| FEIS Home Page |
 |
|
|---|---|
Photo by Laura Erickson, www.lauraerickson.blogspot.com |
AUTHORSHIP AND CITATION:
Meyer, Rachelle. 2012. Aphelocoma coerulescens.
In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: www.fs.usda.gov/database/feis/animals/bird/apco/all.html [].
FEIS ABBREVIATION:
APCO
COMMON NAMES:
Florida scrub-jay
Florida scrub jay
scrub jay
TAXONOMY:
The scientific name of Florida scrub-jay is Aphelocoma coerulescens Bosc (Corvidae) [9].
The Florida scrub-jay was elevated from subspecies status in 1995 [8]. Although rare, hybridization with blue jays has occurred [97].
SYNONYMS:
Aphelocoma coerulescens coerulescens Bosc
ORDER:
Passeriformes
CLASS:
Bird
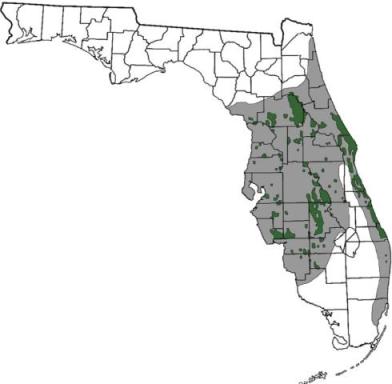 |
|---|
| The current range of the Florida scrub-jay is shown in green (black if viewing in black and white), and the historic range in gray. Map by Monica E. McGarrity in [79], The Florida scrub-jay: A species in peril. |
The Florida scrub-jay is endemic to Florida [127,139]. It occurs in a few large and several small populations, many of which are isolated to varying degrees [126]. Florida scrub-jay historically occurred in 39 of 40 counties of peninsular Florida [146]. By 1983, it was extirpated from several counties, including Broward, Dade, Duval, Pinellas, and St. Johns counties [46]. As of 1993, the Florida scrub-jay was extirpated from Alachua and Clay counties, and 6 counties had less than 10 breeding pairs each [146]. See Population trends for a discussion of Florida scrub-jay population decline.
Florida scrub-jays have been studied for long periods at Archbold Biological Station in central Florida and the Kennedy Space Center/Merritt Island National Wildlife Refuge on the east-central Florida coast. The Archbold Biological Station occupies 5,190 acres (2,100 ha) that overlap a portion of the Lake Wales Ridge metapopulation of Florida scrub-jays, in which about 565 territories where documented in 1992-1993 [126]. The initial study of Florida scrub-jays at Archbold Biological Station began in 1969 [49,146] on a site about 860 to 990 acres (350-400 ha) in size [144]. Starting in the late 1990s, several studies compared individuals at Archbold Biological Station with individuals in suburban areas located about 6 miles (10 km) north, in Placid Lakes, Florida (hereafter, Placid Lakes) [12,13,107,134]. Studies of Florida scrub-jays at the Kennedy Space Center/ Merritt Island National Wildlife Refuge (hereafter Kennedy Space Center) began in the 1980s [24,28]. There are 2 sites within Kennedy Space Center where much of this research occurred. Near the southern boundary of the Kennedy Space Center is a 590-acre (240 ha) [32] scrubby flatwoods site designated Tel-4 or T4 (Tel-4 is used in this review) [28,32]. The Happy Creek site is an 990-acre (400 ha) [24] scrub community about 8 miles (12 km) north of Tel-4 from which fire was excluded for about 20 years before prescribed burning began in 1979 [24]. Because of its overgrown character it provided relatively poor habitat for Florida scrub-jays into the early 2000s [16,24]. A 1992-1993 survey documented 536 Florida scrub-jay territories at Kennedy Space Center [126]. Schmalzer and others [113] provide a detailed characterization of scrub communities at the Kennedy Space Center and other areas within Brevard County.
PLANT COMMUNITIES:
Florida scrub-jays occur in scrub communities of peninsular Florida with low tree cover and high cover of low-growing evergreen oak shrubs such as sand live oak (Quercus geminata), Chapman oak (Q. chapmanii), and myrtle oak (Q. myrtifolia). Scrub communities include oak scrub [5,32,146] and scrubby flatwoods [5,143,146]. Flatwoods have shrub and ground layers similar to those of oak scrub, but also include an overstory of slash (Pinus elliottii) or longleaf pine (P. palustris) [14]. The term scrubby flatwoods may also be used for areas that are mixtures of flatwoods and oak scrub [22]. Florida scrub-jays also use coastal strand in areas where it borders oak scrub. Coastal strand is a shrubby community comprised of species such as twinberry (Myrcianthes fragrans), Hercules-club (Zanthoxylum clava-herculis), and redbay (Persea borbonia) [16]. Florida scrub-jays also use slash pine-turkey oak (Quercus laevis) at Archbold Biological Station [143], oak-saw-palmetto (Quercus spp.-Serenoa repens) and saw-palmetto-oak at Kennedy Space Center [32], and sand pine (P. clausa) scrub on the Ocala National Forest [46]. Florida scrub-jays are occasionally observed in adjacent communities such as swale marshes [20].
Oaks are a critical feature of high-quality Florida scrub-jay habitat. Florida scrub-jays at Archbold Biological Station and nearby areas use oaks for nesting more than would be expected based on their availability [134,143]. Saw palmetto was used less than expected based on availability at Archbold Biological Station [143]. At Placid Lakes and Archbold Biological Station, over 80% of nests occurred in oaks [13]. At Archbold Biological Station, 48% of nests were in sand live oak, 19% in sandhill oak (Q. inopina), 16% in myrtle oak, and 5% in Chapman oak [146]. At Kennedy Space Center, Florida scrub-jays nested in open oak scrub communities more than expected based on availability, but they also selected oak-saw-palmetto communities [27]. Nest success in this area was highest in territories with at least 50% oak cover [37]. Regenerating pasture adjacent to oak scrub at Archbold Biological Station had nest success and yearling production similar to that of the oak-dominated community [49].
Florida scrub-jays forage [146] and roost [80] primarily in oak scrub, but they also forage in palmetto patches [146], grassy road margins [96,146], and regenerating pasture [49,96]. At Archbold Biological Station, an index of nutritional status of young was significantly (P<0.02) greater in territories that had greater proportion of oak cover [70]. Throughout the year, Florida scrub-jays at Archbold Biological Station roost in communities dominated by sandhill oak, sand live oak, and a mixture of oaks more frequently than in saw-palmetto, staggerbush (Lyonia spp), and saw-palmetto-staggerbush communities [80].
Territories with less than about 1 acre (0.4 ha) of open oak scrub often do not provide high-quality habitat [27,32,35]. At the Kennedy Space Center, recruitment typically exceeded mortality in patches of open oak scrub greater than 66 feet wide (20 m) and comprised of scrub more than 3 feet (1 m) tall. In areas of scrub less than 66 feet (20 m) wide, recruitment was less than mortality [27]. At the Tel-4 site, oak scrub ridges less than 1 acre that occurred more than 525 feet (160 m) from larger oak scrub ridges were generally population sinks [32]. In 3 Florida scrub-jay metapopulations along the coast in Brevard County, areas with less than 1 acre of oak scrub had lower reproductive success than those with more oak scrub or areas intersecting well-drained scrub [35].
Oak-dominated communities seem to provide higher quality habitat for Florida scrub-jays than those codominated by saw palmetto [27,32]. For instance, at Kennedy Space Center, nest success was lower in oak-saw-palmetto communities than open oak scrub [27]. Several demographic measures were significantly (P<0.035) greater in oak-dominated territories that overlapped well-drained, oak ridges compared to territories on poorly-drained soils dominated by saw-palmetto and overlapping less than 1 acre (0.4 ha) of oak scrub (saw-palmetto-oak) (Table 1). Mortality exceeded recruitment in saw-palmetto-oak territories [32]. For details of reproductive success in oak scrub see Reproductive output.
| Table 1. Average Florida scrub-jay demographic performance in oak and saw-palmetto oak communities [32] | ||
| Demographic variable | Oak ridges | Saw-palmetto-oak |
| Average number of Florida scrub-jays per family group (P=0.034) | 3.11 | 2.44 |
| Breeder survival (P=0.012) | 83% | 68% |
| First-year nonbreeder survival (P=0.001) | 90% | 31% |
| Demographic performance* (P=0.013) | 0.22 | -0.38 |
| *(yearlings produced−breeder deaths) ÷ territories in a given plant community type; negative values indicate population decline and positive values indicate population growth |
||
See the Fire Regime Table for a list of plant communities in which Florida scrub-jay may occur and information on the fire regimes associated with those communities.
BIOLOGICAL DATA:
Because density is not a reliable indicator of Florida scrub-jay habitat quality [14] or demographic success [26], this review includes few publications that use Florida scrub-jay density as an indicator of population health, demographic success, or similar variables. For instance, Florida scrub-jays may occur at high densities in tall, dense scrub [18] despite Florida scrub-jay mortality rates exceeding reproduction in these communities [26] (see Scrub height). At Kennedy Space Center, habitat suitability models were more strongly correlated with indicators of demographic success, including breeder survival and fledgling production, than they were to Florida scrub-jay density [26,54].
Most of the information on life history and diet presented below was obtained from reviews, including a comprehensive review from Birds of North American written in 1996 [146].
Life history: Florida scrub-jays are nonmigratory, cooperative breeders that typically breed once a year, with first eggs laid from March to May [146]. Florida scrub-jays do not disperse far from their natal territory [35,125,146] and live up to 15.5 years. Predation is the major cause of mortality [146].Cooperative breeding: Florida scrub-jays are cooperative breeders with up to 6 nonbreeding helpers assisting the breeding pair with territory defense, predator mobbing, and sentinel activity. In many instances, they also help feed nestlings and fledglings. Several studies have shown helpers increase reproductive success of breeders [20,28,66,67,98]. At Archbold Biological Station, helpers were also associated with greater breeder survival [144]. Data from Kennedy Space Center suggest that the benefit helpers provide may be negated in poor quality habitat, such as dense vegetation and forest edges (See Preferred Habitat). In these areas effectiveness of visual detection and mobbing of predators may be reduced and some predators that avoid detection, such as nocturnal snakes, may be more common (see Tree cover) [37]. Because Florida scrub-jay sentinel behavior is more effective when there are Florida scrub-jays in adjacent areas [24,27], small populations supported by low-quality habitat may be less effective at detecting predators [24].
Helpers range in age from 1 to 7 years old, but are frequently yearlings that help in their natal territory [146]. At Happy Creek, most Florida scrub-jays delayed breeding for 1 to 2 years. The male to female sex ratio of nonbreeders was 3:1, with 1-year-old females breeding more frequently (P=0.025) than 1-year-old males [24]. Models based on data collected from 1997 to 2005 in 20 Florida scrub-jay populations along central Florida's Atlantic coast showed that yearlings and males delayed breeding more frequently than older nonbreeders and females. The probability of remaining a helper decreased by nearly half when age changed from juvenile to adult and other variables were unchanged. Delayed breeding was lowest at intermediate Florida scrub-jay densities. The authors suggest that ability to find a mate likely limits breeding opportunities at low densities, and competition for territories likely limits breeding opportunities at high densities [34]. During the period of delayed breeding, helpers foray into neighboring territories to detect and fill breeding vacancies [126].
Florida scrub-jay survival is higher, breeder turnover lower, and delayed breeding more common in high-quality habitat [29]. Mortality of breeders leads to breeding vacancies which allow Florida scrub-jays to breed earlier than would otherwise be the case [21,28,34]. At Happy Creek, an area of low-quality habitat and declining populations, breeding yearling females were 10 times more common than at Archbold Biological Station, an area of high-quality habitat [24] and relatively stable populations. At the Tel-4 site, territories in comparatively poor palmetto-oak habitat had lower survival and a greater proportion of yearling breeders than territories in higher quality habitat [32].
More detailed descriptions of Florida scrub-jay social interactions, including mobbing behavior [65], dominance hierarchies, territory defense, time budgets, and communication, including use of certain vocalizations in specific contexts [144,146], are available.
Territories/Density: Florida scrub-jays typically remain on a territory from the time they become breeders until their death. Territories are acquired in 1 of 4 ways. The most common for both sexes is to replace a lost breeder on a nearby territory. Another fairly common method is to "bud" off a portion of the natal territory into a new territory. More common in males than females is the direct inheritance of the natal territory following the death of breeders. In a few cases a new territory is established between existing territories or in unoccupied scrub. Habitat destruction, extensive fire, or, rarely, the loss of a mate results in Florida scrub-jays abandoning territories; the latter is more common among females than males [146].
Florida scrub-jays defend territories that typically range from 10 to 45 acres (4-18 ha) [61], with territory size in good habitat averaging about 23 acres (9 ha) [31,68,146]. The smallest average territory size reported was 6 acres (2.4 ha) just north of Archbold Biological Station [138], and the largest territory size reported was 119.6 acres (48.4 ha) at Kennedy Space Center [68]. Fitzpatrick and others [61] note that 23 acres is the average amount of useable oak scrub in a territory, and most territories contain additional patches of unused habitat. Territory size varies with group size, availability of supplemental food [146], community type [61,68,146], and, in some instances, density of Florida scrub-jays [24].
Due to space requirements, density of Florida scrub-jay territories averages about 4 to 5 territories per 100-acre (40 ha) area, with densities declining in scrub that is sparse or overgrown [146]. Minimum territory density for a stable population is 2.0 territories per 100 acres of optimal habitat, and maximum territory density is 6.5 territories per 100 acres [61].
Population growth may be affected by Florida scrub-jay density. There are many reasons that low densities could be detrimental to Florida scrub-jays, including fewer helpers to contribute to reproductive success (see Cooperative breeding), longer dispersal distances needed to find mates, and smaller populations being more vulnerable to extinction from stochastic events such as hurricanes [23] or disease epidemics. However, demographic performance per pair was highest at the lowest densities observed (2.0 pairs/40 ha) in oak-palmetto territories at Tel-4. The Florida scrub-jay population at this site was growing when densities were less than 2.8 pairs/40 ha, and declining at higher densities [32].
Breeding: Florida scrub-jays form monogamous pairs [24,146] typically when 2 or 3 years old, although this ranges from 1 to 7 years old [146]. Divorce rates were less than 1% at Kennedy Space Center [24] and 6% at Archbold Biological Station [146]. Following the death of a mate, 87% of Florida scrub-jays at Happy Creek remained breeders on their territory, and 8% bred on a neighboring territory. A small proportion of Florida scrub-jays do not breed the year following loss of a mate [24,146]. Based on over 20 years of data from Archbold Biological Station, breeding Florida scrub-jays average 4.2 breeding seasons [146].
Florida scrub-jays typically raise 1 brood a year [143,146]. They will lay replacement clutches after unsuccessful nesting attempts. At Archbold Biological Station, clutches of 3 or 4 eggs were most common, with a range of 1 to 6 eggs [146]. True second broods are rare [143,146].
Novice breeders at Archbold Biological Station bred later and had smaller clutches than experienced breeders [143]. However, a later analysis found pair bond duration had a stronger influence on Florida scrub-jay clutch initiation date (and in turn clutch size [104,119,120]) than breeder age or breeder experience [10].
Available discussions of breeding behaviors include information on pair formation [138], mating displays, nest construction, nestling care, sentinel and mobbing behavior, time budgets [144,146], helper behaviors, and territorial interactions [138,144,146].
Timing of breeding, nesting, and early development: Pair formation occurs year-round but is most frequent in spring before nesting and in fall. Two miles north of Archbold Biological Station, nest building began as early as 20 February and continued until early June [138]. From 1970 to 1994 at Archbold Biological Station, 55% of nests were incubated in March, 41% in April, and 4% in May [146]. If the nest fails, the first egg of a replacement nest is often laid after about 10 days. Young typically fledge by early June [143] and are capable fliers after about 32 days [146].
Reproductive output: Based on over 20 years of data from Archbold Biological Station, 88% of breeders produced at least 1 fledgling, but 70% of fledglings were produced by 30% of breeders. On average, 49% of Florida scrub-jay nests produced at least 1 fledgling, although annually this ranged from 29% to 75%. Average annual production of independent young was 1.12 per pair [146]. At Kennedy Space Center, only 35% of nests successfully produced at least 1 fledgling, with 94% of failures due to predation [37]. On the Ocala National Forest, pairs without helpers produced an average of 1.71 fledglings, and pairs with helpers produced an average of 2.24 fledglings. Predation was the likely cause of the low nest success, with 64% of 13 video-monitored Florida scrub-jay nests being partially or totally depredated [66].
Reproductive success of Florida scrub-jays is variable and influenced by several factors including presence or absence of helpers (see Cooperative breeding), annual variation [67,146], experience of breeders, duration of pair bond [10,141,146], timing of laying and hatching [37,67], and habitat (see paragraph below). Annual variation is substantial. In optimal habitat at Archbold Biological Station, average annual reproductive success ranged from 1.05 to 2.71 fledglings/pair [61]. Rainfall has been shown to influence nest success, although in different ways at different locations [37,141]. It is not likely that rainfall's effects on nest success are due to its influence on food availability because few nestlings die from starvation [37]. Inexperienced breeders had lower reproductive success than experienced breeders [28,46], and established pairs had better success than new pairs at Archbold Biological Station [141,146]. Nest success declined as the breeding season progressed at both Kennedy Space Center [37] and on the Ocala National Forest [67].
