| FEIS Home Page |
 |
|
| Squirreltail in fruit in Wind Cave National Park. Wikimedia Commons image by Jim Pisarowicz. |
AUTHORSHIP AND CITATION:
Simonin, Kevin A. 2001. Elymus elymoides.
In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: https://www.fs.usda.gov
/database/feis/plants/graminoid/elyely/all.html
[].
ABBREVIATION:
ELYELY
SYNONYMS:
Sitanion hystrix (Nutt.) J. G. Smith [51,82,115,123,132]
NRCS PLANT CODE [193]:
ELEL5
COMMON NAMES:
squirreltail
bottlebrush squirreltail
TAXONOMY:
The scientific name of squirreltail is Elymus elymoides
(Raf.) Swezey [60,87,113] (Poaceae). Barkworth and Dewey [12] realigned Sitanion hystrix (Nuttall) J. G. Smith
in the Elymus genus as Elymus elymoides. Realignment of the Elymus genus
is based upon morphological and genomic characters [12,56].
The following subspecies are currently recognized: Elymus elymoides ssp. brevifolius,
E. e. ssp. californicus, E. e. ssp. elymoides, and E.
e. ssp. hordeoides [93].
Squirreltail hybridizes frequently with other
Elymus species and infrequently with Hordeum species [200].
Squirreltail also hybridizes with saline wildrye (Leymus salinus)
[106].
LIFE FORM:
Graminoid
FEDERAL LEGAL STATUS:
No special status
OTHER STATUS:
No entry
GENERAL DISTRIBUTION:
Squirreltail is found from British Columbia to Saskatchewan, south throughout
the western and central United States and into Mexico [200]. The PLANTS database
provides a distributional map for squirreltail.
Elymus elymoides subsp. brevifolius occurs in the San Bernardino
Mountains,
Peninsular Ranges, Modoc Plateau, and Mojave Desert of California to Oregon, the Great Plains
and south to northern Mexico. Elymus elymoides subsp. californicus is found in the
Klamath Range, Cascade Range, Sierra Nevada, San Gabriel Mountains, San Bernardino Mountains,
east Sierra Nevada of California to Washington, Montana and Utah. Elymus elymoides subsp.
elymoides is found in the Transverse Ranges, San Jacinto Mountains, and Great Basin floristic Province from
California to Washington, Wyoming and Colorado. Elymus elymoides subsp.
hordeoides occurs in Klamath Range from California to Washington and Nevada.
ECOSYSTEMS [80]:
FRES20 Douglas-fir
FRES21 Ponderosa pine
FRES23 Fir-spruce
FRES26 Lodgepole pine
FRES28 Western hardwoods
FRES29 Sagebrush
FRES30 Desert shrub
FRES32 Texas savanna
FRES33 Southwestern shrubsteppe
FRES34 Chaparral-mountain shrub
FRES35 Pinyon-juniper
FRES36 Mountain grasslands
FRES38 Plains grasslands
FRES40 Desert grasslands
FRES44 Alpine
| AZ | CA | CO | ID |
| IL | KS | KY | MI |
| MT | NE | NV | NM |
| ND | OK | OR | SD |
| TX | UT | WA | WY |
| AB | BC | MB | SK |
| MEXICO |
BLM PHYSIOGRAPHIC REGIONS [22]:
3 Southern Pacific Border
4 Sierra Mountains
5 Columbia Plateau
6 Upper Basin and Range
7 Lower Basin and Range
8 Northern Rocky Mountains
9 Middle Rocky Mountains
10 Wyoming Basin
11 Southern Rocky Mountains
12 Colorado Plateau
13 Rocky Mountain Piedmont
14 Great Plains
15 Black Hills Uplift
KUCHLER [121] PLANT ASSOCIATIONS:
K005 Mixed conifer forest
K007 Red fir forest
K008 Lodgepole pine-subalpine forest
K010 Ponderosa shrub forest
K011 Western ponderosa forest
K012 Douglas-fir forest
K016 Eastern ponderosa forest
K017 Black Hills pine forest
K018 Pine-Douglas-fir forest
K019 Arizona pine forest
K020 Spruce-fir-Douglas-fir forest
K021 Southwestern spruce-fir forest
K022 Great Basin pine forest
K023 Juniper-pinyon woodland
K024 Juniper steppe woodland
K026 Oregon oakwoods
K030 California oakwoods
K031 Oak-juniper woodland
K032 Transition between K031 and K037
K037 Mountain-mahogany-oak scrub
K038 Great Basin sagebrush
K039 Blackbrush
K040 Saltbush-greasewood
K041 Creosotebush
K046 Desert: vegetation largely lacking
K050 Fescue-wheatgrass
K052 Alpine meadows and barren
K053 Grama-galleta steppe
K054 Grama-tobosa prairie
K055 Sagebrush steppe
K056 Wheatgrass-needlegrass shrubsteppe
K057 Galleta-threeawn shrubsteppe
K059 Trans-Pecos shrub savanna
K060 Mesquite savanna
K064 Grama-needlegrass-wheatgrass
K065 Grama-buffalo grass
K066 Wheatgrass-needlegrass
K085 Mesquite-buffalo grass
K086 Juniper-oak savanna
SAF COVER TYPES [71]:
66 Ashe juniper-redberry (Pinchot) juniper
68 Mesquite
207 Red fir
209 Bristlecone pine
210 Interior Douglas-fir
217 Aspen
218 Lodgepole pine
220 Rocky Mountain juniper
229 Pacific Douglas-fir
237 Interior ponderosa pine
238 Western juniper
239 Pinyon-juniper
241 Western live oak
242 Mesquite
243 Sierra Nevada mixed conifer
244 Pacific ponderosa pine-Douglas-fir
246 California black oak
250 Blue oak-foothills pine
256 California mixed subalpine
SRM (RANGELAND) COVER TYPES [176]:
101 Bluebunch wheatgrass
102 Idaho fescue
104 Antelope bitterbrush-bluebunch wheatgrass
105 Antelope bitterbrush-Idaho fescue
106 Bluegrass scabland
107 Western juniper/big sagebrush/bluebunch wheatgrass
108 Alpine Idaho fescue
109 Ponderosa pine shrubland
110 Ponderosa pine-grassland
201 Blue oak woodland
207 Scrub oak mixed chaparral
210 Bitterbrush
211 Creosotebush scrub
212 Blackbush
301 Bluebunch wheatgrass-blue grama
310 Needle-and-thread-blue grama
314 Big sagebrush-bluebunch wheatgrass
315 Big sagebrush-Idaho fescue
318 Bitterbrush-Idaho fescue
320 Black sagebrush-bluebunch wheatgrass
321 Black sagebrush-Idaho fescue
322 Curlleaf mountain-mahogany-bluebunch wheatgrass
401 Basin big sagebrush
403 Wyoming big sagebrush
405 Black sagebrush
406 Low sagebrush
407 Stiff sagebrush
408 Other sagebrush types
409 Tall forb
410 Alpine rangeland
412 Juniper-pinyon woodland
413 Gambel oak
414 Salt desert shrub
415 Curlleaf mountain-mahogany
416 True mountain-mahogany
417 Littleleaf mountain-mahogany
501 Saltbush-greasewood
502 Grama-galleta
503 Arizona chaparral
504 Juniper-pinyon pine woodland
509 Transition between oak-juniper woodland and mahogany-oak association
607 Wheatgrass-needlegrass
611 Blue grama-buffalo grass
612 Sagebrush-grass
614 Crested wheatgrass
615 Wheatgrass-saltgrass-grama
704 Blue grama-western wheatgrass
712 Galleta-alkali sacaton
713 Grama-muhly-threeawn
714 Grama-bluestem
715 Grama-buffalo grass
718 Mesquite-grama
727 Mesquite-buffalo grass
735 Sideoats grama-sumac-juniper
HABITAT TYPES AND PLANT COMMUNITIES:
Squirreltail is a common component of sagebrush (Artemisia spp.)/grass
communities of the Intermountain shrubsteppe [109,212].
Within rangelands of Utah, Nevada, southern Idaho, and western Wyoming, squirreltail
commonly grows under and adjacent to shadscale (Atriplex confertifolia), black greasewood
(Sarcobatus vermiculatus), and green rabbitbrush (Chrysothamnus viscidiflorus)
[161]. Bottlebrush
squirreltail is a common component of pinyon-juniper (Pinus spp.-Juniperus spp.)
communities of the Great Basin. It occurs mostly within the mountain ranges of Nevada and Utah,
and to a lesser extent in California and Idaho [191].
Arizona:
Squirreltail occurs in northern desert shrub communities [137] and
ponderosa pine (Pinus ponderosa) forests [39,137]. Within aspen (Populus tremuloides)-bunchgrass
communities of northern Arizona, squirreltail commonly occurs with
Arizona fescue (Festuca arizonica), mountain muhly (Muhlenbergia montana), western yarrow
(Achillea millefolium), lupine (Lupinus spp.), fleabane (Erigeron spp.) and
American vetch (Vicia americana) [86]. Squirreltail is occasionally found
in openings and under shrub canopies within shrub live oak (Quercus turbinella)-mixed shrub
communities [173].
California:
Squirreltail is native to California's central valley
and is commonly associated with purple tussock grass (Nassella
pulchra), nodding tussock grass (N. cernua), smallflower tussockgrass
(N. lepida), and shooting star (Dodecatheon
spp.) [14].
It is a minor component of blue oak (Quercus douglasii) [28,126] and interior live oak
(Q. wislizenii) habitats [126].
Within sagebrush scrub of the White Mountains, prairie Junegrass
(Koeleria macrantha), muhly (Muhlenbergia spp.) and timberline bluegrass (Poa
glauca spp. rupicola) are common associates [130]. Within alluvial
fans of desert shrub communities, squirreltail is commonly associated
with Indian
ricegrass (Achnatherum hymenoides)
and desert needlegrass (A. speciosum). Shrub associates of squirreltail
include California jointfir (Ephedra californica), goldenfleece (Ericameria arborescens),
white burrobrush (Hymenoclea salsola), and purple sage (Salvia dorrii) [135].
Squirreltail occasionally occurs in alpine flora of the Sierra Nevada eastern
slope [40]. It is also an occasional understory species of California red fir (Abies magnifica)
forests in the Sierra Nevada [155].
Colorado:
Squirreltail is a member
of north-central Colorado's short-grass prairie [45,57,175]. Within the short-grass
prairie, squirreltail is commonly associated with western wheatgrass (Pascopyrum smithii),
purple threeawn (Aristida purpurea), sideoats grama (Bouteloua curtipendula),
blue grama (Bouteloua gracilis), buffalo grass (Buchloe dactyloides), and needle-and-thread
grass (Hesperostipa comata) [57]. It occurs in blue grama ranges of Colorado
along with sun sedge (Carex heliophila), sand dropseed (Sporobolus cryptandrus),
and purple threeawn [139].
Within Colorado pinyon-Utah juniper (Pinus edulis-Juniperus osteosperma) habitats of Colorado, squirreltail
is associated with Gambel oak (Quercus gambelii), Utah serviceberry (Amelanchier utahensis),
true mountain-mahogany (Cercocarpus montanus), fendlerbush (Fendlera rupicola),
banana yucca (Yucca baccata), and antelope bitterbrush (Purshia tridentata). Grass
associates include cheatgrass (Bromus tectorum), Indian ricegrass, mutton grass
(Poa fendleriana), and brome grasses (Bromus spp.) [67].
Squirreltail is an occasional associate of Rocky Mountain bristlecone pine
(Pinus aristata) [162].
Idaho:
Squirreltail is a dominant species in shadscale communities of south-central
Idaho [174], along with Indian ricegrass [189].
Montana:
Squirreltail generally occurs as scattered plants on rangelands [150].
In the eastern plains of Montana, squirreltail is a dominant species
of saline rangelands in association with alkali sacaton (Sporobolus airoides),
western wheatgrass (Pascopyrum smithii), thickspike wheatgrass (Elymus lanceolatus), inland saltgrass
(Distichlis stricta), Sandberg bluegrass (Poa secunda), and basin wildrye
(Leymus cinereus). Shrub associates include black greasewood and
Nuttall's saltbush (Atriplex nuttallii).
Nevada:
Squirreltail occurs
in gray low sagebrush (Artemisia arbuscula ssp. arbuscula) and big sagebrush (A. tridentata)
communities. Principal grass associates include cheatgrass [24], Sandberg bluegrass [24,25],
bluebunch wheatgrass (Pseudoroegneria spicata), and Thurber needlegrass (Achnatherum thurberianum).
Common shrub associates include green rabbitbrush and
gray horsebrush (Tetradymia canescens). Forb associates include bird's-beak
(Cordylanthus ramosus), mourning milkvetch (Astragalus atratus),
woollypod milkvetch (Astragalus purshii),
desert yellow fleabane (Erigeron linearis), lava aster (Lonactis alpina),
Heerman's buckwheat (Eriogonum heermanii), tail cup lupine (Lupinus caudatus)
and phlox (Phlox longifolia) [25].
