| FEIS Home Page |
2024, December 20].
Whitebark pine is an important tree species in high-elevation ecosystems of western North America, but has suffered widespread mortality throughout its range from the combined effects of mountain pine beetle outbreaks and white pine blister rust infection. Fire exclusion amplifies these impacts by allowing succession to shade tolerant species, stressing mature whitebark pines, and limiting opportunities for seedling establishment. Projected warming and drying trends will likely further exacerbate this decline. Because of widespread concern for whitebark pine ecosystems, there is a fast growing body of literature on whitebark pine ecology, threats, and management options. This FEIS Species Review was written in 2002; for more recent information on whitebark pine ecology and management guidelines, see Restoring whitebark pine ecosystems in the face of climate change and other resources provided by the Whitebark Pine Ecosystem Foundation.
Revisions: On 20 December 2024 federal legal status was updated.
 |
 |
||
| Whitebark pine in Yosemite National Park. | A Clark's nutcracker in whitebark pine. | ||
| Photos by Diana Tomback, Whitebark Pine Ecosystem Foundation. | |||
Whitebark and limber pine (P. flexilis) have been crossed in the laboratory, and putative hybrids between the 2 species have been identified on the Rocky Mountain Front of Montana. Such entities are rare and apparently infertile [51,59].
LIFE FORM: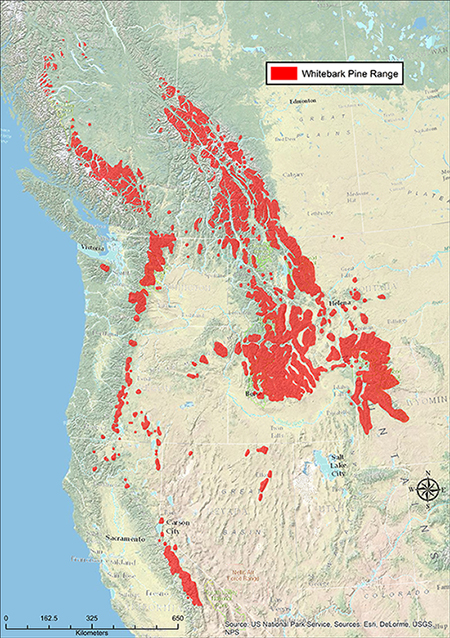 |
|
| Distribution of whitebark pine. Map courtesy of Whitebark Pine Ecosystem Foundation, Whitebark pine range maps, 2014. [2018, November 28]. |
| CA | ID | MT | NV |
| OR | WA | WY |
| AB | BC |
Several other conifer species may share dominance with whitebark pine. At midelevation, whitebark pine throughout its range is associated with lodgepole pine (Pinus contorta) [75,87,126,185,230]. Whitebark pine is not commonly perceived as a midelevation species, but stand reconstruction studies show that whitebark pine was an important historical component of midelevation forests. On the Bitterroot National Forest of western Montana, whitebark pine dominated 14% of midelevation (6,500-7,500 feet (1,950-2,250 m)) stands from the 1700s to 1900 [18,77]. Where not dominant, it was a common component of midelevation Rocky Mountain lodgepole pine (P. c. var. latifolia) forest. By the time of the study (1991), whitebark pine dominated none of the midelevation study sites. At its lowest elevations throughout its range, whitebark pine overlaps with Douglas-fir (Pseudotsuga menziesii) [194]. In the coastal states, it associates with mountain hemlock (Tsuga mertensiana), with increasing codominance of the 2 species to the south [87]. Subalpine fir (Abies lasiocarpa) and Engelmann spruce (Picea engelmannii) co-occur in northwestern states; subalpine fir is the most frequent codominant where its range overlaps with that of whitebark pine [54,126,185,230]. In the Northwest, whitebark pine types between 6,600 and 7,800 feet (2,000-2,400 m) are often found adjacent to alpine larch (Larix lyallii) communities. The 2 species tend to be complementary rather than competitive in distribution, with whitebark pine occupying dry south and west aspects and alpine larch restricted to more mesic sites [9,16,50,126]. Sadly, whitebark pine communities of the northern Cascades [126], eastern Washington [229], Idaho, and Montana [171] are often characterized as "ghost forests" of whitebark that have been dead for decades, with little regeneration [130].
Washington: Whitebark pine communities in the Cascade Range are often mixed with or adjacent to mountain big sagebrush (Artemisia tridentata ssp. vaseyana) or mountain grassland communities. On some sites, whitebark pine patches form "islands" within shrubland or grassland communities: these mixed communities form highly diverse mosaics. At lower elevations (~ 6,230 feet (1,900 m)), whitebark pine grades into subalpine fir, or sometimes (at ~ 5,720 feet), coast Douglas-fir (Pseudotsuga menziesii var. menziesii) communities. Depending on elevation, subalpine fir, Engelmann spruce, lodgepole shore pine (Pinus contorta var. contorta), and coast Douglas-fir are common components of whitebark pine communities; subalpine fir is the most common co-dominant. Shrubs typically show low cover; Oregon boxwood (Paxistima myrsinites) is the only constant shrub associate. The herb layer is often diverse. Whitebark pine forms fringe forests and woodlands at timberline. It is an important component of alpine larch communities occurring below ~ 7,330 feet (2,230 m), and persists as krummholz in higher-elevation alpine larch communities [126]. In Mt. Rainier National Park, krummholz whitebark pine/common juniper (Juniperus communis) forests dominate high, rocky ridges above Yakima Park [69].
In eastern Washington and northern Idaho, whitebark pine is a seral component of subalpine fir communities and dominates the highest peaks and ridges (> 6,000 ft (1,800 m)). Understory cover is typically discontinuous on these high-elevation sites. Engelmann spruce, Rocky Mountain lodgepole pine, and Rocky Mountain Douglas-fir (Pseudotsuga menziesii var. glauca) may associate, especially on midelevation sites [54,229]. Grouse whortleberry (Vaccinium scoparium) is the most widespread and constant dominant shrub in whitebark pine communities throughout the Rocky Mountains [17]. Pinegrass (Calamagrostis rubescens) is the most common and constant herb; smooth woodrush (Luzula hitchcockii) and Drummond's rush (Juncus drummondii) also occur in and sometimes dominate whitebark pine understories [229]. Oregon boxwood and common juniper are characteristic shrubs [54,229].
Oregon: In the Blue Mountains of eastern Oregon and Washington, whitebark pine codominates with subalpine fir between 7,600 and 8,500 feet (2,300-2,550 m). Whitebark pine assumes increasing dominance with elevation; it is the only tree on the highest sites. Rocky Mountain lodgepole pine, Engelmann spruce, and Rocky Mountain Douglas-fir co-occur. Alpine larch assumes dominance on cold, moist sites. Elk sedge (Carex geyeri) is usually dominant on the ground layer; it is the most constant herb across sites [75,230]. In the Cascade Range yellow sedge (C. pensylvanica) and Wheeler bluegrass (Poa nervosa) are dominant ground layer species [17].
Idaho: Limber pine, subalpine fir, and/or Rocky Mountain lodgepole pine co-occur in whitebark pine communities. Elk sedge, Ross' sedge (C. rossii), or cushion plants such as subalpine fleabane (Erigeron peregrinus) and rosy pussytoes (Antennaria rosea) dominate the sparse understory of high-elevation whitebark pine/barrengrounds [185]. On lower-elevation sites (< 9,000 feet (2,700 m)), grouse whortleberry, common juniper, pink mountainheath (Phyllodoce empetriformis), Oregon boxwood, Idaho fescue (Festuca idahoensis), and/or smooth woodrush are understory dominants [50,194].
Wyoming: Whitebark pine occurs in the Absaroka, Teton, and Wind River ranges. Best development of whitebark pine habitats occurs on the relatively dry Wind River Range, where whitebark pine is at the edge of its distribution. Rocky Mountain lodgepole pine is seral in this type. Rocky Mountain Douglas-fir, subalpine fir, and Engelmann spruce occur occasionally, but rarely reproduce well. Grouse whortleberry, heartleaf arnica (Arnica cordifolia), Ross' sedge, and Wheeler's bluegrass (Poa wheeleri) are common dominant understory components; common juniper and russet buffaloberry (Shepherdia canadensis) are occasional dominants [194].
California: Pure or nearly pure whitebark pine communities occur at treeline in the Sierra Nevada and Cascade Range. Western hemlock typically codominates with whitebark pine in the Cascades and northern Sierra Nevada but is increasingly replaced by Sierra Nevada lodgepole pine (P. c. var. murrayana) from the central Sierra Nevada southward. In upper montane and subalpine forests (6,000-11,000 (1,830-3,350 m)), whitebark pine is common in mixed stands with Sierra Nevada lodgepole pine, mountain hemlock, and/or foxtail pine (P. balfouriana) [87,136,182]. At lower elevations (7,500 feet in the north and 9,000 feet in the south), whitebark pine merges with mixed Sierra Nevada lodgepole pine, red fir (Abies magnifica), and/or Jeffrey pine (P. jeffreyi) forest. Krummholz whitebark pine merges into alpine fell-fields at high elevations (9,500-11,100 feet (2,900-3,4900 m), depending on latitude) [17,32,87,202].
In the Warner Mountains, whitebark pine co-occurs with Sierra Nevada lodgepole pine, Washoe pine (P. washoensis), white fir (A. concolor), and Jeffrey pine [136,179].
Klamath Mountain associates in whitebark pine/oceanspray (Holodiscus discolor) communities include mountain hemlock, Shasta red fir (A. m. var. shastensis), western white pine (P. monticola), Jeffrey pine, foxtail pine, and Sierra Nevada lodgepole pine [156,184].
Nevada: Limber pine is the primary codominant [17]. Limber pine dominates the lower subalpine zone
(8,000-9,000 feet (2,400-2,700 m)) of the Ruby and Humboldt mountains, while whitebark pine
dominates vegetation in the upper subalpine zone (8,550 to 10,600 feet
(2,610-3,230 m)) [125]. In the Ruby Mountains, whitebark pine forms subalpine communities
with
Great Basin bristlecone (P. longaeva) and limber pines; it is the only area where
the 3 Strobus species
overlap [117,125].
Due to inaccessibility and previously low interest in managing whitebark pine types, whitebark pine communities are not well described compared to other subalpine types. There is agreement in the literature that whitebark pine understories are diverse, and more whitebark pine types exist than have been classified [50,54,194,195]. Accurate descriptions of whitebark pine communities are further confounded by loss of the overstory dominant to insects and disease, moving successional pathways onto new trajectories. The following classifications present preliminary descriptions of whitebark pine plant communities.
California [87,110,136,179,182,184,202]Bark thickness is thin to moderate, seldom reaching over 0.4 inch (1 cm) [21]. Branches of mature trees are ascending [89]. Needles are in bundles of 5. They may reach 7 inches (18 cm) in length, or as little as 1.5 inches (3.8 cm). Mature female cones are 1.6 to 3 inches (4-8 cm) long [64]. They are located mostly at the tops of the upswept branches and are readily recognizable from the air, probably an adaptation to encourage seed foraging by Clark's nutcrackers. The purple cast of mature cones may further aid Clark's nutcrackers in finding ripe seed. Cone color is also the easiest way for humans to distinguish between whitebark and the morphologically similar limber pine [116,117]. A female whitebark pine cone contains an average of 75 seeds. Whitebark pine's wingless seeds are large and heavy for Pinus species: 7 to 10 mm in length and an average mass of 72 mg (+ 13 mg) per seed [21,89].
Tree stocking in whitebark pine communities can be low [110], but whitebark pine attains considerable cover on some sites. A stand on the Wenatchee National Forest of Washington supported 143 to 358 whitebark pine/acre with 41-214 feet2/acre basal area [126]. On the Okanogan National Forest of Washington, mean overstory cover in a whitebark pine/pinegrass community was 27% [230].
Whitebark pines at high elevations often attain extreme age. Stands in the Wind River Range, Wyoming, and Jasper National Park, Alberta, have been aged at > 600 and 700 years, respectively [133,194]. The oldest documented individual is on the Sawtooth National Forest of Idaho; it is >1,270 years old [169].
Whitebark pine is extremely wind firm. High-elevation trees on shallow, undeveloped soils frequently endure near-hurricane-force winds [32].
RAUNKIAER [176] LIFE FORM:On a fine scale, genetic structure of whitebark pine consists of clusters of close relatives. As a consequence of Clark's nutcracker's habit of planting seeds from a parent tree in the same cache, individuals within clusters often cross- or self-pollinate. This results in inbreeding. Trees within clumps are usually related as half-siblings, full siblings, or selfed. Neighboring clumps (probably planted by a different bird and/or collected from a different parent tree) are not closely related to each other [61,127,208,213].
Seed production: Cone production requires 2 years, as is typical for pines (Pinus spp.). Cones are 1st produced at 20 to 30 years of age on good sites. Trees do not reach full cone production until 60 to 100 years of age on most sites [125,146]. Peak cone production extends for another 250 years, then gradually declines. Some 1,000-year-old trees still reproduce [207]. Cone production is characterized by frequent years of small cone crops and less frequent years of moderate to heavy crops [104]. In the Greater Yellowstone area, moderate or large whitebark pine conecrop years occurred 2 or 3 times a decade (1980-1990) [159]. Best reproduction occurs when day/night July temperatures are above 68/39 degrees Fahrenheit (20o/4o C), and there is no summer water stress [226].
Factors limiting reproduction: A number of agents reduce natural regeneration in whitebark pine. White pine blister rust, fire exclusion, bark beetles, animals, and fungal diseases reduce ability of mature trees to reproduce. White pine blister rust is the greatest threat to whitebark pine regeneration [28]. In blister rust-infected trees, branch die-off 1st occurs on the ends of large, cone-producing branches. Although tree mortality may not occur for decades, infected trees rapidly loose ability to produce seed [17]. By reducing the gene pool, genetic consequence of white pine blister rust is inbreeding depression (expression of maladaptive or lethal genes) [84,232]. However, other factors also contribute to poor regeneration and decline (see Other Management Considerations). Using historical stand reconstruction studies on the Bitterroot National Forest, Arno and others [18] determined that whitebark pine dominated 14% of the landscape in 1900. By the end of that century, combined effects of fire exclusion and white pine blister rust had reduced whitebark pine to the point that whitebark pine longer dominated any of the study sites. Remaining stands with cone-bearing whitebark pine were one-half their former size. Mountain pine beetle epidemics can depress whitebark pine regeneration for decades by killing mature, cone-bearing trees [23]. On the Sundance Burn in northern Idaho, scant whitebark pine regeneration has been attributed to mountain pine beetle attacks prior to large-scale wildfire coupled with blister rust damage to whitebark pines on the burn's periphery [210].