Some habitat attributes impact Florida scrub-jay reproductive output, while others have not been shown to have a significant influence. Table 2 shows several measures of reproductive output from sites of varying quality. Based on data from Archbold Biological Station collected from 1969 to 1984, lifetime reproductive success of Florida scrub-jays in unburned, overgrown habitat is 0.4 breeding offspring per individual, while in periodically burned habitat it is 1.1 breeding offspring per individual [59]. See Influence of fire on Florida scrub-jay demographics for more information. Data from the same area through 1994 show Florida scrub-jays in high-quality habitat produced an average of 7.4 fledglings, 4.2 independent young, and 2.2 yearlings over their lifetimes [146]. Stand age was an important influence on daily nest survival rate during the incubation phase on the Ocala National Forest (see Influence of fire characteristics on Florida scrub-jay response for details) [67]. Bowman [13] found that nest success was higher in oaks than in other vegetation at Placid Lakes and Archbold Biological Station. In contrast, an earlier study of these sites found species of shrub used for nesting did not have a significant influence on nest success [134]. Although differences were not significant, Florida scrub-jays in territories with a substantial component of palmetto had lower nest success [27] and produced fewer offspring [32] than those in open, oak-dominated territories. At Archbold Biological Station, nest success and yearling production of Florida scrub-jay nests in territories comprised of regenerating pasture bordering oak scrub were not significantly different from those in intact oak scrub [49].
| Table 2. Comparison of average Florida scrub-jay demographic rates in 3 areas | |||||
| Demographic parameter | Fragmented suburban populations in southern Brevard County [21]* | Kennedy Space Center [28] | Archbold Biological Station (1969-1986) [145] | ||
| Tel-4 site (1989-1993) | Happy Creek site (1988-1993) |
Periodically burned | Unburned | ||
| Fledglings/pair | No data | 1.83 | 1.11 | 1.97 | 1.58 |
| Juveniles/pair | 0.41 | 0.96 | 0.47 | 1.17 | 0.8 |
| Yearlings/pair | 0.22 | 0.62 | 0.32 | 0.60 | 0.36 |
| Breeder survival | 0.79 | 0.76 | 0.8 | 0.79 | 0.72 |
| Fledgling survival | 0.34 | 0.29 | 0.31 | 0.23 | |
| Nonbreeding adult survival | 0.48 | 0.72** | 0.73** | 0.74 | No data |
| Family size | 2.2 | 3.2 | 2.8 | 3.0 | No data |
| *Average of 5 years, 1 an epidemic year (see Disease for details of Florida scrub-jay epidemics). **This is the minimal survival for this site, because a few nonbreeders may have become breeders outside the study area. |
|||||
Reproduction in suburban areas: Compared to wildland populations, Florida scrub-jays in the suburban area of Placid Lakes began breeding earlier, laid larger clutches, attempted more nest starts per pair, and had more true second broods; however, they had 50% less juvenile recruitment than wildland Florida scrub-jays [12]. One possible reason for lower reproductive success is that fewer eggs hatch from nests in suburban territories [6]. Data from Placid Lakes suggest that longer exposure of first-laid eggs to warm temperatures before the start of incubation leads to fewer first-laid eggs hatching in these areas [6,86]. In addition, brood size is reduced over the nesting period [12,122]. Broods at Placid Lakes were reduced on average about 30% [122], while at Archbold Biological Station brood reduction was about 5% [12,122]. One suggested reason for this difference is the longer laying period in suburban areas; on average the nesting season at Placid Lakes was 30 days longer than at Archbold Biological Station [12]. The earlier laying and larger clutch sizes that lengthen the nesting period also lead to greater size differences between the first and last hatchlings. This leaves the smaller, last-hatched nestlings at a disadvantage [6,122]. Boughton [11] found reduced immune response in nestlings from larger broods. However, he suggests that the poor quality of an anthropogenic diet common in the suburbs was a likely cause (see Diet for more detail). As of 2012, the links between food availability and quality, parental care, and brood reduction are unclear. Fewer helpers in suburban habitat might also contribute to reduced nest success in suburban habitat [122]. In addition to reduced hatching and fledging rates, mortality of fledged juveniles was higher at Placid Lakes than at Archbold Biological Station (see Survival). The levels of recruitment observed in the suburban population were not sufficient to offset mortality [12].
Earlier laying in wildland territories that were provided supplemental food led to larger clutch sizes [104,120] and greater frequency of second broods (up to 30% of pairs attempted) (Bowman and others 1995 cited in [146]). See Diet for more on the impacts of supplemental feeding and its management potential.
Dispersal: Most Florida scrub-jays disperse less than 1.9 miles (3 km) from their natal territory [35,125,146]. For instance, at Tel-4 Florida scrub-jays dispersed into marginal palmetto-oak vegetation less than 1.2 miles (2 km) away from a predominantly oak community that was at optimal height [32]. The mean dispersal distances of male and female Florida scrub-jays in 3 metapopulations along the coast in Brevard County were 1 mile (1.6 km) and 1.7 miles (2.8 km), respectively. From 82% to 92% of all breeding vacancies were filled by individuals from the same territory cluster [35]. From 1979 to 1993, 80% of Florida scrub-jays at Archbold Biological Station dispersed less than 1.1 miles (1.7 km) and 99% dispersed less than 5.2 miles (8.3 km) [125]. At Archbold Biological Station [60,146], Tel-4 [32], and Happy Creek [24] most Florida scrub-jays dispersed ≤3 territories away from their natal territory. The longest reported dispersal distance was 23.6 miles (38 km) [146]. Although genetic analysis suggested individual dispersal events as long as 47 miles (75 km), only 6 Florida scrub-jays out of 1,028 genotyped individuals had dispersed from their natal metapopulation [43]. At Archbold Biological Station, Florida scrub-jays that dispersed the longest distances tended to have dispersed by the time they were 2 years old (P<0.01). This suggests that some Florida scrub-jays do not exhaust local breeding opportunities before dispersing [60]. Nonbreeders have been observed to range up to 3 miles (5 km) from the natal territory on dispersal forays before permanently dispersing [146].
Female Florida scrub-jays disperse farther than males. Females dispersed significantly (P≤0.044) farther from their natal territory than males on a barrier island in southern Brevard County [21], at Happy Creek [24], and in 3 metapopulations located along the coast in Brevard County [35]. Males at Archbold Biological Station breed from 0 to 2 territories distant from their natal territory significantly (P≤0.05) more often than 3 or more territories away, while the differences in these dispersal distances were not significant for females [60]. Males become breeders in their natal territory more often than females [24,32,146]. Along the coast in Brevard County, only 30% of the individual Florida scrub-jays that dispersed outside of their natal territory cluster were males [35].
Landscape factors influence dispersal distances and "effective dispersal" (dispersal that results in the exchange of genetic material between areas) of Florida scrub-jays. Open water, dense forests, open fields, and most urban areas are barriers to Florida scrub-jay dispersal [14,126,146]. Florida scrub-jays rarely travel as far as 0.6 mile (1 km) over or through dense forest [146]. Florida scrub-jays disperse through fragmented environments with some shrub cover, including suburbs with trees, citrus groves, pastures with scattered oaks [126], and poor-quality, unoccupied scrub [14]. Florida scrub-jay dispersal distances in these types of landscapes were farther than in unfragmented landscapes of Sarasota (P<0.01) [129], Brevard [21,35], and Highland counties [44]. However, Florida scrub-jay dispersal distance in fragmented and unfragmented habitat at Archbold Biological Station [60] and Brevard County [35] are similar when expressed as number of occupied territories crossed. Because Florida scrub-jay dispersal strategy involves monitoring neighboring territories for breeding opportunities, Florida scrub-jays in fragmented habitat have to disperse farther than those in unfragmented habitat [35,60,129]. In low-quality habitat with occupied habitat fragments no more than 2.6 miles (4.2 km) apart, females may disperse up to 9.3 miles (15 km) through urban landscapes, at least temporarily [21]. At Archbold Biological Station, Florida scrub-jays that dispersed farther had lower survival [60] and lower lifetime reproductive success than short-distance dispersers [44,60]. Those that disperse through vegetation communities and land cover other than scrub also have lower survival [126].
In Sarasota County, 1-year-old females in suburban areas dispersed more frequently than those in wildland areas. Timing of dispersal also differed significantly (P≤0.001) between the 2 areas. Eighty percent of Florida scrub-jays in unfragmented habitat dispersed from February to April, while those in fragmented habitat dispersed year-round [129].
Survival: As of the mid-1990s, from 10% to 20% of the breeding population at Archbold Biological Station was at least 10 years old, and the maximum observed lifespan of Florida scrub-jays was 15.5 years [146]. Although the major source of mortality is predation [146], road mortality [100,146] and disease also impact Florida scrub-jay populations [146]. Most mortality occurs when food is relatively abundant, suggesting that starvation is not a major source of mortality [28,144].
Like reproduction, survival exhibits substantial variation and is influenced by several factors, including age, family, and habitat. Survival of Florida scrub-jays from fledgling to 1 year averaged 33% with a range of 1% to 63% at Archbold Biological Station. Survival of nonbreeders ages 1 to 2 was about 81% for males and 65% for females, which are more likely to go on dispersal forays. Survival of breeding Florida scrub-jays averages 78% and decreases with increasing age. Average survival of breeders at Tel-4 over 5 years was 72% for females and 79% for males, and at Happy Creek over 6 years it was 75% for females and 85% for males [28]. Survival of nonbreeding Florida scrub-jays more than 1 year old was typically more than 80% at Happy Creek [24]. Florida scrub-jays in remnants of scrub surrounded by urban areas exhibited faster decline than populations in overgrown scrub on a barrier island in southern Brevard County. Possible explanations included greater mortality from cars and domestic cats in areas of human development [21]. In contrast, survival rates in regenerating pasture adjacent to scrub were similar to those in scrub at Archbold Biological Station [49]. Fitzpatrick and others [61] reported large annual variation in breeder survival, which ranged from 55% to 96% in optimal habitat at Archbold Biological Station. Over 35 years of data from this site indicated that differences between families explained more variation in survival than differences between years. The degree to which genetics or inheritance of high quality territories influenced this trend could not be determined [64]. Variation in predation may also influence annual variability in Florida scrub-jay survival [29].
Predation: Birds prey on Florida scrub-jays at all life stages. American crows (Corvus brachyrhynchos) [61,66], fish crows (C. ossifragus), and blue jays (Cyanocitta cristata) eat Florida scrub-jay eggs and nestlings. The decline of Florida scrub-jays in dense scrub and wooded areas may be due in part to the greater abundance of blue jays in these areas [61]. Red-tailed hawks (Buteo jamaicensis) and eastern screech owls (Megascops asio) have been noted to take young Florida scrub-jays, while sharp-shinned hawks (Accipiter striatus), Cooper's hawks (A. cooperii), and merlins (Falco columbarius) prey on adult Florida scrub-jays. Northern harriers (Circus cyaneus) and great horned owls (Bubo virginianus) prey on young and adult Florida scrub-jays [61,146]. Florida scrub-jays at Kennedy Space Center are highly susceptible to avian predators during their spring and fall migrations [28].
Adult Florida scrub-jays are less vulnerable to predation from mammals and snakes than young Florida scrub-jays [146], although brooding females may be susceptible to nocturnal predators, particularly snakes. Mammalian predators of Florida scrub-jay nests and young include eastern spotted skunks (Spilogale putorius), gray foxes (Urocyon cinereoargenteus) [66], hispid cotton rats (Sigmodon hispidus) [61], black rats (Rattus rattus), raccoons (Procyon lotor), and probably long-tailed weasels (Mustela frenata), eastern gray squirrels (Sciurus carolinensis), and southern flying squirrels (Glaucomys volans) [146]. Bobcats (Lynx rufus) [146] and domestic cats (Felis catus) [61,146] likely prey on young and adult Florida scrub-jays. Predation by domestic cats is one of many factors likely to reduce demographic success of Florida scrub-jays in suburban areas [21,146]. Snakes, including the eastern coachwhip (Masticophis flagellum flagellum), eastern indigo snake (Drymarchon couperi) [146], and yellow rat snakes (Elaphe obsoleta) are Florida scrub-jay nest predators. At Happy Creek 75% of egg and nestling losses observed were attributed to the yellow rat snake [38]. Eastern diamond-backed rattlesnakes (Crotalus adamenteus), pinesnakes (Pituophis melanoleucus), and eastern racers (Coluber constrictor) are also potential predators of young Florida scrub-jays [146]. Coachwhips and indigo snakes have also been reported to eat adult Florida scrub-jays [61,146]. The majority (70% to 88%) of recorded nest predation on the Ocala National Forest [66] and at Happy Creek [38] occurred at night, and snakes were responsible for more nest predation than mammals or birds at both sites.
The Florida scrub-jay's predator defense system is not effective against nocturnal predators, so brooding females are at risk of being depredated [28,38]. The association of primarily nocturnal predators with forests that do not provide good quality Florida scrub-jay habitat (see Tree cover) suggests that habitat management, such as reducing tree density, may reduce predation rates. If predation rates are not reduced by habitat management, Carter and others [38] suggest considering management actions to reduce populations of the yellow rat snake.
Road mortality: Despite higher mortality near roads than away from roads [100], Florida scrub-jays often use roadside areas [96,100]. Breeding Florida scrub-jays with territories near a 2-lane highway at Archbold Biological Station had a significantly (P<0.0001) lower survival rate (62.5%) than those with territories away from the highway (77.4%). Florida scrub-jays that recently (≤2 years) immigrated to roadside areas had the lowest survival rate observed (50-55%). Mortality was greater than reproduction in roadside territories [100]. Florida scrub-jays likely use roadside habitats because of greater food availability [96]. In southern Brevard County, mortality from cars may have caused the faster decline in Florida scrub-jay populations in scrub remnants surrounded by urban areas than is typical for low-quality, overgrown scrub [21].
Disease: Three episodes of elevated mortality over 26 years [133], including 70% mortality of breeding Florida scrub-jays from June 1979 to May 1980 [61] and 39% mortality of breeders from 2008 to 2009 [140] at Archbold Biological Station, suggest that disease epidemics periodically impact Florida scrub-jay populations [133,146]. Epidemics have been reported in Brevard County [29]. It seems likely that in situations where Florida scrub-jay abundance and habitat are not limited, these epidemics would have little long-term impact [133,146]. However, risk of local extinction from these events is increasing with habitat loss and declining populations [133]. More information on Florida scrub-jay parasites and diseases is available [133,146].
Diet: Florida scrub-jays primarily eat arthropods and acorns [50,146]. Arthropods taken from leaves or leaf litter [61] are most common in the summer diet [50]. Young in wildland areas are only fed arthropods, particularly Lepidoptera and Orthoptera. Florida scrub-jays have been observed foraging on the backs of ungulates, presumably eating ticks [146]. A foraging study at Archbold Biological Station performed from January 1974 to May 1975 found that fresh acorns are eaten from August to December and cached primarily from September to November. Cached acorns are eaten throughout the year, most commonly from September through February. Each Florida scrub-jay cached from 6,500 to 8,000 acorns [50]. Florida scrub-jays cache acorns on sand or vegetation, such as moss and palmetto fronds [146]. Florida scrub-jays at Archbold Biological Station were more likely to recache acorns stored in bare sand or sites with low soil moisture compared to those in more mesic microenvironments. Florida scrub-jays often cache out of view of conspecifics [83]. Fitzpatrick [61] suggests that Florida scrub-jay habitat selection is driven by the birds' reliance on acorns and sandy areas for caching them. Small vertebrates such as frogs, lizards, and small snakes comprise the majority of vertebrate prey. Other small vertebrates, berries, seeds, carrion, and snails are rarely eaten [146].
Low-quality food available to suburban Florida scrub-jays may contribute to poor condition and low survival of young in these areas, although other factors may be involved (See Reproduction in suburban areas). Individuals from Archbold Biological Station and Placid Lakes that obtained food the fastest bred the earliest, suggesting that food availability, and not necessarily food quality, provides a cue to begin breeding [62]. There is limited evidence that the predictability of food availability in the suburbs may also influence initiation of breeding [119]. Despite greater foraging rates, adult Florida scrub-jays at Placid Lakes had lower net energy gain compared to individuals at Archbold Biological Station [62]. The lower abundance of high-quality invertebrate food in the suburbs, the relative ease of obtaining human-provided food, the lack of a difference between food delivery rates in suburban and wildland areas [107,122], and greater brood reduction in areas and years with low food abundance [122] suggest that Florida scrub-jays in suburbs provide nestlings a detrimental amount of low-quality food.
Experimental supplementation with high-quality food at Archbold Biological Station may improve fledging success and survival to independence, particularly in difficult years [103,120]. Earlier laying in supplemented territories led to larger clutch sizes [104,119,120]. Based on these findings Schoech and others [120] suggest that supplemental feeding could be a useful management option to quickly increase effective carrying capacity or assist in translocation efforts, although they caution that it could also lead to predator entrainment and increased disease transmission.
PREFERRED HABITAT:
The character of high-quality Florida scrub-jay habitat–composition, structure, and spatial arrangement of vegetation–is highly specific. Well-drained oak scrub provides high-quality habitat [35,146]; however, large expanses of well-drained scrub are not necessarily required for viable Florida scrub-jay populations [32]. Florida scrub-jay populations are generally viable in oak-dominated scrub communities (see Plant Communities) where each territory has at least 0.75 to 1.0 acre (0.3-0.4 ha) of 3- to 7-foot tall scrub [32,35], less than 1 acre of tall scrub [24], scattered open areas, and few trees (see Structural features). Good-quality habitat also borders open, native vegetation (see Landscape features). Oak scrub of this nature historically developed under a regime of frequent fires (as often as every 5 to 10 years). These fires were often patchy, with burned patches usually smaller than Florida scrub-jay territories (see Fire Regimes for details).
Presence of Florida scrub-jays does not necessarily indicate good-quality habitat. In some cases, areas with Florida scrub-jays cannot support a population over the long-term [26,32], while in optimal habitat recruitment generally exceeds mortality [24]. Florida scrub-jays will defend territories in marginal habitat, as was the case at Happy Creek from 1988 to 2000 [24]. Optimal habitat was selected by Florida scrub-jays at Kennedy Space Center, suggesting that these are the first areas occupied when Florida scrub-jays are at low densities [24,27]. In contrast, a lack of colonization of high-quality habitat at Avon Park Air Force Range led Stith [126] to suggest that dispersing Florida scrub-jays may cue on the presence of other Florida scrub-jays more than aspects of habitat quality when establishing territories.
