Index of Species Information
SPECIES: Gleditsia triacanthos
|
|
 |
| Honeylocust. Creative Commons image by Richard Webb, Bugowood.org. |
Introductory
SPECIES: Gleditsia triacanthos
AUTHORSHIP AND CITATION:
Sullivan, Janet. 1994. Gleditsia triacanthos. In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station,
Fire Sciences Laboratory (Producer). Available:
https://www.fs.usda.gov/database/feis/plants/tree/gletri/all.html [].
ABBREVIATION:
GLETRI
SYNONYMS:
NO-ENTRY
SCS PLANT CODE:
GLTR
COMMON NAMES:
honeylocust
common honeylocust
honey shucks locust
sweet bean locust
TAXONOMY:
The currently accepted scientific name for honeylocust is Gleditsia
triacanthos L. (Cesalpiniaceae) [11,14,16,27,42]. Thornless
honeylocust (G. t. forma inermis Schneid.) is occasionally found wild
[27,42].
Natural hybridization between honeylocust and water-locust (G.
aquatica) has been reported [27].
LIFE FORM:
Tree
FEDERAL LEGAL STATUS:
No special status
OTHER STATUS:
NO-ENTRY
DISTRIBUTION AND OCCURRENCE
SPECIES: Gleditsia triacanthos
GENERAL DISTRIBUTION:
The natural range of honeylocust extends from central Pennsylvania
through extreme southern Ontario, extreme southern Michigan, southern
Wisconsin, and extreme southeastern Minnesota to extreme southeastern
South Dakota; south through eastern Nebraska to eastern Texas; east to
Alabama; and northeast along the western slopes of the Appalachians.
Isolated populations occur in northwestern Florida. Honeylocust is
naturalized east of the Appalachians as far north as Nova Scotia [16,27].
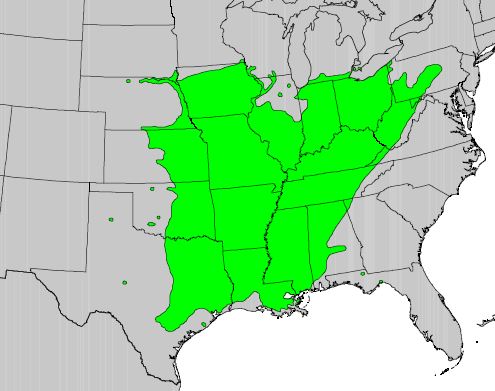 |
| Honeylocust distribution. 1971 USDA, Forest Service map provided by Thompson and others [46]. |
ECOSYSTEMS:
FRES14 Oak - pine
FRES15 Oak - hickory
FRES16 Oak - gum - cypress
FRES17 Elm - ash - cottonwood
FRES18 Maple - beech - birch
STATES:
AL AR CT DE FL GA IN IL IA KS
KY LA OK MD MI MN MS MO NE NY
NC OH PA RI SC SD TN TX VA WI
WV NS ON
BLM PHYSIOGRAPHIC REGIONS:
14 Great Plains
KUCHLER PLANT ASSOCIATIONS:
K098 Northern floodplain forest
K100 Oak - hickory forest
K101 Elm - ash forest
K102 Beech - maple forest
K103 Mixed mesophytic forest
K104 Appalachian oak forest
K106 Northern hardwoods
K111 Oak - hickory - pine forest
K112 Southern mixed forest
K113 Southern floodplain forest
SAF COVER TYPES:
27 Sugar maple
42 Bur oak
62 Silver maple - American elm
82 Loblolly pine - hardwood
88 Willow oak - water oak - diamondleaf oak
92 Sweetgum - willow oak
93 Sugarberry - American elm - green ash
94 Sycamore - sweetgum - American elm
SRM (RANGELAND) COVER TYPES:
NO-ENTRY
HABITAT TYPES AND PLANT COMMUNITIES:
Honeylocust is usually only a minor component of natural forest stands.
