: Genetics/silviculture workshop proceedings; 1990 August 27-31; Wenatchee, WA. Washington, DC: U.S. Department of Agriculture, Forest Service, Timber Management Staff: 46-84. [16562]
70. Hann, David W. 1997. Equations for predicting the largest crown width of stand-grown trees in western Oregon. Research Contribution 17. Corvallis, OR: Oregon State University, College of Forestry, Forest Research Laboratory. 14 p. [65649]
71. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org/niftt/released/FRCC_Guidebook_2010_final.pdf. [81749]
72. Harrington, Timothy B.; Tappeiner, John C., II; Warbinton, Ralph. 1992. Predicting crown sizes and diameter distributions of tanoak, Pacific madrone, and giant chinkapin sprout clumps. Western Journal of Applied Forestry. 7(4): 103-107. [19614]
73. Hawk, Glenn M. 1979. Vegetation mapping and community description of a small western Cascade watershed. Northwest Science. 53(3): 200-212. [8677]
74. Hayes, G. L. 1959. Forest and forest-land problems of southwestern Oregon. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 54 p. [8595]
75. Heavilin, Danny. 1977. Conifer regeneration on burned and unburned clearcuts on granitic soils of the Klamath National Forest. Res. Note PSW-321. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 3 p. [4981]
76. Helgerson, Ole T. 1990. Response of underplanted Douglas-fir to herbicide injection of sclerophyll hardwoods in southwest Oregon. Western Journal of Applied Forestry. 5(3): 86-89. [11768]
77. Hemstrom, Miles A.; Emmingham, W. H.; Halverson, Nancy M.; Logan, Shiela E.; Topik, Christopher. 1982. Plant association and management guide for the Pacific silver fir zone, Mt. Hood and Willamette National Forests. R6-Ecol 100-1982a. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 104 p. [5784]
78. Hemstrom, Miles A.; Logan, Sheila E. 1986. Plant association and management guide: Siuslaw National Forest. R6-Ecol 220-1986a. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 121 p. [10321]
79. Hemstrom, Miles A.; Logan, Sheila E.; Pavlat, Warren. 1987. Plant association and management guide: Willamette National Forest. R6-Ecol 257-B-86. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 312 p. [13402]
80. Hershey, Katherine T.; Meslow, E. Charles; Ramsey, Fred L. 1998. Characteristics of forests at spotted owl nest sites in the Pacific Northwest. The Journal of Wildlife Management. 62(4): 1398-1410. [66175]
81. Hickman, James C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p. [21992]
82. Hitchcock, C. Leo; Cronquist, Arthur. 1964. Vascular plants of the Pacific Northwest. Part 2: Salicaceae to Saxifragaceae. Seattle, WA: University of Washington Press. 597 p. [1166]
83. Hjelmqvist, H. 1960. Notes on some names and combinations within the Amentiferae. Botaniska Notiser. 113(4): 373-380. [7536]
84. Holland, Robert F. 1986. Preliminary descriptions of the terrestrial natural communities of California. Sacramento, CA: California Department of Fish and Game. 156 p. [12756]
85. Hooven, Edward F. 1973. Effects of vegetational changes on small forest mammals. In: Hermann, Richard K.; Lavender, Denis P., eds. Even-age management: Proceedings of a symposium; 1972 August 1; [Corvallis, OR]. Paper 848. Corvallis, OR: Oregon State University, School of Forestry: 75-97. [16241]
86. Hopkins, William E. 1979. Plant associations of South Chiloquin and Klamath Ranger Districts--Winema National Forest. R6-Ecol-79-005. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 96 p. [7339]
87. Hubbard, R. L. 1974. Castanopsis (D. Don) Spach chinkapin. In: Schopmeyer, C. S., technical coordinator. Seeds of woody plants in the United States. Agric. Handb. 450. Washington, DC: U.S. Department of Agriculture, Forest Service: 276-277. [7573]
88. Hunter, John C. 1997. Correspondence of environmental tolerance with leaf and branch attributes for six co-occurring species of broadleaf evergreen trees in northern California. Trees. 11(3): 169-175. [27687]
89. Hunter, John C. 1997. Fourteen years of change in two old-growth Pseudotsuga-Lithocarpus forests in northern California. Journal of the Torrey Botanical Society. 124(4): 273-279. [66479]