In northeastern Nevada squirreltail is commonly found with black sagebrush
(Artemisia nova) [107,219], shadscale, Nevada ephedra (Ephedra nevadensis),
Sandberg bluegrass and Indian ricegrass [107].
Squirreltail
also occurs in shadscale communities [24,145]. Common shrub associates include green molly
(Kochia americana), winterfat (Krascheninnikovia lanata), budsage
(Artemisia spinescens) and spiny hopsage (Grayia spinosa )
[145]. Common grass associates are cheatgrass, Indian ricegrass
[24] and galleta (Pleuraphis jamesii) [145]. Several common forb associates are
salt lover
(Halogeton glomeratus), Adonis blazingstar (Mentzelia multiflora) and gooseberryleaf
globemallow (Sphaeralcea grossulariifolia). Squirreltail is also common to
juniper (Juniperus spp.) and greasewood communities [24].
Squirreltail is found in Wyoming big sagebrush (Artemisia tridentata ssp.
wyomingensis) rangelands of Nevada [23].
Oregon:
Eastern Oregon grass associates of squirreltail include bluebunch wheatgrass,
prairie Junegrass [31], Idaho fescue (Festuca idahoensis),
Thurber needlegrass [31,59], Sandberg bluegrass and
cheatgrass. Common forbs include Hood's phlox (Phlox hoodii) and
maiden blue eyed Mary (Collinsia parviflora) [59]. Squirreltail
occurs in lodgepole pine stands in the
Cascades of Oregon [61], along with western needlegrass (Achnatherum occidentale)
and Ross' sedge (Carex rossii) [65].
Texas:
In western Texas squirreltail occurs in Pinchot juniper (Juniperus pinchotii)
rangelands with sideoats grama, buffalo grass,
slim tridens (Tridens muticus), awnless bushsunflower (Simsia calva) and
plains fleabane (Erigeron modestus) [143].
Utah:
Squirreltail is common in salt-desert shrub ranges along with the shrubs
shadscale (Atriplex confertifolia), mat saltbush (A. corrugata), fourwing
saltbush (A. canescens), valley saltbush (A. cunneata), greasewood,
winterfat, spiny hopsage, budsage, black sagebrush and
green rabbitbrush. Common grasses include Indian ricegrass,
Sandberg bluegrass, galleta,
alkali sacaton, sand dropseed, and
blue grama. Squirreltail is also
found in pinyon-juniper woodlands [29], ponderosa pine savannas [148] along with
dry Douglas-fir (Pseudotsuga menziesii) and mixed conifer habitats [29].
Wyoming:
Squirreltail commonly occurs in big sagebrush steppe along with
aspen, big sagebrush, mountain snowberry (Symphoricarpos oreophilus),
Utah serviceberry, rose (Rosa spp.),
Scouler's willow (Salix scouleriana),
and Oregon-grape (Mahonia repens). Common forb associates include
rosy pussytoes (Antennaria microphylla), arrowleaf buckwheat
(Eriogonum compositum), pineywoods geranium (Geranium caespitosum) and
northern bedstraw (Galium boreale). Graminoid associates include needle-and-thread grass,
Columbia needlegrass (Achnatherum nelsonii), and elk sedge (Carex geyeri) [36].
Classifications describing plant communities in which squirreltail is a dominant species are as
follows:
Idaho [97]
California [218]
Colorado [119]
New Mexico [94,146]
Nevada [24,25,107,125,192,221]
Oregon [59,101,197]
Utah [151,203]
Wyoming [186]
IMPORTANCE TO LIVESTOCK AND WILDLIFE:
Squirreltail is a dietary component of several wildlife species.
It is a minor component of bison and cattle
summer diets within sagebrush rangelands of southern Utah [195].
Although of little importance, squirreltail may provide forage for mule deer [122,124].
Pronghorn of western Utah feed upon squirreltail [16]. Townsend's ground squirrels [211],
Nuttall's cottontails [111,127], and black-tailed
jackrabbits [5,72,112,127] all feed upon squirreltail.
In southeastern Oregon salt desert-shrub ranges, squirreltail is an
important component of domestic livestock seasonal diets. Winter months show greatest use
[83,140].
PALATABILITY:
Squirreltail is a very palatable winter forage for domestic sheep of
Intermountain ranges. Domestic sheep relish the green foliage [104]. Overall, bottlebrush
squirreltail is considered moderately palatable to livestock.
When present, the long sharp awns of squirreltail greatly reduce
its palatability [150]. Mature awns may penetrate flesh around the mouth of grazing
animals, producing inflammation [51,115]. Eye and ear injury may also occur [51].
NUTRITIONAL VALUE:
Clary [44] compared chemical constituents (%) of squirreltail within
open and timbered ponderosa pine
overstories in Arizona.
Greater digestibility and significantly (p<0.05) higher crude protein
were found in open versus timbered overstories:
| Open | Timbered | |
| Crude protein (%) | 16.0 | 9.7 |
| Phosphorus (%) | 0.25 | 0.26 |
| Ash (%) | 12.3 | 13.7 |
| Digestibility (%) | 66.7 | 61.0 |
| Composition (%) | |
| Ether Extract | 2.6 |
| Total protein | 4.5 |
| Ash | 17.1 |
| Lignin | 8.7 |
| Cellulose | 37.5 |
| Other Carbohydrates | 29.6 |
| Phosphorus | 0.07 |
| Gross energy | 1730 (kcal/lb) |
| Carotene | 0.5 (mg/lb) |
COVER VALUE:
The degree of environmental protection provided by squirreltail for wildlife species is as follows
[58]:
| Utah | Wyoming | |
| Pronghorn | Poor | Poor |
| Elk | Poor | Poor |
| Mule deer | Poor | Poor |
| White-tailed deer | ---- | Poor |
| Small mammals | Good | Good |
| Small nongame birds | Fair | Good |
| Upland game birds | Fair | Fair |
| Waterfowl | Poor | Fair |
VALUE FOR REHABILITATION OF DISTURBED SITES:
Squirreltail is tolerant of disturbance [133]. The Bureau of Land Management,
U.S. Department of the Interior, identifies bottlebrush
squirreltail as a high priority species for restoring native plant diversity in the Great
Basin and the Columbia River
Plateau [90]. Squirreltail naturally colonizes disturbed sites
in Yellowstone National Park and is a component of seed mixtures used
for restoration of
lodgepole pine communities [129]. Brown and Amacher [34] recommend
squirreltail for use in restoration of disturbed arid to semi-arid,
desert shrub and pinyon-juniper
systems. Squirreltail is well adapted for seeding of Wyoming, black and low sagebrush communities
of the Intermountain West, receiving 9 to 13 inches (229-330 mm) annual precipitation. Squirreltail
grows well under rabbitbrush canopies in south-central Idaho rangelands [149].
Squirreltail
inhabits xeric sandy soils (73.9% sand, 16.8% silt, 9.2% clay,
1.3% organic matter) of a 50-year-old abandoned tailings pond from a Pb-Zn-processing mill [41], and is
recommended for seed mixtures used to reclaim strip mines in southeastern Montana [64].
The
large ecological amplitude of squirreltail lends to ecotypic
differentiation. Phenological differences in growth rate, root:shoot ratios, leaf area, and overall plant size
exist between subspecies of squirreltail. Differences are directly related to
subspecies distribution
[100]. Squirreltail seed source
should be considered when implementing revegetation projects. Arredondo and others [9] observed
a higher root length-to-leaf area ratio in plants grown from seed
collected from different environments. Differences in phenology between
individuals of different habitats are common (see: SEASONAL DEVELOPMENT
within the Botanical and Ecological Characteristics section for further information).
Squirreltail seed is available commercially [103,104,134].
The United States Department of Agriculture (USDA), Utah Division of Wildlife Resources,
in conjunction with the Intermountain Research Station, Forest Service, USDA,
established squirreltail seed quality standards. Seed quality standards
as of 1990 are summarized below [181]:
| Seed unit1 | Acceptable purity (%)2 | Acceptable viability (%)2 |
| spikelet with or without awns | 90 | 85 |
Germinability of primed squirreltail seed significantly (p<0.05)
decreases when dried and stored [89].
Competition with invasive weeds:
The
persistence of squirreltail in areas invaded by exotic weeds is well recognized. Bottlebrush
squirreltail persists in areas infested with cheatgrass [9,18,99,100,103,188],
medusahead (Taeniatherum caput-medusae) [9,96,169,213,216], and Japanese brome (Bromus japonicus) [166].
Squirreltail naturally invades rangelands
dominated by cheatgrass and medusahead [9]. However, mechanisms behind
squirreltail's ability to occupy weed-infested areas are
not completely understood. Several studies have evaluated the persistence
of squirreltail within cheatgrass infested ranges. Beckstead [18] found recently harvested bottlebrush
squirreltail seeds from mountain brush and meadow sites to possess lower levels of
dormancy than cheatgrass at higher temperatures, 68/86 degrees Fahrenheit (20/30 C), whereas the opposite was
true of lower temperatures, 41/59 degrees Fahrenheit (5/15 C).
Squirreltail at lower elevations (4,100 feet (1,250 m)) have a greater
probability of autumn germination than cheatgrass [2].
Established squirreltail plants generally
initiate growth before the rosettes of cheatgrass in desert rangelands of Nevada
[188].
Beckstead [18] suggests fall seeding of squirreltail into cheatgrass
infested rangelands.
Early spring growth and ability to grow at low temperatures contribute to the
persistence of squirreltail among cheatgrass dominated ranges [100]. Bottlebrush
squirreltail seedlings have the ability to grow roots at low soil
temperatures, allowing for soil penetration similar to medusahead and cheatgrass in the
northern regions of
the Great Basin. Root development at low temperatures promotes squirreltail
seedling establishment
and effective competition with medusahead [96].
Bottlebrush
squirreltail has potential to outcompete medusahead. Management goals often concentrate
on protecting squirreltail seedlings from livestock and
rabbits, along with maintaining a natural supply
of seed [169]. Hironaka and Sindelar [98] evaluated squirreltail growth under
greenhouse conditions,
when closely associated with medusahead. Squirreltail plants (10 plants)
were observed in combination
with 0, 4, 12, 36, 108, and 324 medusahead/foot2.
Squirreltail growth was not affected by medusahead until 5 weeks old, grown under densities of 108 and 324
medusahead/foot2. Although stunted, no
squirreltail mortality was seen at all densities tested, whereas a large
amount of medusahead mortality
was observed in the 324 medusahead/foot2 level. Squirreltail
acquired greater
root carbohydrate reserves than medusahead under competitive conditions.
Under proper management, Hironaka [96] suggests a successional
sequence of cheatgrass to medusahead to squirreltail dominated sites for northern Great Basin areas receiving greater than 11 inches
(279 mm) precipitation.
Rome and Eddelman [166] compared squirreltail seedling growth in competition
with Japanese brome at densities of 0,
50, 100, 200, 400 Japanese brome/m2. Observations were
made in Missoula, Montana at 23, 42, 56, 82, and 97 days following an 8
April seeding of squirreltail and Japanese brome. Squirreltail averaged
85% survival in areas without Japanese brome, compared to an average of 66% survival from areas
with 100 to 400 Japanese brome/m2 (p<0.05).
Overall, squirreltail under competition with Japanese brome showed the
greatest competitive ability at 100 Japanese brome/m2.
Martlette and Anderson [131] observed poor squirreltail seed dispersal into
adjacent crested wheatgrass (Agropyron cristatum) stands. Plant cover acted as a barrier restricting the
dispersal capabilities of squirreltail.
Under greenhouse conditions, Schlatterer and Tisdale [172] found sagebrush leaf litter
to significantly (p<0.05)
decrease squirreltail germination compared to moss and rabbitbrush
(Chrysothamnus spp.) litter. The average number of squirreltail seeds (20 seeds/pot)
germinating under different litter treatments is summarized below:
| Big sagebrush | Moss | Rabbitbrush | No litter |
| 11.25 | 18.75 | 18.25 | 18.25 |
OTHER USES AND VALUES:
No entry
OTHER MANAGEMENT CONSIDERATIONS:
The addition of nitrogen to disturbed sagebrush communities in Colorado [141] and mountain meadows of Nevada [62]
had no positive effect on
squirreltail establishment.
Squirreltail decreased after the addition of nutrients in the form of stabilized sewage
sludge [78].
Squirreltail reproductive potential is adversely affected by jointworm larvae.
Spears and Barr [179]
found culm length, seed weight, germination (%),
and germination rate all significantly lower (p<0.01) on squirreltail
infested with jointworms compared to uninfested plants. Results are summarized below
:
| Variable | Infested | Uninfested |
| Culm length (cm) | 30.0 | 33.7 |
| Leaf length (cm) | 22.0 | 23.1 |
| # of spikelets | 5 | 9 |
| Seed weight (mg 25 seeds) | 108.2 | 162.5 |
| Germination (%) | 20.0 | 66.0 |
| Growth rate (Seedlings day -1 100 seeds -1) | 2.8 | 7.8 |
Squirreltail's total available root carbohydrate reserves are lowest in early spring
(approximately 3rd leaf stage), and at the beginning of fall regrowth. Total available carbohydrates are highest after
anthesis [50].