Animal seed predation on whitebark pine seed is high. Except following good conecrop years, whitebark pine seedling establishment is probably incidental due to high rates of seed predation [214,229]. Even Clark's nutcracker harvesting of whitebark pine seed, often presented as a classic example of animal-plant mutualism [204], may be detrimental on some sites. Although individual Clark's nutcrackers only remove seeds that they plant themselves [147], researchers fear that in areas of high blister rust infection, whitebark pine seed will become so rare that Clark's nutcrackers will consume most of the seed they cache, leaving few seed reserves for regeneration [215]. Clark's nutcrackers were the most efficient harvesters of whitebark pine seed on the Bridger-Teton National Forest of Wyoming, showing a 97% forage success rate (measured as time spent harvesting/seeds collected). Other important predators that harvested directly from whitebark pine cones included pine grosbeaks (92% success rate), ravens (79%), red squirrels (60%), and chipmunks (35%) [89]. Similarly, vertebrates harvested 100% of mature whitebark pine seeds on the slopes of Bachelor Butte in the Cascade Range of Oregon. Most successful seed collectors were Clark's nutcrackers, Douglas' squirrels, least chipmunks, and golden-mantled ground squirrels, respectively [134]. Mammalian and bird seed predation reduced the amount of soil-cached seed significantly (p<0.01) on the Gallatin National Forest of Montana. Northern pocket gophers were the most important seed predator [147].
Little is known of insect cone predators and their possible effects on whitebark pine regeneration. Further studies are needed in this area. Anderton and Jenkins [8] have documented whitebark pine seed predation by seed bugs (Leptoglossus occidentalis) and larch cone flies (Strobilomyia macalpinei) on the Bitterroot National Forest, Montana. Insect damage ranged from 0.4 to 7.1% of total seed crop in their study. A study across California, Oregon, Washington, Idaho, and Montana found that seed bugs were the most serious insect pest (27% of total whitebark pine seedcrop destroyed), with fir coneworms (Dioryctria abietivorella) damaging up to 13% of whitebark pine seeds [104].
Seed dispersal: Whitebark pine cones are indehiscent, meaning they do not open when seeds are ripe. Because cones are indehiscent, seed caching by Clark's nutcrackers is the only important means of dispersal [89]. Clark's nutcrackers break through the cone scales with their beaks to remove the seeds, then bury the seeds in shallow caches for use as future food [203,204]. In good conecrop years, the birds cache many more seeds than they recover for food [204]. Hutchins and Lanner [89] estimated that 1 Clark's nutcracker caches 98,000 seeds in a good conecrop year. Many unretrieved seeds germinate and produce new trees [89,116,120]. The birds prefer burns and other open, disturbed areas as cache sites, although they also select closed, shady sites that are unfavorable for whitebark pine regeneration [203,204]. Norment and Conner [166] found that Clark's nutcrackers are most abundant on small (0.1- to 2-ha), disturbed patches or nonforested patches. Approximately 40% of caches on plots in the Sierra Nevada were on sites favorable for whitebark pine regeneration [204].
Germination: Germination and the 1st few weeks of seedling life may be the most critical stages of whitebark pine's life history. Seedlings do not emerge until (a) embryonic development has occurred and (b) the seedbed is moist [212]. Clark's nutcrackers often cache whitebark pine seeds before they are fully ripe and developed [117]. Embryonic development continues after planting and requires stratification and weathering of the seedcoat before germination occurs [124]. Germinants typically emerge 2 or more years after caching, when embryos are mature and seedbeds are moist long enough for seeds to fully imbibe (> 4 days under laboratory conditions) [124,208]. Some germination occurs in fresh seed the 1st growing season after caching. Germination of 1st-year, mature seed collected on the Bridge-Teton National Forest, Wyoming, ranged from 6.7 to 56.7% [89]. Above-average precipitation may favor emergence. On the Gallatin National Forest, seeds that were hand planted in 1988, a dry year, showed reduced 1st-year emergence compared to seeds planted in 1989, a moist year. Emergence is best on burned or other mineral soils compared to soils with litter [147]. Light-severity burns do not prepare as good a seedbed as more severe burns [147,225]. Because they are relatively free from competition, seedlings on burns have the best chance of growing into mature trees [145].
Seed banking: Whitebark pine appears to be the only North American pine (Pinaceae) with a seed bank. Due to seed caching by Clark's nutcrackers and delayed seed germination, whitebark pine may show good seedling establishment even if the previous year's cone crop was poor. Studies conducted after the 1988 fires on the Gallatin National Forest and Yellowstone National Park found that germination rates of natural regeneration were greatest 2 years after good cone crops. Some seeds germinated the spring after Clark's nutcracker planting, while others germinated in the 3rd (and last) year of the study. Synchronous germination occurred in both seedling clusters and single germinants. As of 1995, mean survivorship of seedling clusters > 1 year of age was 25%. The role of precipitation was unclear, but favorable precipitation was positively correlated (r=0.935) with good seedling establishment on the Yellowstone site [208]. Clark's nutcrackers have been observed caching seed as far as 13 miles (22 km) from parent trees [223]. They sometimes relocate cached seed to new sites [89], so actual dispersal distances may be greater. Longer travel distances may translate to fewer seedlings, however. Seedling density on the Sleeping Child Burn of western Montana decreased significantly (p > 0.05) as distance from seed source increased [199].
Seedling establishment and growth: Due to delayed germination and Clark's nutcracker caching habits, good seedling establishment requires many years. Clark's nutcrackers continue to cache seeds on burns and other disturbed sites as long as sites remain open and soils are bare. Burns where fire was exceptionally hot may not show good establishment for several postfire decades [11]. For example, the Sleeping Child and Saddle Mountain burns of western Montana 1st showed whitebark pine establishment 5 and 7 years after fire, respectively, with best establishment occurring 2 or more years after favorable summer rains promoted cone production [214].
 |
| Whitebark pine emergents on the Gallatin National Forest. Image by Garon Smith, used with permission. |
Whitebark pine seedlings are generally considered hardy after their 1st few weeks of life [17,208]. Seedlings rapidly grow deep roots and thick, drought-resistant stems [40], enabling whitebark pine seedlings to better survive drought compared to their more sun-intolerant conifer associates. Even so, droughty, coarse-textured soils may reduce whitebark pine establishment. Light shade improves seedling survivorship; however, McCaughey [147] found that heavy shade increased drought-related seedling mortality on the Gallatin National Forest. He suggested that in dry years, increased cover might intercept critical precipitation. Shrub nurse plants may increase whitebark pine seedling survivorship, but herbaceous species with abundant fibrous roots appear to inhibit establishment. Based upon relative species abundance, whitebark pine seedlings on the Sleeping Child and Saddle Mountain burns were most frequently associated with grouse whortleberry, and seldom associated with smooth woodrush and beargrass (Xerophyllum tenax) [199,214].
Whitebark pine survivorship is generally considered best on burns [147]; however, given open conditions and mineral soil, seedlings may show good survivorship on a variety of sites. In Yellowstone National Park, whitebark pine seedlings showed best establishment on moist, moderately to severely burned sites compared to moist, unburned sites and dry burned/unburned sites. On the Gallatin National Forest, however, seedling establishment was similar on burned and unburned sites with similar moisture regimes [208].
Most seedlings gain rapid root growth, acquiring top-growth more slowly. First-year germinants on the Gallatin National Forest showed root lengths ranging from 2 to 7.1 inches (5-18 cm) [146]. In Yosemite National Park, mean top-growth rate of seedlings at 10,000 feet (3,050 m) elevation was 0.9 inch (2.3 cm)/year, while seedlings at 10,810 (3,295 m) gained an average 0.7 inch (1.7 cm) per year [204].
A number of agents may damage or kill seedlings. Heat damage to unshaded stem tissue is the common cause of death. Browsing animals also kill seedlings. Northern pocket gophers cause highest mortality on whitebark pine seedlings on the Gallatin National Forest, although browsing elk, chipmunks, and birds - including Clark's nutcrackers - also consume seedlings [146]. Tomback [204] found that 2 years after emergence, survivorship of natural whitebark pine regeneration on 2 Sierra Nevada sites averaged 41 and 65% of 1st-year cohorts.
Most growth occurs in midsummer [87]. Growth on cold sites may be very slow [229], taking as long as 17 years to produce a 5-inch-long (12-cm) branch [197]. Tree-ring data from the central and southern Sierra Nevada show that best growth occurs following warm, wet winters, and slowest growth occurs after cool, dry winters [70].
Asexual regeneration: Whitebark pine reproduces by layering where long-lasting snowloads bend lower branches and thin, flexible stems onto soil. Layering is most common in krummholz whitebark pine [17,146]. Krummholz whitebark pine rarely sets seed and when it does, the seed often shows poor germination. Krummholz patches usually originate from lower-elevation seed transported into the upper subalpine by Clark's nutcrackers. Once krummholz is established, layering is its primary method of patch expansion [205]. Except in the upper subalpine, layering is not an important method of whitebark pine regeneration [17].
SITE CHARACTERISTICS:Topography: Whitebark pine is most common on rocky, well-drained sites [87,126]. Best development occurs on sheltered, north-facing slopes and basins [139]. In the southern Sierra Nevada, whitebark pine is confined to moist north slopes. Topography is rolling to rough with moderate to steep slope [75,126]. Slopes on the Blue Mountains ranged from 5 to 60% [87]. Whitebark pine occurs on all exposures but is most common on south- and west-facing slopes [20,75,105,126,194,230].
Soils: Soils in whitebark pine communities are classified as cryochrepts [76]. Soils are moderately to poorly developed and well drained. Coarse fragments are well represented [75,126]. Whitebark pine soils are nutrient poor and usually derived from granite or basalt [76,87,126,230], although whitebark pine occasionally grows on sedimentary soils [76,185]. Soil pH is usually strongly acidic, although whitebark pine also occurs on basic soils [65,171,227]. Whitebark pine occurs on serpentine soils in the Blue, Klamath, and Siskiyou mountains [75,184]. Soils textures include coarse sands, sandy loams, and loams [75,230]. Rooting depth is typically shallow; for example, maximum rooting depth at 1 site on the Okanogan National Forest of Oregon was < 12 inches (30 cm) [230]. Krummholz or matted whitebark pine grows mostly on high-elevation sites where glacial scouring has eliminated most of the soil [182].
Elevation: Whitebark pine occurs from 4,300 to 12,100 feet (1,300-3,700 m) elevation [64]. Ranges by state or province are as follows:
| Location | Elevation |
| CA | 7,000 to 9,500 feet (2,100-2,900 m) in the Cascades 10,000 to 12,100 feet (3,050-3,700 m) in the Sierra Nevada [17,32,87,172] |
| ID | 7,300-10,500 feet (2,225-3,200 m) [185,194] |
| OR | 5,810 -8,500 feet (2,500 m) in the Blue Mts. [48,230] 5,400 to 9,500 feet (1,620-2,900 m) in the Cascades [17,134,200] Lowest natural range reported anywhere for whitebark occurs at 3,600 feet (1,110 m) on Mt. Hood |
| MT | 5,900 to 9,300 feet (1,800-2,830 m) [17] |
| NV | 6,400 to 10,00 feet (2,000-3,000 m) [52] |
| WA | 5,700 to 8,500 (1,700-2,600 m) in the Cascades and Olympic Mountains [17,126] |
| WY | 7,300-10,500 feet (2,225-3,200 m) [17,194] |
| BC | 5,200 feet (1,600 m) in the Coast Ranges 5,643 to 7,999 feet (1,720-2,438 m) in the Rocky Mountains [17,86] |
| SK | 6,500 to 7,500 feet (6,500-7,500 m) [17] |
Whitebark pine in the Cascade Range occupies a seral role in subalpine parklands and forests [2,69,126]. Early to midseral conditions in these associations are mostly maintained by occasional stand-replacement fire. In the absence of fire, subalpine fir usually forms closed stands of mature trees [126]. In high-elevation whitebark pine communities, stands are typically open even in "near-climax" conditions [4,67]. Subalpine fir is sometimes present in the understory, suggesting eventual replacement in even these high-elevation types. However, successional patterns in high-elevation ecosystems are largely undocumented and difficult to predict [4]. On Mount Rainier, whitebark pine and subalpine fir are invading subalpine meadows simultaneously [68].
In Crater Lake National Park, Oregon, whitebark pine is the dominant tree on Wizard Island. Sierra Nevada lodgepole pine is invading the island and appears to be replacing whitebark pine in importance [93].
SEASONAL DEVELOPMENT:| Event | Date |
| bud break | late May - mid-June |
| branch, needle, & cone elongation | July |
| pollination | 8-11 Aug. |
| male cone drop | 17 Aug., after 1st snow |
| seed ripening | late Aug. |
Mean timing for whitebark pine phenological and animal interactions on the Bridger-Teton National Forest [89]:
| Event | Date | Total seedcrop harvested |
| red squirrels begin foraging | mid-July | 4% |
| Clark's nutcrackers begin foraging | early August | 5% |
| 1st germinable seed | early August | 10% |
| Clark's nutcrackers begin caching | mid-August | 18% |
| cones mature | early Sept. | 35% |
| chipmunks begin harvesting | late Sept. | 65% |
| Clarks nutcrackers recache seed from 1 site to another | mid-October | 90% |
Whitebark pine seedlings establish on open sites created by mixed-severity and stand-replacement fires [115,214,223]. Late-successional species dominate when fire-return intervals are long, but fires were historically likely to return before whitebark pine was successionally replaced [159]. Although whitebark pine recruitment is depressed in many areas, whitebark pine seedlings were historically highly competitive with other conifer species in the postfire environment. For example, 25 years after an 1892 stand-replacement wildfire on Mt. Adams in Washington, whitebark pine seedling establishment was equal to that of western hemlock (9% of total recruitment) and better than that of lodgepole pine and Engelmann spruce. Hofmann [85] noted that whitebark pine seedlings were fairly evenly distributed over the 80-acre (32 ha) burn, even though parent seed trees were at least a mile away.
Fuels: Whitebark pine wood is highly flammable even when green. However, fire is usually unable to spread widely in whitebark pine stands due to discontinuous canopies and sparse understory fuels [34,38,194]. Understory species such as pinegrass and grouse whortleberry provide fine fuels that spread surface fires [164].
Fire regimes: Whitebark pine ecosystems have a mixed-severity fire regime of widely ranging fire intensities and frequencies [2,17,25,157]. Mixed-severity fires create complex landscapes of dead whitebark pine stands intermingled with live stands of different ages [18,38,97]. Whitebark pine stands also experience nonlethal surface fires and infrequent stand-replacement fires [2,10,97,159]. Under whitebark pine's highly variable fire regime, fire-return intervals range from 30 to 350+ years [17,25,157]. In a review paper, Agee [2] lists mean fire-return intervals of 29 to 300 years in whitebark pine habitats, with moderate-severity fire-return interval means of 25 to 75 years and stand-replacement fire-return interval means of 140+ years.