It is considered an accessory species in four SAF cover types: bur oak
(Quercus macrocarpa), willow oak (Q. phellos)-water oak (Q.
nigra)-diamondleaf (laurel) oak (Q. laurifolia), sweetgum (Liquidambar
styraciflua)-willow oak, and sugarberry (Celtis laevigata)-American elm
(Ulmus americana). Honeylocust is a secondary species in all other SAF
cover types listed above [8,17].
Mesophytic species commonly associated with honeylocust include red
maple (Acer rubrum), persimmon (Diospyros virginiana), black tupelo
(Nyssa sylvatica), sweet pecan (Carya illinoensis), boxelder (Acer
negundo), Kentucky coffeetree (Gymnocladus dioica), and black walnut
(Juglans nigra) [8].
MANAGEMENT CONSIDERATIONS
SPECIES: Gleditsia triacanthos
WOOD PRODUCTS VALUE:
Honeylocust wood is dense, hard, coarse-grained, strong, stiff,
shock-resistant, takes a high polish, and is durable in contact with
soil [11,14,16,22,42]. Honeylocust wood is used locally for posts,
pallets, crates, general construction, furniture, interior finish,
turnery, and firewood [8,36]. It is useful, but is too scarce to be of
economic importance [8].
IMPORTANCE TO LIVESTOCK AND WILDLIFE:
Honeylocust pods are eaten by cattle, goats, white-tailed deer,
Virginia opossum, eastern gray squirrel, fox squirrel, rabbits, quail
(including northern bobwhite), crows, and starling [8,11]. White-tailed
deer frequently strip and eat the soft bark of young trees in winter
[36]; rabbits also consume honeylocust bark in winter [8]. Livestock
and white-tailed deer consume young vegetative growth [8,36].
Honeylocust is a source of pollen and nectar for honey [36].
In Virginia, honeylocust and other species were planted for mast
production on the margins of plots cleared and revegetated for wildlife
[28]. Honeylocust is planted into currently operating pastures and
hayfields to provide high-protein mast for livestock (a management
system termed browse agroforestry). Cattle do not digest the seeds and
thus do not derive full nutritional benefit from consuming whole pods,
but ground honeylocust pods do provide a high-protein feed for cattle.
Sheep do digest the seeds, and therefore obtain more of the available
protein when consuming whole pods. The open canopy of honeylocust
allows good growth of pasture grasses [43].
PALATABILITY:
NO-ENTRY
NUTRITIONAL VALUE:
Ground honeylocust seeds and pods contained 16.1 percent crude fiber
(as fed) and 9.3 percent protein [30].
COVER VALUE:
NO-ENTRY
VALUE FOR REHABILITATION OF DISTURBED SITES:
Honeylocust pioneers on strip-mine spoil banks in the Midwest. It
is often planted for erosion control [8].
OTHER USES AND VALUES:
Thornless honeylocust is widely planted as an ornamental [11],
particularly on dry sites [23]. Honeylocust is also widely used in
windbreaks and shelterbelts [8,36].
Honeylocust pods are being fermented for ethanol production in studies
to explore the feasibility of biomass fuels [4].
Honeylocust was one of a number of species planted to assess biomass
yield potential for short-rotation cropping. Honeylocust showed good
survival through the fourth annual harvest [21].
Honeylocust pods are edible [5].
OTHER MANAGEMENT CONSIDERATIONS:
Rows of honeylocust planted for windbreaks showed a positive response
to release [9].
In some areas honeylocust invades rangelands. Honeylocust is
susceptible to triclopyr and to a mixture of picloram and 2,4,-D [29].
Honeylocust is not usually subject to serious insect and disease
problems; however, with the increase in plantations of honeylocust,
there has been a concomitant increase in insect pests. Honeylocust is
host to a number of leaf feeders including spider mites, white marked
tussock moth, and honeylocust plant bug. The only serious disease of
honeylocust is a canker which is occasionally fatal [8].
Damage to young honeylocust is caused by rabbits gnawing the bark [8]
and by livestock and white-tailed deer browsing [8,36].