By the 4th leaf stage, squirreltail has replaced the carbohydrate
reserves found in roots at the beginning of the growing season [20]. Wright [206]
found squirreltail most tolerant to herbage removal at
time of seed maturity, declining slightly after maturity before fall regrowth.
In eastern Oregon, squirreltail is resistant to late season defoliation [31]
Squirreltail generally increases in abundance when moderately grazed
or protected on the foothills of intermountain winter ranges [104].
Moderate trampling by livestock in big sagebrush rangelands of central Nevada enhanced
squirreltail seedling emergence compared to untrampled conditions. Heavy trampling
destroys germination sites and significantly
(p<0.05) reduces germination, whereas moderate trampling may
enhance germination [63].
Squirreltail is tolerant of grazing in big sagebrush rangelands of
southeastern Idaho [4].
In sagebrush rangelands of western Utah, Cook and Child [46] found winter harvesting
to have a minor effect on crown cover, whereas early spring (April 1, May 1) harvest greatly
reduced squirreltail cover.
Squirreltail vegetative vigor was evaluated over 25 years within a sagebrush rangeland
of southeastern Oregon excluded from grazing. Vigor of squirreltail increased
significantly over the 25 year period, with the 1st decade showing slower growth than the
2nd. The average annual precipitation over the 25 years equaled 8.3 inches (210 mm) with 40%
falling during April, May, and June. Winters were cold with snow cover from December to March.
Summers were hot, occasionally exceeding 100 degrees Fahrenheit (38 °C) [3].
Squirreltail is commonly found in heavily grazed and browsed
(cattle and deer) aspen stands of big sagebrush steppe in Wyoming [36].
McPherson and Wright [144] observed significantly (p<0.01) greater coverage of bottlebrush
squirreltail on ungrazed versus grazed Pinchot juniper rangelands in western Texas.
Within the ponderosa pine bunchgrass ranges of the central Rocky Mountains, squirreltail
production is greatest under light and moderate grazing regimes [52].
Squirreltail is tolerant of heavy grazing in the ponderosa pine zone of the Coconino Plateau, Arizona, since its long, sharp
awns are usually present to discourage grazing [8].
On shortgrass ranges of the central plains squirreltail is very tolerant of light
to moderate grazing [118].
Silviculture:
Climax western juniper stands are of mixed age, consisting of 1st year seedlings to trees
several hundred years old. Seral stands are composed of predominately younger aged trees.
In central
Oregon, Vaitkus and Eddleman [194] observed significantly greater (p<0.05) squirreltail
production when associated with large (older) trees compared to small trees. Production of bottlebrush
squirreltail was also significantly greater (p<0.05)
under juniper canopies compared to intercanopy zones. McPherson and others [143]
observed significantly greater (p<0.01) squirreltail
under Pinchot juniper canopies and at canopy edges compared to areas
beyond canopy, within grazed and relict grasslands
of western Texas. Evaluations by Tueller and Platou [190] lend
supporting evidence (see: SUCCESSION within the Botanical and Ecological Characteristics section).
Squirreltail does not reduce ponderosa pine seedling growth. Two-year-old pine
seedlings that were planted the 1st postfire spring, after a June wildfire in northern Arizona,
were not affected in height or diameter by competition with squirreltail [66].
In Arizona ponderosa pine forests, seedlings
normally gain dominance over squirreltail within 5 years [8].
Squirreltail drastically increased 4 years after a clear-cut within
a lodgepole pine forest of northeastern Utah at 8,800 feet (2,700 m). Bottlebrush
squirreltail
showed the largest increase in vegetative production out of all grasses present [10]:
| 1976 (kg/ha) | 1980 (kg/ha) | |
| Ross' sedge | 56.8 | 42.0 |
| elk sedge | 2.1 | 4.4 |
| Poa spp. | 10.2 | 40.7 |
| squirreltail | 3.3 | 47.7 |
| 5 others | 0.0 | 13.9 |
GENERAL BOTANICAL CHARACTERISTICS:
Squirreltail is a cool season, [8] perennial bunchgrass native to
the Intermountain West [18]. It is solitary [200], possessing solid, mostly flowering
culms [210], with flat leaf blades. The inflorescence is
a spike 0.8 to 6.7 inches (2-17 cm) long [82,150,200].
Ecotypic variation is common among squirreltail populations [9].
 |
| Squirreltail growth form. Image by Dave Powell, USDA Forest Service (retired), Bugwood.org. |
Reynolds and Fraley [164] found bottlebrush squirreltail roots to achieve depths of 39.4 inches (100 cm) below the soil surface. Depths below 39.4 inches (100 cm) were not seen due to a subsurface layer of basalt, suggesting rooting depths greater than 39.4 inches (100 cm) are possible. A lateral root extension of 16 inches (40 cm) was observed at 9.8, 20, 24 and 39.4 inch (25, 50, 60 and 100 cm) depths.
RAUNKIAER [163] LIFE FORM:
Hemicryptophyte
REGENERATION PROCESSES:
Squirreltail regenerates from surviving root crown [29,201] and seed [18].
Vegetative propagation is nonexistent [18].
Squirreltail has the ability to produce large numbers [99,214]
of highly germinable seeds, with relatively rapid germination [214]
when exposed to the correct environmental cues.
Plants are self-fertilizing [55].
Seeds are readily dispersed by wind [15,99] a few days following maturation [18].
Dispersal is a function of squirreltail's long reflexed awns and
disarticulating, mature inflorescence [99,131,148]. Seeds are dispersed when the
spike inflorescence is carried along the ground by wind catching the long awns [131].
Although squirreltail has the potential for long distance seed dispersal, Martlette and
Anderson [131] found natural plant cover to act as a barrier to dispersal. Wind dispersal of squirreltail
seed did not exceed 131 feet (40 m), with viable seed remaining relatively close to mature squirreltail plants.
Dormancy protects squirreltail seeds from germinating during
seasonal dry periods. Dry seeds require a period of afterippening, which widens
environmental conditions conducive to germination [18].
Allen and others [2] found germination rate increased and dormancy levels decreased
as the duration of dry storage increased.
Desert squirreltail seed commonly show higher levels
of dormancy than seed from mountain populations [18].
Squirreltail seeds may germinate without a
period of afterippening, showing a partial state of dormancy. However mean germination time
for recently harvested seeds is longer than for afterippened seeds.
Beckstead [19]
evaluated the germination temperature requirements of recently harvested bottlebrush
squirreltail seeds obtained from mountain and desert habitats. The greatest germination occurred
primarily at 50/68 degrees Fahrenheit (10/20 °C)
and 59/77 degrees Fahrenheit (15/25 °C), with higher temperatures of 68/86 degrees Fahrenheit (20/30 °C)
inhibiting germination.
Environmental conditions and timing of phenological events greatly affect the probability of
recently harvested squirreltail seed germination. Temperatures of
50/68 degrees Fahrenheit (10/20 °C) and 59/77 degrees Fahrenheit (15/25 °C) are unlikely to occur during
summer months in desert
habitats. In higher, mountain habitats, summer temperatures of 50/68 degrees Fahrenheit (10/20 °C)
and 59/77 degrees Fahrenheit (15/25 °C)
may occur; however, squirreltail usually ripens later at higher elevations [19].
In general, recently harvested
squirreltail seeds at lower elevations have a much greater
probability of fall germination than seeds from higher elevations [2].
Chabet and Billings [40] observed germination of squirreltail seeds from
alpine sites (10,793 feet (3,290 m)) in the Sierra Nevada. The greatest germination (%)
occurred at day/night temperatures of 81/73 degrees Fahrenheit (27/23 °C (96%)) and 90/82 degrees Fahrenheit
(32/28 °C (92%)).
SITE CHARACTERISTICS:
Squirreltail has wide ecological amplitude [161], but it most commonly
occurs in disturbed areas of deserts, valleys, foothills, and mountain meadows [18].
Regional:
Squirreltail is
found throughout Colorado on dry hills, plains, and rocky slopes, and within open woods and
meadows [92].
In Montana, squirreltail occurs in dry, open habitats, from valley to
timberline [123].
Throughout the western Great Plains, squirreltail is commonly found on
dry soils of pastures and roadsides [82].
In Utah, squirreltail prefers dry to moist vegetation types, from salt desert shrub to alpine
grassland [200]. Plains, rocky hills, or montane slopes are common sites
in New Mexico [132].
In Arizona, open sandy ground, rocky hills, and open pine woods are common sites
[115].
Squirreltail is common to dry rangeland areas of Kansas [154].
In central Washington, squirreltail prefers disturbed sites. Within these sites plant density is negatively
correlated with individual plant size [153]. In California, squirreltail is found in scattered stands
at high elevations on dry, gravelly soils. It is also common to hillsides and brush associations [168].
Soils:
Squirreltail inhabits a wide variety of soil types and
is tolerant of saline [108] and alkaline soils [168].
It is widely distributed in salt-desert shrub ranges of the west, on dry, gravelly soils,
or within alkaline conditions. Squirreltail is found on clayey soils of northeastern
California sagebrush communities [27]. Throughout Montana it occurs predominantly
on dry, gravelly soils, in saline or alkaline areas [150].
Within alpine areas of Olympic National Park, Washington, squirreltail prefers
well-drained, undifferentiated, disturbed, shallow and stony soils [21].
Passey and Hugie [158] found squirreltail to achieve better growth on
Newdale silt loam soils than on Brunt silt loam, in areas with similar climate, slope, and
exposure. Squirreltail may also occur on loose, ashy soil [11].
Squirreltail is not common within wet areas such as river lowlands and soil along
irrigation canals [153].
Elevation by state:
| Arizona | 2,000 to 11,500 (609-3,505 m) [115] |
| west-central Montana | 7,000 to 9,200 feet (2,135-2,805 m) [123] |
| New Mexico | 4,500 to 11,500 feet (1,372-3,505 m) [132] |
| Utah | 3,510 to 11,400 feet (1,070-3,500 m) [200] |
SUCCESSIONAL STATUS:
Depending upon habitat type, squirreltail may occur as an early,
mid-, or late successional species.
Shrub rangelands:
Squirreltail is generally a dominant component of seral big sagebrush/bunchgrass communities [217].
Squirreltail is represented in early seral and climax stages of big sagebrush/bluebunch
wheatgrass associations in Nevada. Tueller and Platou observed the most pronounced bottlebrush
squirreltail during early and climax stages of big sagebrush/bluebunch wheatgrass
associations in Nevada [190].
Squirreltail is found within seral and climax stages of big sagebrush rangelands in southeastern Idaho [4].
It is a component of climax big sagebrush communities in Idaho [205]
and is a member of climax big sagebrush/western wheatgrass communities of Colorado [183].
Within shrub-steppe ecosystems of western Colorado, squirreltail is an early seral
species [117]. Squirreltail also occurs in climax shadscale communities [100].
Pinyon-juniper communities:
Squirreltail is common in mid-seral and climax pinyon-juniper communities of Mesa Verde, Colorado [67,68].
Squirreltail is a component of seral and climax western juniper
(Juniper occidentalis) communities of the Pacific Northwest [54].
Ponderosa pine communities:
Squirreltail is a member of interior ponderosa pine climax communities
within the central and southern
Rocky Mountains [209].
Prior to invasion of nonnative annuals in the Snake River Plain, Idaho, squirreltail
occupied a mid to late seral status, suppressing the early seral fescues, sixweeks fescue (Vulpia octoflora), and foxtail fescue
(Vulpia myuros) [160].
SEASONAL DEVELOPMENT:
The wide ecological amplitude of squirreltail leads to differential timing of phenological events between
individuals of differing habitats [43,109]. Flowering generally occurs in spring or
early summer [18,57]. Lower elevation
populations (that is, cold desert, salt desert habitats) usually mature early June with
higher elevation populations (that is, mountain brush, mountain meadows) reaching maturity in
late July [18].
Hironaka and Tisdale observed phenological differences between the subspecies Elymus
elymoides ssp. elymoides
and ssp. californicum. In a common garden experiment E. e. ssp. elymoides
developed 10 to 14 days earlier than
ssp. californicum [100].
Between 1960 and 1969, Murray and others evaluated squirreltail phenology in southern
Idaho. Growth began from mid-March to mid-April. Flower stalks began to form late-April to mid-May,
with anthesis occurring in early to mid-June. Plants were dormant from the middle of July to the end of August
with fall regrowth occurring through October [152].
Clary [43] evaluated squirreltail phenology and rate of growth
from different environments using a transplant garden and growth chamber.