90. Jenkinson, James L. 1980. Jeffrey pine. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 123. [50058]
91. Jenny, H.; Arkley, R. J.; Schultz, A. M. 1969. The pygmy forest-podsol ecosystem and its dune associates of the Mendocino Coast. Madrono. 20(2): 60-74. [10726]
92. Kartesz, John T. 1999. A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. 1st ed. In: Kartesz, John T.; Meacham, Christopher A. Synthesis of the North American flora (Windows Version 1.0), [CD-ROM]. Chapel Hill, NC: North Carolina Botanical Garden (Producer). In cooperation with: The Nature Conservancy; U.S. Department of Agriculture, Natural Resources Conservation Service; U.S. Department of the Interior, Fish and Wildlife Service. [36715]
93. Kauffman, J. B.; Martin, R. E. 1990. Sprouting shrub response to different seasons and fuel consumption levels of prescribed fire in Sierra Nevada mixed conifer ecosystems. Forest Science. 36(3): 748-764. [13063]
94. Kauffman, John Boone. 1986. The ecological response of the shrub component to prescribed burning in mixed conifer ecosystems. Berkeley, CA: University of California. 235 p. Dissertation. [19559]
95. Keeler-Wolf, Todd. 1988. The role of Chrysolepis chrysophylla (Fagaceae) in the Pseudotsuga hardwood forest of the Klamath Mountains of California. Madrono. 35(4): 285-308. [6449]
96. King, David A. 1991. Tree allometry, leaf size and adult tree size in old-growth forests of western Oregon. Tree Physiology. 9(3): 369-381. [48473]
97. Kruckeberg, A. R. 1980. Golden chinquapin (Chrysolepis chrysophylla) in Washington state: a species at the northern limit of its range. Northwest Science. 54(1): 9-16. [8796]
98. Kruckeberg, A. R. 1982. Gardening with native plants of the Pacific Northwest. Seattle, WA: University of Washington Press. 252 p. [9980]
99. Kruckeberg, Arthur R. 1977. Manzanita (Arctostaphylos) hybrids in the Pacific Northwest: effects of human and natural disturbance. Systematic Botany. 2(4): 233-250. [13561]
100. Kuchler, A. W. 1964. California mixed evergreen forest (Quercus-Arbutus-Pseudotsuga). In: Kuchler, A. W. Manual to accompany the map of potential vegetation of the conterminous United States. Special Publication No. 36. New York: American Geographical Society: 29. [67226]
101. LaHaye, William S.; Gutierrez, R. J. 1999. Nest sites and nesting habitat of the northern spotted owl in northwestern California. The Condor. 101(2): 324-330. [36109]
102. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: http://www.landfire.gov/downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
103. LANDFIRE Rapid Assessment. 2007. Rapid assessment reference condition models, [Online]. In: LANDFIRE. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; The Nature Conservancy (Producers). Available: http://www.landfire.gov/models_EW.php [2008, April 18] [66533]
104. Larsen, David R.; Hann, David W. 1987. Height-diameter equations for seventeen tree species in southwest Oregon. Research Paper 49. Corvallis, OR: Oregon State University, College of Forestry, Forest Research Lab. 16 p. [49458]
105. Leech, Hugh B. 1959. Notes on a few species of Pacific Coast Cerambycidae. The Coleopterists Bulletin. 13(2): 42-46. [84127]
106. Liang, Jingjing; Buongiorno, Joseph; Monserud, Robert A.; Kruger, Eric L.; Zhou, Mo. 2007. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. Forest Ecology and Management. 243(1): 116-127. [68913]
107. Lutz, James A.; Halpern, Charles B. 2006. Tree mortality during early forest development: a long-term study of rates, causes, and consequences. Ecological Monographs. 76(2): 257-275. [83796]
108. Martin, Robert E. 1982. Shrub control by burning before timber harvest. In: Baumgartner, David M., compiler. Site preparation and fuels management on steep terrain: Proceedings of a symposium; 1982 February 15-17; Spokane, WA. Pullman, WA: Washington State University, Cooperative Extension: 35-40. [18528]
109. McDonald, Philip M. 2008. Chrysolepis Hjelmqvist: chinquapin. In: Bonner, Franklin T.; Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 404-406. [79137]