BOTANICAL AND ECOLOGICAL CHARACTERISTICS
SPECIES: Gleditsia triacanthos
 |
|
 |
|
Honeylocust flowerbuds and fruits. Creative Commons images by Paul Wray, Iowa State University, Bugwood.org/.
|
GENERAL BOTANICAL CHARACTERISTICS:
Honeylocust is a native, deciduous tree. Mature heights usually range
from 49 to 98 feet (15-30 m) [11,36], with a maximum height of 140 feet
(43 m) [14]. In natural stands honeylocust averages 70 to 80 feet
(21-24 m) in height [8]. Honeylocust is armed with heavy branched
thorns on the lower branches and trunk [11]. The crown is plumelike and
open [14,42]. The bole is usually short and often divided near the
ground. The bark of mature trunks is usually 0.25 to 0.75 inches
(0.6-3.5 cm) thick with narrow ridges divided by fissures. The bark
peels in strips [14]. The thick, fibrous roots are deep and
wide-spreading [14,39]. The tree is sturdy and windfirm [14]. The
fruit is a legume 8 to 16 inches (15-40 cm) long and 1 to 1.4 inches
(2.5-3.5 cm) wide [8,11,22].
Honeylocust is usually described as rapid-growing [8,39]. Average
longevity for honeylocust is 125 years [8].
Unlike most leguminous species, honeylocust does not form Rhizobium
nodules on its roots, and does not fix nitrogen [12].
RAUNKIAER LIFE FORM:
Phanerophyte
REGENERATION PROCESSES:
The minimum seed-bearing age of honeylocust is 10 years. Optimum seed
production occurs from about 25 to 75 years of age. Seeds are produced
until about age 100. Large crops usually occur every other year but can
be produced annually. Some seed is usually produced every year.
Honeylocust seed is viable for long periods due to an impermeable
seedcoat. Seeds are dispersed by birds and mammals, including cattle.
Germination of honeylocust seeds is apparently enhanced by passage
through the digestive tract of animals. Germination is artificially
enhanced by scarification (both hot water and acid treatments are
effective) [8]. Honeylocust seeds showed the broadest germination
response of five species tested (honeylocust, white ash (Fraxinus
americana), sycamore (Platanus occidentalis), red mulberry (Morus
rubra), and black cherry (Prunus serotina). Honeylocust showed a high
rate of emergence under all temperatures tested, and under all but the
driest conditions. It was also the only species of the five that had a
higher proportion of variance in germination rate explained by moisture
than by temperature [13].
Honeylocust seedlings grew faster on clay soils than on loess and
alluvium. There was no growth difference between sun and shade on clay
soils, but on the other two soil types honeylocust seedlings exhibited
retarded growth in the shade. Seedling root depths were 5 to 5.25 feet
(1.5-1.6 m) on clay and 20 to 24 inches (50.8-61 cm) in moist alluvial
soil [7].
Honeylocust can be propagated by grafting, budding, and cuttings
(hardwood, softwood, and root cuttings) [8].
SITE CHARACTERISTICS:
Honeylocust is adapted to a variety of soils and climates [14]. It is
common in both bottomlands and uplands, in the open or in open woods
[16]. Honeylocust occurs on well-drained sites, upland woodlands and
borders, old fields, fencerows, river floodplains, hammocks [22], rich,
moist bottomlands [8], and rocky hillsides [36]. It is most commonly
found on moist, fertile soils near streams and lakes [8]. Best growth
occurs in small stream valleys in southern Indiana and Illinois [14].
It has been rated highly tolerant to flooding [24]. It is also
drought-resistant and somewhat tolerant of salinity [37,39].
Honeylocust tolerates both alkaline and acid soils, but its best growth
occurs on soils with pH between 6.0 and 8.0 [8]. Honeylocust grew
better on low nitrogen sites than many other tree species [1].
The natural range of honeylocust is generally below 2,500 feet (760 m)
elevation, although the upper limit appears to be 5,000 feet (1,520 m).
A 20-year-old plantation of honeylocust had good survival at 6,900 feet
(2,100 m) in Colorado, but the trees were small [8,16].
