Index of Species Information
SPECIES: Vaccinium ovalifolium
|
|
 |
| Oval-leaf huckleberry. Image by Rob Routledge, Sault College, Bugwood.org. |
Introductory
SPECIES: Vaccinium ovalifolium
AUTHORSHIP AND CITATION:
Tirmenstein, D. 1990. Vaccinium ovalifolium. In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station,
Fire Sciences Laboratory (Producer). Available:
https://www.fs.usda.gov/database/feis/plants/shrub/vacovl/all.html [].
Images were added on 28 August 2018.
ABBREVIATION:
VACOVL
SYNONYMS:
Vaccinium chamissonis
NRCS PLANT CODE:
VAOV
COMMON NAMES:
ovalleaf huckleberry
ovalleaf blueberry
TAXONOMY:
The scientific name of oval-leaf huckleberry is Vaccinium ovalifolium Sm.
(Ericaceae) [38].
Oval-leaf huckleberry readily hybridizes with a number of species,
including Alaska huckleberry (V. alaskaense) [47], and forms
intermediate to oval-leaf and Alaska huckleberry have been widely
reported. Oval-leaf huckleberry-dwarf huckleberry (V. caespitosum) and
oval-leaf huckleberry-grouse whortleberry (V. scoparium) hybrids also
occur and may have contributed genetic material to blue huckleberry (V.
membranaceum) [8,44]. Intermediates between oval-leaf huckleberry and
dwarf huckleberry (V. caesoitosum) have been reported in parts of
eastern North America [8].
LIFE FORM:
Shrub
FEDERAL LEGAL STATUS:
No special status
OTHER STATUS:
NO-ENTRY
DISTRIBUTION AND OCCURRENCE
SPECIES: Vaccinium ovalifolium
GENERAL DISTRIBUTION:
Oval-leaf huckleberry grows from Alaska to the Cascades of Washington and
Oregon, eastward to Idaho and Montana [34]. This shrub also occurs
across much of the Pacific Rim, from the Aleutians to Japan, and reaches
parts of mainland eastern Asia [63]. Disjunct populations are common
throughout eastern Canada and the Great Lakes Region [4]. In eastern
North America, oval-leaf huckleberry occurs sporadically from northern
Quebec, Nova Scotia, and Newfoundland, southwestward to northern Michigan
[34].
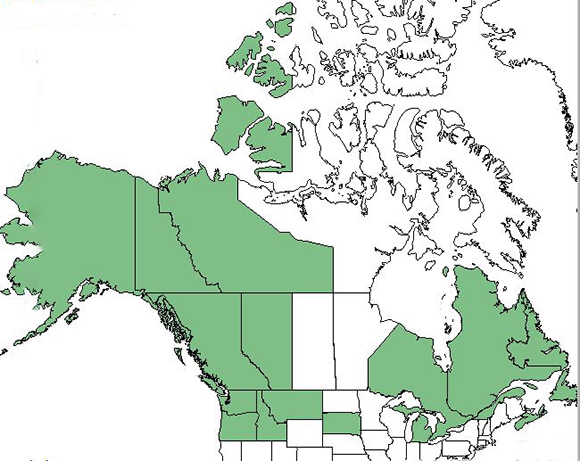 |
| Distribution of oval-leaf huckleberry in North America. Map courtesy of USDA, NRCS. 2018. The PLANTS Database.
National Plant Data Team, Greensboro, NC. [2018, August 28] [81]. |
ECOSYSTEMS:
FRES11 Spruce - fir
FRES18 Maple - beech - birch
FRES19 Aspen - birch
FRES23 Fir - spruce
FRES24 Hemlock - Sitka spruce
FRES26 Lodgepole pine
STATES:
AK CA ID MI MT OR WA AB BC NF
NS ON PQ
BLM PHYSIOGRAPHIC REGIONS:
1 Northern Pacific Border
2 Cascade Mountains
KUCHLER PLANT ASSOCIATIONS:
K001 Spruce - cedar - hemlock forest
K002 Cedar - hemlock - Douglas-fir forest
K003 Silver fir - Douglas-fir forest
K004 Fir - hemlock forest
K015 Western spruce - fir forest
K106 Northern hardwoods
SAF COVER TYPES:
005 Balsam fir
201 White spruce
205 Mountain hemlock
206 Engelmann spruce - subalpine fir
218 Lodgepole pine
223 Sitka spruce
224 Western hemlock
225 Western hemlock - Sitka spruce
226 Coastal true fir - hemlock
227 Western redcedar - western hemlock
228 Western redcedar
229 Pacific Douglas-fir
230 Douglas-fir - western hemlock
HABITAT TYPES AND PLANT COMMUNITIES:
In the West, oval-leaf huckleberry occurs in coastal montane or interior
forests dominated by western redcedar (Thuja plicata), western hemlock
(Tsuga heterophylla), mountain hemlock (Tsuga mertensiana), Sitka spruce
(Picea sitchensis), Pacific silver fir (Abies amabilis), and
yellow-cedar (Chamaecyparis nootkatensis). Common understory
codominants include blue huckleberry (V. membranaceum), Alaska
huckleberry (V. alaskaense), Oregon oxalis (Oxalis oregana), western
swordfern (Polystichum munitum), and bog Labrador tea (Ledum
glandulosum). In the East, oval-leaf huckleberry occurs in montane
forests dominated by such species as balsam fir (A. balsamea) and paper
birch (Betula papyrifera) [49].
Published classifications including oval-leaf huckleberry as an indicator
or dominant in habitat types, community types, plant associations, or
ecosystem associations are listed below.