110. McDonald, Philip M.; Helgerson, Ole T. 1990. Mulches aid in regenerating California and Oregon forests: past, present, and future. Gen. Tech. Rep. PSW-123. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 19 p. [15105]
111. McDonald, Philip M.; Huber, Dean W. 1995. California's hardwood resource: managing for wildlife, water, pleasing scenery, and wood products. Gen. Tech. Rep. PSW-GTR-154. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 23 p. [27152]
112. McDonald, Philip M.; Litton, R. Burton, Jr. 1998. Combining silviculture and landscape architecture to enhance the roadside view. Res. Pap. PSW-RP-235. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 20 p. [48517]
113. McDonald, Philip M.; Minore, Don; Atzet, Tom. 1983. Southwestern Oregon-northern California hardwoods. In: Burns, Russell M., comp. Silvicultural systems for the major forest types of the United States. Agric. Handb. 445. Washington, DC: U.S. Department of Agriculture: 29-32. [7142]
114. McIntire, Patrick W. 1984. Fungus consumption by the Siskiyou chipmunk within a variously treated forest. Ecology. 65(1): 137-146. [8456]
115. McIntosh, Anne C. S.; Gray, Andrew N.; Garman, Steven L. 2009. Canopy structure on forest lands in western Oregon: differences among forest types and stand ages. Gen Tech. Rep. PNW-GTR-794. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 35 p. [81006]
116. McKee, Arthur. 1990. Castanopsis chrysophylla (Dougl.) A. DC. giant chinkapin. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Vol. 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 234-239. [13962]
117. Means, Joseph Earl. 1980. Dry coniferous forests in the western Oregon Cascades. Corvallis, OR: Oregon State University. 264 p. Dissertation. [5767]
118. Miller, Richard E.; Lavender, Denis P.; Grier, Charles C. 1976. Nutrient cycling in the Douglas-fir type--silvicultural implications. In: America's renewable resource potential--1975: The turning point; 1975 Society of American Foresters national convention; 1975 September 28 - October 2; Washington, DC: Society of American Foresters: 359-390. [8514]
119. Minore, Don. 1972. A classification of forest environments in the South Umpqua basin. Res. Pap. PNW-129. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 28 p. [1660]
120. Minore, Don. 1986. Effects of madrone, chinkapin, and tanoak sprouts on light intensity, soil moisture, and soil temperature. Journal of Forest Research. 16(3): 654-658. [4985]
121. Mirov, N. T.; Kraebel, C. J. 1937. Collecting and propagating the seeds of California wild plants. Res. Note No. 18. Berkeley, CA: U.S. Department of Agriculture, Forest Service, California Forest and Range Experiment Station. 27 p. [9787]
122. Munz, Philip A.; Keck, David D. 1973. A California flora and supplement. Berkeley, CA: University of California Press. 1905 p. [6155]
123. Newton, Michael; Roberts, Catherine A. 1977. Brush control alternatives for forest site preparation. In: Proceedings and research progress report, 28th annual Oregon weed control conference; [Date of conference unknown]; Salem, OR. [Place of publication unknown]. [Publisher unknown]. 1-10. [21477]
124. Niemiec, Stanley S.; Ahrens, Glenn R.; Willits, Susan; Hibbs, David E. 1995. Hardwoods of the Pacific Northwest. Research Contribution 8. Corvallis, OR: Oregon State University, College of Forestry, Forest Research Laboratory. 115 p. [65435]
125. Nissley, S. D.; Zasoski, R. J.; Martin, R. E. 1980. Nutrient changes after prescribed surface burning of Oregon ponderosa pine stands. In: Martin, Robert E.; Edmonds, Donald A.; Harrington, James B.; Fuquay, Donald M.; Stocks, Brian J.; Barr, Sumner, eds. Proceedings, 6th conference of fire and forest meteorology; 1980 April 22-24; Seattle, WA. Washington, DC: Society of American Foresters: 214-219. [10207]
126. Odion, Dennis C.; Frost, Evan J.; Strittholt, James R.; Jiang, Hong; DellaSala, Dominick A. 2004. Patterns of fire severity and forest conditions in the western Klamath Mountains, California. Conservation Biology. 18(4): 927-936. [50401]