The timing of squirreltail phenological
events and overall growth rate was closely related to
homesite environmental conditions. Squirreltail
individuals from higher elevations were limited by cold temperatures
whereas individuals from lower elevations were limited by water availability and warm
temperatures. Under the same
environmental constraints, squirreltail from areas with low moisture
stress and cool climates showed higher growth rates, attaining maximum height earlier
than individuals from warmer drier sites. Squirreltail requires the
longest time to flower in areas of relatively moderate temperature and moisture
regimes:
Time to flowering in days for squirreltail individuals from different habitats is shown below. Plants were
grown at 6,490 feet (1,980 m) on a clay loam with an annual precipitation of 21.4 inches (544 mm)
and annual temperature of 49 degree Fahrenheit (9.5 oC).
| Squirreltail collection site description | Days to flower |
| 7,410 feet (2,260 m), silt loam, ponderosa pine | 205.5 |
| 4,990 feet (1,520 m), stony clay loam, ponderosa pine | 201.2 |
| 7,200 feet (2,200 m), loam, pinyon-juniper | 193.8 |
| 7,810 feet (2,380 m), clay loam, ponderosa pine | 192.5 |
| 9,780 feet (2,980 m), gravelly loam, spruce-fir | 172.5 |
| 9,320 feet (2,840 m), gravelly sandy loam, mountain grassland | 166.8 |
| 4,530 feet (1,380 m), loamy fine sand, short grass | 165.8 |
| 4,720 feet (1,440 m), cobble clay, pinyon-juniper | 162.2 |
| 4,990 feet (1,520 m), stony clay loam, ponderosa pine | 159.5 |
| 5,510 feet (1,680 m), silty clay loam, sagebrush-greasewood | 158.0 |
| 4,530 feet (1,380 m), stony loam, oak savannah | 153.5 |
FIRE ECOLOGY OR ADAPTATIONS:
Squirreltail's small size, coarse stems, and sparse leafy material aid in its tolerance
of fire [31]. Postfire regeneration occurs from surviving root crowns and from on- and off-site
seed sources [29]. Frequency of disturbance greatly influences postfire response of squirreltail. Undisturbed
plants within a 6 to 9 year age class generally contain large amounts of dead material,
increasing squirreltail's susceptibility to fire [210].
Koniak [120] found squirreltail to be a major component of postfire pinyon-juniper communities of the Great Basin
at any time during succession. Greatest occurrence and
coverage of squirreltail are generally achieved during mid-seral stages.
| Successional stage | Occurrence (%) | Percent of areas achieving > 5% cover |
| Early (1 year old) | 43 | 3 |
| Early-mid (4-8 years old) | 58 | 15 |
| Mid (15-17 years old) | 49 | 28 |
| Late-mid (22-60 years old) | 90 | 0 |
| Late > 60 years old | 44 | 0 |
| Community or Ecosystem | Dominant Species | Fire Return Interval Range (years) |
| silver fir-Douglas-fir | Abies amabilis-Pseudotsuga menziesii var. menziesii | > 200 |
| sagebrush steppe | Artemisia tridentata/Pseudoroegneria spicata | 20-70 [33] |
| basin big sagebrush | A. t. var. tridentata | 12-43 [170] |
| mountain big sagebrush | A. t. var. vaseyana | 20-60 [7,37] |
| Wyoming big sagebrush | A. t. var. wyomingensis | 10-70 (40)** [196,215] |
| saltbush-greasewood | Atriplex confertifolia-Sarcobatus vermiculatus | < 35 to < 100 |
| desert grasslands | Bouteloua eriopoda and/or Pleuraphis mutica | 5-100 |
| plains grasslands | Bouteloua spp. | < 35 |
| blue grama-needle-and-thread grass-western wheatgrass | B. g.-Hesperostipa comata-Pascopyrum smithii | < 35 |
| blue grama-buffalo grass | B. g.-Buchloe dactyloides | < 35 |
| grama-galleta steppe | B. g.-Pleuraphis jamesii | < 35 to < 100 |
| blue grama-tobosa prairie | B. g.-P. mutica | < 35 to < 100 |
| cheatgrass | Bromus tectorum | < 10 | mountain-mahogany-Gambel oak scrub | Cercocarpus ledifolius-Quercus gambelii | < 35 to < 100 |
| western juniper | Juniperus occidentalis | 20-70 |
| Rocky Mountain juniper | J. scopulorum | < 35 |
| Sierra lodgepole pine* | Pinus contorta var. murrayana | 35-200 |
| Rocky Mountain ponderosa pine* | P. ponderosa var. scopulorum | 2-10 |
| Arizona pine | P. p. var. arizonica | 2-10 |
| galleta-threeawn shrubsteppe | Pleuraphis jamesii-Aristida purpurea | < 35 to < 100 |
| mesquite-buffalo grass | Prosopis glandulosa-Buchloe dactyloides | < 35 |
| Texas savanna | P. g. var. glandulosa | < 10 [33] |
| mountain grasslands | Pseudoroegneria spicata | 3-40 (10)** [6] |
| Rocky Mountain Douglas-fir* | Pseudotsuga menziesii var. glauca | 25-100 |
| interior live oak | Quercus wislizenii | < 35 [33] |
POSTFIRE REGENERATION STRATEGY [182]:
Crown residual colonizer (on-site, initial community)
Secondary colonizer (on-site or off-site seed sources)
IMMEDIATE FIRE EFFECT ON PLANT:
Although squirreltail is generally top-killed by fire, its small size and
low density of coarse fuel per unit basal area make it relatively fire tolerant [31,198,208].
Low density of above ground plant tissue produces a quick, "hot" flame, transferring
little
heat
to growing points below the soil surface [208,210]. The solid culms of squirreltail
do not readily burn, compared to those of perennial grass associates
[210].
DISCUSSION AND QUALIFICATION OF FIRE EFFECT:
No entry
PLANT RESPONSE TO FIRE:
Squirreltail sprouts from surviving root crown [29,201] and colonizes from seed [29].
Seasonal trends in squirreltail root carbohydrate reserves greatly affect
postfire response. Burning is generally harmful
during late spring and early summer [30,208] coinciding with low points in carbohydrate reserves
[20]. Squirreltail is most tolerant of late summer (anthesis) or mid-fall (before regrowth)
fires [30,49,79], coinciding with relatively high carbohydrate reserves [20]:
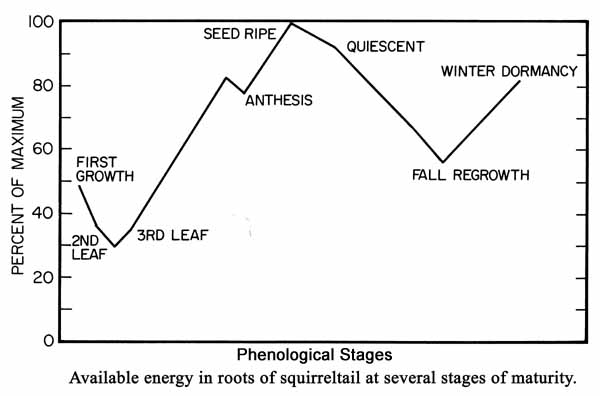
A difference in phenological traits of surviving postfire individuals may exist between
small (1 to 3 inch (2.5-7.6 cm) crown diameter) and large (>3.5 inches (8.9 cm) crown
diameter) squirreltail plants. Wright [210] found large plants to produce
significantly (p<0.01)
higher numbers of flowering stalks than small plants after fire.
DISCUSSION AND QUALIFICATION OF PLANT RESPONSE:
Wright [208] compared squirreltail response to burning and
clipping near Boise, Idaho. Heat was applied by propane burner for 20 to 30 seconds to raise soil
surface temperature to 400 or 800 degrees Fahrenheit. The 800 degree Fahrenheit treatment killed 25%
of squirreltail plants during July and August. No other treatment caused mortality.
Burning and clipping during all seasons reduced yields 1 year after treatment. Burning reduced
yield most during May. Average herbage weight (in grams) per squirreltail plants
in relation to season and treatment at 1 postfire year is summarized below:
| Season | 400 °F | 800 °F | Clipped | Control |
| May | 3.94a | 5.48 | 7.41 | 22.58 |
| June | 6.96 | 8.50 | 7.26 | 22.09 |
| July | 8.51a | 4.32ab | 13.25 | 14.01 |
| August | 7.50a | 9.42 | 11.58 | 16.61 |
| September | 10.44 | 6.11ab | 10.21 | 21.97 |
| 19 May | 10 June | 21 July | 20 August | 21 September |
| 4.00 | 5.50 | 18.50 | 28.00 | 33.50 |
| Moderate burn 1972 | Severe burn 1972 | Control (logged, not burned) 1972 |
| 7.2 | 0 | 0 |
| Moderate burn 1974 | Severe burn 1974 | Control (logged, not burned) 1974 |
| 18.1 | 0.1 | 1.1 |
Although frequency of squirreltail was too low for statistical analysis, Champlin [42] reported no damage to squirreltail basal cover and height 2 postfire years after a spring fire in a big sagebrush community of northern California. Squirreltail vigor increased the 1st and 2nd postfire growing season in central Oregon, following a spring fire within a sagebrush-bitterbrush/bunchgrass plant community [1].
Summer:
Squirreltail increased following an August wildfire in a big sagebrush
community with an understory dominated by cheatgrass and Lyall's milkvetch (Astragalus
lyallii) [95]. Significantly (p<0.01) greater biomass was achieved 1 postfire
year after a 19 July prescribed fire in Oregon. At time of burn, bottlebrush
squirreltail had entered summer quiescence with no green shoot material evident. Mean
shoot biomass of burned plants was greater per unit crown area, compared to
control. Burned plants also averaged 49% higher root
biomass per unit crown area, producing a shoot:root biomass ratio of 1.73 compared to
control plots at 0.43 shoot:root biomass. Burning also increased the proportion of reproductive
culms; 74.8% of all shoots of burned plants produced reproductive culms compared to 14.3% for unburned plants
[220].
Squirreltail showed a negative postfire response to summer (July) wildfire
within a sagebrush rangeland in Utah, for the 2nd and 3rd postfire years compared to
control [202].
Squirreltail decreased in abundance 1 postfire year after a summer
(July) prescribed fire and after a lightning fire within a mountain mahogany-big sagebrush community [187].
Fall:
Squirreltail maintained previous levels of production (kg/ha) 1 postfire
year after an October fire in an aspen-bunchgrass community of northern Arizona.
Although total vegetative production remained constant, percent cover and density
of squirreltail were significantly higher. The October fire resulted in a
large squirreltail population consisting of small individuals whose
combined vegetative biomass equaled or exceeded preburn levels. Associated
dominants, Arizona fescue and mountain muhly, decreased [86].
For further information on squirreltail response to fire, see Fire Case Studies, Lyon's Research Paper (Lyon 1971), and the following Research Project Summaries:
Seeding:
Aerially applied seed mixture of mutton grass, prairie Junegrass, Indian ricegrass, slender
wheatgrass (Elymus trachycaulus) and squirreltail aided in the
reestablishment of squirreltail after a summer (August)
wildfire within Mesa Verde National Park, Colorado [74]. Squirreltail was an
important component 1, 2, [76] and 3 postfire years [75] in seeded areas, whereas no
squirreltail was observed in unseeded areas [74].
Postfire recovery of squirreltail occurred after a summer (June 1956)
wildfire in Arizona chaparral, aerially seeded with weeping lovegrass
(Eragrostis curvula) and
crested wheatgrass. Results shown that percent frequency of squirreltail within
9.6 foot (2.9 m) square plots increased steadily for 4 years postfire [157]:
| 1956 | 1957 | 1958 | 1960 | 1961 | |
| squirreltail | 0 | 2.5 | 4.0 | 10.5 | 21.5 |
| crested wheatgrass | 0 | 14.0 | 20.5 | 17.5 | 13.0 |
| weeping lovegrass | 0.5 | 2.0 | 1.5 | 4.0 | 6.0 |
FIRE CASE STUDY CITATION:
Simonin, Kevin, compiler. 2001.
Squirreltail postfire response in a ponderosa pine forest of Arizona.
In: Elymus elymoides. In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: https://www.fs.usda.gov/database/feis/plants/graminoid/elyely/all.html#FireCaseStudies [
].
REFERENCE:
Vose, James M.; White, Alan S. 1991. Biomass response mechanisms of understory species
the first year after prescribed burning in an Arizona ponderosa pine community. Forest Ecology and Management.
40(3/4): 175-187. [199].
SEASON/SEVERITY CLASSIFICATION:
This was a late October fire. The fire smoldered for several days and
consumed the entire litter layer, with a total heat yield of 115,830
calories/foot2 (1600 kJ/m2).
STUDY LOCATION:
The prescribed fire took place within open sawtimber sites, pole
sites, and sapling sites within a ponderosa pine (Pinus ponderosa)/Arizona fescue
(Festuca arizonica) habitat type on the Fort Valley Experiment Forest
near Flagstaff.
| Saw timber sites | 296 trees/acre (120 trees/ha) |
| Pole sites | average tree diameter of 5.9 inches (15cm) at 1,730 trees/ha |
| Sapling sites | average tree diameter 1.8 inches (4.5 cm) at 10,070 trees/ha |
SITE DESCRIPTION:
ponderosa pine/Arizona fescue habitat type
FIRE DESCRIPTION:
The day of initiation,
average temperature was between 57.2 and 64.4 degrees Fahrenheit (14-18 °C)
and relative humidity was 21%.