Old-growth forests of the Canadian Rocky Mountain national parks [1]
Structure of coniferous forest communities in western Washington:
diversity and ecotype properties [15]
Classification of montane forest community types in the Cedar River
Drainage of western Washington, U.S.A. [16]
Vegetation and soils in the subalpine forests of the southern Washington
Cascade Range [19]
Natural vegetatin of Oergon and Washington [21]
Plant communities in the old-growth forests of north coastal Oregon [33]
Forest ecosystems of Mount Rainer National Park [48]
Understory associates: Species which commonly occur with oval-leaf
huckleberry in western North America include menziesia (Menziesia
ferruginea), five leaf bramble (Rubus pedatus), queencup beadlily
(Clintonia uniflora), blue huckleberry, beargrass (Xerophyllum tenax),
red huckleberry (V. parvifolium), devil's club (Oplopanax horridus),
western swordfern (Polystichum munitum), lady fern (Athyrium
filix-femina), threeleaf foamflower (Tiarella trifoliata), and Oregon
oxalis (Oxalis oregana) [25,33,51,67]. Overall species diversity is low
on many drier oval-leaf huckleberry sites [26]. Common eastern
understory associates include bunchberry (Cornus canadensis), woodfern
(Dryopteris spinulosa), twinflower (Linnaea borealis), yellow beadlily
(Clintonia borealis), sedges (Carex spp.), American starflower
(Trientalis borealis), Canada beadruby (Maianthemum canadense), and
mountain cranberry (Vaccinium vitis-idaea) [49].
MANAGEMENT CONSIDERATIONS
SPECIES: Vaccinium ovalifolium
IMPORTANCE TO LIVESTOCK AND WILDLIFE:
Browse: Oval-leaf huckleberry provides at least some browse for elk,
deer, and mountain goats [26]. On the Olympic Peninsula of Washington,
it is considered a moderately important elk browse [50]. In many areas,
elk feed on the leaves and twigs year-round, but this shrub is generally
of greatest importance during winter and summer [59]. In parts of
eastern North America, deer browse buds and tender young twigs in early
spring, but use is often heaviest in winter [8]. In western Washington,
oval-leaf huckleberry is a preferred browse of black-tailed deer, with
greatest utilization reported in April, May, and October1w. This food
source may be unavailable for winter use wherever foliage is heavily
browsed in summer [7]. Livestock use of oval-leaf huckleberry appears
limited although domestic sheep and goats feed on this shrub in some
locations [14,65].
Fruit: Fruits of oval-leaf huckleberry are eaten by many birds and
mammals [26,29]. In Alaska, both leaves and berries are important fall
foods of the spruce grouse [17]. The scarlet tanager, thrushes,
thrashers, towhees, ptarmigans, ring-necked pheasant, ruffed, blue, and
sharp-tailed grouse all consume huckleberry (Vaccinium spp.) fruit
[45,65]. Many mammals including chipmunks, black bear, red fox, skunks,
squirrels, gray fox, and raccoon also eat Vaccinium berries [45,65].
Oval-leaf huckleberry fruit is an important grizzly bear food in parts of
British Columbia [3,46].
PALATABILITY:
Palatability of oval-leaf huckleberry browse apparently varies with site
[7] and seasonal development. However, overall palatability to big game
is generally described as moderate [28]. It is preferred by
black-tailed deer in parts of western Washington during the winter
months [7] and tender buds and twigs are readily consumed by
white-tailed deer during early spring or late winter in parts of the
East [8]. Palatability of oval-leaf huckleberry browse to elk in
Washington is rated as good [59]. Fruit is preferred by many birds and
mammals.
NUTRITIONAL VALUE:
Browse: Huckleberry (Vaccinium spp.) foliage is relatively high in
carotene, manganese, and energy content [12,30]. The nutrient content
of oval-leaf huckleberry browse varies seasonally. A composite analysis
of oval-leaf and red huckleberry (V. parvifolium) browse in western
Washington revealed the following values [7]:
crude ether crude N-free total Ca K PO4
protein extract fiber extract ash
(percent)
7.57 3.56 35.71 46.90 4.38 1.032 0.535 0.434
Fruit: Fruits of Vaccinium spp. are sweet and contain high
concentrations of both mono- and di-saccharides [62]. Berries are rich
in vitamin C and energy content but contain little fat [36,55].
COVER VALUE:
Oval-leaf huckleberry presumably provides cover for a variety of wildlife
species. Taller plants, which grow in forest openings, may serve as
favorable hiding places for large mammals.
VALUE FOR REHABILITATION OF DISTURBED SITES:
Species within the genus Vaccinium can be propagated from hardwood
cuttings or by seed. Oval-leaf huckleberry averages 1,606,200 cleaned
seeds per pound (3,538/g). Seedlings grown in the greenhouse can be
transplanted 6 to 7 weeks after emergence. Seed collection and storage
techniques have been examined in detail [11].
OTHER USES AND VALUES:
Berries of oval-leaf huckleberry are tart but flavorful [29,35]. Fruit
is eaten fresh, cooked, or dried [28,29,63]. Berries also make
excellent jelly and wine [28,31]. Fruit of the oval-leaf and Alaska
huckleberries are the most commonly gathered wild berries along the
Pacific Coast of Alaska [2]. Approximately 8.5 ounces (250 ml) of fruit
can be harvested within a 10-minute period [70].
The oval-leaf huckleberry was traditionally an important food source for
Native peoples of present-day Alaska and British Columbia [63,70].
Berries were eaten fresh or preserved for winter use [70]. Preserved
fruit provided a good source of vitamin C during the winter months.
Oval-leaf huckleberry was first cultivated in 1880 [11]. Many species of
huckleberries (Vaccinium spp.) have value as ornamentals or as
fruit-producers in backyard gardens. However, Schultz [58] reports that
oval-leaf huckleberry does not appear well-suited for horticultural
breeding purposes.
OTHER MANAGEMENT CONSIDERATIONS:
Chemical control: Huckleberries (Vaccinium spp.) exhibit variable
susceptibility to herbicides such as 2,4-D, 2,4,5-T, glyphosate,
karbutilate, and picloram [6].