The dry, windswept upper slopes where whitebark pine grows are predisposed to lightning strikes. Whitebark pine and mixed-conifer communities with a whitebark component may experience fire frequently [34,38,194]. Fire severity is low where surface fuels are sparse, and the resulting underburn kills mostly small trees and fire-susceptible overstory species, leaving live, mature whitebark pine [164]. On 3 sites on the Bitterroot National Forest, Montana, Arno [10] reported minimum/maximum fire-return intervals of 2/68 (x̄ = 33), 4/78 (x̄ = 30), and 8/50 (x̄ = 41) years. Brown and others [38] found fires in the subalpine mixed-conifer zone of the Selway-Bitterroot Wilderness of Montana and Idaho were mostly mixed severity, with patchy, fine-grained patterns. Return intervals ranged from 25 to 60 years. Fires were still patchy and of mixed severity in pure whitebark pine stands at high elevations, but fire-return interval lengthened to a 115-year mean.
Infrequent stand-replacement fires are an important component of whitebark pine's fire ecology [11,97]. Occasional stand-replacement fire maintains whitebark pine as an early to midseral species in subalpine fir communities in Washington's Cascade Range [126] and elsewhere. Arno [11] found that in the Bitterroot Mountains, subalpine communities on moist, north-facing slopes were most likely to experience long return-interval, stand-replacement fires. He stated, "stand-replacement fire is essential to maintain whitebark on moist slopes because of the rapid rate of succession" [12].
Both large and small, patchy fires are also important to whitebark pine regeneration, especially where whitebark pine is seral. Fires create openings for seed caching by Clark's nutcrackers, recycle nutrients and biomass, and helps prevent successional replacement of mature whitebark pines by shade-tolerant conifers such as Engelmann spruce [158]. Fires historically started in summer and fall and burned over many weeks [97]. Extent of stand-replacement burns varies; ranges from 2.5 to 120 acres (1-50 ha) are typical [166,211]. Small-acreage fires were, and are, more common. For example, a 12-year study (1979-1990) in the Selway-Bitterroot Wilderness Area showed that 84% of wildland fires for resource benefit (prescribed natural fires) burned less than 4 hectares. A single year (1988) accounted for 39% of the area burned [38]. Large fires typically occurred in drought years, burned through lower-elevation plant communities as well the subalpine, and lasted from weeks to months [158].
Fire regime examples: Fire histories of Yellowstone National Park show the mean fire-return interval in underburned whitebark pine stands ranged widely, from 66 to 204 years. Underburns were often patchy and restricted to 1 or several stands. Whitebark pine and mixed-conifer communities from 6,000 to 11,000 feet (2,000-3,300 m) experienced stand-replacement fire every 350 years or more. Slow fuel accretion and moist fuels restricted fire spread, and large fires occurred only in extreme fire weather years such as 1988 [25,181].
In the Sierra Nevada fuel loadings were historically light between 7,500 to 10,000 feet (2,300-3,050 m), and fires were usually of low severity. Large, stand-replacing fires occurred rarely but played an important successional role: fires created openings in which whitebark pine and the nonserotinous Sierra Nevada lodgepole pine established. In the absence of fire, red fir is successionally replacing the 2 pines at elevations below 10,000 feet. Fire records for Yosemite National Park from 1931 to 1978 show that most subalpine fires occurred in the lower, red fir zone (representing 10% of total Park area but 37% of total Park fires), where whitebark pine is seral. Although fires were not as common in the mid-subalpine lodgepole pine-mountain hemlock zone - where mature, cone-bearing whitebark pine is most prevalent - the fires burned over larger areas (14% of total fires, equaling 19,677 acres). The upper subalpine zone (> 10,000 feet) - where whitebark occurs in pure to nearly pure stands - represents 14% of total Park area and experienced no fires between 1931 and 1978. Fires were rare in the upper subalpine, but were "intense" when they did occur, especially in krummholz whitebark pine [34].
On the Cascade Range, fire regime of westside whitebark pine forests is typically stand replacement. Forests with a lodgepole pine component burn more frequently and severely; stands without lodgepole pine burn less frequently and have greater potential for creeping ground fire [126]. On the east side, whitebark pine forests appear to have the shortest fire-return interval of high-elevation forests [2]. Stand-replacement fires are rare on the east side [126].
Fire exclusion: Fire exclusion has favored shade-tolerant, late-successional species throughout whitebark pine's range [100,157,191]. Because fire-return intervals are often long where whitebark pine is climax, fire exclusion has affected high-elevation whitebark pine less than whitebark pine at mid-elevations, where whitebark pine was historically a highly productive seral species [100]. At the landscape level, however, fire exclusion in the upper subalpine has shifted succession away from whitebark pine to later-successional species [3]. Murray and others [164] suggest that livestock grazing in the 19th century reduced fire frequency even before fire suppression was practiced. Small, isolated mountain ranges may be most affected by anthropogenically altered fire regimes. Subalpine portions of the West Bighole Range, an isolated spur of the Bitterroot Range on the Montana-Idaho border, have experienced reduced fire frequencies and an 87% decrease in area burned since European-American settlement. From 1754 to 1873, actual fire rotation was 184 years; modeling predicts a fire rotation of 1,364 years based upon fire frequencies from 1874 to 1993.
The following table provides fire return intervals for plant communities and ecosystems where whitebark pine is dominant or common. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
| Community or Ecosystem | Dominant Species | Fire-Return Interval Range (years) |
| silver fir-Douglas-fir | Abies amabilis-Pseudotsuga menziesii var. menziesii | >200 |
| grand fir | Abies grandis | 35-200 [13] |
| mountain big sagebrush | Artemisia tridentata var. vaseyana | 15-40 [15,41,154] |
| Wyoming big sagebrush | Artemisia tridentata var. wyomingensis | 10-70 (40**) [224,235] |
| western larch | Larix occidentalis | 25-100 [13] |
| whitebark pine | Pinus albicaulis | 50-300+ [2] |
| Rocky Mountain lodgepole pine* | Pinus contorta var. latifolia | 25-300+ [11,13,181] |
| Sierra lodgepole pine* | Pinus contorta var. murrayana | 35-200 |
| Jeffrey pine | Pinus jeffreyi | 5-30 |
| western white pine* | Pinus monticola | 50-200 |
| Pacific ponderosa pine* | Pinus ponderosa var. ponderosa | 1-47 [13] |
| interior ponderosa pine* | Pinus ponderosa var. scopulorum | 2-30 [13,22,123] |
| quaking aspen (west of the Great Plains) | Populus tremuloides | 7-120 [13,74,153] |
| Rocky Mountain Douglas-fir* | Pseudotsuga menziesii var. glauca | 25-100 [13] |
| coastal Douglas-fir* | Pseudotsuga menziesii var. menziesii | 40-240 [13,160,180] |
| western redcedar-western hemlock | Thuja plicata-Tsuga heterophylla | > 200 |
| mountain hemlock* | Tsuga mertensiana | 35 to > 200 [13] |
| Percent fire kill of mature whitebark pine compared to mature overstory associates [24] | |
| whitebark pine | 35 |
| Rocky Mountain lodgepole pine | 36 |
| Engelmann spruce | 47 |
| subalpine fir | 59 |
Postfire growth: Few studies have been conducted on postfire growth rates of whitebark pine. Sund [199] found that on the Sleeping Child Burn described below, whitebark pine seedling heights at postfire year 26 ranged from 0.4 to 94 inches (1-238 cm), with seedling height highly correlated (p < 0.001) with seedling age. Seedling height was greatest on ridges (x̄ = 10.3 in. (36.2 cm)), followed by south and north slopes (x̄ = 12.0 and 11.9 in. (30.6 and 29.7 cm)), and was least on roadside plots (x̄ = 7.6 in. (19.2 cm)).
Effects of fire size and other disturbance agents: Large stand-replacement fires can favor whitebark pine over wind-dispersed conifers if cone-bearing whitebark pine are nearby. For example, in 1961 the lightning-ignited Sleeping Child Fire burned over 27,900 acres (11,300 ha) on the Bitterroot National Forest, Montana. Blister rust infection in the area was light, so whitebark pine seed sources were not limited. Clark's nutcrackers primarily collected whitebark pine seed from trees in the adjacent unburned forest; however, some birds traveled 5 miles (8 km) or more to collect seed. Conifer re-establishment was assessed at postfire year 26 (1987). Whitebark pine and Rocky Mountain lodgepole pine dominated study plots, with whitebark pine showing dominance on north slopes and lodgepole pine showing dominance on south slopes and ridgetops. Lodgepole pine was absent from many upper subalpine plots. Although the oldest conifers in the burn were 25 years of age, the oldest whitebark pine were 21 years old, demonstrating both whitebark pine's tendency to delay postfire establishment and its ability to compete with other conifers despite the delay. Typical of species with wind-dispersed seed on large burns, subalpine fir showed good establishment at the burn's perimeter, and poor establishment in the burn's interior [205].
The combination of mountain pine beetle attacks and blister rust infection followed by large, stand-replacing fire is harmful because whitebark pine seed sources are severely reduced. The Sundance Fire in northern Idaho provides an example. A history of bark beetle infestation and high incidence of blister rust in the area had already reduced whitebark pine seed sources prior to the wildfire. A survey conducted at postfire year 25 revealed a 27% blister rust infection rate in mature whitebark pine adjacent to the burn. Most whitebark pine in unburned plots were snags with mountain pine beetle galleries. On unburned plots, mean density of live whitebark pine > 0.4 inch (1 cm) in diameter was 0.008 tree site (single tree or cluster)/m2. In contrast, seed source density for the Sleeping Child Burn was 0.064 tree site/m2, and the Sleeping Child Burn had more whitebark pine regeneration. Comparison of whitebark pine regeneration on the 2 burns is given below. Data are mean densities (1 standard deviation, range) [210].
| Burn area | Burn year | Study year | Density (tree site/m2) |
| Sundance | 1967 | 1992 | 0.0077 (0.0131, 0-0.0800) |
| Sleeping Child | 1961 | 1987 | 0.0700 (0.1041, 0-0.5120) |
Tomback and others [210] state that whitebark pine seed production near the Sundance Burn was so low that Clark's nutcrackers were not caching many seeds. Without active management including artificial regeneration, the long-term outlook for whitebark pine in the area does not look good. Twenty-nine percent of the seedlings on the Sundance Burn show symptoms of blister rust; actual rate of seedling infection is probably higher.
Fire scorching may increase whitebark pine's susceptibility to mountain pine beetle attack [62,72]. A 1990s investigation of 2nd-order fire effects following the 1988 wildfires in Yellowstone National Park revealed the following trends in whitebark pine mortality at postfire year 7 [175]:
| Tree condition | Percent |
| green (survived fire) | 36.1 |
| fire killed | 59.7 |
| postfire insect kill (mostly mt. pine & pine engraver beetles) |
2.8 |
| unknown mortality | 1.4 |
Wilderness: Across its range, the proportion of whitebark pine habitat that falls within Wilderness boundaries is greater than that of nearly any other tree species [187]. Whitebark pine in Wilderness Areas is not immune to decline: Kendall and Arno [107] estimated that as of 1990, 90% of whitebark pine in Glacier National Park, much of it in wilderness boundaries, had died from white pine blister rust. Fire management of whitebark pine is particularly problematic in small Wilderness Areas, where management-ignited fires are seldom an option [101,163]. Yet conservation of whitebark pine may be impossible without reintroduction of fire to Wilderness Areas [109]. Since firelines in Wilderness areas are costly, damaging, difficult to construct in remote areas, and often in violation of the Wilderness Act, wilderness fires for resource benefit present the best management option [97]. As of this writing (2002), studies are underway to determine fire histories and explore management options in small, isolated Wilderness Areas [163]. Renkin and Despain [178] summarized a 17-year trend (1972-1988) of fire occurrence under the prescribed natural fire program in Yellowstone National Park. They found that the high moisture levels in whitebark pine ecosystems did not favor crown fires in most years (1988 being an exception), and fire occurrence was less than expected (based on amount of unburned area available) in whitebark pine communities. In mixed-conifer forests where whitebark pine was a component of the vegetation, fire occurrence was greater than expected in subalpine fir-Engelmann spruce and old-growth lodgepole pine with a subalpine fir-Engelmann spruce understory, and less than expected in seral lodgepole pine. Keane [97] states "the most important management action for conserving and maintaining vital whitebark pine ecosystems is to allow fires to burn in wilderness areas and play a more natural role in the ecosystem."
Restoring whitebark pine with fire: Long-term outlook for whitebark pine is not without hope, but restoring whitebark pine ecosystems cannot be accomplished without returning fire to subalpine landscapes. Keane and Arno [100] state "maintenance of native fire regimes is the single most important management action to ensure conservation of whitebark pine." Whitebark pine will continue to decline in the short term, but natural selection will probably increase genetic blister-rust resistance in whitebark pine populations [108,198]. It is also likely that future disturbances, particularly large fires and mountain pine beetle attacks, will kill many of these genetically valuable trees. Despite the dangers of landscape-level fire to whitebark pine populations, returning fire to the landscape is best way to restore whitebark pine. Kendall and Keane [108] state "It is important to note that fire exclusion has a far greater negative than positive consequence for whitebark pine. In the absence of fire, atypical amounts of fuel accumulate that foster more fires that are lethal to mature whitebark pine trees." Reintroduction of stand-replacing fire fosters whitebark pine regeneration by providing open sites suitable for Clark's nutcracker caching and seedling establishment. It also reduces impacts of mountain pine beetle infestations by creating mosaics of mutiaged stands that are less conducive to beetle epidemics [78]. It is encouraging that 40% of the progeny of healthy trees in stands otherwise heavily infested with blister rust show some genetic resistance to blister rust [84]. Without intervention, it is likely that the small proportion of whitebark pine resistant to white pine blister rust will be killed in stand-replacement fires before they can reproduce [108].
Management-ignited fires can be used for fire hazard reduction and whitebark pine restoration treatments [37]. Fire researchers emphasize that it is less important to reconstruct historic stand structures than to reintroduce fire to whitebark pine ecosystems. It is crucial to create open sites that are favorable for Clark's nutcracker caching and growth of natural and artificial regeneration. Six Demonstration Areas have been established in the Selway-Bitterroot Wilderness Complex of Idaho and Montana as part of the Restoring Whitebark Pine Ecosystems Project. Ongoing restoration treatments include prescribed fire and silvicultural cuttings. Since the research is ongoing, conclusions and recommendations are based on limited data, and further suggestions will be forthcoming as the project continues [100].
Large, stand-replacement fires are not recommended in areas where whitebark pine is in severe decline (for example, northern Idaho and northwestern Montana). Small-scale prescribed burning is recommended; otherwise, natural whitebark pine regeneration will be extremely slow [210]. Prescribed burning is best conducted in fall, after an early frost (<25oF (-4oC)) kills herbaceous plants and shrub foliage. Such foliage quickly cures and can propagate fire. In other seasons whitebark pine ecosystems are usually too wet to burn, or in extreme fire years, downslope vegetation is so dry that spotting may ignite fire in lower elevations. Aids for conducting stand inventories, prioritizing whitebark pine habitat for prescribed fire, designing and implementing treatments, and posttreatment monitoring and available [100]. Follow-up thinning treatments, especially of subalpine fir, are usually needed to encourage whitebark pine growth [210]. Augmenting natural regeneration with blister-rust resistant seed sources is recommended in areas where whitebark pine seed sources are absent or greatly reduced [84,210].