FIRE EFFECTS ON TARGET SPECIES:
Vegetative
biomass within open saw timber sites, including surviving squirreltail plants
and new seedlings, was approximately
3 times greater (P<0.05) on burn plots compared to control, 247 lbs (112 kg/ha) and
88 lbs (40 kg/ha) respectively. Surviving squirreltail plants within burned, open saw
timber areas had more than twice the vegetative production. Seedling
recruitment within open saw timber sites was also greater on burned than control plots. Burned
open saw timber plots produced an average seedling biomass 15 times greater (p<0.05) than on control plots.
Squirreltail in pole sites
and sapling sites showed a more negative response to fire than in open sawtimber
sites. Results
for squirreltail are summarized below:
| Burn biomass (kg/ha) | Control biomass (kg/ha) | Density (plants/m2) | Control density (plants/m2 ) | |
| Open saw timber sites | ||||
| all plants | 112.45 | 40.06 | 9.06 | 5.74 |
| residual plants | 96.00 | 40.23 | 4.34 | 4.56 |
| seedlings | 15.84 | 1.03 | 4.98 | 0.57 |
| Pole sized sites | ||||
| all plants | 18.97 | 21.86 | 2.27 | 5.56 |
| residual plants | 18.23 | 18.64 | 2.05 | 3.41 |
| seedlings | 0.78 | 3.17 | 0.28 | 1.99 |
| Sapling sites | ||||
| all plants | 14.08 | 11.72 | 2.91 | 4.18 |
| residual plants | 12.77 | 10.64 | 1.96 | 2.97 |
| seedlings | 1.36 | 0.77 | 1.14 | 0.52 |
FIRE MANAGEMENT IMPLICATIONS:
Overall response of squirreltail within pole and sapling stands
was less than in open saw timber areas; however, the response of surviving plants in
pole and sapling stands
remained strong. Seedlings are generally more vulnerable to environmental
changes than established plants. The postfire response of surviving
squirreltail plants may result in an increased presence of bottlebrush
squirreltail within later postfire stages.
1. Adams, Glenn R. 1980. Results of range/wildlife prescribed burning on the Fort Rock Ranger District in central Oregon. R-6 Fuels Management Notes. September 24, 1980. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region, Aviation and Fire Management. 6 p. [292]
2. Allen, Phil S.; Debaene-Gill, Susan B.; Meyer, Susan E. 1994. Regulation of germination timing in facultatively fall-emerging grasses. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 215-219. [24284]
3. Anderson, Jay E.; Holte, Karl E. 1981. Vegetation development over 25 years without grazing on sagebrush-dominated rangeland in southeastern Idaho. Journal of Range Management. 34(1): 25-29. [319]
4. Anderson, Jay E.; Jeppson, R. J.; Wildosz, R. J.; [and others]. 1978. Trends in vegetation development on the Idaho National Engineering Laboratory Site. In: Markham, O. D., ed. Ecological studies on the Idaho National Engineering Laboratory Site: 1978 Progress Report. IDO-112087. Idaho Falls, ID: U.S. Department of Energy, Environmental Sciences Branch, Radiological and Environmental Sciences Lab: 144-166. [320]
5. Anderson, Jay E.; Shumar, Mark L. 1986. Impacts of black-tailed jackrabbits at peak population densities on sagebrush vegetation. Journal of Range Management. 39(2): 152-155. [322]
6. Arno, Stephen F. 1980. Forest fire history in the Northern Rockies. Journal of Forestry. 78(8): 460-465. [11990]
7. Arno, Stephen F.; Gruell, George E. 1983. Fire history at the forest-grassland ecotone in southwestern Montana. Journal of Range Management. 36(3): 332-336. [342]
8. Arnold, Joseph F. 1950. Changes in ponderosa pine bunchgrass ranges in northern Arizona resulting from pine regeneration and grazing. Journal of Forestry. February: 118-126. [352]
9. Arredondo, J. Tulio; Jones, Thomas A.; Johnson, Douglas A. 1998. Seedling growth of Intermountain perennial and weedy annual grasses. Journal of Range Management. 51(5): 584-589. [35483]
10. Austin, D. D.; Urness, Philip J. 1982. Vegetal responses and big game values after thinning regenerating lodgepole pine. The Great Basin Naturalist. 42(4): 512-516. [8354]
11. Bailey, Warren Hutchinson. 1963. Revegetation in the 1914-1915 devastated area of Lassen Volcanic National Park. Corvallis, OR: Oregon State University. 195 p. Dissertation. [29203]
12. Barkworth, Mary E.; Dewey, Douglas R. 1985. Genomically based genera in the perennial Triticeae of North America: identification and membership. American Journal of Botany. 72(5): 767-776. [393]
13. Barney, Milo A.; Frischknecht, Neil C. 1974. Vegetation changes following fire in the pinyon-juniper type of west-central Utah. Journal of Range Management. 27(2): 91-96. [397]
14. Barry, W. James. 1972. The Central Valley prairie. Vol 1. Sacramento, CA: State of California, Department of Parks and Recreation. 82 p. [28344]
15. Bates, Jon D.; Miller, Richard F.; Svejcar, Tony J. 1998. Understory dynamics in a cut juniper woodland (1991-1997). In: Annual report: Eastern Oregon Agricultural Research Center. Corvallis, OR: Oregon State University, Agricultural Experiment Station: 24-33. [29191]
16. Beale, Donald M.; Smith, Arthur D. 1970. Forage use, water consumption, and productivity of pronghorn antelope in western Utah. Journal of Wildlife Management. 34(3): 570-582. [6911]
17. Beaulieu, Jean Thomas. 1975. Effects of fire on understory plant populations in a northern Arizona ponderosa pine forest. Flagstaff, AZ: Northern Arizona University. 38 p. Thesis. [29095]
18. Beckstead, Julie. 1994. Between-population differences in the germination ecophysiology of cheatgrass (Bromus tectorum) and squirreltail (Elymus elymoides) during afterripening. Provo, UT: Brigham Young University. 96 p. Thesis. [27522]
19. Beckstead, Julie; Meyer, Susan E.; Allen, Phil S. 1995. Effects of afterripening on cheatgrass (Bromus tectorum) and squirreltail (Elymus elymoides) germination. In: Roundy, Bruce A.; McArthur, E. Durant; Haley, Jennifer S.; Mann, David K, compilers. Proceedings: wildland shrub and arid land restoration symposium; 1993 October 19-21; Las Vegas, NV. Gen. Tech. Rep. INT-GTR-315. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 165-172. [24843]
20. Bedell, Thomas E. 1980. Range plant growth and development. Extension Circular 1038. Corvallis, OR: Oregon State University, Extension Service. 4 p. [6518]
21. Belsky, J.; Del Moral, R. 1982. Ecology of an alpine-subalpine meadow complex in the Olympic Mountains, Washington. Canadian Journal of Botany. 60: 779-788. [6740]
22. Bernard, Stephen R.; Brown, Kenneth F. 1977. Distribution of mammals, reptiles, and amphibians by BLM physiographic regions and A.W. Kuchler's associations for the eleven western states. Tech. Note 301. Denver, CO: U.S. Department of the Interior, Bureau of Land Management. 169 p. [434]
23. Bethlenfalvay, Gabor J.; Dakessian, Suren. 1984. Grazing effects on mycorrhizal colonization and floristic composition of the vegetation on a semiarid range in northern Nevada. Journal of Range Management. 37(4): 312-316. [439]
24. Blackburn, Wilbert H.; Eckert, Richard E., Jr.; Tueller, Paul T. 1969. Vegetation and soils of the Cow Creek Watershed. R-49. Reno, NV: University of Nevada, Agricultural Experiment Station. 77 p. In cooperation with: U.S. Department of the Interior, Bureau of Land Management. [458]
25. Blackburn, Wilbert H.; Eckert, Richard E., Jr.; Tueller, Paul T. 1971. Vegetation and soils of the Rock Springs Watershed. R-83. Reno, NV: University of Nevada, Agricultural Experiment Station. 116 p. In cooperation with: U.S. Department of the Interior, Bureau of Land Management. [457]
26. Blank, Robert R.; Allen, Fay; Young, James A. 1994. Growth and elemental content of several sagebrush-steppe species in unburned and post-wildfire soil and plant effects on soil attributes. Plant and Soil. 164: 35-41. [26887]
27. Blank, Robert R.; Trent, James D.; Young, James A. 1992. Sagebrush communities on clayey soils of northeastern California: a fragile equilibrium. In: Clary, Warren P.; McArthur, E. Durant; Bedunah, Don; Wambolt, Carl L., compilers. Proceedings--symposium on ecology and management of riparian shrub communities; 1991 May 29-31; Sun Valley, ID. Gen. Tech. Rep. INT-289. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 198-202. [19121]
28. Borchert, Mark; Davis, Frank W.; Allen-Diaz, Barbara. 1991. Environmental relationships of herbs in blue oak (Quercus douglasii) woodlands of central coastal California. Madrono. 38(4): 249-266. [17067]
29. Bradley, Anne F.; Noste, Nonan V.; Fischer, William C. 1992. Fire ecology of forests and woodlands of Utah. Gen. Tech. Rep. INT-287. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 128 p. [18212]
30. Britton, C. M.; Sneva, F. A.; Clark, R. G. 1979. Effect of harvest date on five bunchgrasses of eastern Oregon. In: 1979 Progress report...research in rangeland management. Special Report 549. Corvallis, OR: Oregon State University, Agricultural Experiment Station: 16-19. In cooperation with: U.S. Department of Agriculture, SEA-AR. [2743]
31. Britton, Carlton M.; McPherson, Guy R.; Sneva, Forrest A. 1990. Effects of burning and clipping on five bunchgrasses in eastern Oregon. The Great Basin Naturalist. 50(2): 115-120. [12371]
32. Britton, Carlton M.; Ralphs, Michael H. 1979. Use of fire as a management tool in sagebrush ecosystems. In: The sagebrush ecosystem: a symposium: Proceedings; 1978 April; Logan, UT. Logan, UT: Utah State University, College of Natural Resources. 101-109. [518]
33. Brown, James K.; Smith, Jane Kapler, eds. 2000. Wildland fire in ecosystems: Effects of fire on flora. Gen. Tech Rep. RMRS-GRT-42-vol. 2. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 257 p. [36581]
34. Brown, Ray W.; Amacher, Michael C. 1999. Selecting plant species for ecological restoration: a perspective for land managers. In: Holzworth, Larry K.; Brown, Ray W., comps. Revegetation with native species: Proceedings, 1997 Society for Ecological Restoration annual meeting; 1997 November 12-15; Fort Lauderdale, FL. Proc. RMRS-P-8. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 1-16. [30341]
35. Bunting, Stephen C. 1987. Use of prescribed burning in juniper and pinyon-juniper woodlands. In: Everett, Richard L., compiler. Proceedings--pinyon-juniper conference; 1986 January 13-16; Reno, NV. Gen. Tech. Rep. INT-215. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 141-144. [4836]
36. Burke, Ingrid C.; Reiners, William A.; Olson, Richard K. 1989. Topographic control of vegetation in a mountain big sagebrush steppe. Vegetatio. 84(2): 77-86. [11178]
37. Burkhardt, Wayne J.; Tisdale, E. W. 1976. Causes of juniper invasion in southwestern Idaho. Ecology. 57: 472-484. [565]
38. Busse, M. D.; Cochran, P. H.; Barrett, J. W. 1996. Changes in ponderosa pine site productivity following removal of understory vegetation. Soil Science Society of America Journal. 60: 1614-1621. [28434]
39. Campbell, R. E.; Baker, M. B., Jr.; Ffolliott, P. F.; [and others]. 1977. Wildfire effects on a ponderosa pine ecosystem: an Arizona case study. Res. Pap. RM-191. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 12 p. [4715]
40. Chabot, Brian F.; Billings, W. D. 1972. Origins and ecology of the Sierran alpine flora and vegetation. Ecological Monographs. 42(2): 163-199. [11228]
41. Chambers, Jeanne C.; Sidle, Roy C. 1991. Fate of heavy metals in an abandoned lead-zinc tailings pond: I. Vegetation. Journal of Environmental Quality. 20(4): 745-751. [34948]
42. Champlin, Mark R. 1982. Big sagebrush (Artemisia tridentata) ecology and management with emphasis on prescribed burning. Corvallis, OR: Oregon State University. 136 p. Dissertation. [9484]
43. Clary, Warren P. 1975. Ecotypic adaptation in Sitanion hystrix. Ecology. 56(6): 1407-1415. [34923]
44. Clary, Warren P. 1975. Range management and its ecological basis in the ponderosa pine type of Arizona: the status of our knowledge. Res. Pap. RM-158. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 35 p. [4688]
45. Coffin, D. P.; Lauenroth, W. K. 1989. Small scale disturbances and successional dynamics in a shortgrass plant community: interactions of disturbance characteristics. Phytologia. 67(3): 258-286. [34887]
46. Cook, C. Wayne; Child, R. Dennis. 1971. Recovery of desert plants in various states of vigor. Journal of Range Management. 24: 339-343. [677]
47. Cook, C. Wayne; Harris, Lorin E. 1968. Nutritive value of seasonal ranges. Bulletin 472. Logan, UT: Utah State University, Agricultural Experiment Station. 55 p. [679]
