Index of Species Information
SPECIES: Celtis laevigata
|
|
 |
| Creative Commons image by Vern Wilkins, Indiana University, Bugwood.org. |
Introductory
SPECIES: Celtis laevigata
AUTHORSHIP AND CITATION:
Sullivan, Janet. 1993. Celtis laevigata. In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station,
Fire Sciences Laboratory (Producer). Available:
https://www.fs.usda.gov/database/feis/plants/tree/cellae/all.html [].
ABBREVIATION:
CELLAE
SYNONYMS:
For Celtis laevigata:
Celtis mississippiensis Bosc
For Celtis laevigata var. laevigata:
C. l. var. brachyphylla Sarg., Uvalde sugar hackberry
C. l. var. anomala Sarg., scrub sugar hackberry
For Celtis laevigata var. reticulata:
Celtis reticulata Torr.
Celtis rugulosa Rydb.
For Celtis laevigata var. smallii:
Celtis smallii Beadle
NRCS PLANT CODE:
CELA
CELAB
CELAL
CELAR
CELAS3
CELAT8
COMMON NAMES:
sugarberry
hackberry
lowland hackberry
sugar hackberry
Arizona sugarberry
netleaf hackberry
Small's hackberry
southern hackberry
Texas sugarberry
TAXONOMY:
The accepted scientific name for sugarberry is Celtis laevigata Willd. (Ulmaceae) [17,59].
Recognized varieties are as follows [59]:
Celtis laevigata var. brevipes Sarg., Arizona sugarberry
Celtis laevigata var. laevigata, sugarberry
Celtis laevigata var. reticulata (Torr.) L.D. Benson, netleaf hackberry
Celtis laevigata var. smallii (Beadle) Sarg., Small's hackberry
Celtis laevigata var. texana (Scheele) Sarg., Texas sugarberry
See the FEIS review of netleaf hackberry for detailed information on that variety.
LIFE FORM:
Tree
FEDERAL LEGAL STATUS:
No special status
OTHER STATUS:
NO-ENTRY
DISTRIBUTION AND OCCURRENCE
SPECIES: Celtis laevigata
GENERAL DISTRIBUTION:
Sugarberry is native to the southeastern part of the United States,
ranging south from southeastern Virginia to southern Florida; west to
central Texas and including northeastern Mexico; north to western
Oklahoma and southern Kansas; and east to Missouri, extreme southern
Illinois, and Indiana. It occurs locally in Maryland [5,17,36].
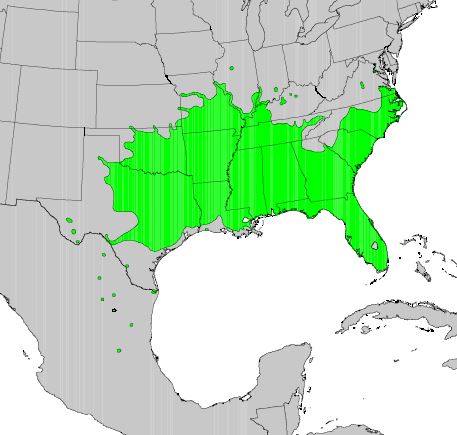 |
| Distribution of sugarberry. 1971 USDA, Forest Service map digitized by Thompson and others [67]. |
ECOSYSTEMS:
FRES12 Longleaf - slash pine
FRES13 Loblolly - shortleaf pine
FRES14 Oak - pine
FRES15 Oak - hickory
FRES16 Oak - gum - cypress
FRES17 Elm - ash - cottonwood
STATES:
AL AR FL GA IL IN KY LA MD MS
MO NC SC TN TX MEXICO
BLM PHYSIOGRAPHIC REGIONS:
NO-ENTRY
KUCHLER PLANT ASSOCIATIONS:
K084 Cross Timbers
K089 Black Belt
K092 Everglades
K111 Oak - hickory - pine forest
K112 Southern mixed forest
K113 Southern floodplain forest
SAF COVER TYPES:
70 Longleaf pine
75 Shortleaf pine
80 Loblolly pine - shortleaf pine
81 Loblolly pine
82 Loblolly pine - hardwood
83 Longleaf pine - slash pine
84 Slash pine
85 Slash pine - hardwood
87 Sweet gum - yellow-poplar
88 Willow oak - water oak - diamondleaf oak
89 Live oak
92 Sweetgum - willow oak
93 Sugarberry - American elm - green ash
94 Sycamore - sweetgum - American elm
95 Black willow
96 Overcup oak - water hickory
105 Tropical hardwoods
111 South Florida slash pine
SRM (RANGELAND) COVER TYPES:
NO-ENTRY
HABITAT TYPES AND PLANT COMMUNITIES:
In many areas, sugarberry occurs as scattered individuals. After
disturbances, a seral sugarberry-American elm (Ulmus americana)-green
ash (Fraxinus pennsylvanica) forest cover type may develop, with
sugarberry as a codominant. This type intermixes with sweetgum
(Liquidambar styraciflua)-willow oak (Quercus phellos) types, which
contain essentially the same species in different densities. The
sugarberry-American elm-green ash type occurs most often on the central
coastal plain of the Gulf of Mexico, heavily concentrated on the
Mississippi alluvial plain, and along major river basins [21,36].