SUCCESSIONAL STATUS:
Honeylocust is intolerant of shade. Reproduction establishes only in
open areas, gaps, and at the edges of woods [8]. The ability of
honeylocust to invade open prairie is thought to be related to its
tolerance of xeric conditions [3]. Both top and root growth are
retarded by shade. Lower limbs die back in excessive shade.
Honeylocust is a fast-growing member of early seral stands [8]. Hupp
[45] classes honeylocust as an upland disturbance species which is
sometimes found on the most severely degraded stream channels (streams
disturbed by stream channelization projects). The presence of
honeylocust and similar species suggests that these streambanks are now
so high as to be above most fluvial activity, and that these sites are
highly disturbed [45]. Honeylocust is also described as a
mid-successional species [41] and is found in gaps or on the edges of
old-growth forests [10]. The distribution of honeylocust appears to be
related to the serendipitous combination of openings (disturbance) and
seed dispersal.
In southeastern Iowa, honeylocust was one of the major dominants in
pioneer forests that developed on abandoned fields and pastures [44].
Honeylocust is also a pioneer in the rocky limestone glades of
Tennessee and Kentucky that are later populated by eastern redcedar
(Juniperus virginiana) [8]. In Mississippi, honeylocust was a
volunteer on an 11-year-old hardwood stand planted to Nuttall oak
(Quercus nuttallii). At 20 feet (8.8 m), it was the tallest tree in the
stand. It is likely that honeylocust will eventually be overtopped and
shaded out by other species as the stand matures [25]. In Tennessee,
honeylocust was present on a 12-year-old site (oldfield succession),
but not on 3-, 28-, 30-, 40-, and 45-year-old sites [34].
In southeastern Texas, honeylocust was present at very low density on a
47-year-old gravel pit, but was not present in 3- and 5-year-old pits or
in adjacent undisturbed forest [31]. In southwestern Ohio, honeylocust
was common in 50-year-old forests (on old fields), and present but not
common in 90-year-old and old-growth (over 200 years old) forests
[41,41]. In Ohio, honeylocust was an occasional member of the canopy
of 40- and 60-year-old oak (Quercus spp.)-sugar maple (Acer saccharum)
stands [15].
In central Indiana, honeylocust was present in edge plots but not
interior plots in an old-growth forest [10]. In Kansas, honeylocust
grew in patches on the edges of Konza Prairie gallery forests, reaching
heights of up to 20 feet (6 m); under the canopy it was rarely over 6 to
8 feet (1.8-2.4 m) tall [33]. Large honeylocust trees were present in
a mature shingle oak (Quercus imbricaria)- bur oak community in Kansas,
suggesting that they were relics of an earlier successional stage.
There was no honeylocust in the reproduction layer [44].
SEASONAL DEVELOPMENT:
Honeylocust begins to flower when its leaves are nearly full grown,
from around May 10 in the southern parts of its range to around June 25
in the northern parts of its range [8,42]. The legumes ripen from
September to October, usually falling after ripening but sometimes
remaining on the tree through February [8,16,39,42].
FIRE ECOLOGY
SPECIES: Gleditsia triacanthos
FIRE ECOLOGY OR ADAPTATIONS:
Honeylocust appears to be excluded from prairies by frequent fire, and
expands where fire is excluded. On bluestem (Andropogon spp. and/or
Schizachyrium spp.) prairie in Kansas, honeylocust was one of a number
of woody species invading undisturbed prairie that had not burned since
1947 [18].
On the Konza Prairie, sites adjacent to gallery forests that had
remained unburned for 10 or more years were converting to woodlands
dominated by junipers (Juniperus spp.), elms (Ulmus spp.), honeylocust,
and hackberries (Celtis spp.). In areas farther from gallery forests,
fire exclusion leads to increased density of species, including
honeylocust, that otherwise persist only at low densities along stream
margins of frequently burned prairies [3].
Honeylocust also occurs in bottomland forests that experience fire
infrequently. Fire may create openings for honeylocust reproduction in
these forests.