Unfortunately, restoration treatments may increase bark beetle predation on whitebark pine. On the Beaver Ridge Demonstration Area in northern Idaho, Six [190] found that Pityogenes fossifrons beetles were the most serious posttreatment pest species: they preferred young, apparently healthy whitebark pine in Clark's nutcracker openings, but also attacked a few fire-damaged mature trees. Ips spp. colonized slash heavily and attacked a few fire-damaged mature trees, but mostly left healthy trees alone. Mountain pine beetle numbers, which are rising in the study area, rose on the treatment sites but did not significantly respond to treatments. To reduce Pityogenes fossifrons infestation, Six [190] recommended spraying high-value whitebark pine in Clark's nutcracker openings with carbaryl for 2 posttreatment years (refer to Fire Case Studies).
Fuels: Information on tree biomass is useful in determining fuel loads and predicting potential fire behavior. Moeur [155] provides a model for estimating crown widths and foliage weights of whitebark pine and other northern Rocky Mountain conifers. Regression equations[35,36] and tables [193] are available for estimating whole-tree weight and weights of boles, branches, branchwood with foliage, and live and dead crowns of whitebark pine and other western conifers. Van Wagtendonk and others [220,221,222] provide models for calculating weight, depth, heat content, and other fuel properties of whitebark pine and other Sierra Nevada conifers.
 |
| Downed woody fuels resulting from a 2017 avalanche on the Gallatin National Forest. Image by Garon Smith, used with permission. |
 |
 |
|
Prescribed surface fire. |
Nutcracker opening (harvest/burn). |
|
U.S. Forest Service, Fire Sciences Laboratory photos taken at the Smith Creek site. |
||
A total of 4 treatments were used on the site. Two fire treatments were used: underburning (surface fire) and silvicultural harvest/burning. Other treatments were silvicultural thinning to promote seral whitebark pine, and an undisturbed control. Each unit averaged 2 hectares. The surface fire unit was left undisturbed prior to fire treatment. Objective of the underburn was to develop fire treatments that kill late-successional subalpine fir and release whitebark pine. Objective of the harvest/burn treatment was to develop silvicultural and fire treatments that promote natural whitebark pine regeneration by creating burned openings for Clark's nutcracker seed caching. For the harvest/burn treatment, three 0.2-ha circular areas were designated for cutting, with slash left in place, to create fire-scarified areas. Nearly all trees within the 3 target openings were harvested during late August to mid-September of 1995. The rest of the unit was thinned in late summer of 1995 for underburning, with some slash removed but most left in place as fuel. Healthy whitebark and lodgepole pine outside the target openings were not cut, but all dying whitebark, dying lodgepole pine, and all subalpine fir and Engelmann spruce were harvested. Fires were ignited on 2 Oct., 1996. It was mostly sunny with light winds. Both fire-treatment units were burned with 3-meter-wide strip headfires to keep intensities low. Conditions on the day of burning were:
| Temperature | 10-16 oC |
| relative humidity | 21-31% |
| windspeed | 0-8 km/h |
| log moisture content | 16-28% |
| fine woody fuel (< 1-in.) moisture content | 14-33% |
The fires smoldered for 9 days, until a snowfall extinguished them. The fire burned 52% of the underburn unit, resulting in 19.5% mortality of trees (all species).
FIRE EFFECTS ON TARGET SPECIES:| Postfire Characteristics | Underburn | Harvest/Burn |
Tree |
||
| whitebark pine mortality (%) | 16.8 | 20.0 |
| subalpine fir mortality (%) | 29.9 | 20.0 |
| scorch height (m) | 6.3 | 9.1 |
| crown volume scorch (%) | 32.9 | 59.9 |
| bole char height (m) | 3.4 | 3.2 |
| FUEL | ||
| fire coverage (% of area) | 56.0 | 81.2 |
| fuel consumption (%) | 32.0 | 44.6 |
| fuel consumption (kg/m2) | 1.4 | 2.9 |
| log consumption (%) | 28.1 | 33.3 |
Ground Cover |
||
| soil cover (%) | 18.4 | 38.7 |
| rock cover (%) | 2.4 | 5.4 |
| wood cover (%) | 10.0 | 11.0 |
The nutcracker openings were created in the summer of 1995, a heavy cone crop year for whitebark pine. Even though slash covered 20 to 30% of the ground after harvest treatments, open spots were available for caching. Clark's nutcrackers were observed caching whitebark pine seeds in the harvested treatments over a 2-week period in late August and early September of 1995. The birds planted most heavily in the 3 open areas, which has not yet been burned. The cone crop was poor after the 1996 fire treatments, but some Clark's nutcrackers were observed caching seeds in the underburned areas as well as the 3 burned openings. Clark's nutcrackers have not observed caching seed in the untreated control unit. The study site is being monitored for postfire establishment of whitebark pine and other conifer seedlings.
As of this writing (2002), the Restoring Whitebark Pine Ecosystems Project has conducted prescribed burning and thinning treatments on 2 other whitebark pine sites in the northern Rocky Mountains. Burning on those sites was conducted in 1999, a good cone crop year. Clark's nutcracker use of the burns was heavy. The birds began caching seed within 5 days after burning (Keane 2001, personal communication).
FIRE MANAGEMENT IMPLICATIONS:The harvest/burn treatment continues to be attractive to seed-caching Clark's nutcrackers as of this writing (2002). Further monitoring is needed to determine the rate of posttreatment whitebark pine establishment [100]. Consumption of downed logs on the harvest/burn unit was comparable to that of natural mixed-severity burns [14]. In retrospect, researchers could have harvested all mature whitebark on the harvest/burn unit. Most were killed by the fire, and it would have been difficult to reduce fire intensity enough to minimize mortality of mature whitebark and still create openings for Clark's nutcrackers.
As of 2002, posttreatment information on the silvicultural (thinning) unit has not been published.
It is likely that subalpine fir will regenerate in the treatments more quickly than whitebark pine. Mature subalpine fir are abundant adjacent to the study units, and the majority of nearby mature whitebark pine are damaged by blister rust, and thus have limited ability to produce cones. All stands would benefit from understory cuttings of subalpine fir at about 20 posttreatment years to release new whitebark pine saplings [100].Bears: Whitebark pine ecosystems provide critical habitat for grizzly and black bears. Agee and others [5] found that grizzly bear sightings in North Cascades National Park, Washington, were more frequent than statistically expected in whitebark pine/alpine larch habitats. Whitebark pine seeds are a high-quality bear food [141,143,152]. Red and Douglas' squirrels provide an important ecological link between whitebark pine and bears by making the seeds more readily available. Grizzly and black bears rarely harvest whitebark pine cones from trees; they raid squirrel middens laden with whitebark pine seeds [105,143,204]. Bear consumption of whitebark pine seed peaks just before hibernation in late October and early November. Bears feeding on whitebark pine seeds tend to feed on nothing else, and a good supply of seeds increases bear fecundity. In Yellowstone National Park, female grizzly bears were less likely to abort, and more likely to have larger litters (3 cubs compared to 1-2 cubs) in good conecrop years. Most importantly, grizzly bear death rate was nearly double when bear consumption of whitebark pine seeds was low [142]. Grizzly bears and humans have fewer troublesome encounters when whitebark pine seeds crops are large. In Yellowstone National Park, grizzly bear summer and fall movement is related to availability of whitebark pine seed. In good crop years, grizzly bear tend to congregate in remote whitebark pine communities. When seed is limited, they tend to forage in more populated, lower-elevation sites. Yearling cubs and females with cubs-of-the-year are most likely to be displaced to lower elevations in poor conecrop years [33,141,142].
 |
| A red squirrel midden of whitebark pine seeds. Image by Steve Arno, used with permission. |
Large ungulates: Whitebark pine is a minor browse species for big game, but whitebark pine understories often provide valuable forage. Rocky Mountain mule deer consume trace amounts of whitebark pine [113]. Productivity in whitebark pine understories is highly variable. Stands with grassy understories are usually most productive. Herbage production on the Wenatchee National Forest averaged 204 lbs/acre in whitebark pine/pinegrass communities and 115 lbs/acre in whitebark pine/green fescue communities. In contrast, a whitebark pine/grouse whortleberry/smooth woodrush community site showed herbage production of 22 lbs/acre [126,229].
Birds: Many bird species use whitebark pine ecosystems. Tomback and Kendall [212] provide a list of year-round and neotropical species that nest in or otherwise use whitebark pine ecosystems.
Whitebark pine provides ecologically critical linkage between Clark's nutcracker and lower-elevation, Clark's nutcracker-dependent pines (i.e., limber pine and pinyon pines (Cembroides)). When the seed crop of 1 pine species is insufficient, Clark's nutcrackers migrate up- or downslope to harvest species with more bountiful seed crops. Loss of whitebark pine, their preferred species, reduces Clark's nutcracker populations, and may have negative consequences for other pine species with seeds dispersed by Clark's nutcrackers [212].
Palatability/nutritional value: Whitebark pine seeds are highly nutritious. They are especially high in lipids. Content of seed collected from the Gallatin National Forest was 52% fat, 21% carbohydrate, 21% protein, 3% ash, and 3% water. Major minerals present were copper, zinc, iron, manganese, magnesium, and calcium [121]. Energy content of fresh, mature whitebark pine seed collected on the Bridger-Teton National Forest ranged from 5,526 to 7,308 calories/g (µ=6,800 calories/g) [89,115]. Tomback [204] reported similar energy values (x̄ = 7,716 calories/g) for whitebark pine seed from the Sierra Nevada.
Cover value: Wildlife and livestock use whitebark pine/shrub communities for shade and bedding cover [126]. In Silverbow County, Montana, elk primarily used whitebark pine with a subalpine fir component as fall cover. Female mule deer used whitebark pine communities 15% of their time in summer and 3% in fall. Male mule deer used whitebark pine communities 4% of their time in summer; insufficient data precluded estimates of their fall usage [131]. Whitebark pine ridgetops are prime calving habitat for woodland caribou. Additional fire-created openings in whitebark pine ecosystems might be an asset to caribou reproduction [186].
VALUE FOR RESTORATION OF DISTURBED SITES:1. Inventory stands to document tree age and health, stand structure, cone-production potential, and project the time
frame of successional replacement [12,18,19].
2. Apply and evaluate management-ignited and wildland for resource benefit fires designed to kill late-successional
trees and favor whitebark pine (see Fire Management Considerations).
3. Conduct seed trials with blister rust-resistant stock in areas where natural whitebark pine seed sources
have disappeared [12].
Artificial regeneration: Whitebark pine can be grown in the nursery from seed [94], and there is considerable interest in outplanting nursery-grown, blister-rust resistant stock to replace dead and dying mature whitebark pine [119]. Transplanted whitebark pine has shown fair survivorship rates; further studies are needed to determine which habitat/aspect/elevational combinations are best for artificial regeneration. Whitebark pine appears to tolerate broad, possibly regional seed transfer. Transfer guidelines are available [135,217]. Planting seed may be a good restoration option, as natural dormancy of whitebark pine seed may help ensure germination under conditions favorable for establishment (see Regeneration Processes). On the Gallatin National Forest, seedlings established from hand-planted seed showed mean 1st-year survivorship rates of 73% in a drought year (1988) and 90% in a wet year (1989) [147]. Kendall [106] recommends developing a cold-stored genetic seed bank for whitebark pine, emphasizing that collections from small, isolated populations should be a priority. Authorities from the U.S. Forest Service Nursery in Coeur d'Alene, Idaho, [42] provide guidelines for collecting whitebark pine seed in the field, growing whitebark pine in the greenhouse, and transporting seedlings to planting sites.
Genetic considerations: Hoff and others [83,84] provide advice on managing whitebark pine in the field to promote genetic resistance for blister rust. Experts on whitebark pine genetics have raised the possibility of establishing "seed orchards" similar to those successfully used for western white pine, an important timber species that has also been decimated by blister rust. Seed orchard trees are started from seed collected from parent trees showing blister-rust resistance. The seedlings are planted in greenhouses for later planting on favorable wildland sites. Given ideal growth conditions, age of reproduction of parent trees can be greatly reduced. Some whitebark on moist sites on the Kootenai National Forest have produced female cones at 10 years of age (personal observation); trees in greenhouses may set seed as early as 7 years. Natural and artificial cross-breeding of symptomless whitebark parents will enhance genetic selection for blister-rust resistance and provide opportunities for restocking blister rust-decimated landscapes with orchard progeny, many of which will inherit mechanisms for blister-rust resistance [84].
Yanchuk [233,234] provides guidelines for prioritizing conifer species for genetic conservation programs in British Columbia. Selection is based on species protection status, conservation breeding programs already in place, and relative capacity for regeneration. As of this writing (2002), whitebark pine was rated the species in greatest need of genetic conservation management.
Due to its clumping habit, whitebark pine has an unusual genetic population structure (see Breeding system). Planting whitebark pine without regard for its natural habit of growing in clumped family groups may have long-term consequences on natural selection processes operating on whitebark pine [207].
OTHER USES:Wood Products: Mainly due to the species' inaccessibility, whitebark pine wood is not considered commercially valuable. Large-diameter whitebark pine in mixed stands were harvested in the past. Whitebark pine is classified as a soft-wood pine, and its wood has bending, compression, and shearing properties similar to eastern (P. strobus) and western white pine. Wood density is slightly higher than most white pines, and is similar to Douglas-fir [55,96].
Timber: Whitebark pine reforestation techniques are still in the trial stage. Whitebark pine sites are not recommended for timber production at this time [126,229].
Grazing: A short growing season, drought, and commonly shallow, rocky soils make whitebark pine habitats slow to recover from grazing [126,229]. Whitebark pine/grouse whortleberry habitats seem more tolerant of grazing than whitebark/bunchgrass types [194]. "Moderate overgrazing" on whitebark pine sites may increase lupines (Lupinus spp.), luina (Luina nardosima), and other unpalatable herbs at the expense of bunchgrasses. "Severe overgrazing" can create large, highly erosive patches of bare soil [229]. Willard [228] provides guidelines for assessing range condition in whitebark pine ecosystems, including plant species indicators and soil condition indicators.
Moderate grazing on subalpine mixed conifer-meadow ecotones may encourage invasion of whitebark pine and other conifers into meadows. Conifer invasion into meadows on the Wind River Mountains of Wyoming began about 1890, concurrent with cattle grazing. Tree invasion ceased in 1963, concurrent with cessation of cattle grazing. Dunwiddie [58] suggests that moderate cattle grazing favors whitebark pine and other conifers by reducing competition with meadow vegetation.
Wilderness: Half of whitebark pine's distribution lies in designated Wilderness Areas or Parks [97]. See Fire Management Considerations/Wilderness for further information.