Publications in which sugarberry is listed as a dominant or codominant
include:
Woody vegetation of an old-growth creekbottom forest in north-central
Texas. [41]
Quadrat study of a bottomland forest in St. Martin Parish, Louisiana. [50]
Woody species composition of the upper San Antonio River gallery
forest. [6]
Productivity and composition of a bald cypress-water tupelo site and a
bottomland hardwood site in a Louisiana swamp. [10]
Vegetative analysis of the floodplain of the Trinity River, Texas. [42]
Plant communities of the Santa Ana National Wildlife Refuge, Texas. [62]
The distribution of woody species in the Guadalupe River floodplain
forest in the Edwards Plateau of Texas. [20]
MANAGEMENT CONSIDERATIONS
SPECIES: Celtis laevigata
WOOD PRODUCTS VALUE:
The wood of sugarberry is close grained, soft, and of medium strength.
It is used mostly for furniture but also is used for dimension stock,
flooring, crating, fuel, cooperage, and fence posts [5,59].
IMPORTANCE TO LIVESTOCK AND WILDLIFE:
The fruits of sugarberry are eaten by many birds, including the
ring-necked pheasant, waterfowl, quail, and ruffed grouse. They are a
preferred food of turkeys in fall and winter. Squirrels occasionally
eat the fruit, and will also consume buds and bark, but do so rarely.
Other game and nongame animals consume the fruit. Cattle will browse
sugarberry heavily, especially in winter on poor ranges [12].
White-tailed deer will browse sugarberry, but it has a low preference
rating [4,8].
PALATABILITY:
NO-ENTRY
NUTRITIONAL VALUE:
A study of the nutritional value of a number of fruits and nuts included
sugarberry fruits in the following analysis [49]. This study reported
only the combined averages for particular types of fruits and nuts. The
following data are percentages of dry weight for all fleshy fruits
tested, except for crude fat which is the average for drupes only:
crude protein 8.4
crude fat 14.2
crude fiber 24.1
estimated true
dry matter digestibility 64.4
COVER VALUE:
NO-ENTRY
VALUE FOR REHABILITATION OF DISTURBED SITES:
NO-ENTRY
OTHER USES AND VALUES:
Sugarberry is planted as an ornamental and as a street tree [5].
OTHER MANAGEMENT CONSIDERATIONS:
In dense even-aged stands, sugarberry will self-prune and produce a
straight stem [5].
In cottonwood (Populus spp.) stands on alluvium, sugarberry (usually
with poor growth forms) will take over openings created when cottonwoods
are cut, and control sites that managers would prefer to be in more
valuable species [30]. On a site that was logged then seeded with Nuttall
oak (Quercus nuttallii), sugarberry (probably carried in by animals)
naturally established in sufficient numbers to make up one of four species
accounting for 83 percent of stems [33,39].
Sugarberry is susceptible to damage by ice, which breaks main stems and
branches [5].
Defoliation of sugarberry by hackberry butterfly (Asterocampa celtis)
has been reported, though no tree death or crown die-back was observed.
Hackberry butterfly can be controlled by spraying trees with
insecticides [5].
Sugarberry is used as an ornamental, even though leaf leachate can
reduce growth of grasses under the trees due to the presence of ferulic,
caffeic, and p-coumaric acids [5].
Good stands of sugarberry are able to establish naturally after logging
[22]. In a study of logging practices in Mississippi, sugarberry
reached the highest densities in regeneration after all sawtimber-sized
stems were removed and either all stems greater than 2 inches in d.b.h.