48. Cook, C. Wayne; Stoddart, L. A.; Harris, Lorin E. 1954. The nutritive value of winter range plants in the Great Basin as determined with digestion trials with sheep. Bulletin 372. Logan, UT: Utah State University, Agricultural Experiment Station. 56 p. [682]
49. Countryman, Clive M.; Cornelius, Donald R. 1957. Some effects of fire on a perennial range type. Journal of Range Management. 10: 39-41. [699]
50. Coyne, Patrick I.; Cook, C. Wayne. 1970. Seasonal carbohydrate reserve cycles in eight desert range species. Journal of Range Management. 23: 438-444. [707]
51. Cronquist, Arthur; Holmgren, Arthur H.; Holmgren, Noel H.; Reveal, James L. 1972. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Vol. 1. New York: Hafner Publishing Company, Inc. 270 p. [717]
52. Currie, Pat O. 1975. Grazing management of ponderosa pine-bunchgrass ranges of the central Rocky Mountains. Res. Pap. RM-159. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 24 p. [12600]
53. Currie, Pat O.; Goodwin, D. L. 1966. Consumption of forages by black-tailed jackrabbits on salt-desert ranges of Utah. Journal of Wildlife Management. 30(2): 304-311. [25015]
54. Dealy, J. Edward; Geist, J. Michael; Driscoll, Richard S. 1978. Western juniper communities on rangelands of the Pacific Northwest. Hyder, Donald, ed. Proceedings, 1st international rangeland congress; 1978 August 14-18; Denver, CO. Denver, CO: Society for Range Management: 201-204. [785]
55. Dewey, D. R. 1988. The U.S. living collection of perennial Triticeae grasses. Utah Science. Fall: 71-76. [11384]
56. Dewey, Douglas R. 1983. Historical and current taxonomic perspectives of Agropyron, Elymus, and related genera. Crop Science. 23: 637-642. [793]
57. Dickinson, C. E.; Dodd, Jerrold L. 1976. Phenological pattern in the shortgrass prairie. The American Midland Naturalist. 96(2): 367-378. [799]
58. Dittberner, Phillip L.; Olson, Michael R. 1983. The plant information network (PIN) data base: Colorado, Montana, North Dakota, Utah, and Wyoming. FWS/OBS-83/86. Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service. 786 p. [806]
59. Doescher, P. S.; Miller, R. F.; Swanson, S. R.; Winward, A. H. 1986. Identification of the Artemisia tridentata ssp. wyomingensis/Festuca idahoensis habitat type in eastern Oregon. Northwest Science. 60(1): 55-60. [815]
60. Dorn, Robert D. 1984. Vascular plants of Montana. Cheyenne, WY: Mountain West Publishing. 276 p. [819]
61. Dyrness, C. T. 1976. Effect of wildfire on soil wettability in the high Cascades of Oregon. PNW-202. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 18 p. [8573]
62. Eckert, R. E. 1975. Improvement of mountain meadows in Nevada. Research Report. Reno, NV: U.S. Department of Agriculture, Bureau of Land Management. 45 p. [8124]
63. Eckert, Richard E., Jr.; Peterson, Frederick F.; Emmerich, Fay L. 1987. A study of factors influencing secondary succession in the sagebrush [Artemisia spp. L.] type. In: Frasier, Gary W.; Evans, Raymond A., eds. Proceedings of the symposium: "Seed and seedbed ecology of rangeland plants"; 1987 April 21-23; Tucson, AZ. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service: 149-168. [3544]
64. Eddleman, Lee E.; Doescher, Paul S. 1978. Selection of native plants for spoils revegetation based on regeneration characteristics and successional status. In: Land Reclamation Program, Annual Report July 1976-October 1977. ANL/LRP-2. Argonne, IL: Argonne National Laboratory, Energy & Environmental Systems Division: 132-138. [5729]
65. Edgerton, Paul J.; McConnell, Burt R.; Smith, Justin G. 1975. Initial response of bitterbrush to disturbance by logging and slash disposal in a lodgepole pine forest. Journal of Range Management. 28(2): 112-114. [16009]
66. Elliott, Katherine J.; White, Alan S. 1987. Competitive effects of various grasses and forbs on ponderosa pine seedlings. Forest Science. 33(2): 356-366. [860]
67. Erdman, James A. 1970. Pinyon-juniper succession after natural fires on residual soils of Mesa Verde, Colorado. Brigham Young University Science Bulletin: Biological Series. 11(2): 1-26. [11987]
68. Erdman, James Allen. 1969. Pinyon-juniper succession after fires on residual soils of the Mesa Verde, Colorado. Boulder, CO: University of Colorado. 81 p. Dissertation. [11437]
69. Everett, Richard L. 1987. Allelopathic effects of pinyon and juniper litter on emergence and growth of herbaceous species. In: Frasier, Gary W.; Evans, Raymond A., eds. Proceedings of symposium: "Seed and seedbed ecology of rangeland plants"; 1987 April 21-23; Tucson, AZ. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service: 62-67. [3353]
70. Everett, Richard L.; Sharrow, Steven H. 1983. Understory seed rain on tree-harvested and unharvested pinyon-juniper sites. Journal of Environmental Management. 17(4): 349-358. [35997]
71. Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters. 148 p. [905]
72. Fagerstone, Kathleen A.; Lavoie, G. Keith; Griffith, Richard E., Jr. 1980. Black-tailed jackrabbit diet and density on rangeland and near agricultural crops. Journal of Range Management. 33(3): 229-233. [21756]
73. Fernau, R. F.; Rey Benayas, J. M.; Barbour, M. G. 1998. Early secondary succession following clearcuts in red fir forests of the Sierra Nevada, California. Madrono. 45(2): 131-136. [30094]
74. Floyd-Hanna, Lisa; DaVega, Anne; Hanna, David; Romme, William H. 1997. Chapin 5 fire vegetation monitoring and mitigation first year report. Unpublished report. Washington, DC: U.S. Department of the Interior, National Park Service, Mesa Verde National Park. 7 p. (+ Appendices). On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [34181]
75. Floyd-Hanna, Lisa; Hanna, David. 1999. Chapin 5 fire vegetation monitoring and mitigation annual report, year 3. Unpublished report. Washington, DC: U.S. Department of the Interior, National Park Service, Mesa Verde National Park. 8 p. (+Appendices). On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [34569]
76. Floyd-Hanna, Lisa; Hanna, David; Romme, William H. 1998. Chapin 5 Fire vegetation monitoring and mitigation annual report, year 2. Unpublished report. Washington, DC: U.S. Department of the Interior, National Park Service, Mesa Verde National Park. 7 p. (+ Appendices). On file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. [34460]
77. Foxx, Teralene S. 1996. Vegetation succession after the La Mesa Fire at Bandelier National Monument. In: Allen, Craig D., ed. Fire effects in southwestern forests: Proceedings, 2nd La Mesa fire symposium; 1994 March 29-31; Los Alamos, NM. RM-GTR-286. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 47-69. [27283]
78. Fresquez, P. R.; Francis, Richard E.; Dennis, G. L. 1990. Soil and vegetation responses to sewage sludge on a degraded semiarid broom snakeweed/blue grama plant community. Journal of Range Management. 43(4): 325-331. [34945]
79. Gaines, Edward M.; Kallander, Harry R.; Wagner, Joe A. 1958. Controlled burning in southwestern ponderosa pine: results from the Blue Mountain plots, Fort Apache Indian Reservation. Journal of Forestry. 56: 323-327. [988]
80. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; [and others]. 1977. Vegetation and environmental features of forest and range ecosystems. Agric. Handb. 475. Washington, DC: U.S. Department of Agriculture, Forest Service. 68 p. [998]
81. Gates, Robert J. 1985. Observations of the formation of sage grouse lek. Wilson Bulletin. 97(2): 219-221. [25625]
82. Great Plains Flora Association. 1986. Flora of the Great Plains. Lawrence, KS: University Press of Kansas. 1392 p. [1603]
83. Green, Lisle R.; Sharp, Lee A.; Cook, C. Wayne; Harris, Lorin E. 1951. Utilization of winter range forage by sheep. Journal of Range Management. 4: 233-241. [7891]
84. Gregg, Michael A.; Crawford, John A.; Drut, Martin S. 1993. Summer habitat use and selection by female sage grouse (Centrocercus urophasianus) in Oregon. The Great Basin Naturalist. 53(3): 293-298. [22057]
85. Grey, William E.; Quimby, Paul C., Jr.; Mathre, Donald E.; Young, James A. 1995. Potential for biological control of downy brome (Bromus tectorum) and medusahead (Taeniatherum caput-medusae) with crown and root rot fungi. Weed Technology. 9(2): 362-365. [35196]
86. Haisley, James R. 1984. The effects of prescribed burning on four aspen-bunchgrass communities in northern Arizona. Flagstaff, AZ: Northern Arizona University. 47 p. Thesis. [27667]
87. Hallsten, Gregory P.; Skinner, Quentin D.; Beetle, Alan A. 1987. Grasses of Wyoming. 3rd ed. Research Journal 202. Laramie, WY: University of Wyoming, Agricultural Experiment Station. 432 p. [2906]
88. Hardegree, S. P.; Van Vactor, S. S. 1999. Predicting germination response of four cool-season range grasses to field-variable temperature regimes. Environmental and Experimental Botany. 41(3): 209-217. [35229]
89. Hardegree, Stuart P. 1994. Drying and storage effects on germination of primed grass seeds. Journal of Range Management. 47(3): 196-199. [34943]
90. Hardegree, Stuart P. 1994. Germination enhancement of perennial grasses native to the Intermountain region. In: Monsen, Stephen B.; Kitchen, Stanley G, compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 229-232. [24287]
91. Hedrick, D. W.; Hyder, D. N.; Sneva, F. A.; Poulton, C. E. 1966. Ecological response of sagebrush-grass range in central Oregon to mechanical and chemical removal of Artemisia. Ecology. 47(3): 432-439. [1115]
92. Herzman, Carl W.; Everson, A. C.; Mickey, Myron H.; [and others]. 1959. Handbook of Colorado native grasses. Bull. 450-A. Fort Collins, CO: Colorado State University, Extension Service. 31 p. [10994]
93. Hickman, James C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p. [21992]
94. Hill, Alison; Pieper, Rex D.; Southward, G. Morris. 1992. Habitat-type classification of the pinyon-juniper woodlands in western New Mexico. Bulletin 766. Las Cruces, NM: New Mexico State University, College of Agriculture and Home Economics, Agricultural Experiment Station. 80 p. [37374]
95. Hinds, W. T.; Sauer, R. H. 1976. Soil erodibility, soil erosion, and revegetation following wildfire in a shrug-steppe community. In: Atmosphere-surface exchange of particulate and gaseous pollutants; proceedings of a symposium; [Date of conference unknown]; Richland, WA. [Place of publication unknown]. [Publisher unknown]. 571-590. [8092]
96. Hironaka, M. 1994. Medusahead: natural successor to the cheatgrass type in the northern Great Basin. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 89-91. [24259]
97. Hironaka, M.; Fosberg, M. A.; Winward, A. H. 1983. Sagebrush-grass habitat types of southern Idaho. Bulletin Number 35. Moscow, ID: University of Idaho, Forest, Wildlife and Range Experiment Station. 44 p. [1152]
98. Hironaka, M.; Sindelar, Brian W. 1975. Growth characteristics of squirreltail seedlings in competition with medusahead. Journal of Range Management. 28(4): 283-285. [1159]
99. Hironaka, M.; Tisdale, E. W. 1963. Secondary succession in annual vegetation in southern Idaho. Ecology. 44(4): 810-812. [1160]
100. Hironaka, M.; Tisdale, E. W. 1972. Growth and development of Sitanion hystrix and Poa sandbergii. Research Memorandum RM 72-24. U.S. International Biological Program, Desert Biome. 15 p. [1161]
101. Hopkins, William E. 1979. Plant associations of the Fremont National Forest. R6-ECOL-79-004. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 106 p. [7340]
102. Hosten, P. E.; West, N. E. 1994. Cheatgrass dynamics following wildfire on a sagebrush semidesert site in central Utah. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 56-62. [24253]
103. Humphrey, L. David; Schupp, Eugene W. 1999. Temporal patterns of seeding emergence and early survival of Great Basin perennial plant species. The Great Basin Naturalist. 59(1): 35-49. [29654]
104. Hutchings, Selar S.; Stewart, George. 1953. Increasing forage yields and sheep production on Intermountain winter ranges. Circular No. 925. Washington, DC: U.S. Department of Agriculture. 63 p. [1227]
105. Jameson, Donald A. 1966. Competition in a blue grama-broom snakeweed-actinea community and responses to selective herbicides. Journal of Range Management. 19: 121-124. [1250]