127. Odion, Dennis C.; Moritz, Max A.; DellaSala, Dominick A. 2010. Alternative community states maintained by fire in the Klamath Mountains, USA. Journal of Ecology. 98(1): 96-105. [80808]
128. Overholser, J. L. 1977. Oregon hardwood timber. Corvallis, OR: Oregon State University, Forest Research Laboratory. 43 p. [16165]
129. Paul, Benson H. 1962. Choose the right wood: Properties and uses of some western hardwoods. Woodworking Digest. 64(3): 47-49. [49497]
130. Pickart, Andrea J.; Barbour, Michael G. 2007. Beach and Dune. In: Barbour, Michael G.; Keeler-Wolf, Todd; Schoenherr, Allan A., eds. Terrestrial vegetation of California. Berkeley, CA: University of California Press: 155-179. [82696]
131. Pierovich, John M.; Clarke, Edward H.; Pickford, Stewart G.; Ward, Franklin R. 1975. Forest residues management guidelines for the Pacific Northwest. Gen. Tech. Rep. PNW-33. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 273 p. [44131]
132. Rambo, Thomas R.; Muir, Patricia S. 1998. Bryophyte species associations with coarse woody debris and stand ages in Oregon. The Bryologist. 101(3): 366-376. [84231]
133. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
134. Raven, Peter H.; Axelrod, Daniel I. 1978. Origin and relationships of the California flora. University of California Publications in Botany. Berkeley, CA: University of California Press. 72: 1-134. [61422]
135. Raymond, Crystal L.; Peterson, David L. 2005. Fuel treatments alter the effects of wildfire in a mixed-evergreen forest, Oregon, USA. Canadian Journal of Forest Research. 35(12): 2981-2995. [63656]
136. Regal, Philip J. 1982. Pollination by wind and animals: ecology of geographic patterns. Annual Review of Ecology and Systematics. 13: 497-524. [71104]
137. Roach, A. W. 1952. Phytosociology of the Nash Crater lava flows, Linn County, Oregon. Ecological Monographs. 22(3): 169-193. [8759]
138. Roof, J. B. 1969. Some brief acquaintances with chinquapins. Four Seasons. 3(1): 16-19. [7535]
139. Roof, J. B. 1970. Some brief acquaintances with chinquapins-II. Four Seasons. 3(2): 15-19. [8094]
140. Roy, Douglass F. 1979. Shelterwood cuttings in California and Oregon. In: The shelterwood regeneration method: Proceedings of the national silviculture workshop; 1979 September 17-21; Charleston, SC. Washington, DC: U.S. Department of Agriculture, Forest Service, Division of Timber Management: 143-165. [11665]
141. Roy, Douglass F. 1980. Redwood. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 109-110. [50047]
142. Ruha, T. L. A.; Landsberg, J. D.; Martin, R. E. 1996. Influence of fire on understory shrub vegetation in ponderosa pine stands. In: Barrow, Jerry R.; McArthur, E. Durant; Sosebee, Ronald E.; Tausch, Robin J., comps. Proceedings: shrubland ecosystem dynamics in a changing environment; 1995 May 23-25; Las Cruces, NM. Gen. Tech. Rep. INT-GTR-338. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 108-113. [27036]
143. Russel, D. W. 1974. The life history of vine maple on the H. J. Andrews Experimental Forest. Corvallis, OR: Oregon State University. 167 p. Thesis. [4974]
144. Saavedra, A.; Hansen, E. M.; Goheen, D. J. 2007. Phytophthora cambivora in Oregon and its pathogenicity to Chrysolepis chrysophylla. Forest Pathology. 37(6): 409-419. [84128]
145. Sarr, D. A.; Hibbs, D. E. 2007. Woody riparian plant distributions in western Oregon, USA: comparing landscape and local scale factors. Plant Ecology. 190(2): 291-311. [84241]
146. Sawyer, John O. 2007. Forests of northwestern California. In: Barbour, Michael G.; Keeler-Wolf, Todd; Schoenherr, Allan A., eds. Terrestrial vegetation of California. Berkeley, CA: University of California Press: 253-295. [82703]
147. Sawyer, John O.; Sillett, Stephen C.; Popenoe, James H.; LaBanca, Anthony; Sholars, Teresa; Largent, David L.; Euphrat, Fred; Noss, Reed F.; Van Pelt, Robert. 2000. Characteristics of redwood forests. In: Noss, Reed F., ed. The redwood forest: History, ecology, and conservation of the coast redwoods. Washington, DC: Island Press: 39-79. [40464]