In most cases, suburbs do not provide good Florida scrub-jay habitat. A population of Florida scrub-jays surrounded by urban areas on a barrier island in southern Brevard County declined at a faster rate than typical for low-quality unburned scrub [21]. Mortality from vehicle collisions [21,100,146] and predation by domestic cats [21,146] (see Survival) have been suggested as possible causes of poor demographic performance in areas of human development. Human-provided food may contribute to reduced reproductive success (see Diet). Due to these and other issues, territories near human development often have mortality rates that exceed reproduction [12,21,100]. Florida scrub-jays in preserve areas of Sarasota [129] and Highlands [12] counties did not disperse into nearby suburban Florida scrub-jay populations. Despite these general trends, fledgling success in suburbs has been good in some years [142], and territories next to suburban development in Brevard County with optimal scrub height and openings had greater reproduction than mortality. Although these conditions rarely occurred, the researchers suggest maintaining optimal habitat conditions in edge territories to increase their potential to contribute to population increases [35].
 |
|
|---|---|
Photo by Laura Erickson, www.lauraerickson.blogspot.com |
Landscape features: Florida scrub-jay territories are comprised of a mosaic of oak scrub and openings within a matrix (area surrounding Florida scrub-jay habitat) of rarely- or never-used vegetation [27,35,126]. Potential population sizes may be greatly underestimated if medium to small patches of open scrub habitat imbedded in non-habitat communities, such as mesic flatwoods, are not included [22,32,35].
Although rarely used, matrix vegetation influences the quality of Florida scrub-jay habitat patches [21,26,27,49]. In Brevard County, Florida scrub-jay use of scrub patches was influenced by the landscape cover surrounding them; matrix habitats of flatwoods and palmetto allowed for increased capacity in areas where scrub was limited [14]. Open, flammable communities away from human development apparently make the best matrix vegetation because they provide increased distance from forests (see Tree cover), increased opportunity for dispersal, increased occurrence of native prey species (see Diet) [27], potentially fewer predators (see Tree cover), shortened fire-return intervals within the oak scrub [16,27,35] (see Fire Regime), and reduced detrimental impacts associated with suburbs (see Preferred Habitat). The openness and flammability of pastures led Davison and Fitzpatrick [49] to recommend their consideration in habitat conservation planning.
Structural features: Viable Florida scrub-jay populations are comprised of Florida scrub-jays occupying many territories that each have at least 0.75 to 1.0 acre (0.3-0.4 ha) of medium-height scrub (see Scrub height) [24,32], less than 1 acre of tall scrub [24], scattered open areas [14,27,126], and less than 20% pine cover [26,27,46]. Sites occupied by Florida scrub-jays at the Kennedy Space Center had greater percent oak cover (P=0.002), percent open space (P=0.008), and distance to forest (P=0.004) than unoccupied sites [27]. Variation in Florida scrub-jay occupancy in Brevard County was explained mainly by pine canopy, open space, and scrub height [14]. Habitat suitability indices developed in 1992 scored vegetation based on several factors and defined good-quality Florida scrub-jay habitat as being over 330 feet (100 m) from forest vegetation and having >50% oak cover, <15% pine cover, an average shrub height of 4 to 5.5 feet (1.2-1.7 m), and 20% to 50% open space or near proximity (<330 feet (100 m)) to a ruderal edge (Breininger 1992 cited in [26]). Areas with the highest values based on these measures were preferred by Florida scrub-jays for nesting and had the highest fledgling production [26]. These indices were also correlated with demographic performance at Tel-4 (r=0.87; P<0.001) [54] and at Happy Creek when averaged over 5 years (P<0.001) [26]. Florida scrub-jays had greater genetic diversity in vegetation with more than 50% oak cover that was 4 to 5.5 feet (1.2-1.7 m) tall and had open sandy areas [82].
Scrub height: Medium-height scrub (4 to 5.5 feet (1.2-1.7 m) tall) was positively associated with Florida scrub-jay demographic variables in several studies in Brevard County [24,29,32,35], although 1 study found that scrub height was not important to nest success [37]. At the Happy Creek site the only territories in which recruitment potential exceeded breeder mortality were those with at least 0.32 acre (0.13 ha) of medium-height scrub, no more than 1 acre (0.4 ha) of tall scrub (>5.5 feet (1.7 m) tall), and the remainder of the territory comprised primarily of short (<4 feet tall) scrub. In territories meeting these habitat criteria, demographic performance (P=0.001) and fledgling survival (P=0.039) were significantly greater than in territories in marginal habitat. Marginal habitat was typically due to large (>1 acre) patches of tall scrub [24]. At Tel-4, Florida scrub-jays in territories with 2.2 to 3 acres (0.9-1.2 ha) of medium-height scrub had better average demographic performance/pair than territories with either more or less medium-height scrub. Territories with less than 0.7 acre (0.3 ha) of medium-height scrub typically had mortality rates that exceeded recruitment [32]. In 3 metapopulations along the Atlantic Coast, territories comprised of short and medium-size oaks had greater yearling production than breeder mortality. Well-drained oak scrub ridges had lower demographic performance than territories with a mixture of scrub ridge and palmetto-scrub vegetation, possibly because of lower flammability and consequently taller shrub heights on scrub ridges [35]. Another extensive study of Florida scrub-jays in coastal Brevard County found that survival rates ranged from 71% in short scrub habitat to 82% in medium-height oak scrub. Survival in tall scrub was intermediate to these values [29]. In contrast, nest success at Kennedy Space Center may have been influenced more by a lack of openings or the occurrence of nocturnal snakes than by shrub height [37].
Height of scrub communities is influenced by time since disturbance. In 1982 and 1983 on the Ocala National Forest, a significantly greater proportion of sand pine stands that regenerated from 1972 to 1978 (43%) were occupied by Florida scrub-jays than stands that regenerated from 1965 to 1971 (22%, P<0.01). Once the sand pines reached 9.8 feet (3 m) tall or 50% cover, the stands no longer provided good-quality Florida scrub-jay habitat [46]. See Postfire succession and habitat quality for details regarding postfire habitat quality.
The timeframe in which successional vegetation provides optimal Florida scrub-jay habitat is influenced by several factors. These include disturbance history [46], plant community composition [30,77], site characteristics [30,46], and weather [46]. Optimal-height habitat was estimated to remain optimal for 3.7 years without disturbance at Kennedy Space Center [78]. Scrub communities with relatively high proportions of sandhill oak could provide habitat for a longer period than those comprised of other species because sandhill oak has a relatively short lifespan and reaches a median height of 6.6 feet (2 m) despite stands being up to 40 years old [77]. The level of the water table [30], soil nutrients, rainfall, and the severity and extent of previous fires [46] also influence if and when vegetation on a site provides high-quality Florida scrub-jay habitat.
Tree cover: Vegetation with high tree cover provides poor quality Florida scrub-jay habitat and reduces quality of adjacent habitat [26,27]. At Avon Park Air Force Range in central Florida, Florida scrub-jays selected territories with low tree cover, and Florida scrub-jay group size was generally larger in territories with lower tree cover. Florida scrub-jays in areas with more than 20% to 30% tree cover within or adjacent to territories had poor demographic performance [126]. In a model of Florida scrub-jay occupancy for Brevard County, increasing pine canopy cover was a key indicator of poor-quality habitat [14]. Nest success at Kennedy Space Center declined with proximity to forest edges, and daily survival increased in areas with greater oak cover and greater distance to the nearest forest edge [37]. Recent analyses suggest that high-quality habitat has no more than 1 tree/acre, and that habitat within 1,000 feet (300 m) of forested areas may provide low-quality Florida scrub-jay habitat (Burgman and others 2001 as cited in [81]). Nest survival was reduced within 2,400 feet (730 m) of forests at Kennedy Space Center (Carter unpublished cited in [81]). In the Ocala National Forest, Florida scrub-jays occupy sand pine communities where oaks are dense and sand pines are short (9.8 feet (<3 m)) and comprise <50% cover [46]. Patches of good-quality scrub less than 2.5 acres (1 ha) were occupied by Florida scrub-jays at Kennedy Space Center, except when they were surrounded by forests [27].
Florida scrub-jay's poor performance in areas with high tree cover may be related to predators. Many avian predators are more common in forests [126] and may be harder to detect in forested communities ([14], Breininger and others 1995 cited in [26]). Dense forest growth may also provide cover for ground predators, increasing the likelihood of successful ambush of Florida scrub-jays and their nests [45]. Snakes that prey on Florida scrub-jay nests often use forest edges (reviewed in [37]). Carter and others [37] suggest that habitat changes due to fire exclusion at Kennedy Space Center may have increased the density of snakes.
Openings: Oak scrub communities that provide high-quality Florida scrub-jay habitat have a complex mosaic of openings comprising less than 50% of the area. Openings are areas with bare sand [26,146] and open canopies [17,37]. Several predators are associated with dense vegetation (see Tree cover). In contrast, openings likely allow for easier predator detection [16,37,144] and are used for caching acorns (see Diet). Florida scrub-jay use increased as open space increased from 0% to 30% at Kennedy Space Center [27]. At Avon Park Air Force Range, Florida scrub-jay territories had more open sand than areas outside of Florida scrub-jay territories [126]. Nest success in the scrubby flatwoods vegetation at Tel-4 was greater than in the scrub at Happy Creek. The difference in nest success is likely related to the dense nature of the scrub community at Happy Creek, which could make detection of predators more difficult [37]. It has been suggested that the simple edges and dense scrub at Happy Creek provide lower quality habitat than the complex mosaic of openings at Tel-4 [26]. Simple edges were systematically patrolled by predators of loggerhead shrikes (Lanius ludovicianus) at Archbold Biological Station (Yosef 1994 cited in [54]). Carter and others [37] recommend more research into the relationship between openings and Florida scrub-jay nest success. Natural openings are apparently of higher quality than ruderal areas [26], but the reasons for this are not well understood.
Nest site characteristics: Florida scrub-jay nests are off the ground [13,134,146] and well concealed from above [134,138,146]. In suburban habitat at Placid Lakes, nests were commonly associated with greenbriar (Smilax spp.), which seemed to increase concealment [13,134]. Nests at Archbold Biological Station were built at an average of 3 feet (0.9 m) above ground and ranged from 1.6 to 8.2 feet (0.5-2.5 m) above ground [146]. Nest trees at Placid Lakes averaged 10.2 feet (3.1 m) tall, and nest trees at Archbold Biological Station averaged 7.2 feet (2.2 m) tall [134]. Nests at both Archbold Biological Station and Placid Lakes occurred at greater heights in taller trees. This trend was stronger in the suburbs, where nests in high shrubs seemed more susceptible to wind damage [13]. Nest sites are almost never reused [146].
Federal legal status:
Threatened [132]
Other status: Information on state- and province-level protection status of animals in the United States and Canada is available at NatureServe, although recent changes in status may not be included.
Other management information: The Florida scrub-jay is in decline and subject to continuing threats, including loss of optimal habitat. Several population and habitat management options have been proposed to address these concerns.
Population trends: Rangewide, Florida scrub-jays have declined substantially in both the long and short-terms. A 1982 survey estimated Florida scrub-jay population size at 15,330 to 22,530 individuals [46]. A 1992-1993 survey found 4,000 pairs [125]; adding helpers resulted in a total population estimate of about 10,000 individuals [146]. Woolfenden and Fitzpatrick [146] suggest this 1992 estimate is 10% of the presettlement Florida scrub-jay population level, at most. For information on the associated range contraction, see General Distribution. From 1988 to 2005 Florida scrub-jay populations in existing and proposed conservation areas in Brevard and northeastern Indian River counties declined [29]. From the early 1990s to 2010, surveys at 63 sites throughout Florida found an overall decline of 18%, with 54% of sites exhibiting declines [130]. Data available as of 2007 led to estimates that all or portions of 10 Florida scrub-jay metapopulations had declined 37.5% to 65.0% since the 1992-1993 survey [133].
Threats: As of 2012, the biggest threat to Florida scrub-jay is a loss of high-quality habitat, which is caused primarily by fire exclusion [133,146]. A 1992-1993 rangewide survey found that 64% of scrub patches occupied by Florida scrub-jay were in fire-excluded, "overgrown" scrub (i.e., too tall and/or dense to maintain viable populations of Florida scrub-jay). Overgrown scrub becomes progressively less suitable (see Postfire succession and habitat quality) and more fire resistant (see Impacts of fire exclusion) with time. Thirty percent of occupied scrub patches were in suburban areas [125]. These areas provide low-quality habitat partly due to the reduced fire frequency associated with fragmentation [56]. In 1995 and 1996, most of the Florida scrub-jay habitat in Brevard County was overgrown due to fire exclusion. Researchers classified 2,879 acres (1,165 ha) as "slightly overgrown", 6,788 acres (2,747 ha) as "moderately overgrown", and 3,398 acres (1,375 ha) as "very overgrown". Only 235.7 acres (95.4 ha) were in optimal condition (see Preferred Habitat) [106]. As of 2003, 38% of scrubby flatwoods and 83% of sand pine scrub at Archbold Biological Station were due or overdue for burning based on their modal fire-return interval [75]. The intensive management required to keep scrub on public lands from degrading (see Fire Management Considerations) has been difficult to implement, and scrub communities on private lands are rarely managed for maintaining high-quality Florida scrub-jay habitat [133].
Direct loss of habitat also threatens Florida scrub-jay populations [101,146]. From 1920 to 1990, over 65% of scrub habitat was converted to other land cover types in the Indian River Lagoon watershed [55]. From 1989 to 2003, there was a 19% decline in land cover classes potentially suitable for Florida scrub-jays (Burns 2006 cited in [133]), and the U.S. Fish and Wildlife Service expects future habitat destruction with increasing human population size [133]. Continued acquisition of scrub communities by conservation and land management organizations and region-wide Habitat Conservation Plans are the main strategies for addressing this issue (see Habitat management).
Although there is concern regarding potential impacts of hurricanes on Florida scrub-jays and their habitat (e.g., [126]), available information suggests neutral [21] or positive impacts of hurricanes [133]. A 1995 hurricane that hit the southern barrier island of Brevard County, Florida, did not increase Florida scrub-jay mortality [21]. An environmental assessment of the Umbrella Habitat Conservation Plan describes minimal impacts on Florida scrub-jays from Hurricane Charley and the hurricanes of 2004 and 2005. It also includes evidence for increased occurrence of Florida scrub-jays following heavy damage to pine canopy cover that resulted in increases in oak scrub. In the short-term, however, reductions in acorn production and oak cover could be detrimental [133]. Managing for potential negative impacts of hurricanes focuses on maintaining or increasing population size and availability of high-quality habitat [23].
Inbreeding may threaten Florida scrub-jays. Based on an investigation of populations throughout peninsular Florida, Coulon and others [43] concluded that substantial genetic variation has been lost through the extinction of isolated populations. In Brevard County, there is limited gene flow and some level of inbreeding is occurring [82].
The tameness of Florida scrub-jays, especially in areas where they are fed by humans, makes them vulnerable to shooting [146].
Habitat management: Increasing the amount of high-quality habitat is critical to the recovery of the Florida scrub-jay. They are dependent on large expanses of scrub [133], and the amount of scrub habitat available strictly limits the number of Florida scrub-jay territories [146]. Modeling Florida scrub-jay populations based on the habitat available in 1992-1993 suggests that only 3 metapopulations were likely to remain viable without further habitat acquisition. Habitat acquisition would "greatly" or "moderately" reduce risk of falling below 10 pairs in 4 relatively stable metapopulations and in 11 of 14 highly vulnerable metapopulations [124]. A population model of Florida scrub-jays indicated that 30 to 40 contiguous territories are needed to achieve a 90% chance of the population persisting for 100 years [61]. Mapping techniques used to identify potential Florida scrub-jay habitat and associated population sizes were discussed by Breininger [22]. As of March of 2007, over 280,000 acres (113,300 ha) of scrub habitat had been purchased and protected [133]. An umbrella habitat conservation plan has streamlined the process for alterations to property on small lots occupied by Florida scrub-jays that are not part of viable populations. In 2007 this was expected to generate enough mitigation funds over 7 years to purchase an additional 29,856 acres (12,080 ha) [131]. Analyses of habitat conservation plans that describe their function and limitations are provided by James [76] and Watchman and others [135].
The quality of available habitat is critical because of its influence on the demographic performance of Florida scrub-jays (see Preferred Habitat). For instance, the 35% population decline per decade observed in Brevard County could not be explained by the 5% loss of habitat alone [35]. Modeling of a Florida scrub-jay population with demographic rates observed in optimal habitat predicted a 5% chance of the population falling below 100 pairs within 50 years, while a population modeled with the demographic rates observed at Happy Creek in the mid-1990s predicted a 90% chance of falling below 100 pairs in the next 50 years [16]. As of 2003, the overgrown Happy Creek site was declining, likely because too few territories had optimal habitat to offset declines in sink territories [24]. Other simulations of Florida scrub-jay populations in Brevard County found that only those in optimal or slightly overgrown habitat are sustainable [106]. Data from Archbold Biological Station suggest that risk of extinction can be reduced through improvements to habitat quality [23]. In contrast, simulations of implementing an optimal management strategy based on vegetation height data from Kennedy Space Center did not predict increasing Florida scrub-jay populations [78]. A focus on territory-scale restoration is needed to ensure that most territories have all the required structural features [24]. Treatments recommended to improve habitat quality include prescribed burning. See Recommendations for fire characteristics for details.
Preserving large expanses of scrub with minimal fragmentation [21,61,146], minimizing the distance between optimal patches of habitat (see Dispersal) [16,21,43,126], and improving matrix quality (see Landscape features) [14] are common Florida scrub-jay habitat management recommendations. Fitzpatrick and others suggest that on average, 750 acres (300 ha) of periodically-burned oak scrub are required to support a viable Florida scrub-jay population. This size area would allow for 30 contiguous territories at 25 acres (10 ha) each. Smaller populations are adequately protected if they occur within 3 to 5 miles (5-8 km) of a population supporting at least 30 territories [61]. Because of Florida scrub-jays' limited dispersal abilities, preserves more than 7.5 miles (12 km) apart without any Florida scrub-jay habitat in between are fully isolated from one another. Distances of 5 miles (8 km) and farther significantly restrict dispersal [146]. Stith [126] suggests removal of dispersal barriers and research into methods for facilitating dispersal such as creating partially cleared rights-of-way between patches. Boyle [14] also recommended managing the matrix to maintain communities readily traversed by dispersing Florida scrub-jays. Matrix quality can be improved by minimizing interspersion [27] and boundaries between scrub and roads, areas of human development (see Preferred Habitat), and dense forest (see Tree cover) [16,61].
Concerns have been raised over the impacts of managing areas for a single species and the assumption that other scrub species will also benefit [93]. A long-term multi-species monitoring plan may address this concern by providing information that can be incorporated into future management decisions [128]. Variable management prescriptions (see Recommendations for fire characteristics) also address aspects of this concern.