Other values: As a long-lived species, whitebark pine tree-ring chronologies are a valuable source of long-term climate information. Perkins and Swetnam [169] have correlated patterns of tree growth and climate for more than 1,000 years from whitebark pine stands in the Sawtooth Mountains of Idaho.
Whitebark pine is valued for its beauty, and whitebark pine ecosystems are popular backcountry recreation sites [49,149,209].
Whitebark pine is planted worldwide as an ornamental [162].
Whitebark pine seeds are a traditional Native American food [216]. The easternmost population of whitebark pine, isolated in the Sweetwater Mountains of Montana, may have originally been planted by Native Americans as a food source [17].
OTHER MANAGEMENT CONSIDERATIONS:| Agent | Percent of snags |
| blister rust | 36 |
| successional suppression | 28 |
| bark beetles | 18 |
| mammals | 8 |
| weather | 6 |
| insects other than bark beetles | 2 |
| root disease | 2 |
| dwarf-mistletoe | 0 |
| fire | 0 |
Blister rust: White pine blister rust is an extremely serious threat to whitebark pine. Accidentally introduced from Europe, the fungal pathogen has infected white pine (Strobus) species in the United States and Canada. It has affected whitebark pine of all age classes throughout the species' range [28,192,192]. Whitebark pine's genetic resistance to the rust is low compared to other North American white pines: it has variously been ranked from most susceptible [28,31] to 4th most susceptible [80] to rust mortality in greenhouse trials with white pine seedlings. Resistance of mature whitebark pine in the field averages about 5%. Long-term mortality may be even greater than 95%, as some trees that have apparently resisted the rust for decades are now succumbing [215]. Whitebark pine infection rates are highest in the Pacific Northwest, where relatively cool, moist climate favors fungal growth and reproduction. A quarter to half of all whitebark pine in the region are dead [108], and some stands have lost nearly all the mature, cone-bearing trees [12,44]. A 1999 survey of whitebark pine stands in British Columbia showed a 17% mortality rate, 46% of which was directly attributable to blister rust. Thirty-two percent of live trees were infected, with 64% mortality of infected trees expected within a few years [236,237]. Keane and Arno [99] estimated a 2.1% average decrease in basal area of whitebark pine per year on infected sites in western Montana, resulting in a 65% mortality rate over 20 years. There is a distinct reduction in white pine blister rust infection to the south; however, populations in southern portions of whitebark pine's range are not immune to infection because substantial tree infection can occur in a single moist year. Infected trees may take from 2 years to decades to succumb, but infection is always fatal [82,83,99].
Gooseberries and currants (Ribes spp.) are the primary host of white pine blister rust. Life cycle of white pine blister rust is complex. Gitzendanner and others [73] and McDonald and Hoff [150] provide details of the rust's life history and ecology. Hoff [81] provides a diagnostic guide to aid managers in recognizing symptoms of blister rust infection in white pines.
There are no known methods of controlling blister rust [100]. Fungicide application, pruning infected tree branches, and/or removing Ribes spp. have neither eliminated nor controlled white pine blister rust [45,150], and such treatments have undesirable ecological effects [100].
Bark beetles: Mountain pine beetle is the most destructive insect pest of mature whitebark pine. Ponderosa pine (P. ponderosa) and lodgepole pine are often described as the primary hosts of mountain pine beetle; however, mountain pine beetle populations have thrived using whitebark pine as their primary host [167,170]. Endemic levels of mountain pine beetle are common in whitebark pine stands. Limited studies suggest that when mountain pine beetle numbers are low, beetles may concentrate their attacks on trees weakened by other stressors such as fungi or fire. However, little research has been conducted on effects of endemic mountain pine beetle populations on whitebark pine, and further research is needed [23]. Epidemic outbreaks cause high whitebark pine mortality [231]. Millions of acres of Rocky Mountain lodgepole pine-whitebark pine forests were destroyed in the 1901-1942 outbreaks in northern Idaho and western Montana [9,46,129,130]. Climate may trigger epidemics: mountain pine beetles are favored by warm, droughty summers and mild winters. Temperatures at low- and mid-elevations have historically been most favorable for mountain pine beetle broods [46,129,130].
Periodic mountain pine beetle outbreaks are a natural component of whitebark ecosystems [170]. Decaying whitebark pine "ghost forests" provide woody fuels that spread fire, and the openings created by fire provide favorable Clark's nutcracker caching sites [46,167]. It is the interactive effects of mountain pine beetle infestation, fire exclusion, and white pine blister rust that have devastated whitebark pine populations [46]. Warming of subalpine sites due to climate change may accelerate upward shifts of mountain pine beetle populations into whitebark pine habitats [129,130].
Because mountain pine beetle populations have historically been larger in lower-elevation forests, it has been assumed that lower-elevation forests serve as source points for attacks, with upper-elevation whitebark pine acting as "spill-over" sinks for mountain pine beetles [12,27,46,129]. However, experimental evidence is lacking. Even if once true, fugitive status of mountain pine beetle in whitebark pine appears to be changing. Six [190] found that in Rocky Mountain lodgepole pine and whitebark pine stands in western Montana, mountain pine beetle preferred trees with relatively thin phloem thickness, thin bark, and low sapwood moisture. Whitebark pine generally fulfills these requirements better than lodgepole pine. Larval survivorship and adult beetle emergence were significantly greater in whitebark pine compared to Rocky Mountain lodgepole pine of similar DBH. Previous studies have also found that whitebark pine is a better host for mountain pine beetle than lodgepole pine [7]. In Six's study, initial outbreaks occurred in whitebark, not lodgepole, pine stands. Mountain pine beetle larvae matured and emerged in 1 year, regardless if the host tree was whitebark or lodgepole pine. Six concludes that mountain pine beetles will attack high-elevation whitebark pine without a source population of mountain pine beetles in lower-elevation lodgepole pine [190].
Interactive effects of mountain pine beetles and white pine blister rust are unclear, and further research is needed. In a related study on sites in northern Idaho and western Montana, Six [190] found that whitebark pine attacked by mountain pine beetle had a significantly lower moisture content than trees that were not attacked. Further, blister rust-infected trees had an overall higher rate of mountain pine beetle attack compared to uninfected trees. However, mountain pine beetle choice was related to beetle population density: at low numbers, beetles preferred apparently healthy whitebark pine. As populations grew, beetles tended to select infected whitebark pine over healthy trees.
Secondary beetles, which usually cause mortality only in whitebark pine already stressed by other factors such as root rot fungi, include red turpentine beetles, Ips spp., and Pityogenes spp. [23].
Mistletoes: Whitebark pine is an alternate host for limber pine dwarf mistletoe (Arceuthobium cyanocarpum), which has caused heavy localized losses of whitebark pine. A survey on Mt. Shasta in the southern Cascade Range of California found a 96% infection rate in whitebark pine, with 58% mortality [140]. Larch dwarf mistletoe (A. laricis) and mountain hemlock dwarf mistletoe (A. tsugense ssp. mertensianae) sometimes infect whitebark pine [138,139].
Other pathogens: A needle blight (Dothistroma septospora) has caused serious localized disease in whitebark pine of the Crazy Mountains, south-central Montana [201]. Hoff and Hagle [82] provide a review of other pathogens affecting whitebark pine.
The Whitebark and Limber Pine Information System provides a database for storing and analyzing data on site characteristics, stand structure, regeneration, and mortality and infection rates from white pine blister rust and other damaging agents.
Climate effects: Modeling mostly predicts a decline in whitebark pine due to global increases in temperature and more frequent summer droughts [142,144,146]. Climate modeling for Yellowstone National Park predicts that independent of other agents of decline such as blister rust, whitebark pine is the most at-risk conifer in the Park due to drying conditions in high-elevation habitats [26]. However, impact of climate change on whitebark pine is inconclusive: Keane and others [103] predict expansion of whitebark pine in Glacier National Park due to more frequent fire return intervals resulting from global warming.
Old growth: Whitebark pine is rapidly losing its oldest age classes. Arno and others [106] found mean diameter of overstory whitebark pine on the Bitterroot National Forest was 7 inches (18 cm) in 1991. Most of the largest trees had died during a severe mountain pine beetle outbreak in the 1930s.
Nutcracker Notes provides details on whitebark pine management and ecology.1. Achuff, Peter L. 1989. Old-growth forests of the Canadian Rocky Mountain national parks. Natural Areas Journal. 9(1): 12-26. [7442]
2. Agee, James K. 1994. Fire and weather disturbances in terrestrial ecosystems of the eastern Cascades. In: Everett, Richard L.; Hessburg, Paul F., tech. eds. [Vol. 3: Assessment]. Gen. Tech. Rep. PNW-GTR-320. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 52 p. [23656]
3. Agee, James K. 1996. Fire in the Blue Mountains: A history, ecology, and research agenda. In: Jaindl, R. G.; Quigley, T. M., eds. Search for a solution: Sustaining the land, people and economy of the Blue Mountains. Washington, DC: American Forests: 119-145. [28827]
4. Agee, James K.; Kertis, Jane. 1987. Forest types of the North Cascades National Park Service Complex. Canadian Journal of Botany. 65: 1520-1530. [6327]
5. Agee, James K.; Stitt, Susan C. F.; Nyquist, Maurice; Root, Ralph. 1989. A geographic analysis of historical grizzly bear sightings in the North Cascades. Photogrammetric Engineering and Remote Sensing. 55(11): 1637-1642. [14672]
6. American Forests. 2002. Whitebark pine: Pinus albicaulis. In: National register of big trees, [Online]. Available: http://www.americanforests.org/resources/bigtrees/ [2002, April 29]. [40931]
7. Amman, Gene D. 1982. Characteristics of mountain pine beetles reared in four pine hosts. Environmental Entomology. 11(3): 590-593. [40258]
8. Anderton, Laurel K.; Jenkins, Michael J. 2001. Cone entomofauna of whitebark pine and alpine larch (Pinaceae): potential impact of Leptoglossus occidentalis (Hemiptera: Coreidae) and a new record of Strobilomyia macalpinei (Diptera: Anthomyiidae). The Canadian Entomologist. 133(3): 399-406. [40080]
9. Arno, Stephen F. 1970. Ecology of alpine larch (Larix lyallii Parl.) in the Pacific Northwest. Missoula, MT: University of Montana. 264 p. Dissertation. [16708]
10. Arno, Stephen F. 1976. The historical role of fire on the Bitterroot National Forest. Res. Pap. INT-187. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 29 p. [15225]
11. Arno, Stephen F. 1980. Forest fire history in the Northern Rockies. Journal of Forestry. 78(8): 460-465. [11990]
12. Arno, Stephen F. 1986. Whitebark pine cone crops--a diminishing source of wildlife food? Western Journal of Applied Forestry. 3: 92-94. [341]
13. Arno, Stephen F. 2000. Fire in western forest ecosystems. In: Brown, James K.; Smith, Jane Kapler, eds. Wildland fire in ecosystems: Effects of fire on flora. Gen. Tech. Rep. RMRS-GTR-42-vol. 2. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 97-120. [36984]
14. Arno, Stephen F. 2001. Community types and natural disturbance processes. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 74-88. [36694]
15. Arno, Stephen F.; Gruell, George E. 1983. Fire history at the forest-grassland ecotone in southwestern Montana. Journal of Range Management. 36(3): 332-336. [342]
16. Arno, Stephen F.; Habeck, James R. 1972. Ecology of alpine larch (Larix lyallii Parl.) in the Pacific Northwest. Ecological Monographs. 42: 417-450. [16451]
17. Arno, Stephen F.; Hoff, Raymond J. 1990. Pinus albicaulis Engelm. whitebark pine. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 1. Conifers. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 268-279. [13390]
18. Arno, Stephen F.; Reinhardt, Elizabeth D.; Scott, Joe H. 1993. Forest structure and landscape patterns in the subalpine lodgepole pine type: a procedure for quantifying past and present conditions. Gen. Tech. Rep. INT-294. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 17 p. [20711]
19. Arno, Stephen F.; Sneck, Kathy M. 1977. A method for determining fire history in coniferous forests of the Mountain West. INT-42. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 28 p. [18579]
20. Ash, Maria; Lasko, Richard J. 1990. Postfire vegetative response in a whitebark pine community, Bob Marshall Wilderness, Montana. In: Schmidt, Wyman C.; McDonald, Kathy J., comps. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen. Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 360-361. [11705]
21. Bailey, D. K. 1975. Pinus albicaulis. Curtis's Botanical Magazine. 180(3): 140-147; 1975. [376]
22. Baisan, Christopher H.; Swetnam, Thomas W. 1990. Fire history on a desert mountain range: Rincon Mountain Wilderness, Arizona, U.S.A. Canadian Journal of Forest Research. 20: 1559-1569. [14986]
23. Baker, Kristen M. 2000. Effects of whitebark pine (Pinus albicaulis) restoration treatments on the distribution of bark beetle attacks. Missoula, MT: The University of Montana. 57 p. Thesis. [37569]
24. Barmore, William J., Jr.; Taylor, Dale; Hayden, Peter. 1976. Ecological effects and biotic succession following the 1974 Waterfalls Canyon Fire in Grand Teton National Park. Research Progress Report 1974-1975. Unpublished report on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 99 p. [16109]
25. Barrett, Stephen W. 1994. Fire regimes on andesitic mountain terrain in northeastern Yellowstone National Park, Wyoming. International Journal of Wildland Fire. 4(2): 65-76. [23608]
26. Bartlein, Patrick J.; Whitlock, Cathy; Shafer, Sarah L. 1997. Future climate in the Yellowstone National Park region and its potential impact on vegetation. Conservation Biology. 11(3): 782-792. [40063]
27. Bartos, Dale L.; Gibson, Kenneth E. 1990. Insects of whitebark pine with emphasis on mountain pine beetle. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 171-178. [11532]