148. Schoonmaker, Peter; McKee, Arthur. 1988. Species composition and diversity during secondary succession of coniferous forests in the western Cascade Mountains of Oregon. Forest Science. 34(4): 960-979. [6214]
149. Schubert, Gilbert H.; Adams, Ronald S.; Moran, Lewis A. 1971. Reforestation practices for conifers in California. Sacramento, CA: State of California, The Resources Agency, Department of Conservation, Division of Forestry. 359 p. [6994]
150. Seaver, Fred J. 1945. Photographs and descriptions of cup-fungi--XXXIX. The genus Godronia and its allies. Mycologia. 37(3): 333-359. [83703]
151. Sheridan, Chris D.; Spies, Thomas A. 2005. Vegetation-environment relationships in zero-order basins in coastal Oregon. Canadian Journal of Forest Research. 35(2): 340-355. [60361]
152. Skinner, Carl N.; Taylor, Alan H.; Agee, James K. 2006. Klamath Mountains bioregion. In: Sugihara, Neil G.; van Wagtendonk, Jan W.; Shaffer, Kevin E.; Fites-Kaufman, Joann; Thode, Andrea E., eds. Fire in California's ecosystems. Berkeley, CA: University of California Press: 170-194. [65539]
153. Slauson, Keith M. 2004. Habitat selection by American martens (Martes americana) in coastal northwestern California. Corvallis, OR: Oregon State University. 111 p. Thesis. [76883]
154. Slauson, Keith M.; Zielinski, William J. 2009. Characteristics of summer and fall diurnal resting habitat used by American martens in coastal northwestern California. Northwest Science. 83(1): 35. [76044]
155. Spies, Thomas A. 1991. Plant species diversity and occurrence in young, mature, and old-growth Douglas-fir stands in western Oregon and Washington. In: Ruggiero, Leonard F.; Aubry, Keith B.; Carey, Andrew B.; Huff, Mark H., technical coordinators. Wildlife and vegetation of unmanaged Douglas-fir forests. Gen. Tech. Rep. PNW-GTR-285. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 111-121. [17309]
156. Steen, Harold K. 1966. Vegetation following slash fires in one western Oregon locality. Northwest Science. 40(3): 113-120. [5671]
157. Stein, William A. 1980. Port Orford-cedar. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 108-109. [50046]
158. Stein, William I. 1981. Regeneration outlook on BLM lands in the southern Oregon Cascades. Res. Pap. PNW-284. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 68 p. [5031]
159. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in Northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
160. Strothmann, R. O.; Roy, Douglass F. 1984. Regeneration of Douglas-fir in the Klamath Mountains Region, California and Oregon. Gen. Tech. Rep. PSW-81. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station. 35 p. [5640]
161. Stuart, John D.; Salazar, Lucy A. 2000. Fire history of white fir forests in the coastal mountains of northwestern California. Northwest Science. 74(4): 280-285. [38929]
162. Stuart, John D.; Stephens, Scott L. 2006. North Coast bioregion. In: Sugihara, Neil G.; van Wagtendonk, Jan W.; Shaffer, Kevin E.; Fites-Kaufman, Joann; Thode, Andrea E., eds. Fire in California's ecosystems. Berkeley, CA: University of California Press: 147-169. [65538]
163. Swanson, John; Johnson, Robert C.; Merrifield, Dave; Dennestan, Alan. 1982. Fire management plan: Lassen fire management planning area: Lassen Volcanic National Park-Caribou Wilderness Unit: Implementation plan. Mineral, CA: U.S. Department of the Interior, National Park Service, Lassen Volcanic National Park; Susanville, CA: U.S. Department of Agriculture, Forest Service, Lassen National Forest. 66 p. [21407]
164. Swedberg, Kenneth Charles. 1961. The coniferous ecotone of the east slope of the northern Oregon Cascades. Corvallis, OR: Oregon State College. 118 p. Dissertation. [40934]
165. Tappeiner, John C., II; McDonald, Philip M.; Newton, Michael; Harrington, Timothy B. 1992. Ecology of hardwoods, shrubs, and herbaceous vegetation: effects on conifer regeneration. In: Hobbs, Stephen D.; Tesch, Steven D.; Owston, Peyton W.; Stewart, Ronald E.; Tappeiner, John C., II; Wells, Gail E., eds. Reforestation practices in southwestern Oregon and northern California. Corvallis, OR: Oregon State University, Forest Research Laboratory: 136-164. [22157]