Historical and current land uses influence Florida scrub-jay habitat quality and the ease of restoration. For instance, creating scrub in areas used as citrus groves is difficult [114] while areas of periodically-burned pastures responded well to restoration efforts at Tel-4 [53]. Regenerating pasture that borders scrub has conservation value for Florida scrub-jays as an open matrix landcover type without the risks associated with dense forests or suburbs. Regenerating pastures may also serve as dispersal corridors and ignition sources. Because of this, acquisition and restoration of pasture near native scrub was recommended in cases where large tracts of scrub are not available [49]. At Kennedy Space Center, soil disturbance due to vegetation clearing resulted in establishment of trees on flatwoods sites and occurrence of sandy openings in disturbed xeric scrub [53]. Trees in disturbed flatwoods communities likely reduce the suitability of habitat in these areas, while sandy openings could improve habitat quality for Florida scrub-jays in disturbed xeric scrub. History of fire exclusion makes restoration with fire alone difficult (see Impacts of fire exclusion) and often necessitates mechanical treatment prior to burning (see Restoration of overgrown scrub).
Timber harvesting is not used to manage Florida scrub-jay habitat at most sites, but is used on the Ocala National Forest. Sand pine is managed as Florida scrub-jay habitat using clearcuts of no more than 25 acres (10 ha) performed on a 50-year rotation [67]. Oaks in this community reach 3 to 7 feet (1-2 m) tall within 3 to 5 years of harvesting the sand pine. If the oaks are dense [46], Florida scrub-jays will occupy these areas for up to 20 years after harvesting; after 20 years, sand pine reaches heights and densities that reduce Florida scrub-jay habitat quality. In other areas occupied by Florida scrub-jays, sand pine is actively suppressed [67].
Population management: Population management goals generated to assist in the recovery of the Florida scrub-jay identify minimum population sizes and maximum distances between populations. Based on dispersal information and population viability analysis, Stith and others [125] suggest the following: maintain the 3 core metapopulations above 400 pairs each, preserve metapopulations with habitat that supports more than 10 pairs, preserve twice as much Florida scrub-jay habitat in viable populations as that lost in areas that do not have enough habitat to support 10 pairs, ensure that patches of habitat within a metapopulation are no more than 7.5 miles (12 km) apart, and ensure that no subpopulation is more than 5 miles (8 km) from occupied habitat. To preserve the remaining levels of genetic variation it is necessary to achieve sustainable metapopulations throughout the Florida scrub-jay's distribution, especially in small populations near the edge of the current range [43,92]. For details on the role of habitat management to achieve these goals, see Habitat management. For information on the use of fire to improve habitat quality, see Fire Management Considerations.
Although stable or growing populations are ideal, declining subpopulations, or sinks, may benefit Florida scrub-jay metapopulations. Individuals migrating from sinks occasionally become breeders in optimal territories [24], can offset losses in larger populations [21], and provide a source of colonizers for restored habitat [21,129]. In Sarasota County, 83% of female suburban Florida scrub-jays became breeders by colonizing a recently restored scrubby flatwood. No other group of females became breeders by establishing in the restored area [129]. In cases when large populations are at capacity, sink populations provide vacancies for dispersing Florida scrub-jays. This buffering of larger and typically better performing populations by the smaller, typically declining populations could contribute to the longevity of the metapopulation as a whole (Howe and Davis 1991 cited in [126], Pulliam 1988 cited in cited in [22,27]). In addition, habitat supporting population sinks may transition into [24] or be restored to [14] high-quality habitat.
Florida scrub-jay population recovery and/or establishment in restored habitat may be slow [17,23]. Short dispersal distances (see Dispersal) and little recruitment in many source populations (see Population trends) limit the number of potential colonizers [130,133]. For instance, in the late 1990s there was a lack of male dispersal into restored areas in the Valkaria area of southern Brevard County [17]. Helpers are often the first to occupy restored areas [129]. This could reduce the occurrence of delayed breeding [46], since more breeding vacancies lead to earlier breeding [21,28,34]. This reduction in helpers could reduce reproductive success in source areas (see Cooperative breeding). In addition, Florida scrub-jays may not always recognize the habitat potential of restored areas [17] and may cue on occupied habitat to a greater extent than high-quality habitat [126]. Despite these issues, Florida scrub-jays have established in restored habitat in areas of Volusia, southern Brevard, Indian River, and Sarasota counties where there was a source of colonizers near restored habitat [133]. At Lyonia Preserve in Volusia County, Florida scrub-jays established within months of vegetation removal, probably because they occurred in a nearby area with declining habitat quality [130].
Translocation of Florida scrub-jays into restored areas has shown some success. A translocation experiment at Rookery Bay National Estuarine Research Reserve produced a population with nest success, juvenile survival, and adult survival similar to that at Archbold Biological Station beginning about 2 years after the first translocation. However, nearly half the Florida scrub-jays that were introduced were lost within 8 weeks, and emigration and mortality of females fledged on the site resulted in a male-biased sex ratio [99] that persisted for several years after the translocation [146]. Translocation is not a viable option for managing existing populations because properly managed translocation sites are rare, initial loss of translocated Florida scrub-jays is high, and source populations may be impacted [99].
| This photo was taken in May 2009 following a prescribed burn in October 2008. Photo by Christina Macon; Florida Department of Environmental Protection. |
DIRECT FIRE EFFECTS:
Little information is available on the direct impacts of fire on Florida scrub-jays. Florida scrub-jays are unlikely to suffer direct mortality from fire. It is generally accepted that large, fast-moving fires may result in mortality, but adult birds typically have the mobility to avoid fire [39,51,90]. Despite negative short-term impacts, only the smallest Florida scrub-jay populations are at risk of extirpation from severe wildland fires [17].
Limited evidence suggests that in most years, fire has little impact on nest success. From 1969 to 1978, 3 Florida scrub jay eggs and 1 nestling were killed by fire at Archbold Biological Station. For both eggs and nestlings, fire mortality represented less than 1% of losses. Descriptions of the study area suggest that portions were periodically burned under prescription while other areas were not burned during this period [144]. Because wildfires in Florida are most common in the summer (see Fire Regimes), it is likely that most Florida scrub-jays will fledge before the fire season starts. Fledgling Florida scrub-jays may still be weak fliers in early June and may therefore be more vulnerable to early summer fires (see Timing of breeding, nesting, and early development). Literature reviews [90,105] have used fire characteristics and life history of species to speculate on possible effects of fire on nest success and bird populations. Based on these generalizations, Florida scrub-jays' selection of shrubby vegetation for nest sites suggest that fires during the breeding season could result in considerable egg, nestling, and/or fledgling mortality. Renesting following failed nesting attempts (see Breeding) may mitigate the direct effects of early spring fire on Florida scrub-jay recruitment. However, because Florida scrub-jays typically rear only 1 brood per year, fires in the mid- to late-breeding season (April to June) may impact recruitment [90,105].
Typically, Florida scrub-jays only abandon territories following large and uniformly severe fires [95,138,146]. Without patches of unburned scrub, extensively burned territories do not provide the acorns, nesting cover, or escape cover required by Florida scrub-jays [17,95]. Territories with as little as 10% to 25% of unburned scrub may still be used by Florida scrub-jays (Faulhaber 2012 personal communication [57]). Florida scrub-jays at Happy Creek remained on territories following restoration treatments of overgrown, fire-resistant areas on the site; restoration treatments included mechanical treatment from August 1992 to January 1993 and prescribed burning in Feburary 1993. All breeders made at least one nesting attempt within a few months of the prescribed fire [114]. In contrast, after prescribed burning from 1988 to 2000 at Happy Creek, Florida scrub-jays shifted their territories from severely burned areas to areas where tall scrub remained. Prescribed fires burned 40% to 60% of the study area every 3 to 5 years and did not burn entire territories in any year [24]. Recolonization by Florida scrub-jays following habitat restoration is most likely in cases where Florida scrub-jays occur in adjacent areas and the population is increasing and/or the habitat quality of the adjacent area is declining (see Population management).
INDIRECT FIRE EFFECTS:
Considerable research has been conducted on the relationships between fire, Florida scrub-jays, and their habitat, but readers should be aware of its limitations. Some studies on Florida scrub-jays have the same limitations as those addressing bird response to fire in southwestern ponderosa pine (Pinus ponderosa) forests, such as being restricted in spatial or temporal scale, having small sample sizes, or including confounding factors [58]. Some use Florida scrub-jay density to compare sites, which has its own limitations (see Biological Data). Although several studies compare Florida scrub-jay demography between habitats with varying characteristics (see Preferred Habitat), many focus on shrub height and do not include information on the fire or other treatments that initiated scrub development. Few studies describe the history of the site, such as how often a site has been burned in recent years. Also, several of the studies at Archbold Biological Station [49,143,144,145,146] and surrounding areas (e.g. [49,134]; [12,13]; and [12,134]), Tel-4 [16,26,28,30,32], Happy Creek [16,24,26,28,30], and along the Atlantic Coast of central Florida [17,29,34,35] apparently have overlapping datasets.
Although the negative impacts of fire exclusion on Florida scrub-jay and their habitat are well-documented, data supporting specific management alternatives are relatively scarce [133]. Recommendations to avoid homogeneous and fixed burning regimes (see Fire Management Considerations) and too frequent fire (see Postfire succession and habitat quality) are common [81,94]. Patchy fires that recur at variable intervals based on habitat conditions and site characteristics are likely to maintain high-quality Florida scrub-jay habitat, while scrub that has not burned in about 20 years or more becomes overgrown, difficult to burn (See Fire Regimes), and poor habitat. In these cases, high-severity fire or mechanical treatments followed by relatively frequent fire may be needed until the scrub is successfully restored (see Restoration of overgrown scrub).
Florida scrub-jay use of burned areas: Several studies have shown that Florida scrub-jays establish in scrub communities treated with prescribed burning or combinations of mechanical removal and prescribed burning. Although a 1-year study at Kennedy Space Center found no difference in Florida scrub-jay density between burned and unburned sites [33], the use of density, a short timeframe, and small survey stations limit inference. Florida scrub-jays were absent from overgrown scrub in Blue Springs State Park in 1989 when the first clearcut and burn treatment was implemented [133]. As of 2011, 10 Florida scrub-jay families occurred there (Faulhaber 2012 personal communication [57]). Similar results have been observed following similar treatments and maintenance burning in scrub communities in Oscar Scherer State Park in Sarasota County, Lyonia Preserve in Volusia County, and Halpata Tastanaki Preserve in Marion County [133]. At Kennedy Space Center, Florida scrub-jays used treated areas for foraging, caching acorns, and nesting within 18 months of prescribed burning [16]. At the Savannas Preserve State Park along the Atlantic Coast, a Florida scrub-jay established a territory within about a year of a "trial" burn in an area that had been overgrown [123].
At Kennedy Space Center, density of Florida scrub-jays in areas burned by a wildfire (13.2 birds/40 acre) and in areas burned by prescribed fire (9.91 birds/40 ha) did not differ significantly. Areas were at a similar stage of postfire succession [68].
Existing territories may shift following fire. At Kennedy Space Center, most territories were not "optimal" after cutting and burning treatments. Because much of the vegetation was initially too short to provide high-quality habitat, Florida scrub-jays fought to obtain remaining unburned tall patches [24].
Influence of fire on Florida scrub-jay demographics: Although populations often decline just after fire (see Postfire succession and habitat quality), especially after uniformly severe fire covering extensive areas (see Influence of fire characteristics on Florida scrub-jay response), Florida scrub-jays in areas that are regularly burned typically have good demographic performance. In periodically burned areas of Archbold Biological Station, lifetime reproductive success was 1.1 to 1.3 yearlings, while in unburned areas it was only 0.4 to 0.6 yearlings [59,145]. Pairs in unburned habitat produced an average of 1.6 fledglings and 0.3 yearling per year, and pairs in periodically burned habitat produced 2 fledglings and 0.76 yearlings per year [144,145]. Breeder survival was also lower in unburned than burned habitat [145] (see Table 2). At Archbold Biological Station, fledgling production was greater in burned than unburned habitat in 14 of 18 years, and survival was greater in burned than in unburned habitat in 14 of 15 years [61]. Statistical significance of these differences was not tested. Causes of the reduced success in overgrown habitat have not been identified. Arthropod abundance in June and July was similar in burned and unburned habitat at Archbold Biological Station, so that is not a likely explanation. Other possible explanations include reduced abundance of arthropods in unburned habitat at other times of year, vegetation structure and/or composition that led to increased difficulty detecting or capturing prey (see Structural features), decreased acorn production [47], lack of bare ground to cache acorns, and increased vulnerability to predators [47,146].
Preliminary data following the reintroduction of fire to Kennedy Space Center from 1979 to 1985 suggest that Florida scrub-jay response varies among plant communities. Florida scrub-jays decreased significantly during this time in flatwoods communities but not in coastal scrub or coastal strand; variation was high in this study and sample sizes were relatively small [88].
Florida scrub-jays are not capable of long-term survival in areas where fire is excluded [23,106,145]. Increased shrub height and tree cover associated with reduced fire frequency in fragmented habitat was likely the major contributor to a 34% decline over 10 years in 3 metapopulations in Brevard County [35]. At Savannas Preserve State Park, the Florida scrub-jay population began declining as time since fire increased, with a 22% decline occurring between postfire years 14 and 16 [45]. At Archbold Biological Station, the annual variation in fledgling and yearling production was greater in unburned than burned habitat. The greater fluctuation suggests that populations in marginal habitat are more vulnerable than populations in optimal habitat [61]. A simulation based on data from Florida scrub-jays in unburned habitat of Archbold Biological Station predicted population declines of 25% every 5 years [23].
Although Florida scrub-jays may select burned areas for nesting, the relationship between burned areas and nest success is unclear. Florida scrub-jays selected (P<0.001) burned habitat for nesting on the Ocala National Forest, with 68% of nests occurring in burned areas that comprised only 6.6% of the study area (Table 3). Nest success during the incubation stages was greatest in areas less than 5 years old and in areas 16 to 20 years old. The authors speculated that lower nest success in 6- to 15-year-old stands could be explained by greater density of Florida scrub-jays, low food availability, and/or increased predator densities. Stand age was not associated with nest success during the nestling stage [67]. Nest success was similar in Florida scrub-jay habitat at Archbold Biological Station, which is primarily fire-maintained scrub, and unburned scrub at Placid Lakes [134].
| Table 3. Distribution of Florida scrub-jay nests in sand pine scrub stands ≤20 years old in the Ocala National Forest based on method used to regenerate sand pine and the availability of each stand type. Distribution of nests was different from that expected based on site availability (P<0.001) [67]. | ||
| Reforestation method | Percent of nests | Percentage of the area treated with each method |
| No site preparation | 17.5 | 33.7 |
| Seed only | 7.7 | 28.7 |
| Wildfire burn | 39.3 | 5.1 |
| Post-harvest burn | 28.6 | 1.5 |
| Chop and seed (burned or not) | 6.9 | 30.9 |
Influence of fire characteristics on Florida scrub-jay response: Frequency of fire influences Florida scrub-jay response. Due to the high frequency of wildfire in oak-palmetto communities, Florida scrub-jays may be more vulnerable in these communities than in oak scrub communities at Kennedy Space Center [19]. In areas of Kennedy Space Center where fire was introduced after being excluded for >30 years, preliminary results suggested a trend for lower abundance of Florida scrub-jays on transects burned more than once in a seven year period than those burned less frequently [88].
Growing season fires may have less direct impact on Florida scrub-jays than winter fires, because they allow for territory shifts while acorns are still available for caching and before hawk migration [146]. However, because mortality may occur at the nest, prescribed burns at Archbold Biological Station are typically avoided while Florida scrub-jays are nesting in March and April [91] (see Timing of breeding, nesting, and early development).
Florida scrub-jay populations have declined following large and uniformly severe fires at Kennedy Space Center [31,32,88]. From 1988 to 2005, large, severe fires converted territories with optimal scrub height to territories with short scrub, which cannot maintain Florida scrub-jays [29] (see Postfire succession and habitat quality). Large, widely distributed Florida scrub-jay populations at Kennedy Space Center typically recover from such fires in a few years, whereas small fragmented populations may not recover from such fires (Breininger unpublished data cited in [29]). Preliminary data following the reintroduction of fire at Kennedy Space Center suggest that extensive fires led to declines in Florida scrub-jay populations over the 6-year study period [88].
Postfire succession and habitat quality: Oak scrub provides optimal Florida scrub-jay habitat for a limited period, typically sometime between 5 and 20 years following disturbance [21,27,146], depending on weather, site characteristics (see Scrub height), community composition (see below), and location [32,46]. For instance, recovery on the Atlantic coast is typically (though not always) faster than at interior sites along the Lake Wales Ridge [17,110,116] so habitat may be optimal earlier in succession in coastal areas. The duration habitat remains optimal may be short in the absence of disturbance. Table 4 shows the probabilities that 10-acre plots will transition from one height class to another in 1 year with and without fire. Habitat of optimal height develops into mixed or tall habitat after about 3.7 years in the absence of disturbance, but prescribed burning does not guarantee that short and optimal habitat remain in these height classes. Even when you burn an optimal-height stand there is a 14.8% chance it will still grow enough in 1 year to reach a mixed height class. The transition probabilities for burned areas were based on a smaller sample size and have larger standard errors [78]. For fire responses of plant species common to scrub communities occupied by Florida scrub-jay see Abrahamson and others [2,4] and the FEIS reviews of turkey oak, sand pine, and saw-palmetto.
| Table 4. Probability of transitioning from one habitat height class to another (within 1 year) with and without prescribed burning [78] | ||||||||
| Habitat height class | Short | Optimal | Mixed | Tall | ||||
| Burned | Unburned | Burned | Unburned | Burned | Unburned | Burned | Unburned | |
| From short to | 63.3% | 38.3% | 30.0% | 51.7% | 6.7% | 10.1% | 0 | 0 |
| From optimal to | 14.8% | 1.2% | 70.4% | 73.2% | 14.8% | 25.6% | 0 | 0 |
| From mixed to | 3.6% | 0 | 0.8% | 0 | 94.6% | 99.7% | 0.8% | 0.3% |
| From tall to | 0 | 0 | 0 | 0 | 11.4% | 3.1% | 88.6% | 97.0% |
Generally, it takes from 3 to 12 years after fire for regenerating vegetation develop into high-quality Florida scrub-jay habitat, although there are cases where fire improved Florida scrub-jay habitat quality in the short-term [1], for instance, when pine cover was substantially reduced [16]. Florida scrub-jays often occupy sand pine scrub on the Ocala National Forest as early as 2 (Faulhaber 2012 personal communication [57]) to 5 years after clearcutting [46]. In a sand pine woodland on the Ocala National Forest, Florida scrub-jays began occupying stands 5 to 7 years after a severe wildfire and salvage logging [69]. In oak scrub the growth rate is variable ([52], Schmalzer and Adrian 2001 cited in [110]), and it may take 4 to 12 years after fire for scrub to reach heights optimal for Florida scrub-jays [17,52,110]. Florida scrub-jay populations at Kennedy Space Center declined in open oak scrub for 1 to 8 years following extensive fires due to the short stature of the recovering vegetation [27,32]. Several studies at Kennedy Space Center found that scrub had not reached heights optimal for Florida scrub-jays 3 to 6 years after fire [27,68,110,115,116]. In Brevard County, Florida, scrub-jays in territories burned <3 years previously and composed of short (<4 feet, 1.2 m) scrub had the lowest Florida scrub-jay survival of all vegetation height classes [29]. For the first 3 to 5 years after fire in oak scrub at Archbold Biological Station, there was not enough cover or acorns to support Florida scrub-jays [146]. According to the Nature Conservancy, recovery of Florida scrub-jay habitat takes 3 to 5 years or longer on sites with infertile soil [130].