28. Benedict, W. V. 1973. White pine blister rust: Cronartium ribicola J. C. Fischer. In: Report 1180. Ottawa, Canada: Environment Canada, Forestry Service, Publications Department: 185-188. [22728]
29. Bernard, Stephen R.; Brown, Kenneth F. 1977. Distribution of mammals, reptiles, and amphibians by BLM physiographic regions and A.W. Kuchler's associations for the eleven western states. Tech. Note 301. Denver, CO: U.S. Department of the Interior, Bureau of Land Management. 169 p. [434]
30. Billings, W. D. 1951. Vegetational zonation in the Great Basin of western North America. Union of International Science: Biological Series B. 9: 101-122. [443]
31. Bingham, R. T. 1972. Taxonomy, crossability, and relative blister rust resistance of 5-needled white pines. In: In: Biology of rust resistance in forest trees: Proceedings of a NATO-IUFRO advanced study institute; 1969 August 17-24; Moscow, ID. Miscellaneous Publication No. 1221. Washington, DC: U.S. Department of Agriculture, Forest Service: 271-279. [41123]
32. Biswell, Harold H. 1967. The Sierra Nevada: range of light. The forests - a closely woven vesture. [Lecture series given at Sierra College, Rocklin]. Davis, CA: University of California, University Extension. 19 p. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [19073]
33. Blanchard, Bonnie M.; Knight, Richard R. 1991. Movements of Yellowstone grizzly bears. Biological Conservation. 58: 41-67. [22719]
34. Botti, Stephen. 1979. Natural, conditional, and prescribed fire management plan. Washington, DC: U.S. Department of the Interior, National Park Service, Yosemite National Park. 51 p. [20901]
35. Brown, James K. 1976. Predicting crown weights for 11 Rocky Mountain conifers. In: Oslo biomass studies: Proceedings of the 16th IUFRO congress [Working party on the mensuration of forest biomass]; 1976 June 22; Oslo, Norway. Orono, ME: University of Maine, College of Life Sciences and Agriculture: 103-115. [26460]
36. Brown, James K. 1978. Weight and density of crowns of Rocky Mountain conifers. Res. Pap. INT-197. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 56 p. [13188]
37. Brown, James K. 1991. Should management ignitions be used in Yellowstone National Park? In: Keiter, Robert B.; Boyce, Mark S, eds. The Greater Yellowstone Ecosystem: Redefining America's wilderness heritage. New Haven, CT: Yale University Press: 137-148. [16500]
38. Brown, James K.; Arno, Stephen F.; Barrett, Stephen W.; Menakis, James, P. 1994. Comparing the prescribed natural fire program with presettlement fires in the Selway-Bitterroot Wilderness. International Journal of Wildland Fire. 4(3): 157-168. [25485]
39. Bruederle, Leo P.; Roger, Deborah L.; Krutovskii, Konstantin V.; Politov, Dmitri V. 2001. Population genetics and evolutionary implications. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 137-157. [36701]
40. Bruederle, Leo P.; Tomback, Diana F.; Kelly, Kathleen K.; Hardwick, Renea C. 1998. Population genetic structure in a bird-dispersed pine, Pinus albicaulis (Pinaceae). Canadian Journal of Botany. 7: 83-90. [28591]
41. Burkhardt, Wayne J.; Tisdale, E. W. 1976. Causes of juniper invasion in southwestern Idaho. Ecology. 57(3): 472-484. [565]
42. Burr, Karen E.; Eramian, Aram; Eggleston, Kent. 2001. Growing whitebark pine seedlings for restoration. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 325-345. [36709]
43. Callaway, Ragan M. 1998. Competition and facilitation on elevational gradients in subalpine forests of the northern Rocky Mountains, USA. Oikos. 82(3): 561-573. [6447]
44. Campbell, Elizabeth M.; Antos, Joseph A. 2000. Distribution and severity of white pine blister rust and mountain pine beetle in British Columbia. Canadian Journal of Forest Research. 30(7): 1051-1059. [38112]
45. Carlson, Clinton E. 1978. Noneffectiveness of Ribes eradication as a control of white pine blister rust in Yellowstone National Park. Rep. No. 78-18. Missoula, MT: U.S. Department of Agriculture, Forest Service, Northern Region, State & Private Forestry, Forest Insect & Disease Management. 6 p. [22749]
46. Ciesla, William M.; Furniss, Malcolm M. 1975. Idaho's haunted forest. American Forests. 81(8): 32-35. [24231]
47. Clausen, Jens. 1965. Population studies of alpine and subalpine races of conifers and willows in the California high Sierra Nevada. Evolution. 9: 56-68. [28086]
48. Cole, David N. 1982. Vegetation of two drainages in Eagle Cap Wilderness, Wallowa Mountains, Oregon. Res. Pap. INT-288. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 42 p. [658]
49. Cole, David N. 1990. Recreation in whitebark pine ecosystems: demand, problems, and management strategies. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen. Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 305-309. [346]
50. Cooper, Stephen V.; Neiman, Kenneth E.; Steele, Robert; Roberts, David W. 1987. Forest habitat types of northern Idaho: a second approximation. Gen. Tech. Rep. INT-236. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 135 p. [867]
51. Critchfield, William B. 1986. Hybridization and classification of the white pines (Pinus section Strobus). Taxon. 35(4): 647-656. [7858]
52. Critchfield, William B.; Allenbaugh, Gordon L. 1969. The distribution of Pinaceae in and near northern Nevada. Madrono. 20(1): 12-25. [714]
53. Critchfield, William B.; Little, Elbert L., Jr. 1966. Geographic distribution of the pines of the world. Misc. Publ. 991. Washington, DC: U.S. Department of Agriculture, Forest Service. 97 p. [20314]
54. Daubenmire, Rexford F.; Daubenmire, Jean B. 1968. Forest vegetation of eastern Washington and northern Idaho. Tech. Bull. 60. Pullman, WA: Washington State University, Agricultural Experiment Station. 104 p. [749]
55. Day, R. J. 1967. Whitebark pine in the Rocky Mountains of Alberta. Forestry Chronicle. September: 278-283. [766]
56. del Moral, Roger; Fleming, Richard S. 1979. Structure of coniferous forest communities in western Washington: diversity and ecotype properties. Vegetatio. 41(3): 143-154. [7495]
57. Duffield, J. W. 1953. Pine pollen collection dates--annual and geographic variation. For. Res. Notes No. 85. Berkeley, CA: U.S. Department of Agriculture, Forest Service, California Forest and Range Experiment Station. 9 p. [17970]
58. Dunwiddie, Peter W. 1977. Recent tree invasion of subalpine meadows in the Wind River Mountains, Wyoming. Arctic and Alpine Research. 9(4): 393-399. [3293]
59. Ericson, John E. 1965. A suspected hybrid between Pinus albicaulis Engelm. and Pinus flexilis James. Proceedings of the Montana Academy of Sciences. (25): 58- 59. [871]
60. Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters. 148 p. [905]
61. Farnes, Phillip E. 1990. Snotel and snow course data: describing the hydrology of white bark pine ecosystems. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen. Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 302-304. [11699]
62. Fellin, David G. 1980. A review of some interactions between harvesting, residue management, fire, and forest insects and diseases. In: Environmental consequences of timber harvesting in Rocky Mountain coniferous forests: Symposium proceedings; 1979 September 11-13; Missoula, MT. Gen. Tech. Rep. INT-90. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 335-414. [10310]
63. Flanagan, Paul T.; Morgan, Penelope; Everett, Richard L. 1998. Snag recruitment in subalpine forests. Northwest Science. 72(4): 303-309. [30821]
64. Flora of North America Editorial Committee, eds. 2019. Flora of North America north of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
65. Forcella, Frank. 1978. Flora and chorology of the Pinus albicaulis--Vaccinium scoparium association. Madrono. 25: 139-150. [935]
66. Forcella, Frank; Weaver T. 1980. Food production in the Pinus albicaulis--Vaccinium scoparium association. Proceedings of the Montana Academy of Science. 39: 73-80. [936]
67. Franklin, Jerry F.; Dyrness, C. T. 1973. Natural vegetation of Oregon and Washington. Corvallis, OR: Oregon State University Press. 452 p. [961]
68. Franklin, Jerry F.; Mitchell, Russel G. 1967. Successional status of subalpine fir in the Cascade Range. Research Paper PNW-46. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 15 p. [963]
69. Franklin, Jerry F.; Moir, William H.; Hemstrom, Miles A.; Greene, Sarah E.; Smith, Bradley G. 1988. The forest communities of Mount Rainier National Park. Scientific Monograph Series No. 19. Washington, DC: U.S. Department of the Interior, National Park Service. 194 p. [12392]
70. Garfin, Gregg M. 1998. Relationships between winter atmospheric circulation patterns and extreme tree growth anomalies in the Sierra Nevada. International Journal of Climatology. 18(7): 725-740. [40071]
71. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; Lewis, Mont E.; Smith, Dixie R. 1977. Vegetation and environmental features of forest and range ecosystems. Agric. Handb. 475. Washington, DC: U.S. Department of Agriculture, Forest Service. 68 p. [998]
72. Geiszler, D. R.; Gara, R. I.; Littke, W. R. 1984. Bark beetle infestations of lodgepole pine following a fire in south central Oregon. Zeitschrift fur Angewandte Entomologie. 98(4): 389-394. [15046]
73. Gitzendanner, Matthew A.; White, Eleanor E.; Foord, Bret M.; Dupper, Gayle E.; Hodgskiss, Paul D.; Kinlock, Bohun B., Jr. 1996. Genetics of Cronartium ribicola. III. Mating system. Canadian Journal of Botany. 74(22): 1952-1859. [28084]
74. Gruell, G. E.; Loope, L. L. 1974. Relationships among aspen, fire, and ungulate browsing in Jackson Hole, Wyoming. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 33 p. In cooperation with: U.S. Department of the Interior, National Park Service, Rocky Mountain Region. [3862]
75. Hall, Frederick C. 1973. Plant communities of the Blue Mountains in eastern Oregon and southeastern Washington. R6 Area Guide 3-1. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 82 p. [1059]
76. Hansen-Bristow, Katherine,; Montagne, Cliffore; Schmid, Ginger. 1990. Geology, geomorphology, and soils within whitebark pine ecosystems. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 62-71. [11676]
77. Hartwell, Michael G.; Alaback, Paul; Arno, Stephen F. 2000. Comparing historic and modern forests on the Bitterroot Front. In: Smith, Helen Y., ed. The Bitterroot Ecosystem Management Research Project: What we have learned: Symposium proceedings; 1999 May 18-20; Missoula, MT. Proceedings RMRS-P-17. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 11-18. [37129]
78. Hessberg, P. S.; Mitchell, R. G.; Filip, G. M. 1994. Historic and current roles of insects and pathogens in eastern Oregon and Washington forested lands. Gen. Tech. Rep. PNW-GTR-327. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. [Number of pages unknown] [41610]
79. Hickman, James C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p. [21992]
80. Hoff, R.; Bingham, R. T.; McDonald, G. I. 1980. Relative blister rust resistance of white pines. European Journal of Forest Pathology. 10(5): 307-316. [1177]
81. Hoff, Ray J. 1992. How to recognize blister rust infection on whitebark pine. Res. Note INT-406. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 7 p. [19509]
82. Hoff, Ray; Hagle, Susan. 1990. Diseases of whitebark pine with special emphasis on white pine blister rust. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 179-190. [5936]
83. Hoff, Raymand K.; Hagle, Susan K.; Krebill, Richard G. 1994. Genetic consequences and research challenges of blister rust in whitebark pine forests. In: Schmidt, Wyman C.; Holtmeier, Fredrich-Karl, compilers. Proceedings--international workshop on subalpine stone pines and their environment: the status of our knowledge; 1992 September 5-11; St. Moritz, Switzerland. Gen. Tech. Rep. INT-GRT-309. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 118-126. [23769]
84. Hoff, Raymond J.; Ferguson, Dennis E.; McDonald, Geral I.; Keane, Robert E. 2001. Strategies for managing whitebark pine in the presence of white pine blister rust. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 346-366. [36710]
85. Hofmann, J. V. 1917. Natural reproduction from seed stored in the forest floor. Journal of Agricultural Research. 11(1): 1-26. [12446]
86. Hogg, Edward H. 1993. An arctic-alpine flora at low elevation in Marble Canyon, Kootenay National Park, British Columbia. Canadian Field-Naturalist. 107: 282-292. [24145]
87. Holland, Robert F. 1986. Preliminary descriptions of the terrestrial natural communities of California. Sacramento, CA: California Department of Fish and Game. 156 p. [12756]
88. Hungerford, R. D.; Nemani, R. R.; Running, S. W.; Couglan, J. C. 1989. MTCLIM: a mountain microclimate simulation model. Res. Pap. INT-414. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 52 p. [41611]
89. Hutchins, H. E.; Lanner, R. M. 1982. The central role of Clark's nutcracker in the dispersal and establishment of whitebark pine. Oecologia. 55: 192-201. [1228]
90. Hutchins, Harry E. 1994. Role of various animals in dispersal and establishment of whitebark pine in the Rocky Mountains, U.S.A. In: Schmidt, Wyman C.; Holtmeier, Friedrich-Karl, compilers. Proceedings--international workshop on subalpine stone pines and their environment: the status of our knowledge; 1992 September 5-11; St. Moritz, Switzerland. Gen. Tech. Rep. INT-GTR-309. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 163-171. [24638]
91. International Union for Conservation of Nature. 2016. IUCN red list of threatened species, [Online]. Version 2010.1. International Union for Conservation of Nature (Producer). Available: http://www.iucnredlist.org [2016, August 4]. [48717]
92. Irwin, Larry L.; Hammond, Forrest M. 1985. Managing black bear habitats for food items in Wyoming. Wildlife Society Bulletin. 13: 477-483. [3034]
93. Jackson, M. T.; Faller, Adolph. 1973. Structural analysis and dynamics of the plant communities of Wizard Island, Crater Lake National Park. Ecological Monographs. 43: 441-461. [40060]
94. James, R. L.; Burr, K. E. 2000. Diseases associated with whitebark pine seedling production: USDA Forest Service Nursery, Coeur D'Alene, Idaho. Forest Health Protection Report 00-8. Missoula, MT: U.S. Department of Agriculture, Forest Service, Northern Region. 11 p. [40083]
95. Kartesz, John T. 1999. A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. 1st ed. In: Kartesz, John T.; Meacham, Christopher A. Synthesis of the North American flora (Windows Version 1.0), [CD-ROM]. Chapel Hill, NC: North Carolina Botanical Garden (Producer). In cooperation with: The Nature Conservancy; U.S. Department of Agriculture, Natural Resources Conservation Service; U.S. Department of the Interior, Fish and Wildlife Service. [36715]
96. Kasper, J. B.; Szabo, T. 1970. The physical and mechanical properties of whitebark pine. Forestry Chronicle. August: 315-316. [1310]
97. Keane, Robert E. 2001. Can the fire-dependent whitebark pine be saved? Fire Management Today. 61(3): 17-20. [40079]