166. Taylor, Alan H.; Skinner, Carl N. 1998. Fire history and landscape dynamics in a late-successional reserve, Klamath Mountains, California, USA. Forest Ecology and Management. 111(2-3): 285-301. [30321]
167. Taylor, Alan H.; Skinner, Carl N. 2003. Spatial patterns and controls on historical fire regimes and forest structure in the Klamath Mountains. Ecological Applications. 13(3): 704-719. [52969]
168. Temesgen, Hailemarian; Hann, David W.; Monleion, Vincente J. 2007. Regional height-diameter equations for major tree species of southwest Oregon. Western Journal of Applied Forestry. 22(3): 213-219. [68857]
169. The Jepson Herbarium. 2012. Jepson online interchange for California floristics, [Online]. In: Jepson Flora Project. Berkeley, CA: University of California, The University and Jepson Herbaria (Producers). Available: http://ucjeps.berkeley.edu/interchange.html [70435]
170. Thomas, David F. 1984. The use of sheep to control competing vegetation in conifer plantations. In: Proceedings of the 5th annual forest vegetation management conference; 1983 November 2-3; Sacramento, CA. Redding, CA: Forest Vegetation Management Conference: 138-143. [28339]
171. Thompson, Joel; Diller, Lowell; Golightly, Richard; Klug, Richard. 2007. Fisher (Martes pennanti) use of a managed forest in coastal northwest California. In: Gregory A.; Valachovic, Yana; Zielinski, William J.; Furniss, Michael J., tech. eds. Proceedings of the redwood region forest science symposium: What does the future hold; 2004 March 15-17; Rohnert Park, CA. Gen. Tech. Rep. RSW-GTR-194. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: 245-246. [71156]
172. Thorne, Robert F. 1976. The vascular plant communities of California. In: Latting, June, ed. Symposium proceedings: plant communities of southern California; 1974 May 4; Fullerton, CA. Special Publication No. 2. Berkeley, CA: California Native Plant Society: 1-31. [3289]
173. Topik, Christopher; Hemstrom, Miles A., comps. 1982. Guide to common forest-zone plants: Willamette, Mt. Hood, and Siuslaw National Forests. R6-Ecol 101-1982. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 95 p. [3234]
174. Traut, Bibit Halliday; Muir, Patricia S. 2000. Relationships of remnant trees to vascular undergrowth communities in the western Cascades: a retrospective approach. Northwest Science. 74(3): 212-223. [39449]
175. U.S. Department of Agriculture, Natural Resources Conservation Service. 2012. PLANTS Database, [Online]. Available: https://plants.usda.gov /. [34262]
176. Uzoh, Fabian C. C.; Richie, Martin W. 1996. Crown area equations for 13 species of trees and shrubs in northern California and southwestern Oregon. Res. Paper PSW-RP-227. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 13 p. [38453]
177. Valachovic, Y. S.; Caldwell, B. A.; Cromack, K., Jr.; Griffiths, R. P. 2004. Leaf litter chemistry controls on decomposition of Pacific Northwest trees and woody shrubs. Canadian Journal of Forest Research. 34(10): 2131-2147. [62192]
178. Van Dyke, Eric; Holl, Karen D.; Griffin, James R. 2001. Maritime chaparral community transition in the absence of fire. Madrono. 48(4): 221-229. [41368]
179. Veirs, Stephen D., Jr. 1982. Coast redwood forest: stand dynamics, successional status, and the role of fire. In: Means, Joseph E., ed. Forest succession and stand development research in the Northwest: Proceedings of the symposium; 1981 March 26; Corvallis, OR. Corvallis, OR: Oregon State University, Forest Research Laboratory: 119-141. [4778]
180. Volland, Leonard A. 1974. Relation of pocket gophers to plant communities in the pine region of central Oregon. In: Black, Hugh C., ed. Wildlife and forest management in the Pacific Northwest: Proceedings of a symposium; 1973 September 11-12; Corvallis, OR. Corvallis, OR: Oregon State University, School of Forestry, Forest Research Laboratory: 149-166. [8003]
181. Volland, Leonard A. 1985. Plant associations of the central Oregon pumice zone. R6-ECOL-104-1985. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 138 p. [7341]