(5 cm) were injected with 2,4-D or stems of desirable species left
untreated with 2,4-D. Sugarberry was considered a desirable species in
this study [29]. Seven years after clearcutting on a site where
sugarberry was a canopy dominant, sugarberry accounted for 32 percent of
total regeneration stems [23]. After patch clearcutting, sugarberry
dominated both sapling and seedling regeneration on a site where, prior
to harvest, it had been second in basal area (after sweetgum) [25].
Sugarberry has no major diseases of the twigs and leaves, but eastern
mistletoe (Phoradendron flavescens) may cause serious damage in the
western part of sugarberry's range [5].
BOTANICAL AND ECOLOGICAL CHARACTERISTICS
SPECIES: Celtis laevigata
GENERAL BOTANICAL CHARACTERISTICS:
Sugarberry is a moderately tall (60 to 100 feet [18-30 m]), native
deciduous tree [2,5,36]. Mature trees are typically 18 inches (46 cm)
in d.b.h., 80 feet (24 m) tall, with 30 feet (9 m) clear of branches in
good stands [36]. The crown is spreading and round-topped or oblong.
The bark of young trees is gray and smooth; mature trees develop corky
outgrowths that are scattered to dense with smooth areas in between
[15]. The roots of sugarberry are relatively shallow; it does not form
a distinct taproot and has only average resistance to windthrow.
Sugarberry has a moderately long life span, not usually living over 150
years [5].
RAUNKIAER LIFE FORM:
Phanerophyte
REGENERATION PROCESSES:
Sexual reproduction: Sugarberry is polygamo-monoecious [2,5].
Individuals usually first produce seeds at 15 years; optimum seedbearing
years are from 30 to 70 years of age. Good seed crops are produced most
years, some individuals produce good crops every year. There are
approximately 2,000 to 2,400 cleaned seeds per pound (4,400- 5,300/kg)
[5]. Seeds have an innate dormancy, requiring cold stratification at 41
degrees Fahrenheit ( 5 deg C) for 60 to 90 days [2]. Vora [60] found
that sugarberry seeds germinated best with no treatments other than cold
stratification (i.e. acid scarification or gibberellic acid addition).
Sugarberry seeds are dispersed by mammals, birds and by water.
Seedlings are intolerant of flooding [5,36].
Sugarberry tends to grow slowly; the average 10-year diameter increase
in natural stands is 1.5 inches (3.8 cm). The best growth rates are
found in dominant trees at 2.5 inches (6.4 cm) in 10 years [36].
Vegetative reproduction: Sugarberry can be propagated by cuttings.
Small stumps sprout readily [5,36].
SITE CHARACTERISTICS:
Sugarberry is found in moist alluvial woods and slough margins (but not
deep swamps) up to 600 feet (180 m) elevation [15,43]. It also occurs
on upland sites, although rarely. It occurs on any soil type with fair
drainage, from sandy loams and rocky or alluvial soils to heavy black
clay [47]. Sugarberry is most often found on clay soils in the orders
Iceptisols and Entisols on broad flats or shallow sloughs within the
floodplains of major rivers, and on deep moist soils derived from
limestones, but will grow under a considerable range of soil and
moisture conditions [5].
Sugarberry cannot tolerate prolonged flooding or water-saturated soils
[28]. Hook [27] listed sugarberry as weakly tolerant to waterlogging,
and capable of living from seedling to maturity in soils temporarily
waterlogged for 1 to 4 weeks of the year, or about 10 percent of the
growing season. In forested wetlands sugarberry grows best in the drier
areas. Rising water levels (due to sea level rise, flooding,
impoundments etc.) will reduce sugarberry basal area in these forests
[10].
Sugarberry occurs in cedar (Jumiperus spp.) glades in the Nashville
basin, Tennessee, in mesophytic forests of the Mississippi embayment
section, and in the Oachita mountains of Louisiana on elevated rocky
surfaces subject to frequent floods [3]. Sugarberry is present as an
occasional component of hydric hammocks in Florida [58].
Sugarberry is found in humid climates, except in the extreme western
portion of its range in Texas and Oklahoma. Average annual
precipitation ranges from 20 to 60 inches (510-1,520 mm). Summer
temperatures average 80 degrees Fahrenheit (27 deg C) with extremes of
115 degrees Fahrenheit (46 deg C), and temperatures average 30 to 50
degrees Fahrenheit (-1 to 10 deg C) with extremes of -20 degrees
Fahrenheit (-29 deg C). The average frost-free period ranges from 150
to 270 days [5,36].