Timber harvest: Oval-leaf huckleberry commonly persists on cutover sites
[24,42]. On thinned stands in southeastern Alaska, oval-leaf huckleberry
seedlings were scattered where trees were spaced at 7.9 by 7.9 foot (2.4
x 2.4 m) intervals [26]. However, where trees were spaced at 16.5 by
16.5 foot (4.9 x 4.9 m) intervals, oval-leaf huckleberry seedlings were
much more abundant and produced flowers [26].
Wildlife: Huckleberries are an extremely important food source for
grizzly bears [44]. Both black and grizzly bears typically exploit
areas with dense concentrations of berries. The habitat value of
huckleberry shrubfields to grizzly bears can be increased by permanent
or at least seasonal road closures, by coordinating timber harvest dates
to have minimal impact on habitat use patterns, and by considering the
cumulative effects of habitat modification across a broad area. In
general, site preparation should include minimizing soil compaction,
using cooler broadcast burns rather than hot slash burns, or by
eliminating site preparation entirely wherever possible. Grizzly use
can be favored where hiding cover is retained by treating small,
irregular patches instead of large contiguous areas, and by leaving
stringers of timber within larger cuts [68].
Seasonal trail closures have been implemented in major oval-leaf or blue
huckleberry fields in British Columbia's Kokanee Glacier Provincial Park
in an attempt to reduce the likelihood of hiker-grizzly encounters [46].
In many areas, bear-human conflicts are much more likely to occur during
years of huckleberry crop failure [44,56] as wider-ranging hungry bears
encounter recreationists or wildland residents. Damage to crops and
beehives, and livestock losses also typically increase during poor
huckleberry years [56].
BOTANICAL AND ECOLOGICAL CHARACTERISTICS
SPECIES: Vaccinium ovalifolium
GENERAL BOTANICAL CHARACTERISTICS:
Oval-leaf huckleberry is a stout, erect or spreading, diffusely branched
deciduous shrub [24,26,35,58], which grows from 1.3 to 12 feet (0.4-4 m)
in height [35,47]. Plants often become low and broomy in response to
repeated browsing or under arctic and subarctic conditions where snow
depth limits height [1,8,31]. Height is also reduced under a dense
forest canopy where little light reaches the forest floor; tallest
individuals are generally found under openings in the forest canopy
[33]. Maximum annual growth rates in Alaska reportedly average 12
inches per year (30 cm) [26].
The slender, yellowish-green, glabrous twigs of oval-leaf huckleberry are
conspicuously angled [2,47]. Twigs turn a bright red when exposed to
sunlight [58]. Bark of older branches is grayish or grayish-brown
[29,35]. The thin, alternate leaves are entire or have inconspicuously
serrate margins [14,29,47]. Leaves are oval to elliptical but rounded
at the base and tip [26,35,58]. Leaves are glabrous and generally light
and glaucous below [8,35].
Pink, urn-shaped flowers are borne singly in the axils of leaves
[26,58]. Flowers are generally pollinated by long-tongued bees, such as
bumblebees [29]. The floral morphology of oval-leaf huckleberry has been
examined in detail [53]. Fruit of the oval-leaf huckleberry is a
bluish-purple, dark blue, or black berry with a distinct whitish bloom
[11,29]. The relatively large berries are round, spherical, or slightly
oblate, and seedy [2,26,47,63]. Each berry contains an average of 26
seeds [76], but individual berries may contain up to 150 seeds [26].
RAUNKIAER LIFE FORM:
Phanerophyte
Geophyte
REGENERATION PROCESSES:
Oval-leaf huckleberry is capable of reproducing vegetatively or through
seed. Vegetative regeneration appears to be of primary importance in
most western huckleberries (Vaccinium spp.) [44].
Vegetative regeneration: Oval-leaf huckleberry commonly sprouts from
dormant basal buds after repeated browsing or disturbances which damage
the crown [1,8,31]. Layering, which occurs in the absence of
disturbance, has also been reported [52]. Oval-leaf huckleberry is
rhizomatous and sprouting of these structures is reportedly the primary
means by which colonies expand [26].
Germination: Seeds of oval-leaf huckleberry have a short dormant period
and exhibit good germination when exposed to 15 days of warm
temperatures followed by 15 days of chilling [11,26]. Properly stored
seeds exhibit good germination under a regime of 14 hours of light at 82
degrees F (28 degrees C) followed by 10 hours of darkness at
temperatures averaging 55 degrees F (13 degrees C). Fresh seeds also
germinate successfully under these conditions, or when alternately
exposed to temperatures of 71 degrees F (22 degrees C) and 41 degrees F
(5 degrees C) [76]. Huckleberry seedlings first emerge in approximately
1 month and continue to emerge for long periods of time in the absence
of cold stratification [11]. Oval-leaf huckleberry exhibits 50 to 60
percent germination under favorable conditions and up to 93 percent
germination has been observed under optimal laboratory conditions [26].
A minimum of only 2 percent full light is required for germination and
seedlings can develop beneath a forest canopy. Seeds germinate on a
variety of substrates including decaying wood, humus, moss, and mineral
soil. Oval-leaf huckleberry typically produces seed annually, but large
amounts of seed are generally produced only in relatively open areas
such as in clearings or at forest margins [26]. Seeds are readily
dispersed by a wide variety of birds and mammals [29].
Seedbanking: Seedbanking does not appear to be an important
regenerative strategy in oval-leaf huckleberry. Although seeds can
remain viable for up to 12 years in storage, longevity under natural
conditions is believed to be limited [26]. In montane balsam fir (Abies
balsamea)-paper birch (Betula papyrifera) forests of Quebec, an average
of 6.25 oval-leaf huckleberry seeds per square meter was found within the
top 1.2 inches (3 cm) of soil, but none of the seeds were viable [49].
Seedling morphology and establishment: Seedling morphology of species
within the section Myrtillus is poorly known. In the oval-leaf
huckleberry, transition from immature to mature foliage can be either
abrupt or gradual [76]. Seedlings which develop gradually may be easily
confused with seedlings of the closely related Alaska huckleberry [76].
Evidence suggests that oval-leaf huckleberry is a "seedling banker."