Because extensive areas of recently burned scrub do not sustain Florida scrub-jay populations, burning large areas on short fire-return intervals can be detrimental. Short intervals may fail to allow oaks sufficient time to grow to heights optimal for Florida scrub-jay [46,115]. Fire frequencies of 2 to 3 years in scrubby flatwoods and oak-palmetto typically result in short oaks that do not bear acorns [145] and may encourage increased palmetto dominance [115,145].
The time required for recovery of habitat structure varies with community type, whereas species composition changes little after fire, even in frequently burned areas, because herbs and shrubs in oak and flatwoods communities commonly sprout after fire ([1], Schmalzer and others 2003 cited in [29]). At Kennedy Space Center, saw-palmetto had nearly returned to preburn cover in a year, while oak cover had not recovered in 3 years [115]. At Archbold Biological Station, acorn production following fire varied across species; Chapman oak and sand live oak produced acorns the year following a May prescribed fire, while myrtle oak and turkey oak produced acorns 4 years following fire [4]. At Kennedy Space Center, results of prescribed fires from 1994 to 2004 suggested that burning oak scrub at least once every 5 years would increase habitat quality compared with less frequent burning. This trend was not observed in flatwoods [30].
Openings in scrub vegetation, which are an important component of Florida scrub-jay habitat, may close quickly following fire. Fire exclusion compounds this problem, making it difficult to restore openings in overgrown habitat [17], especially in scrubby flatwoods in coastal areas (Faulhaber 2012 personal communication [57]). In oak scrub of Kennedy Space Center that was cut and burned, openings often lasted less than 2 years and the canopy closed before shrubs grew to optimal height for Florida scrub-jays [37]. In mixed oak-palmetto at Kennedy Space Center, bare ground cover was <2% within 3 years after fire [110]. Xeric scrub retains its openings longer [17], and closing of sandy openings in sand pine scrub on the Ocala National Forest was described as gradual [36]. Frequent fires in undisturbed flatwoods and the possibility of hotspots from limbs and needles may explain the fact that openings in a scrubby flatwood site persisted longer than an oak scrub site at Kennedy Space Center [37]. Growing-season fires [52], burning downed logs and snags [16], and piling and burning to produce hot spots [111] have been suggested as possible ways to restore openings in scrubby vegetation. The area of openings created by burning piled fuels was only reduced by 50% 7 years after fire on the Shiloh site at Kennedy Space Center [111]. Openings can also be restored by repeated burning, which is sometimes accomplished by starting fires in more flammable neighboring vegetation, such as mesic flatwoods [17].
As scrub vegetation ages it becomes unsuitable for Florida scrub-jays [5,24,29,89,144,146]. This can occur as early as 10 to 15 years after cutting and burning sand pine stands on the Ocala National Forest [46] and as late as 20 years in oak scrub at Kennedy Space Center [21,27] and Archbold Biological Station [146]. The ability of Florida scrub-jays to remain in vegetation that was unburned for over 40 years at Archbold Biological Station may have been influenced by the high numbers of Florida scrub-jays and the availability of optimal habitat nearby. However, about 20 years after fire, reproductive success and survival of adults and juveniles began to decline [146]. The Florida scrub-jay population began to decline in the 1970s in a fire-excluded area of Archbold Biological Station; in the 1980s the area was abandoned [61]. In areas of Archbold Biological Station burned twice during this approximately 15-year period, abundance of Florida scrub-jays fluctuated without an obvious trend [144].
Characteristics typical of fire-excluded areas with poor Florida scrub-jay habitat quality include increased tree or scrub height [17,46,47,134,145], a loss of openings in the canopy [17,146], increased ground cover (see Openings), reduced oak density (see Oak scrub) [47], and increased tree density (see Tree cover) [46,146]. In Brevard County, landscapes where fire did not occur transitioned from optimal scrub habitat to areas where scrub was too tall to provide high-quality Florida scrub-jay habitat [29]. A model based on data from Kennedy Space Center [54] predicted that vegetation would reach 5.5 feet (1.7 m), which is too tall to provide optimal habitat, by 20 years after fire [17]. Oak-palmetto stands at Kennedy Space Center that had not burned in 25 years were dense, and shrub height typically ranged from 7 to 10 feet (2-3 m) tall [108,116]. Shrubs were as tall as 16 to 20 feet (5-6 m) in some areas [108]. In contrast, fire exclusion from flatwoods communities may allow for a scrubby structure that is more favorable to Florida scrub-jays than regularly burned flatwoods [88]. However, this effect is unlikely to persist. Florida scrub-jays in unburned late-successional scrubby flatwoods at Archbold Biological Station had mortality that exceeded recruitment [61]. For information on other impacts of fire exclusion, see Fire Regimes.
FIRE REGIMES:
Lightning-caused fires in Florida generally occur during the summer rainy season [1,52,145] and are most common in July [52]. At Archbold Biological Station there is an average of 2.1 lightning fires per year [1]. A study of the fire regime at Kennedy Space Center determined that lightning-caused fires were generally small, likely because they occur during the summer rainy season [52]. From 1984 to 2004, under the Kennedy Space Center's managed fire regime, winter was the season with most acres burned, and November was the month with the most acres burned [52].
Oak scrub is a fire-maintained community [5] and can burn as frequently as every 5 to 10 years ([5], Myers 1990, Archbold Biological Station unpublished data cited in [146]), although historic fire-return intervals may have been as long as 30 to 70 years in some areas [1]. As of 2009, the mean fire-return interval for a 73,000-acre (29,540 ha) area at Kennedy Space Center and surrounding federal lands was 14.4 years [52], although portions of this area have fire-return intervals of 3 to 5 years [30]. Oak scrub fires are typically severe [5,95,113] and stand-replacing [5]. Rapid spread rates and flame lengths of 40 to 50 feet (12-15 m) are typical in oak scrub at Kennedy Space Center [5]. There is usually insufficient fuel in oak scrub to burn within 5 years of the previous fire, and on xeric sites fuel accumulation is slower [95]. Oak leaves accumulate in the litter layer but do not substantially contribute to fueling fires [1]. For information on biomass in oak-saw-palmetto communities of Kennedy Space Center see Schmalzer and Hinkle [117].
Other communities occupied by Florida scrub-jay burn regularly, but at widely differing frequencies. Scrubby flatwoods at Kennedy Space Center with "natural" fuel loadings experience moderate severity fires every 3 to 7 years, with the shrubby understory consumed. The pine overstory only burns in extreme weather conditions [5]. Fire-return intervals in coastal scrub and coastal strand are typically more than 10 years [87]. In sand pine communities, fires historically occurred every 30 to 60 years [41]. Sand pines are killed when the fires crown, but their seeds are released from serotinous cones [36]. The Fire Regime Table summarizes characteristics of fire regimes for vegetation communities in which Florida scrub-jays may occur. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find Fire Regimes".
Oak scrub communities are less flammable and burn less frequently than many other native plant communities such as sandhills [136], swale marshes [25], and flatwoods [5,25,52]. Oak scrub is difficult to ignite [5]. For instance, an oak-saw-palmetto scrub community at Kennedy Space Center could not be ignited aerially during a wet winter, and fires were patchy under other conditions [116]. It is common for fires to stop at the edge of oak scrub habitats [36,136]. Factors that contribute to this comparative lack of flammability in oak scrub include evergreen leaves, lack of fine fuels such as grasses and forbs, and slow litter accumulation [136]. At Kennedy Space Center, oak scrub burned less frequently than mesic flatwoods and swale marshes [25]. Mesic flatwoods and swale marshes are comprised of vegetation such as saw-palmetto and grasses that are more flammable, and fuel accumulates more quickly in these communities than in oak scrub vegetation [1,2,3,101,115,118]. When oak scrub communities iginite, it is often due to fire spreading from adjacent, comparatively flammable communities [5,52]. This ignition source is an important factor influencing the frequency of fire in oak scrub [16,95].
Fires in oak scrub are generally patchy [87,95], with burned patches usually smaller than Florida scrub-jay territories [146]. The patchiness of fire is influenced by weather conditions and scrub type, with xeric scrubs experiencing more heterogeneous fires [95]. Fires in scrub at Archbold Biological Station are more patchy than stand-replacement fires in sand pine scrub on the Ocala National Forest, because fire-return intervals are longer and more fuel accumulates between fires in sand pine scrub [46]. In an area at Kennedy Space Center including oak scrub that was burned under prescription 3 times and burned in a wildfire once in 20 years, only 30% of the area was burned 3 or 4 times. The patchiness of fire in scrub communities contributes to the mosaic of openings and mixed-age oak cover that provide high-quality Florida scrub-jay habitat. The patchily burned scrub had areas not burned in more than 10 years that provided oak cover and areas that had burned more frequently that provided open sandy areas [25]. Patchiness of fire influences recovery of scrub vegetation, partly because some plant species colonize burned areas from unburned patches [95].
Although the management fire regime and the natural fire regime have similar fire-return intervals at Kennedy Space Center, the size distributions differ. The natural fire regime is characterized by many frequent small fires and a few large fires, which are up to 4 times the size of prescribed fires. Medium-sized wildfires are most often suppressed, since they are big enough to detect and generate concern but small enough to effectively control. The management fire regime is less variable, with more medium-sized fires [52].
Habitat fragmentation due to land conversion has resulted in reduced fire frequency in Florida scrub-jay habitats. This is due to the reduction in flammable neighboring habitats (see above), succession to fire resistant communities resulting from fire exclusion (see below), and directly through conversion to land uses that impede fire spread, such as roads [1,30], or require fire exclusion, such as suburban development [1,146]. At Kennedy Space Center, edges made vegetation transitions to taller states more likely and transitions to shorter states less likely, because edges reduced both fire spread and fire frequency [30]. In Brevard County, disrupted fire regimes caused by fragmentation reduced habitat quality, contributed to Florida scrub-jay population decline, and magnified the impacts of direct habitat loss [35]. Even minimal land conversion can fragment vegetation sufficiently to disrupt fire spread. For instance, modeling simulations predicted a 50% reduction in fire extent with as little as 10% anthropogenic landcover [56].
Detrimental impacts of fire exclusion in scrub vegetation of Florida include persistent increases in tree height and density [5,72] that contribute to increased fire resistance [110,114], reduced flammability [5,24,32], and decreased fire frequency and heterogeneity. This may increase the potential for severe fire [5,46,113], due to increased amounts [5] and continuous distribution [114] of fuels. However, prolonged fire exclusion can result in a conversion to fire resistant vegetation; for example, scrub communities may succeed to xeric hammock vegetation [113,114] and marshes to forest [30,53]. Once vegetation becomes fire resistant, restoration using prescribed fire is difficult and/or ineffective [15,24,29,30,108,114], at least under typical burning conditions [24,111]. In these cases, restoration commonly requires mechanical treatments combined with repeated prescribed fire [17] (see Restoration of overgrown scrub). For information on the impacts of fire exclusion on Florida scrub-jay habitat quality, see Postfire succession and habitat quality.
FIRE MANAGEMENT CONSIDERATIONS:
The effects of fire on Florida scrub-jays depend on many factors including site characteristics, time since fire (see Postfire succession and habitat quality), characteristics of the fire (see Influence of fire characteristics on Florida scrub-jay response), and dynamics of the Florida scrub-jay populations that are affected. Although Florida scrub-jays will use recently burned areas (see Florida scrub-jay use of burned areas), fire commonly results in a short-term decrease in habitat suitability or availability. When fires are severe and extensive they may increase extinction probability of small and isolated Florida scrub-jay populations (Breininger unpublished data cited in [29]). In the long-term, however, fire can improve Florida scrub-jay habitat quality by increasing openings and reducing tree densities and shrub height (see Postfire succession and habitat quality). Fires that burn in a mosaic at the local scale or that kill many tall shrubs and trees may immediately benefit Florida scrub-jay habitat quality [17]. Areas that are not burned at least every 20 years do not support healthy Florida scrub-jay populations (see Influence of fire on Florida scrub-jay demographics), and fire exclusion leads to changes in vegetation that render scrub unsuitable for Florida scrub-jays (see Postfire succession and habitat quality).
Prioritizing treatment: A substantial amount of burning is required to maintain oak scrub and adjacent native vegetation. At Kennedy Space Center, 202 prescribed fires averaging 460 acres each were performed from 1993 to 2002, for a total of 93,402 acres burned in fire-maintained habitats [5]. In the early 2000s a Fire Strike Team was established to assist land managers in carrying out burns throughout the Florida scrub-jay's range. In its 4th year, 11 organizations had submitted requests for assistance in burning 34 conservation areas, and the team had assisted in burning 140 fire management units in 13 conservation areas [73]. See Threats for information on the prevalence of overgrown scrub as of the mid-1990s and Habitat management for discussion of topics not directly related to fire such as reserve design considerations and the importance of acquisition and maintenance of high-quality Florida scrub-jay habitat.
Given the amount of vegetation requiring restoration, prioritizing areas for treatment has been recommended. Breininger [22] states that achieving optimal habitat conditions in every territory is "unreasonable", implying that it is more realistic to seek a balance of optimal and suboptimal habitat that allows for Florida scrub-jay population growth. Scrub management guidelines for peninsular Florida recommend providing optimal habitat in 70% of potential territories and keeping most of the remainder shorter than optimal [81]. Restoration of overgrown habitat is difficult and may produce less benefit than maintenance of existing Florida scrub-jay habitat (see Restoration of overgrown scrub). Focusing on maintaining optimal areas and restoring areas that are slightly overgrown is likely to maximize benefits [106]. However, restoration of overgrown scrub to increase carrying capacity of conservation areas and/or available habitat for Florida scrub-jays in small populations is citical for long-term recovery (Faulhaber 2012 personal communication [57]). Generalized fire management recommendations, such as performing most fires in summer [17] or burning several small areas [46], may not be practical given the large amount of area that needs treatment. Breininger and others [17] suggest burning year-round until the amount of scrub needing treatment is reduced.
Characteristics of the surrounding landscape may help to prioritize treatments. Locating treatments near areas occupied by Florida scrub-jay increases the likelihood of success ([35], Faulhaber 2012 personal communication [57]). Small restoration treatments in areas within landscapes comprised primarily of good-quality Florida scrub-jay habitat are likely more beneficial for Florida scrub-jays than similar efforts in areas dominated by dense overgrown scrub or forests [114]. Alternative reserve designs that categorize vegetation based on habitat suitability, potential habitat suitability (with and without mechanical treatment), distance to human development, and the economics of restoration and fire management would assist in efficient allocation of available resources [16].
An adaptive management program that incorporates vegetation structural characteristics [30] and Florida scrub-jay and other specialist species' population status [24,128] into decision-making is likely needed to successfully restore scrub communities and provide for stable Florida scrub-jay populations [16,22]. This would require monitoring [22,37,128] and incorporation of these factors into reporting [30]. A technique using remote sensing to track fire occurrence is described by Shao and Duncan [121]. An adaptive management system could also increase knowledge about topics that are not well understood, such as the role of openings in Florida scrub-jay demography [37].
Recommendations for fire characteristics: Spatial arrangement of burned areas on the landscape influences treatment effectiveness. At Kennedy Space Center, recommendations included placing treatments so that oak scrub that is occupied by Florida scrub-jays does not border forests [17]. In Brevard County, oak scrub that is burned frequently to maintain openings may be best located adjacent to mesic flatwood vegetation [17]. Vegetation adjacent to scrub requires maintenance fires [17] for many of the same reasons as scrub (e.g., preventing fuel accumulation [16,17,111]) and also because frequently burned matrix vegetation generally increases the habitat value of the adjacent scrub for Florida scrub-jays [17,35] (see Landscape features). Matrix vegetation typically burns more frequently than oak scrub. For instance, at the Kennedy Space Center, mesic flatwoods are burned within the recommended frequency of every 1 to 8 years [25], and these fires are occationally used to ignite neighboring oak scrub [22] (see Fire Regimes).
The need for open areas and optimal height scrub may be met by performing patchy burns [17,61,110,116,146]. Performing patchy burns on variable rotations based on habitat characteristics [94], such as vegetation height, may allow for habitat maintenance over the large areas required. Repeated patchy fires result in some areas burning more frequently than others [32]; frequently burned sites provide open areas, while those that burn less often provide oak cover needed for nesting and cover [46,61]. Mosaic burns are appropriate in cases meeting the following criteria: the Florida scrub-jay population is small and isolated, there is no Florida scrub-jay habitat adjacent to the burn area, there are resources to burn again within a year, there are resources for spatially explicit mechanical cutting to contain severe fire to localized areas, and/or the vegetation is a mixture of tall and optimal habitat and the Florida scrub-jay population is stable and near capacity. In cases where interspersion of the tall scrub is low, it may be possible to burn only the tall patches [22]. Burning late in the growing-season, when rains assist in keeping fires small, may result in a mosaic of recently burned and various-aged scrub [52]. Burning several small patches within a given area on a rotation, such as 10% every year on a 10-year rotation, could achieve the desired outcome, although this would require burning every year or two which may be impractical [46]. Similarly, performing small burns (<500 acres (200 ha)) every year while avoiding burning any area in consecutive years has been recommended for experimental areas. These experimental burns on small areas may speed restoration, and results could inform future habitat management decisions [16].