Overstory associates not listed in Distribution and Occurrence include
winged elm (Ulmus alata), cedar elm (U. crassifolia), water oak (Quercus
nigra), southern red oak (Q. falcata), blackgum (Nyssa sylvatica),
persimmon (Diospyros virginiana), honeylocust (Gleditsia tricantuos),
red maple (Acer rubrum), boxelder (A. negundo), pecan (Carya
illinoensis), bumelia (Bumelia lanuginosa), persimmon (Diospyros
virginiana), and red mulberry (Morus rubra). Shrub associates include
swamp-privet (Forestiera acuminata), roughleaf dogwood (Cornus
drummondii), swamp dogwood (C. stricta), hawthorn (Crataegus spp.), and
buttonbush (Cephalanthus occidentalis) [5,7,19,36,45]. Lianas occurring
with sugarberry include eastern poison-ivy (Toxicodendron radicans)
[19].
SUCCESSIONAL STATUS:
Seedlings of sugarberry can establish under most stands of southern
bottomland hardwoods; sugarberry is shade tolerant. It will respond
when released, and can outgrow more desirable forest species. When
established in the understory it has a very poor form (limby, short-
boled, crooked or forked) [5,36].
Sugarberry will naturally invade oak plantations, establishing at a rate
of up to 43 stems per acre (105/ha) on 4- to 8-year-old sites [1].
Sugarberry commonly follows eastern cottonwood (Populus deltoides var.
deltoides) and black willow (Salix nigra) in succession on new land
created by rivers [31,48,54]. In succession on land disturbed by gravel
pit operations, sugarberry codominated 47-year-old sites with eastern
redcedar (Juniperus virginiana) but did not occur in large numbers on
younger sites, and may be replaced by winged elm and post oak (Quercus
stellata) on more advanced sites [40]. On Florida tree hammocks,
disturbances such as fire, hurricanes or logging that do not destroy the
roots of young hardwoods are likely to result in canopies containing
sweetgum, hornbeam (Ostrya virginiana), oaks (Quercus spp.), and
sugarberry [58].
In a well documented series of studies, Van Auken, Bush and their
associates [6,7,53,55,56,57] have demonstrated that sugarberry is an
important species in secondary succession on terraces of the San Antonio
River in Texas. Abandoned farmland is colonized first by huisache
(Acacia smallii), a light-requiring leguminous shrub. Sugarberry is
present in early seres, but its growth is suppressed by the low nitrogen
levels of the soils (but not, as is often the case, by the low light
levels). In fact, sugarberry grows better under huisache canopies than
in the open. As huisache matures, the soil nitrogen levels increase,
and sugarberry grows faster and eventually overtops huisache, which dies
out due to high nitrogen and low light levels. Sugarberry either
remains dominant, or is eventually overtopped by other tolerant
hardwoods. They conclude that sugarberry is a late successional species
that needs high soil nitrogen, and is capable of growing in shade, but
can grow in disturbed areas or grasslands at reduced rates depending on
the presence of competition and soil nitrogen levels.
Old-growth stands may include sugarberry as an important overstory
species [41]. However, Robertson and Weaver [46] found that in an
Illinois old-growth stand of sweetgum, green ash, and red maple,
sugarberry was represented in the overstory but not in the reproduction
layers (no seedlings or saplings). An adjacent plot in the later stages
of secondary succession (about 75 years old) had some seedlings, but no
saplings in the reproduction layer. Both the old-growth (implied climax
vegetation) and the seral plots had similar basal areas of mature
sugarberry. One can infer from these reports that perhaps sugarberry
regeneration does not occur at a rate sufficient to maintain its
numbers. Once the canopy is mature and other tolerant hardwoods are
recruited, sugarberry numbers will decrease.
SEASONAL DEVELOPMENT:
Sugarberry flowers when the leaves first appear in spring, from March to
May, depending on latitude. Fruit appears in July and August, ripening
into October. The fruit is retained on the tree until midwinter [2].
Most or all leaves are lost by mid-December in the Rio Grande Valley,
Texas [63].
FIRE ECOLOGY
SPECIES: Celtis laevigata
FIRE ECOLOGY OR ADAPTATIONS:
The bark of sugarberry is thin and easily damaged by fire. When
top-killed, sugarberry will sprout from the root collar [5].