Seedlings are capable of surviving in the forest understory until
disturbance creates conditions favorable for development. Large numbers
of slow-growing seedlings are commonly observed. Growth is typically
slow beneath a forest canopy, and seedlings often remain in the
cotyledon stage for more than two growing seasons. Best seedling
survival occurs in open old growth stands and in clearcuts. Survival is
often poor in immature forests [26].
SITE CHARACTERISTICS:
Oval-leaf huckleberry grows in cool, moist, submontane to subalpine
forests, on open slopes, and at the edges of bogs, meadows, and swamps
[14,29,32,35,78]. It often occurs on elevated microsites in poorly
drained areas [26]. Oval-leaf huckleberry is commonly absent from major
valley bottoms but does occur on subhydric, colluvial, and morainal
sites in smaller valley bottoms [1,26].
Soil: Most huckleberries (Vaccinium spp.) require acidic conditions and
can grow on infertile soils which have relatively small amounts of many
essential elements [40]. Oval-leaf huckleberry thrives on soils low in
nitrogen [78]. Oval-leaf huckleberry grows well on well-drained,
nutrient-poor to nutrient-rich soils with a pH of 4.0 to 5.0 [26,51,65].
Soils are derived from a wide variety of parent materials [26].
Climate: Sites range from dry to moist, but oval-leaf huckleberry is
generally most abundant on moderately moist sites [24,26,61]. Along the
coast of British Columbia, this shrub is associated with a cool
mesothermal climatic regime [78].
Elevation: Oval-leaf huckleberry grows from sea level to timberline [26]
with elevation ranging from 0 to 5,500 feet (0-1,678 m) [14]. In many
parts of the Northwest, it is particularly common at middle elevations
[47,58]. Elevational range by geographic location is as follows
[58,61]:
from 0 to 4,500 feet (0-1,364 m) Cascades
2,000 to 5,000 feet (606-1,515 m) w OR
SUCCESSIONAL STATUS:
Oval-leaf huckleberry is shade tolerant and can persist in undisturbed
forests dominated by species such as western hemlock or white spruce
(Picea glauca) [26,72]. It is a common constituent of climax old growth
Douglas-fir-western hemlock, Pacific silver fir, and moist western
hemlock forests of the Pacific Northwest [21,33,60,70], and of western
hemlock-western redcedar-Sitka spruce forests of southeastern Alaska
[75]. Seedlings grow in open, old growth stands or in clearcuts, but
often do poorly in dense, immature forests [26].
Oval-leaf huckleberry commonly appears soon after disturbance in parts of
western Washington [7] and elsewhere. Sprouts were observed on mudflow
surfaces in scorch and blowdown areas soon after the eruption of Mount
Saint Helens [73,74]. Oval-leaf huckleberry is also one of the first
"forest species" to colonize bog margins in parts of southeastern Alaska
[51]. This shrub often persists on cutover sites throughout its range
and frequently forms a "nearly continuous layer" on newly harvested
sites [24,26,42].
Oval-leaf huckleberry is prevalent in young stands which develop in
avalanche zones in parts of the northwestern Cascades of Washington.
Plants pioneer these sites through layering and sprouting after
aboveground portions of the parent plants are damaged. The ability to
sprout gives species such as oval-leaf huckleberry a competitive
advantage during early succession in these shrub communities. Stem
numbers of oval-leaf huckleberry reportedly reach a minimum 60 to 150
years after the initial disturbance. Advance regeneration subsequently
develops and replaces initial pioneers, producing a subsequent increase
in stem density [52].
Establishment of oval-leaf huckleberry may be slow where parent plants
were absent prior to disturbance. Clement [71] observed oval-leaf
huckleberry in mature climax forests and in young seral stands on
floodplain gravel bars along the west coast of Vancouver Island.
However, it was absent in early seral stands. No parent plants were
present prior to disturbance and establishment on the newly exposed
gravel bars proceeded slowly from offsite seed.
SEASONAL DEVELOPMENT:
In coastal Alaska, bud burst of oval-leaf huckleberry begins in May.
Growth proceeds rapidly, and most vegetative elongation is completed by
mid-June. Stem diameters continue to increase until mid-July, and leaf
senescence usually occurs by September. Along the coast of central
British Columbia, leaf senescence can begin in early September, although
some leaves remain on the shrubs until the end of October [26].
Oval-leaf huckleberry flowers before leaves reach one-half of their full
size [35]. Flowering and fruiting by geographic area has been
documented as follows [11,26,49,70]:
location flowering fruiting
coastal AK April-May mid-July-August
BC coast ---- late June
BC ---- June-August
w OR May-July ----
PQ May July-August
Pacific Northwest May-July ----
FIRE ECOLOGY
SPECIES: Vaccinium ovalifolium
FIRE ECOLOGY OR ADAPTATIONS:
Plants presumably sprout from the stem base or underground rhizomes
[26,31] after aboveground vegetation is destroyed by fire. Limited
seedling establishment may occasionally occur from offsite seed
dispersed by birds and mammals. However, seedling establishment is of
limited importance in most western huckleberries (Vaccinium spp.) [44].
Fire may occur infrequently on some moist sites occupied by oval-leaf
huckleberry. Martin [44] notes that "the role of fire in establishing
populations of western species [of huckleberry] or in maintaining new
ones, is not well-documented."
FIRE REGIMES:
Find fire regime information for the plant communities in which this
species may occur by entering the species name in the FEIS home page under
"Find Fire Regimes".
POSTFIRE REGENERATION STRATEGY:
Tall shrub, adventitious-bud root crown
Geophyte, growing points deep in soil
Initial-offsite colonizer (off-site, initial community)
FIRE EFFECTS
SPECIES: Vaccinium ovalifolium
IMMEDIATE FIRE EFFECT ON PLANT:
Basal portions of the stem sometimes survive after aboveground
vegetation is damaged by fire. Underground rhizomes [26] are presumably
afforded some protection by overlying soil and may survive fires which
consume the crown. As with many other species of huckleberry (Vaccinium
spp.), plants are most likely to be killed by hot, duff-consuming fires
[44]. Seeds of most huckleberries are susceptible to heat and onsite
seed is typically eliminated by fire [44].