FIRE REGIMES:
Find fire regime information for the plant communities in which this
species may occur by entering the species name in the FEIS home page under
"Find Fire Regimes".
POSTFIRE REGENERATION STRATEGY:
Tree with adventitious-bud root crown/soboliferous species root sucker
FIRE EFFECTS
SPECIES: Gleditsia triacanthos
IMMEDIATE FIRE EFFECT ON PLANT:
Honeylocust is easily injured by fire due to its thin bark [8,39].
In south-central Iowa, grassland dominated by Kentucky bluegrass (Poa
pratense) that was undergoing invasion by coralberry (Symphoricarpos
orbiculatus), honeylocust, and elms was prescribed burned with a series
of fires to observe the effect of fire season on brush control.
Prescribed fires were conducted in February, April, June, and September
in order to include all stages of plant phenology. Some large
honeylocust trees suffered bark damage and subsequent insect injury.
Many honeylocust trees under 10 feet (3 m) in height were top-killed
and sprouted the following year [20].
DISCUSSION AND QUALIFICATION OF FIRE EFFECT:
NO-ENTRY
PLANT RESPONSE TO FIRE:
Honeylocust sprouts after top-kill by fire [39].
In the south-central Iowa study, there was an increase in the number of
honeylocust stems in the first season following the April prescribed
fire, but the number of honeylocust stems declined to prefire levels by
the second postfire year [20].
In Kansas, a bur oak-dominated gallery forest was prescribed burned in
1983. There was no apparent fire-caused mortality to the overstory.
The reproduction layer was dominated by elm seedlings, both before and
after the fire. Although honeylocust seedling mortality was not
reported directly, 100 honeylocust seedlings were present before the
fire, and 50 were recorded in each of the 2 years following the fire [2].
FIRE MANAGEMENT CONSIDERATIONS:
No entry
References for species: Gleditsia triacanthos
1. Abrams, Marc D. 1985. Age-diameter relationships of Quercus species in relation to edaphic factors in gallery forests of northeast Kansas. Forest Ecology and Management. 13: 181-193. [10377]
2. Abrams, Marc D. 1986. Ecological role of fire in gallery forests in eastern Kansas. In: Koonce, Andrea L., ed. Prescribed burning in the Midwest: state-of-the-art: Proceedings of a symposium; 1986 March 3-6; Stevens Point, WI. Stevens Point, WI: University of Wisconsin, College of Natural Resources, Fire Science Center: 73-80. [16271]
3. Abrams, Marc D.; Gibson, David J. 1991. Effects of fire exclusion on tallgrass prairie and gallery forest communities in eastern Kansas. In: Nodvin, Stephen C.; Waldrop, Thomas A., eds. Fire and the environment: ecological and cultural perspectives: Proceedings of an international symposium; 1990 March 20-24; Knoxville, TN. Gen. Tech. Rep. SE-69. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southeastern Forest Experiment Station: 3-10. [16627]
4. Avgerinos, George C.; Wang, Daviel I. C. 1980. Utilization of mesquite and honey locust pods as feedstocks for energy production. In: Proceedings, workshop on tree crops for energy co-production on farms; [Date unknown]; [Location unknown]. Golden, CO: Solar Energy Research Institute: 209-217. [23163]
5. Batzell, Peter. 1985. Edible pods. Bio-dynamics. 155: 55-58. [23161]
6. Bernard, Stephen R.; Brown, Kenneth F. 1977. Distribution of mammals, reptiles, and amphibians by BLM physiographic regions and A.W. Kuchler's associations for the eleven western states. Tech. Note 301. Denver, CO: U.S. Department of the Interior, Bureau of Land Management. 169 p. [434]
7. Biswell, Harold H. 1935. Effects of environment upon the root habits of certain deciduous forest trees. Botanical Gazette. 96(4): 676-708. [3076]