98. Keane, Robert E. 2001. Successional dynamics: modeling an anthropogenic threat. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 159-192. [36702]
99. Keane, Robert E.; Arno, Stephen F. 1993. Rapid decline of whitebark pine in western Montana: evidence from 20-year remeasurements. Western Journal of Applied Forestry. 8(2): 44-47. [20850]
100. Keane, Robert E.; Arno, Stephen F. 2001. Restoration concepts and techniques. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 367-400. [36711]
101. Keane, Robert E.; Arno, Stephen F.; Brown, James K.; Tomback, Diana F. 1990. Modelling stand dynamics in whitebark pine (Pinus albicaulis) forests. Ecological Modelling. 51: 73-95. [11837]
102. Keane, Robert E.; Morgan, Penelope; Running, Steven W. 1996. FIRE-BGC -- a mechanistic ecological process model for simulating fire succession on coniferous forest landscapes of the Northern Rocky Mountains. Res. Pap. INT-RP-484. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 122 p. [26890]
103. Keane, Robert E.; Ryan, Kevin C.; Finney, Mark A. 1998. Simulating the consequences of fire and climate regimes on a complex landscape in Glacier National Park. In: Pruden, Teresa L.; Brennan, Leonard A., eds. Fire in ecosystem management: shifting the paradigm from suppression to prescription: Proceedings, Tall Timbers fire ecology conference; 1996 May 7-10; Boise, ID. No. 20. Tallahassee, FL: Tall Timbers Research Station: 310-324. [35651]
104. Kegley, Sandra; Sturdevant, Nancy; Stein, Jack; Willhite, Beth; Flanagan, Paul; Weatherby, Julie; Marsden, Michael. 2001. Cone and seed insects and their impact on whitebark pine. Forest Health Protection Report 01-6. Missoula, MT: U.S. Department of Agriculture, Forest Service, Northern Region. 13 p. [40084]
105. Kendall, Katherine C. 1983. Use of pine nuts by grizzly and black bears in the Yellowstone area. In: Meslow, E. Charles, ed. Bears--their biology and management: Proceedings, 5th international conference on bear research and management; 1980 February; Madison, WI. [Place of publication unknown]: International Association for Bear Research and Management: 166-173. [8385]
106. Kendall, Katherine C. 1994. Whitebark pine conservation in North American national parks. In: Schmidt, Wyman C.; Holtmeier, Friedrich-Karl, compilers. Proceedings--international workshop on subalpine stone pines and their environment: the status of our knowledge; 1992 September 5-11; St. Moritz, Switzerland. Gen. Tech. Rep. INT-GTR-309. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 302-307. [24658]
107. Kendall, Katherine C.; Arno, Stephen F. 1990. Whitebark pine--an important but endangered wildlife resource. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen. Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 264-273. [11694]
108. Kendall, Katherine C.; Keane, Robert E. 2001. Whitebark pine decline: infection, mortality, and population trends. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 221-242. [36704]
109. Kilgore, Bruce M.; Heinselman, Miron L. 1990. Fire in wilderness ecosystems. In: Hendee, John C.; Stankey, George H.; Lucas, Robert C., eds. Wilderness management. 2d ed. (Revised). Golden, CO: North American Press: 297-335. In cooperation with U.S. Department of Agriculture, Forest Service. [41423]
110. Klikoff, Lionel G. 1965. Microenvironmental influence on vegetational pattern near timberline in the central Sierra Nevada. Ecological Monographs. 35(2): 187-211. [40072]
111. Knight, Richard R; Blanchard, Bonnie M. 1983. Yellowstone grizzly bear investigations: Annual report of the Interagency Study Team--1982. Washington, DC: U.S. Department of the Interior, National Park Service. 45 p. [20703]
112. Kuchler, A. W. 1964. United States: Map, [Potential natural vegetation of the conterminous United States]. Special Publication No. 36. New York: American Geographical Society. 1:3,168,000; colored. [3455]
113. Kufeld, Roland C.; Wallmo, O. C.; Feddema, Charles. 1973. Foods of the Rocky Mountain mule deer. Res. Pap. RM-111. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 31 p. [1387]
114. Lackschewitz, Klaus. 1991. Vascular plants of west-central Montana--identification guidebook. Gen. Tech. Rep. INT-227. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 648 p. [13798]
115. Lanner, Ronald M. 1980. Avian seed dispersal as a factor in the ecology and evolution of limber and whitebark pines. In: Dancik, Bruce; Higginbotham, Kenneth, eds. Proceedings, 6th North American forest biology workshop; 1980 August 11-13; Edmonton, AB. Edmonton, AB: University of Alberta: 15-48. [1404]
116. Lanner, Ronald M. 1982. Adaptations of whitebark pine for seed dispersal by Clark's nutcracker. Canadian Journal of Forest Research. 12: 391-402. [1403]
117. Lanner, Ronald M. 1983. Trees of the Great Basin: A natural history. Reno, NV: University of Nevada Press. 215 p. [1401]
118. Lanner, Ronald M. 1990. Biology, taxonomy, evolution, and geography of stone pines of the world. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 14-24. [11672]
119. Lanner, Ronald M. 1993. Is it doomsday for whitebark pine? Western Journal of Applied Forestry. 8(2): 47, 70. [20851]
120. Lanner, Ronald M. 1996. Made for each other: a symbiosis of birds and pines. New York: Oxford University Press. 160 p. [29914]
121. Lanner, Ronald M.; Gilbert, Barrie K. 1994. Nutritive value of whitebark pine seeds and the question of their variable dormancy. In: Schmidt, Wyman C.; Holtmeier, Fredrich-Karl, compilers. Proceedings--international workshop on subalpine stone pines and their environment: the status of our knowledge; 1992 September 5-11; St. Mortiz, Switzerland. Gen. Tech. Rep. INT-GRT-309. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 206-211. [23809]
122. Larsen, J. A. 1930. Forest types of the northern Rocky Mountains and their climatic controls. Ecology. 11(4): 631-672. [12449]
123. Laven, R. D.; Omi, P. N.; Wyant, J. G.; Pinkerton, A. S. 1980. Interpretation of fire scar data from a ponderosa pine ecosystem in the central Rocky Mountains, Colorado. In: Stokes, Marvin A.; Dieterich, John H., tech. coords. Proceedings of the fire history workshop; 1980 October 20-24; Tucson, AZ. Gen. Tech. Rep. RM-81. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 46-49. [7183]
124. Leadem, Carole L. 1986. Seed dormancy in three Pinus species of the Inland Mountain West. In: Shearer, Raymond D., compiler. Proceedings--conifer tree seed in the Inland West symposium; 1985 August 5-6; Missoula, MT. Gen. Tech. Rep. INT-203. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 117-195. [1429]
125. Lewis, Mont E. 1971. Flora and major plant communities of the Ruby-East Humboldt Mountains with special emphasis on Lamoille Canyon. Elko, NV: U.S. Department of Agriculture, Forest Service, Region 4, Humboldt National Forest. 62 p. [1450]
126. Lillybridge, Terry R.; Kovalchik, Bernard L.; Williams, Clinton K.; Smith, Bradley G. 1995. Field guide for forested plant associations of the Wenatchee National Forest. Gen. Tech. Rep. PNW-GTR-359. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 335 p. In cooperation with: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region, Wenatchee National Forest. [29851]
127. Linhart, Yan B.; Tomback, Diana F. 1985. Seed dispersal by nutcrackers causes multi-trunk growth form in pines. Oecologia. 67: 07-110. [1459]
128. Little, Elbert L., Jr. 1971. Atlas of United States trees. Volume 1. Conifers and important hardwoods. Misc. Publ. 1146. Washington, DC: U.S. Department of Agriculture, Forest Service. 320 p. [1462]
129. Logan, J. A.; Bolstad, P. V.; Bentz, B. J.; Perkins, D. L. 1995. Assessing the effects of changing climate on mountain pine beetle dynamics. In: Tinus, Richard W., tech. ed. Interior West global change workshop: Proceedings; 1995 April 25-27; Fort Collins, CO. Gen. Tech. Rep. RM-GTR-262. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 92-105. [26007]
130. Logan, Jesse A.; Powell, James A. 2001. Ghost forests, global warming, and the mountain pine beetle (Coleoptera: Scolytidae). American Entomologist. 47(3): 160-173. [40343]
131. Lonner, Terry N.; Pac, David F. 1990. Elk and mule deer use of whitebark pine forests in southwest Montana: an ecological perspective. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen. Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 237-244. [11691]
132. Loope, Lloyd Lee. 1970. Subalpine and alpine vegetation of northeastern Nevada. Durham, NC: Duke University. 292 p. Dissertation. [41432]
133. Luckman, B. H.; Jozsa, L. A.; Murphy, P. J. 1984. Living seven-hundred-year-old Picea engelmannii and Pinus albicaulis in the Canadian Rockies. Arctic and Alpine Research. 16(4): 419-422. [1481]
134. Lueck, Dennis. 1980. Ecology of Pinus albicaulis on Bachelor Butte, Oregon. Corvallis, OR: Oregon State University. 90 p. Thesis. [28477]
135. Mahalovich, M. F.; Hoff, R. J. 2000. Whitebark pine operational cone collection instructions and seed transfer guidelines. Nutcracker Notes, [Online]. Number 11 (2000, January 1). Available: http://www.nrmsc.usgs.gov/nutnotes/number11.htm [2002, July 16]. [41425]
136. Major, Jack; Taylor, Dean W. 1977. Alpine. In: Barbour, Michael G.; Malor, Jack, eds. Terrestrial vegetation of California. New York: John Wiley and Sons: 601-675. [7213]
137. Marshall, William H. 1946. Cover preferences, seasonal movements, and food habits of Richardson's grouse and ruffed grouse in southern Idaho. The Wilson Bulletin. 58(1): 42-52. [16204]
138. Mathiasen, Robert L. 1994. Natural infection of new hosts by hemlock dwarf mistletoes. Research Note RM-RN-530. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 6 p. [26680]
139. Mathiasen, Robert L. 1998. Comparative susceptibility of conifers to larch dwarf mistletoe in the Pacific Northwest. Forest Science. 44(4): 559-568. [40076]
140. Mathiasen, Robert L.; Hawksworth, Frank G. 1988. Dwarf mistletoes on western white pine and whitebark pine in northern California and southern California. Forest Science. 34(2): 429-440. [5034]
141. Mattson, D. J.; Reinhart, D. P. 1997. Excavation of red squirrel middens by grizzly bears in the whitebark pine zone. Journal of Applied Ecology. 34(4): 926-940. [40061]
142. Mattson, David J.; Kendall, Katherine C.; Reinhart, Daniel P. 2001. Whitebark pine, grizzly bears, and red squirrels. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 121-136. [36700]
143. Mattson, David J.; Reinhart, Daniel P. 1990. Whitebark pine on the Mount Washburn massif, Yellowstone National Park. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen. Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 106-117. [11618]
144. McCaughey, Ward W. 1994. The regeneration process of whitebark pine. In: Schmidt, Wyman C.; Holtmeier, Friedrich-Karl, compilers. Proceedings--international workshop on subalpine stone pines and their environment: the status of our knowledge; 1992 September 5-11; St. Moritz, Switzerland. Gen. Tech. Rep. INT-GTR-309. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 179-187. [24640]
145. McCaughey, Ward W.; Schmidt, Wyman C. 1990. Autecology of whitebark pine. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 85-96. [11679]
146. McCaughey, Ward W.; Tomback, Diana F. 2001. The natural regeneration process. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 105-120. [36699]
147. McCaughey, Ward Wells. 1990. Biotic and microsite factors affecting Pinus albicaulis establishment and survival. Bozeman, MT: Montana State University. 78 p. Dissertation. [40073]
148. McClelland, B. Riley. 1979. Cavity nesters: part of Montana's bird heritage. Montana Outdoors. 10(4): 34-37. [15176]
149. McCool, Stephen F.; Freimund, Wayne A. 2001. Threatened landscapes and fragile experiences: conflict in whitebark pine restoration. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 263-287. [36706]
150. McDonald, Geral I.; Hoff, Raymond J. 2001. Blister rust: an introduced plague. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 193-220. [36703]
151. McKenzie, Donald; Peterson, David L.; Agee, James K. 2000. Fire frequency in the interior Columbia River basin: building regional models from fire history data. Ecological Applications. 10(5): 1497-1516. [38748]
152. Mealey, Stephen P.; Jonkel, Charles J.; Demarchi, Ray. 1977. Habitat criteria for grizzly bear management. In: Peterie, T., ed. Proceedings, 13th international congress of game biologists; 1977 March 11-15; Atlanta, GA. No. 13. [Place of publication unknown]: [Publisher unknown]: 276-289. On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [17030]
153. Meinecke, E. P. 1929. Quaking aspen: A study in applied forest pathology. Tech. Bull. No. 155. Washington, DC: U.S. Department of Agriculture. 34 p. [26669]
154. Miller, Richard F.; Rose, Jeffery A. 1995. Historic expansion of Juniperus occidentalis (western juniper) in southeastern Oregon. The Great Basin Naturalist. 55(1): 37-45. [25666]
155. Moeur, Melinda. 1981. Crown width and foliage weight of northern Rocky Mountain conifers. Res. Pap. INT-283. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 14 p. [13244]
156. Mohr, Jerry A.; Whitlock, Cathy; Skinner, Carl N. 2000. Postglacial vegetation and fire history, eastern Klamath Mountains, California. The Holocene. 10(4): 587-601. [38493]
157. Morgan, Penelope; Bunting, Stephen C.; Keane, Robert E.; Arno, Stephen F. 1994. Fire ecology of whitebark pine forests of the Northern Rocky Mountains, U.S.A. In: Schmidt, Wyman C.; Holtmeier, Friedrich-Karl, compilers. Proceedings--international workshop of subalpine stone pines and their environment: the status of our knowledge; 1992 September 5-11; St. Moritz, Switzerland. Gen. Tech. Rep. INT-GRT-309. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 136-141. [23768]
158. Morgan, Penelope; Murray, Michael P. 2001. Landscape ecology and isolation: implications for conservation of whitebark pine. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 289-309. [36707]
159. Morgan, Penny; Bunting, Stephen C. 1990. Fire effects in whitebark pine forests. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 166-170. [165]
160. Morrison, Peter H.; Swanson, Frederick J. 1990. Fire history and pattern in a Cascade Range landscape. Gen. Tech. Rep. PNW-GTR-254. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 77 p. [13074]
161. Moseley, Robert K.; Bernatas, Susan. 1992. Vascular flora of Kane Lake cirque, Pioneer Mountains, Idaho. The Great Basin Naturalist. 52(4): 335-343. [20212]
162. Muir, Nigel. 1977. White-bark pine. GC&HT. 182(15): 33. [22931]
163. Murray, Michael P.; Bunting, Stephen C.; Morgan, Penelope. 1995. Whitebark pine and fire suppression in small wilderness areas. In: Brown, James K.; Mutch, Robert W.; Spoon, Charles W.; Wakimoto, Ronald H., tech. coords. Proceedings: symposium on fire in wilderness and park management; 1993 March 30-April 1; Missoula, MT. Gen. Tech. Rep. INT-GTR-320. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 237-240. [26224]