106. Jensen, Kevin B.; Redinbaugh, M.; Blood, M.; [and others]. 1999. Natural hybrids of Elymus elymoides x Leymus salinus subsp. salmonis (Poaceae: Triticeae) Crop Science. 39(4): 976-982. [35162]
107. Jensen, M. E.; Peck, L. S.; Wilson, M. V. 1988. A sagebrush community type classification for mountainous northeastern Nevada rangelands. The Great Basin Naturalist. 48: 422-433. [27717]
108. Jensen, M. E.; Simonson, G. H.; Dosskey, M. 1990. Correlation between soils and sagebrush-dominated plant communities of northeastern Nevada. Soil Science Society of America Journal. 54: 902-910. [15502]
109. Jirik, Steven. 1989. A study on the post-fire defoliation response of Agropyron spicatum and Sitanion hystrix. Moscow, ID: University of Idaho. 42 p. Thesis. [15509]
110. Johnsen, Thomas N., Jr. 1987. Seeding pinyon-juniper sites in the Southwest. In: Everett, Richard L., compiler. Proceedings--pinyon-juniper conference; 1986 January 13-16; Reno, NV. Gen. Tech. Rep. INT-215. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 465-472. [29497]
111. Johnson, Mark K.; Hansen, Richard M. 1979. Foods of cottontails and woodrats in south-central Idaho. Journal of Mammalogy. 60(1): 213-215. [23859]
112. Johnson, Randal D.; Anderson, Jay E. 1984. Diets of black-tailed jack rabbits in relation to population density and vegetation. Journal of Range Management. 37(1): 79-83. [21837]
113. Jones, Stanley D.; Wipff, Joseph K.; Montgomery, Paul M. 1997. Vascular plants of Texas. Austin, TX: University of Texas Press. 404 p. [28762]
114. Kartesz, John T. 1994. A synonymized checklist of the vascular flora of the United States, Canada, and Greenland. Volume I--checklist. 2nd ed. Portland, OR: Timber Press. 622 p. [23877]
115. Kearney, Thomas H.; Peebles, Robert H.; Howell, John Thomas; McClintock, Elizabeth. 1960. Arizona flora. 2d ed. Berkeley, CA: University of California Press. 1085 p. [6563]
116. Kitchen, Stanley G.; McArthur, E. Durant; Jorgensen, Gary L. 1999. Species richness and community structure along a Great Basin elevational gradient. In: McArthur, E. Durant; Ostler, W. Kent; Wambolt, Carl L., compilers. Proceedings: shrub ecotones; 1998 August 12-14; Ephraim, UT. Proceedings RMRS-P-11. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 59-65. [36063]
117. Klein, D. A.; Frederick, B. A.; Redente, E. F. 1989. Fertilizer effects on soil microbial communities and organic matter in the rhizosphere of Sitanion hystrix and Agropyron smithii. Arid Soil Research and Rehabilitation. 3: 397-404. [11097]
118. Klipple, G. E.; Costello, David F. 1960. Vegetation and cattle responses to different intensities of grazing on short-grass ranges on the Central Great Plains. Technical Bulletin No. 1216. Washington, DC: U.S. Department of Agriculture. 82 p. [4284]
119. Komarkova, Vera; Alexander, Robert R.; Johnston, Barry C. 1988. Forest vegetation of the Gunnison and parts of the Uncompahgre National Forests: a preliminary habitat type classification. Gen. Tech. Rep. RM-163. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 65 p. [5798]
120. Koniak, Susan. 1985. Succession in pinyon-juniper woodlands following wildfire in the Great Basin. The Great Basin Naturalist. 45(3): 556-566. [1371]
121. Kuchler, A. W. 1964. United States [Potential natural vegetation of the conterminous United States]. Special Publication No. 36. New York: American Geographical Society. 1:3,168,000; colored. [3455]
122. Kufeld, Roland C.; Wallmo, O. C.; Feddema, Charles. 1973. Foods of the Rocky Mountain mule deer. Res. Pap. RM-111. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 31 p. [1387]
123. Lackschewitz, Klaus. 1991. Vascular plants of west-central Montana--identification guidebook. Gen. Tech. Rep. INT-227. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 648 p. [13798]
124. Leckenby, Donavin A.; Adams, Arthur W. 1969. Ecological study of mule deer. Project No.: W-53-R-11. Job Progress Report No. 1. July 1, 1968 to June 30, 1969. Portland, OR: Oregon Game Commission, Research Division. 51 p. [16754]
125. Lewis, Mont E. 1971. Flora and major plant communities of the Ruby-East Humboldt Mountains with special emphasis on Lamoille Canyon. Elko, NV: U.S. Department of Agriculture, Forest Service, Region 4, Humboldt National Forest. 62 p. [1450]
126. Lytle, Dennis J.; Finch, Sherman J. 1987. Relating cordwood production to soil series. In: Plumb, Timothy R.; Pillsbury, Norman H., technical coordinators. Proceedings of the symposium on multiple-use management of California's hardwood resources; 1986 November 12-14; San Luis Obispo, CA. Gen. Tech. Rep. PSW-100 .w. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 260-267. [5380]
127. MacCracken, James G.; Hansen, Richard M. 1984. Seasonal foods of blacktail jackrabbits and Nuttall cottontails in southeastern Idaho. Journal of Range Management. 37(3): 256-259. [25010]
128. Madany, Michael H.; West, Neil E. 1984. Vegetation of two relict mesas in Zion National Park. Journal of Range Management. 37(5): 456-461. [6883]
129. Majerus, Mark. 1999. Collection and production of indigenous plant material for National Park restoration. In: Revegetation with native species: Proceedings, 1997 Society for Ecological Restoration annual meeting; 1997 November 12-15; Fort Lauderdale, FL. Proceedings RMRS-P-8. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 17-21. [30375]
130. Marchand, Denis E. 1973. Edaphic control of plant distribution in the White Mountains, eastern California. Ecology. 54(2): 233-250. [1521]
131. Marlette, Guy M.; Anderson, Jay E. 1986. Seed banks and propagule dispersal in crested-wheatgrass stands. Journal of Applied Ecology. 23: 161-175. [1526]
132. Martin, William C.; Hutchins, Charles R. 1981. A flora of New Mexico. Volume 1. Germany: J. Cramer. 647 p. [37175]
133. Maser, Chris; Strickler, Gerald S. 1978. The sage vole, Lagurus curtatus, as an inhabitant of subalpine sheep fescue, Festuca ovina, communities on Steens Mountain--an observation and interpretation. Northwest Science. 52(3): 276-284. [15507]
134. McArthur, E. Durant; Young, Stanford A. 1999. Development of native seed supplies to support restoration of pinyon-juniper sites. In: Monsen, Stephen B.; Stevens, Richard, compilers. Proceedings: ecology and management of pinyon-juniper communities within the Interior West: Sustaining and restoring a diverse ecosystem; 1997 September 15-18; Provo, UT. Proc. RMRS-P-9. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 327-330. [30591]
135. McCarten, Niall; Van Devender, Thomas R. 1988. Late Wisconsin vegetation of Robber's Roost in the western Mohave Desert, California. Madrono. 35(3): 226-237. [6183]
136. McClaran, Mitchel P.; Allen, Larry S.; Ruyle, George B. 1992. Livestock production and grazing management in the encinal oak woodlands of Arizona. In: Ffolliott, Peter F.; Gottfried, Gerald J.; Bennett, Duane A.; [and others], technical coordinators. Ecology and management of oak and associated woodlands: perspectives in the southwestern United States and northern Mexico: Proceedings; 1992 April 27-30; Sierra Vista, AZ. Gen. Tech. Rep. RM-218. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 57-64. [19743]
137. McClaran, Mitchel P.; Brady, Ward W. 1994. Arizona's diverse vegetation and contributions to plant ecology. Rangelands. 16(5): 208-217. [29721]
138. McDonald, Philip M. 1999. Diversity, density, and development of early vegetation in a small clear-cut environment. Res. Pap. PSW-RP-239. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 22 p. [36204]
139. McGinnies, W. J.;Laycock, W. A.;Tsuchiya, T.,Yonker, C.M.;Edmunds, D. A. 1988. Variability within a native stand of blue grama. Journal of Range Management. 41(5): 391-395. [5971]
140. McInnis, Michael L.; Vavra, Martin. 1987. Dietary relationships among feral horses, cattle, and pronghorn in southeastern Oregon. Journal of Range Management. 40(1): 60-66. [1605]
141. McLendon, Terry; Redente, Edward F. 1994. Role of nitrogen availability in the transition from annual-dominated to perennial-dominated seral communities. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 352-362. [24309]
142. McPherson, Guy R. 1992. Ecology of oak woodlands in Arizona. In: Ffolliott, Peter F.; Gottfried, Gerald J.; Bennett, Duane A.; [and others], technical coordinators. Ecology and management of oak and associated woodlands: perspectives in the southwestern United States and northern Mexico: Proceedings; 1992 April 27-30; Sierra Vista, AZ. Gen. Tech. Rep. RM-218. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 24-33. [19737]
143. McPherson, Guy R.; Rasmussen, G. Allen; Wester, David B.; Masters, Robert A. 1991. Vegetation and soil zonation associated with Juniperus pinchoth Sudw. trees. The Great Basin Naturalist. 51(4): 316-324. [18105]
144. McPherson, Guy R.; Wright, Henry A. 1990. Effects of cattle grazing and Juniperus pinchotii canopy cover on herb cover and production in western Texas. The American Midland Naturalist. 123: 144-151. [11148]
145. Medin, Dean E. 1990. Birds of a shadscale (Atriplex confertifolia) habitat in east central Nevada. The Great Basin Naturalist. 50(3): 295-298. [14989]
146. Medina, Alvin L. 1987. Woodland communities and soils of Fort Bayard, southwestern New Mexico. Journal of the Arizona-Nevada Academy of Science. 21: 99-112. [3978]
147. Moir, W. H. 1993. Alpine tundra and coniferous forest. In: Dick-Peddie, William A., ed. New Mexico vegetation: Past, present, and future. Albuquerque, NM: University of New Mexico Press: 47-84. [21099]
148. Monsen, Stephen B.; Anderson, Val Jo. 1995. A 52-year ecological history of selected introduced and native grasses planted in central Idaho. In: Proceedings, 17th international grassland congress; 1993 February 8-21; Palmerston North, New Zealand. [Place of publication unknown]: [Publisher unknown]: 1740-1741. [25664]
149. Monsen, Stephen B.; Shaw, Nancy L. 1983. Seeding antelope bitterbrush with grasses on south-central Idaho rangelands--a 39-year response. In: Tiedemann, Arthur R.; Johnson, Kendall L., compilers. Proceedings--research and management of bitterbrush and cliffrose in western North America; 1982 April 13-15; Salt Lake City, UT. Gen. Tech. Rep. INT-152. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 126-136. [1684]
150. Morris, H. E.; Booth, W. E.; Payne, G. F.; Stitt, R. E. 1950. Important grasses on Montana ranges. Bull. No. 470. Bozeman, MT: Montana Agricultural Experiment Station. 52 p. [5520]
151. Mueggler, Walter F.; Campbell, Robert B., Jr. 1986. Aspen community types of Utah. Res. Pap. INT-362. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 69 p. [1714]
152. Murray, R. B.; Mayland, H. F.; Van Soest, P. J. 1978. Growth and nutritional value to cattle of grasses on cheatgrass range in southern Idaho. Research Paper INT-199. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 57 p. [1723]
153. Newsome, R.D.; Sauer, R.H. 1983. The ecology of Sitanion (Graminaceae) in Benton County, Washington. In: Brewer, Richard, ed. Proceedings of the eighth North American prairie conference; 1982 August 1-4; Kalamazoo, MI. Kalamazoo, MI: Western Michigan University, Department of Biology: 15-20. [3116]
154. Ohlenbuseh, Paul D.; Hodges, Elizabeth P.; Pope, Susan. 1983. Range grasses of Kansas. Manhattan, KS: Kansas State University, Cooperative Extension Service. 23 p. [5316]
155. Oosting, H. J.; Billings, W. D. 1943. The red fir forest of the Sierra Nevada: Abietum magnificae. Ecological Monographs. 13(3): 260-273. [11521]
156. Pase, Charles P. 1982. Sierran subalpine conifer forest. In: Brown, David E., ed. Biotic communities of the American Southwest--United States and Mexico. Desert Plants. 4(1-4): 40-41. [8883]
157. Pase, Charles P.; Pond, Floyd W. 1964. Vegetation changes following the Mingus Mountain burn. Res. Note RM-18. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 8 p. [5700]
158. Passey, H. B.; Hugie, V. K. 1963. Some plant-soil relationships on an ungrazed range area of southeastern Idaho. Journal of Range Management. 16: 113-118. [1831]
159. Pearson, Henry A. 1965. Studies of forage digestibility under ponderosa pine stands. In: Proceedings: Society of American Foresters meeting; 1964 September 27 - October 1; Denver, CO. Washington, DC: Society of American Foresters: 71-73. [11513]