8. Blair, Robert M. 1990. Gleditsia triacanthos L. honeylocust. In: Burns, Russell M.; Honkala, Barbara H., technical coordinators. Silvics of North America. Volume 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S. Department of Agriculture, Forest Service: 358-364. [21819]
9. Bratton, Gerald F. 1990. Windbreak renovation studies--update, 1964-1989. In: Great Plains Agricultural Council, compiler. Windbreaks: Living with the wind: Proceedings, windbreak renovation workshop; 1990 October 23-25; Hutchinson, KS. Great Plains Agriculture Council Publ. No. 133. Manhattan, KS: Kansas State University, Cooperative Extension Service: 17-20. [15253]
10. Brothers, Timothy S. 1993. Fragmentation and edge effects in central Indiana old-growth forests. Natural Areas Journal. 13(4): 268-275. [22356]
11. Brown, Russell G.; Brown, Melvin L. 1972. Woody plants of Maryland. Baltimore, MD: Port City Press. 347 p. [21844]
12. Burton, Joseph C. 1972. Nodulation and symbiotic nitrogen fixation by prairie legumes. In: Zimmerman, James H., ed. Proceedings, 2nd Midwest prairie conference; 1970 September 18-20; Madison, WI. Madison, WI: University of Wisconsin Arboretum: 116-121. [2909]
13. Burton, Philip J.; Bazzaz, F. A. 1991. Tree seedling emergence on interactive temperature and moisture gradients and in patches of old-field vegetation. American Journal of Botany. 78(1): 131-149. [13443]
14. Collingwood, G. H.; Brush, Warren D.; [revised and edited by Butcher, Devereux]. 1964. Knowing your trees. 2nd ed. Washington, DC: The American Forestry Association. 349 p. [22497]
15. DeMars, Brent G.; Runkle, James R. 1992. Groundlayer vegetation ordination and site-factor analysis of the Wright State University Woods (Greene County, Ohio). Ohio Journal of Science. 92(4): 98-106. [19823]
16. Duncan, Wilbur H.; Duncan, Marion B. 1988. Trees of the southeastern United States. Athens, GA: The University of Georgia Press. 322 p. [12764]
17. Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters. 148 p. [905]
18. Fitch, Henry S.; Kettle, W. Dean. 1983. Ecological succession in vegetation and small mammal populations on a natural area of northeastern Kansas. In: Kucera, Clair L., ed. Proceedings, 7th North American prairie conference; 1980 August 4-6; Springfield, MO. Columbia, MO: University of Missouri: 117-121. [3211]
19. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; [and others]. 1977. Vegetation and environmental features of forest and range ecosystems. Agric. Handb. 475. Washington, DC: U.S. Department of Agriculture, Forest Service. 68 p. [998]
20. George, Ronnie R.; Farris, Allen L.; Schwartz, Charles C.; [and others]. 1978. Effects of controlled burning on selected upland habitats in southern Iowa. Iowa Wildlife Research Bulletin No. 25. Des Moines, IA: Iowa Conservation Commission Wildlife Section. 38 p. [4422]
21. Geyer, Wayne A. 1989. Biomass yield potential of short-rotation hardwoods in the Great Plains. Biomass. 20: 167-175. [10135]
22. Godfrey, Robert K. 1988. Trees, shrubs, and woody vines of northern Florida and adjacent Georgia and Alabama. Athens, GA: The University of Georgia Press. 734 p. [10239]
23. Gutknecht, Kurt W. 1989. Xeriscaping: an alternative to thirsty landscapes. Utah Science. 50(4): 142-146. [10166]
24. Hook, D. D. 1984. Waterlogging tolerance of lowland tree species of the South. Southern Journal of Applied Forestry. 8: 136-149. [19808]
25. Krinard, R. M.; Johnson, R. L. 1981. Description and yields of an 11-year-old hardwood stand on Sharkey clay soil. Res. Note SO-265. New Orleans, LA: U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station. 2 p. [4229]
26. Kuchler, A. W. 1964. Manual to accompany the map of potential vegetation of the conterminous United States. Special Publication No. 36. New York: American Geographical Society. 77 p. [1384]