It has been recommended that the size of burned patches be smaller than Florida scrub-jay territories. In areas with clusters of Florida scrub-jay territories, Breininger and others [32] recommend leaving at least 2.5 acres (1 ha) of optimal-height scrub in every territory. Other recommendations include creating a mosaic with 60% of the scrub unburned [16] and burning no more than 25% of a refuge occupied by Florida scrub-jays in a single fire [61]. Florida scrub-jays may remain on recently burned territories if at least 10% to 25% of the scrub remains unburned (Faulhaber 2012 personal communication [57]). Burning entire territories likely has the least impact on large populations and in areas where adjacent areas can provide Florida scrub-jay habitat. This strategy is generally not recommended in areas occupied by small Florida scrub-jay populations [81]. Restoration that focuses on the territory-scale is important, since that is the landscape unit related to demography [24].
Recommended fire frequency in oak scrub ranges from about 5 to 20 years [16,17,29,146], including 10 to 20 years at Archbold Biological Station [61] and Brevard County [106]. Fire frequency for specific areas should be based on plant community height, structure, and composition; management history; and site characteristics. For example, at Kennedy Space Center burning is recommended before scrub taller than 5.5 feet (1.7 m) accumulates, because it provides low-quality Florida scrub-jay habitat and becomes increasingly difficult to burn [32]. In scrub with less than 5% bare ground at Archbold Biological Station, burning was recommended when scrub reached 3.3 to 5 feet (1.0-1.5 m) tall. In areas with more open space, shrubs may be allowed to reach greater heights before burning [46]. More frequent burning may be required to restore overgrown areas (see Restoration of overgrown scrub). Several researchers have suggested basing fire frequency on site characteristics that influence the growth rate of oaks and other Florida scrub-jay habitat characteristics [17,19,46]. For instance, more rapid growth along the Atlantic Coastal Ridge compared to interior Florida scrub-jay habitat may result in a shorter fire-return interval being optimal in costal scrub communities [17,110]. Given the importance of the state of the vegetation in influencing management decisions, a monitoring program is essential.
Limited evidence suggests growing season fires may have greater benefits for Florida scrub-jay habitat than fall or winter fires. However, year-round burning may be required in areas with large acreages needing treatment [17]. At Archbold Biological Station the majority of burning is performed in summer to mimic the natural fire regime, and winter fires are mainly performed in overgrown vegetation [91]. At Kennedy Space Center from 1984 to 2004, the greatest number of acres, on average, was burned in November [52]. The consequences of prescribed burning in fall and winter are not well understood. Differences in scrub vegetation response following winter and summer prescribed burns at Kennedy Space Center were slight and suggest season does not have a significant effect on the postfire response of scrub vegetation [63]. Reductions in postfire sprouting of hardwoods [114] and cover of oaks (reviewed by [17]) are greater following growing season fires than following winter fires. The effect this has on openings is unclear; some research suggests growing season fires result in persistent openings [52]. Prescribed fire on a site in central Kennedy Space Center in November did not result in persistent openings [18]. Fall fires may cause greater pine mortality than fires in other seasons (Menges and Deyrup in preparation cited in [91]).
Variability in prescribed burning has been recommended for shrublands [42] including Florida scrub [16,81,91,93,94,113]. At Archbold Biological Station, spatial and temporal heterogeneity are incorporated into fire management by burning units of various sizes at fire-return intervals ranging from 6 to 9 years in scrubby flatwoods to 15 to 59 years in rosemary (Ceratiola ericoides) scrub [91]. Mosaics of varying stand ages of sand pine are recommended on the Ocala National Forest [67]. A mosaic of variable fire-return intervals also benefits scrub species other than Florida scrub-jay [94]. Management that is identical in all stands of the same community results in homogenization that does not allow for preservation of natural variability or species diversity [102]. In areas where it is feasible, management should attempt to mimic the full range of variability in the natural fire regime [93], including season, with most fires occurring in summer [16,81]. Extensive fires may be used in some situations, such as when scrub has been degraded by fire exclusion and either the site is not occupied by Florida scrub-jays or the population is large and widespread (see Restoration of overgrown scrub).
Optimal burning conditions for scrub are narrow, with a small window between conditions where oak scrub will not burn and those where fire is impossible to control [95]. A description of prescription conditions and associated behavior of prescribed fires performed on 2 sites at Kennedy Space Center is available. Flame lengths on these 2 sites ranged from 2.0 to 16.4 feet (0.6-5 m), rates of spread from 20 to 400 m/hr, and extent ranged from 60% to over 90% of the areas being burned. Fuel distribution and weather differences account for most of the differences between the fires [114]. The small window for burning may be an obstacle to use of fire for habitat improvement. For instance, during the first 2 years of the Fire Strike Team's operation, only 18 prescribed fires were performed due to drought conditions [74]. Custer and Thorsen [48] describe the prescribed fire conditions for a successful 30-acre stand-replacement fire in sand pine-scrub on the Ocala National Forest.
Restoration of overgrown vegetation: Fire exclusion may result in changes that cannot be treated with prescribed fire alone (see Impacts of fire exclusion). Based on data from aerial imagery from Kennedy Space Center, the probability of tall vegetation transitioning to short vegetation or optimal vegetation after prescribed fire was zero, and the odds of mixed optimal and tall vegetation transitioning to short or optimal after prescribed fire were less than 4.5% [78] (see Table 4). After about 20 years of fire exclusion at Happy Creek [24], scrub became fire resistant and species of hardwood hammock forests began to establish [53]. Prescribed burning at Happy Creek did not restore desired scrub characteristics that were present before fire exclusion [26]. Repeated winter fires did not restore openings in areas of fire-excluded oak scrub at Kennedy Space Center [114]. Prescribed fire reversed some but not all of the trends associated with fire exclusion at Kennedy Space Center. For instance, pine stands on disturbed areas of Tel-4 and hardwood swamps at Happy Creek persisted, and openings were not restored in oak scrub at Happy Creek [53].
Severe [16,29] large fires about every 20 years may restore overgrown scrub vegetation. These fires consume fuels [29] and can burn areas that are resistant to fire under typical prescription conditions [24]. Current status of the habitat and Florida scrub-jay population are important factors to include when considering this option [29], since small or fragmented populations are unlikely to recover from extensive fires (See Influence of fire characteristics on Florida scrub-jay response). Long-term negative impacts are unlikely following uniformly severe fire that burns a few territories of a large population, while that is probably not the case in small populations (Faulhaber 2012 personal communication [57]). A description of ignition techniques and prescriptions for large, severe fires is available [16]. Such fires may be useful when the area adjacent to the large burn is Florida scrub-jay habitat, there are few resources available to treat the area mechanically, the area of tall scrub is extensive, frequent burning is impractical, and/or severe fire is need to create open, sandy areas [22]. In tall areas that are still occupied by Florida scrub-jays, it may be possible to burn after clearing vegetation around the best remaining habitat patches. These unburned areas should not be near roads, forests, or areas with dense trees or tall shrubs [22]. Firebreaks may be needed for fire control [16,52,61], but they could result in simple edges that would increase the risk of predation on Florida scrub-jays [16,17,52,114].
Overgrown scrub is typically restored by mechanical treatments followed by prescribed fire [30,106,113]. Preliminary results suggest these treatments may restore overgrown scrub [109,114,137] and create openings that persist longer than those created by burn-only treatments [15,111] (see below). At the Kennedy Space Center habitat quality was not expected to improve without mechanical cutting [30]. In areas where overgrown vegetation is next to human development, mechanical techniques may be the only method available for opening scrub habitat [146]. Equipment and techniques used for mechanical treatment in scrubby vegetation at Kennedy Space Center are described by Schmalzer and others [111,114].
Mechanical treatment followed by burning is generally effective in creating persistent openings in oak scrub. Burning slash piles at Kennedy Space Center [15,111] and mowing followed by burning 1 year later near Placid Lakes [137] resulted in more persistent openings than fire alone. At Kennedy Space Center, area of openings declined by about half 7 years after burning slash piles, while openings in fire-only treatments were nearly gone within a few years. The researchers suggest that the heat produced by burning slash kills roots and rhizomes of sprouting species, requiring colonization of other vegetation to close the opening [111]. Use of caution in applying this method has been recommended because repeated burning required in oak scrub requires fuel continuity [111]. At a site near Placid Lakes, bare sand cover was significantly (P<0.001) greater after mowing followed by burning 1 year later, than in mow-only, burn-only, and control treatments. On a site without the year delay, cover of open sand in mow-and-burn areas did not differ significantly from that in burn-only treatments, but both had greater cover of bare sand than mow-only and control treatments [137]. Using repeated fire on a 2- to 4-year return interval over 15 years may also create openings when shrubs are killed by reduction of carbohydrate reserves [16,116]. Because it may take up to 4 years for enough fuel accumulation to carry a fire and repeated fires are needed, this method takes longer than mechanical treatment. In communities with a substantial pine component, burning downed logs and snags may create openings, although this may produce smoke issues [16].
Scrub recovery time after mechanical treatment can be rapid. Vigorous recovery after mechanical treatment has been reported [93,109], with height growth in cut-and-burn treatments exceeding that in burn-only treatments by 50% or more in some cases [111]. Sand pines may also recover faster after cut-and-burn than burn-only treatments [46]. However, oak cover was similar in cut-and-burn and burn-only plots 4 years following treatment at Happy Creek [112], and both mow-and-burn and burn-only treatments near Placid Lakes reduced cover and height of woody vegetation relative to controls 5 years after treatment [137]. Saw-palmetto cover is often reduced to a greater extent and for a longer duration after mechanical treatment than after burning alone [15,111]. This may be especially relevant to flatwoods, where saw-palmetto increases in dominance with fire exclusion and is typically not top-killed when fire is reintroduced [30]. Since saw-palmetto can be important in providing fuel to allow for burning following mechanical treatment, large reductions of saw-palmetto can interfere with Florida scrub-jay habitat management [111]. Breininger and others [17] note that treatments that reduce pine canopy such as salvage logging with minimal soil disturbance are likely to improve Florida scrub-jay habitat quality.
Reported impacts of mechanical treatments on Florida scrub-jays have been positive or neutral. At 2 sites at Kennedy Space Center, no Florida scrub-jays abandoned their territories during mechanical treatment followed by prescribed fires [114]. On the Ocala National Forest, regenerating sand pine adjacent to occupied Florida scrub-jay habitat is typically colonized by Florida scrub-jays when oaks on the regenerating site are about 3 feet (1 m) tall [46]. Although the number of Florida scrub-jay pairs was greater in burned treatments of Cape Canaveral Air Force Station, the number of fledglings was greatest in cut treatments (Stevens and Knight 2004 cited in [128]). An oak-palmetto scrub site at Kennedy Space Center that was mechanically cleared developed into oak scrub that was densely occupied by Florida scrub-jays 20 years later [18]. Spatially arranging treatments to prevent treating too much area within territories [114] and performing treatments outside the breeding season [7] are strategies for minimizing direct detrimental effects of mechanical treatments on Florida scrub-jays. Mechanical treatments can have negative impacts on other species, including gopher tortoises. Mitigation of these impacts should be incorporated into treatment plans [81].
Mechanical treatments should be used with caution for several reasons. Mechanical restoration is expensive [30,40], costing an estimated $3,390 to $19,310 more per hectare than maintenance fires as of 2001 [40]. Mechanical treatments have the potential to cause soil damage [16,17,46,93], which may impact fuels [16,17] and increase establishment and spread nonnative invasive plants [17,93]. If these treatments are performed over areas as large as Florida scrub-jay territories they would result in too much short vegetation, which could impact Florida scrub-jay demography [78]. Mechanical treatments may lead to proliferation of vines that require spot control, may result in simple edges that could increase predation risk [114], have uncertain long-term impacts [61,93], and raise other concerns regarding replacing fire with mechanical treatments in fire-adapted systems [93].
Because of the disadvantages of mechanical treatments and the advantages of using fire, mechanical treatments are recommended as preparation for maintenance fires [40,81,93,112,114,137]. According to a review of studies using mechanical treatment in scrub, the positive effects of a mechanical treatments may be lost if it is not followed by repeated prescribed fires, because of vigorous sprouting by many species [93]. Burning within 3 months of mechanical treatment has been recommended, since flammability begins to decline within about 6 months of treatment [81]. Relatively short fire-return intervals may be needed following mechanical treatment in long unburned scrub until the vegetation is fully restored [111,113]. Because of this, it is important that mechanical treatment does not reduce the potential for frequent burning, such as causing excessive loss of saw-palmetto [111]. For information on fires used to maintain scrub vegetation, see Recommendations for fire characteristics.