PLANT RESPONSE TO FIRE:
Vegetative response: Oval-leaf huckleberry often sprouts from the stem
base [31] after aboveground vegetation is consumed by fire. Rhizome
sprouting may occur after fires remove or damage all aboveground
vegetation, including the stem base [26]. In related species of
Vaccinium, sprouting is much less likely after hot, duff-consuming fires
[44].
Seedling establishment: Limited postfire seedling establishment may
occur on some sites. Seedbanking does not appear to be an important
regenerative strategy in oval-leaf huckleberry [26]. Seeds of most
huckleberries appear to be of short viability and are readily killed by
heat [44]. Birds and mammals may transport some seed from offsite [29].
Rate of postfire recovery: The postfire recovery rate of oval-leaf
huckleberry appears variable. In many areas recovery is very slow [79].
Oval-leaf huckleberry was absent during the first growing season after a
moderate fire in southwestern British Columbia, and plants had not
regained preburn vigor by the third growing season [26]. However, in
parts of the Cascades, this shrub may be common on recently burned sites
[50]. Recovery has been documented as follows on two burned sites in
coastal British Columbia [42]:
1969 1970 1971
(preburn) (postburn) (postburn)
% frequency 10.0 10.0 1.7
% cover 0.1 0.2 0.1
1968 1969 1970 1971
(preburn) (postburn) (postburn) (postburn)
% frequency 47.0 8.0 23.9 20.5
% cover 2.8 0.1 0.5 0.2
FIRE MANAGEMENT CONSIDERATIONS:
Wildlife: Evidence suggests that fire suppression may be having an
adverse impact on bear habitat in some areas [64,68]. Once productive
seral berry fields are being invaded by conifers. Since plants beneath
a forest canopy generally produce few berries, fruit production has been
steadily declining [47]. Logging treatments which include severe soil
scarification or slash burns may also reduce berry production. Even
where timber harvest favors berry production, lack of cover in early
years can limit bear use. Wildfires often create diverse habitat
mosaics which incorporate elements of hiding cover and favor bear use
[68].
Prescribed fire: Prescribed fire has long been used to increase yields
in commercial low sweet blueberry (V. angustifolium) fields of the East
by naturally pruning decadent shoots [47,77]. Flower buds generally
tend to be more numerous on new shoots and periodic removal of old
shoots can increase fruit yield as well as enhance overall vigor [47].
Spring burns, conducted when the soil is moist, tend to be most
effective in promoting fruit production [77]. In the Great Lakes
Region, where disjunct populations of oval-leaf huckleberry occur, Krautz
[77] recommends burning huckleberry (Vaccinium spp.) stands with 4 to 5
years fuel accumulation during the early afternoon on warm, clear, sunny
days with average windspeeds of 5 to 10 miles per hour (6-8 km/hour).
Fast-moving fire fronts which burn aboveground parts but leave
underground regenerative structures intact generally produce best
results. Therefore, when increased huckleberry fruit production is a
primary management objective, head fires are preferable to backing
fires. Supportive ignition (repeated ignitions) is generally required
when burning huckleberry stands in the East. In the Great Lakes Region,
areas to be burned should be rotated over a 4- to 5-year interval to
maintain adequate berry production for recreationists and wildlife [77].
Minore [47] has considered the effects of prescribed fire on the blue
huckleberry (V. membranaceum) in the Northwest [see VACMEM], but little
is known about the specific effects of prescribed fire on fruit
production in oval-leaf huckleberry.
Berry production: Berry production in most western huckleberries is
generally reduced for at least 5 years after fire [44]. On some sites,
berry production may be significantly reduced for 20 to 30 years or more
[44]. Reduced initial berry production is probable after fires in
oval-leaf huckleberry fields of western North America. Abundance is
often reduced after fires used for site preparation in British Columbia
[79].
REFERENCES
SPECIES: Vaccinium ovalifolium
REFERENCES:
1. Achuff, Peter L. 1989. Old-growth forests of the Canadian Rocky Mountain
national parks. Natural Areas Journal. 9(1): 12-26. [7442]
2. Anderson, J. P. 1959. Flora of Alaska and adjacent parts of Canada.
Ames, IA: Iowa State University Press. 543 p. [9928]
3. Banner, Allen; Pojar, Jim; Trowbridge, Rick; Hamilton, Anthony. 1986.
Grizzly bear habitat in the Kimsquit River Valley, coastal British
Columbia: classification, description, and mapping. In: Contreras, Glen
P.; Evans, Keith E., compilers. Proceedings--grizzly bear habitat
symposium; 1985 April 30 - May 2; Missoula, MT. Gen. Tech. Rep. INT-207.
Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain
Research Station: 36-49. [10810]
4. Barclay-Estrup, P. 1987. A new shrub for Ontario: mountain bilberry,
Vaccinium membranaceum, in Pukaskwa National Park. Canadian
Field-Naturalist. 101(4): 526-531. [6233]
5. Bernard, Stephen R.; Brown, Kenneth F. 1977. Distribution of mammals,
reptiles, and amphibians by BLM physiographic regions and A.W. Kuchler's
associations for the eleven western states. Tech. Note 301. Denver, CO:
U.S. Department of the Interior, Bureau of Land Management. 169 p.