160. Peters, Erin F.; Bunting, Stephen C. 1994. Fire conditions pre- and postoccurrence of annual grasses on the Snake River Plain. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 31-36. [24249]
161. Plummer, A. Perry; Hull, A. C., Jr.; Stewart, George; Robertson, Joseph H. 1955. Seeding rangelands in Utah, Nevada, southern Idaho and western Wyoming. Agric. Handb. 71. Washington, DC: U.S. Department of Agriculture, Forest Service. 73 p. [11736]
162. Ranne, Brigitte M.; Baker, William L.; Andrews, Tom; Ryan, Michael G. 1997. Natural variability of vegetation, soils, and physiography in the bristlecone pine forests of the Rocky Mountains. The Great Basin Naturalist. 57(1): 21-37. [27383]
163. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford: Clarendon Press. 632 p. [2843]
164. Reynolds, Timothy D.; Fraley, Leslie, Jr. 1989. Root profiles of some native and exotic plant species in southeastern Idaho. Environmental and Experimental Botany. 29(2): 241-248. [36276]
165. Robertson, Joseph H. 1947. Responses of range grasses to different intensities of competition with sagebrush (Artemisia tridentata Nutt.). Ecology. 28(1): 1-16. [2008]
166. Romo, J. T.; Eddleman, L. E. 1987. Effects of Japanese brome on growth of bluebunch wheatgrass, Junegrass, and squirreltail seedlings. Reclamation and Revegetation Research. 6: 207-218. [262]
167. Ross, Robert L.; Hunter, Harold E. 1976. Climax vegetation of Montana: Based on soils and climate. Bozeman, MT: U.S. Department of Agriculture, Soil Conservation Service. 64 p. [2028]
168. Sampson, Arthur W.; Chase, Agnes; Hedrick, Donald W. 1951. California grasslands and range forage grasses. Bull. 724. Berkeley, CA: University of California College of Agriculture, California Agricultural Experiment Station. 125 p. [2052]
169. Sanders, Kenneth D. 1994. Can annual rangelands be converted and maintained as perennial grasslands through grazing management? In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 19-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 412-413. [24321]
170. Sapsis, David B. 1990. Ecological effects of spring and fall prescribed burning on basin big sagebrush/Idaho fescue--bluebunch wheatgrass communities. Corvallis, OR: Oregon State University. 105 p. Thesis. [16579]
171. Schlatterer, Edward F.; Hironaka, M. 1972. Some factors influencing tolerance to moisture stress of three range grasses. Journal of Range Management. 25(5): 364-367. [35440]
172. Schlatterer, Edward F.; Tisdale, E.W. 1969. Effects of litter of Artemisia, Chrysothamnus, and Tortula on germination and growth of three perennial grasses. Ecology. 50(5): 869-873. [2078]
173. Severson, Kieth E.; DeBano, Leonard F. 1991. Influence of Spanish goats on vegetation and soils in Arizona chaparral. Journal of Range Management. 44(2): 111-117. [15770]
174. Sharp, Lee A.; Sanders, Ken; Rimbey, Neil. 1990. Forty years of change in a shadscale stand in Idaho. Rangelands. 12(6): 313-328. [15527]
175. Shaw, R. B.; Anderson, S. L.; Schultz, K. A.; Diersing, V. E. 1989. Floral inventory for the U. S. Army Pinon Canyon Maneuver Site, Colorado. Phytologia. 67(1): 1-42. [12137]
176. Shiflet, Thomas N., ed. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p. [23362]
177. Sneva, Forrest A. 1971. Species succession on sprayed range under continuously deferred grazing. Progess report. Burns, OR: Squaw Butte Experiment Station: 61-96. [2187]
178. Sneva, Forrest A.; Rittenhouse, L. R.; Tueller, P. T.; Reece, P. 1984. Changes in protected and grazed sagebrush-grass in eastern Oregon, 1937 to 1974. Station Bulletin 663. Corvallis, OR: Oregon State University, Agricultural Experiment Station. 11 p. [2195]
179. Spears, Brian M.; Barr, William F. 1985. Effect of jointworms on the growth and reproduction of four native range grasses of Idaho. Journal of Range Management. 38(1): 44-46. [2205]
180. Stevens, Richard. 1983. Species adapted for seeding mountain brush, big, black, and low sagebrush, and pinyon-juniper communities. In: Monsen, Stephen B.; Shaw, Nancy, compilers. Managing Intermountain rangelands--improvement of range and wildlife habitats: Proceedings; 1981 September 15-17; Twin Falls, ID; 1982 June 22-24; Elko, NV. Gen. Tech. Rep. INT-157. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 78-82. [2240]
181. Stevens, Richard; Meyer, Susan E. 1990. Seed quality testing for range and wildland species. Rangelands. 12(6): 341-346. [14163]
182. Stickney, Peter F. 1989. Seral origin of species originating in northern Rocky Mountain forests. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Intermountain Research Station, Fire Sciences Laboratory, Missoula, MT; RWU 4403 files. 10 p. [20090]
183. Strong, Laurence L.; Dana, Robert W.; Carpenter, Len H. 1985. Estimating phytomass of sagebrush habitat types from microdensitometer data. Photogrammetric Engineering and Remote Sensing. 51(4): 467-474. [2267]
184. Sturges, David L. 1986. Responses of vegetation and ground cover to spraying a high elevation, big sagebrush watershed with 2,4-D. Journal of Range Management. 39(2): 141-146. [2276]
185. Tausch, Robin J.; West, Neil E. 1977. Competition and structural changes during secondary succession in juniper-pinyon woodlands. Unpublished report on file at: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, Intermountain Fire Sciences Laboratory, Missoula, MT. [2307]
186. Thilenius, John F.; Brown, Gary R.; Medina, Alvin L. 1995. Vegetation on semi-arid rangelands, Cheyenne River Basin, Wyoming. Gen. Tech. Rep. RM-GTR-263. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 60 p. [26478]
187. Tiagwad, Tamara E.; Olson, Craig M.; Martin, Robert E. 1982. Single-year response of breeding bird populations to fire in a curlleaf mountainmahogany-big sagebrush community. In: Starkey, Edward E.; Franklin, Jerry F.; Matthews, Jean W., technical coordinators. Ecological research in national parks in the Pacific Northwest; [Date of conference unknown]; [Location of conference unknown]. Corvallis, OR: Oregon State University, Forest Research Lab: 101-110. [8087]
188. Tipton, F. H. 1994. Cheatgrass, livestock, and rangeland. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 19-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 414-416. [24322]
189. Tisdale, E. W. 1986. Native vegetation of Idaho. Rangelands. 8(5): 202-207. [2339]
190. Tueller, P. T.; Platou, K. A. 1991. A plant succession gradient in a big sagebrush/grass ecosystem. Vegetatio. 94(1): 57-68. [16576]
191. Tueller, Paul T.; Beeson, C. Dwight; Tausch, Robin J.; [and others]. 1979. Pinyon-juniper woodlands of the Great Basin: distribution, flora, vegetal cover. Res. Pap. INT-229. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 22 p. [2367]
192. Tueller, Paul T.; Eckert, Richard E., Jr. 1987. Big sagebrush (Artemisia tridentata vaseyana) and longleaf snowberry (Symphoricarpos oreophilus) plant associations in northeastern Nevada. The Great Basin Naturalist. 47(1): 117-131. [3015]
193. U.S. Department of Agriculture, Soil Conservation Service. 1994. Plants of the U.S.--alphabetical listing. Washington, DC: U.S. Department of Agriculture, Soil Conservation Service. 954 p. [23104]
194. Vaitkus, Milda R.; Eddleman, Lee E. 1991. Tree size and understory phytomass production in a western juniper woodland. The Great Basin Naturalist. 51(3): 236-243. [16869]
195. Van Vuren, Dirk. 1984. Summer diets of bison and cattle in southern Utah. Journal of Range Management. 37(3): 260-261. [24531]
196. Vincent, Dwain W. 1992. The sagebrush/grasslands of the upper Rio Puerco Area, New Mexico. Rangelands. 14(5): 268-271. [19698]
197. Volland, Leonard A. 1985. Plant associations of the central Oregon Pumice Zone. R6-ECOL-104-1985. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 138 p. [7341]
198. Volland, Leonard A.; Dell, John D. 1981. Fire effects on Pacific Northwest forest and range vegetation. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Region, Range Management and Aviation and Fire Management. 23 p. [2434]
199. Vose, James M.; White, Alan S. 1991. Biomass response mechanisms of understory species the first year after prescribed burning in an Arizona ponderosa pine community. Forest Ecology and Management. 40(3/4): 175-187. [35011]
200. Welsh, Stanley L.; Atwood, N. Duane; Goodrich, Sherel; Higgins, Larry C., eds. 1987. A Utah flora. The Great Basin Naturalist Memoir No. 9. Provo, UT: Brigham Young University. 894 p. [2944]
201. West, Neil E. 1994. Effects of fire on salt-desert shrub rangelands. In: Monsen, Stephen B.; Kitchen, Stanley G., compilers. Proceedings--ecology and management of annual rangelands; 1992 May 18-22; Boise, ID. Gen. Tech. Rep. INT-GTR-313. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 71-74. [24256]
202. West, Neil E.; Hassan, M. A. 1985. Recovery of sagebrush-grass vegetation following wildfire. Journal of Range Management. 38(2): 131-134. [2513]
203. West, Neil E.; Tausch, Robin J.; Tueller, Paul T. 1998. A management-oriented classification of pinyon-juniper woodlands of the Great Basin. Gen. Tech. Rep. RMRS-GTR-12. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 42 p. [29131]
204. White, Alan S.; Cook, James E.; Vose, James M. 1991. Effects of fire and stand structure on grass phenology in a ponderosa pine forest. The American Midland Naturalist. 126(2): 269-278. [16885]
205. Winward, Alma H. 1970. Taxonomic and ecological relationships of the big sagebrush complex in Idaho. Moscow, ID: University of Idaho. 79 p. Ph.D. dissertation. [2583]
206. Wright, Henry A. 1967. Contrasting responses of squirreltail and needleandthread to herbage removal. Journal of Range Management. 20: 398-400. [2606]
207. Wright, Henry A. 1970. A method to determine heat-caused mortality in bunchgrasses. Ecology. 51(4): 582-587. [2609]
208. Wright, Henry A. 1971. Why squirreltail is more tolerant to burning than needle-and-thread. Journal of Range Management. 24: 277-284. [2610]
209. Wright, Henry A. 1978. The effect of fire on vegetation in ponderosa pine forests: A state-of-the-art review. Lubbock, TX: Texas Tech University, Department of Range and Wildlife Management. 21 p. In cooperation with: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. [4425]
210. Wright, Henry A.; Klemmedson, James O. 1965. Effect of fire on bunchgrasses of the sagebrush-grass region in southern Idaho. Ecology. 46(5): 680-688. [2624]
211. Yensen, Eric; Quinney, Dana L. 1992. Can Townsend's ground squirrels survive on a diet of exotic annuals? The Great Basin Naturalist. 52(3): 269-277. [20990]
212. Young, James A. 1989. Intermountain shrubsteppe plant communities--pristine and grazed. In: Western raptor management symposium and workshop: Proceedings; 1987; Boise, ID. Scientific Technical Series No. 12. Washington, DC: National Wildlife Federation: 3-14. [25377]
213. Young, James A.; Evans, Raymond A. 1970. Invasion of medusahead into the Great Basin. Weed Science. 18(1): 89-97. [2647]
214. Young, James A.; Evans, Raymond A. 1977. Squirreltail seed germination. Journal of Range Management. 30(1): 33-36. [280]
215. Young, James A.; Evans, Raymond A. 1981. Demography and fire history of a western juniper stand. Journal of Range Management. 34(6): 501-505. [2659]
216. Young, James A.; Evans, Raymond A.; Eckert, Richard E., Jr. 1969. Wheatgrass establishment with tillage and herbicides in a mesic medusahead community. Journal of Range Management. 22: 151-155. [2666]
217. Young, James A.; Evans, Raymond A.; Major, J. 1972. Alien plants in the Great Basin. Journal of Range Management. 25: 194-201. [2674]
218. Young, James A.; Evans, Raymond A.; Major, Jack. 1977. Sagebrush steppe. In: Barbour, Michael G.; Major, Jack, eds. Terrestrial vegetation of California. New York: John Wiley & Sons: 763-796. [4300]
219. Young, James A.; Palmquist, Debra E. 1992. Plant age/size distributions in black sagebrush (Artemisia nova): effects on community structure. The Great Basin Naturalist. 52(4): 313-320. [20180]
220. Young, Richard P.; Miller, Richard F. 1985. Response of Sitanion hystrix (Nutt.) J. G. to prescribed burning. The American Midland Naturalist. 113(1): 182-187. [2682]
221. Zamora, B.; Tueller, Paul T. 1973. Artemisia arbuscula, A. longiloba, and A. nova habitat types in northern Nevada. The Great Basin Naturalist. 33(4): 225-242. [2688]