| Fire regime information on vegetation communities in which Florida scrub-jay may occur. This information is taken from the LANDFIRE Rapid Assessment Vegetation Models [85], which were developed by local experts using available literature, local data, and/or expert opinion. This table summarizes fire regime characteristics for each plant community listed. The PDF file linked from each plant community name describes the model and synthesizes the knowledge available on vegetation composition, structure, and dynamics in that community. Cells are blank where information is not available in the Rapid Assessment Vegetation Model. | |||||
| Southeast | |||||
| Vegetation Community (Potential Natural Vegetation Group) | Fire severity* | Fire regime characteristics | |||
| Percent of fires | Mean interval (years) |
Minimum interval (years) |
Maximum interval (years) |
||
| Southeast Woodland | |||||
| South Florida slash pine flatwoods | Replacement | 6% | 50 | 50 | 90 |
| Surface or low | 94% | 3 | 1 | 6 | |
| Southeast Forested | |||||
| Mesic-dry flatwoods | Replacement | 3% | 65 | 5 | 150 |
| Surface or low | 97% | 2 | 1 | 8 | |
| Sand pine scrub | Replacement | 90% | 45 | 10 | 100 |
| Mixed | 10% | 400 | 60 | ||
| *Fire Severities— Replacement: Any fire that causes greater than 75% top removal of a vegetation-fuel type, resulting in general replacement of existing vegetation; may or may not cause a lethal effect on the plants. Mixed: Any fire burning more than 5% of an area that does not qualify as a replacement, surface, or low-severity fire; includes mosaic and other fires that are intermediate in effects. Surface or low: Any fire that causes less than 25% upper layer replacement and/or removal in a vegetation-fuel class but burns 5% or more of the area [71,84]. |
|||||
1. Abrahamson, Warren G. 1984. Post-fire recovery of Florida Lake Wales Ridge vegetation. American Journal of Botany. 71(1): 9-21. [9509]
2. Abrahamson, Warren G. 1984. Species response to fire on the Florida Lake Wales Ridge. American Journal of Botany. 71(1): 35-43. [9608]
3. Abrahamson, Warren G.; Hartnett, David C. 1990. Pine flatwoods and dry prairies. In: Myers, Ronald L.; Ewel, John J., eds. Ecosystems of Florida. Orlando, FL: University of Central Florida Press: 103-149. [17388]
4. Abrahamson, Warren G.; Layne, James N. 2002. Post-fire recovery of acorn production by four oak species in southern ridge sandhill association in south-central Florida. American Journal of Botany. 89(1): 119-123. [85219]
5. Adrian, Frederic W. 2006. Fire management in the inter galatic interface or 30 years of fire management at Merritt Island National Wildlife Refuge/Kennedy Space Center, Florida. In: Andrews, Patricia L.; Butler, Bret W., comps. Fuels management--how to measure success: conference proceedings; 2006 March 28-30; Portland, OR. Proceedings RMRS-P-41. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 739-749. [65185]
6. Aldredge, Robert A. 2002. Hatching asynchrony occurs as a byproduct of maintaining egg viability in the Florida scrub-jay. Orlando, FL: University of Central Florida. 87 p. Thesis. [85326]
7. Alshouse, Alan W.; Shaw, Susan; Neal, Harry V.; Lala, Ruth. 2001. Florida scrub-jay habitat restoration utilizing a fuel wood timber harvest: the planning phase. In Cannizzaro, Patrick J., ed. Proceedings of the 28th annual conference on ecosystems restoration and creation; 2001 May; Plant City, FL. Tampa, FL: Hillsborough Community College; Institute of Florida Studies: 93-99. Available online: http://digicoll.library.wisc.edu/cgi-bin/EcoNatRes/EcoNatRes-idx?type=article&did=EcoNatRes.Wetlands28.AAlshouse&id=EcoNatRes.Wetlands28&isize=text [2012, September 13]. [84836]
8. American Ornithologists' Union. 1995. Fortieth supplement to the American Ornithologists' Union check-list of North American Birds. The Auk. 112(3): 819-830. [60876]
9. American Ornithologists' Union. 2012. The A.O.U. check-list of North American birds, 7th ed., [Online]. American Ornithologists' Union (Producer). Available: http://checklist.aou.org/. [50863]
10. Borst, Elizabeth Marie. 1998. Effects of breeder age, breeder experience, and pair-bond duration on clutch initiation date and clutch size in Florida scrub-jays. Villanova, PA: Villanova University. 63 p. Thesis. [84838]
11. Boughton, R. K.; Bowman, R.; Kearns, L.; Schoech, S. J. 2005. Variation between suburban and wildland populations in nestling immune response of Florida scrub-jays (Aphelocoma coerulescens). Integrative and Comparative Biology. 45(6): 968. Abstract. [84839]
12. Bowman, Reed. 1998. Population dynamics, demography, and contributions to metapopulation dynamics by suburban populations of the Florida scrub-jay, Aphelocoma coerulescens. Final report: Project Number NG94-032. Lake Placid, FL: Archbold Biological Station. 143 p. [85325]
13. Bowman, Reed; Woolfenden, Glen E. 2002. Nest site selection by Florida scrub-jays in natural and human-modified habitats. The Wilson Bulletin. 114(1): 128-135. [85324]
14. Boyle, Shannon R. 1996. A landscape approach to Florida Scrub-Jay (Aphelocoma coerulescens) habitat use in Brevard County, Florida. Melbourne, FL: Florida Institute of Technology. 134 p. Thesis. [84841]
15. Boyle, Shannon R.; Schmalzer, Paul A.; Adrian, Frederic W. 2000. Methods for restoring long-unburned Florida oak-saw palmetto scrub using mechanical cutting and prescribed burning. In: Moser, W. Keith; Moser, Cynthia F., eds. Fire and forest ecology: innovative silviculture and vegetation management: Proceedings of the 21st Tall Timbers fire ecology conference: an international symposium; 1998 April 14-16; Tallahassee, FL. No. 21. Tallahassee, FL: Tall Timbers Research: 190. Abstract. [37664]
16. Breininger, D. R.; Larson, V. L.; Schaub, R.; Duncan, B. W.; Schmalzer, P. A.; Oddy, D. M.; Smith, R. B.; Adrian, F.; Hill, H., Jr. 1996. A conservation strategy for the Florida scrub-jay on John F. Kennedy Space Center/Merritt Island National Wildlife Refuge: an initial scientific basis for recovery. NASA-TM-111676. Kennedy Space Center, FL: National Aeronautics and Space Administration, John F. Kennedy Space Center. 113 p. [85882]
17. Breininger, D. R.; Oddy, D. M.; Legare, M. L.; Duncan, B. W. 1999. Developing biological criteria for the recovery of Florida scrub-jay populations on public lands in Brevard County: patterns of fire history, habitat fragmentation, habitat use and demography. Final report: Contract No. 1448-40181-97-C-002. Jacksonville, FL: U.S. Fish and Wildlife Service, Endangered Species Office. 97 p. [85921]
18. Breininger, D. R.; Schmalzer, P. A. 1990. Effects of fire and disturbance on plants and birds in Florida oak/ palmetto scrub community. The American Midland Naturalist. 123(1): 64-74. [9875]
19. Breininger, D. R.; Schmalzer, P. A.; Rydene, D. A.; Hinkle, C. R. 1988. Burrow and habitat relationships of the gopher tortoise in coastal scrub and slash pine flatwoods on Merritt Island, Florida. Final report: Project GFC-84-016. Tallahassee, FL: Florida Game and Fresh Water Fish Commission, Nongame Wildlife Program. 238 p. [74246]
20. Breininger, David R. 1992. Birds of swale marshes on John F. Kennedy Space Center. Florida Field Naturalist. 20(2): 36-41. [21095]
21. Breininger, David R. 1999. Florida scrub-jay demography and dispersal in a fragmented landscape. The Auk. 116(2): 520-527. [85030]
22. Breininger, David R. 2004. An adaptive approach to managing Florida scrub-jay habitat. NASA/TM-2004-211532. Kennedy Space Center, FL: National Aeronautics and Space Administration. 33 p. [86203]
23. Breininger, David R.; Burgman, Mark A.; Stith, Bradley M. 1999. Influence of habitat quality, catastrophes, and population size on extinction risk of the Florida scrub-jay. Wildlife Society Bulletin. 27(3): 810-822. [37574]
24. Breininger, David R.; Carter, Geoffrey M. 2003. Territory quality transitions and source-sink dynamics in a Florida scrub-jay population. Ecological Applications. 13(2): 516-529. [85021]
25. Breininger, David R.; Duncan, Brean W.; Dominy, Nathaniel J. 2002. Relationships between fire frequency and vegetation type in pine flatwoods of east-central Florida, USA. Natural Areas Journal. 22(3): 186-193. [43152]
26. Breininger, David R.; Larson, Vickie L.; Duncan, Brean W.; Smith, Rebecca B. 1998. Linking habitat suitability to demographic success in Florida scrub-jays. Wildlife Society Bulletin. 26(1): 118-128. [85044]
27. Breininger, David R.; Larson, Vickie L.; Duncan, Brean W.; Smith, Rebecca B.; Oddy, Donna M.; Goodchild, Michael F. 1995. Landscape patterns of Florida scrub jay habitat use and demographic success. Conservation Biology. 9(6): 1442-1453. [29090]
28. Breininger, David R.; Larson, Vickie L.; Oddy, Donna M.; Smith, Rebecca B.; Barkaszi, Mary Jo. 1996. Florida scrub-jay demography in different landscapes. The Auk. 113(3): 617-625. [85025]
29. Breininger, David R.; Nichols, James D.; Carter, Geoffrey M.; Oddy, Donna M. 2009. Habitat-specific breeder survival of Florida scrub-jays: inferences from multistate models. Ecology. 90(11): 3180-3189. [85031]
30. Breininger, David R.; Nichols, James D.; Duncan, Brean W.; Stolen, Eric D.; Carter, Geoffrey M.; Hunt, Danny K.; Drese, John H. 2010. Multistate modeling of habitat dynamics: factors affecting Florida scrub transition probabilities. Ecology. 91(11): 3354-3364. [85022]
31. Breininger, David R.; Oddy, Donna M. 2001. Fire and Florida scrub-jay source-sink dynamics in mesic flatwoods. In: Zattau, Dawn P., ed. Proceedings of the Florida scrub symposium 2001; 2001 June 5-7; Orlando, FL. Jacksonville, FL: U.S. Fish and Wildlife Service: 3-7. [85318]
32. Breininger, David R.; Oddy, Donna M. 2004. Do habitat potential, population density, and fires influence scrub-jay source-sink dynamics? Ecological Applications. 14(4): 1079-1089. [85024]
33. Breininger, David R.; Smith, Rebecca B. 1992. Relationships between fire and bird density in coastal scrub and slash pine flatwoods in Florida. The American Midland Naturalist. 127(2): 233-240. [17993]
34. Breininger, David R.; Stolen, Eric D.; Carter, Geoffrey M.; Oddy, Donna M.; Hunt, Danny K. 2010. A model-selection approach to predicting whether Florida scrub-jays delay breeding. The Condor. 112(2): 378-389. [85023]
35. Breininger, David R.; Toland, Brian; Oddy, Donna M.; Legare, Michael L. 2005. Landcover characterizations and Florida scrub-jay (Aphelocoma coerulescens) population dynamics. Biological Conservation. 128: 169-181. [85035]
36. Campbell, Howard W.; Christman, Stephen P. 1982. The herpetological components of Florida sandhill and sand pine scrub associations. In: Scott, Norman J., Jr., ed. Herpetological communities: a symposium of the Society for the Study of Amphibians and Reptiles and the Herpetologists' League: Proceedings; 1977 August; [Meeting location unknown]. Wildlife Research Report 13. Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service: 163-171. [28592]
37. Carter, Geoffrey M.; Breininger, David R.; Stolen, Eric D.; Oddy, Donna M. 2011. Determinants of nest survival in a manged Florida scrub-jay population. The Condor. 113(3): 629-636. [84846]
38. Carter, Geoffrey M.; Legare, Mike L.; Breininger, David R.; Oddy, Donna M. 2007. Nocturnal nest predation: a potential obstacle to recovery of a Florida scrub-jay population. Journal of Field Ornithology. 78(4): 390-394. [85340]
39. Chandler, Craig; Cheney, Phillip; Thomas, Philip; Trabaud, Louis; Williams, Dave. 1983. Fire in forestry: Vol. I. Forest fire behavior and effects. New York: John Wiley & Sons. 450 p. [12241]
40. Chen, Linus Y. 2001. Cost savings from properly managing endangered species habitats. Natural Areas Journal. 21(2): 197-203. [40130]
41. Christensen, Norman L. 1981. Fire regimes in southeastern ecosystems. In: Mooney, H. A.; Bonnicksen, T. M.; Christensen, N. L.; Lotan, J. E.; Reiners, W. A., technical coordinators. Fire regimes and ecosystem properties: Proceedings of the conference; 1978 December 11-15; Honolulu, HI. Gen. Tech. Rep. WO-26. Washington, DC: U.S. Department of Agriculture, Forest Service: 112-136. [4391]
42. Christensen, Norman L. 1985. Shrubland fire regimes and their evolutionary consequences. In: Pickett, S. T. A.; White, P. S., eds. The ecology of natural disturbance and patch dynamics. Orlando, FL: Academic Press: 85-100. [12152]
43. Coulon, A.; Fitzpatrick, J. W.; Bowman, R.; Stith; B. M.; Makarewich, A.; Stenzler, L. M.; Lovette, I. J. 2008. Congruent population structure inferred from dispersal behaviour and intensive genetic surveys of the threatened Florida scrub-jay (Aphelocoma coerulescens). Molecular Ecology. 17(7): 1685-1701. [84852]
44. Coulon, Aurelie; Fitzpatrick, John W.; Bowman, Reed; Lovette, Irby J. 2010. Effects of habitat fragmentation on effective dispersal of Florida scrub-jays. Conservation Biology. 24(4): 1080-1088. [85333]
45. Cowan, Ernest M. 2005. Reproductive success, territory size, and predation pressures of the Florida scrub-jay (Aphelocoma coerulescens) at Savannas Preserve State Park. Boca Raton, FL: Florida Atlantic University. 63 p. Thesis. [85877]
46. Cox, Jeffrey A. 1984. Distribution, habitat, and social organization of the Florida scrub jay, with a discussion of the coopertive breeding in New World jays. Gainesville, FL: University of Florida. 271 p. Dissertation. [17410]
47. Curry, Robert L.; Hannon, Michelle E.; Fitzpatrick, John W.; Woolfenden, Glen E. 1992. Correlates of succession in unburned scrub: what prevents scrub jays from persisting? In: History and ecology of the Florida scrub: Program and abstracts; 1992 April 29-May 2; Lake Placid, FL. Lake Placid, FL: Archbold Biological Station: [page unknown]. Abstract. [84855]
48. Custer, George; Thorsen, James. 1996. Stand-replacement burn in the Ocala National Forest--a success. Fire Management Notes. 56(2): 7-12. [50617]
49. Davison, Marita A.; Fitzpatrick, John W. 2010. Role of human-modified habitat in protecting specialist species: a case study in the threatened Florida scrub-jay. Biological Conservation. 143(11): 2815-2822. [81405]
50. DeGange, Anthony R.; Fitzpatrick, John W.; Layne, James N.; Woolfenden, Glen E. 1989. Acorn harvesting by Florida scrub-jays. Ecology. 70(2): 348-356. [85346]
51. Dickson, James G. 2002. Fire and bird communities in the South. In: Ford, W. Mark; Russell, Kevin R.; Moorman, Christopher E., eds. The role of fire in nongame wildlife management and community restoration: traditional uses and new directions: Proceedings of a special workshop; 2000 December 15; Nashville, TN. Gen. Tech. Rep. NE-288. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northeastern Research Station: 52-57. [41557]
52. Duncan, Brean W. 2009. Native fire regime as a reference for establishing management practices. Orlando, FL: University of Central Florida. 171 p. Dissertation. [85945]
53. Duncan, Brean W.; Boyle, Shannon; Breininger, David R.; Schmalzer, Paul A. 1999. Coupling past management practice and historic landscape change on John F. Kennedy Space Center, Florida. Landscape Ecology. 14: 291-309. [85926]
54. Duncan, Brean W.; Breininger, David R.; Schmalzer, Paul A.; Larson, Vickie L. 1995. Validating a Florida scrub jay habitat suitability model using demographic data on Kennedy Space Center. Photogrammetric Engineering and Remote Sensing. 61(11): 1361-1370. [84856]
55. Duncan, Brean W.; Larson, Vickie L.; Schmalzer, Paul A. 2004. Historic landcover and recent landscape change in the north Indian River Lagoon watershed, Florida, USA. Natural Areas Journal. 24(3): 198-215. [84857]
56. Duncan, Brean W.; Schmalzer, Paul A. 2004. Anthropogenic influences on potential fire spread in a pyrogenic ecosystem of Florida, USA. Landscape Ecology. 19: 153-165. [47826]
57. Faulhaber, Craig. 2012. [Personal communication]. November 5. Regarding Florida scrub-jay response to fire and management of Florida scrub-jay habitat. Ocala, FL: Florida Fish and Wildlife Conservation Commission. On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [86240]
58. Finch, Deborah M.; Ganey, Joseph L.; Yong, Wang; Kimball, Rebecca T.; Sallabanks, Rex. 1997. Effects and interactions of fire, logging, and grazing. In: Block, William M.; Finch, Deborah M., tech. eds. Songbird ecology in southwestern ponderosa pine forests: a literature review. Gen. Tech. Rep. RM-GTR-292. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 103-136. [27990]
59. Fitzpatrick, John W.; Woolfenden, Glen E. 1986. Demographic routes to cooperative breeding in some New World jays. In: Nitecki, Matthew H.; Kitchell, Jennifer A., eds. Evolution of animal behavior: Paleontological and field approaches. New York: Oxford University Press: 137-160. [84885]
60. Fitzpatrick, John W.; Woolfenden, Glen E.; Bowman, Reed. 1999. Dispersal distance and its demographic consequences in the Florida scrub-jay. In: Adams, Nigel J.; Slotow, Robert H., eds. Proceedings, 22nd international ornithological congress; 1998 August; Durban, South Africa. Johannesburg, South Africa: Bird Life: 2465-2479. Available online: http://www.int-ornith-union.org/files/proceedings/durban/Symposium/S41/S41.2.htm. [84859]
61. Fitzpatrick, John W.; Woolfenden, Glen E.; Kopeny, Mark T. 1991. Ecology and development-related habitat requirements of the Florida scrub-jay (Aphelocoma coerulescens coerulescens). Nongame Wildlife Program Technical Report No. 8. Tallahassee, FL: Florida Game and Fresh Water Fish Commission. 49 p. [85317]
62. Fleischer, Arthur L., Jr.; Bowman, Reed; Woolfenden, Glen E. 2003. Variation in foraging behavior, diet, and time of breeding of Florida scrub-jays in suburban and wildland habitats. The Condor. 105(3): 515-527. [85348]
63. Foster, Tammy E.; Schmalzer, Paul A. 2003. The effect of season of fire on the recovery of Florida scrub, [Online]. In: Proceedings, 2nd international wildland fire ecology and fire management congress held concurrently with the 5th symposium on fire and forest meteorology; 2003 November 16-20; Orlando, FL. Boston, MA: American Meteorology Society (Producer): available online https://ams.confex.com/ams/pdfpapers/65301.pdf [2012, September 5]. [85948]
64. Fox, Gordon A.; Kendall, Bruce E.; Fitzpatrick, John W.; Woolfenden, Glen E. 2006. Consequences of heterogeneity in survival probability in a population of Florida scrub-jays. Journal of Animal Ecology. 75: 921-927. [85335]
65. Francis, Ann Marie; Hailman, Jack P.; Woolfenden, Glen E. 1989. Mobbing by Florida scrub jays: behaviour, sexual asymmetry, role of helpers and ontogeny. Animal Bahaviour. 38: 795-816. [16520]
66. Franzreb, Kathleen E. 2007. Reproductive success and nest depredation of the Florida scrub-jay. The Wilson Journal of Ornithology. 119(2): 162-169. [85314]
67. Franzreb, Kathleen E.; Zarnoch, Stanley J. 2011. Factors affecting Florida scrub-jay nest survival on Ocala National Forest, Florida. Journal of Wildlife Management. 75(5): 1040-1050. [85313]
68. Gipe, Todd Gregory. 1987. Effects of prescribed burning on the suitability of habitat for the Florida scrub jay Aphelocoma coerulescens coerulescens (Bosc). Melbourne, FL: Florida Institute of Technology. 49 p. Thesis. [62065]