[434]
6. Bovey, Rodney W. 1977. Response of selected woody plants in the United
States to herbicides. Agric. Handb. 493. Washington, DC: U.S. Department
of Agriculture, Agricultural Research Service. 101 p. [8899]
7. Brown, Ellsworth R. 1961. The black-tailed deer of western Washington.
Biological Bulletin No. 13. [Place of publication unknown]: Washington
State Game Commission. 124 p. [8843]
8. Camp, W. H. 1942. A survey of the American species of Vaccinium,
subgenus Euvaccinium. Brittonia. 4: 205-247. [6950]
9. Camp, W. H. 1942. On the structure of populations in the genus
Vaccinium. Brittonia. 4(2): 189-204. [9512]
10. Camp, W. H. 1945. The North American blueberries with notes on other
groups of Vacciniaceae. Brittonia. 5(3): 203-275. [9515]
11. Crossley, John A. 1974. Vaccinium L. Blueberry. In: Schopmeyer, C. S.,
ed. Seeds of woody plants in the United States. Agric. Handb. 450.
Washington, DC: U.S. Department of Agriculture, Forest Service: 840-843.
[7774]
12. Dahlgreen, Matthew Craig. 1984. Observations on the ecology of Vaccinium
membranaceum Dougl. on the southeast slope of the Washington Cascades.
Seattle, WA: University of Washington. 120 p. Thesis. [2131]
13. Daubenmire, R. 1969. Ecologic plant geography of the Pacific Northwest.
Madrono. 20: 111-128. [740]
14. Dayton, William A. 1931. Important western browse plants. Misc. Publ.
101. Washington, DC: U.S. Department of Agriculture. 214 p. [768]
15. del Moral, Roger; Fleming, Richard S. 1979. Structure of coniferous
forest communities in western Washington: diversity and ecotype
properties. Vegetatio. 41(3): 143-154. [7495]
16. del Moral, Roger; Long, James N. 1977. Classification of montane forest
community types in the Cedar River drainage of western Washington,
U.S.A. Canadian Journal of Forest Research. 7: 217-225. [8778]
17. Ellison, Laurence. 1966. Seasonal foods and chemical analysis of winter
diet of Alaskan spruce grouse. Journal of Wildlife Management. 30(4):
729-735. [9735]
18. Eyre, F. H., ed. 1980. Forest cover types of the United States and
Canada. Washington, DC: Society of American Foresters. 148 p. [905]
19. Franklin, Jerry Forest. 1966. Vegetation and soils in the subalpine
forests of the southern Washington Cascade Range. Pullman, WA:
Washington State University. 132 p. Thesis. [10392]
20. Franklin, Jerry F. 1983. Ecology of noble fir. In: Oliver, Chadwick
Dearing; Kenady, Reid M., eds. Proceedings of the biology and management
of true fir in the Pacific Northwest symposium; 1981 February 24-26;
Seattle-Tacoma, WA. Contribution No. 45. Seattle, WA: University of
Washington, College of Forest Resources: 59-69. [7783]
21. Franklin, Jerry F.; Dyrness, C. T. 1973. Natural vegetation of Oregon
and Washington. Gen. Tech. Rep. PNW-8. Portland, OR: U.S. Department of
Agriculture, Forest Service, Pacific Northwest Forest and Range
Experiment Station. 417 p. [961]
22. Fonda, R. W. 1974. Forest succession in relation to river terrace
development in Olympic National Park, Washington. Ecology. 55(5):
927-942. [6746]
23. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; [and others].
1977. Vegetation and environmental features of forest and range
ecosystems. Agric. Handb. 475. Washington, DC: U.S. Department of
Agriculture, Forest Service. 68 p. [998]
24. Green, R. N.; Courtin, P. J.; Klinka, K.; [and others]. 1984. Site
diagnosis, tree species selection, and slashburning guidelines for the
Vancouver Forest Region. Land Management Handbook Number 8. Abridged
version. Burnaby, BC: Ministry of Forests, Vancouver Forest Region. 143
p. [9475]
25. Grier, Charles C. 1978. A Tsuga heterophylla- Picea sitchensis ecosystem
of coastal Oregon: decomposition and nutrient balances of fallen logs.
Canadian Journal of Forest Research. 8: 198-206. [8512]
26. Haeussler, S.; Coates, D. 1986. Autecological characteristics of
selected species that compete with conifers in British Columbia: a
literature review. Land Management Report No. 33. Victoria, BC: Ministry
of Forests, Information Services Branch. 180 p. [1055]
27. Haeussler, S.; Pojar, J.; Geisler, B. M.; [and others]. 1985. A guide to
the interior cedar-hemlock zone, northwestern transitional subzone
(ICHg), in the Prince Rupert Forest Region, British Columbia. Land
Management Report Number 26; ISSN 0702-9861. Victoria, BC: British
Columbia, Ministry of Forests. 263 p. [6930]
28. Hall, Frederick C. 1974. Prediction of plant community development and
its use in management. In: Black, Hugh C., ed. Wildlife and forest
management in the Pacific Northwest: Proceedings of a symposium; 1973
September 11-12; Corvallis, OR. Corvallis, OR: Oregon State University,
School of Forestry, Forest Research Laboratory: 113-119. [7998]
29. Halverson, Nancy M., compiler. 1986. Major indicator shrubs and herbs on
National Forests of western Oregon and southwestern Washington.
R6-TM-229. Portland, OR: U.S. Department of Agriculture, Forest Service,
Pacific Northwest Region. 180 p. [3233]
30. Hanley, Thomas A.; McKendrick, Jay D. 1983. Seasonal changes in chemical
composition and nutritive values of native forages in a spruce-hemlock
forests, southeastern Alaska. Res. Pap. PNW-312. Portland, OR: U.S.
Department of Agriculture, Forest Service, Pacific Northwest Forest and
Range Experiment Station. 41 p. [8770]
31. Hayes, Doris W.; Garrison, George A. 1960. Key to important woody plants
of eastern Oregon and Washington. Agric. Handb. 148. Washington, DC:
U.S. Department of Agriculture, Forest Service. 227 p. [1109]
32. Hebda, Richard J. 1979. Size productivity and paleoecological
implications of ericaceous pollen from Burns Bog, southern Fraser River
Delta, British Columbia. Canadian Journal of Botany. 57(16): 1712-1717.