Sugarberry occurs in areas that have undergone a shift from grassland to
hardwoods (central Texas and western Oklahoma) or from pines to
hardwoods as a result of fire suppression [26]. The moist bottomlands
in which sugarberry occurs do not have frequent fire regimes.
FIRE REGIMES:
Find fire regime information for the plant communities in which this
species may occur by entering the species name in the FEIS home page under
"Find Fire Regimes".
POSTFIRE REGENERATION STRATEGY:
Tree with adventitious-bud root crown/root sucker
Ground residual colonizer (on-site, initial community)
Secondary colonizer - off-site seed
FIRE EFFECTS
SPECIES: Celtis laevigata
IMMEDIATE FIRE EFFECT ON PLANT:
Light-severity fires will kill or top-kill seedlings and saplings of
sugarberry, and top-kill larger trees; severe fires may kill even the
largest trees [5].
DISCUSSION AND QUALIFICATION OF FIRE EFFECT:
NO-ENTRY
PLANT RESPONSE TO FIRE:
Fire-damaged seedlings and saplings sprout from the root collar [5].
After an April wildfire in Texas that top-killed all vegetation,
sugarberry was observed to be sprouting from the root collar by July
[61].
Wounding by fire increases susceptibility to butt rot (any of 30 species
of Fomes, Polyporus, Hericium or Pleurotus).
In a study of 55 years of postfire succession in a Florida mixed
hardwood forest, sugarberry, while not an important species, increased
in frequency [26]. Sugarberry is often a component of areas that have
undergone some type of disturbance, including fire, although it is not
an initial colonizer of disturbed areas (usually establishing by 4 or 5
years) [33].
DISCUSSION AND QUALIFICATION OF PLANT RESPONSE:
NO-ENTRY
FIRE MANAGEMENT CONSIDERATIONS:
Sugarberry occurs as scattered individuals in Florida pine flatwoods
that are usually maintained by fire. When fire is eliminated,
succession usually proceeds to either southern mixed hardwoods or
bayhead communities, with a concomitant increase in basal area of
sugarberry [38].
REFERENCES
SPECIES: Celtis laevigata
REFERENCES:
1. Allen, James A. 1990. Establishment of bottomland oak plantations on the
Yazoo National Wildlife Refuge Complex. Southern Journal of Applied
Forestry. 14(4): 206-210. [14615]
2. Bonner, F. T. 1974. Celtis L. Hackberry. In: Schopmeyer, C. S.,
technical coordinator. Seeds of woody plants in the United States.
Agric. Handb. 450. Washington, DC: U.S. Department of Agriculture,
Forest Service: 298-300. [7579]
3. Braun, E. Lucy. 1950. Deciduous forests of eastern North America.
Philadelphia, PA: Blakiston Books. [pages unknown]. [19812]
4. Bryant, F. C.; Kothmann, M. M. 1979. Variability in predicting edible
browse from crown volume. Journal of Range Management. 32(2): 144-146.
[10292]
5. Burns, Russell M.; Honkala, Barbara H., tech. coords. 1990. Silvics of
North America. Vol 2. Hardwoods. Agric. Handb. 654. Washington, DC: U.S.
Department of Agriculture, Forest Service. 877 p. [13955]
6. Bush, J. K.; Van Auken, O. W. 1984. Woody species composition of the
upper San Antonio River gallery forest. Texas Journal of Science.
36(2&3): 139-148. [12481]
7. Bush, J. K.; Van Auken, O. W. 1986. Changes in nitrogen, carbon, and
other surface soil properties during secondary succession. Soil Science
Society of America Journal. 50: 1597-1601. [19805]
8. Chamrad, Albert D.; Box, Thadis W. 1968. Food habits of white-tailed
deer in south Texas. Journal of Range Management. 21: 158-164. [10857]
9. Collins, Scott L.; Klahr, Sabine C. 1991. Tree dispersion in
oak-dominated forests along an environmental gradient. Oecologia. 86(4):
471-477. [17584]
10. Conner, W. H.; Day, J. W., Jr. 1976. Productivity and composition of a
baldcypress-water tupelo site and a... American Journal of Botany. 63:
1354-1364. [19807]
11. Conner, William H.; Day, John W., Jr. 1989. Responses of coastal wetland
forests to human and natural changes in the environment with emphasis on
hydrology. In: Hook, Donal D.; Lea, Russ, eds. The forested wetlands of
the southern United States: Proceedingsl of the symposium; 1988 July
12-14; Orlando, FL. Gen. Tech. Rep. SE-50. Asheville, NC: U.S.