27. Little, Elbert L., Jr. 1979. Checklist of United States trees (native and naturalized). Agric. Handb. 541. Washington, DC: U.S. Department of Agriculture, Forest Service. 375 p. [2952]
28. McGinnes, Burd S.; Ripley, Thomas H. 1962. Evaluation of wildlife response to forest-wildlife management--a preliminary report. In: Southern forestry on the march: Proceedings, Society of American Foresters meeting; [Date of conference unknown]; Atlanta, GA. [Place of publication unknown]. [Publisher unknown]. 167-171. [16735]
29. Melichar, M. W.; Geyer, W. A.; Ritty, P. M. 1986. Hardwood tree control with herbicide applications. In: Proceedings, 40th annual meeting of the Northeastern Weed Science Society; [Date unknown]; [Location unknown]. [Place of publication unknown]: Northeastern Weed Science Society: 210-211. [10484]
30. National Academy of Sciences. 1971. Atlas of nutritional data on United States and Canadian feeds. Washington, DC: National Academy of Sciences. 772 p. [1731]
31. Nixon, Elray S. 1975. Successional stages in a hardwood bottomland forest near Dallas, Texas. The Southwestern Naturalist. 20: 323-335. [12250]
32. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
33. Reichman, O. J. 1987. Forests. In: Konza Prairie: A tallgrass natural history. Lawrence, KS: University Press of Kansas: 115-124. [4255]
34. Shankman, David. 1990. Forest regeneration on abandoned agricultural fields in western Tennessee. Southeastern Geographer. 30(1): 36-47. [17640]
35. Stickney, Peter F. 1989. Seral origin of species originating in northern Rocky Mountain forests. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Fire Sciences Laboratory, Missoula, MT; RWU 4403 files. 10 p. [20090]
36. Stubbendiek, James; Conard, Elverne C. 1989. Common legumes of the Great Plains: an illustrated guide. Lincoln, NE: University of Nebraska Press. 330 p. [11049]
37. Townsend, A. M. 1989. The search for salt tolerant trees. Arboricultural Journal. 13(1): 67-73. [13061]
38. U.S. Department of Agriculture, Soil Conservation Service. 1982. National list of scientific plant names. Vol. 1. List of plant names. SCS-TP-159. Washington, DC. 416 p. [11573]
39. Van Dersal, William R. 1938. Native woody plants of the United States, their erosion-control and wildlife values. Washington, DC: U.S. Department of Agriculture. 362 p. [4240]
40. Vankat, John L.; Carson, Walter P. 1991. Floristics of a chronosequence corresponding to old field-deciduous forest succession in sw Ohio. III. Post-disturbance vegetation. Bulletin of the Torrey Botanical Club. 118(4): 385-391. [17754]
41. Vankat, John L.; Snyder, Gary W. 1991. Floristics of a chronosequence corresponding to old field-diciduous forest succession in southwestern Ohio. I. Undisturbed vegetation. Bulletin of the Torrey Botanical Club. 118(4): 365-376. [18758]
42. Vines, Robert A. 1960. Trees, shrubs, and woody vines of the Southwest. Austin, TX: University of Texas Press. 1104 p. [7707]
43. Wilson, A. A. 1991. Browse agroforestry using honeylocust. Forestry Chronicle. 67(3): 232-235. [23162]
44. McBride, Joe. 1973. Natural replacement of disease-killed elms. The American Midland Naturalist. 90(2): 300-306. [8868]
45. Hupp, Cliff R. 1992. Riparian vegetation recovery patterns following stream channelization: a geomorphic perspective. Ecology. 73(4): 1209-1226. [19499]
46. Thompson, Robert S.; Anderson, Katherine H.; Bartlein, Patrick J. 1999. Digital representations of tree species range maps from "Atlas of United States trees" by Elbert L. Little, Jr. (and other publications), [Online]. In: Atlas of relations between climatic parameters and distributions of important trees and shrubs in North America. Denver, CO: U.S. Geological Survey, Information Services (Producer). Available: esp.cr.usgs.gov/data/little/ [2015, May 12]. [82831]
FEIS Home Page
https://www.fs.usda.gov/database/feis/plants/tree/gletri/all.html