[10154]
33. Hines, William Wester. 1971. Plant communities in the old-growth forests
of north coastal Oregon. Corvallis, OR: Oregon State University. 146 p.
Thesis. [10399]
34. Hitchcock, C. Leo; Cronquist, Arthur. 1973. Flora of the Pacific
Northwest. Seattle, WA: University of Washington Press. 730 p. [1168]
35. Hitchcock, C. Leo; Cronquist, Arthur; Ownbey, Marion. 1959. Vascular
plants of the Pacific Northwest. Part 4: Ericaceae through
Campanulaceae. Seattle, WA: University of Washington Press. 510 p.
[1170]
36. Hunn, Eugene S.; Norton, Helen H. 1984. Impact of Mt. St. Helens ashfall
on fruit yields of mountain huckleberry Vaccinium membranaceum,
important Native American food. Economic Botany. 38(1): 121-127. [9501]
37. Iwagaki, H.; Ishikawa, S.; Tamada, T.; Koike, H. 1977. The present
status of blueberry work and wild Vaccinium species in Japan. Acta
Horticulturae. 61: 331-334. [9701]
38. Kartesz, John T.; Kartesz, Rosemarie. 1980. A synonymized checklist of
the vascular flora of the United States, Canada, and Greenland. Volume
II: The biota of North America. Chapel Hill, NC: The University of North
Carolina Press; in confederation with Anne H. Lindsey and C. Richie
Bell, North Carolina Botanical Garden. 500 p. [6954]
39. Kessell, Stephen R. 1979. Comparison of community stratification methods
in Mount Rainier National Park and Glacier National Park. Unpublished
preliminary report on file with: U.S. Department of Agriculture, Forest
Service, Intermountain Research Station, Fire Sciences Lab, Missoula,
MT. 154 p. [6678]
40. Korcak, Ronald F. 1988. Nutrition of blueberry and other calcifuges.
Horticultural Reviews. 10: 183-227. [9612]
41. Kuchler, A. W. 1964. Manual to accompany the map of potential vegetation
of the conterminous United States. Special Publication No. 36. New York:
American Geographical Society. 77 p. [1384]
42. Lafferty, R. R. 1972. Regeneration & plant success. as related to fire
intensity on clear-cut logged areas in coastal cedar-hemlock type: an
interim report. Internal Report BC-33. Victoria, BC: Department of the
Environment, Canadian Forestry Service, Pacific Forest Research Centre.
129 p. Unpublished report on file with: U.S. Department of Agriculture,
Forest Service, Intermountain Research Station, Fire Sciences Lab,
Missoula, MT. [9985]
43. Lyon, L. Jack; Stickney, Peter F. 1976. Early vegetal succession
following large northern Rocky Mountain wildfires. In: Proceedings, Tall
Timbers fire ecology conference and Intermountain Fire Research Council
fire and land management symposium; 1974 October 8-10; Missoula, MT. No.
14. Tallahassee, FL: Tall Timbers Research Station: 355-373. [1496]
44. Martin, Patricia A. E. 1979. Productivity and taxonomy of the Vaccinium
globulare, V. membranaceum complex in western Montana. Missoula, MT:
University of Montana. 136 p. Thesis. [9130]
45. Martin, Alexander C.; Zim, Herbert S.; Nelson, Arnold L. 1951. American
wildlife and plants. New York: McGraw-Hill Book Company, Inc. 500 p.
[4021]
46. McCrory, Wayne; Herrero, Stephen; Whitfield, Phil. 1986. Using grizzly
bear habitat information to reduce human-grizzly bear conflicts in
Kokanee Glacier and Valhalla Provincial Parks, B. C. In: Contreras, Glen
P.; Evans, Keith E., compilers. Proceedings--grizzly bear habitat
symposium; 1985 April 30 - May 2; Missoula, MT. Gen. Tech. Rep. INT-207.
Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain
Research Station: 24-30. [10809]
47. Minore, Don. 1972. The wild huckleberries of Oregon and Washington -- a
dwindling resource. PNW-143. Portland, OR: U.S. Department of
Agriculture, Forest Service, Pacific Northwest Forest and Range
Experiment Station. 20 p. [8952]
48. Moir, W. H.; Hobson, F. D.; Hemstrom, M.; Franklin, J. F. 1979. Forest
ecosystems of Mount Rainier National Park. In: Linn, Robert M., ed.
Proceedings, 1st conference on scientific research in the National
Parks: Vol I; 1976 Nov. 9-12; New Orleans, LA. National Park Service
Transactions and Proceedings Series No. 5. Washington, DC: U.S.
Department of the Interior, National Park Service: 201-207. [1674]
49. Morin, Hubert; Payette, Serge. 1988. Buried seed populations in the
montane, subalpine, and alpine belts of Mont Jacques-Cartier, Quebec.
Canadian Journal of Botany. 66: 101-107. [6376]
50. Morris, William G. 1958. Influence of slash burning on regeneration,
other plant cover, and fire hazard in the Douglas-fir region (A progress
report). Res. Pap. PNW-29. Portland, OR: U.S. Department of Agriculture,
Forest Service, Pacific Northwest Forest and Range Experiment Station.