Department of Agriculture, Forest Service, Southeastern Forest
Experiment Station: 34-43. [9227]
12. Crawford, Hewlette S.; Kucera, Clair L.; Ehrenreich, John H. 1969. Ozark
range and wildlife plants. Agric. Handb. 356. Washington, DC: U.S.
Department of Agriculture, Forest Service. 236 p. [18602]
13. Daubenmire, Rexford. 1990. The Magnolia grandiflora-Quercus virginiana
forest of Florida. American Midland Naturalist. 123: 331-347. [10871]
14. Dittberner, Phillip L.; Olson, Michael R. 1983. The plant information
network (PIN) data base: Colorado, Montana, North Dakota, Utah, and
Wyoming. FWS/OBS-83/86. Washington, DC: U.S. Department of the Interior,
Fish and Wildlife Service. 786 p. [806]
15. Duncan, Wilbur H.; Duncan, Marion B. 1988. Trees of the southeastern
United States. Athens, GA: The University of Georgia Press. 322 p.
[12764]
16. Eyre, F. H., ed. 1980. Forest cover types of the United States and
Canada. Washington, DC: Society of American Foresters. 148 p. [905]
17. Fernald, Merritt Lyndon. 1950. Gray's manual of botany. [Corrections
supplied by R. C. Rollins]. Portland, OR: Dioscorides Press. 1632 p.
(Dudley, Theodore R., gen. ed.; Biosystematics, Floristic & Phylogeny
Series; vol. 2). [14935]
18. Fisher, Richard F. 1980. Allelopathy: a potential cause of regeneration
failure. Journal of Forestry. 78: 1980. [9049]
19. Fitzgerald, Charles H.; Belanger, Roger P.; Lester, William W. 1975.
Characteristics and growth of natural green ash stands. Journal of
Forestry. 73: 486-488. [5122]
20. Ford, Allen L.; Van, Auken, O. W. 1982. The distribution of woody
species in the Guadalupe River floodplain forest in the Edwards Plateau
of Texas. Southwestern Naturalist. 27(4): 383-392. [19806]
21. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; [and others].
1977. Vegetation and environmental features of forest and range
ecosystems. Agric. Handb. 475. Washington, DC: U.S. Department of
Agriculture, Forest Service. 68 p. [998]
22. Georgia Chapter, Society of American Foresters. 1979. Silvicultural
guidelines for forest owners in Georgia. Georgia Forest Research Paper
6. [Place of publication unknown]: Georgia Forestry Commission, Research
Division. 35 p. [15405]
23. Golden, Michael S.; Loewenstein, Edward F. 1991. Regeneration of tree
species 7 years after clearcutting in a river bottom in central Alabama.
In: Coleman, Sandra S.; Neary, Daniel G., compilers. Proceedings, 6th
biennial southern silvicultural research conference: Volume I; 1990
October 30 - November 1; Memphis, TN. Gen. Tech. Rep. SE-70. Asheville,
NC: U.S. Department of Agriculture, Forest Service, Southeastern Forest
Experiment Station: 76-83. [17464]
24. Great Plains Flora Association. 1986. Flora of the Great Plains.
Lawrence, KS: University Press of Kansas. 1392 p. [1603]
25. Gresham, Charles A. 1985. Pine and hardwood regeneration alternatives
for harvested bottomland hardwood stands. In: Shoulders, Eugene, ed.
Proceedings of the Third Biennial Southern Silvicultural Research
Conference; 1984 November 7 - November 8; Atlanta. General Technical
Report SO-54. New Orleans: U.S. Department of Agriculture, Forest
Service, Southern Forest Experiment Station: 87-92. [7387]
26. Hartnett, David C.; Krofta, Douglas M. 1989. Fifty-five years of
post-fire succession in a southern mixed hardwood forest. Bulletin of
the Torrey Botanical Club. 116(2): 107-113. [9153]
27. Hook, D. D. 1984. Waterlogging tolerance of lowland tree species of the
South. Southern Journal of Applied Forestry. 8: 136-149. [19808]
28. Hosner, John F.; Boyce, Stephen G. 1962. Tolerance to water saturated
soil of various bottomland hardwoods. Forest Science. 8(2): 180-186.