164. Murray, Michael P.; Bunting, Stephen C.; Morgan, Penny. 1998. Fire history of an isolated subalpine mountain range of the Intermountain Region, United States. Journal of Biogeography. 25(6): 1071-1080. [30130]
165. Murray, Michael P.; Bunting, Stephen C.; Morgan, Penny. 2000. Landscape trends (1753-1993) of whitebark pine (Pinus albicaulis) forests in the West Big Hole Range of Idaho/Montana, U.S.A. Arctic, Antarctic, and Alpine Research. 32(4): 412-418. [38643]
166. Norment, Christopher J.; Conner, Charles R. 1991. Bird use of forest patches in the subalpine forest-alpine tundra ecotone of the Beartooth Mountains, Wyoming. Northwest Science. 65(1): 1-9. [13556]
167. Parker, Douglas L.; Stipe, Lawrence E. 1993. A sequence of destruction: mountain pine beetle and wildfire: Yellowstone National Park. Albuquerque, NM: U.S. Department of Agriculture, Forest Service, Southwestern Region. 19 p. [25737]
168. Payne, Gene F. 1973. Vegetative rangeland types in Montana. Bull. 671. Bozeman, MT: Montana State University, Montana Agricultural Experiment Station. 15 p. [1847]
169. Perkins, Dana L.; Swetnam, Thomas W. 1996. A dendroecological assessment of whitebark pine in the Sawtooth--Salmon River region, Idaho. Canadian Journal of Forest Research. 26: 2123-2133. [27299]
170. Perkins, Dana Lee. 1995. A dendroecological assessment of whitebark pine in the Sawtooth Salmon River region Idaho. Tucson, AZ: The University of Arizona. 56 p. Thesis. [26771]
171. Pfister, Robert D.; Kovalchik, Bernard L.; Arno, Stephen F.; Presby, Richard C. 1977. Forest habitat types of Montana. Gen. Tech. Rep. INT-34. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 174 p. [1878]
172. Pinder, J. E., III; Kroh, G. C.; White, J. D.; May, A. M. Basham. 1997. The relationships between vegetation type and topography in Lassen Volcanic National Park. Plant Ecology. 131(1): 17-29. [40062]
173. Pinol, J.; Sala, A. 2000. Ecological implications of xylem cavitation for several Pinaceae in the Pacific northern USA. Functional Ecology. 14(5): 538-545. [40064]
174. Primack, Richard B. 1998. What is biological diversity? In: Primack, Richard B. Essentials of conservation biology. 2d ed. Sutherland, MA: Sinauer Associates: 23-53. [41346]
175. Rasmussen, Lynn A.; Amman, Gene D.; Vandygriff, James C.; Oakes, Robert D.; Munson, A. Steven. 1996. Bark beetle and wood borer infestation in the Greater Yellowstone Area during four postfire years. Res. Pap. INT-RP-487. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 10 p. [26586]
176. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford, England: Clarendon Press. 632 p. [2843]
177. Reid, Elbert H.; Strickler, Gerald S.; Hall, Wade B. 1980. Green fescue grassland: 40 years of secondary succession. Res. Pap. PNW-274. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 39 p. [11394]
178. Renkin, Roy A.; Despain, Don G. 1992. Fuel moisture, forest type, and lightning-caused fire in Yellowstone National Park. Canadian Journal of Forest Research. 22: 37-45. [17974]
179. Riegel, Gregg M.; Thornburgh, Dale A.; Sawyer, John O. 1990. Forest habitat types of the South Warner Mountains, Modoc County, California. Madrono. 37(2): 88-112. [11466]
180. Ripple, William J. 1994. Historic spatial patterns of old forests in western Oregon. Journal of Forestry. 92(11): 45-49. [33881]
181. Romme, William H. 1982. Fire and landscape diversity in subalpine forests of Yellowstone National Park. Ecological Monographs. 52(2): 199-221. [9696]
182. Rundel, Philip W.; Parsons, David J.; Gordon, Donald T. 1977. Montane and subalpine vegetation of the Sierra Nevada and Cascade Ranges. In: Barbour, Michael G.; Major, Jack, eds. Terrestrial vegetation of California. New York: John Wiley & Sons: 559-599. [4235]
183. Sala, Anna; Carey, Eileen V.; Keane, Robert E.; Callaway, Ragan M. 2001. Water use by whitebark pine and subalpine fir: potential consequences of fire exclusion in the northern Rocky Mountains. Tree Physiology. 21(11): 717-725. [40067]
184. Sawyer, John O.; Thornburgh, Dale A. 1977. Montane and subalpine vegetation of the Klamath Mountains. In: Barbour, Michael G.; Major, Jack, eds. Terrestrial vegetation of California. New York: John Wiley & Sons: 699-732. [685]
185. Schlatterer, Edward F. 1972. A preliminary description of plant communities found on the Sawtooth, White Cloud, Boulder and Pioneer Mountains. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Region. Unpublished paper on file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 111 p. [2076]
186. Schwandt, John. 2000. Whitebark pine on the Idaho Panhandle: excerpts from a planning meeting. Nutcracker Notes, [Online]. Number 11 (2000, January 1). Available: http://www.nrmsc.usgs.gov/nutnotes/number11.htm [2002, July 16]. [41426]
187. Scott, J. Michael; Murray, M.; Wright, R. G.; Csuti, B.; Morgan, P.; Pressey, R. L. 2001. Representation of natural vegetation in protected areas: capturing the geographic range. Biodiversity and Conservation. 10: 1297-1301. [40075]
188. Selby, Corinne J.; Pitt, Michael D. 1984. Classification and distribution of alpine and subalpine vegetation in the Chilcotin Mountains of southern British Columbia. Syesis. 17: 13-41. [41429]
189. Shiflet, Thomas N., ed. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p. [23362]
190. Six, Diana L. 2002. Interactions between mountain pine beetle and white pine blister rust in whitebark pine. [Fire Lab seminar presented March 14, 2002]. Summary on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; RWU 4403 files. [40935]
191. Skovlin, Jon M.; Strickler, Gerald S.; Peterson, Jesse L.; Sampson, Arthur W. 2001. Interpreting landscape change in high mountains of northeastern Oregon from long-term repeat photography. Gen. Tech. Rep. PNW-GTR-505. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 78 p. [40256]
192. Smith, J. P.; Hoffman, J. T.; Sullivan, K. F.; Van Arsdel, E. P.; Vogler, D. 2000. First report of white pine blister rust in Nevada. Plant Disease. 84(5): 594. [38915]
193. Snell, J. A. Kendall; Brown, James K. 1980. Handbook for predicting residue weights of Pacific Northwest conifers. Gen. Tech. Rep. PNW-103. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 44 p. [20464]
194. Steele, Robert; Cooper, Stephen V.; Ondov, David M.; Roberts, David W.; Pfister, Robert D. 1983. Forest habitat types of eastern Idaho-western Wyoming. Gen. Tech. Rep. INT-144. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 122 p. [2230]
195. Steele, Robert; Pfister, Robert D.; Ryker, Russell A.; Kittams, Jay A. 1981. Forest habitat types of central Idaho. Gen. Tech. Rep. INT-114. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 138 p. [2231]
196. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 10 p. [20090]
197. Storer, Tracy I.; Usinger, Robert L. 1963. Sierra Nevada natural history: An illustrated handbook. Berkeley, CA: University of California Press. 374 p. [41612]
198. Stuart-Smith, Geoffrey Jones. 1998. Conservation of whitebark pine in the Canadian Rockies: blister rust and population genetics. Edmonton, AB: University of Alberta. 125 p. Thesis. [41434]
199. Sund, Sharren Kay. 1988. Post-fire regeneration of Pinus albicaulis in western Montana: patterns of occurrence and site characteristics. Boulder, CO: University of Colorado. 63 p. Thesis. [25933]
200. Swedberg, Kenneth Charles. 1961. The coniferous ecotone of the east slope of the northern Oregon Cascades. Corvallis, OR: Oregon State College. 118 p. Dissertation. [40934]
201. Taylor, J. E.; Walla, J. A. 1999. First report of Dothistroma septospora on native limber and whitebark pine in Montana. Plant Disease. 83(6): 590. [39470]
202. Thorne, Robert F. 1976. The vascular plant communities of California. In: Latting, June, ed. Symposium proceedings: Plant communities of southern California; 1974 May 4; Fullerton, CA. Special Publication No. 2. Berkeley, CA: California Native Plant Society: 1-31. [3289]
203. Tomback, Diana F. 1978. Foraging strategies of Clark's nutcracker. The Living Bird. 16: 123-161. [2349]
204. Tomback, Diana F. 1982. Dispersal of whitebark pine seeds by Clark's nutcracker: a mutualism hypothesis. Journal of Animal Ecology. 51: 451-467. [2346]
205. Tomback, Diana F. 1986. Post-fire regeneration of krummholz whitebark pine: A consequence of nutcracker seed caching. Madrono. 33(2): 100-110. [2347]
206. Tomback, Diana F. 1989. The broken circle: fire, birds and whitebark pine. In: Walsh, Tom, compiler. Wilderness and wildfire: Proceedings; 1989 June; Missoula, MT. Misc. Pub. No. 50. Missoula, MT: University of Montana, School of Forestry, Wilderness Institute; Montana Forest and Range Experiment Station: 14-17. [8201]
207. Tomback, Diana F. 2001. Clark's nutcracker: Agent of regeneration. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 88-104. [36698]
208. Tomback, Diana F.; Anderies, Angela J.; Carsey, Katherine S.; Powell, Mary L.; Mellmann-Brown, Sabine. 2001. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology. 82(9): 2587-2600. [39446]
209. Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E. 2001. The compelling case for management intervention. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 3-25. [36691]
210. Tomback, Diana F.; Clary, Jane Kees; Koehler, James; Hoff, Raymond J.; Arno, Stephen F. 1995. The effects of blister rust on post-fire regeneration of whitebark pine: the Sundance Burn of northern Idaho (U.S.A.). Conservation Biology. 9(3): 654-664. [26002]
211. Tomback, Diana F.; Hoffmann, Lyn A.; Sund, Sharren K. 1990. Coevolution of whitebark pine and nutcrackers: implications for forest regeneration. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 118-129. [11682]
212. Tomback, Diana F.; Kendall, Katherine C. 2001. Biodiversity losses: the downward spiral. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 243-262. [36705]
213. Tomback, Diana F.; Schuster, William S. 1994. Genetic population structure and growth form distribution in bird-dispersed pines. In: Schmidt, Wyman C.; Holtmeier, Friedrich-Karl, compilers. Proceedings--international workshop on subalpine stone pines and their environments: the status of our knowledge; 1992 September 5-11; St. Mortiz, Switzerland. Gen. Tech. Rep. INT-GRT-309. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 43-50. [23807]
214. Tomback, Diana F.; Sund, Sharren K.; Hoffmann, Lyn A. 1993. Post-fire regeneration of Pinus albicaulis: height-age relationships, age structure, and microsite characteristics. Canadian Journal of Forest Research. 23: 113-119. [20856]
215. Tomback, Diana. 2002. [Personal communication]. January 28. Regarding Clark's nutcrackers and seed caching. Boulder, CO: University of Colorado. In: FEIS log book. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [41614]
216. Turner, Nancy J. 1988. Ethnobotany of coniferous trees in Thompson and Lillooet Interior Salish of British Columbia. Economic Botany. 42(2): 177-194. [4542]
217. U.S. Department of Agriculture, Regions 1, 2 and 4, Forest Service. 2001. Skills for tree improvement personnel: Workshop proceedings; 2001 March 6-8; Coeur d'Alene, ID. Moscow, ID: U.S. Department of Agriculture, Forest Service. [Variously paginated]. [41125]
218. USDA, NRCS. 2019. The PLANTS Database, [Online]. U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team, Greensboro, NC (Producer). Available: https://plants.usda.gov/. [34262]
219. USDI, Department of Interior, Fish and Wildlife Service. 2019. Endangered Species Program, [Online]. U.S. Department of the Interior, Fish and Wildlife Service (Producer). Available: https://www.fws.gov/endangered/ [2019, June 20]. [86564]
220. van Wagtendonk, Jan W.; Benedict, James M.; Sydoriak, Walter M. 1996. Physical properties of woody fuel particles of Sierra Nevada conifers. International Journal of Wildland Fire. 6(3): 117-123. [29412]
221. van Wagtendonk, Jan W.; Benedict, James M.; Sydoriak, Walter M. 1998. Fuel bed characteristics of Sierra Nevada conifers. Western Journal of Applied Forestry. 13(3): 73-84. [28859]
222. van Wagtendonk, Jan W.; Sydoriak, Walter M.; Benedict, James M. 1998. Heat content variation of Sierra Nevada conifers. International Journal of Wildland Fire. 8(3): 147-158. [29410]
223. Vander Wall, Stephen B.; Balda, Russell P. 1977. Coadaptations of the Clark's nutcracker and the pinon pine for efficient seed harvest and dispersal. Ecological Monographs. 47: 89-111. [2424]
224. Vincent, Dwain W. 1992. The sagebrush/grasslands of the upper Rio Puerco area, New Mexico. Rangelands. 14(5): 268-271. [19698]
225. Vogl, Richard J.; Ryder, Calvin. 1969. Effects of slash burning on conifer reproduction in Montana's Mission Range. Northwest Science. 43(3): 135-147. [8546]
226. Weaver, T. 2001. Whitebark pine and its environment. In: Tomback, Diana F.; Arno, Stephen F.; Keane, Robert E., eds. Whitebark pine communities: Ecology and restoration. Washington, DC: Island Press: 41-73. [36693]
227. Weaver, T.; Dale, D. 1974. Pinus albicaulis in central Montana: environment, vegetation and production. The American Midland Naturalist. 92(1): 222-230. [2470]
228. Willard, E. Earl. 1990. Use and impact of domestic livestock in whitebark pine forests. In: Schmidt, Wyman C.; McDonald, Kathy J., compilers. Proceedings--symposium on whitebark pine ecosystems: ecology and management of a high-mountain resource; 1989 March 29-31; Bozeman, MT. Gen. Tech. Rep. INT-270. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 201-207. [11687]
229. Williams, Clinton K.; Kelley, Brian F.; Smith, Bradley G.; Lillybridge, Terry R. 1995. Forest plant associations of the Colville National Forest. Gen. Tech. Rep. PNW-360. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 375 p. [27360]
230. Williams, Clinton K.; Lillybridge, Terry R. 1983. Forested plant associations of the Okanogan National Forest. R6-Ecol-132b. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 116 p. [2566]
231. Wood, C. S.; Unger, L. 1996. Mountain pine beetle: A history of outbreaks in pine forests in British Columbia, 1910 to 1995. Victoria, BC: Canadian Forest Service, Natural Resources Canada, Pacific Forestry Centre. 61 p. [40260]
232. Wright, J. W. 1976. Introduction to forest genetics. New York: Academic Press. 463 p. [41609]
233. Yanchuk, Alvin D. 1997. Conservation issues and priorities for the conifer genetic resources of British Columbia, Canada. Forest Genetic Resources. 25: 2-9. [41615]
234. Yanchuk, Alvin D.; Lester, Donald T. 1996. Setting priorities for conservation of the conifer genetic resources of British Columbia. The Forestry Chronicle. 72(4): 406-415. [40065]
235. Young, James A.; Evans, Raymond A. 1981. Demography and fire history of a western juniper stand. Journal of Range Management. 34(6): 501-505. [2659]
236. Zeglen, S. 2000. Whitebark pine and white pine blister rust in southwestern British Columbia - 1998. Canadian Plant Disease Survey. 80: 148-149. [40596]
237. Zeglen, S. 2001. Whitebark pine and white pine blister rust in British Columbia - 1999. Canadian Plant Disease Survey. 81: 169. [40257]