49 p. [4803]
51. Neiland, Bonita J. 1971. The forest-bog complex of southeast Alaska.
Vegetatio. 22: 1-64. [8383]
52. Oliver, Chadwick D.; Adams, A. B.; Zasoski, Robert J. 1985. Disturbance
patterns and forest development in a recently deglaciated valley in the
northwestern Cascade Range of Washington, U.S.A. Canadian Journal of
Forest Research. 15: 221-232. [6387]
53. Palser, Barbara F. 1961. Studies of floral morphology in the Ericales.
V. Organography and vascular anatomy in several United States species of
the Vacciniaceae. Botanical Gazette. 123(2): 79-111. [9032]
54. Raunkiaer, C. 1934. The life forms of plants and statistical plant
geography. Oxford: Clarendon Press. 632 p. [2843]
55. Reich, Lee. 1988. Backyard blues. Organic Gardening. 35(6): 28-34.
[9179]
56. Rogers, Lynn. 1976. Effects of mast and berry crop failures on survival,
growth, and reproductive success of black bears. Transactions, North
American Wildlife Conference. 41: 431-438. [8951]
57. Rowe, J. S.; Scotter, G. W. 1973. Fire in the boreal forest. Quaternary
Research. 3: 444-464. [72]
58. Schultz, Joseph Herbert. 1944. Some cytotaxonomic and germination
studies in the genus Vaccinium. Pullman, WA: Washington State
University. 115 p. Thesis. [10285]
59. Schwartz, John E., II; Mitchell, Glen E. 1945. The Roosevelt elk on the
Olympic Peninsula, Washington. Journal of Wildlife Management. 9(4):
295-319. [8878]
60. Sonnenfeld, Nancy L. 1987. A guide to the vegetative communities at the
Valley of the Giants, Outstanding Natural Area, northwestern Oregon,
USA. Arboricultural Journal. 11: 209-225. [7453]
61. Topik, Christopher; Hemstrom, Miles A., compilers. 1982. Guide to common
forest-zone plants: Willamette, Mt. Hood, and Siuslaw National Forests.
Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific
Northwest Region. 95 p. [3234]
62. Stiles, Edmund W. 1980. Patterns of fruit presentation and seed
dispersal in bird-disseminated woody plants in the Eastern deciduous
forest. American Naturalist. 116(5): 670-688. [6508]
63. U.S. Department of Agriculture, Forest Service. 1937. Range plant
handbook. Washington, DC. 532 p. [2387]
64. Unsworth, James W.; Beecham, John J.; Irby, Lynn R. 1989. Female black
bear habitat use in west-central Idaho. Journal of Wildlife Management.
53(3): 668-673. [8407]
65. Van Dersal, William R. 1938. Native woody plants of the United States,
their erosion-control and wildlife values. Washington, DC: U.S.
Department of Agriculture. 362 p. [4240]
66. Viereck, L. A.; Dyrness, C. T. 1979. Ecological effects of the
Wickersham Dome Fire near Fairbanks, Alaska. Gen. Tech. Rep. PNW-90.
Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific
Northwest Forest and Range Experiment Station. 71 p. [6392]
67. Williams, Carroll B.; Dyrness, C. T. 1967. Some characteristics of
forest floors and soils under true fir-hemlock stands in the Cascade
Range. PNW-37. Portland, OR: U.S. Department of Agriculture, Forest
Service, Pacific Northwest Forest and Range Experiment Station. 19 p.
[8181]
68. Zager, Peter Edward. 1980. The influence of logging and wildfire on
grizzly bear habitat in northwestern Montana. Missoula, MT: University
of Montana. 131 p. Dissertation. [5032]
69. Odell, A. E.; Vander Kloet, S. P.; Newell, R. E. 1989. Stem anatomy of
Vaccinium section Cyanococcus and related taxa. Canadian Journal of
Botany. 67(8): 2328-2334. [8944]
70. Lepofsky, Dana; Turner, Nancy J.; Kuhnlein, Harriet V. 1985. Determining
the availability of traditional wild plant foods: an example of Nuxalk
foods, Bella Coola, British Columbia. Ecology of Food and Nutrition. 16:
223-241. [7002]
71. Clement, C. J. E. 1985. Floodplain succession on the west coast of
Vancouver Island. Canadian Field-Naturalist. 99(1): 34-39. [8928]
72. Eis, S. 1981. Effect of vegetative competition on regeneration of white
spruce. Canadian Journal of Forest Research. 11: 1-8. [10104]
73. Halpern, Charles B.; Harmon, Mark E. 1983. Early plant succession on the
Muddy River mudflow, Mount St. Helens, Washington. American Midland
Naturalist. 110(1): 97-106. [8870]
74. Means, Joseph E.; McKee, W. Arthur; Moir, William H.; Franklin, Jerry F.
1982. Natural revegetation of the northeastern portion of the devestated
area. In: Keller, S. A, C.; ed. Mount St. Helens: one year later:
Proceedings of a symposium; 1981 May 17-18; Cheney, WA. Cheney, WA:
Eastern Washington University Press: 93-103. [5977]
75. Taylor, R. F. 1932. The successional trend and its relation to
second-growth forests in southeastern Alaska. Ecology. 13(4): 381-391.
[10007]
76. Vander Kloet, S. P. 1983. Seed and seedling characters in Vaccinium
Myrtillus. Naturaliste Canadien. 110: 285-292. [10592]
77. Kautz, Edward W. 1987. Prescribed fire in blueberry management. Fire
Management Notes. 48(3): 9-12. [9848]
78. Klinka, K.; Krajina, V. J.; Ceska, A.; Scagel, A. M. 1989. Indicator
plants of coastal British Columbia. Vancouver, BC: University of British
Columbia Press. 288 p. [10703]
79. Hawkes, B. C.; Feller, M. C.; Meehan, D. 1990. Site preparation: fire.
In: Lavender, D. P.; Parish, R.; Johnson, C. M.; [and others], eds.
Regenerating British Columbia's forests. Vancouver, BC: University of
British Columbia Press: 131-149. [10712]
80. Stickney, Peter F. 1989. Seral origin of species originating in northern
Rocky Mountain forests. Unpublished draft on file at: U.S. Department of
Agriculture, Forest Service, Intermountain Research Station, Fire
Sciences Laboratory, Missoula, MT; RWU 4403 files. 7 p. [20090]
81. U.S. Department of Agriculture, Natural Resources Conservation Service. 2018.
PLANTS Database, [Online]. U.S. Department of Agriculture, Natural Resources
Conservation Service (Producer). Available: https://plants.usda.gov/. [34262]
82. U.S. Department of the Interior, National Biological Survey. [n.d.]. NP
Flora [Data base]. Davis, CA: U.S. Department of the Interior, National
Biological Survey. [23119]
FEIS Home Page