[18950]
29. Hurst, George A.; Bourland, Thomas R. 1980. Hardwood density and species
composition in bottomland areas treated for regeneration. Southern
Journal of Applied Forestry. 4(3): 122-127. [7839]
30. Johnson, Robert L. 1965. Regenerating cottonwood from natural seedfall.
Journal of Forestry. 63(1): 33-36. [6290]
31. Johnson, R. L.; Shropshire, F. W. 1983. Bottomland hardwoods. In: Burns,
Russell M., tech. comp. Silvicultural systems for the major forest types
of the United States. Agric. Handb. 445. Washington, DC: U.S. Department
of Agriculture, Forest Service: 175-179. [18953]
32. Kartesz, John T.; Kartesz, Rosemarie. 1980. A synonymized checklist of
the vascular flora of the United States, Canada, and Greenland. Volume
II: The biota of North America. Chapel Hill, NC: The University of North
Carolina Press; in confederation with Anne H. Lindsey and C. Richie
Bell, North Carolina Botanical Garden. 500 p. [6954]
33. Krinard, R. M.; Johnson, R. L. 1981. Description and yields of an
11-year-old hardwood stand on Sharkey clay soil. Res. Note SO-265. New
Orleans, LA: U.S. Department of Agriculture, Forest Service, Southern
Forest Experiment Station. 2 p. [4229]
34. Kuchler, A. W. 1964. Manual to accompany the map of potential vegetation
of the conterminous United States. Special Publication No. 36. New York:
American Geographical Society. 77 p. [1384]
35. Lyon, L. Jack; Stickney, Peter F. 1976. Early vegetal succession
following large northern Rocky Mountain wildfires. In: Proceedings, Tall
Timbers fire ecology conference and Intermountain Fire Research Council
fire and land management symposium; 1974 October 8-10; Missoula, MT. No.
14. Tallahassee, FL: Tall Timbers Research Station: 355-373. [1496]
36. McKnight, J. S. 1965. Sugarberry. Agric. Handb. 271. Washington, DC:
U.S. Department of Agriculture, Forest Service. 2 p. [5123]
37. McWilliams, William H.; Rosson, James R., Jr. 1990. Composition and
vulnerability of bottomland hardwood forests of the Coastal Plain
Province in the south central United States. Forest Ecology and
Management. 33/34: 485-501. [11814]
38. Monk, Carl D. 1968. Successional and environmental relationships of the
forest vegetation of north central Florida. American Midland Naturalist.
79(2): 441-457. [10847]
39. Newling, Charles J. 1990. Restoration of bottomland hardwood forests in
the lower Mississippi Valley. Restoration & Management Notes. 8(1):
23-28. [14611]
40. Nixon, Elray S. 1975. Successional stages in a hardwood bottomland
forest near Dallas, Texas. Southwestern Naturalist. 20: 323-335.
[12250]
41. Nixon, E. S.; Ward, J. R.; Fountain, E. A.; Neck, J. S. 1991. Woody
vegetation of an old-growth creekbottom forest in north-central Texas.
Texas Journal of Science. 43(2): 157-164. [15407]
42. Nixon, Elray S.; Willett, R. Larry. 1974. Vegetative analysis of the
floodplain of the Trinity River, Texas. Contract No. DACW6-74-C-0030.
Prepared for U.S. Army Corps of Engineers, Fort Worth District, Fort
Worth, Texas. [Place of publication unknown]: [Publisher unknown]. 267
p. On file at: U.S. Department of Agriculture, Forest Service,
Intermountain Research Station, Fire Sciences Laboratory, Missoula, MT.
[20420]
43. Radford, Albert E.; Ahles, Harry E.; Bell, C. Ritchie. 1968. Manual of
the vascular flora of the Carolinas. Chapel Hill, NC: The University of
North Carolina Press. 1183 p. [7606]
44. Raunkiaer, C. 1934. The life forms of plants and statistical plant
geography. Oxford: Clarendon Press. 632 p. [2843]
45. Risser, Paul G.; Rice, Elroy L. 1971. Phytosociological analysis of
Oklahoma upland forest species. Ecology. 52(5): 940-945. [7868]
46. Robertson, Philip A.; Weaver, George T.; Cavanaugh, James A. 1978.
Vegetation and tree species patterns near the northern terminus of the
southern floodplain forest. Ecological Monographs. 48(3): 249-267.