69. Greenberg, Cathryn H.; Harris, Lawrence D.; Neary, Daniel G. 1995. A comparison of bird communities in burned and salvage-logged, clearcut, and forested Florida sand pine scrub. The Wilson Bulletin. 107(1): 40-54. [26024]
70. Grubb, Thomas C., Jr.; Woolfenden, Glen, E.; Fitzpatrick, John W. 1998. Factors affecting nutritional conditions of fledgling Florida scrub-jays: a ptilochronology approach. The Condor. 100(4): 753-756. [85864]
71. Hann, Wendel; Havlina, Doug; Shlisky, Ayn; [and others]. 2010. Interagency fire regime condition class (FRCC) guidebook, [Online]. Version 3.0. In: FRAMES (Fire Research and Management Exchange System). National Interagency Fuels, Fire & Vegetation Technology Transfer (NIFTT) (Producer). Available: http://www.fire.org. [81749]
72. Huff, Mark H.; Smith, Jane Kapler. 2000. Fire effects on animal communities. In: Smith, Jane Kapler, ed. Wildland fire in ecosystems: Effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 35-42. [44446]
73. Huffman, Mary R.; Morrison, S. C.; Peterson, A. R.; Pace-Aldana, B. 2003. The Florida scrub-jay fire strike team, [Online]. In: Proceedings, 2nd international wildland fire ecology and fire management congress held concurrently with the 5th symposium on fire and forest meteorology; 2003 November 16-20; Orlando, FL. Boston, MA: American Meteorology Society (Producer). Abstract. Available: https://ams.confex.com/ams/FIRE2003/techprogram/paper_67091.htm [2012, March 23]. [85946]
74. Huffman, Mary; Hebb, Mark. 2001. The Florida Scrub-jay Fire Strike Team: working together to burn the overgrown scrub. In: Zattau, Dawn P., ed. Proceedings of the Florida scrub symposium 2001; 2001 June 5-7; Orlando, FL. Jacksonville, FL: U.S. Fish and Wildlife Service: 52. Abstract. [85925]
75. Hutchinson, Jeffrey T; Menges, Eric S.; Pickert, Roberta L.; Swain, Hilary M. 2003. Fire management at Archbold Biological Station: burning to promote heterogeneity, conservation, research, and education, [Online]. In: Proceedings, 2nd international wildland fire ecology and fire management congress held concurrently with the 5th symposium on fire and forest meteorology; 2003 November 16-20; Orlando, FL. Boston, MA: American Meteorology Society (Producer). Available: https://ams.confex.com/ams/pdfpapers/65539.pdf [2012, September 5]. [85950]
76. James, Frances C. 1999. Lessons learned from a study of habitat conservation planning. BioScience. 49(11): 871-874. [84861]
77. Johnson, Ann F.; Abrahamson, Warren G. 2002. Stem turnover in the rhizomatous scrub oak, Quercus inopina, from south-central Florida. The American Midland Naturalist. 147(2): 237-246. [45940]
78. Johnson, Fred A.; Breininger, David R.; Duncan, Brean W.; Nichols, James D.; Runge, Michael C; Williams, B. Ken. 2011. A Markov decision process for managing habitat for Florida scrub-jays. Journal of Fish and Wildlife Management. 2(2): 234-246. [86179]
79. Johnson, Steve A.; Miller, Karl E.; Blunden, Travis. 2009. The Florida scrub-jay: a species in peril, [Online]. WEC261. Gainesville, FL: University of Florida, Institute of Food and Agricultural Sciences, Florida Cooperative Extension Service, Wildlife Ecology and Conservation Department (Producer). Available: http://edis.ifas.ufl.edu/uw306 [2012, September 5]. [85904]
80. Keating, William J. 1999. Nocturnal roosting behavior of the Florida scrub-jay. Tampa, FL: University of South Florida. 67 p. Thesis. [84862]
81. Kent, Adam; Kindell, Carolyn. 2010. Scrub management guidelines for peninsular Florida: using the scrub-jay as an umbrella species, [Online]. Tallahassee, FL: Florida Natural Areas Inventory, Division of Habitat and Species Conservation. 10 p. In: Central Florida Cooperative Invasive Species Management Area--Educational resources. Forida Invasive Species Partnership (Producer). Available: http://www.floridainvasives.org/central/ScrubMgmt_6-09.pdf [2012, November 15]. [86270]
82. Khodadad, Christina Louise Myers. 2008. Genetic variation, population substructure, and sex ratio of the Florida scrub-jay, Aphelocoma coerulescens coerulescens. Melbourne, FL: Florida Institute Of Technology. 128 p. Dissertation. [85863]
83. Kulahci, Ipek G.; Bowman, Reed. 2011. Recaching decisions of Florida scrub-jays are sensitive to ecological conditions. Ethology. 117: 700-707. [85347]
84. LANDFIRE Rapid Assessment. 2005. Reference condition modeling manual (Version 2.1), [Online]. In: LANDFIRE. Cooperative Agreement 04-CA-11132543-189. Boulder, CO: The Nature Conservancy; U.S. Department of Agriculture, Forest Service; U.S. Department of the Interior (Producers). 72 p. Available: https://www.landfire.gov/downloadfile.php?file=RA_Modeling_Manual_v2_1.pdf [2007, May 24]. [66741]
85. LANDFIRE Rapid Assessment. 2007. Rapid assessment reference condition models, [Online]. In: LANDFIRE. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Lab; U.S. Geological Survey; The Nature Conservancy (Producers). Available: https://www.landfire.gov/models_EW.php [2008, April 18] [66533]
86. LeClair, Sonya Christine. 2005. Comparison of hatching failure in a wildland and suburban population of the Florida scrub-jay (Aphelocoma coerulescens). Tampa, FL: University of South Florida. 51 p. Thesis. Available online: http://scholarcommons.usf.edu/etd/736 [2012, September 5]. [85328]
87. Leenhouts, Bill. 1997. Number of Florida scrub jays detected along census routes in habitats with different fire severities. In: Greenlee, Jason M., ed. Proceedings, 1st conference on fire effects on rare and endangered species and habitats; 1995 November 13-16; Coeur d'Alene, ID. Fairfield, WA: International Association of Wildland Fire: 77-79. [28125]
88. Leenhouts, Bill. [n.d.]. Fire effects on the Florida scrub jay at the Merritt Island National Wildlife Refuge, Florida. Boise, ID: U.S. Fish and Wildlife Service; Boise Interagency Fire Center. Unpublished manuscript on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT. 9 p. [18491]
89. Lyon, L. Jack; Huff, Mark H.; Telfer, Edmund S.; Schreiner, David Scott; Smith, Jane Kapler. 2000. Fire effects on animal populations. In: Smith, Jane Kapler, ed. Wildland fire in ecosystems: Effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 25-34. [44436]
90. Lyon, L. Jack; Telfer, Edmund S.; Schreiner, David Scott. 2000. Direct effects of fire and animal responses. In: Smith, Jane Kapler, ed. Wildland fire in ecosystems: Effects of fire on fauna. Gen. Tech. Rep. RMRS-GTR-42-vol. 1. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 17-23. [44435]
91. Main, Kevin N.; Menges, Eric S. 1997. Archbold Biological Station Station Fire Management Plan. Land Management Publication 97-1. Lake Placid, FL: Archbold Biological Station. 96 p. [85943]
92. McDonald, David B.; Potts, Wayne K; Fitzpatrick, John W.; Woolfenden, Glen E. 1999. Contrasting genetic structures in sister species of North American scrub-jays. Proceedings of the Royal Society of London: Series B. 266: 1117-1125. [85918]
93. Menges, Eric S.; Gordon, Doria R. 2010. Should mechanical treatments and herbicides be used as fire surrogates to manage Florida's uplands? A review. Florida Scientist. 73(2): 147-174. [85928]
94. Menges, Eric S.; Kohfeldt, Nancy. 1995. Life history strategies of Florida scrub plants in relation to fire. Bulletin of the Torrey Botanical Club. 122(4): 282-297. [26976]
95. Menges, Eris S. 1999. Ecology and conservation of Florida scrub. In: Anderson, Roger C.; Fralish, James S.; Baskin, Jerry M., eds. Savannas, barrens, and rock outcrop plant communities of North America. Boston, MA: Cambridge University Press: 7-22. [84866]
96. Morgan, Gina M.; Boughton, Raoul K.; Rensel, Michelle A.; Schoech, Stephan J. 2010. Road effects on food availability and energetic intake in Florida scrub-jays (Aphelocoma coerulescens). The Auk. 127(3): 581-589. [85341]
97. Morgan, M. C. ; Morgan, Amanda. 1997. A case of hybridization. Living Bird. 16(1): 33. [84867]
98. Mumme, Ronald L. 1992. Do helpers increase reproductive success? An experimental analysis in the Florida scrub-jay. Behavioral Ecology and Sociobiology. 31(5): 319-328. [85032]
99. Mumme, Ronald L.; Below, Theodore H. 1999. Evaluation of translocation for the threatened Florida scrub-jay. The Journal of Wildlife Management. 63(3): 833-842. [85919]
100. Mumme, Ronald L.; Schoech, Stephan J.; Woolfenden, Glen E.; Fitzpatrick, John W. 2000. Life and death in the fast lane: demographic consequences of road mortality in the Florida scrub-jay. Conservation Biology. 14(2): 501-512. [85334]
101. Myers, Ronald L. 1990. Scrub and high pine. In: Myers, Ronald L.; Ewel, John J., eds. Ecosystems of Florida. Orlando, FL: University of Central Florida Press: 150-193. [17389]
102. Myers, Ronald L.; Ewel, John J. 1990. Problems, prospects, and strategies for conservation. In: Myers, Ronald L.; Ewel, John J., eds. Ecosystems of Florida. Orlando, FL: University of Central Florida Press: 619-632. [17397]
103. Reynolds, S. James; Schoech, Stephan J.; Bowman, Reed. 2003. Diet quality during pre-laying and nestling periods influences growth and survival of Florida scrub-jay (Aphelocoma coerulescens) chicks. Journal of Zoology. 261: 217-226. [85351]
104. Reynolds, S. James; Schoech, Stephan J.; Bowman, Reed. 2003. Nutritional quality of prebreeding diet influences breeding performance of the Florida scrub-jay. Oecologia. 134(3): 308-316. [85330]
105. Robbins, Louise E.; Myers, Ronald L. 1992. Seasonal effects of prescribed burning in Florida: a review. Misc. Publ. No. 8. Tallahassee, FL: Tall Timbers Research. 96 p. [21094]
106. Root, Karen V. 1998. Evaluating the effects of habitat quality, connectivity, and catastrophes on a threatened species. Ecological Applications. 8(3): 854-865. [85906]
107. Sauter, Annette; Bowman, Reed; Schoech, Stephan J.; Pasinelli, Gilberto. 2006. Does optimal foraging theory explain why suburban Florida scrub-jays (Aphelocoma coerulescens) feed their young human-provided food? Behavioral Ecology and Sociobiology. 60(4): 465-474. [84870]
108. Schmalzer, Paul A. 1993. Characteristics of long unburned stands of oak-saw palmetto scrub. Bulletin of the Ecological Society of America . 74(2): 428. (Supplement). Abstract. [22944]
109. Schmalzer, Paul A. 1994. Restoring unburned shrublands: an example using cutting and burning in oak-saw palmetto scrub. In: Science and public policy: Proceedings, 79th annual meeting of the Ecological Society of America; 1994 August 7-11; Knoxville, TN. In: Bulletin of the Ecological Society of America. 75(2): 206. (Supplement). Abstract. [23745]
110. Schmalzer, Paul A. 2003. Growth and recovery of oak-saw palmetto scrub through ten years after fire. Natural Areas Journal. 23(1): 5-13. [43567]
111. Schmalzer, Paul A.; Adrian, Frederic W. 2001. Scrub restoration on Kennedy Space Center/Merritt Island National Wildlife Refuge, 1992-2000. Zattau, Dawn P., ed. Proceedings of the Florida scrub symposium 2001; 2001 June 5-7; Orlando, FL. Jacksonville, FL: U.S. Fish and Wildlife Service: 17-20. [85858]
112. Schmalzer, Paul A.; Boyle, Shannon R. 1998. Restoring long-unburned oak-saw palmetto scrub requires mechanical cutting and prescribed burning. Restoration and Management Notes. 16: 96-97. [84872]
113. Schmalzer, Paul A.; Boyle, Shannon R.; Swain, Hilary M. 1999. Scrub ecosystems of Brevard County, Florida: a regional characterization. Florida Scientist. 62(1): 13-47. [84871]
114. Schmalzer, Paul A.; Breininger, David R.; Adrian, Frederic W.; Schaub, Ron; Duncan, Brean W. 1994. Development and implementation of a scrub habitat compensation plan for Kennedy Space Center. Technical Memorandum 109202. Washington, DC: National Aeronautics and Space Administration. 55 p. [85929]
115. Schmalzer, Paul A.; Hinkle, C. Ross. 1992. Recovery of oak-saw palmetto scrub after fire. Castanea. 57(3): 158-173. [19718]
116. Schmalzer, Paul A.; Hinkle, C. Ross. 1992. Species composition and structure of oak-saw palmetto scrub vegetation. Castanea. 57(4): 220-251. [21018]
117. Schmalzer, Paul A.; Hinkle, C. Ross. 1996. Biomass and nutrients in aboveground vegetation and soils of Florida oak-saw palmetto scrub. Castanea. 61(2): 168-193. [28634]
118. Schmalzer, Paul A.; Hinkle, C. Ross; Mailander, Joseph L. 1991. Changes in species composition and biomass in Juncus roemerianus Scheele and Spartina bakeri Merr. marshes one year after fire. Wetlands. 11(1): 67-86. [85972]
119. Schoech, S. J.; Bowman, R.; Reynolds, S. J. 2004. Corticosterone, nutrition, and timing of reproduction in Florida scrub-jays. Hormones and Behavior. 46(1): 121. Abstract. [84874]
120. Schoech, Stephan J.; Bridge, Eli S.; Boughton, Raoul K.; Reynolds, S. James; Atwell, Jonathan W.; Bowman, Reed. 2008. Food supplementation: a tool to increase reproductive output? A case study in the threatened Florida scrub-jay. Biological Conservation. 141: 162-173. [85331]
121. Shao, Guofan; Duncan, Brean W. 2007. Effects of band combinations and GIS masking on fire-scar mapping at local scales in east-central Florida, USA. Canadian Journal of Remote Sensing. 33(4): 250-259. [85947]
122. Shawkey, Matthew D.; Bowman, Reed; Woolfenden, Glen E. 2004. Why is brood reduction in Florida scrub-jays higher in suburban than in wildland habitats? Canadian Journal of Zoology. 82: 1427-1435. [85329]
123. Smith, Theresa E. 2007. Territory size of the Florida scrub-jay (Aphelocoma coerulescens) at Savannas Preserve State Park. Jupiter, FL: Florida Atlantic University. 38 p. Senior thesis. [85941]
124. Stith, Bradley M. 2001. Metapopulation viability analysis of the Florida scrub-jay. Zattau, Dawn P., ed. Proceedings of the Florida scrub symposium 2001; 2001 June 5-7; Orlando, FL. Jacksonville, FL: U.S. Fish and Wildlife Service: 1-2. [85923]
125. Stith, Bradley M.; Fitzpatrick, John W.; Woolfenden, Glen E.; Pranty, Bill. 1996. Classification and conservation of metapopulations: a case study of the Florida scrub jay. In: McCullough, Dale R., ed. Metapopulations and wildlife conservation. Washington DC: Island Press: 187-215. [28045]
126. Stith, Bradley Morris. 1999. Metapopulation dynamics and landscape ecology of the Florida scrub-jay, Aphelocoma coerulescens. Gainesville, FL: University of Florida. 383 p. Dissertation. [85315]
127. Stout, I. Jack; Marion, Wayne R. 1993. Pine flatwoods and xeric pine forests of the southern (lower) Coastal Plain. In: Martin, William H.; Boyce, Stephen G.; Echternacht, Arthur C., eds. Biodiversity of the southeastern United States: Lowland terrestrial communities. New York: John Wiley & Sons: 373-446. [22015]
128. Suazo, Alexis A.; Fauth, John E.; Roth, James D.; Parkinson, Christopher L.; Stout, I. Jack. 2009. Responses of small rodents to habitat restoration and management for the imperiled Florida scrub-jay. Biological Conservation. 142: 2322-2328. [85927]
129. Thaxton, J. E.; Hingtgen, T. M. 1996. Effects of suburbanization and habitat fragmentation on Florida scrub-jay dispersal. Florida Field Naturalist. 24(2): 25-37. [85332]
130. The Nature Conservancy. 2010. Jay Watch: Annual report 2010, [Online]. In: Jay Watch: Monitoring Florida's only endemic bird. In: Florida--Volunteer. Arlington, VA: The Nature Conservancy (Producer). Available: http://www.nature.org/ourinitiatives/regions/northamerica/unitedstates/florida/jay-watch-report-2010.pdf [2012, September 13]. [85905]
131. U. S. Fish and Wildlife Service. 2007. Florida scrub-jay umbrella Habitat Conservation Plan and Environmental Assessment, [Online]. In: Key Florida species. In: Jacksonville, FL: U.S. Fish and Wildlife Service, Southeast Region, North Florida Ecological Services Office (Producer). Available: http://www.fws.gov/northflorida/Scrub-Jays/Docs/Umbrella/20110800_ver_FSJ_Umbrella_HCP_EA.pdf [2012, March 19]. [85342]
132. U.S. Department of the Interior, Fish and Wildlife Service. 2016. Endangered Species Program, [Online]. Available: http://www.fws.gov/endangered/. [86564]
133. U.S. Fish and Wildlife Service, Southeast Region, Ecological Services,. 2007. Florida scrub-jay (Aphelocoma coerulescens)--5-year review: Summary and evaluation, [Online]. In: Five-year reviews for federally listed species. In: Endangered species recovery program. Jacksonville, FL: Ecosystem Services Office (Producer). Available: http://www.fws.gov/southeast/5yearReviews/5yearreviews/Florida-scrub-jay.pdf [2012, March 23]. [85344]
134. Walton, Lee Mitchell. 1997. Florida scrub-jay nest-site selectivity in unfragmented, periodically burned vs. fragmented, overgrown habitat. Villanova, PA: Villanova University. 144 p. Thesis. [84878]
135. Watchman, Laura H.; Groom, Martha; Perrine, John D. 2001. Science and uncertainty in habitat conservation planning. American Scientist. 89: 351-359. [84879]
136. Webber, H. J. 1935. The Florida scrub, a fire-fighting association. American Journal of Botany. 22: 344-361. [18493]
137. Weekley, Carl W.; Menges, Eric S.; Berry-Greenlee, Dawn; Rickey, Marcia A.; Clarke, Gretel L.; Smith, Stacy A. 2011. Burning more effective than mowing in restoring Florida scrub. Ecological Restoration. 29(4): 357-373. [84692]
138. Westcott, Peter Walter. 1970. Ecology and behavior of the Florida scrub jay. Gainesville, FL: The University of Florida. 94 p. Dissertation. [17411]
139. Whitney, Ellie; Means, D. Bruce; Rudloe, Anne. 2004. Interior scrub. In: Priceless Florida: Natural ecosystems and native species. Sarasota, FL: Pineapple Press: 67-84. [84714]
140. Wilcoxen, Travis E.; Boughton, Raoul K.; Schoech, Stephan J. 2010. Selection on innate immunity and body condition in Florida scrub-jays throughout an epidemic. Biology Letters. 6: 552-554. [85345]
141. Wilcoxen, Travis E.; Bridge, Eli S.; Boughton, Raoul K.; Rensel, Michelle A.; Reynolds, S. James; Schoech, Stephan J. 2011. Parental, social, and environmental factors associated with hatching failure in Florida scrub-jays, (Aphelocoma coerulescens). Ibis. 153: 70-77. [85323]
142. Wilkinson, Todd Stewart. 1989. Nest site vegetational characteristics of the Florida scrub-jay in disturbed and undisturbed areas. Melbourne, FL: Florida Institute of Technology. 66 p. Thesis. [84881]
143. Woolfenden, Glen E. 1973. Nesting and survival in a population of Florida scrub jays. Living Bird. 12: 25-49. [16723]
144. Woolfenden, Glen E.; Fitzpatrick, John W. 1984. The Florida scrub-jay: Demography of a cooperative breeding bird. Princeton, NJ: Princeton University Press. 406 p. [84813]
145. Woolfenden, Glen E.; Fitzpatrick, John W. 1991. Florida scrub jay ecology and conservation. In: Perrins, C. M.; Lebreton, J. D.; Hirons, G. J. M., eds. Bird population studies: relevance to conservation and management. New York: Oxford University Press: 542-565. [84886]
146. Woolfenden, Glen E.; Fitzpatrick, John W. 1996. Florida scrub-jay (Aphelocoma coerulescens). In: Poole, A.; Gill, F., eds. The birds of North America. No. 228. Philadelphia, PA: The Acedemy of Natural Sciences; Washington, DC: The American Ornithologists' Union: 1-28. Available online: http://bna.birds.cornell.edu/bna/species/228 [2012, June 21]. [84791]