182. Volland, Leonard A.; Dell, John D. 1981. Fire effects on Pacific Northwest forest and range vegetation. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region, Range Management and Aviation and Fire Management. 23 p. [2434]
183. Wallace, Michael Wayne. 1976. The effects of fire on nutrient conditions in the Pinus ponderosa zone of central Oregon. Seattle, WA: University of Washington. 73 p. Thesis. [34456]
184. Waring, R. H. 1969. Forest plants of the eastern Siskiyous: their environment and vegetational distribution. Northwest Science. 43(1): 1-17. [9047]
185. Weatherspoon, C. Phillip; Skinner, Carl N. 1995. An assessment of factors associated with damage to tree crowns from the 1987 wildfires in northern California. Forest Science. 41(3): 430-451. [26005]
186. Weisberg, Peter J. 1998. Fire history, fire regimes, and development of forest structure in the central western Oregon Cascades. Corvallis, OR: Oregon State University. 256 p. Dissertation. [40273]
187. Weisberg, Peter J. 2009. Historical fire frequency on contrasting slope facets along the McKenzie River, western Oregon Cascades. Western North American Naturalist. 69(2): 206-217. [81463]
188. Wellersdick, Marilee; Zalunardo, Ray. 1978. Characteristics of snags used by wildlife for nesting and feeding in the western Cascades, Oregon. Roseburg, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region, Umpqua National Forest. 30 p. [17180]
189. Wells, Philip V. 1962. Vegetation in relation to geological substratum and fire in the San Luis Obispo quadrangle, California. Ecological Monographs. 32(1): 79-103. [14183]
190. Westman, W. E. 1975. Edaphic climax pattern of the pygmy forest region of California. Ecological Monographs. 45(2): 109-135. [10695]
191. Westman, W. E.; Whittaker, R. H. 1975. The pygmy forest region of northern California: studies on biomass and primary productivity. Journal of Ecology. 63(2): 493-520. [8186]
192. Whittaker, R. H. 1953. A consideration of climax theory: the climax as a population and pattern. Ecological Monographs. 23(1): 41-78. [64492]
193. Whittaker, R. H. 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs. 30(3): 279-338. [6836]
194. Whittaker, R. H. 1961. Vegetation history of the Pacific Coast states and the "central" significance of the Klamath region. Madrono. 16(1): 5-23. [7467]
195. Wickman, Boyd E.; Kline, LeRoy N. 1985. New Pacific Northwest records for the California oakworm. Pan-Pacific Entomologist. 61(2): 152. [83707]
196. Williamson, Richard L. 1980. Pacific Douglas-fir. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 106-107. [50044]
197. Witt, Joseph A. 1972. A change in name for the golden chinquapin. American Horticultural Magazine. 51(2): 24-25. [19599]
198. Yerkes, Vern P. 1960. Occurrence of shrubs and herbaceous vegetation after clear cutting old-growth Douglas-fir. Res. Pap. PNW-34. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 12 p. [8937]
199. Young, James A.; Young, Cheryl G. 1986. Collecting, processing and germinating seeds of wildland plants. Portland, OR: Timber Press. 236 p. [12232]
200. Youngblood, Andrew; Johnson, Kim; Schlaich, Jim; Wickman, Boyd. 2004. Silvicultural activities in Pringle Falls Experimental Forest, central Oregon. In: Shepperd, Wayne D.; Eskew, Lane G., compilers. Silviculture in special places: proceedings of the 2003 National Silviculture Workshop; 2003 September 8-11; Granby, CO. Proceedings RMRS-P-34. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 31-48. [53519]
201. Zielinski, William J.; Truex, Richard L.; Schmidt, Gregory A.; Schlexer, Fredrick V.; Schmidt, Kristin N.; Barrett, Reginald H. 2004. Resting habitat selection by fishers in California. The Journal of Wildlife Management. 68(3): 475-492. [64029]
202. Zobel, Donald B.; McKee, Arthur; Hawk, Glenn M.; Dyrness, C. T. 1976. Relationships of environment to composition, structure, and diversity of forest communities of the central western Cascades of Oregon. Ecological Monographs. 46(2): 135-156. [8767]