[10381]
47. Simpson, Benny J. 1988. A field guide to Texas trees. Austin, TX: Texas
Monthly Press. 372 p. [11708]
48. Shelford, V. E. 1954. Some lower Mississippi valley flood plain biotic
communities; their age and elevation. Ecology. 35(2): 126-142. [4329]
49. Short, Henry L.; Epps, E. A., Jr. 1976. Nutrient quality and
digestibility of seeds and fruits from southern forests. Journal of
Wildlife Management. 40(2): 283-289. [10510]
50. Thieret, John W. 1971. Quadrat study of a bottomland forest in St.
Martin Parish, Louisiana. Castanea. 36: 174-181. [9923]
51. U.S. Department of Agriculture, Forest Service, Forest Products
Laboratory. 1974. Wood handbook: wood as an engineering material. Agric.
Handb. No. 72. Washington, DC. 415 p. [16826]
52. U.S. Department of Agriculture, Soil Conservation Service. 1982.
National list of scientific plant names. Vol. 1. List of plant names.
SCS-TP-159. Washington, DC. 416 p. [11573]
53. Van Auken, O. W.; Bush, J. K. 1985. Secondary succession on terraces of
the San Antonio River. Bulletin of the Torrey Botanical Club. 112(2):
158-166. [19810]
54. Van Auken, O. W.; Bush, J. K. 1988. Dynamics of establishment, growth,
and development of black willow and cottonwood in the San Antonio River
Forest. Texas Journal of Science. 40(3): 269-277. [11138]
55. Van Auken, O. W.; Bush, J. K. 1991. Influence of shade and herbaceous
competition on the seedling growth of two woody species. Madrono. 38(3):
149-157. [16572]
56. Van Auken, O. W.; Gese, E. M.; Connors, K. 1985. Fertilization response
of early and late successional species: Acacia smallii and Celtis
laevigata. Botanical Gazette. 146(4): 564-569. [19811]
57. Whisenant, Steven G.; Uresk, Daniel W. 1989. Burning upland, mixed
prairie in Badlands National Park. Prairie Naturalist. 21(4): 221-227.
[11151]
58. Vince, Susan W.; Humphrey, Stephen R.; Simons, Robert W. 1989. The
ecology of hydric hammocks: A community profile. Biological Rep.
85(7.26). Washington, DC: U.S. Department of the Interior, Fish and
Wildlife Service, Research and Development. 82 p. [17977]
59. Vines, Robert A. 1960. Trees, shrubs, and woody vines of the Southwest.
Austin, TX: University of Texas Press. 1104 p. [7707]
60. Vora, Robin S. 1989. Seed germination characteristics of selected native
plants of the lower Rio Grande Valley, Texas. Journal of Range
Management. 42(1): 36-40. [6101]
61. Vora, Robin S. 1989. Fire in an old field adjacent to a sabal palm grove
in south Texas. Texas Journal of Science. 41(1): 107-108. [7063]
62. Vora, Robin S. 1990. Plant communities of the Santa Ana National
Wildlife Refuge, Texas. Texas Journal of Science. 42(2): 115-128.
[11944]
63. Vora, Robin S. 1990. Plant phenology in the lower Rio Grande Valley,
Texas. Texas Journal of Science. 42(2): 137-142. [11832]
64. Williams, Robert D.; Hanks, Sidney H. 1976. Hardwood nurseryman's guide.
Agric. Handb. 473. Washington, DC: U.S. Department of Agriculture,
Forest Service. 78 p. [4182]
65. Wheeler, E. A.; LaPasha, C. A.; Miller, R. B. 1989. Wood anatomy of elm
(Ulmus) and hackberry (Celtis) species native to the United States.
International Association of Wood Anatomy Bulletin. 10(1): 5-26.
[11552]
66. Wright, Henry A.; Bailey, Arthur W. 1982. Fire ecology: United States
and southern Canada. New York: John Wiley & Sons. 501 p. [2620]
67. Thompson, Robert S.; Anderson, Katherine H.; Bartlein, Patrick J. 1999.
Digital representations of tree species range maps from "Atlas of United
States trees" by Elbert L. Little, Jr. (and other publications). In:
Atlas of relations between climatic parameters and distributions of
important trees and shrubs in North America. Denver, CO: U.S. Geological
Survey, Information Services (Producer). On file at: U.S. Department of
Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences
Laboratory, Missoula, MT; FEIS files. [92575]
FEIS Home Page
https://www.fs.usda.gov/database/feis/plants/tree/cellae/all.html

